Abstract
Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors that regulate various aspects of central nervous system processing in normal physiology and in disease. They are thought to function as disulfide-linked homodimers, but studies have suggested that mGluRs can form functional heterodimers in cell lines. Using selective allosteric ligands, ex vivo brain slice electrophysiology, and optogenetic approaches, we found that two mGluR subtypes—mGluR2 and mGluR4 (or mGlu2 and mGlu4)—exist as functional heterodimers that regulate excitatory transmission in a synapse-specific manner within the rodent medial prefrontal cortex (mPFC). Activation of mGlu2/mGlu4 heterodimers inhibited glutamatergic signaling at thalamo-mPFC synapses but not at hippocampus-mPFC or amygdala-mPFC synapses. These findings raise the possibility that selectively targeting these heterodimers could be a therapeutic strategy for some neurologic and neuropsychiatric disorders involving specific brain circuits.
INTRODUCTION
The metabotropic glutamate (mGlu) receptors include eight major subtypes (mGlu1-mGlu8) that differentially regulate synaptic transmission and other aspects of brain function based on their signaling pathways and cellular and synaptic localization (1, 2). Selective ligands for individual mGlu receptor subtypes have the potential as treatments for multiple brain disorders, including schizophrenia, depression, anxiety, Alzheimer’s disease, Parkinson’s disease, and addiction, as well as others (3). Historically, the eight mGlu receptor subtypes have been thought to function primarily as obligate homodimers (4, 5). However, time-resolved fluorescence resonance energy transfer (trFRET) and single-molecule fluorescence resonance energy transfer (smFRET) studies have revealed that mGlu receptors can also form heterodimers in HEK293 cells (6, 7). These findings could be critically important for therapeutically targeting these receptors.
Recent efforts have been successful in establishing unique pharmacological profiles for heterodimers between at least two mGlu receptor subtypes, mGlu2 and mGlu4, relative to mGlu2 and mGlu4 homodimers using selective positive allosteric modulators (PAMs) [7,9-15]. These include selective mGlu4 PAMs that potentiate mGlu4 homodimers, but not mGlu2 homodimers or mGlu2/mGlu4 (mGlu2/4) heterodimers (8–10) and other mGlu4 PAMs that potentiate responses to activation of either mGlu4 homodimers or mGlu2/4 heterodimers (9, 10). Furthermore, novel nanobodies have been identified that act as selective PAMs at mGlu2 homodimers but have no effects on mGlu4 homodimers or mGlu2/4 heterodimers (11). Identifying pharmacological differences between mGlu2/4 heterodimers versus homodimeric forms of these receptors has enabled studies that provide evidence that mGlu2/4 heterodimers may play a role in regulation of transmission at corticostriatal (10) and lateral perforant path-dentate gyrus synapses (12). However, the pharmacological tools used in these previous studies relied on evaluation of synergistic effects of orthosteric agonists (12) or compounds that acted on a single mGlu receptor protomer, rather than having complementary compounds that have independent actions on each protomer of a heterodimer complex (10). Thus, results of these previous studies in native systems could be explained by mechanisms other than activation of functional mGlu2/4 heterodimers. Furthermore, none of these previous studies included tools that would differentiate between mGlu2/4 and mGlu3/4 in native systems.
Both mGlu2 and mGlu4 are expressed in the medial prefrontal cortex (mPFC). The effect of mGlu2 activation has been extensively studied in the mPFC, where mGlu2 inhibits excitatory synaptic transmission at the thalamo-mPFC synapse. In addition, group III mGlu receptor agonists also inhibit excitatory synaptic transmission in the mPFC (13), which raises the possibility that mGlu2 and mGlu4 are co-expressed at the thalamo-mPFC synapse and that the effects of mGlu2 agonists in regulating this synapse are mediated by mGlu2/4 heterodimers. However, roles for mGlu4 in the effects of the group III mGlu receptor agonist in mPFC have not been established, and it is not clear whether this effect is mediated by specific actions at the thalamo-mPFC synapse. If mGlu2 and mGlu4 act as heterodimers at thalamo-mPFC synapses, this could have important implications for possible strategies for treatment of schizophrenia and related disorders. Over activity at this synapse has been postulated to play an important role in the pathophysiology that may underlie some symptoms associated with schizophrenia, including hallucinations and some aspects of cognitive dysfunction (14). Furthermore, serotonergic hallucinogens, such as 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) increase spontaneous activity at this synapse, and this can be reversed by activation of mGlu2 (15–18), and by newer atypical antipsychotic agents that have antagonist activity to the serotonin receptor 5-HT2. Thus, it will be critical to determine whether the ability of mGlu2 and mGlu4 agonists to reduce transmission at this synapse is due to activation of an mGlu2/4 heterodimeric form of the receptors.
Here, we characterized a highly selective negative allosteric modulator (NAM) that can act at the mGlu2 protomer, but not mGlu3, to inhibit activation of the mGlu2/4 heterodimer by an orthosteric agonist acting selectively at the mGlu4 protomer. This selective NAM, along with the previously available mGlu4 PAMs that can differentiate mGlu4 homomer from heteromer, allowed for a more definitive evaluation of potential functional roles of mGlu2/4 heterodimers at identified synapses in the CNS than was previously possible. With these tools, we uncovered exciting new evidence that mGlu2 and mGlu4 receptors operate as functional heterodimers to reduce transmission in a synapse-specific manner.
RESULTS
L-AP4 has different effects on optogenetically isolated thalamo-, hippocampo-, and amygdala-mPFC synaptic responses
The mPFC receives synaptic inputs from multiple cortical and subcortical regions, including the thalamus (particularly medial thalamic structures, such as mediodorsal (MD) and the midline/intralaminar nuclei), ventral hippocampus, and basolateral amygdala (19–26). To determine whether mGlu4 activation differentially modulates synaptic transmission at thalamo-cortical synapses and at synapses from other synaptic inputs to mPFC, we used optogenetic approaches to selectively activate the synaptic input of the mPFC from these regions. Optically evoked EPSCs (oEPSCs) were recorded from layer V pyramidal cells in coronal brain slices at a holding potential of −70mV and elicited by a blue light. Bath application of the group III mGlu agonist L-2-amino-4-phosphonobutyric acid (L-AP4; 30 μM) caused a robust inhibition of oEPSCs on thalamo-mPFC synapses measured at a time point when the drug was still present, right before drug washout (Fig. 1A). However, at the same time point, it only had a slight and non-significant acute effect on hippocampo-mPFC synaptic responses (Fig. 1B) and no effect on amygdala-mPFC synapses (Fig. 1C). These results suggest that L-AP4-induced acute inhibition of excitatory synaptic transmission in the mPFC is input specific, with a significant effect only on thalamo-mPFC synapses (Fig. 1, A to C). We note that L-AP4 did appear to induce a delayed-onset, prolonged inhibition at hippocampo-mPFC synapses (Fig. 1B). At around 14 to 15 min, the inhibition of oEPSCs was statistically significant, but there was no significant change in paired-pulse ratio (PPR; Fig. 1B), suggesting a postsynaptic mechanism was involved in the delayed inhibition. At other synapses, acute and more long-term depression of synaptic transmission are mechanistically distinct (27), and, for the current studies, we focused on mGlu receptors involved in acute regulation of excitatory synaptic transmission.
Fig. 1. Effects of L-AP4 on optogenetically evoked glutamatergic transmission at thalamo-, hippocampo-, and amygdala-mPFC synapses.
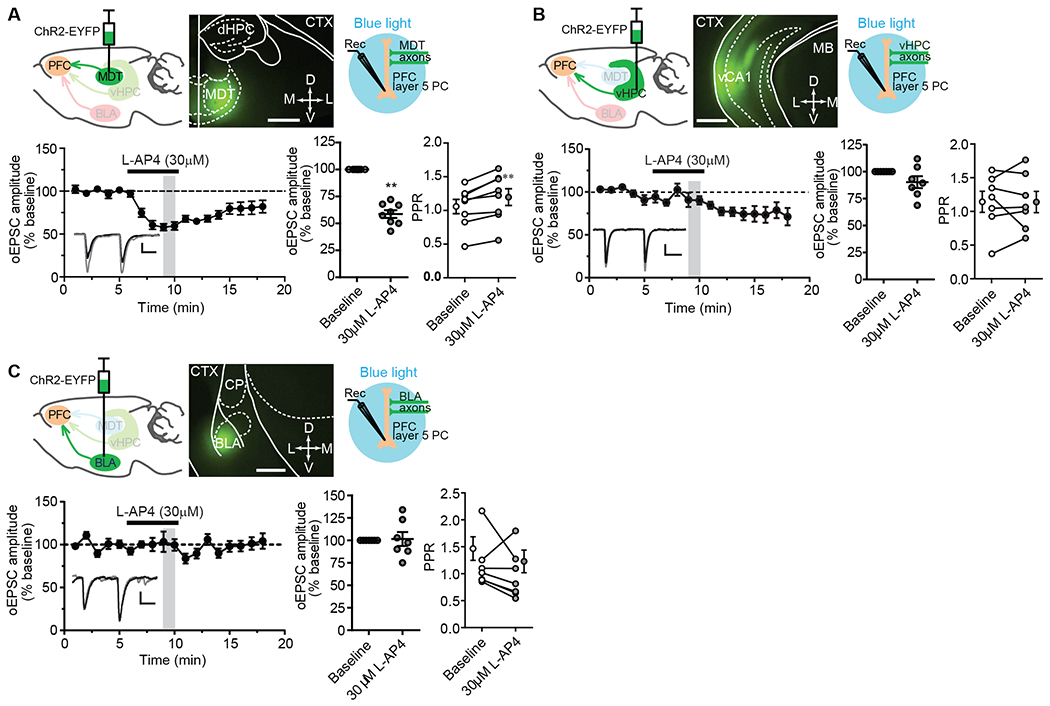
(A to C) Schematic diagram showing the injection of AAV5-CaMKIIa-hChR2(E123T/T159C)-EYFP into the mediodorsal thalamic nucleus (MDT; A), ventral hippocampus (vHPC; B), or basolateral amygdala (BLA; C), each with a representative image of EYFP-tagged ChR2 expression in the injection site 3 to 5 weeks after viral injection (calibration bar, 200μm), and a second schematic (right) depicting the acquisition of ex vivo whole-cell recordings from mPFC layer V pyramidal cells in response to light stimulation of ChR2:EYFP expressed exons. Bottom panels show the effect of L-AP4 on both oEPSCs over time and paired-pulse ratios (PPR) at each synapse type. Data are mean ± SEM from N = 8 neurons from total of 7 mice (A), and 7 neurons from 5 (B) or 4 (C) mice. Synapse-specific oEPSCs versus baselines were compared by Wilcoxon matched-pairs tests: **P <0.01 (A), P > 0.1 (B), and P > 0.9 (C). PPRs were also compared by Wilcoxon matched-pairs tests: **P < 0.01 (A), P > 0.9 (B), and P > 0.2 (C). Abbreviations: dHPC = dorsal hippocampus, CTX = cortex, vCA1= ventral CA1, MB = midbrain, CP = caudal putamen. Calibration bars for oEPSC traces: 100pA/20ms (A), 50pA/20ms (B), and 20pA/20ms (C). The gray-shaded vertical bar in the time course indicates the time points used to be averaged for statistical comparisons to baseline. For reference but not marked in the figure is the statistical comparison of oEPSCs and PPR at 14-15 min after L-AP4 application at hippocampo-mPFC synapses (B): P < 0.05 and P > 0.9, respectively, by Wilcoxon matched-pairs test.
The effect of L-AP4 on thalamo-mPFC synaptic responses is mediated by mGlu4
L-AP4 activates all group III mGlu receptor subtypes (mGlu4,6,7,8), though mGlu6 receptor expression is limited to the retina (28, 29). Thus, we used a panel of pharmacological tools to determine the specific group III mGlu receptor subtype(s) involved in the effect of L-AP4 on thalamo-mPFC synaptic responses. L-AP4 inhibited oEPSC amplitudes in a concentration-dependent manner at thalamo-mPFC synapses in layer V pyramidal cells of prefrontal cortical slices (Fig. 2A). LSP4-2022, an agonist that is selective for mGlu4 at the 5 μM concentration used (30), mimicked the inhibitory effect of L-AP4 at this synapse (Fig. 2B), while the mGlu8 agonist (S)-3,4-dicarboxyphenylglycine (DCPG; 1 μM) (31) did not (Fig. 2C). In addition, the mGlu7 NAM ADX71743 (3 μM) (32) did not block the L-AP4 induced inhibition of oEPSCs (Fig. 2D). These results suggest that L-AP4-induced inhibition of excitatory synaptic transmission at thalamo-mPFC synapses is mediated by mGlu4. Similar results were obtained in electrically evoked EPSCs in mPFC layer V pyramidal cells with L-AP4 in conjunction with LSP4-2022, DCPG, and ADX71743 (fig. S1, A to E). This was consistent with the optogenetic studies and suggests the effect of mGlu4 on thalamo-cortical EPSCs is clearly observed with electrical stimulation of multiple excitatory synapses.
Fig. 2. Effects of L-AP4 and mGlu4 PAMs with different pharmacologic profiles on glutamatergic transmission at thalamo-mPFC synapse.
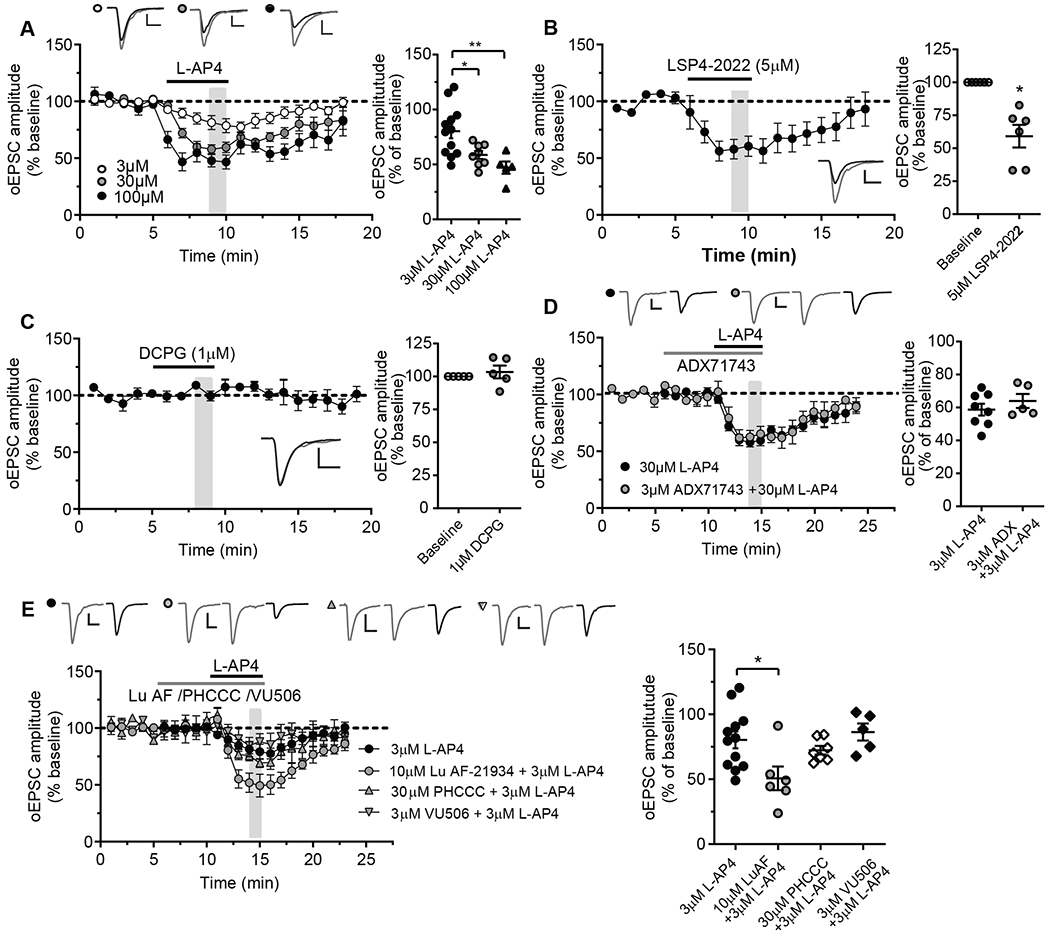
(A) Time courses (left) and dot plot (right) summarizing the effects of L-AP4 at different concentration on oEPSCs at thalamo-mPFC synapses (N = 12 neurons from 10 mice for 3 μM L-AP4, 8 neurons from 7 mice for 30 μM L-AP4 [data are from Fig.1A], and 5 neurons from 3 mice for 100 μM L-AP4. *P < 0.05 and **P < 0.01 by one-way ANOVA, [F(2,22)=7.30, P = 0.0037] with Bonferroni’s post-test. (B) The effect of LSP4-2022 on oEPSCs at thalamo-mPFC synapses. N = 6 neurons from 5 mice; *P < 0.05 by Wilcoxon matched-pairs test. (C) The effect of mGlu8 agonist DCPG on oEPSCs. N = 5 neurons from 3 mice; P > 0.4 by Wilcoxon matched-pairs test. (D) The effect of mGlu7 NAM ADX71743 with 30 μM L-AP4 on oEPSCs (N = 5 neurons from 3 mice) compared to the effect of 30 μM L-AP4 alone (same data as in Fig. 1A). P > 0.4 by Mann-Whitney test. (E and F) The effect of mGlu4 PAM Lu AF21934, PHCCC or VU0418506 with 3 μM L-AP4 on oEPSCs (N = 6 neurons from 4 mice, 7 per 5, and 5 per 4, respectively) compared to the effect of 3 μM L-AP4 on oEPSCs (same data as in panel A). *P < 0.05 by one-way ANOVA [F(3,26) = 4.14, P = 0.0159] with Dunnett’s post-test. Calibration bars for oEPSC traces: 100pA/10ms, 100pA/10ms, 40pA/10ms (A); 100pA/10ms (B, C); 200pA/10ms (D); 100pA/10ms (E).
mGlu2 and mGlu4 can function as heterodimers in the modulation of thalamo-mPFC synaptic transmission
In addition to mGlu4, previous studies suggest that mGlu2 agonists can also inhibit transmission at thalamo-mPFC synapses (15, 17, 18). To determine whether mGlu2 and mGlu4 can function as heterodimers in the modulation of synaptic transmission at thalamo-mPFC synapses, we took advantage of pharmacological reagents that selectively modulate signaling by mGlu2/4 heteromers relative to homomeric forms of the receptors. First, we used Lu AF21934, an mGlu4 PAM that is efficacious at both mGlu4 homodimers and mGlu2/4 heterodimers (10), to determine if it could potentiate the effect of a sub-maximal concentration of L-AP4 on oEPSCs at thalamo-mPFC synapses in layer V pyramidal cells of prefrontal cortical slices. In the absence of Lu AF21934, 3 μM L-AP4 inhibited oEPSCs by ~20%. However, 10 μM Lu AF21934 significantly potentiated the response to L-AP4, and the same concentration of L-AP4 inhibited oEPSCs by ~50% in the presence of the mGlu2/4 PAM (Fig. 2E). In contrast, 30 μM N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC), an mGlu4 PAM that is only active at mGlu4 homodimers but not mGlu2/4 heterodimers (10), failed to potentiate the L-AP4 induced inhibition of oEPSCs (Fig. 2E). Similar to PHCCC, another mGlu4 PAM, VU0418506 (3 μM), that has no efficacy at mGlu2/4 heterodimers (9), failed to potentiate the effect of L-AP4 on oEPSCs (Fig. 2E). Together, these results suggest that functional mGlu2/4 heterodimers might be present at thalamo-mPFC synapses and play an important role in the regulation of glutamate release at this synapse. Curiously, these results also argue against a functional role for mGlu4/4 homodimers at this synapse.
The mGlu2 NAM MRK-8-29 differentiates mGlu4 homodimers from mGlu2/4 heterodimers
To more definitively test the hypothesis that mGlu2/4 heterodimers are involved in regulation of transmission at the thalamo-mPFC synapse, it would be important to have a selective NAM that acts at the mGlu2 protomer to block mGlu2/4 activity. If a NAM acting on the mGlu2 protomer blocks responses to orthosteric agonists acting at the mGlu4 protomer, this combined with the heterodimer PAMs (which act at the mGlu4 protomer) used above would provide a clear demonstration that the two protomers must interact to induce mGlu2/4-mediated responses at the thalamo-mPFC synapse. We previously reported the characterization of MRK-8-29 as a novel mGlu2-selective NAM that has no effects on responses to L-AP4 at mGlu4 or on other mGlu receptor subtypes expressed as homodimers (33–36). To evaluate MRK-8-29 as a potential mGlu2/4 NAM, we determined the effects of this compound on responses to L-AP4 in human embryonic kidney (HEK) cell lines expressing only mGlu4 or both mGlu2 and mGlu4 (and presumably, therefore, at least some mGlu2/4 heterodimers) by assessing effects on G-protein-gated inwardly rectifying potassium (GIRK) channels using a well-established thallium flux assay (37). MRK-8-29 had no effect on L-AP4-induced thallium influx in the cell line expressing mGlu4 alone (Fig. 3A and table S1), but inhibited L-AP4-induced thallium influx in a concentration-dependent manner, reduced the L-AP4 maximal response and shifted the concentration-response-curves (CRC) to the right in the cell line expressing both mGlu2 and mGlu4 (Fig. 3B, table S1).
Fig. 3. Effect of mGlu2 NAM MRK-8-29 on L-AP4 induced inhibition of thalamo-mPFC synaptic transmission.
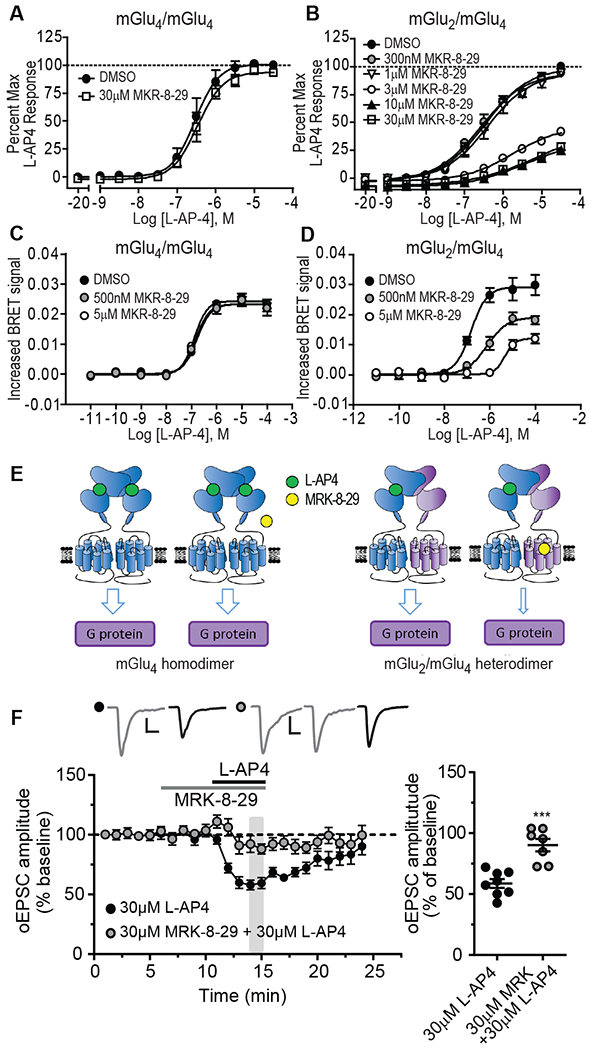
(A and B) The effects of MRK-8-29 on L-AP4 CRC in HEK cells expressing mGlu4 homodimers (A) or mGlu2/4 heterodimers (B) by GIRK assay. (C and D) The effects of MRK-8-29 on L-AP4 CRC in HEK cells expressing mGlu4 homodimers (C) or mGlu2/4 heterodimers (D) by CODA-RET assay. (E) The schematic diagrams illustrate the effect of MRK-8-29 on L-AP4 induced response at mGlu4 homodimers (left) or mGlu2/4 heterodimers (right). (F) The effect of 30 μM MRK-8-29 with L-AP4 on oEPSCs (N = 7 neurons from 5 mice) compared to the effect of 30 μM L-AP4 alone (same data as in Fig. 1A). ***P < 0.001 by Mann-Whitney test. Calibration bars for oEPSC traces: 100pA/10ms.
While studies with native mGlu2 and mGlu4 are critical for assessing effects of MRK-8-29 on native receptors, cell lines expressing mGlu2 and mGlu4 include homodimers of each receptor as well as mGlu2/4 heterodimers (9). Thus, the ability of MRK-8-29 to inhibit L-AP4-induced activation of mGlu2/4 heterodimers was further confirmed by the complemented donor-acceptor resonance energy transfer (CODA-RET) assay, which allows selective detection of a signal from heterodimer activation without contamination of the signal from homodimeric receptors (9, 38). Using this technique, we confirmed that MRK-8-29 does not inhibit L-AP4-induced responses in cultured HEK-293T cells expressing mGlu4/4 homodimers (Fig. 3C; table S2), but concentration-dependently inhibited the L-AP4-induced responses in the cells expressing mGlu2/4 heterodimers with reduced maximal responses and rightward shift of the L-AP4 CRC (Fig. 3D; table S2). The effects of MRK-8-29 on the L-AP4 induced response at mGlu4 homodimers and mGlu2/4 heterodimers are illustrated schematically (Fig. 3E).
L-AP4-induced inhibition of synaptic responses at thalamo-mPFC synapse is blocked by mGlu2 NAM MRK-8-29
Having established the effect of MRK-8-29 on mGlu2/4 heterodimers, we used this compound to further test the hypothesis that L-AP4-induced inhibition of oEPSCs at the thalamo-mPFC synapse is mediated by activation of mGlu2/4. In the presence of MRK-8-29 (30 μM), the ability of L-AP4 (30 μM) to inhibit oEPSCs at thalamo-mPFC synapse in prefrontal cortical slices was greatly diminished (Fig. 3F). Notably, L-AP4 acts only on the mGlu4 protomer, whereas MRK-8-29 acts selectively at the mGlu2 protomer to inhibit mGlu2/4 heterodimer signaling. Taken together with the finding that heterodimer, but not homodimer-selective, mGlu4 PAMs potentiate this response, these data provide strong support for the hypothesis that functional mGlu2/4 heterodimers are involved in inhibition of transmission at thalamo-mPFC synapses.
Activation of mGlu2/4 heterodimers inhibits DOI-induced increases in sEPSCs in mPFC layer V pyramidal cells
We and others have previously shown that 5-HT2 receptor activation with serotonergic hallucinogens (including DOI) induces an increase in frequency of spontaneous EPSCs (sEPSCs) in layer V pyramidal cells (15, 17, 18, 39, 40). Furthermore, lesions of the medial thalamus reduce the ability of 5-HT2 receptor activation to induce increases in sEPSCs (17), suggesting that the thalamo-mPFC synapse is the primary synapse that increases glutamate release in response to 5-HT2 receptor activation. The increase in sEPSCs can be suppressed by activation of mGlu2 (15, 16) or group III mGlu receptors (13, 39, 40). These previous studies, along with the present findings described above, raise the possibility that functional mGlu2/4 heterodimers at thalamo-mPFC synapses might be engaged in regulation of 5-HT2 receptor-mediated increases in sEPSCs in layer V pyramidal cells. To test this hypothesis, we used L-AP4 in combination with the mGlu2/4 NAM MRK-8-29 to determine if MRK-8-29 could reverse the L-AP4 effect on DOI-induced sEPSCs. Bath application of 10 μM DOI, a 5-HT2 receptor agonist, significantly increased the frequency of sEPSCs in layer V pyramidal cells of prefrontal cortical slices (Fig. 4A). In the presence of L-AP4 (3 μM), the same concentration of DOI (10 μM) had no effect on sEPSC frequency (Fig. 4B), suggesting that activation of group III mGlu receptors, likely mGlu4, is able to inhibit sEPSCs induced by 5-HT2 receptor activation. In addition, the inhibitory effect of L-AP4 on DOI-induced sEPSCs was antagonized by the mGlu2 NAM, MRK-8-29 (Fig. 4C). Together, these data demonstrated that L-AP4 inhibited DOI-induced sEPSCs (Fig. 4Band D), which could be reversed by the mGlu2 NAM MRK-8-29 that is efficacious at mGlu2/4 heterodimers (Fig. 4C and D).
Fig. 4. Effects of L-AP4 and MRK-8-29 on 5-HT2 agonist DOI induced increase in sEPSCs in mPFC layer V pyramidal cells.
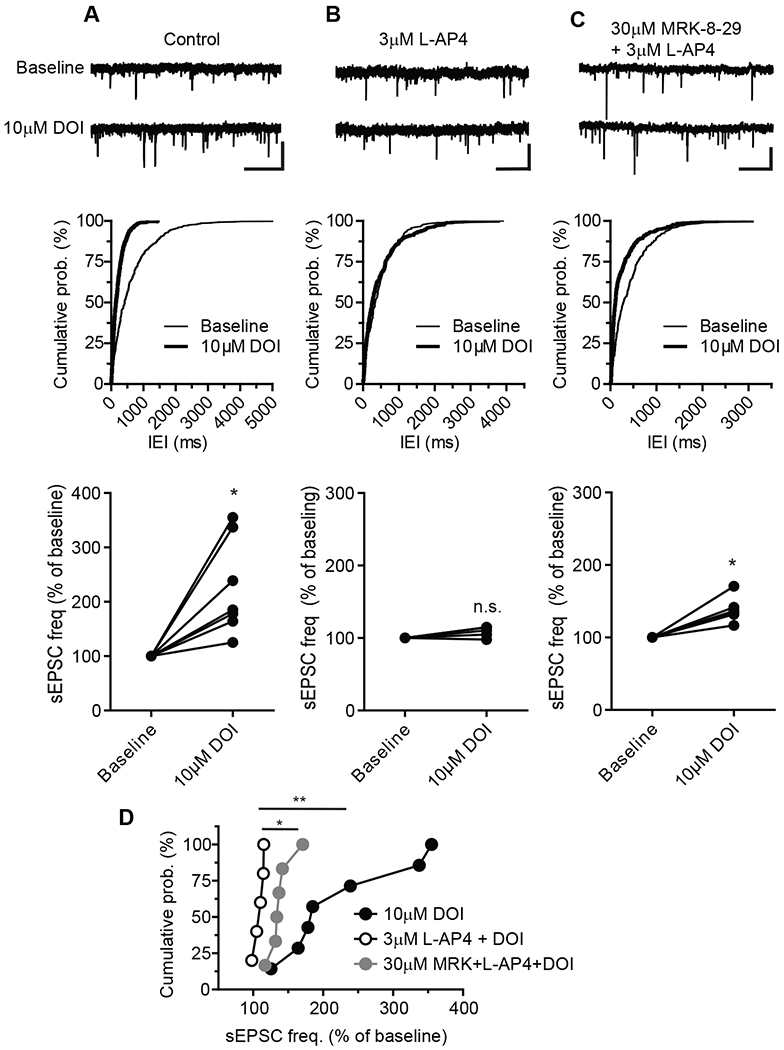
(A to C) Sample traces (top), cumulative probability plots of inter-event interval (IEI) of sEPSCs (middle) from three representative cells and summary plot of normalized group data (bottom) before and during application of 10μM DOI (A), before and during application of 10μM DOI in the presence of 3 μM L-AP4, and before and during application of 10μM DOI in the presence of 30 μM MRK-8-29 and 3 μM L-AP4. Calibration bars for sEPSC traces: 20pA/1s (A and B), and 30pA/1s (C). Normalized sEPSC frequency after application of 10μM DOI verse baseline under different conditions were compared using Wilcoxon matched-pairs tests: *P < 0.05, N = 7 neurons from 5 mice (A, bottom); P > 0.1, N = 5 per 4 (B, bottom); *P < 0.05, N = 6 per 4 (C, bottom). (D) Cumulative probability plots summarizing DOI-induced sEPSC frequency change in control, in the presence of L-AP4 and in the combination of L-AP4 and MRK-8-29. * P < 0.05 and ** P < 0.01 by one-way ANOVA [F(2,15)=7.113, P = 0.0067] with Bonferroni’s post-test.
To further confirm the role of mGlu2/4 in the DOI-induced increase in sEPSCs, we used two mGlu4 PAMs, Lu AF21934 that is active at Glu2/4 heterodimers (10) and VU0418506 that is selective for mGlu4 homodimers and is inactive at mGlu2/4 heterodimers (9), to determine if they had differential effects on L-AP4 modulation of DOI-induced sEPSCs. Bath application of a low concentration of L-AP4 (0.3 μM) did not block DOI-induced sEPSCs (fig. S2A). However, in the presence of 10 μM Lu AF21934, the same low concentration of 0.3 μM L-AP4 was able to suppress DOI-induced sEPSCs, compared to 0.3 μM L-AP4 alone (fig. S2, B and D). In contrast, in the presence of VU0418506 (3 μM), the low concentration of L-AP4 was not able to suppress DOI-induced sEPSCs, compared to 0.3 μM L-AP4 alone (fig. S2, C and D). These results, combined with the studies of oEPSCs outlined above, suggest that activation of mGlu2/4 heterodimers can reduce both evoked and DOI-induced EPSCs at the thalamo-mPFC synapse.
DISCUSSION
Since the initial reports suggesting that mGlu4 and mGlu2 can form heterodimers in cell lines and transfected neurons (6, 8), emerging evidence has suggested the presence of functional mGlu2/4 heterodimers in native brain tissues. Previous studies identified two synapses that may be regulated by mGlu2/4 heterodimers. These include corticostriatal synapses (10) and hippocampal lateral perforant path-dentate gyrus synapses (12). However, these studies relied on showing synergistic effects of orthosteric agonists (12) or on the use of agents acting solely on the mGlu4 protomer to differentiate between mGlu2/4 heteromers and mGlu4 homomers (10). The previous lack of allosteric modulators that can differentiate between mGlu2 and mGlu3, as well as compounds that modulate mGlu2/4 activity by actions at the mGlu2 protomer, allows for alternate interpretations that may not require activity of mGlu2/4 heteromeric receptors. To more definitively establish a role for mGlu2/4 heterodimers, it is important to demonstrate that ligands acting at either the mGlu2 or mGlu4 protomer of a heteromeric complex have effects that are consistent with their actions on the heteromeric receptor. In the present studies, we took advantage of the highly selective allosteric modulators that act on mGlu4 protomers and have distinct pharmacological profiles for mGlu4 homodimers and mGlu2/4 heterodimers. In addition, we report the discovery that MRK-8-29 is a novel mGlu2/4 NAM that acts on the mGlu2 protomer, but not mGlu3, to inhibit responses to L-AP4, which provides a major advance in more firmly establishing a clear role for mGlu2/4 and not mGlu3/4 heterodimers at an identified synapse in the CNS than did any previous studies. Furthermore, each of these tools has been characterized for activity at mGlu2/4 heteromeric receptors using CODA-RET, which allows definitive demonstration of activities at defined homomeric and heteromeric receptors.
Using these novel molecular probes in conjunction with electrophysiology and optogenetic studies, we provide strong evidence that mGlu2/4 heterodimers selectively inhibit transmission at the thalamo-mPFC synapse, a key synapse that plays a critical role in multiple aspects of mPFC function. Specifically, we used a range of mGlu4 PAMs that show distinct pharmacologic profiles and found that the homodimer-selective mGlu4 PAMs PHCCC and VU0418506 do not potentiate L-AP4-mediated inhibition of thalamo-mPFC oEPSCs. In contrast, Lu AF21934, which is efficacious at both mGlu4 homodimers and mGlu2/4 heterodimers, potentiates the L-AP4 effect. Furthermore, the mGlu2/4 NAM MRK-8-29, which shows no efficacy at mGlu4 homodimers, inhibited L-AP4-induced inhibition of oEPSCs at this synapse. Consistent with these findings, previous anatomical studies suggest that both mGlu2 and mGu4 are co-expressed at thalamo-mPFC synapses (41–44). The findings that the homodimer-selective mGlu4 PAMs PHCCC and VU0418506 failed to potentiate L-AP4 induced inhibition of thalamo-mPFC synaptic transmission suggested that there are probably no functional mGlu4 homodimers present at this synapse. However, the current studies could not rule out the possibility of the presence of functional mGlu2 homodimers at thalamo-mPFC synapses, which remains to be determined in future studies.
It is particularly interesting that the role of mGlu2/4 heterodimers is synapse-specific and active at thalamo-mPFC synapses, but not at synapses in the same neuronal population from projections originating in the hippocampus or amygdala. Based on this, mGlu2/4 heterodimers could selectively modulate specific functions of the mPFC associated with specific synapses. For instance, the thalamic input to mPFC is critically engaged in many different cognitive processes, such as regulation of goal-directed behaviors, working memory, and social dominance behavior (45–48). In contrast, the information flow between the ventral hippocampus and mPFC is important for spatial working memory, fear learning and anxiety-related behaviors (49–51) and amygdala-mPFC synaptic interactions have been implicated in regulation of emotional process, stress, fear learning and anxiety-related behaviors (52–54). The exquisite synapse specificity of mGlu2/4 is very exciting and provides a novel opportunity to exert highly selective modulation of activity at a specific input to a critical neuronal population. It is especially interesting in light of previous studies suggesting that modulation of activity at thalamo-mPFC synapses plays a major role in actions of hallucinogenic drugs and inhibition of transmission at this synapse could provide a benefit in schizophrenia patients (13, 15–17, 55, 56). Thus, the mGlu2/4 heterodimer at thalamo-mPFC synapses could be a novel therapeutic target for treatment of positive symptoms of schizophrenia. In addition, integrity of the mediodorsal thalamic prefrontal cortical loop is essential for cognitive control and motivation (57, 58), and interruption of integrity of the MD thalamic nucleus and PFC has been implicated in psychiatric symptoms related to cognitive deficits and negative symptoms of schizophrenia (59, 60). However, it remains to be determined whether and how the mGlu2/4 heterodimer at thalamo-mPFC synapses could contribute to treatment of cognitive disturbance and negative symptoms of schizophrenia. Nonetheless the potential relevance of the current findings to potential strategies for treatment of schizophrenia highlights the exciting breakthrough of identifying a mechanism that may enable highly synapse-specific pharmacological manipulations in a given brain structure.
Previous studies with animal models have shown that Lu AF21934, a mGlu4 PAM with activity at mGlu2/4, can have effects that are relevant for treatment of symptoms associated with schizophrenia, including reversal of hyperactivity induced by MK-801 or amphetamine, and head twitches induced by DOI (39). In light of our current understanding, it is possible that some of these previously reported effects of Lu AF21934 could be mediated by actions on mGlu2/4. In future studies, it will be important to fully evaluate the behavioral effects of mGlu4 and mGlu2 homomer-selective allosteric modulators relative to mGlu2/4 modulators to develop a more complete understanding of the potential utility of selectively targeting homomeric versus heteromeric forms of these receptors.
Our findings here along with previous reports altogether suggest that selectively targeting heteromeric forms of the receptors could provide a novel approach to precisely modulate specific inputs and functional responses. The findings that mGlu2 and mGlu4 only form heterodimers at selective synapses in the CNS raise the exciting possibility that developing novel therapeutics that targets these heterodimeric receptors might provide a unique therapeutic profile for treatment of brain disorders.
MATERIALS AND METHODS
Animals
For ex vivo electrophysiology studies, both male and female C57BL/J6 mice (7 to 14 weeks old) were used. As we did not note any obvious differences between male and female mice in electrophysiological responses to pharmacological agents, the data from both sexes were pooled and analyzed together. Mice were group-housed in an animal facility maintained at 23 °C with 12-hour light/dark cycles and food and water available ab libitum in their home cages. All experimental procedures were approved by the Vanderbilt University Animal Care and Use committee and followed the guidelines set forth by the Guide for the Care and Use of Laboratory animals.
Drugs and virus
L-AP4 was obtained from Abcam (Cambridge MA). DOI was obtained from Sigma-Aldrich (St Luis, MO). DCPG was obtained from Tocris (Minneapolis, MN). ADX71743, VU0418506, Lu AF21934, LSP4-2022, PHCCC and MRK-8-29 were synthesized in house at Vanderbilt Center for Neuroscience Drug Discovery. AAV5/CaMKIIa-hChR2 (H134R)-EYFP-WPRE (61) (tilter > 4 X 1012 vg/ml) was obtained from University of North Carolina Vector Core.
Stereotaxic surgery.
A subset of mice underwent stereotaxic surgeries. Adeno-associated virus serotype 5 (AAV5) carrying fusion genes for ChR2 and EYFP under the CaMKIIa promoter (AAV5/CaMKIIa-hChR2 (H134R)-EYFP-WPRE, UNC Vector Core) were bilaterally injected into the mediodorsal thalamic nucleus, ventral hippocampus or basolateral amygdala. Mice (5 to 7 weeks old) were anaesthetized with 2-3% isoflurane and placed on a motorized stereotaxic apparatus (Neurostar, Tubingen, Germany). A small craniotomy was made on the skull over the injection location. Viral particles were injected through a 28-gauge needle using a Hamilton syringe placed on a Microinjection Robot mounted on the Neurostar motorized stereotaxic apparatus at a rate of 0.2 μL/min. Injection volumes were 200-500nl each location. The following coordinates relative to Bregma were used (in mm), for thalamus, the mediodorsal nuclei were targeted, A/P:−1.5, M/L: ±0.36, D/V:−3.5; for the ventral hippocampus, A/P:−3.4, M/L: ±3.2, D/V:−4.0; for the basolateral amygdala, A/P: +1.2, M/L: ±3.2, D/V:−4.9. Three to seven weeks following surgery, brains were removed for ex vivo slice electrophysiology recordings.
Brain slice preparation
Coronal brain slices (300 μm) containing the mPFC were prepared from mice using a procedure similar to those described previously (15, 34). Specifically, mice were anesthetized with isoflurane and perfused transcardially with ice-cold, oxygenated (95% O2/5% CO2) cutting solution. Brains were removed from the skull and submerged in cold, oxygenated cutting solution, which was composed of (in mM): 220 glucose, 2.5 KCl, 8 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 D-glucose. Coronal brain slices (300 μm) containing the prefrontal cortex were cut using a Leica VT1200S microtome (Leica Microsystems Inc), incubated at 32 °C for 30 min in artificial cerebrospinal fluid (ACSF) with addition of 12 mM N-acetyl-L-cysteine (pH adjusted to 7.3-7.4 with KOH and osmolality to 300-310 with deionized water), and then maintained at room temperature afterward until transferred to a recording chamber. The chamber was continuously superfused with oxygenated regular ACSF at a rate of ~2 mL/min. The ACSF contained (in mM): 126 NaCl, 2.5 KCl, 2.0 CaCl2, 1.0 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, and 10 D-glucose.
Ex vivo electrophysiology
Whole cell recordings were made from visually identified layer V pyramidal cells in mPFC slices under an Olympus BX50WI upright microscope (Olympus, Lake Success, NY). Recording electrodes were prepared from borosilicate glass (Sutter Instruments, Novato, CA) using a Narishige puller (model PP-830; Narishige International USA, East Meadow, NY) and had resistance of 3–4 MΩ when filled the following electrode solution (in mM): 123 K-gluconate, 7 KCl, 1 MgCl2, 0.025 CaCl2, 10 HEPES, 0.1 EGTA, 2 ATP and 0.2 GTP at a pH of 7.3 and osmolarity of ~295 mOsm. The cell was voltage-clamped at −70 to −75 mV, closed to the calculated equilibrium potential of Cl−such that GABAA receptor mediated inhibitory currents were undetectable under this recording condition. For electrically evoked excitatory postsynaptic currents (eEPSCs), a concentric bipolar electrode (Frederick Haer Company, Bowdoinham, ME) was placed at layer 2/3. For optically evoked EPSCs (oEPSCs), a blue light (470nm LED, CoolLED) was delivered through a 40x water immersion objective lens on the Olympus BX50WI upright microscope. The electrophysiological signal was amplified using an Axon Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 3 kHz, digitized at 20 kHz and acquired using a Clampex10/DigiData1440A system (Molecular Devices). Recordings with change in access resistance greater than 20% that affected EPSC kinetics were excluded from the final analysis.
Analysis of electrophysiology data
Clampfit 10 (Molecular Devices) was used for analyzing evoked EPSCs; MiniAnalysis (Synaptosoft Inc) for detecting and analyzing spontaneous EPSCs; Excel (Microsoft) for data management; and Prism 5.0 (GraphPad Software) for statistical analysis. Data were presented as mean ± SEM and median with interquartile interval. Wilcoxon matched-pairs test was used for testing the change after drug application; Mann-Whitney test for comparing changes between two groups; one-way ANOVA plus Bonferroni adjusted post-test for comparing changes in more than two settings.
Thallium flux assays
Cell culture for thallium flux assays were established and cultured as described previously (9, 10, 62). Rat mGlu2 or rat mGlu4 was cloned into the pIRESpuro3 vector, transfected into human embryonic kidney (HEK)/GIRK cells, and selected with puromycin. Polyclonal rat mGlu2/HEK/GIRK and rat mGlu4/HEK/GIRK cells were cultured in growth media as previously described (63), supplemented with nonessential amino acids. Rat mGlu4 was also subcloned into the pIREShyg3 vector, and the resulting plasmid was transfected into rat mGlu2/HEK/GIRK cells; cells were then selected with 200 μg/ml hygromycin B. Polyclonal cells were cultured in growth media supplemented with 100 μg/ml hygromycin B.
Thallium flux assays were then performed according to methods described previously (37) with minor modifications. For dye loading, media was exchanged with assay buffer (HBSS, containing 20 mm HEPES, pH 7.4) using an ELX405 microplate washer (BioTek), leaving 20 μL/well, followed by addition of 20 μL/well 2× FluoZin-2 AM (330 nm final) indicator dye, (Invitrogen, prepared as a DMSO stock and mixed in a 1:1 ratio with pluronic acid F-127) in assay buffer. After 1 hour incubation at room temperature, dye was exchanged with assay buffer, leaving 20 μL/well. Thallium flux was measured at room temperature using a Functional Drug Screening System 7000 (Hamamatsu). Baseline readings were taken (2 images at 1 Hz; excitation, 470 ± 20 nm; emission, 540 ± 30 nm), and test compounds (2X) were added in a 20 μL volume and incubated for 140 s before the addition of 10 μl of thallium buffer with or without agonist (5X). Data were collected for an additional 2.5 min and analyzed using Excel (Microsoft) as previously described (63), and the concentration–response curves were fitted to a four-parameter logistic equation to determine potency estimates using GraphPad Prism as follows:
where A is the molar concentration of the compound, bottom and top denote the lower and upper plateaus of the concentration–response curve, HillSlope is the Hill coefficient that describes the steepness of the curve; and pEC50 is the negative logarithm of the molar concentration of compound required to generate a response halfway between the top and bottom.
CODA-RET assays
CODA-RET assays were performed in cultured HEK-293T cells that were transfected with rat mGlu2 and mGlu4 cDNAs tagged with a hemagglutinin (HA) epitope tag at the N-terminal using standard molecular biology procedures as previously described (9). Specific procedures were as follows: cDNAs encoding the L1 (residues 1– 229) or L2 (residues 230–311) fragments of Renilla Luciferase 8 (RLuc8, a gift from Sam Gambhir, Stanford) were fused in frame to the C terminus of mGlu4 and mGlu2 following the linker “GSPPARAT” in the pcDNA3.1 vector. The following G-protein constructs were also used: Gαi-mVenus with the mVenus inserted at position 91, untagged Gβ1, and untagged Gγ2. The integrity of all the constructs was confirmed with sequencing analysis. Cultured HEK-293T cells were transfected with a constant amount of plasmid cDNA using polyethylenimine (Polysciences Inc.) in a 1:1 ratio in 10 cm dishes. The ratio of transfected plasmids was optimized to maximize the luminescence of the complemented donor as well as the dynamic range of the BRET response to L-AP4. In the case of mGlu4 homodimers, the ratio of mGlu4-L1, mGlu4-L2, Gαi-mVenus, Gβ1 and Gγ2 was 4:4:1:1:1 (for a 10 cm dish, 4, 4, 1, 1, and 1 μg respectively). For the mGlu2 and mGlu4 heterodimer, the ratio of mGlu4-L1, mGlu2L2, Gαi-mVenus, Gβ1, and Gγ2 was 8:4:1:1:1 (for a 10 cm dish, 8, 4, 1, 1, and 1 μg respectively). Cells were maintained in culture with DMEM supplemented with 10% FBS. Forty-eight hours after transfection, cells were harvested, washed twice, and resuspended in PBS. Approximately 200,000 cells per well were distributed in 96-well plates and stimulated by the indicated drugs dissolved in prewarmed PBS for 5 min at 37 °C. A concentration of 5 μM coelenterazine H (the substrate for luciferase) was added to each well (Dalton Pharma Services). Two minutes after the addition of coelenterazine H, the fluorescence and luminescence was quantified (Pherastar, BMG Labtech) and the BRET signal was determined by calculating the ratio of the emission of mVenus (510–540 nm) over the emission of Rluc8 (485 nm). This technique combines luciferase complementation and bioluminescence resonance energy transfer (BRET) to directly measure the signal induced by activating a defined receptor heterodimer (mGlu2/4 in the present studies). A modified Renilla reniformis luciferase (Rluc8) is split into N-terminal (L1) and C-terminal (L2) fragments which have no function alone but complement to form a functional luciferase when in close proximity. The fragments are fused to the C tail of the target receptors, here mGlu2 and mGlu4, to control the complemented protomers. In a homodimer, only one fragment of the donor molecule will be present, and no signal will be generated in response to substrate. By monitoring the BRET signal between a complemented pair of defined protomers (as a donor) and mVenus labeled Gα subunit (as an acceptor), we can selectively determine G protein recruitment by the activated heterodimer.
Supplementary Material
Acknowledgements:
The authors would like to thank Weimin Peng, Doug Shaw and Jennifer Zachry for excellent assistant in stereotaxic viral injection, and Dr. Wu Gong for providing invaluable consultation for statistical analysis. We also thank Sam Gambhir (Stanford) for gifting us the RLuc8 fragments.
Funding:
This work is supported by grants from National Institute of Health: NS031373 (P.J.C.), and MH108498 (C.M.N. and C.W.L.), and R21NS113614 (J.A.J.).
Footnotes
Competing interests: D.E.O. is currently an employee of AbbVie, Inc. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Plasmids for CODA-RET are available upon request by standardized, universally acceptable Materials Transfer Agreement (the UBMTA-Master) through Columbia University.
References and Notes
- 1.Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP, Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041 (2011); published online Epub6/2011 (S0028-3908(10)00292-3 [pii]; 10.1016/j.neuropharm.2010.10.022 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niswender CM, Conn PJ, Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual review of pharmacology and toxicology 50, 295–322 (2010); published online Epub2010 ( 10.1146/annurev.pharmtox.011008.145533). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickols HH, Conn PJ, Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol.Dis 61, 55–71 (2014); published online Epub1/2014 (S0969-9961(13)00259-3 [pii]; 10.1016/j.nbd.2013.09.013 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K, Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977 (2000); published online Epub10/26/2000 ( 10.1038/35039564 [doi]). [DOI] [PubMed] [Google Scholar]
- 5.Romano C, Yang WL, O’Malley KL, Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J.Biol.Chem 271, 28612–28616 (1996); published online Epub11/8/1996 ( [DOI] [PubMed] [Google Scholar]
- 6.Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP, A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 25, 66–77 (2011); published online Epub1/2011 (fj.10-163147 [pii]; 10.1096/fj.10-163147 [doi]). [DOI] [PubMed] [Google Scholar]
- 7.Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, Isacoff EY, Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron 92, 143–159 (2016); published online Epub10/5/2016 (S0896-6273(16)30529-3 [pii]; 10.1016/j.neuron.2016.08.036 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kammermeier PJ, Functional and pharmacological characteristics of metabotropic glutamate receptors 2/4 heterodimers. Mol Pharmacol 82, 438–447 (2012); published online Epub9/2012 (mol.112.078501 [pii]; 10.1124/mol.112.078501 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niswender CM, Jones CK, Lin X, Bubser M, Thompson GA, Blobaum AL, Engers DW, Rodriguez AL, Loch MT, Daniels JS, Lindsley CW, Hopkins CR, Javitch JA, Conn PJ, Development and Antiparkinsonian Activity of VU0418506, a Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor 4 Homomers without Activity at mGlu2/4 Heteromers. ACS Chem.Neurosci 7, 1201–1211 (2016); published online Epub9/21/2016 ( 10.1021/acschemneuro.6b00036 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin S, Noetzel MJ, Johnson KA, Zamorano R, Jalan-Sakrikar N, Gregory KJ, Conn PJ, Niswender CM, Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J.Neurosci 34, 79–94 (2014); published online Epub1/1/2014 (34/1/79 [pii]; 10.1523/JNEUROSCI.1129-13.2014 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholler P, Nevoltris D, De BD, Bossi S, Moreno-Delgado D, Rovira X, Moller TC, El MD, Mathieu M, Blanc E, McLean H, Dupuis E, Mathis G, Trinquet E, Daniel H, Valjent E, Baty D, Chames P, Rondard P, Pin JP, Allosteric nanobodies uncover a role of hippocampal mGlu2 receptor homodimers in contextual fear consolidation. Nat.Commun 8, 1967 (2017); published online Epub12/6/2017 ( 10.1038/s41467-017-01489-1 [doi]; 10.1038/s41467-017-01489-1 [pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno DD, Moller TC, Ster J, Giraldo J, Maurel D, Rovira X, Scholler P, Zwier JM, Perroy J, Durroux T, Trinquet E, Prezeau L, Rondard P, Pin JP, Pharmacological evidence for a metabotropic glutamate receptor heterodimer in neuronal cells. Elife. 6, (2017); published online Epub6/29/2017 ( 10.7554/eLife.25233 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Marek GJ, Group III metabotropic glutamate receptor agonists selectively suppress excitatory synaptic currents in the rat prefrontal cortex induced by 5-hydroxytryptamine2A receptor activation. J.Pharmacol.Exp.Ther 320, 437–447 (2007); published online Epub1/2007 (jpet.106.107490 [pii]; 10.1124/jpet.106.107490 [doi]). [DOI] [PubMed] [Google Scholar]
- 14.Marek GJ, Metabotropic glutamate2/3 (mGlu2/3) receptors, schizophrenia and cognition. Eur.J.Pharmacol 639, 81–90 (2010); published online Epub8/10/2010 (S0014-2999(10)00252-9 [pii]; 10.1016/j.ejphar.2010.02.058 [doi]). [DOI] [PubMed] [Google Scholar]
- 15.Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E, A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol 72, 477–484 (2007); published online Epub2007 (mol.107.035170 [pii] 10.1124/mol.107.035170). [DOI] [PubMed] [Google Scholar]
- 16.Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK, Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292, 76–87 (2000); published online Epub2000 ( [PubMed] [Google Scholar]
- 17.Marek GJ, Wright RA, Gewirtz JC, Schoepp DD, A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 105, 379–392 (2001); published online Epub2001 (S0306-4522(01)00199-3 [pii]). [DOI] [PubMed] [Google Scholar]
- 18.Joffe ME, Centanni SW, Jaramillo AA, Winder DG, Conn PJ, Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies. ACS Chem.Neurosci 9, 2188–2204 (2018); published online Epub9/19/2018 ( 10.1021/acschemneuro.8b00200 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacon SJ, Headlam AJ, Gabbott PL, Smith AD, Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 720, 211–219 (1996); published online Epub5/13/1996 (0006-8993(96)00155-2 [pii]; 10.1016/0006-8993(96)00155-2 [doi]). [DOI] [PubMed] [Google Scholar]
- 20.DeNardo LA, Berns DS, DeLoach K, Luo L, Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat.Neurosci. 18, 1687–1697 (2015); published online Epub11/2015 ( 10.1038/nn.4131 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jay TM, Witter MP, Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J.Comp Neurol 313, 574–586 (1991); published online Epub11/22/1991 ( 10.1002/cne.903130404 [doi]). [DOI] [PubMed] [Google Scholar]
- 22.Krettek JE, Price JL, Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J.Comp Neurol. 172, 687–722 (1977); published online Epub4/15/1977 ( 10.1002/cne.901720408 [doi]). [DOI] [PubMed] [Google Scholar]
- 23.Krettek JE, Price JL, The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J.Comp Neurol. 171, 157–191 (1977); published online Epub1/15/1977 ( 10.1002/cne.901710204 [doi]). [DOI] [PubMed] [Google Scholar]
- 24.McDonald AJ, Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. J.Comp Neurol. 262, 46–58 (1987); published online Epub8/1/1987 ( 10.1002/cne.902620105 [doi]). [DOI] [PubMed] [Google Scholar]
- 25.Takagishi M, Chiba T, Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 566, 26–39 (1991); published online Epub12/6/1991 (0006-8993(91)91677-S [pii]). [DOI] [PubMed] [Google Scholar]
- 26.Thierry AM, Gioanni Y, Degenetais E, Glowinski J, Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus 10, 411–419 (2000); published online Epub2000 ( [doi]). [DOI] [PubMed] [Google Scholar]
- 27.Gladding CM, Fitzjohn SM, Molnar E, Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol.Rev. 61, 395–412 (2009); published online Epub12/2009 (pr.109.001735 [pii]; 10.1124/pr.109.001735 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conn PJ, Pin JP, Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37, 205–237 (1997); published online Epub1997 ( [DOI] [PubMed] [Google Scholar]
- 29.Pin JP, Duvoisin R, The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34, 1–26 (1995); published online Epub1995 ( [DOI] [PubMed] [Google Scholar]
- 30.Goudet C, Vilar B, Courtiol T, Deltheil T, Bessiron T, Brabet I, Oueslati N, Rigault D, Bertrand HO, McLean H, Daniel H, Amalric M, Acher F, Pin JP, A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. Faseb J 26, 1682–1693 (2012); published online Epub4/2012 (fj.11-195941 [pii]; 10.1096/fj.11-195941 [doi]). [DOI] [PubMed] [Google Scholar]
- 31.Thomas NK, Wright RA, Howson PA, Kingston AE, Schoepp DD, Jane DE, (S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology 40, 311–318 (2001); published online Epub3/2001 (S0028390800001696 [pii]). [DOI] [PubMed] [Google Scholar]
- 32.Kalinichev M, Le PE, Bolea C, Girard F, Campo B, Fonsi M, Royer-Urios I, Browne SE, Uslaner JM, Davis MJ, Raber J, Duvoisin R, Bate ST, Reynolds IJ, Poli S, Celanire S, Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J.Pharmacol.Exp.Ther. 350, 495–505 (2014); published online Epub9/2014 (jpet.114.214437 [pii]; 10.1124/jpet.114.214437 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien DE, Shaw DM, Cho HP, Cross AJ, Wesolowski SS, Felts AS, Bergare J, Elmore CS, Lindsley CW, Niswender CM, Conn PJ, Differential Pharmacology and Binding of mGlu2 Receptor Allosteric Modulators. Mol.Pharmacol. 93, 526–540 (2018); published online Epub5/2018 (mol.117.110114 [pii]; 10.1124/mol.117.110114 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, Lindsley CW, Conn PJ, Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc.Natl.Acad.Sci.U.S.A 112, 1196–1201 (2015); published online Epub1/27/2015 (1416196112 [pii]; 10.1073/pnas.1416196112 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bungard CJ, Converso A, De Leon P, Hanney B, Hartingh TJ, Manikowski JJ, Manley PJ, Meissner R, Meng Z, Perkins JJ, Rudd MT, Shu Y, Quinoline Carboxamide and Quinoline Carbonitrile Derivatives as mGluR2-Negative Allosteric Modulators, Compositions, and Their Use. (2013). [Google Scholar]
- 36.Celanire S, Sebhat I, Wichmann J, Mayer S, Schann S, Gatti S, Novel metabotropic glutamate receptor 2/3 antagonists and their therapeutic applications: a patent review (2005-Present). Expert.Opin.Ther.Pat 25, 69–90 (2015); published online Epub1/2015 ( 10.1517/13543776.2014.983899 [doi]). [DOI] [PubMed] [Google Scholar]
- 37.Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD, A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol 73, 1213–1224 (2008); published online Epub2008 (mol.107.041053 [pii] 10.1124/mol.107.041053). [DOI] [PubMed] [Google Scholar]
- 38.Urizar E, Yano H, Kolster R, Gales C, Lambert N, Javitch JA, CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat.Chem.Biol.7, 624–630 (2011); published online Epub7/24/2011 (nchembio.623 [pii]; 10.1038/nchembio.623 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slawinska A, Wieronska JM, Stachowicz K, Marciniak M, Lason-Tyburkiewicz M, Gruca P, Papp M, Kusek M, Tokarski K, Doller D, Pilc A, The antipsychotic-like effects of positive allosteric modulators of metabotropic glutamate mGlu4 receptors in rodents. Br.J.Pharmacol. 169, 1824–1839 (2013); published online Epub8/2013 ( 10.1111/bph.12254 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wozniak M, Golembiowska K, Noworyta-Sokolowska K, Acher F, Cieslik P, Kusek M, Tokarski K, Pilc A, Wieronska JM, Neurochemical and behavioral studies on the 5-HT1A-dependent antipsychotic action of the mGlu4 receptor agonist LSP4-2022. Neuropharmacology 115, 149–165 (2017); published online Epub3/15/2017 (S0028-3908(16)30278-7 [pii]; 10.1016/j.neuropharm.2016.06.025 [doi]). [DOI] [PubMed] [Google Scholar]
- 41.Gu G, Lorrain DS, Wei H, Cole RL, Zhang X, Daggett LP, Schaffhauser HJ, Bristow LJ, Lechner SM, Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Res 1197, 47–62 (2008); published online Epub2008 (S0006-8993(07)03022-3 [pii] 10.1016/j.brainres.2007.12.057). [DOI] [PubMed] [Google Scholar]
- 42.Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N, Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci.Lett. 202, 197–200 (1996); published online Epub1/5/1996 (0304394095122486 [pii]). [DOI] [PubMed] [Google Scholar]
- 43.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N, Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience 53, 1009–1018 (1993); published online Epub4/1993 (0306-4522(93)90485-X [pii]). [DOI] [PubMed] [Google Scholar]
- 44.Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N, Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J.Comp Neurol. 360, 555–570 (1995); published online Epub10/2/1995 ( 10.1002/cne.903600402 [doi]). [DOI] [PubMed] [Google Scholar]
- 45.Mitchell AS, Chakraborty S, What does the mediodorsal thalamus do? Front Syst.Neurosci. 7, 37 (2013); published online Epub2013 ( 10.3389/fnsys.2013.00037 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parnaudeau S, Bolkan SS, Kellendonk C, The Mediodorsal Thalamus: An Essential Partner of the Prefrontal Cortex for Cognition. Biol.Psychiatry 83, 648–656 (2018); published online Epub4/15/2018 (S0006-3223(17)32193-5 [pii]; 10.1016/j.biopsych.2017.11.008 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe Y, Funahashi S, Thalamic mediodorsal nucleus and working memory. Neurosci.Biobehav.Rev. 36, 134–142 (2012); published online Epub1/2012 (S0149-7634(11)00085-6 [pii]; 10.1016/j.neubiorev.2011.05.003 [doi]). [DOI] [PubMed] [Google Scholar]
- 48.Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, Yang Z, Zhang L, Lin L, Zhan Y, Wang Z, Hu H, History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 357, 162–168 (2017); published online Epub7/14/2017 (357/6347/162 [pii]; 10.1126/science.aak9726 [doi]). [DOI] [PubMed] [Google Scholar]
- 49.Jones MW, Wilson MA, Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS.Biol. 3, e402 (2005); published online Epub12/2005 (05-PLBI-RA-0687R2 [pii]; 10.1371/journal.pbio.0030402 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA, Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron 89, 857–866 (2016); published online Epub2/17/2016 (S0896-6273(16)00012-X [pii]; 10.1016/j.neuron.2016.01.011 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ, Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011); published online Epub1/2011 (npp2010184 [pii]; 10.1038/npp.2010.184 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM, Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat.Neurosci. 15, 1736–1741 (2012); published online Epub12/2012 (nn.3257 [pii]; 10.1038/nn.3257 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Likhtik E, Paz R, Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 38, 158–166 (2015); published online Epub3/2015 (S0166-2236(14)00235-5 [pii]; 10.1016/j.tins.2014.12.007 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marusak HA, Martin KR, Etkin A, Thomason ME, Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology 40, 1250–1258 (2015); published online Epub3/13/2015 (npp2014311 [pii]; 10.1038/npp.2014.311 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marek GJ, Kinon BJ, McKinzie DL, Schkeryantz JM, Monn JA, in Targets and Emerging Therapies for Schizophrenia, Albert JS, Wood MW, Eds. (Wiley, Hoboken, New Jersey, 2012), chap. 6, pp. 143–185. [Google Scholar]
- 56.Marek GJ, Interactions of Hallucinogens with the Glutamatergic System: Permissive Network Effects Mediated Through Cortical Layer V Pyramidal Neurons. Curr.Top.Behav.Neurosci, (2017); published online Epub8/23/2017 ( 10.1007/7854_2017_480 [doi]). [DOI] [PubMed] [Google Scholar]
- 57.Kouneiher F, Charron S, Koechlin E, Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12, 939–945 (2009); published online EpubJul ( 10.1038/nn.2321). [DOI] [PubMed] [Google Scholar]
- 58.Pergola G, Danet L, Pitel AL, Carlesimo GA, Segobin S, Pariente J, Suchan B, Mitchell AS, Barbeau EJ, The Regulatory Role of the Human Mediodorsal Thalamus. Trends Cogn Sci 22, 1011–1025 (2018); published online EpubNov ( 10.1016/j.tics.2018.08.006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakurai T, Gamo NJ, Hikida T, Kim SH, Murai T, Tomoda T, Sawa A, Converging models of schizophrenia--Network alterations of prefrontal cortex underlying cognitive impairments. Prog Neurobiol 134, 178–201 (2015); published online EpubNov ( 10.1016/j.pneurobio.2015.09.010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strauss GP, Waltz JA, Gold JM, A review of reward processing and motivational impairment in schizophrenia. Schizophr.Bull. 40 Suppl 2, S107–S116 (2014); published online Epub3/2014 (sbt197 [pii]; 10.1093/schbul/sbt197 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K, Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010); published online Epub6/10/2010 (nature09108 [pii]; 10.1038/nature09108 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, Engers DW, Italiano K, Bode J, Daniels JS, Lindsley CW, Hopkins CR, Conn PJ, Niswender CM, The Metabotropic Glutamate Receptor 4-Positive Allosteric Modulator VU0364770 Produces Efficacy Alone and in Combination with L-DOPA or an Adenosine 2A Antagonist in Preclinical Rodent Models of Parkinson’s Disease. J Pharmacol Exp Ther 340, 404–421 (2012); published online Epub2/2012 (jpet.111.187443 [pii]; 10.1124/jpet.111.187443 [doi]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ, Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol 74, 1345–1358 (2008); published online Epub2008 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


