Abstract
Background
Mass vaccination campaigns to prevent coronavirus disease 2019 (Covid-19) are occurring in many countries; estimates of vaccine effectiveness are urgently needed to support decision making. A countrywide mass vaccination campaign with the use of an inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (CoronaVac) was conducted in Chile starting on February 2, 2021.
Methods
We used a prospective national cohort, including participants 16 years of age or older who were affiliated with the public national health care system, to assess the effectiveness of the inactivated SARS-CoV-2 vaccine with regard to preventing Covid-19 and related hospitalization, admission to the intensive care unit (ICU), and death. We estimated hazard ratios using the extension of the Cox proportional-hazards model, accounting for time-varying vaccination status. We estimated the change in the hazard ratio associated with partial immunization (≥14 days after receipt of the first dose and before receipt of the second dose) and full immunization (≥14 days after receipt of the second dose). Vaccine effectiveness was estimated with adjustment for individual demographic and clinical characteristics.
Results
The study was conducted from February 2 through May 1, 2021, and the cohort included approximately 10.2 million persons. Among persons who were fully immunized, the adjusted vaccine effectiveness was 65.9% (95% confidence interval [CI], 65.2 to 66.6) for the prevention of Covid-19 and 87.5% (95% CI, 86.7 to 88.2) for the prevention of hospitalization, 90.3% (95% CI, 89.1 to 91.4) for the prevention of ICU admission, and 86.3% (95% CI, 84.5 to 87.9) for the prevention of Covid-19–related death.
Conclusions
Our results suggest that the inactivated SARS-CoV-2 vaccine effectively prevented Covid-19, including severe disease and death, a finding that is consistent with results of phase 2 trials of the vaccine. (Funded by Agencia Nacional de Investigación y Desarrollo and others.)
The coronavirus disease 2019 (Covid-19) pandemic has imposed an enormous disease burden worldwide, with more than 159 million cases and approximately 3.3 million deaths reported as of May 10, 2021.1 Covid-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and the severity ranges from mild symptoms to life-threatening disease.2 Older age and underlying conditions substantially increase the case fatality rate.3,4 Nonpharmaceutical interventions, such as social distancing, face masks, and contact tracing, have so far been the mainstay of health policy strategies to reduce viral spread and limit demands on health care.5,6 New Covid-19 vaccines are beginning to change this situation. On December 2, 2020, the first vaccine tested in a large, randomized clinical trial was approved in the United Kingdom,7,8 although some countries began vaccinations before clinical results were available. Several effective vaccines against Covid-19 have been developed and approved in record time,8-12 and numerous new vaccines are in the final stages of clinical trials.13
Mass vaccination campaigns to prevent Covid-19 are now occurring in many countries.14 Preliminary results of the effectiveness of other Covid-19 vaccines across different populations have been published, including studies at the national level in Israel15 and Scotland16 and studies involving essential frontline workers at specific locations in the United States.17-19 Estimates of vaccine effectiveness in the prevention of Covid-19 are essential because they reflect real-world challenges, such as logistics, cold chains, vaccination schedules, and follow-up, and also involve more diverse populations than those selected in randomized clinical trials, such as older or immunocompromised persons or those with coexisting conditions. Despite being the standard for assessing vaccine efficacy, phase 3 clinical trials have some limitations, such as restrictive inclusion criteria and implementation under strict experimental conditions that may not resemble a mass vaccination rollout.20 Thus, large observational studies to estimate the effectiveness of new vaccines in real-world settings are an essential complement to randomized, controlled trials.21
Existing vaccine-effectiveness estimates have focused on the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech), the ChAdOx1 nCoV-19 vaccine (Oxford–AstraZeneca), and the mRNA-1273 vaccine (Moderna).15-19 Several countries are conducting vaccination campaigns with the use of an inactivated SARS-CoV-2 vaccine (CoronaVac) amid a record surge of Covid-19 cases worldwide.1,13 A total of 22 primarily low- and middle-income countries have approved the CoronaVac vaccine for emergency use. Despite its global importance, limited evidence is available on the efficacy or effectiveness of this vaccine.
Phase 1–2 trials of the CoronaVac vaccine22 were carried out in China among participants 18 to 59 years of age23 and in participants 60 years of age or older.24 The findings suggested that the vaccine was safe and immunogenic in most patients 14 days after receipt of the second dose. Phase 3 clinical trials are taking place in Brazil, Chile, Indonesia, and Turkey (ClinicalTrials.gov numbers, NCT04456595, NCT04651790, NCT04508075, and NCT04582344, respectively). Efficacy results from these trials have not yet been published, but reported efficacy estimates from the manufacturers with regard to mild Covid-19 have varied substantially among the sites: 50.7% (95% confidence interval [CI], 35.6 to 62.2) in Brazil, 65.3% in Indonesia, and 83.5% (95% CI, 65.4 to 92.1) in Turkey.25-28, In addition, preliminary estimates from an observational study involving vaccinated health care workers (from a preprint server) suggested that at least one dose of the CoronaVac vaccine was 49.6% (95% CI, 11.3 to 71.4) effective against Covid-19 in Manaus, Brazil, a location where the P.1 (or gamma) variant, which is considered to be a variant of concern by the Centers for Disease Control and Prevention,29 is predominant (occurred in approximately 75% of the test results).30 No estimates of the effectiveness of the CoronaVac vaccine with regard to preventing Covid-19 in the general population or in persons who have received full vaccination are publicly available.
On February 2, 2021, Chile began a mass vaccination campaign with the CoronaVac vaccine (Section S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).31 The Public Health Institute of Chile approved the CoronaVac vaccine for emergency use on January 20, 2021; the vaccine is to be administered in a two-dose schedule, with doses separated by 28 days. The vaccination campaign prioritized older adults, beginning at 90 years of age or older; frontline health care workers; and persons with underlying conditions. The government relied on the existing health care infrastructure to roll the vaccines out to the eligible population where they lived. Vaccination rollout was organized by means of a publicly available national schedule that assigned specific dates to eligible groups. Eligible persons needed to show up at the nearest vaccination site with their identification; they did not need to make an appointment (Figs. S3 and S4). A national immunization registry keeps track of the vaccination schedules. As of May 10, 2021, the Ministry of Health has administered 13.98 million doses of the CoronaVac vaccine (7.62 million first doses and 6.36 million second doses).32 Vaccine introduction and scale-up of the campaign occurred during a period with the highest incidence rates of Covid-19 since the beginning of the pandemic in Chile.
We used a rich administrative observational data set to provide estimates of the effectiveness of the CoronaVac vaccine in preventing Covid-19 and related hospitalization, admission to the intensive care unit (ICU), and death in the Chilean population. We estimated the effectiveness of the administration of one vaccine dose and of two doses (the complete schedule), with adjustment for relevant demographic and clinical confounders of the association between vaccination and Covid-19 outcomes. We conducted robustness checks to test whether vaccine effectiveness would be affected by differences in health care access between the vaccinated and unvaccinated groups, and we provide vaccine-effectiveness estimates among persons 16 to 59 years of age and among those 60 years of age or older.
Methods
Study Population and Design
We used a prospective observational cohort at the national level. The study cohort included participants 16 years of age or older who were affiliated with Fondo Nacional de Salud (FONASA), the national public health insurance program, which includes approximately 80% of the Chilean population. A detailed description of the vaccination campaign is provided in the Supplementary Appendix. Eligibility criteria included an age of 16 years or more, affiliation with FONASA, and receipt of at least one dose of the CoronaVac vaccine between February 2 and May 1, 2021, or no receipt of any Covid-19 vaccination. We excluded participants with a probable or confirmed SARS-CoV-2 infection, as assessed by reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay or antigen testing, on or before February 2, 2021, and persons who had received at least one dose of the BNT162b2 vaccine. We did not focus on the effectiveness of the BNT162b2 vaccine because these estimates have been provided elsewhere.15,17 We focused on the results regarding the CoronaVac vaccine because they are the mainstay of the vaccination strategy in Chile. However, we provide estimates of the effectiveness of the BNT162b2 vaccine in the Supplementary Appendix as a validation of the procedures used here.
All persons 16 years of age or older are eligible to receive the vaccine, according to the national vaccination schedule. We classified participants into three groups: those who were not vaccinated, those who were partially immunized (≥14 days after receipt of the first vaccine dose and before receipt of the second dose), and those who were fully immunized (≥14 days after receipt of the second dose).
The study team was entirely responsible for the design of the study and for the collection and analysis of the data. The authors vouch for the accuracy and completeness of the data. The first, second, and last authors wrote the first draft of the manuscript.
Outcomes and Covariates
We estimated vaccine effectiveness using four primary outcomes: laboratory-confirmed Covid-19, hospitalization for Covid-19, admission to the ICU for Covid-19, and Covid-19–related death. For all the outcomes, we considered the time from the beginning of follow-up (February 2, 2021) to the onset of symptoms as the end point. Vaccine-effectiveness estimates regarding Covid-19 cases included the more severe outcomes. All suspected cases of Covid-19 in Chile are notified to health authorities by means of an online platform and are confirmed by laboratory testing. In our study, cases of Covid-19 and related deaths were those in persons with laboratory-confirmed infection, which corresponds to code U07.1 in the International Classification of Diseases, 10th Revision.
We controlled for several patient characteristics that could confound the association between vaccination and outcomes, including age, sex, region of residence, income, nationality, and whether the patient had underlying conditions that have been associated with severe Covid-19. These conditions included chronic kidney disease, diabetes, cardiovascular disease, stroke, chronic obstructive pulmonary disease, hematologic disease, autoimmune disease, human immunodeficiency virus infection, and Alzheimer’s disease and other dementias.4,33-35
Statistical Analysis
Our analysis was broadly based on the analytic methods of Thompson et al.17 for estimating vaccine effectiveness in the United States. We determined vaccine effectiveness by estimating the hazard ratio between the vaccinated and unvaccinated groups. On the basis of the observed information regarding the time to symptom onset from February 2, 2021, we estimated hazard ratios using the extension of the Cox proportional-hazards model, which allowed us to account for a time-varying vaccination status of the persons in the study. We evaluated the robustness of the model assumptions by fitting a stratified version of the extended Cox proportional-hazards model using the available predictors. Inference was based on a partial likelihood approach (Section S2).17 We estimated the change in the hazard associated with partial immunization and full immunization, and both time-to-event analyses were performed separately. Because the immunity status induced by the CoronaVac vaccine is unknown during the 13 days between vaccine administration and partial or full immunization, those periods were excluded from the at-risk person-time in our analyses.17
We estimated the vaccine effectiveness as 1 minus the corresponding hazard ratio, obtained from a model including the previously described covariates, which was expressed as a percentage. We also provide the results with adjustment for the effect of sex and age only. To evaluate whether our effectiveness results were affected by potentially different access to health care between vaccinated persons and unvaccinated persons and according to the age distribution, we performed subgroup analyses involving the subgroup of persons with access to RT-PCR or antigen testing for SARS-CoV-2 and subgroups of persons 60 years of age or older and persons 16 to 59 years of age. Statistical analyses were conducted with the use of the survival package of R software, version 4.0.5.36,37
Results
Study Population and Vaccination Rollout
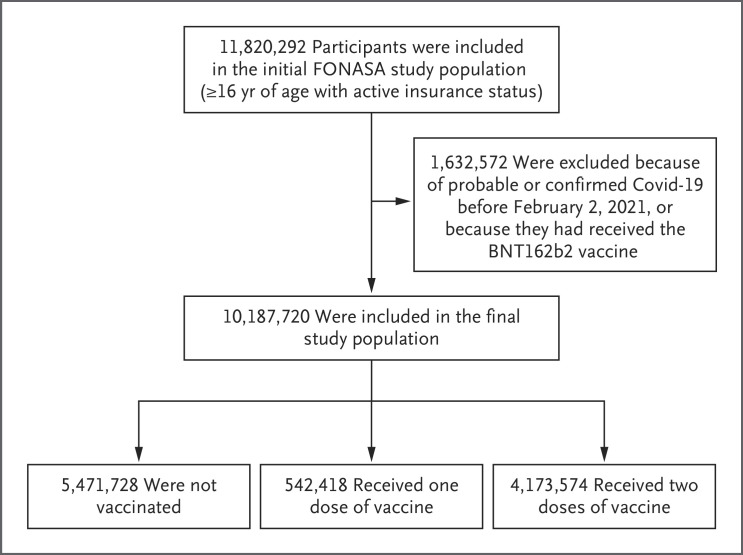
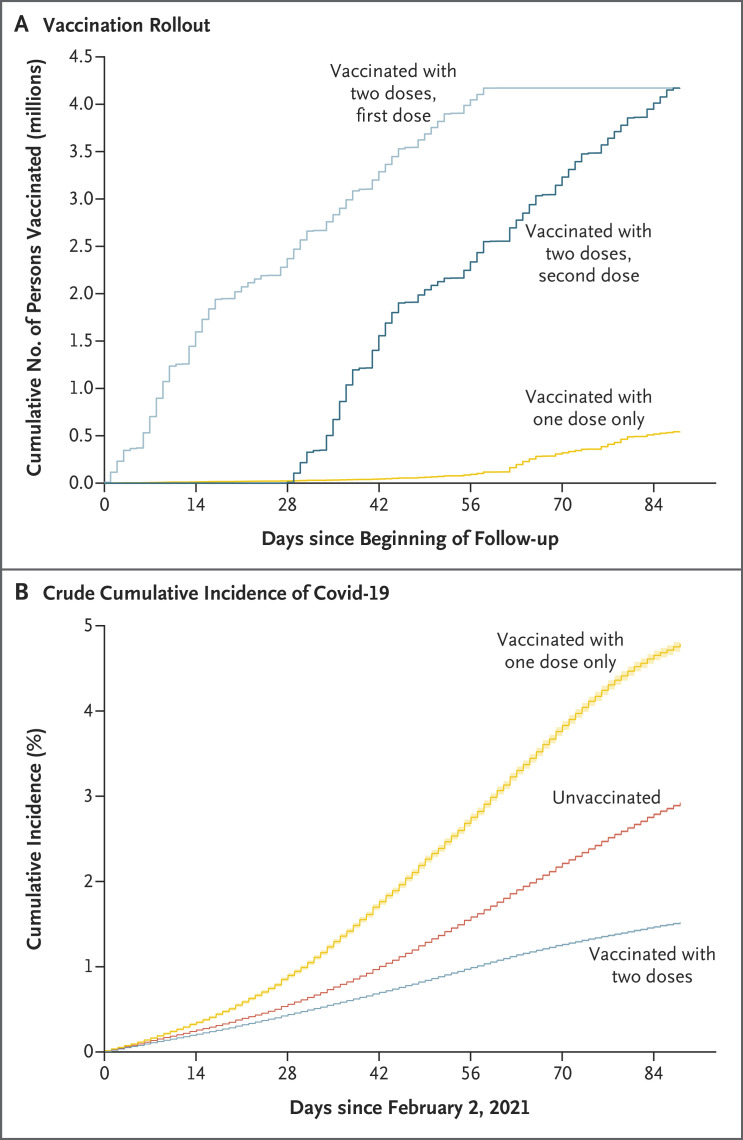
Figure 1 shows the flow diagram of the study cohort. Of the 11,820,292 persons 16 years of age or older who were affiliated with FONASA, 10,187,720 were eligible for inclusion in the study. Table 1 shows the descriptive statistics for the approximately 10.2 million participants included in the study cohort. There were significant differences according to geographic region, sex, age, income group, nationality, and presence of underlying medical conditions, both in the incidence of Covid-19 and according to vaccination status (unvaccinated, vaccinated with only one dose, or vaccinated with two doses). Laboratory confirmation of infection was by RT-PCR assay in 98.1% of the cases and by antigen testing in 1.9%. Figure 2A shows the rapid rollout of the vaccination campaign, which started on February 2, 2021. Details of the vaccination campaign are provided in Section S1 and Figures S5 through S8. Figure 2B shows the crude cumulative incidence of Covid-19 during the study period among persons who had received one or two doses of vaccine or were unvaccinated.
Figure 1. Study Participants and Cohort Eligibility.
Participants were at least 16 years of age, were affiliated with Fondo Nacional de Salud (FONASA; the national public health care system in Chile), and either had received at least one dose of the CoronaVac vaccine between February 2 and May 1, 2021, or had not received any vaccination. We excluded persons who had probable or confirmed coronavirus disease 2019 (Covid-19) according to reverse-transcriptase–polymerase-chain-reaction assay for severe acute respiratory syndrome coronavirus 2 and all persons who had been immunized with the BNT162b2 vaccine.
Table 1. Characteristics of the Study Cohort, Overall and Those with Laboratory-Confirmed Covid-19, According to Vaccination Status.*.
| Characteristic | Cohort Participants |
Persons with Covid-19 |
P Value | Unvaccinated Persons |
Persons Vaccinated with One Dose |
Persons Vaccinated with Two Doses |
P Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | % | no. | % | no. | % | no. | % | no. | % | |||
| Total | 10,187,720 | 100 | 248,645 | 2.4 | — | 5,471,728 | 53.7 | 542,418 | 5.3 | 4,173,574 | 41.0 | — |
| Sex | <0.001 | |||||||||||
| Female | 5,469,202 | 54.0 | 135,311 | 2.5 | <0.001 | 2,775,436 | 50.8 | 272,044 | 5.0 | 2,421,722 | 44.3 | |
| Male | 4,718,518 | 46.0 | 113,334 | 2.4 | 2,696,292 | 57.1 | 270,374 | 5.7 | 1,751,852 | 37.1 | ||
| Age group | <0.001 | |||||||||||
| 16–19 yr | 708,676 | 7.0 | 14,871 | 2.1 | <0.001 | 670,451 | 94.6 | 8,192 | 1.2 | 30,033 | 4.2 | |
| 20–29 yr | 2,017,676 | 20.0 | 59,645 | 3.0 | 1,655,595 | 82.1 | 55,854 | 2.8 | 306,227 | 15.2 | ||
| 30–39 yr | 1,867,491 | 18.0 | 54,480 | 2.9 | 1,446,544 | 77.5 | 59,166 | 3.1 | 361,781 | 19.4 | ||
| 40–49 yr | 1,423,770 | 14.0 | 39,993 | 2.8 | 851,622 | 59.8 | 165,487 | 11.6 | 406,661 | 28.6 | ||
| 50–59 yr | 1,457,564 | 14.0 | 37,539 | 2.6 | 434,694 | 29.8 | 184,268 | 12.6 | 838,602 | 57.5 | ||
| 60–69 yr | 1,365,940 | 13.0 | 23,669 | 1.7 | 221,738 | 16.2 | 41,693 | 3.1 | 1,102,509 | 80.7 | ||
| 70–79 yr | 870,082 | 8.5 | 11,778 | 1.4 | 111,592 | 12.8 | 16,412 | 1.9 | 742,078 | 85.3 | ||
| ≥80 yr | 476,521 | 4.7 | 6,670 | 1.4 | 79,492 | 16.7 | 11,346 | 2.4 | 385,683 | 80.9 | ||
| No. of coexisting conditions† | <0.001 | |||||||||||
| 0 | 6,880,426 | 68.0 | 168,401 | 2.4 | 0.04 | 4,447,684 | 64.6 | 394,030 | 5.7 | 2,038,712 | 29.6 | |
| ≥1 | 3,307,294 | 32.0 | 80,244 | 2.4 | 1,024,044 | 31.0 | 148,388 | 4.5 | 2,134,862 | 64.6 | <0.001 | |
| Nationality | ||||||||||||
| Chilean | 9,497,058 | 93.2 | 233,572 | 2.5 | <0.001 | 4,913,208 | 51.7 | 513,604 | 5.4 | 4,070,246 | 42.9 | |
| Non-Chilean | 690,662 | 6.8 | 15,073 | 2.2 | 558,520 | 80.9 | 28,814 | 4.2 | 103,328 | |||
The study cohort included eligible persons who were affiliated with Fondo Nacional de Salud, the national public health insurance program, which collects, manages, and distributes funds for the public health care system in Chile. The model also included individual-level income and location (16 regions). Additional details are provided in Table S1. Covid-19 denotes coronavirus disease 2019.
Coexisting conditions included chronic kidney disease, diabetes, cardiovascular disease (hypertension or myocardial infarction), stroke, chronic obstructive pulmonary disease, hematologic disease (lymphoma, leukemia, or myeloma), autoimmune disease (rheumatoid arthritis, juvenile idiopathic arthritis, or systemic lupus erythematosus), human immunodeficiency virus infection, and Alzheimer’s disease and other dementias.
Figure 2. Vaccination Rollout and Crude Cumulative Incidence of Covid-19 in the Study Cohort.
Panel A shows the pace and coverage of the vaccination program among persons who received both doses of vaccine (first and second doses shown separately) or only one dose during the study period (February 2 through May 1, 2021). Panel B shows the crude cumulative incidence of Covid-19 during the study period among unvaccinated persons, among persons who had received only one dose of vaccine, and among persons who had received both doses of vaccine. The relatively high cumulative incidence of Covid-19 in the one-dose group should be interpreted with caution. As shown in Panel A, this group initiated vaccination approximately 40 days after the beginning of the vaccination campaign on February 2, 2021. Therefore, the incidence curve includes all cases that occurred from before vaccination up to 13 days after receipt of the first dose. Shading on the lines indicates 95% confidence intervals.
Vaccine Effectiveness
There were approximately 615 million person-days in the unvaccinated group, 70 million person-days in the partially immunized group, and 92 million person-days in the fully immunized group during the study period (Table 2). We documented 218,784 cases of Covid-19, as well as 22,866 hospitalizations, 7873 ICU admissions, and 4042 deaths.
Table 2. Effectiveness of CoronaVac Vaccine in Preventing Covid-19 Outcomes in Overall Study Cohort, According to Immunization Status.*.
| Outcome and Immunization Status | Study Cohort | Persons with Covid-19 | Vaccine Effectiveness (95% CI) | |||
|---|---|---|---|---|---|---|
| No. of Person-Days |
No. of Persons |
Incidence Rate |
Analysis Adjusted for Sex and Age |
Analysis Adjusted for All Covariates† |
Stratified Analysis‡ |
|
| no. of events/ 1000 person-days |
percent | |||||
| Covid-19 | ||||||
| Unvaccinated | 614,868,240 | 185,633 | 0.3019 | — | — | — |
| Partially immunized | 69,788,352 | 20,865 | 0.2990 | 8.0 (6.5–9.4) |
15.5 (14.2–16.8) |
17.2 (15.8–18.6) |
| Fully immunized | 91,671,797 | 12,286 | 0.1340 | 61.2 (60.3–62.0) |
65.9 (65.2–66.6) |
63.7 (62.8–64.6) |
| Hospitalization | ||||||
| Unvaccinated | 620,894,706 | 18,034 | 0.0290 | — | — | — |
| Partially immunized | 70,690,796 | 3,370 | 0.0477 | 31.4 (28.6–34.0) |
37.4 (34.9–39.9) |
40.3 (37.6–42.8) |
| Fully immunized | 92,445,333 | 1,462 | 0.0158 | 86.0 (85.1–86.8) |
87.5 (86.7–88.2) |
86.5 (85.6–87.4) |
| Admission to ICU | ||||||
| Unvaccinated | 621,226,431 | 6,359 | 0.0102 | — | — | — |
| Partially immunized | 70,836,597 | 1,154 | 0.0163 | 37.5 (33.1–41.5) |
44.7 (40.8–48.3) |
45.3 (41.2–49.2) |
| Fully immunized | 92,622,083 | 360 | 0.0039 | 88.8 (87.4–90.0) |
90.3 (89.1–91.4) |
90.2 (88.9–91.4) |
| Confirmed death | ||||||
| Unvaccinated | 621,426,477 | 2,786 | 0.0045 | — | — | — |
| Partially immunized | 70,854,187 | 847 | 0.0120 | 39.8 (34.4–44.7) |
45.7 (40.9–50.2) |
46.0 (40.7–50.8) |
| Fully immunized | 92,514,261 | 409 | 0.0044 | 84.4 (82.4–86.2) |
86.3 (84.5–87.8) |
86.7 (84.9–88.3) |
Participants were classified into three groups: those who were unvaccinated, those who were partially immunized (≥14 days after receipt of the first vaccine dose and before receipt of the second dose), and those who were fully immunized (≥14 days after receipt of the second dose). The 13 days between vaccine administration and partial or full immunization were excluded from the at-risk person-time. ICU denotes intensive care unit.
The analysis was adjusted for age, sex, region of residence, income, nationality, and whether the patient had underlying conditions that have been associated with severe Covid-19.
A stratified version of the extended Cox proportional-hazards model was fit to test the robustness of the estimates to model assumptions, with stratification according to age, sex, region of residence, income, nationality, and whether the patient had underlying conditions that have been associated with severe Covid-19.
We estimated that the vaccine effectiveness among partially immunized persons (14 to 28 days after receipt of the first dose) was 15.5% (95% CI, 14.2 to 16.8) for the prevention of Covid-19 and 37.4% (95% CI, 34.9 to 39.9) for the prevention of hospitalization, 44.7% (95% CI, 40.8 to 48.3) for the prevention of admission to the ICU, and 45.7% (95% CI, 40.9 to 50.2) for the prevention of Covid-19–related death. In the fully immunized group, the estimated adjusted vaccine effectiveness was 65.9% (95% CI, 65.2 to 66.6) for the prevention of Covid-19 and 87.5% (95% CI, 86.7 to 88.2) for the prevention of hospitalization, 90.3% (95% CI, 89.1 to 91.4) for the prevention of ICU admission, and 86.3% (95% CI, 84.5 to 87.9) for the prevention of Covid-19–related death (Table 2). The vaccine-effectiveness estimates in the stratified model were consistent with these results.
We estimated that the adjusted vaccine effectiveness in the subgroup of fully immunized persons 60 years of age or older was 66.6% (95% CI, 65.4 to 67.8) for the prevention of Covid-19 and 85.3% (95% CI, 84.3 to 86.3) for the prevention of hospitalization, 89.2% (95% CI, 87.6 to 90.6) for the prevention of ICU admission, and 86.5% (95% CI, 84.6 to 88.1) for the prevention of Covid-19–related death (Table 3). Vaccine-effectiveness estimates among persons 16 to 59 years of age are provided in Table S3.
Table 3. Effectiveness of CoronaVac Vaccine in Preventing Covid-19 Outcomes among Cohort Participants 60 Years of Age or Older, According to Immunization Status.
| Outcome and Immunization Status | Subgroup Cohort | Persons with Covid-19 | Vaccine Effectiveness (95% CI) | |||
|---|---|---|---|---|---|---|
| No. of Person-Days |
No. of Persons |
Incidence Rate |
Analysis Adjusted for Sex and Age |
Analysis Adjusted for All Covariates* |
Stratified Analysis† |
|
| no. of events/ 1000 person-days |
percent | |||||
| Covid-19 | ||||||
| Unvaccinated | 75,707,905 | 15,597 | 0.2060 | — | — | — |
| Partially immunized | 35,675,604 | 8,333 | 0.2336 | 3.9 (0.9–6.8) |
9.7 (6.9–12.4) |
12.7 (9.8–15.5) |
| Fully immunized | 66,563,272 | 7,510 | 0.1128 | 63.4 (62.0–64.6) |
66.6 (65.4–67.8) |
67.2 (66.0–68.4) |
| Hospitalization | ||||||
| Unvaccinated | 76,047,640 | 5,304 | 0.0697 | — | — | — |
| Partially immunized | 35,961,593 | 2,168 | 0.0603 | 29.2 (25.1–33.1) |
35.0 (31.3–38.6) |
38.6 (34.8–42.2) |
| Fully immunized | 66,986,859 | 1,344 | 0.0201 | 83.4 (82.2–84.5) |
85.3 (84.3–86.3) |
85.4 (84.3–86.4) |
| Admission to ICU | ||||||
| Unvaccinated | 76,194,648 | 1,811 | 0.0238 | — | — | — |
| Partially immunized | 36,062,081 | 672 | 0.0186 | 38.2 (31.9–44.0) |
44.5 (38.7–49.7) |
47.0 (41.2–52.2) |
| Fully immunized | 67,051,769 | 331 | 0.0049 | 87.5 (85.7–89.0) |
89.2 (87.6–90.6) |
89.3 (87.8–90.7) |
| Confirmed death | ||||||
| Unvaccinated | 76,169,386 | 1,999 | 0.0262 | — | — | — |
| Partially immunized | 36,053,806 | 768 | 0.0213 | 39.7 (33.8–45.1) |
45.8 (40.4–50.7) |
46.1 (40.5–51.2) |
| Fully immunized | 67,045,620 | 402 | 0.0060 | 84.4 (82.3–86.2) |
86.5 (84.6–88.1) |
86.8 (85.0–88.4) |
The analysis was adjusted for age, sex, region of residence, income, nationality, and whether the patient had underlying conditions that have been associated with severe Covid-19.
A stratified version of the extended Cox proportional-hazards model was fit to test the robustness of the estimates to model assumptions, with stratification according to sex, age, coexisting conditions, nationality, and income.
To address a potential concern that the observed vaccine effectiveness may have been driven by health care access, we conducted an analysis in the subgroup of persons who had undergone testing with an RT-PCR assay (98.1%) or antigen test (1.9%) during the analysis period. The results, conditional on whether testing was performed, showed larger effects for vaccination than when we included the complete cohort. Among fully immunized persons in this subgroup, the adjusted vaccine effectiveness was 72.9% (95% CI, 72.3 to 73.4) for the prevention of Covid-19 and 89.2% (95% CI, 88.5 to 89.8) for the prevention of hospitalization, 91.6% (95% CI, 90.5 to 92.5) for the prevention of ICU admission, and 87.8% (95% CI, 86.2 to 89.2) for the prevention of Covid-19–related death (Table S4).
Discussion
We provide estimates of the effectiveness of administration of the CoronaVac vaccine in a countrywide mass vaccination campaign for the prevention of laboratory-confirmed Covid-19 and related hospitalization, admission to the ICU, and death. Among fully immunized persons, the adjusted vaccine effectiveness was 65.9% for Covid-19 and 87.5% for hospitalization, 90.3% for ICU admission, and 86.3% for death. The vaccine-effectiveness results were maintained in both age-subgroup analyses, notably among persons 60 years of age or older, independent of variation in testing and independent of various factors regarding vaccine introduction in Chile.
The vaccine-effectiveness results in our study are similar to estimates that have been reported in Brazil for the prevention of Covid-19 (50.7%; 95% CI, 35.6 to 62.2), including estimates of cases that resulted in medical treatment (83.7%; 95% CI, 58.0 to 93.7) and estimates of a composite end point of hospitalized, severe, or fatal cases (100%; 95% CI, 56.4 to 100).27 The large confidence intervals for the trial in Brazil reflect the relatively small sample (9823 participants) and the few cases detected (35 cases that led to medical treatment and 10 that were severe). However, our estimates are lower than the vaccine effectiveness recently reported in Turkey (83.5%; 95% CI, 65.4 to 92.1),27,28 possibly owing to the small sample in that phase 3 clinical trial (10,029 participants in the per-protocol analysis), differences in local transmission dynamics, and the predominance of older adults among the fully or partially immunized participants in our study. Overall, our results suggest that the CoronaVac vaccine had high effectiveness against severe disease, hospitalizations, and death, findings that underscore the potential of this vaccine to save lives and substantially reduce demands on the health care system.
Our study has at least three main strengths. First, we used a rich administrative health care data set, combining data from an integrated vaccination system for the total population and from the Ministry of Health FONASA, which covers approximately 80% of the Chilean population. These data include information on laboratory tests, hospitalization, mortality, onset of symptoms, and clinical history in order to identify risk factors for severe disease. Information on region of residence also allowed us to control for differences in incidence across the country. We adjusted for income and nationality, which correlate with socioeconomic status in Chile and are thus considered to be social determinants of health. The large population sample allowed us to estimate vaccine effectiveness both for one dose and for the complete two-dose vaccination schedule. It also allowed for a subgroup analysis involving adults 60 years of age or older, a subgroup that is at higher risk for severe disease3 and that is underrepresented in clinical trials. Second, data were collected during a rapid vaccination campaign with high uptake and during a period with one of the highest community transmission rates of the pandemic, which allowed for a relatively short follow-up period and for estimation of the prevention of at least four essential outcomes: Covid-19 cases and related hospitalization, ICU admission, and death. Finally, Chile has the highest testing rates for Covid-19 in Latin America, universal health care access, and a standardized, public reporting system for vital statistics, which limited the number of undetected or unascertained cases and deaths.14
Our study has several limitations. First, as an observational study, it is subject to confounding. To account for known confounders, we adjusted the analyses for relevant variables that could affect vaccine effectiveness, such as age, sex, underlying medical conditions, region of residence, and nationality. The risk of misclassification bias that would be due to the time-dependent performance of the SARS-CoV-2 RT-PCR assay is relatively low, because the median time from symptom onset to testing in Chile is approximately 4 days (98.1% of the tests were RT-PCR assays). In this 4-day period, the sensitivity and specificity of the molecular diagnosis of Covid-19 are high.38 However, there may be a risk of selection bias. Systematic differences between the vaccinated and unvaccinated groups, such as health-seeking behavior or risk aversion, may affect the probability of exposure to the vaccine and the risk of Covid-19 and related outcomes.39,40 However, we cannot be sure about the direction of the effect. Persons may be hesitant to get the vaccine for various reasons, including fear of side effects, lack of trust in the government or pharmaceutical companies, or an opinion that they do not need it, and they may be more or less risk-averse. Vaccinated persons may compensate by increasing their risky behavior (Peltzman effect).40 We addressed potential differences in health care access by restricting the analysis to persons who had undergone diagnostic testing, and we found results that were consistent with those of our main analysis.
Second, owing to the relatively short follow-up in this study, late outcomes may not have yet developed in persons who were infected near the end of the study, because the time from symptom onset to hospitalization or death can vary substantially.3,15 Therefore, effectiveness estimates regarding severe disease and death, in particular, should be interpreted with caution. Third, during the study period, ICUs in Chile were operating at 93.5% of their capacity on average (65.7% of the patients had Covid-19).32 If fewer persons were hospitalized than would be under regular ICU operation, our effectiveness estimates for protection against ICU admission might be biased downward, and our effectiveness estimates for protection against death might be biased upward (e.g., if patients received care at a level lower than would usually be received during regular health system operation).
Fourth, although the national genomic surveillance for SARS-CoV-2 in Chile has reported the circulation of at least two viral lineages considered to be variants of concern, P.1 and B.1.1.7 (or the gamma and alpha variants, respectively),41 we lack representative data to estimate their effect on vaccine effectiveness (Table S2). Results from a test-negative design study of the effectiveness of the CoronaVac vaccine in health care workers in Manaus, Brazil, where the gamma variant is now predominant, showed that the efficacy of at least one dose of the vaccine against Covid-19 was 49.6% (95% CI, 11.3 to 71.4).30 Although the vaccine-effectiveness estimates in Brazil are not directly comparable with our estimates owing to differences in the target population, the vaccination schedule (a window of 14 to 28 days between doses is recommended in Brazil42), and immunization status, they highlight the importance of continued vaccine-effectiveness monitoring.
Overall, our study results suggest that the CoronaVac vaccine was highly effective in protecting against severe disease and death, findings that are consistent with the results of phase 2 trials23,24 and with preliminary efficacy data.27,28
Acknowledgments
We thank the attendees of the WHO meeting and working group for suggestions and comments; Drs. Ira Longini (University of Florida), Daniel Cohen (Tel Aviv University), Richard Peto (University of Oxford), Elizabeth Miller (Public Health England), and Myron M. Levine (University of Maryland); Drs. Catterina Ferreccio (Pontificia Universidad Católica de Chile), Ximena Aguilera (Universidad del Desarrollo), Maria T. Valenzuela (Universidad de Los Andes), Pablo Vial (Universidad del Desarrollo), Gonzalo Valdivia (Pontificia Universidad Católica de Chile), Fernando Otaiza (Ministerio de Salud), and Alvaro Erazo (Pontificia Universidad Católica de Chile), from the Chilean Covid-19 Advisory Group, for advice on the study design and interpretation of results; and Drs. José R. Zubizarreta (Harvard University) and Erika M.C. D’Agata (Brown University) for thoughtful suggestions.
Supplementary Appendix
Disclosure Forms
The research protocol was approved by the Comité Ético Científico Clínica Alemana Universidad del Desarrollo. The study was considered exempt from informed consent; no human health risks were identified. Research analysts are employees of the Chilean Ministry of Health; our use of data follows Chilean law 19.628 on private data protection.
This article was published on July 7, 2021, and updated on July 13, 2021, at NEJM.org.
Owing to data privacy regulations, the individual-level data in this study cannot be shared (Law N19.628). Aggregate data on vaccination and incidence are publicly available at https://github.com/MinCiencia/Datos-COVID19/.
Footnotes
Presented in part at the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) meeting on coronavirus disease 2019 (Covid-19) vaccines, April 29, 2021, and the WHO Vaccines Prioritization Working Group, April 21, 2021.
Supported by grants from the Agencia Nacional de Investigación y Desarrollo (ANID) Millennium Science Initiative Program, Millennium Nucleus Center for the Discovery of Structures in Complex Data (MIDAS) (NCN17_059, to Dr. Jara), and Millennium Initiative for Collaborative Research in Bacterial Resistance (MICROB-R) (NCN17_081, to Drs. Undurraga and Araos); the Advanced Center for Chronic Diseases, ANID Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias (FONDAP) (15130011, to Dr. Araos); and the Research Center for Integrated Disaster Risk Management ANID FONDAP (15110017, to Dr. Undurraga).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Dong E, Du H, Gardner L. An interactive Web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782-793. [DOI] [PubMed] [Google Scholar]
- 3.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020;20:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Campbell H, Kulkarni D, et al. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. Lancet Infect Dis 2021;21:193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker PGT, Whittaker C, Watson OJ, et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science 2020;369:413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledford H, Cyranoski D, Van Noorden R. The UK has approved a COVID vaccine — here’s what scientists now want to know. Nature 2020;588:205-206. [DOI] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021;397:671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanne JH. Covid-19: FDA panel votes to approve Pfizer BioNTech vaccine. BMJ 2020;371:m4799-m4799. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer C, Corum J, Wee S-L. Coronavirus vaccine tracker. New York Times, June 10, 2021. (https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html).
- 14.Our World in Data. Coronavirus pandemic (COVID-19). 2021. (https://ourworldindata.org/coronavirus).
- 15.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasileiou E, Simpson CR, Robertson C, et al. Effectiveness of first dose of COVID-19 vaccines against hospital admissions in Scotland: national prospective cohort study of 5.4 million people. February 19, 2021. (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3789264). preprint.
- 17.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers — eight U.S. locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021;70:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel W, Nivet M, Warner J, Podolsky DK. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med 2021;384:1962-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britton A, Jacobs Slifka KM, Edens C, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks — Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep 2021;70:396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doshi P. Will covid-19 vaccines save lives? Current trials aren’t designed to tell us. BMJ 2020;371:m4037-m4037. [DOI] [PubMed] [Google Scholar]
- 21.Lopalco PL, DeStefano F. The complementary roles of phase 3 trials and post-licensure surveillance in the evaluation of new vaccines. Vaccine 2015;33:1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020;369:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021;21:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021;21:803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J, Moutinho S. Third time’s the charm? Brazil scales back efficacy claims for COVID-19 vaccine from China. Science. January 12, 2021. (https://www.sciencemag.org/news/2021/01/third-time-s-charm-brazil-scales-back-efficacy-claims-covid-19-vaccine-china).
- 26.Baraniuk C. What do we know about China’s covid-19 vaccines? BMJ 2021;373:n912-n912. [DOI] [PubMed] [Google Scholar]
- 27.Sinovac. Summary of clinical trial data of Sinovac’s COVID-19 vaccine (CoronaVac). 2021. (In Chinese) (http://www.sinovacbio.com/?optionid=754&auto_id=927).
- 28.Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021. July 8 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. SARS-CoV-2 variant classifications and definitions. 2021. (https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-updates%2Fvariant-surveillance%2Fvariant-info.html).
- 30.Hitchings MDT, Ranzani OT, Scaramuzzini Torres MS, et al. Effectiveness of CoronaVac in the setting of high SARS-CoV-2 P.1 variant transmission in Brazil: a test-negative case-control study. May 1, 2021. (https://www.medrxiv.org/content/10.1101/2021.04.07.21255081v2). preprint. [DOI] [PMC free article] [PubMed]
- 31.Shepherd A. Covid-19: Chile joins top five countries in world vaccination league. BMJ 2021;372:n718-n718. [DOI] [PubMed] [Google Scholar]
- 32.Cifras Oficiales COVID-19. Ministerio de Salud, plan de acción coronavirus COVID-19, 2020. (https://www.gob.cl/coronavirus/cifrasoficiales/).
- 33.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhargava A, Fukushima EA, Levine M, et al. Predictors for severe COVID-19 infection. Clin Infect Dis 2020;71:1962-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Numbers K, Brodaty H. The effects of the COVID-19 pandemic on people with dementia. Nat Rev Neurol 2021;17:69-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Therneau TM. A package for survival analysis in R: R package version 3.1-12. Rochester, MN: Mayo Clinic, April 2021. (https://cran.r-project.org/package=survival/survival.pdf). [Google Scholar]
- 37.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 38.Jara A, Undurraga EA, Araos R. Tool for estimating the probability of having COVID-19 with one or more negative RT-PCR results. January 24, 2021. (https://www.medrxiv.org/content/10.1101/2021.01.16.21249939v1). preprint. [DOI] [PMC free article] [PubMed]
- 39.Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epidemiol 2016;45:2060-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trogen B, Caplan A. Risk compensation and COVID-19 vaccines. Ann Intern Med 2021;174:858-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ministerio de Salud. Reporte circulación de variantes SARS-CoV-2 en Chile. Santiago, Chile: Departamento de Epidemiología Ministerio de Salud, 2021. [Google Scholar]
- 42.Ministério da Saúde. Informe técnico: campanha nacional de vacinação contra a Covid-19. January 19, 2021. (https://www.conasems.org.br/wp-content/uploads/2021/01/1611078163793_Informe_Tecnico_da_Campanha_Nacional_de_Vacinacao_contra_a_Covid_19-1.pdf).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.