Abstract
Masking and social distancing have been adopted to mitigate the SARS-CoV-2 pandemic. We evaluated the indirect impact of SARS-CoV-2 prevention strategies on invasive Staphylococcus aureus, Streptococcus pneumoniae (IPD) and Streptococcus pyogenes (IGAS) in Houston area children. We observed a decline in IPD and IGAS temporally associated with social distancing/masking/school closures.
Keywords: Staphylococcus aureus, Pneumococcus, Group A Streptococcus, SARS-CoV-2, Social Distancing
Introduction
The SARS-CoV-2 pandemic has had a tremendous impact directly and indirectly on child health. Most regions instituted a variety of measures intended to mitigate the spread of the SARS-CoV-2 pandemic including universal masking, closure of in-person school activities, physical distancing and restricted access to public activities. These strategies have been temporally associated with a significant decline in the incidence of common childhood respiratory viral infections,1,2 in addition to bacterial upper respiratory infections such as acute otitis media and streptococcal pharyngitis.3,4
The gram-positive organisms Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes (Group A Streptococcus, GAS), are common childhood pathogens responsible for both non-invasive as well as severe invasive disease. Notably, all three of these pathogens may be asymptomatically harbored in the upper airway including the oro- and nasopharynx and anterior nares. Invasive infection with these organisms has in some cases been associated with antecedent viral upper respiratory infection.5 No data are currently available regarding the impact of SARS-CoV-2 quarantine/infection prevention measures on the frequency of invasive staphylococcal and streptococcal infections. We examined the incidence of invasive community-onset S. aureus (I-CO-SA), GAS (IGAS), and pneumococcal disease (IPD) and its potential relationship to the SARS-CoV-2 pandemic.
Methods
Cases were identified through prospective active surveillance studies ongoing at all Texas Children’s Hospital (TCH) campuses. TCH is a tertiary referral center in Houston, TX with 789 inpatient pediatric beds including the Texas Medical Center (TMC) Campus, West Campus (24 miles west of TMC) and The Woodlands Campus (39 miles north of TMC). All isolates of S. pneumoniae, GAS and S. aureus identified by the clinical microbiology laboratory are captured by the surveillance. For purposes of this study, we examined culture positive invasive infections from January 1, 2017-December 31, 2020. Invasive infections were regarded as those in which the organisms of interest were isolated from a sterile site and/or deep abscess.6
Beginning on March 15, 2020, Houston area public schools (kindergarten-12th grade) were closed to in-person instruction; some daycares/preschools continued usual activities. In Harris County, beginning on March 24, 2020, non-essential businesses were temporarily closed and/or had restricted access and residents were required to wear a face covering in public and were encouraged to limit gatherings.
The annual and monthly number of hospital admissions across all TCH campuses was obtained from TCH administrative data. The number of non-neonatal pediatric admissions (patients < 18 years old) was employed as the denominator in calculations of frequency and presented as rate/10,000 admissions which was used as a surrogate for incidence. The primary comparison of interest was the rate/10,000 admissions of IGAS, IPD or I-CO-SA infection in the period subsequent to social distancing/school closure/masking mandates in the Houston area compared to the prior three years. To adjust for potential trends unrelated to the SARS-CoV-2 pandemic, incidence rates from 2017–2019 were examined using linear regression which was then compared with incidence rates in 2020 using χ2 for trend and reported as p-values and relative risk (RR) with 95% confidence intervals (95% CI).
Results
During the study period, the annual number of hospitalizations were as follows: 2017– 20,840 admissions; 2018– 20,760 admissions; 2019– 22,304 admissions and in 2020 there were 17,348 admissions (total 81,252). During the study period, there were 480 I-CO-SA infections (of which 151 were methicillin-resistant S. aureus [MRSA]), 269 IGAS and 177 IPD. The most common diagnoses are depicted in Figure 1A. The number of cases varied monthly/annually but exhibited a decline beginning in April 2020 (Figure 1B–D).
Figure 1.
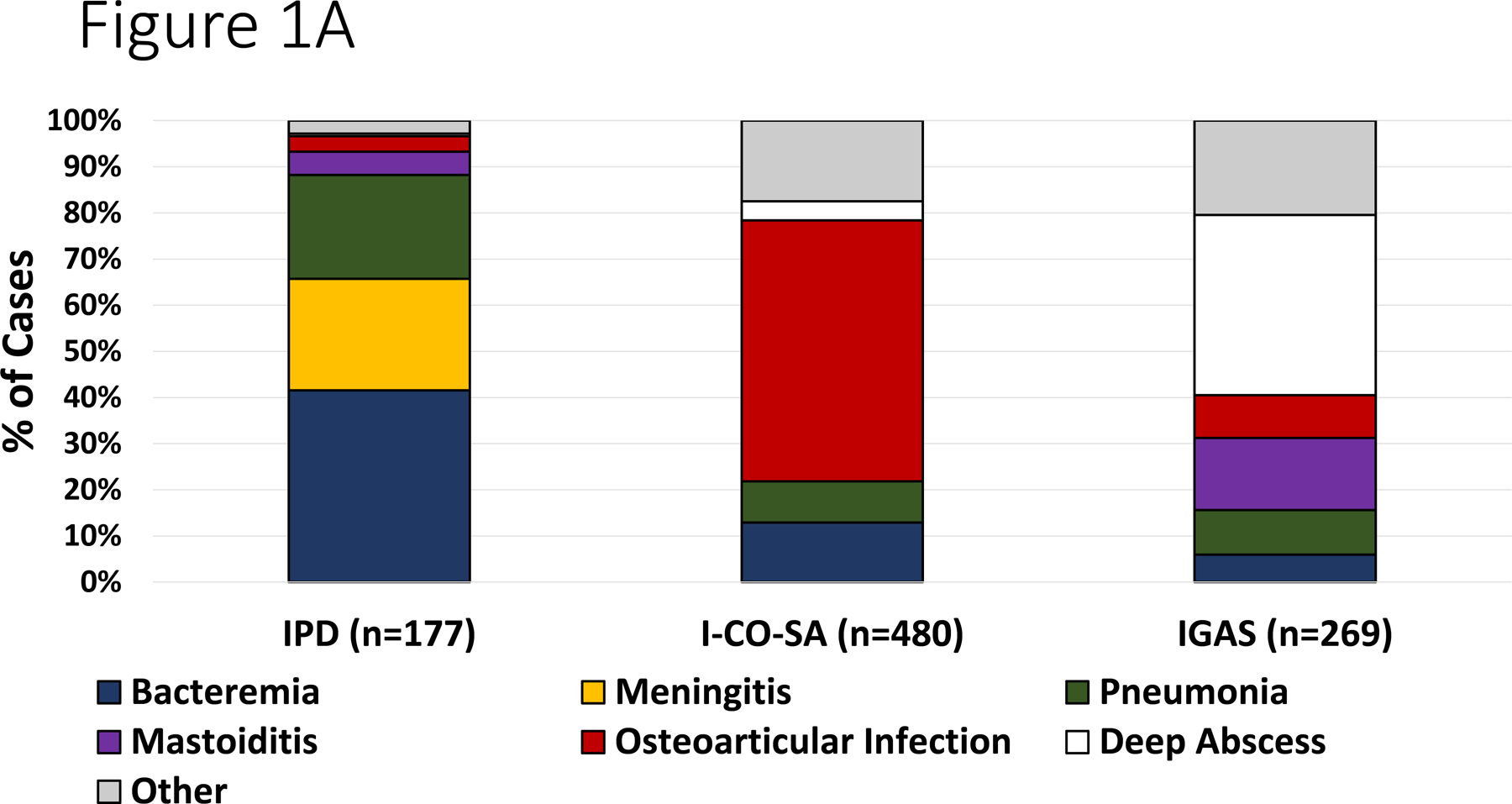
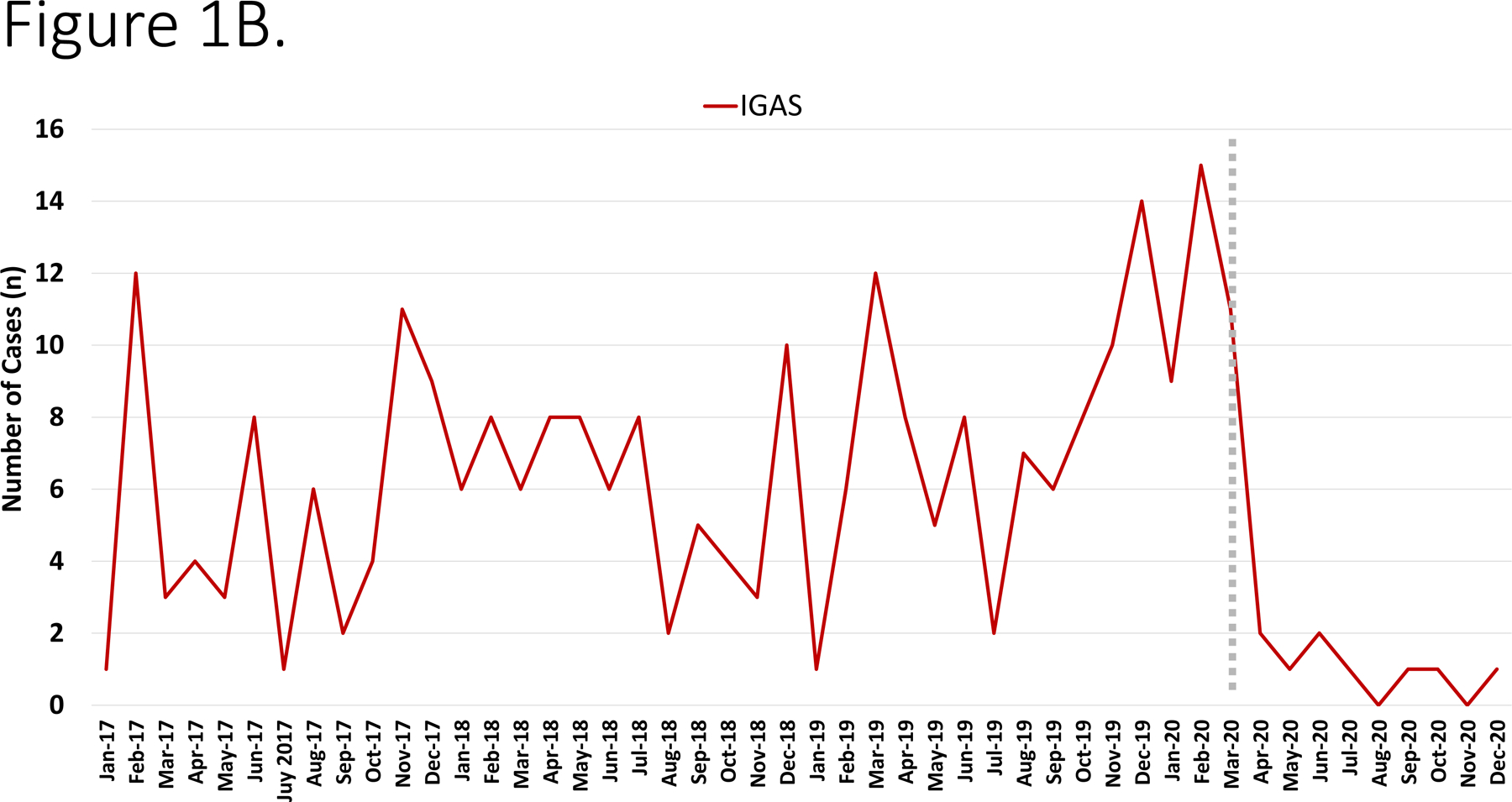
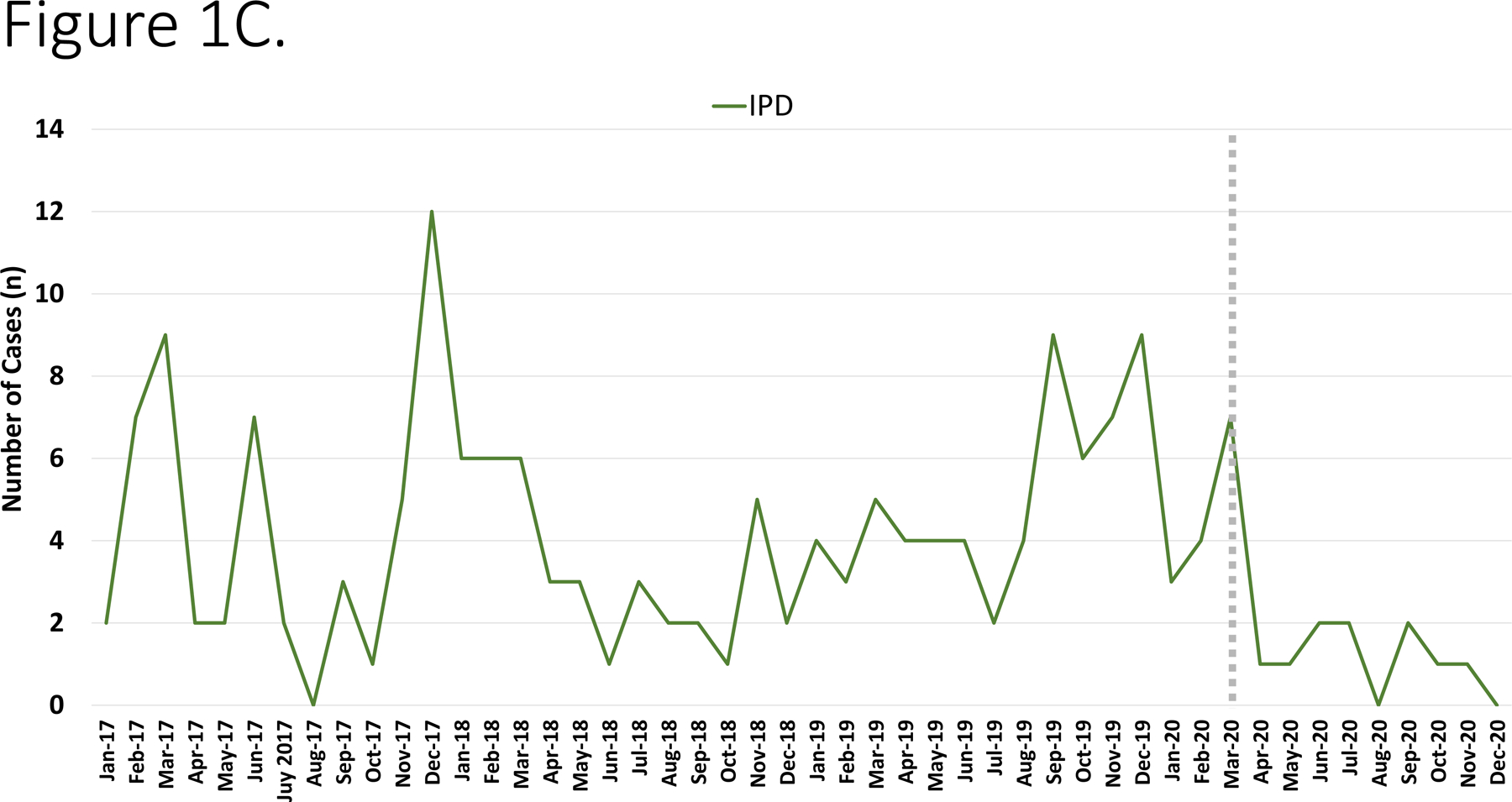
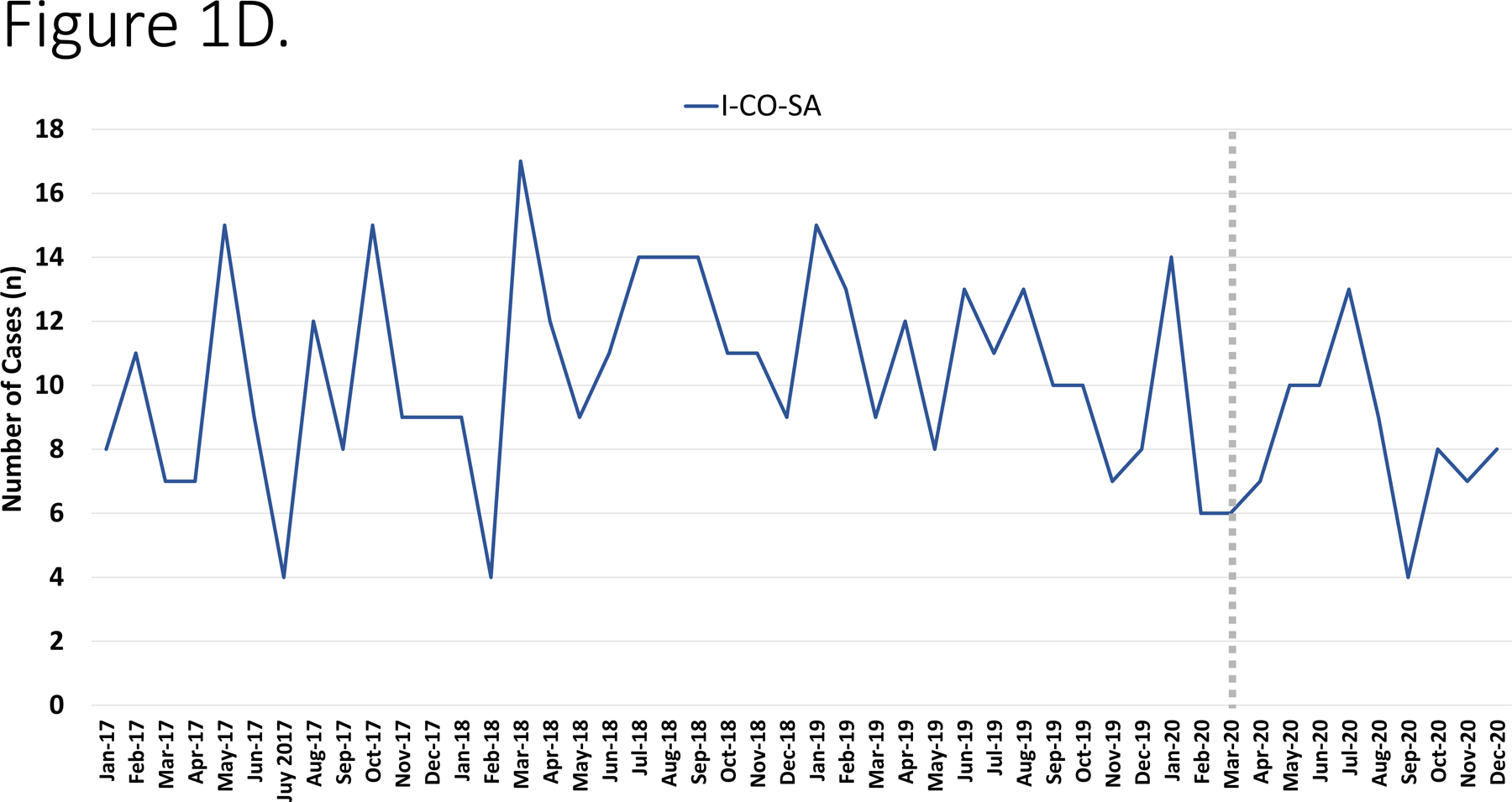
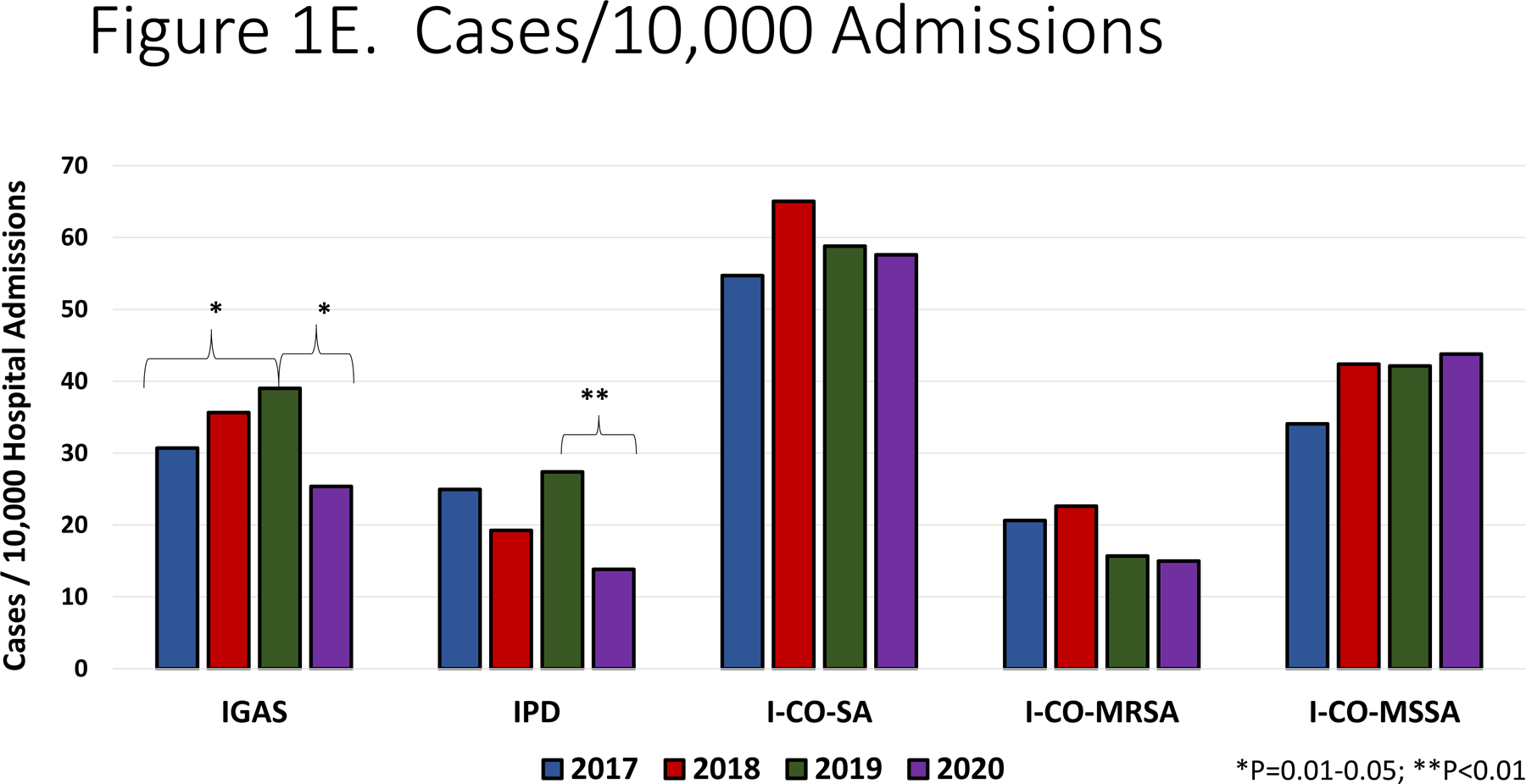
Panel A. Most common infectious diagnoses by organism. Panel B-D. Trend in number of cases of IGAS, IPD and I-CO-SA, respectively. The dashed vertical line corresponds to the initiation of infection mitigation mandates. Panel E. Yearly rate of hospitalization for organisms of interest. *P=0.01–0.05. **P<0.01.
Among IPD, the annual incidence was stable from 2017–2019 (ranging from 19.26–23.39 cases/10,000 admissions, p=0.54, R2=0.43) and then declined in 2020 to 13.83/10,000 admissions, (p=0.004, RR=0.51, 95% CI: 0.32–0.81, Figure 1E). With respect to specific diagnoses of IPD, declines in incidence were most significant for bacteremia (11.21/10,000 in 2019 vs. 5.19/10,000 in 2020, p=0.02, RR=0.46, 95% CI: 0.21–0.99), meningitis (7.62/10,000 in 2019 vs. 2.88/10,000 in 2020, p=0.03, RR=0.37 95% CI: 0.12–0.98) and pneumonia (6.72/10,000 in 2019 vs, 2.88/10,000 in 2020, p=0.06, RR=0.43 95% CI: 0.15–1.17). When I-CO-SA were analyzed, the incidence increased from 2017 to 2018 (54.7/10,000 vs. 65.03/10,000, p=0.08) and then was relatively stable from 2018 −2020 (57.6/10,000 in 2020, p=0.35, R2=0.54); there was no significant difference in incidence of I-CO-SA between 2019 and 2020 (p=0.47, RR=0.99, 95% CI: 0.78–1.32). Similar trends were noted when MRSA was analyzed alone. With regards to IGAS, an increase in incidence was noted from 2017–2019 (30.71/10,000 to 39.01/10,000, p=0.05, R2=0.98) which was followed by a decline in 2020 (25.36/10,000 admissions, p=0.02, RR=0.65, 95% CI: 0.45–93). Among IGAS, the decline in deep abscesses was most significant (15.69/10,000 in 2019 vs. 8.64/10,000 in 2020, p=0.03, RR=0.55 95% CI: 0.29–0.99). Among the cases of pneumonia in 2020 due to any of the three pathogens under study, none was associated with SARS-CoV-2 co-detection.
To adjust for potential seasonality, monthly incidence of disease was compared across study years. IPD exhibited declines in disease incidence beginning in August 2020 and achieved statistical significance in November. By contrast the incidence of IGAS peaked in March 2020 which was followed by a decline that reached statistical significance in June 2020 and largely continued through the remainder of the year.
Discussion
The SARS-CoV-2 pandemic has exacted a tremendous toll on the healthcare system stemming from the sheer number of cases, severity of illness, and psychologic impact on patients and providers. Additionally, social distancing and masking requirements enacted in many jurisdictions have further altered the practice of medicine, including rates of hospital admission and frequency of administration of vaccines, as well as the spectrum of diseases observed.7–9 Numerous authors have reported a temporal decline in the incidence of minor childhood illnesses and viral infections,3 presumably stemming from these infection prevention measures. We sought to evaluate the potential indirect impact of SARS-CoV-2 on invasive staphylococcal and streptococcal infections in children.
Following three years of relatively stable disease incidence, we observed a >40% decrease in IPD in 2020. From 2017–2019, our center observed a gradual increase in IGAS disease which was then followed by a sharp decline in 2020. Interestingly, the highest recorded incidence of IGAS at our center occurred in Jan-March 2020. Upper respiratory viral infection-related mucosal inflammation is hypothesized to promote entry of pneumococci into the bloodstream leading to hematogenous dissemination to distant sites.5 Based on this presumption, it is conceivable that a reduction in viral respiratory infections secondary to infection prevention measures could result in a decline in IPD and IGAS. In addition to the potential impact of reduced viral upper respiratory infections, closure of schools in March 2020 likely impacted student-to-student transmission of GAS and S. pneumoniae and potentially subsequent invasive infection. Approximately 15% of school age children have asymptomatic pharyngeal carriage of GAS and GAS transmission is well recognized to occur in the school environment.10 Our findings suggest that measures to prevent transmission of common respiratory viruses may serve as a useful adjunct in the prevention of IPD and IGAS. While prolonged quarantine and absence from in-person school activities are not feasible for long-term prevention, other strategies to reduce respiratory virus transmission, including aggressive hand hygiene, cough etiquette and staying home when ill, may mitigate secondary invasive bacterial infections.
By contrast, we observed a relatively stable incidence of I-CO-SA in the study period. Based on these findings, it appears that SARS-CoV-2 mitigation policies had little indirect impact on the incidence of I-CO-SA in our center. Interestingly, Wilder observed that while the rates of hospitalization for several diagnoses associated with respiratory viral illnesses (such as bronchiolitis) declined after March 2020, hospitalizations for cellulitis remained relatively stable compared to pre-pandemic data.9 Given that S. aureus is a prominent cause of cellulitis and other soft tissue infections, our findings are consistent with this previous work. Notably, S. aureus transmission dynamics within households are well described, with transmission events frequently occurring from family members as well as contaminated environmental surfaces.11,12 Increased time spent indoors and among household contacts may have contributed to the relatively stable incidence of invasive S. aureus in 2020, potentially mitigating any benefits from public social distancing and reduced respiratory illnesses.
There are limitations to this work which should be acknowledged. Foremost, our results are from a single children’s hospital network and may not reflect the general population. Given the utilization of culture-based surveillance, cases of invasive streptococcal or staphylococcal infection for which a culture was not obtained would not have been captured by our study design resulting in incorrect estimates of disease incidence. However, the reliance on culture provides a higher level of specificity than previous studies which have largely been based on diagnostic coding. Additionally, it is difficult to know if the perceived lower frequency of invasive infection was truly a result of reduced incidence or a change in care-seeking behavior as has been the case for routine immunizations.7 While reductions in non-invasive bacterial infections (e.g., skin infections or pharyngitis) may in part be attributable to patient/parent avoidance of physician offices/hospitals, it seems unlikely that this would impact invasive, potentially life-threatening infections. Finally, these findings represent a temporal association and a causal relationship between social distancing/masking/school closure and invasive streptococcal or staphylococcal infection cannot, strictly speaking, be inferred. Similar findings noted for less severe childhood infections reported by other investigators, however, add a degree of credence to our data.
In summary, we observed a decline in IPD and IGAS temporally associated with the institution of social distancing/masking/school closures in the Greater Houston area. By contrast, I-CO-SA incidence was stable relative to prior study years. Such findings have implications for the pathogenesis of invasive gram-positive infections in children. These trends should continue to be monitored as SARS-CoV-2 vaccines are administered, population immunity increases, and infection prevention measures are relaxed.
Conflicts of Interest:
Dr. McNeil receives grant funding from the Agency for Healthcare Research and Quality (AHRQ R01HS026896). Dr. McNeil is the local PI on a multicentre clinical trial sponsored by Nabriva Therapeutics unrelated to the work under consideration. Dr. McNeil has also received a donation of laboratory materials from Allergan for work unrelated to this manuscript. Dr. Kaplan and Dr. Hulten receive research support through Pfizer. Dr. Flores receives grant funding through NIAID R01AI25216, R21AI153663, R21AI142126, R21AI159059.
References
- 1.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased Influenza Activity During the COVID-19 Pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69: 1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Respiratory and Enteric Virus Surveillance Sytem, Centers for Disease Control and Prevention. RSV National Trends 2020. [CDC Website]; https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html. Accessed Feb 2, 2021. [Google Scholar]
- 3.McBride JA, Eickhoff J, Wald ER. Impact of COVID-19 Quarantine and School Cancelation on Other Common Infectious Diseases. Pediatr Infect Dis J. 2020; 39: e449–e452. [DOI] [PubMed] [Google Scholar]
- 4.Torretta S, Capaccio P, Coro I, et al. Incidental lowering of otitis-media complaints in otitis-prone children during COVID-19 pandemic: not all evil comes to hurt. Eur J Pediatr. 2021; 180: 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ampofo K, Bender J, Sheng X, et al. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics. 2008; 122: 229–237. [DOI] [PubMed] [Google Scholar]
- 6.Flores AR, Chase McNeil J, Shah B, et al. Capsule-Negative emm Types Are an Increasing Cause of Pediatric Group A Streptococcal Infections at a Large Pediatric Hospital in Texas. J Pediatric Infect Dis Soc. 2019; 8: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69: 591–593. [DOI] [PubMed] [Google Scholar]
- 8.Hatoun J, Correa ET, Donahue SMA, et al. Social Distancing for COVID-19 and Diagnoses of Other Infectious Diseases in Children. Pediatrics. 2020;146; e2020006460. [DOI] [PubMed] [Google Scholar]
- 9.Wilder JL, Parsons CR, Growdon AS, et al. Pediatric Hospitalizations During the COVID-19 Pandemic. Pediatrics. 2020; 146: e2020005983. [DOI] [PubMed] [Google Scholar]
- 10.Martin JM, Green M, Barbadora KA, et al. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics. 2004; 114: 1212–1219. [DOI] [PubMed] [Google Scholar]
- 11.Mork RL, Hogan PG, Muenks CE, et al. Comprehensive modeling reveals proximity, seasonality, and hygiene practices as key determinants of MRSA colonization in exposed households. Pediatr Res. 2018; 84: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mork RL, Hogan PG, Muenks CE, et al. Longitudinal, strain-specific Staphylococcus aureus introduction and transmission events in households of children with community-associated meticillin-resistant S aureus skin and soft tissue infection: a prospective cohort study. Lancet Infect Dis. 2020; 20: 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]


