Abstract
The assembly of neuronal circuits involves the migrations of neurons from their place of birth to their final location in the nervous system, as well as the coordinated growth and patterning of axons and dendrites. In screens for genes required for patterning of the nervous system, we identified the catp-8/P5A-ATPase as an important regulator of neural patterning. P5A-ATPases are part of the P-type ATPases, a family of proteins known to serve a conserved function as transporters of ions, lipids and polyamines in unicellular eukaryotes, plants, and humans. While the function of many P-type ATPases is relatively well understood, the function of P5A-ATPases in metazoans remained elusive. We show here, that the Caenorhabditis elegans ortholog catp-8/P5A-ATPase is required for defined aspects of nervous system development. Specifically, the catp-8/P5A-ATPase serves functions in shaping the elaborately sculpted dendritic trees of somatosensory PVD neurons. Moreover, catp-8/P5A-ATPase is required for axonal guidance and repulsion at the midline, as well as embryonic and postembryonic neuronal migrations. Interestingly, not all axons at the midline require catp-8/P5A-ATPase, although the axons run in the same fascicles and navigate the same space. Similarly, not all neuronal migrations require catp-8/P5A-ATPase. A CATP-8/P5A-ATPase reporter is localized to the ER in most, if not all, tissues and catp-8/P5A-ATPase can function both cell-autonomously and non-autonomously to regulate neuronal development. Genetic analyses establish that catp-8/P5A-ATPase can function in multiple pathways, including the Menorin pathway, previously shown to control dendritic patterning in PVD, and Wnt signaling, which functions to control neuronal migrations. Lastly, we show that catp-8/P5A-ATPase is required for localizing select transmembrane proteins necessary for dendrite morphogenesis. Collectively, our studies suggest that catp-8/P5A-ATPase serves diverse, yet specific, roles in different genetic pathways and may be involved in the regulation or localization of transmembrane and secreted proteins to specific subcellular compartments.
Author summary
P-type ATPases are a large family of transporters that are conserved from unicellular eukaryotes and plants to metazoans. Structurally and functionally, they fall into five subfamilies, P1 to P5, of which the latter is further divided into P5A and P5B-type ATPases. Unlike for other P-type ATPases, no mutant phenotypes for the P5A-type ATPases have been described in metazoans. Here, we show that the catp-8/P5A-ATPase in the nematode Caenorhabditis elegans is involved in multiple aspects of neuronal patterning, including neuronal migrations as well as axon guidance and dendrite patterning. A functional fluorescent reporter fusion shows the CATP-8/P5A-ATPase is expressed in most, if not all, tissues in the endoplasmic reticulum and that catp-8 can function both in neurons and surrounding tissues from where it orchestrates neuronal development. Genetically, catp-8 acts in multiple pathways during these processes, including the Wnt signaling and the Menorin pathway. Imaging studies suggest that the catp-8/P5A-ATPase is necessary for proper localization of cell-surface transmembrane proteins to dendrites of sensory neurons, but likely not for their trafficking. In summary, we propose that CATP-8/P5A-ATPase serves a function in the ER during development of select neurons, by localizing certain transmembrane, and possibly, secreted proteins.
Introduction
Development of a nervous system is a crucial step in metazoan development that requires the coordinated interactions of many genetic pathways and tissues. This process can be broken down into at least three components. First, in metazoans, a majority of neurons are actually born distant from their final location in adult animals. Therefore, neurons have to migrate from the place where they are born to their final location in the nervous system, a process that is guided by both intrinsic and extrinsic factors [1,2]. Second, axonal processes, the cellular structures that mediate output of nerve cells, need to grow and navigate, guided by extracellular guidance factors, towards their cellular targets with whom they form specific synaptic connections [3,4]. Third, the often elaborately sculpted dendrites, a neuron’s receiving cellular structures, are patterned by dedicated pathways that originate from different tissues and act both cell-autonomously and non-autonomously [5–7].
The nematode is an excellent system to study basic aspects of conserved processes involved in neural patterning. Several cellular paradigms in worms can be studied at single cell resolution, and often, ample genetic information already exists for these processes. For example, the somatosensory PVD neurons are a powerful system to study conserved mechanisms of dendrite patterning [8,9]. These mechanisms involve the interactions of different tissues that coordinately establish the stereotyped dendritic trees of PVD neurons. A complex of two transmembrane cell adhesion molecules, SAX-7/L1CAM and MNR-1/Menorin, function from the skin in concert with a secreted chemokine LECT-2/Chondromodulin II from muscle to instruct the growing PVD dendrites via a leucine rich transmembrane receptor DMA-1/LRR-TM expressed in dendrites [8,9]. Other well-studied paradigms include neuronal migrations, such as the anteriorly directed embryonic migration of hermaphrodite specific (HSN) neurons from the tail region towards the midbody region [10] or the pair of laterally located Q neuroblasts, which undergo stereotypical divisions and migrations during larval stages [11]. All cell migrations appear to be controlled, at least in part, by the conserved Wnt signaling system, among others [12,13].
The diverse P-type ATPase family of transporters is an evolutionarily highly conserved family of transporters in eukaryotes that, respectively, move multiple molecular species, including ions and lipids across membranes through the hydrolysis of ATP. These P-type ATPases are multi-transmembrane proteins that can be divided into five subfamilies based on structural and functional characteristics, P1 through P5 [14,15], of which the latter can be further divided into the P5A and P5B family [16]. The P1 through P3 subfamilies serve as ion transporters, whereas the P4 subfamily has been shown to function as flippases that move lipids across membranes [15]. The P5B family of transporters was recently reported to transport polyamines across membranes [17]. Mutations in the P5A family of transporters in both plants (A. thaliana) and unicellular eukaryotes (S. cerevisiae) were shown to display pleiotropic phenotypes, including defects in phosphate homeostasis and male gametogenesis [18–20], phospholipid and sterol homeostasis [21], and targeting of mitochondrial outer membrane proteins [22]. However, it remained unclear what functions P5A-ATPases serve in metazoans.
We show here that the sole C. elegans ortholog catp-8/P5A-ATPase is required for specific aspects of nervous system development, including dendrite patterning, axonal guidance decisions and neuronal migrations. A CATP-8/P5A-ATPase reporter shows expression in the endoplasmic reticulum in most if not all tissues albeit at different levels. Moreover, the catp-8/P5A-ATPase can function both cell-autonomously and non-autonomously in multiple genetic pathways in to regulate neuronal development. Additionally, we find that in catp-8/ATPase mutants, the amounts of functional reporters for select transmembrane proteins (the leucine rich transmembrane receptor DMA-1/LRR-TM and the HPO-30/Claudin-like molecule) are strongly reduced on higher order dendritic branches of PVD dendrites. Taken together, these results suggest that the CATP-8/P5A-ATPase functions in multiple pathways during neural patterning and is important for correct localization of transmembrane cell surface proteins and possibly secreted proteins. While this study was in progress, two studies reporting a function for catp-8/P5A-ATPase in PVD patterning in C. elegans were published [23,24]. Feng et al. suggested a role for catp-8/P5A-ATPase in dendrite patterning and targeting of DMA-1/LRR-TM in dendrites [23] and Qin et al. suggested that catp-8 was important for ER homeostasis by preventing mistargeting of mitochondrial proteins independently of ER-associated degradation (ERAD) as well as dendrite patterning [24]. Similarly, a crystal structure of the yeast homolog Spf1 in conjunction with biochemical studies suggested a role for P5A-ATPases in ER and mitochondrial quality control [25].
Results
The P5A-type ATPase CATP-8 is required for somatosensory dendrite patterning
In a genetic screen for animals with defects in dendrite arborization of PVD somatosensory neurons, we isolated the allele dz224 with characteristic defects in dendrite patterning of PVD. Using a combination of whole genome sequencing and mapping (S1 Fig, Materials and Methods)[26]), we found that the molecular lesion in dz224 results in a premature stop codon after 55 amino acids in catp-8, which encodes the sole P5A-type ATPase in the C. elegans genome (Fig 1A–1C). Based on the molecular nature of dz224, we predict this allele to result in strong, if not complete loss of function of the catp-8/P5A-ATPase.
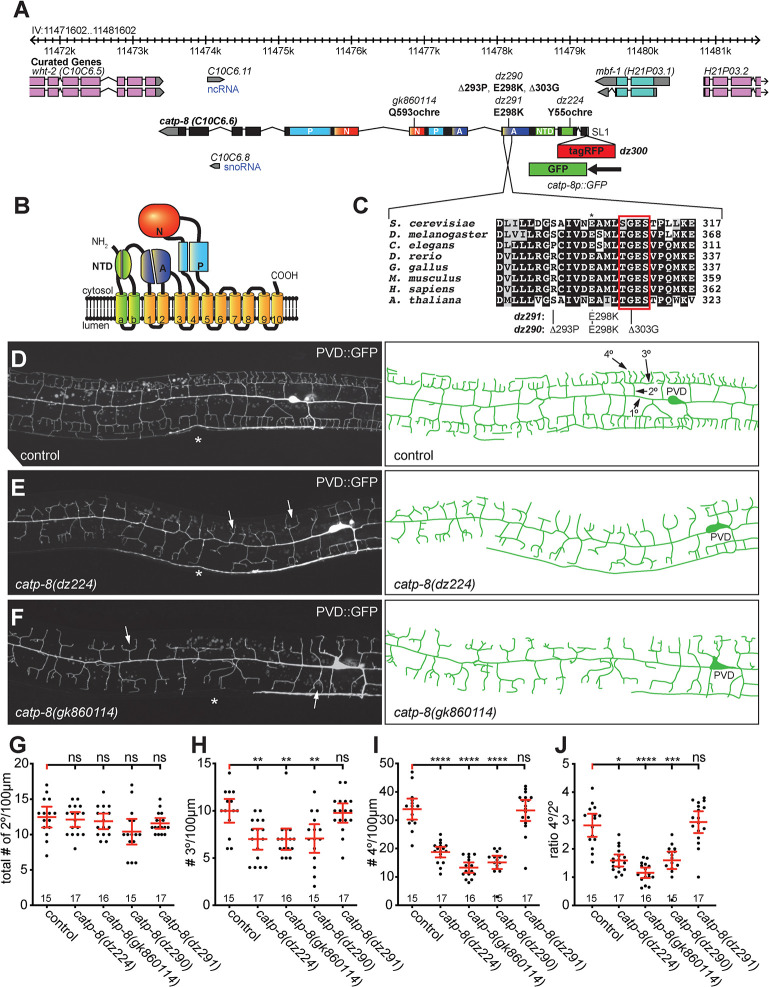
Fig 1. CATP-8/P5A-ATPase is required for PVD dendrite patterning.
A. Genetic environs of the catp-8 locus on chromosome IV. catp-8 is encoded on the minus strand and transcribed from right to left. Alleles used in this study are indicated as are the resulting molecular changes. The catp-8 transcript can be SL1 spliced based on the yk1435b05 EST clone (gift of Y. Kohara). Since catp-8 has also been suggested to be SL2 spliced [23], catp-8 may be part of a hybrid operon. Parts of the exons that encode specific domains are color coded and designated as: NTD: N-terminal domain, A: actuator domain, N: nucleotide binding domain, P: phosphorylation domain. A schematic of the catp-8p::GFP transcriptional reporter is shown as is the genomic location of the tagRFP insertion to create the translational tagRFP::CATP-8 reporter. B. Schematic domain structure and membrane topology of the CATP-8/P5A ATPase with the domains labeled and color coded as in A. C. Alignment of a conserved section of the actuator domain encoding a phosphatase activity, which is required for functional cycling of P-type ATPases [15]. The E298K mutation (asterisk, equivalent to the E349K mutant associated with intellectual disability [32]) is immediately adjacent to the (S/T)GES loop (boxed in red), which is important for A-domain function [25]. Noted on the right is the number of amino acids. Accession numbers of sequences for P5A-type ATPases used in the alignment are P39986 (S. cerevisiae), Q9VKJ6 (D. melanogaster), P90747 (C. elegans), F1R1X4 (D. rerio), Q5ZKB7 (G. gallus), Q9EPE9 (M. musculus), Q9VKJ6 (H. sapiens), Q9LT02 (A. thaliana). D. -F. Fluorescent micrographs of PVD neurons with corresponding schematics on the right in the genotypes indicated. PVD was visualized with wdIs52 (Is[F49H12.4p::GFP]) [28]. Asterisks mark the location of the vulva. In D., primary (1°), secondary (2°), tertiary (3°) and, quaternary (4°) dendrites are indicated. White arrows in E. and F. indicate typical catp-8 mutant defects. G.—J. Quantification of the number of secondary (G), tertiary (H), and quaternary (I) dendrite branches, as well as the ratio of quaternary to secondary (J) branches, 100 μm anterior to the PVD cell body in the genotypes indicated. Data are represented as the mean ± 95% confidence interval. Statistical significance was calculated using one-sided ANOVA with Tukey’s multiple comparison test. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001, ns not significant. n = 15 wild-type control (wdIs52) animals; n = 17 catp-8(dz224) mutant animals; n = 16 catp-8(gk860114); n = 15 catp-8(dz290); n = 17 catp-8(dz291).
To more precisely determine the function of catp-8 in dendrite patterning, we conducted morphometric analyses of PVD somatosensory dendrites. During larval stages two primary dendrites emanate from the PVD cell body on either side of the animal in an anterior and posterior direction, respectively, and through sequential perpendicular branching of secondary, tertiary, and quaternary branches establish highly stereotyped dendritic trees with semblance to Menorah-like candelabras (Fig 1D)[27–29]. We traced dendrites in a region 100μm anterior to the cell body of young adult animals and determined the number and length of secondary, tertiary and quaternary dendritic branches. Compared to wild type animals, we found the total number of secondary branches unchanged, whereas the number of tertiary and quaternary branches were significantly reduced in catp-8(dz224) mutants (Fig 1E and 1G–1I). However, the number of secondary branches that reached the line of the tertiary branches was significantly reduced, resulting in a reduced aggregate length of secondary branches (S1B and S1C Fig). Overall, many dendritic trees displayed a conspicuous defect, where secondary branches appeared to lack proper tertiary branches, and merely bifurcated into two quaternary dendrites rather than forming perpendicular tertiary branches. This observation was also borne out by a reduced number of quaternary branches per secondary branches, approaching an average of two quaternary branches per secondary branch (i.e. per menorah) as opposed to approximately four in control animals, and a decrease in aggregate length of secondary, tertiary and quaternary branches (Figs 1J and S1B–S1E). In addition, we observed increased numbers of ectopic secondary and ectopic tertiary branches, possibly reflective of a lack of a functioning feed-back loop that has been postulated to restrict secondary branching once stable tertiary branches have been formed [30].
To corroborate these findings, we obtained three additional alleles of catp-8/P5A ATPase, including gk860114 from the million mutation project [31], which results in a premature stop codon after 593 amino acids (Fig 1A). We used CRISPR/Cas9 based genome editing to create two additional alleles (Fig 1A and 1C): dz290, which deletes two codons within a perfectly conserved stretch of the actuator domain [15], and a missense mutation (E289K), which changes a perfectly conserved glutamic acid to lysine (E289K) in the actuator domain (Fig 1A and 1C), and is analogous to a mutation described in a pair of sisters with intellectual disability [32]. Finally, we introduced the E289K mutation on its own to create dz291. Of these, all but the dz291 allele displayed strong phenotypes in PVD patterning that were indistinguishable from the dz224 allele (Figs 1G–1J and S1B–S1E). Consistent with the nonsense mutations in dz224 and gk860114, both alleles behaved recessively and failed to complement each other, suggesting loss of catp-8 function (S1F Fig). To test, whether the gk860114 allele behaved genetically as a null allele, we analyzed animals where catp-8(gk860114) was placed in trans to the mDf7 deficiency, which removes the genomic region encoding catp-8 and fails to complement catp-8 mutant alleles (S1A Fig). We found that these animals did not display a more severe phenotype in PVD patterning than catp-8(gk860114) homozygous animals, demonstrating that gk860114 behaves as a genetic null allele (S1G Fig). Animals carrying the catp-8(gk860114) null allele appear superficially normal, have a normal body length at the L1 larval stage (S1H Fig), but seem to grow slower. Taken together these findings suggest that catp-8 function is required for PVD patterning, especially for the extension of tertiary and quaternary dendritic branches. Moreover, the E289K mutation, which is analogous to a mutation shared by the pair of sisters with intellectual disability [32], does not compromise catp-8 sufficiently to result in morphological phenotypes, at least in the context of PVD patterning or neuronal migration (see below). Lastly, the defects due to mutations in the actuator domain are consistent with the interpretation that enzymatic activity of CATP-8/P5A type ATPase is required for PVD patterning.
The P5A-type ATPase CATP-8 is required for specific neuronal and axonal migrations
We next asked whether catp-8 is only necessary for development of the highly branched dendritic trees of PVD dendrites or more generally, for other aspects of neural patterning. To this end, we crossed catp-8 mutants with a panel of GFP reporters that label individual neurons or classes of neurons, including motor neurons, interneurons, sensory neurons and touch receptor neurons. We found that mutant alleles of catp-8 displayed defects in neurite patterning of some but not all neurons. For example, in catp-8 mutant animals the D-type motor neurons displayed neurite extension defects in the ventral and dorsal nerve cords, whereas the DA/DB-motor neurons appeared indistinguishable from control animals (Table 1, S2A–S2D Fig). This is significant insofar as the processes of both types of motor neurons make similar navigational choices and travel, at least in the nerve cords, in the same fascicles. However, neither patterning of the pair of AIY interneurons, nor the amphid sensory neurons or phasmid sensory neurons was obviously dependent on catp-8 function (Table 1, S2E–S2J Fig). Lastly, we investigated axon guidance choices of HSN and PVQ neurons at the ventral midline, where both neurons send axons in the left and right ventral nerve cords from the midbody or tail to the head region, respectively. We observed significant defects in axon midline guidance of HSN neurons with cross overs, but no defects for PVQ neurons (Table 1, S2K–S2N Fig). Taken together, our findings show that axonal patterning of some but not all neurons is dependent on catp-8/P5A-ATPase function. Importantly, even neurons that make similar guidance choices and travel in the same nerve fascicles such as the D-type and DA/DB motor neurons or the HSN and PVQ neurons, can develop either dependent on catp-8 function or, independently.
Table 1. Survey of neuroanatomical defects in catp-8/P5A-ATPase mutant animals.
| Neurons Examined (Marker Used) | control [%] | N | catp-8 [%] | N | P Value1 |
|---|---|---|---|---|---|
| Motor Neurons2 | |||||
| D-Type (juIs76) | |||||
| Gaps in ventral nerve cord | 10.8 | 93 | 41.3 | 109 | **** |
| Gaps in dorsal nerve cord | 12.9 | 93 | 26.6 | 109 | * |
| 0 L/R choice commissure | 60.0 | 40 | 23.6 | 55 | *** |
| 1 L/R choice commissure | 25.0 | 40 | 41.8 | 55 | ** |
| ≥ 2 L/R choice commissure | 15.0 | 40 | 32.7 | 55 | |
| defasciculation of DNC | 0.0 | 31 | 11.4 | 44 | 0.07 |
| defasciculation of VNC | 19.4 | 31 | 36.4 | 44 | 0.13 |
| defasciculation in both | 3.2 | 31 | 2.3 | 44 | ns |
| DA/DB (evIs82b) | |||||
| Gaps in ventral nerve cord | 0 | 102 | 0 | 108 | ns |
| Gaps in dorsal nerve cord | 0 | 102 | 0 | 108 | ns |
| L/R choice of commissures | 4.8 | 102 | 5.7 | 108 | ns |
| HSN (mgIs71) | |||||
| Ventral nerve cord crossover2 | 0.0 | 85 | 12.5 | 72 | *** |
| Interneurons3 | |||||
| PVQ (oyIs14) | |||||
| Ventral cord crossover | 11.8 | 101 | 7 | 100 | ns |
| AIY (mgIs32) | |||||
| Early termination | 0 | 120 | 0 | 124 | ns |
| Ectopic axonal branching | 2 | 120 | 2 | 124 | ns |
| Amphid Neurons4 | |||||
| DiI Stain | |||||
| Defasciculation | 6 | 50 | 4 | 50 | ns |
| Early termination | 0 | 50 | 0 | 50 | ns |
| Anterior process | 0 | 50 | 0 | 50 | ns |
| Anterior nerve ring | 0 | 50 | 0 | 50 | ns |
| Posterior process | 0 | 50 | 0 | 50 | ns |
| Posterior nerve ring | 0 | 50 | 0 | 50 | ns |
| Phasmid Neurons3 | |||||
| DiI Stain | |||||
| Ectopic axons | 0 | 50 | 0 | 50 | ns |
| Axon crossover | 0 | 50 | 0 | 50 | ns |
| Wandering processes | 0 | 50 | 0 | 50 | ns |
1 Statistical significance was calculated using Fisher’s exact test.
*, P ≤ 0.05
**, P ≤ 0.005
****, P ≤ 0.0001; ns, not significant.
2 Note that the cross over defects invariably occurred when HSNL failed to migrate, in which case a process first grew out posteriorly, before turning ventrally. The process then crossed the midline and grew anteriorly in the right ventral nerve cord.
3 Neuroanatomical defects were scored as described [65].
4 Neuro-anatomical defects were scored as described [66].
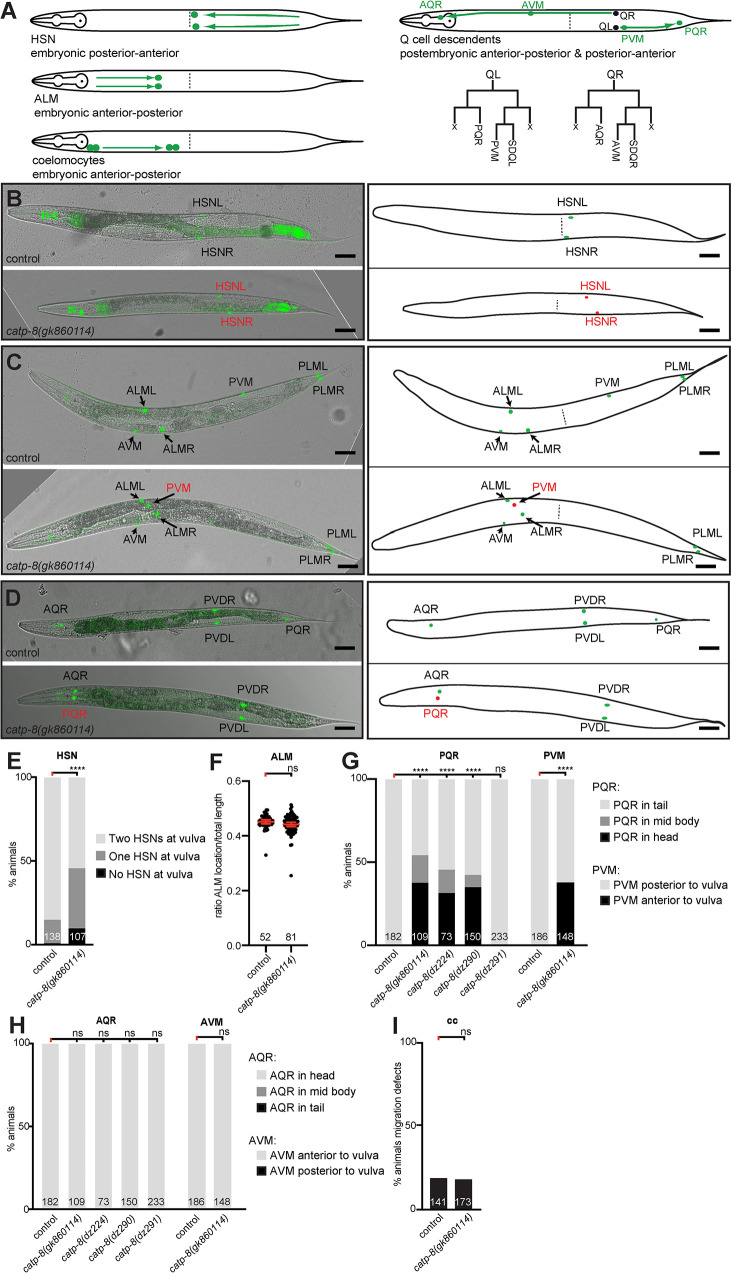
We next investigated a set of stereotyped neuronal and non-neuronal cellular migrations, including both embryonic and postembryonic migrations, as well as anteriorly-posteriorly and posteriorly-anteriorly directed migrations (Fig 2A). We first investigated two embryonic migrations: the pair of ALM mechanosensory neurons, which migrate from the head region towards the midbody region (i.e. anteriorly-posteriorly directed), and the hermaphrodite specific neurons (HSNs), a set of motor neurons that migrate from the tail towards the mid body region (i.e. posteriorly-anteriorly directed)[33]. Loss of catp-8 function resulted in severe migration defects in anteriorly directed HSN migrations, which often failed to reach their final destination around the midbody and instead stopped migration prematurely (Fig 2B and 2E). In contrast, loss of catp-8 had no effect on posteriorly directed ALM neuronal migrations (Fig 2C and 2F). We next investigated a set of postembryonic migrations, specifically of the Q cell descendants [11]. The bilateral pair of Q cells are neuroblast cells, which are born during the mid L1 larval stage. On the right side of the animal, the QR cell migrates anteriorly while undergoing a series of stereotyped cell divisions as follows. The first division results in two more blast cells, of which one divides again and gives rise to the AQR cell and the other undergoes programmed cell death [34]. The other blast cell also gives rise to one cell that undergoes programmed cell death and another that after a further division forms the AVM and SDQR neurons (Fig 2A). The AQR and AVM/SDQR neurons therefore originate from two subbranches of QR cell descendants, and are positioned in the head or a lateral anterior section of the worms, respectively. The Q cell descendants on the left side of the animal undergo a posteriorly directed migration while also giving rise, in a similar pattern of divisions, to PQR and PVM/SDQL neurons (Fig 2A). PQR travels to the tail whereas PVM and SDQL remain in a lateral posterior section of the animals. We found that the anteriorly directed migrations of both AQR and AVM, which represent both sub branches of QR descendants, are not obviously affected in catp-8 mutants, whereas the posteriorly directed migrations of PQR and PVM neurons display defects (Fig 2C, 2D, 2G, and 2H). Specifically, PQR and PVM erroneously migrate anteriorly towards the head instead of posteriorly. This observation suggests that the choice of directionality in migration of QL descendants on the left side of the animals is dependent on catp-8/P5A ATPase function, rather than migratory capacity itself. Moreover, we found that gk860114 homozygous mutants originating from homozygous mothers displayed Q cell migration defects, whereas gk860114 homozygous mutants originating from gk860114/+ heterozygous animals did not display this phenotype, suggesting a maternal contribution of catp-8 during this postembryonic migration (S1I Fig). In contrast, we observed a similar range of defects in HSN migration and PVD dendrite patterning in gk860114 homozygous mutants coming from heterozygous mothers compared to those originating from homozygous mothers (S1F and S1J Fig, cf also Fig 2E), leading us to conclude that Q cell migration requires less catp-8 function compared to HSN and PVD patterning. Lastly, we investigated two pairs of non-neuronal cells, the coelomocytes, which migrate embryonically in a posterior direction [33]. We found that coelomocyte migration proceeds normally in catp-8 mutants (Fig 2I). Collectively, our experiments show that both embryonic and postembryonic cell migrations require catp-8/P5A-ATPase function, including anteriorly and posteriorly directed migrations.
Fig 2. CATP-8/P5A-ATPase is required for neuronal migrations.
A. Schematics of different cellular migrations. Shown on the left are embryonic migrations including HSN (posterior to anterior), ALM (anterior to posterior) and coelomocytes (anterior to posterior). For simplicity, all migrations are illustrated in adult animals. Indicated on the right are the migrations of the descendants of the QL and QR neuroblasts, respectively, which arise in stereotyped cell-divisions as indicated below. Note that Q cells divide into distinct sub-branches, comprising the AQR/PQR or AVM/PVM and SDQR/SDQL neurons, respectively. X denotes cells that undergo programmed cell death [34]. B.—D. Composite of fluorescent and differential interference contrast images of reporters visualizing the HSN neurons (B), touch receptor neurons (C) or AQR/PQR (D). Images of wild type and catp-8(gk860114) mutant animals are shown in top and lower panels, respectively, with corresponding schematics on the right. Correctly positioned neurons are shown in green and incorrectly positioned neurons in red. HSN visualized by dzIs75 II Is[kal-1p9::GFP; ttx-3p::mCherry][47]), AQR/PQR by wdIs52 II Is[F49H12.4p::GFP], and touch receptor neurons by zdIs5 I Is[mec-4p::GFP] [67]. Scale bar = 25μm. E. Quantification of cell migration defects of HSN neurons. Percent animals are shown with neurons in wild type position (light grey bars), partially incomplete migration (dark grey bars) and completely failed migration (black bars). Pairwise statistical significance was calculated using Fisher’s exact test. **** P ≤ 0.0001, ns not significant. F. Quantification of ALM migration by comparing the ratio of ALM distance from the tip of the nose to the total animal length in control versus catp-8(gk860114) mutant animals. Unpaired t test, ns, not significant. G.—H. Quantification of migration of PQR and PVM (G) and AQR and AVM (H) neurons comparing control versus indicated alleles of catp-8. Percent animals are shown with neurons in wild type position (light grey bars), partially incomplete migration (dark grey bars) and completely failed migration (black bars). Pairwise statistical significance was calculated using Chi-squared test or Fisher exact test. **** P ≤ 0.0001; ns, not significant. I. Percentage of animals exhibiting coelomocyte migratory defect in wildtype or catp-8(gk860114) mutant animals. Fisher’s exact test. ns not significant.
CATP-8/P5A ATPase functions both cell-autonomously and non-autonomously in neural patterning
To gain insight into the cellular focus of action of the catp-8/P5A-ATPase, we used CRISPR/Cas9 engineering to insert a tagRFP fluorescent protein at the N-terminus of CATP-8 (Fig 1A). We found these animals to show widespread, if not ubiquitous expression of CATP-8, including in the epidermis, muscle, pharynx, intestine, and the major neuronal ganglia (Fig 3A–3D). The expression appears intracellular in a perinuclear pattern and different tissues appear to express different levels of CATP-8. Of note, we detected expression in both neurons that were phenotypically affected in catp-8 mutants as well as neurons that were not visibly affected. For example, clear expression of the endogenous tagRFP::CATP-8 reporter was seen in HSN, PVM, and PVD neurons, but also in ALM neurons and others (Fig 3E–3H). Importantly, PVD morphology and PQR cell position appeared completely normal in animals carrying the endogenous fluorescent tag, suggesting that the N-terminal tagRFP::CATP-8 fusion is fully functional. We found similar expression patterns in transgenic animals expressing GFP under control of the catp-8 5’ region immediately upstream of the ATG start codon, where we also saw widespread expression from embryonic stages onwards, including in muscle, intestine, pharynx and other tissues (S3 Fig). Collectively, our studies show that catp-8 is widely, if not ubiquitously, expressed albeit at different levels.
Fig 3. CATP-8/P5A-ATPase is widely expressed at apparently different levels.
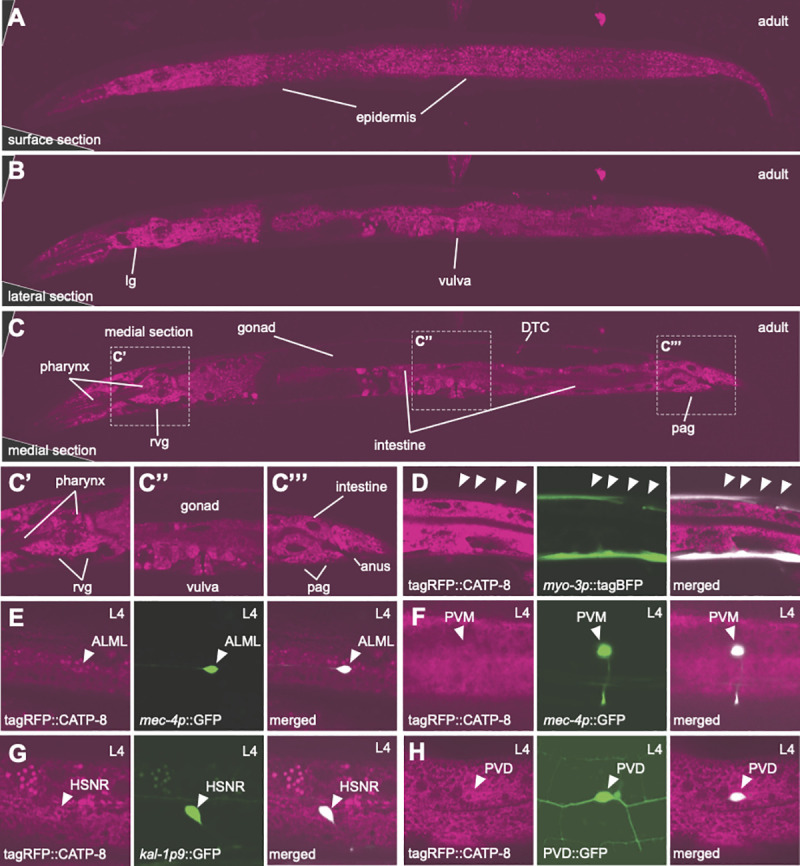
A.- C. Whole body confocal optical sections of tagRFP translational knock-in of catp-8 animals, showing perinuclear expression in the tissues indicated. An optical section near the surface of the animals shows epidermal expression, whereas a more lateral and a medial section reveal many other tissues, including pharynx, intestine, lateral ganglion (lg), retrovesicular ganglion (rvg), distal tip cell (DTC), and preanal ganglion (pag). Insets in C are shown magnified as C’, C”, C”‘. Note that tagRFP::CATP-8 is not visibly expressed in the gonad and that expression levels vary in different tissues (e.g. compare epidermal and muscle expression, confer with D). D.- H. Confocal images showing CATP-8 expression in indicated neurons and tissues, as shown by colocalization of tagRFP::CATP-8 with relevant markers, including dzEx2136 (Ex[myo-3p::tagBFP; mec-4p::catp-8] for muscle, D), zdIs5 (Is[mec-4p::GFP] for ALM and PVM, E, F), dzIs75 (Is[kal-1p9::GFP] for HSN, G), wdIs52 (Is[F49H12.4p::GFP] for PVD, H).
A functional CATP-8/P5A ATPase fusion localizes to the endoplasmic reticulum
Previous work showed that the yeast Spf1 (and Arabidopsis MALE GAMETOGENESIS IMPAIRED ANTHERS (MIA) or PDR2) homologs are localized to the endoplasmic reticulum (ER)[19,20,35]. We therefore aimed to determine the subcellular localization of CATP-8 in C. elegans. To this end, we used the functional N-terminal fusion of tagRFP to CATP-8 and drove expression from a single copy insertion transgene under control of the heterologous dpy-7p promoter, which is specific for the epidermis [36]. We chose to use this single copy, functional transgene over the endogenously tagged catp-8 locus to minimize interference with signal from other tissues (cf. Fig 3). This epidermally-expressed transgene (dzSi3), which also displayed perinuclear staining (and rescued PVD patterning defects) was crossed with a panel of organellar reporters—for (1) the ER, (2) the cis/medial Golgi, (3) early endosomes, (4) late endosomes and lysosomes, (5) autophagosomes, and (6) lysosomes. We found the strongest colocalization with a marker for the ER and no correlation with markers for Golgi, late endosomes or lysosomes (Fig 4). These findings suggest that in metazoans CATP-8/P5A-ATPase is localized to the ER similarly to plants and unicellular eukaryotes. We next tested whether the gross morphology of these cellular compartments was defective in catp-8 mutant animals. We found no apparent defects in the perinuclear localization, patterns or intensity of any of the organelle reporters examined (S4 Fig). Taken together, our findings suggest that the CATP-8/P5A-ATPase serves a function in the ER, which may be conserved from unicellular eukaryotes and plants to animals.
Fig 4. CATP-8/P5A-ATPase localizes primarily to the endoplasmic reticulum.
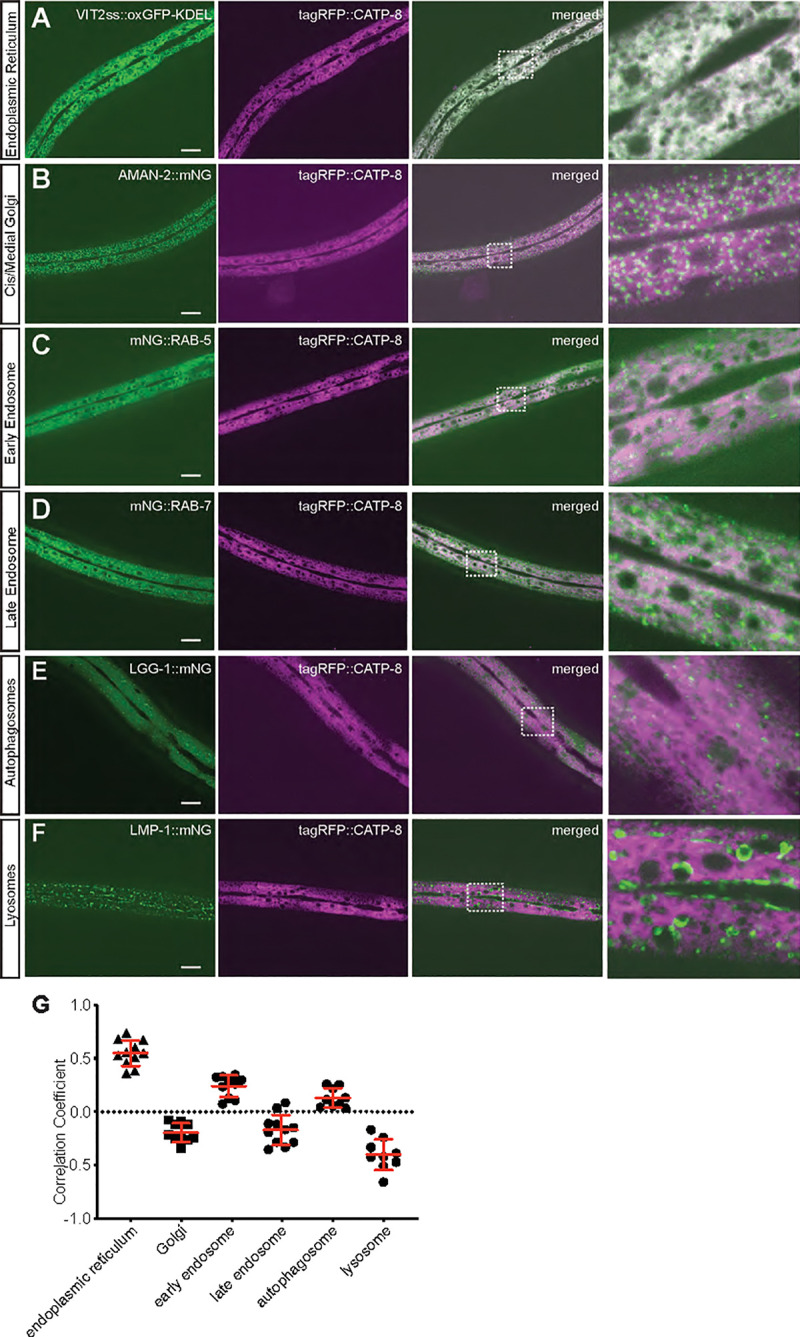
A.—F. Confocal images of animals expressing a functional tagRFP::CATP-8 fusion under control of the epidermal specific promoter dpy-7p from a single copy transgene, in combination with single copy organellar markers for the endoplasmic reticulum (A, pwSi82[Phyp-7::VIT2ss::oxGFP-KDEL]), cis/medial Golgi (B, pwSi202[Phyp7::AMAN-2::mNG;HYG-R]), early endosomes (C, pwSi145[Phyp7::mNG::RAB-5;HYG-R]), late endosomes and lysosomes (D, pwSi140[Phyp7::mNG::RAB-7;HYG-R]), autophagosomes (E, pwSi144[Phyp7::mNG::LGG-1;HYG-R]), and lysosomes (F, pwSi205[Phyp7::LMP-1::mNG;HYG-R]). The right column represents magnifications of the insets indicated. G. Co-localization quantification between tagRFP::CATP-8 and respective cellular compartment markers.
CATP-8/P5A ATPase can serve both cell-autonomous and cell-non-autonomous functions during neural patterning
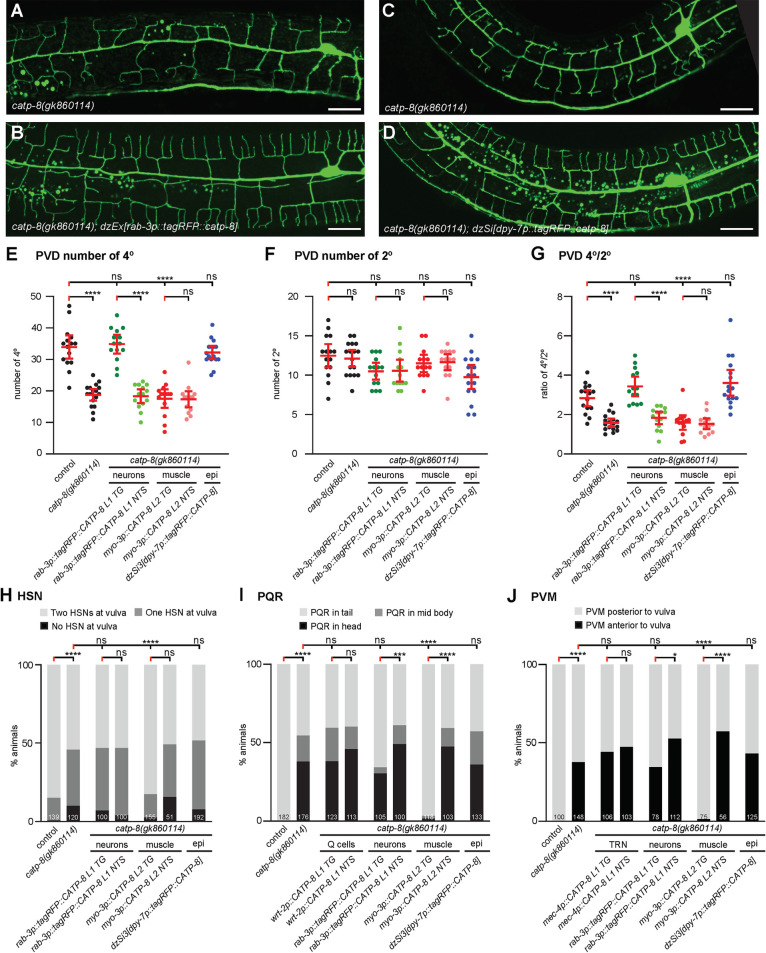
To determine where catp-8 may function during different developmental processes, we performed transgenic rescue experiments, where we expressed a catp-8 cDNA under control of heterologous promoters in catp-8 mutant animals. We found that patterning defects of PVD dendrites were rescued by pan-neuronal transgenic expression of catp-8, but not by expression in muscle (Figs 5A–5G and S5A–S5C). Interestingly, a single copy transgene expressing the functional N-terminal tagRFP::CATP-8 fusion under control of the heterologous epidermis-specific dpy-7p promoter also rescued PVD patterning defects in catp-8 mutants (Figs 5A–5G and S5A–S5C). These observations suggest that catp-8 can serve redundant functions in different tissues, i.e. both cell-autonomously and non-autonomously, but may be sufficient in either neurons or the epidermis. Surprisingly, embryonic and postembryonic cell migration defects of HSN, PQR and, PVM neurons were completely rescued by expression of catp-8 in muscle, but not in the epidermis, the touch receptor neurons (i.e. PVM), or the precursors that give rise to the Q cells (Figs 5H–5J and S5D and S5E). One out of three transgenic lines expressing the catp-8 cDNA under control of a pan-neuronal reporter showed partial rescue of the neuronal migration defects of PVM and PQR neurons (Figs 5I,5J and S5D and S5E), suggesting that overexpression in neurons can partially compensate for loss of catp-8 in other tissues, e.g. muscle. We conclude that at least for these cellular migrations, catp-8 appears to function non-autonomously in muscle. Therefore catp-8 can serve both autonomous and non-autonomous functions during neural patterning, including in dendrite morphogenesis and different neuronal migrations.
Fig 5. CATP-8/P5A-ATPase can function cell autonomously and non-autonomously during neural patterning.
A.—B. Maximum z-projection of confocal images of the PVD::GFP marker wdIs52 from non-transgenic (A) and transgenic (B) sibling animals carrying the pan-neuronal catp-8 rescueing array in a catp-8(gk860114) mutant background, showing restoration of PVD structure upon neuronal expression. Scale bar = 20μm. C.—D. Maximum z-projection of confocal images of the PVD::GFP marker wdIs52 from animals without (C) and with (D) single-copy insertion of epidermal catp-8 rescue under catp-8 mutant background, showing restoration of PVD structure under epidermal rescue. Scale bar = 20μm. E.—G. Quantification of number of quaternary branches (E), secondary branches (F), and the ratio of quaternary to secondary branches (G) 100 μm anterior to the PVD cell body in animals of genotypes indicated, showing complete rescue of catp-8(gk860114) phenotype when catp-8 is expressed from pan-neuronal or epidermal promoter, but not muscle promoter. TG: transgenic; NTS: non-transgenic siblings. Data are represented as the mean ± 95% confidence interval. ****, P<0.0001, ns, not significant, Kruskal-Wallis test with Dunn’s multiple comparisons test. n = 14 animals per genotype. H.—J. Percentage of animals with the indicated migration phenotype of HSN (H), PQR (I) and PVM (J) in the indicated genotypes, showing that all migration phenotypes of catp-8 were rescued completely when catp-8 was expressed from muscle, but not when expressed cell-autonomously, pan-neuronally or epidermally. **** P < 0.0001, ns not significant, Chi-squared test. Sample size indicated on graph.
CATP-8/P5A ATPase functions in the Menorin pathway to pattern PVD dendrites
The conserved Menorin pathway shapes somatosensory patterning of PVD dendrites [8,9]. Briefly, the two putative cell adhesion molecules SAX-7/L1CAM and MNR-1/Menorin form a complex in the epidermis, which, together with the muscle derived chemokine LECT-2/Chondromodulin II forms a high affinity substrate for the leucine rich repeat transmembrane receptor DMA-1/LRR-TM, which functions in PVD neurons [8,9]. Downstream of DMA-1/LRR-TM in PVD, a conserved set of intracellular molecules such as the TIAM-1/GEF guanine nucleotide exchange factor and HPO-30/Claudin act together to regulate the actin cytoskeleton [8,9,37]. Previous genetic experiments established that SAX-7/L1CAM, LECT-2/Chondromodulin II and MNR-1/Menorin function in a linear genetic pathway with the DMA-1/LRR-TM receptor, but also suggested that DMA-1 can serve functions in PVD patterning that are independent of SAX-7/L1CAM, MNR-1/Menorin and LECT-2/Chondromodulin II [30,38]. To determine the genetic relationship between catp-8 and the Menorin pathway, we analyzed double null mutants of catp-8 and various components of the Menorin pathway using morphometric analyses. We traced and quantified the number and length of secondary, tertiary and quaternary dendrites 100μm anterior to the PVD cell bodies as previously described [30]. With regard to the number of secondary dendrites, we found that the catp-8 mutant was epistatic in catp-8; mnr-1 and lect-2; catp-8 double mutants, i.e. the double mutant resembled the catp-8 single mutant rather than displaying the increased number of secondary branches observed in the mnr-1/Menorin and lect-2/Chondromodulin II single mutants (Fig 6A). In double mutants with dma-1, hpo-30 or tiam-1 the phenotypes were not further enhanced by concomitant genetic removal of catp-8. With regard to the number of tertiary branches, we made similar observations, although in some cases the double mutants between catp-8 and for instance mnr-1/Menorin, or hpo-30/Claudin appeared phenotypically stronger than the single mutants (Fig 6B). For the number of quaternary branches, double mutants between catp-8 and genes in the Menorin pathway invariably displayed the stronger phenotype observed in mutants of the Menorin pathway (Fig 6C). We observed similar genetic interactions when investigating the aggregate lengths of secondary, tertiary and quaternary branches (S6 Fig). We conclude that catp-8 functions in the Menorin pathway and likely within both branches, i.e. the mnr-1/Menorin-dependent as well as -independent branches, both of which feed into the DMA-1/LRR-TM receptor in PVD neurons.
Fig 6. The catp-8/P5A ATPase functions with the Menorin pathway during dendrite patterning.
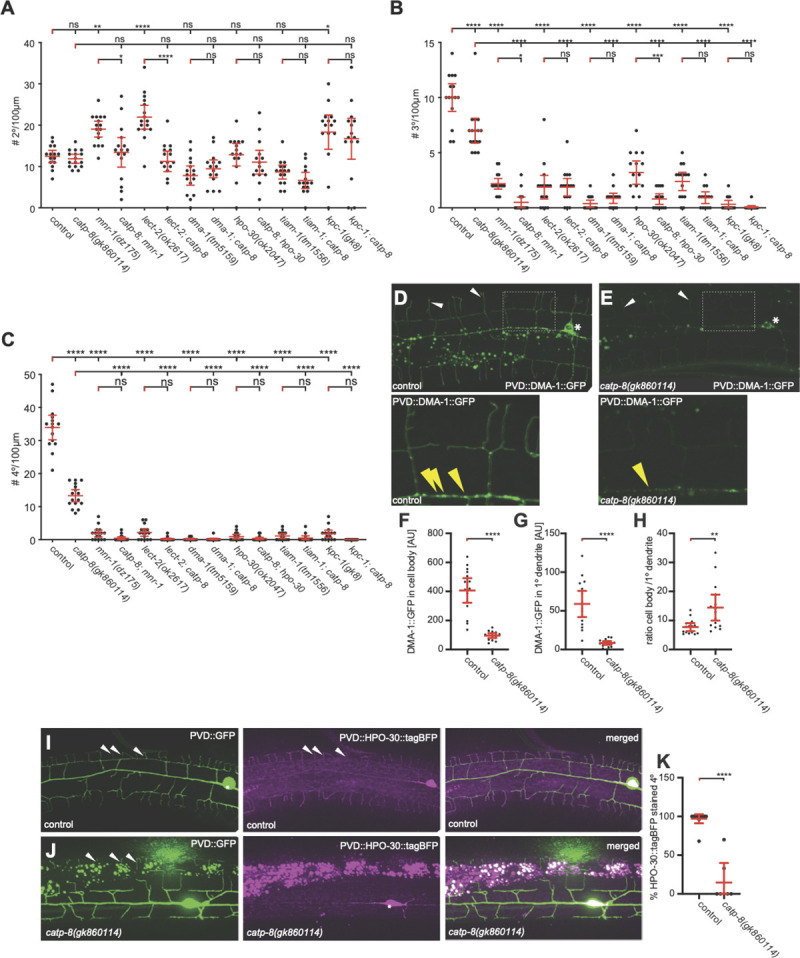
A.—C. Quantification of the number of secondary (A), tertiary (B), and quaternary (C) dendrite branches within 100 μm anterior to the PVD cell body in animals of the indicated genotypes. Note that all alleles are molecular or genetic null alleles. Data are represented as the mean ± 95% confidence interval. * P<0.05, ** P<0.01, *** P<0.001, **** P < 0.0001 ns not significant; one-sided ANOVA with Tukey’s multiple comparison test. n = 15 animals per genotype. D.—E. Maximum z-projection of confocal images and magnified inset of DMA-1::GFP expressed in PVD (qyIs369) in a wildtype or catp-8 mutant background, showing a reduction of DMA-1::GFP puncta in the primary dendrite (yellow arrowheads) in mutant animals. Note also the reduction in overall fluorescence in catp-8 mutant animals. F.—H. Quantification of DMA-1::GFP signal in cell body (F), and on the primary dendrite 100 μm anterior from the cell body (G) in control and catp-8 animals. The signal ratio of cell body to primary dendrite is presented in (H). Data are represented as the mean ± 95% confidence interval. ** P<0.01, **** P<0.0001, Mann-Whitney test. n = 14 animals per genotype. I.—J. Maximum z-projection of apotome images of HPO-30::tagBFP (magenta) expressed in PVD and PVD reporter wdIs52 (green) in control (I) or catp-8 mutant animals (J). Note, that as previously reported [40], overexpression of HPO-30::tagBFP in PVD dendrites results in a reduced number of quaternary dendrites, possibly due to downregulation of DMA-1/LRR-TM. K. Percentage of HPO-30::tagBFP stained quaternary dendrites 100 μm anterior to the cell body in control or catp-8 mutant animals, presented as mean ± 95% confidence interval. **** P<0.0001, Mann-Whitney test. n = 10 animals per genotype.
CATP-8/P5A ATPase is required for localization of some cell surface transmembrane molecules
The functions of catp-8 in PVD neurons and the localization to the ER prompted us to test whether the localization or trafficking of two transmembrane molecules, which are expected to transit the ER, is affected in catp-8 mutants. Specifically, we analyzed the localization of reporters for the single transmembrane receptor DMA-1/LRR-TM (DMA-1::GFP) [39] and the four transmembrane, claudin-like molecule HPO-30/Claudin (HPO-30::tagBFP) [40]. We found that overall DMA-1::GFP expression was noticeably dimmer in catp-8 mutants (Fig 6D and 6E). When we quantified the fluorescence in the cell body and the primary dendrite we found it strongly reduced in catp-8 versus control animals (Fig 6F and 6G). Interestingly, the ratio of fluorescence in the cell body versus the primary dendrite was significantly increased (Fig 6H). These observations could be a reflection of reduced trafficking towards the periphery or reduced amounts of DMA-1::GFP/vesicle. To distinguish between these possibilities, we quantified the number of puncta, previously suggested to represent the vesicular fraction of DMA-1::GFP [41,42], in primary and tertiary dendrites of catp-8 and control animals. We found the number of puncta of DMA-1::GFP in neither primary nor tertiary dendritic branches significantly different in catp-8 mutant versus control animals (S7B and S7C Fig). We therefore propose that catp-8 does not serve a function in vesicle trafficking. Instead, catp-8 may function to facilitate correct membrane localization of DMA-1/LRR-TM. Alternatively, but not mutually exclusive, catp-8 could serve a role in loading DMA-1::GFP into vesicles.
As previously reported, HPO-30::tagBFP localizes primarily to primary and quaternary dendritic branches [40]. We found that HPO-30::tagBFP fluorescence in distal dendrites appeared reduced in catp-8 mutant animals (Fig 6I and 6J). Although HPO-30::tagBFP expression in the cell body did not seem visibly different, HPO-30::tagRFP fluorescence in quaternary dendrite branches was strongly reduced in catp-8 mutant versus control animals (Fig 6K). Yet, the number of HPO-30::tagRFP puncta in the PVD primary branch remained unaffected, implying again that trafficking of HPO-30::tagBFP does not require catp-8 function (S7D Fig).
Because epidermal expression of catp-8/P5A-ATPase could rescue PVD defects in catp-8 mutant animals, we tested, whether another conserved transmembrane cell adhesion molecule SAX-7/L1CAM, which functions in the epidermis to shape PVD dendrites, is affected in catp-8 mutant animals. We found that SAX-7::GFP localization appears qualitatively and quantitatively unchanged in catp-8 mutants compared to control animals, or in animals that overexpressed tagRFP::CATP-8 transgenically in the epidermis (S7E and S7F Fig). Similarly, localization of the secreted LECT-2/Chondromodulin II was not affected in catp-8 mutants (S7G Fig). We conclude, catp-8/P5A-ATPase function is required for the regulation and localization of some but not all transmembrane proteins.
CATP-8/P5A ATPase functions with the Wnt signaling pathway to control neuronal migrations
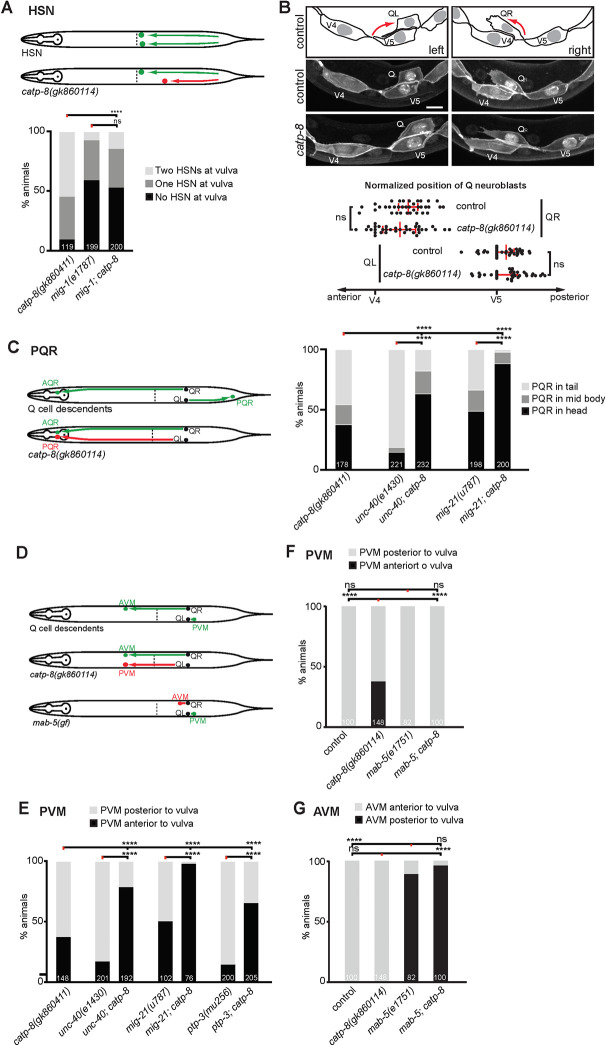
Our studies show that catp-8 is required for both embryonic and postembryonic neuronal migrations, including the anteriorly directed embryonic migrations of HSN neurons and the posteriorly directed postembryonic migrations of the Q cell descendants PVM and PQR. Both of these cell migrations are genetically well-described and depend on, among other pathways, Wnt signaling [10,11,13]. Briefly, the egl-20/Wnt ligand is expressed in posterior tissues, including body wall muscle [43,44], is believed to form a gradient [45] and to function through the G-protein coupled receptor mig-1/Frizzled in HSN neurons to mediate HSN migration [46]. While other Wnts and frizzled receptors play smaller, largely redundant roles in HSN migration [13], most functions appear to be mediated by the MIG-1/Frizzled receptor in HSN neurons, because a mig-1; egl-20 double mutant does not display more severe defects in HSN migration than the single mutants [47]. To investigate the genetic relationship between catp-8/P5A-ATPase and Wnt signaling, we therefore created a double null mutant between catp-8 and mig-1. We found that the HSN migration phenotype in mig-1; catp-8 double mutant animals was not enhanced compared to the more severe of the single mutants (mig-1/Fz) (Fig 7A). This suggests that catp-8 functions genetically in a pathway with mig-1/Frizzled and, by inference, Wnt signaling.
Fig 7. Genetic interactions of catp-8/P5A ATPase during cell migrations.
A. Schematics depicting normal HSN migration (upper panel) and HSN migration defects (lower panel), and quantification of HSN migration defects in the genotypes indicated. Percent animals are shown with neurons in the wild type position (light grey bars), partially incomplete migration (dark grey bars) and completely failed migration (black bars). Pairwise statistical significance was calculated using Chi-squared test or Fisher exact test. **** P ≤ 0.0001, ns not significant. Sample size indicated on graph. B. Schematic, images and quantification of the relative position of initial Q neuroblast migration in the genotypes indicated, imaged 4.5 hours after hatching. Scale bar = 5 μm. Data are represented as the mean ± 95% confidence interval. ns not significant; Mann-Whitney test. n = 35 animals per genotype. C. Schematics depicting normal PQR migration (upper panel) and migration defects (lower panel), and quantification of PQR migration defects in the genotypes indicated. Percent animals are shown with neurons in the wild type position (light grey bars), partially incomplete migration (dark grey bars) and completely failed migration (black bars). Pairwise statistical significance was calculated using Chi-squared test or Fisher exact test. **** P ≤ 0.0001. Sample size indicated on graph. D. Schematics depicting normal AVM and PVM migration (upper panel) and migration defects (middle and lover panels) in the genotypes indicated. E. Quantification of PVM migration defects in the genotypes indicated. Percent animals are shown with neurons in the wild type position (light grey bars), and failed migration (black bars). Pairwise statistical significance was calculated using Chi-squared test or Fisher exact test. **** P ≤ 0.0001. Sample size indicated on graph. F.–G. Quantification of PVM (F) and AVM (G) migration defects in the genotypes indicated. Percent animals are shown with neurons in the wild type position (light grey bars), and failed migration (black bars). Pairwise statistical significance was calculated using Chi-squared test or Fisher exact test. **** P ≤ 0.0001, ns not significant. Sample size indicated on graph.
The migration of Q cell descendants occurs in two phases [11]. In an initial phase, the QL and QR cells, both of which are located laterally on either side of the animals, are polarized and migrate about the distance of one cell diameter over the posteriorly and anteriorly located epidermal blast cells, respectively (Figs 7B and S7A). This is followed by a second phase, in which the QL and QR cells migrate further posteriorly and anteriorly, respectively, while undergoing the stereotyped cell divisions [34](Fig 2A). The first phase is controlled by at least three partially redundant genetic pathways, which are defined by genes encoding: (1) the transmembrane cell adhesion molecule UNC-40/DCC (Deleted in Colorectal Cancer)[48], (2) the transmembrane protein MIG-21 and the conserved, putative C-mannosyl transferase DPY-19/DPY19L [48–50], and (3) the cadherin proteins CDH-3, CDH-4, and the LAR-like receptor tyrosine phosphatase PTP-3/LAR [51–53]. Mutations in any of these genes result in defects in the initial polarization and, consequently, migration. In contrast, we find that initial polarization and migration of QL and QR cells proceeds normally in catp-8 null mutant animals, suggesting that catp-8 functions primarily during the second phase of migration (Figs 7B and S7A). Consistent with this interpretation, we found that unc-40; catp-8 double mutants exhibited a more severe phenotype than either single mutant alone (Fig 7C–7E). Similar results were found in mig-21; catp-8 and ptp-3; catp-8 double mutant animals, which also showed a more severe phenotype compared to the single mutants (Fig 7C–7E). We conclude that catp-8 serves no individual role during the first phase of Q cell migration, but rather functions during the second phase of Q cell migration.
We next focused our analysis on the second phase of migration and in particular on AVM and PVM migrations. The mab-5/HOX-C antennapedia-like homeobox transcription factor is necessary and sufficient in QL cells after the initial polarization to mediate the second phase of their posterior migration [54] and is activated in response to the egl-20/Wnt morphogen that is expressed in posterior tissues [43]. Similar to loss of catp-8, loss of mab-5 results in QL cell migration defects, where QL cell descendants migrate in an anterior rather than posterior direction [54]. Because the mab-5 mutant phenotype is essentially fully penetrant, we could not test for possible enhancement in a mab-5; catp-8 double mutant. However, in a mab-5(gf) gain of function allele mab-5 is activated in both QL and QR cells. Migration of the QL descendants is not affected in the mab-5(gf) gain of function mutants, whereas QR descendants fail to migrate in an anterior direction [54]. We therefore tested the epistatic relationship between the mab-5(gf) allele and the catp-8 loss of function mutant. We found that the aberrant anterior migration of the QL descendant PVM in catp-8 single mutants was completely suppressed in a mab-5(gf); catp-8 double mutant (Fig 7D and 7F). In contrast, the migration defects of QR descendants in a mab-5(gf) remained unaffected by concomitant loss of catp-8 (Fig 7D and 7G). Thus, catp-8/P5A-ATPase functions upstream of or in parallel to the mab-5/HOX-C homeobox transcription factor.
Discussion
In this study we describe multiple functions of the catp-8/P5A-ATPase in metazoan development. Specifically, we show that catp-8 is required for different aspects of nervous system development, including axonal guidance, dendrite patterning and neuronal migrations. A reporter for CATP-8/P5A-ATPase is expressed in the ER of most if not all tissues and can function both cell-autonomously and non-autonomously during neuronal development. Genetically, catp-8/P5A functions in multiple pathways, including the conserved Menorin pathway during dendrite development and Wnt signaling during neuronal migrations. We find a substantial degree of genetic redundancy with different developmental pathways, suggesting that catp-8/P5A-ATPase may serve diverse, yet specific functions in different cellular contexts Lastly, catp-8/P5A-ATPase is necessary to correctly localize reporters for some but not all transmembrane proteins.
Functional redundancy of catp-8/P5A-ATPase in neuronal patterning
Not all neurons are affected by loss of catp-8 function. For example, the PVD somatosensory neurons require catp-8 for the elaboration of dendritic trees, but the dendrites of amphid sensory neurons seem unaffected. Similarly, axonal guidance or extension of HSN and D-type motor neurons requires catp-8 function, whereas guidance of PVQ axons or DA/DB motor neurons appears independent of catp-8, respectively. It is interesting to note that PVQ and HSN (but also D-type and DA/DB motor neurons to some extent) make similar navigational choices, are part of the same fascicles and depend on similar axon guidance pathways, such as Netrin signaling or Slit/Robo signaling [46,55,56], although we cannot exclude that the extracellular or signaling environment is distinct at different times during development. It should be noted that Wnt signaling is also required for correct axon guidance of both HSN and PVQ axons at the midline [46,57] and, at least for the latter cellular context, has been shown to function in parallel with Netrin signaling [57]. A possible explanation is that different genetic redundancies for catp-8 exist in different cells; in other words, in some cells, catp-8 may serve unique roles for which no functional alternative to catp-8 exists whereas in other cells catp-8 has functions that can be partially served by other genes. Such genes could be the related catp-6 or catp-7 ATPases, although at least in PVD patterning they play no redundant roles [23]. Alternatively, the molecules that are required for development of a given cell may be distinct in different neurons, such that some neurons depend on more or less proteins requiring catp-8 function.
Transgenic rescue experiments suggest both cell-autonomous and non-autonomous functions for catp-8. For example, PVD can develop normally with catp-8/P5A-ATPase function present in either neurons or the epidermis. This finding is in contrast to findings by Feng et al. who could rescue catp-8 defects by transgenic expression of catp-8 in neurons, but not in muscle or the epidermis [23]. One possible explanation for this discrepancy is different levels in transgene expression (we use a single copy transgenes for epidermal expression whereas Feng et al. utilized multi copy transgenes [23]). This hypothesis is supported by the fact that the Menorin pathway is known to be dosage sensitive and can be both positively and negatively regulated [58,59].
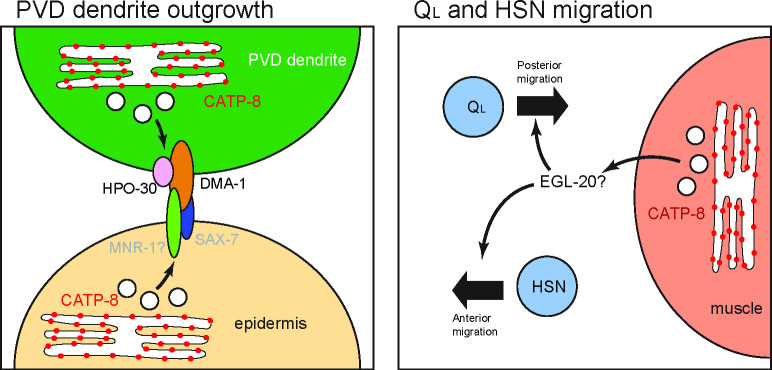
In contrast to this tissue redundancy, the migration defects of Q cell descendants are rescued only by non-autonomous expression of catp-8 in muscle, but not by expression in neurons or other tissues. These results strongly argue for a non-autonomous function of catp-8/P5A-ATPase in muscle during migration. However, no transmembrane proteins are known to function from muscle in Q cell migration, although muscle is clearly important, as both the RNA-Binding Protein ETR-1/CELF1 and the extracellular matrix protein SPON-1/F-spondin can function in muscle cells to mediate Q cell migration [60,61]. It is therefore tempting to speculate that secretion of the EGL-20/Wnt ligand could be regulated by catp-8/P5A-ATPase (Fig 8). First, we found, that during HSN migration (and possibly also Q cell migration), Wnt signaling functions in concert with the catp-8/P5A-ATPase. Second, EGL-20/Wnt has been shown to be expressed from posterior body wall muscle among other posterior tissues [44,45]. Third, the cellular migrations that are affected in catp-8/P5A-ATPase mutant animals (PVM, PQR, HSN) are all dependent on egl-20/Wnt whereas cell migrations that are independent of catp-8/P5A-ATPase (ALM, coelomocytes) are also independent of egl-20/Wnt [13]. Lastly, a mab-5(gf) mutant rescues the catp-8/P5A-ATPase mutant and is therefore likely downstream of catp-8 –just as mab-5 is downstream of egl-20/Wnt [43]. Further experiments are needed to definitively establish whether and how EGL-20/Wnt secretion or localization is dependent on catp-8/P5A-ATPase function.
Fig 8. Graphical Model of CATP-8 functions.
CATP-8 is associated with the endoplasmic reticulum and regulates or facilitates the secretary pathway. In the case of PVD dendritic outgrowth, CATP-8/P5A-ATPase is proposed to ensure the expression and localization of DMA-1 and HPO-30 in PVD, as well as transmembrane factors from the epidermis, of which one candidate is MNR-1. In the case of QL neuroblast and HSN migration, our results strongly suggest that CATP-8 ensures the sufficient secretion of the Wnt ligand EGL-20, which is needed for the posterior migration of QL neuroblasts, and the anterior migration of HSN neurons.
Substrates of the CATP-8/P5A-ATPase
Previous studies in yeast and plants suggested various roles for P5A-ATPases, such as functions in phosphate homeostasis and male gametogenesis [18–20], phospholipid and sterol homeostasis [21] and targeting of mitochondrial outer membrane proteins [22]. Therefore, which functions P5A ATPases may serve in metazoans remains a major question. Here, we provide genetic evidence that catp-8/P5A-ATPase is required for the localization of sufficient amounts of the DMA-1/LRR-TM receptor in the membrane of higher order PVD dendrites during morphogenesis. Similarly, the four transmembrane protein HPO-30/Claudin, which also functions in PVD neurons during dendrite patterning [62] requires catp-8 to be correctly localized to distal, higher order dendrites. Importantly, the number of vesicles containing the respective reporters and, which are presumed to represent endocytic/transport vesicles [41,42] along the dendritic branches, remain unaffected. Intriguingly, localization of the conserved SAX-7/L1CAM cell adhesion molecule in either neurons or the epidermis is not obviously affected. Therefore, catp-8 may be involved in the insertion/localization of certain transmembrane proteins but not others to the plasma membrane (Fig 8). These findings are consistent with another recent study showing similar effects on DMA-1::GFP [23,24], although it was also reported by Qin et al. that the levels of a HPO-30 reporter remained unchanged [24]. A possible explanation for this discrepancy is that we measured fluorescence in distal dendrites rather than the cell body [24]. The findings of a role for the catp-8/P5A ATPase in the localization of some cell surface proteins is seemingly at odds with two other studies in yeast and C. elegans suggesting that P5A-ATPases primarily maintain ER homeostasis by functioning as dislocases to remove mislocalized mitochondrial proteins [24,25]. However, several arguments can be made that P5A-ATPases serve additional functions beyond removing mislocalized mitochondrial proteins. First, genetic manipulations in C. elegans that suppress ER defects in PVD due to mislocalized DRP-1/Drp1 mitochondrial fission protein to the ER in catp-8 mutants, fail to restore the correct dendritic morphology [24] suggesting that catp-8/P5A-ATPase serves additional functions beyond maintaining ER integrity to mediate PVD morphogenesis. Second, proteomic studies in HeLa cells found not only the levels of mitochondrial, but also other transmembrane and secreted proteins changed in ATP13A1/P5A-ATPAse mutant cells. For example, a number of secreted growth factors, including the platelet derived growth factor D (PDGFD) or transforming growth factor beta (TGFB) as well as axon guidance molecules such as ephrin A5 (EFNA5), to name but a few, were significantly reduced in abundance upon loss of ATP13A1 catalytic activity [25]. In the context of these studies, our findings argue for a more diverse, yet likely specific role for catp-8 in the localization of certain transmembrane proteins and regulation of secreted proteins (Fig 8). Further experiments will be required to delineate the full spectrum of genetic and biochemical functions of P5A-type ATPases.
Materials and methods
C. elegans strains and genetics
All C. elegans strains were grown on nematode growth medium plates with E. coli (OP50) as a food source as described [63], usually at 20°C unless otherwise specified. Strains used in this work can be found in S1 Table. The catp-8 alleles dz224 and gk860114 were backcrossed four and three times, respectively, prior to further analysis.
Molecular cloning of catp-8(dz224)
Using a combination of Hawaiian SNP mapping and sequencing [26] the allele dz224 was localized to a region between 8Mb and 13Mb on LG IV, which was further refined by deficiency mapping to between 10.2Mb and 12Mb (S1 Fig). This 1.8Mb interval comprised 7 non-synonymous missense mutations and one non-sense mutation in catp-8, which was confirmed by Sanger sequencing: an A to a T transversion at position 11,479,010 on LG IV (WB270), resulting in an ochre premature stop codon after Y55 in C10C6.6 (catp-8). We found that catp-8(gk860411) failed to complement dz224, further suggesting that both alleles are affecting the same gene.
Molecular biology and transgenesis
Cloning of all constructs were carried out using standard molecular biology methods. A list of primers used can be found in S2 Table. For a list of plasmids used and detailed descriptions of constructs see S3 and S4 Tables. All extrachromosomal arrays were generated by injecting a mixture of the desired plasmids with pBluescript to a final concentration of 100 ng/μl of DNA. A detailed list of all the extrachromosomal transgenic lines generated can be found in S5 Table.
For CRISPR/Cas9 mediated genome editing, appropriate guide RNA sites were selected using IDT Cas9 crRNA design tool and, oligos in the general form of TAATACGACTCACTATA(gRNA)GTTTTAGAGCTAGAAATAGCAAG were ordered, where (gRNA) is the 20nt of the guide RNA sequence before the PAM motif, optimized for T7 promoter transcription. These oligos were used in PCR reactions as a forward primer in conjunction with the universal reverse primer AAAAGCACCGACTCGGTG (oLT337) to generate sgRNA transcription templates from pDD162 (containing the sequence of tracrRNA). sgRNA was then transcribed from this template using the HiScribe T7 in-vitro transcription kit (NEB) and purified with the Monarch RNA cleanup kit (NEB). The resulting sgRNA was used in an injection mix at 20 ng/μl per sgRNA, together with 250 ng/μl of Alt-R Cas9 endonuclease (IDT) and repair templates (100 ng/μl for single-strand oligos, 200 ng/μl for PCR products or plasmids). Injection and CRISPR efficiency were monitored through a Co-CRISPR strategy with dpy-10(cn64) or unc-58(e665) conversions, or through rol-6(su1006) co-injection markers [64]. A list of all genome-edited strains and detailed methodology can be found in S6 Table.
Scoring of migration phenotypes
All worms were immobilized using 1 mM levamisole and mounted on 4% agarose pads for phenotypic analysis.
AQR/PQR migrations were scored in wdIs52 (Is[F49H12.4p::GFP]) in L2 to young adults with a Plan-Apochromat 10x/0.25 objective on a Zeiss Axioimager Z1. Cells were considered in the head if anterior to the intestine, in the tail if posterior to the intestine, and in the mid body otherwise. AVM/PVM migrations were scored in zdIs5 (Is[mec-4p::GFP]) in L4 to young adults with a Plan-Apochromat 10x/0.25 objective on a Zeiss Axioimager Z1. Cells were scored based on their relative position (anterior or posterior) to the vulva. HSNs migrations were scored in dzIs75 (Is[kal-1p9::GFP]) in adult worms with a Plan-Apochromat 16x/0.5 objective on a Zeiss Axioimager Z1. Cells were considered to be at the vulva if the cell bodies were positioned less than or equal to an egg’s length posterior to the vulva. ALM was scored in zdIs5 (Is[mec-4p::GFP]) in synchronized L1 animals. Animals are imaged using a Plan-Apochromat 10x/0.25 objective on a Zeiss Axioimager Z1 Apotome. Using ImageJ, the distance of the more anterior cell body from the nose was measured and divided by the total length of the animal. Coelomocytes were scored in dzEx2140 at the L4 to young adult stage with a Plan-Apochromat 16x/0.5 objective on a Zeiss Axioimager Z1. Coelomocytes were considered to be in the head region if in the anterior quarter of the worm.
Scoring of axon pathfinding
HSN axon guidance at the ventral midline was scored in dzIs75 or mgIs71 at the L4 to adult worms stage with a Plan-Apochromat 40x/1.3 objective on a Zeiss Axioimager Z1. Crossover defects of axons were scored if any portion of the axons of HSNL and HSNR were touching. DD/VD neurons were scored in juIs76 at the L4 to adult worm stage with a Plan-Apochromat 40x/1.3 objective on a Zeiss Axioimager Z1. Axon guidance was categorized into two measurements, L/R choice and gaps in the dorsal or ventral cord. For L/R choice, any worm with 1 or more axons found on the opposite side of the stereotyped location was counted as having an L/R choice defect as indicated. Additionally, any worm with a visible discontinuation of the dorsal and/or ventral cord between any two neurons (gaps) was considered to have a dorsal and/or ventral cord defect respectively. DA/DB motor neurons were scored in evIs82b at the L4 to adult stage with a Plan-Apochromat 40x/1.3 objective on a Zeiss Axioimager. Axon guidance was categorized into two measurements, L/R choice and gaps in the dorsal or ventral cord. For L/R choice, any worm with 1 or more axons found on the opposite side of the stereotyped location was counted as having an L/R choice defect. Any worm with a visible discontinuation of the dorsal and/or ventral cord between any two neurons (gaps) was considered to have a dorsal or ventral cord defect, respectively. AIY axonal morphology was scored with mgIs32 at the L4 to adult stage with a Plan-Apochromat 16x/0.5 objective on a Zeiss Axioimager Z1. Cells were considered to be overbranching if an additional protrusion off of the cell body at least the length of the cell body was present. PVQ axonal guidance was scored in oyIs14 at the L4 to adult stage with a Plan-Apochromat 16x/0.5 objective on a Zeiss Axioimager Z1. Crossover defects were counted if any portion of the axons of PVQL and PVQR anterior to the vulva were not visibly separable. For DiI staining, well-fed adult worms were washed from plates with 1 mL M9, pelleted, resuspendend in 1 mL M9 before 5 μL of DiI stock solution (2 mg/mL DiI (Molecular Probes, #D282) in dimethyl formamide) was added. Animals were incubated with light shaking for 3 hours, spun and washed with M9 two times, followed by transfer to fresh plates. After 30 minutes recovery, animals were immobilized in 1 mM levamisole and neuronal processes were assessed for defects and imaged with a Plan-Apochromat 40x/1.3 or 63x/1.4 objective on a Zeiss Axioimager Z1 Apotome.
Fluorescent microscopy and quantification
PVD morphometric analysis
Fluorescent images were captured in live C. elegans at the L4 larval stage using a Plan-Fluor Nikon 40x/1.3x on a Nikon CSU-W1 Spinning Disk Confocal. At least 15 L4 larval animals were scored per genotype. Optical sections were collected and maximum intensity projections adjusted for optimal contrast to resolve detailed morphology were used for further analysis. For tracing, the 100 μm section of the primary branch anterior to the cell body was used for morphometric analyses using the NeuronJ plugin of the FIJI software. Branches were defined as follows: 2° dendritic branches as any neurite branching out of the primary dendrite; 3° branches as neurites branching at the end of a 2° branch in proximity to the tertiary line along the border of outer body wall muscles and the lateral epidermis; 4° dendritic branches as those originating from 3° dendritic branches and extending towards the dorsal or ventral nerve cords, respectively.
DMA-1::GFP quantifications
All DMA-1::GFP fluorescent images were captured in strains containing qyIs369 at the L4 larval stage using a Plan-Fluor Nikon 60x/1.4x objective and GFP filter setting on a Nikon CSU-W1 Spinning Disk Confocal with identical imaging parameters and appropriate z-stack. Fluorescence measurements of the z-projected image were subsequently performed in ImageJ as follows. For Intensity of GFP in the PVD cell body an outline of the cell body was drawn as Region of Interest (ROI) and the average signal in the ROI was quantified. An ROI of the same shape was placed outside of the cell body to measure the background signal, which was subtracted from the fluorescent signal. For the intensity of GFP in the PVD primary dendrite, the primary dendrite was traced for 100 μm anterior to the cell body, and an ROI was defined as 10 pixels (= 1.6 μm) thick along the traced line, where the average signal in the ROI was quantified. A ROI of the same size was placed next to the dendrite to measure the background signal, which was subtracted from the fluorescent signal. The number of puncta within 100 μm of the primary dendrite anterior to the cell body were counted manually.
HPO-30::tagBFP quantification
All HPO-30::tagBFP fluorescent images were captured in strains containing dzEx2140 and wdIs52 at the L4 larval stage using a 40x/1.3 objective on a Zeiss Axioimager Z1 Apotome with identical imaging parameters for tagBFP and GFP filter setting, and appropriate z-stacks. Quantification of the z-projected images were subsequently performed in ImageJ as follows. For the percentage of quaternary dendrites containing HPO-30::tagBFP, the number of quaternary branches with visible tagBFP signal within 100 μm anterior to the cell body were counted in the tagBFP channel, then divided by the number of quaternary dendrites as imaged in the GFP channel. For the number of HPO-30::tagBFP puncta, quantification was performed by manually counting the puncta within 100 μm anterior to the cell body.
SAX-7::GFP and LECT-2::mNG imaging:
Fluorescent images for the strains containing the ddIs290 fosmid based SAX-7::GFP reporter or dz249[lect-2::GFP] endogenous knock-in were captured at the late L4 larval stage using a Plan-Apochromat 40x/1.3 objective on a Zeiss Axioimager Z1 Apotome at an exposure time of 151 ms, followed by appropriate z-stacks. Neuronal and epidermal patterning were assessed, and the PVD phenotype was observed in a dzIs53 background.
Colocalization assay of subcellular compartment markers
L4 animals carrying dzSi3 and single-copy insertions of GFP subcellular compartment markers were imaged using a Plan-Fluor Nikon 60x/1.4x objective on a Nikon CSU-W1 Spinning Disk. Single planes from a confocal image stack in which both the catp-8 and organellar reporters were clearly visible were manually thresholded, then analyzed using the Correlation Plot application in Metamorph 7.7 to establish a Pearson’s Correlation Coefficient for each image pair.
Analysis of Q neuroblast polarization
Animals carrying the heIs63 transgene were synchronized and imaged 4.5 hours after hatching using a Plan-Fluor Nikon 100x/1.4x objective on a Nikon CSU-W1 Spinning Disk Confocal, before the first cell division of the Q neuroblast. The normalized position of the Q neuroblast was calculated by first drawing a straight line between the nuclei of V4 and V5, then the perpendicular location of nuclei of Q neuroblasts on the line was noted. The normalized position was calculated by subtracting the distance between the nuclei of V5 and Q neuroblasts (negative if posterior to V5) from that between the nuclei of V4 and Q neuroblasts (negative if anterior to V4) and divided by that between the nuclei of V4 and V5.
Statistical analysis
All statistical analyses were performed using the Prism 9 Statistical Software suite (GraphPad). All pairwise migration phenotypes are analyzed with Fisher exact test when applicable, or Chi-squared test as indicated. All comparisons of averages/proportions are analyzed with one-way ANOVA with Tukey correction, the Kruskal-Wallis test, the Mann-Whitney test, or the Z-test as applicable.
Supporting information
S1 Table lists all strains that were used during this study, including strain names and genotypes.
(XLSX)
S2 Table lists all primers and oligos that were used during this study, including name, sequence and their applicationb.
(XLSX)
S3 Table lists all plasmids that were used during this study and had been previously described.
(XLSX)
S4 Table lists all plasmids that were generated and used during this study.
(XLSX)
S5 Table lists all extrachromosomal transgenes that were generated and analyzed during this study.
(XLSX)
S6 Table lists information regarding alleles, strain names and reagents that were generated and used, respectively, during this study for CRISPR/Cas9 mediated genome engineering.
(XLSX)
A. Frequency plot of N2 SNPs from whole genome sequencing results as per Hawaiian SNP mapping [26], indicating dz224 lesions reside between 6 Mbp and 13 Mbp on linkage group (LG) IV. Deficiencies covering the region are indicated below (green, complementation of dz224; red non-complementation of dz224), demonstrating that dz224 lesions lies between 10,202,993, and 12,011,007 bp, the physical positions of the last known genes inside of eDf19 and the first one outside of mDf7, respectively. The table below lists lesions detected within this range, with catp-8 containing the only stop-gain mutation. B.—E. Quantification of the number of secondary branches that reach the tertiary line where muscle and epidermis abut (B)(i.e. excluding ectopic stunted secondary branches), aggregate length of all secondary (C), tertiary (D), and quaternary (E) dendrite branches 100 μm anterior to the PVD cell body in the genotypes indicated. Data are represented as the mean ± 95% confidence interval. Statistical significance was calculated using one-sided ANOVA with Tukey’s multiple comparison test. * P ≤ 0.05, *** P ≤ 0.001, **** P ≤ 0.0001, ns not significant. F. Complementation assay tallying the percentage of animals with PVD defects. Note that there is non-complementation between dz224 and catp-8(gk860114), recessiveness of dz224 and catp-8(gk860114), and absence of maternal effects. G. Quantification of the number of quaternary and secondary branches, and the ratio of quaternary to secondary branches 100 μm anterior to the PVD cell body in catp-8(gk860114) and catp-8(gk860114)/mDf7 transheterozygous animals. No enhancement was observed in the transheterozygous genotype, showing that gk860114 behaves as a genetic null allele. Data are represented as the mean ± 95% confidence interval. ns not significant, Kruskal-Wallis test with Dunn’s multiple comparisons test. n = 14 animals per genotype. H. Body length of wildtype and catp-8(gk860114) L1 larval animals, measured and presented as mean ± 95% confidence interval. ns not significant. Statistical significance was calculated with an unpaired t-test. I. Fraction of catp-8 homozygous mutant animals presenting PQR migration defects originating from homozygous or heterozygous catp-8 mutant parents. PQR migration is completely normal in F1s originating from heterozygous animals, indicating a maternal contribution of catp-8 in PQR migration. Homozygosity of animals was determined by the PVD phenotype, which is not maternally rescued. J. Fraction of descendants from catp-8(gk860114) heterozygous animals with HSN migration defects, suggesting the absence of maternal effects. * P ≤ 0.05, Fisher exact test.
(TIFF)
A.—B. Maximum z-projection confocal images of DA/DB cholinergic motor-neurons (visualized by evIs82b (Is[unc-129p::GFP]) of wildtype (A) and catp-8 mutant animals (B). No difference between the genotypes were observed. Lateral views, ventral up, anterior to the left. C.—D. Maximum z-projection confocal images of D-type GABAergic motor-neurons (visualized by juIs76(Is[unc-25p::GFP]) of wild type (C) and catp-8 mutant animals (D). Occasional gaps caused by under-extension of neurites can be observed in catp-8 mutant animals. Lateral views, dorsal up, anterior to the left. E.—F. Maximum z-projection confocal images of AIY interneurons (visualized by mgIs32 (Is[ttx-3p::GFP]) in wildtype (E) and catp-8 mutant animals (F). No difference between the genotypes was observed. Lateral views, dorsal up, anterior to the left. G.—J. Maximum z-projection apotome images of DiI stained phasmid and amphid sensory neurons, respectively in wildtype (G,I) and catp-8 mutant animals (H,K). No difference between the genotypes was observed. Ventral views, anterior to the left. Lateral views, dorsal up, anterior to the left. K.—N. Maximum z-projection confocal images of PVQ interneurons (visualized by oyIs14 (Is[sra-6p::GFP]) (K,L) and HSN motor-neurons (visualized by dzIs75 (Is[kal-9p::GFP]) (M,N) in wildtype and catp-8 mutant animals. No defects were observed in PVQ axons at the midline, but HSN neurons showed a significant number of midline cross overs in catp-8 mutant animals (see also Table 1). Ventral views, anterior to the left.
(TIFF)
A.—E. Maximum z-projection confocal images of an embryo (A), L2 larval animal (B), L3 larval animal (C-D) and L4 larval animal (E) carrying the catp-8p promoter GFP reporter fusion array dzEx2101. dzEx2101 exhibits a high degree of mosaicism, resulting in staining of different tissues across the population, including muscle, intestine, pharynx, and some unidentified neurons.
(TIFF)
Confocal images of single copy insertion transgenes of epidermally expressed organellar reporters in catp-8(gk860114) mutant and wild type animals, for ER/endoplasmic reticulum (pwSi82 [Phyp-7::VIT2ss::oxGFP-KDEL]), cis/medial Golgi (pwSi202 [Phyp7::AMAN-2::mNG;HYG-R]), early endosomes (pwSi145 [Phyp7::mNG::RAB-5;HYG-R]), late endosomes and lysosomes pwSi140 [Phyp7::mNG::RAB-7;HYG-R]), autophagosomes (pwSi144 [Phyp7::mNG::LGG-1;HYG-R]), and lysosomes (pwSi205 [Phyp7::LMP-1::mNG;HYG-R]).
(TIFF)
A.—C. Quantification of the number of quaternary branches (A), secondary branches (B), and the ratio of quaternary to secondary branches (C) 100 μm anterior to the PVD cell body in animals of all extrachromosomal rescue lines tested. TG: transgenic animals, NTS: non-transgenic siblings. Data are represented as the mean ± 95% confidence interval. **** P < 0.0001, ns not significant, Kruskal-Wallis test with Dunn’s multiple comparisons test. n = 14 animals per genotype. D.—E. Percentage of animals with the indicated migration phenotype of PVM (D) and PQR (E) for all extrachromosomal rescue lines tested. TG: transgenic animals, NTS: non-transgenic siblings. **** P < 0.0001, ns not significant, Chi-squared test. n > 75 animals per genotype.
(TIFF)
A.—C. Quantification of the aggregate length of secondary (A), tertiary (B), and quaternary (C) dendrite branches within 100μm anterior to the PVD cell body in animals of the indicated genotypes. Note that all alleles are molecular or genetic null alleles. Data are represented as the mean ± 95% confidence interval. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 ns not significant; one-sided ANOVA with Tukey’s multiple comparison test. n = 15 animals per genotype.
(TIFF)
A. Fluorescent images of initial Q neuroblasts morphologies, when polarization is just beginning in control and catp-8(gk860114) mutant animals, imaged 1.5 hours after hatching. Left and right sides of the animals are shown as indicated. Scale bar = 5 μm. B.—C. Quantification of DMA-1::GFP puncta either in the primary (B) or on the tertiary (C) dendrite 100μm anterior of the cell body in the genotypes indicated. ns, not significant, Mann-Whitney test. n = 14 animals per genotype. D. Number of HPO-30::tagBFP puncta in the primary dendrite 100 μm anterior to the cell body in control or catp-8 mutant animals, presented as mean ± 95% confidence interval. ns not significant, Mann-Whitney test. n = 10 animals per genotype. E. Fluorescent images of animals expressing a functional fosmid based SAX-7::GFP reporter (ddIs290) in control, catp-8(gk860114) mutants, and animals overexpressing catp-8 in the epidermis (dzSi3). White arrowheads denote neuronal staining, while open arrows indicate examples of epidermal staining at the lateral epidermal ridge. F. Quantification of the percentage of animals with PVD 4° branching defects in the indicated genotypes in a ddIs290 background. Statistical comparisons were performed using the Z-test. ****, P < 0.0005, ns not significant. n = 15 animals per genotype. G. Fluorescent images of animals expressing lect-2::mNG endogenous knock-in (dz249) [38] in control and catp-8(gk860114) mutant animals. White arrowheads denote neuronal staining, while open arrows indicate examples of epidermal staining at the lateral epidermal ridge.
(TIFF)
Acknowledgments
We thank S. Emmons, A. Meléndez, Y. Salzberg, and members of the Bülow laboratory for comments on the manuscript and for helpful discussions during the course of this project. We thank S. Cook for the initial observation of PQR cell migration defects, R. Poole for help with the analysis of whole genome sequencing data and, Yuji Kohara for cDNA clones and M. Harterink for a plasmid; some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by a fellowship from the Croucher Foundation (https://croucher.org.hk/) to L.T.H.T., A Tishman Scholarship to J.F. (https://einsteinmed.org/departments/neuroscience/), the National Institutes of Health (NIH) R01NS096672 & R21NS111145 (https://www.nih.gov) to H.E.B., R01GM135326, R01GM067237 & R01AG047101 to B.D.G., F31NS111939 to M.T., F31NS100370 to M.R., T32GM007491 to C.J.S., P40 OD010440, and to Caenorhabditis Genetics Center at the University of Minnesota, P30CA013330 & P30HD071593 to Albert Einstein College of Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have not financial conflicts of interest to declare.
References
- 1.Marin O, Valiente M, Ge X, Tsai LH. Guiding neuronal cell migrations. Cold Spring Harbor perspectives in biology. 2010;2(2):a001834. doi: 10.1101/cshperspect.a001834 ; PubMed Central PMCID: PMC2828271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis F, Cappello S. Neuronal migration and disorders—an update. Curr Opin Neurobiol. 2020;66:57–68. Epub 2020/10/24. doi: 10.1016/j.conb.2020.10.002 . [DOI] [PubMed] [Google Scholar]
- 3.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298(5600):1959–64. doi: 10.1126/science.1072165 . [DOI] [PubMed] [Google Scholar]
- 4.Sanes JR, Zipursky SL. Synaptic Specificity, Recognition Molecules, and Assembly of Neural Circuits. Cell. 2020;181(6):1434–5. Epub 2020/06/13. doi: 10.1016/j.cell.2020.05.046 . [DOI] [PubMed] [Google Scholar]
- 5.Dong X, Shen K, Bülow HE. Intrinsic and extrinsic mechanisms of dendritic morphogenesis. Annu Rev Physiol. 2015;77:271–300. doi: 10.1146/annurev-physiol-021014-071746 . [DOI] [PubMed] [Google Scholar]
- 6.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11(5):316–28. Epub 2010/04/21. nrn2836 [pii] doi: 10.1038/nrn2836 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre JL. Molecular mechanisms that mediate dendrite morphogenesis. Current Topics in Developmental Biology: Academic Press; 2021. [DOI] [PubMed] [Google Scholar]
- 8.Inberg S, Meledin A, Kravtsov V, Iosilevskii Y, Oren-Suissa M, Podbilewicz B. Lessons from Worm Dendritic Patterning. Annu Rev Neurosci. 2019;42:365–83. Epub 2019/04/03. doi: 10.1146/annurev-neuro-072116-031437 . [DOI] [PubMed] [Google Scholar]
- 9.Sundararajan L, Stern J, Miller DM, 3rd. Mechanisms that regulate morphogenesis of a highly branched neuron in C. elegans. Dev Biol. 2019;451(1):53–67. Epub 2019/04/21. doi: 10.1016/j.ydbio.2019.04.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988;336(6200):638–46. doi: 10.1038/336638a0 [DOI] [PubMed] [Google Scholar]
- 11.Middelkoop TC, Korswagen HC. Development and migration of the C. elegans Q neuroblasts and their descendants. WormBook. 2014:1–23. Epub 2014/10/16. doi: 10.1895/wormbook.1.173.1 ; PubMed Central PMCID: PMC4781233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadsworth WG, Hedgecock EM. Guidance of neuroblast migrations and axonal projections in Caenorhabditis elegans. Curr Opin Neurobiol. 1992;2(1):36–41. doi: 10.1016/0959-4388(92)90159-i [DOI] [PubMed] [Google Scholar]
- 13.Zinovyeva AY, Yamamoto Y, Sawa H, Forrester WC. Complex network of Wnt signaling regulates neuronal migrations during Caenorhabditis elegans development. Genetics. 2008;179(3):1357–71. doi: 10.1534/genetics.108.090290 ; PubMed Central PMCID: PMC2475739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmgren MG, Axelsen KB. Evolution of P-type ATPases. Biochim Biophys Acta. 1998;1365(1–2):37–45. Epub 1998/08/07. doi: 10.1016/s0005-2728(98)00041-3 . [DOI] [PubMed] [Google Scholar]
- 15.Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys. 2011;40:243–66. Epub 2011/03/01. doi: 10.1146/annurev.biophys.093008.131331 . [DOI] [PubMed] [Google Scholar]
- 16.Sørensen DM, Buch-Pedersen MJ, Palmgren MG. Structural divergence between the two subgroups of P5 ATPases. Biochim Biophys Acta. 2010;1797(6–7):846–55. Epub 2010/04/27. doi: 10.1016/j.bbabio.2010.04.010 . [DOI] [PubMed] [Google Scholar]
- 17.van Veen S, Martin S, Van den Haute C, Benoy V, Lyons J, Vanhoutte R, et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature. 2020;578(7795):419–24. Epub 2020/01/31. doi: 10.1038/s41586-020-1968-7 . [DOI] [PubMed] [Google Scholar]
- 18.Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J. 2004;37(6):801–14. Epub 2004/03/05. doi: 10.1111/j.1365-313x.2004.02005.x . [DOI] [PubMed] [Google Scholar]
- 19.Ticconi CA, Lucero RD, Sakhonwasee S, Adamson AW, Creff A, Nussaume L, et al. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proc Natl Acad Sci U S A. 2009;106(33):14174–9. Epub 2009/08/12. doi: 10.1073/pnas.0901778106 ; PubMed Central PMCID: PMC2723163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsen MK, Poulsen LR, Schulz A, Fleurat-Lessard P, Moller A, Husted S, et al. Pollen development and fertilization in Arabidopsis is dependent on the MALE GAMETOGENESIS IMPAIRED ANTHERS gene encoding a type V P-type ATPase. Genes Dev. 2005;19(22):2757–69. Epub 2005/11/18. doi: 10.1101/gad.357305 ; PubMed Central PMCID: PMC1283967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sørensen DM, Holen HW, Pedersen JT, Martens HJ, Silvestro D, Stanchev LD, et al. The P5A ATPase Spf1p is stimulated by phosphatidylinositol 4-phosphate and influences cellular sterol homeostasis. Mol Biol Cell. 2019;30(9):1069–84. Epub 2019/02/21. doi: 10.1091/mbc.E18-06-0365 ; PubMed Central PMCID: PMC6724510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krumpe K, Frumkin I, Herzig Y, Rimon N, Ozbalci C, Brugger B, et al. Ergosterol content specifies targeting of tail-anchored proteins to mitochondrial outer membranes. Mol Biol Cell. 2012;23(20):3927–35. Epub 2012/08/25. doi: 10.1091/mbc.E11-12-0994 ; PubMed Central PMCID: PMC3469509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Z, Zhao Y, Li T, Nie W, Yang X, Wang X, et al. CATP-8/P5A ATPase Regulates ER Processing of the DMA-1 Receptor for Dendritic Branching. Cell Rep. 2020;32(10):108101. Epub 2020/09/10. doi: 10.1016/j.celrep.2020.108101 . [DOI] [PubMed] [Google Scholar]
- 24.Qin Q, Zhao T, Zou W, Shen K, Wang X. An Endoplasmic Reticulum ATPase Safeguards Endoplasmic Reticulum Identity by Removing Ectopically Localized Mitochondrial Proteins. Cell Rep. 2020;33(6):108363. Epub 2020/11/12. doi: 10.1016/j.celrep.2020.108363 . [DOI] [PubMed] [Google Scholar]
- 25.McKenna MJ, Sim SI, Ordureau A, Wei L, Harper JW, Shao S, et al. The endoplasmic reticulum P5A-ATPase is a transmembrane helix dislocase. Science. 2020;369(6511). Epub 2020/09/26. doi: 10.1126/science.abc5809 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minevich G, Park DS, Blankenberg D, Poole RJ, Hobert O. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics. 2012;192(4):1249–69. doi: 10.1534/genetics.112.144204 ; PubMed Central PMCID: PMC3512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albeg A, Smith CJ, Chatzigeorgiou M, Feitelson DG, Hall DH, Schafer WR, et al. C. elegans multi-dendritic sensory neurons: morphology and function. Mol Cell Neurosci. 2011;46(1):308–17. Epub 2010/10/26. S1044-7431(10)00246-0 [pii] doi: 10.1016/j.mcn.2010.10.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CJ, Watson JD, Spencer WC, O’Brien T, Cha B, Albeg A, et al. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev Biol. 2010;345(1):18–33. Epub 2010/06/12. S0012-1606(10)00805-5 [pii] doi: 10.1016/j.ydbio.2010.05.502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oren-Suissa M, Hall DH, Treinin M, Shemer G, Podbilewicz B. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science. 2010;328(5983):1285–8. doi: 10.1126/science.1189095 ; PubMed Central PMCID: PMC3057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salzberg Y, Diaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, et al. Skin-Derived Cues Control Arborization of Sensory Dendrites in Caenorhabditis elegans. Cell. 2013;155(2):308–20. doi: 10.1016/j.cell.2013.08.058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson O, Edgley M, Strasbourger P, Flibotte S, Ewing B, Adair R, et al. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 2013;23(10):1749–62. doi: 10.1101/gr.157651.113 ; PubMed Central PMCID: PMC3787271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anazi S, Maddirevula S, Salpietro V, Asi YT, Alsahli S, Alhashem A, et al. Expanding the genetic heterogeneity of intellectual disability. Hum Genet. 2017;136(11–12):1419–29. Epub 2017/09/25. doi: 10.1007/s00439-017-1843-2 . [DOI] [PubMed] [Google Scholar]
- 33.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100(1):64–119. doi: 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- 34.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56(1):110–56. doi: 10.1016/0012-1606(77)90158-0 [DOI] [PubMed] [Google Scholar]
- 35.Cronin SR, Rao R, Hampton RY. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J Cell Biol. 2002;157(6):1017–28. Epub 2002/06/12. doi: 10.1083/jcb.200203052 ; PubMed Central PMCID: PMC2174042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilleard JS, Barry JD, Johnstone IL. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol Cell Biol. 1997;17(4):2301–11. doi: 10.1128/MCB.17.4.2301 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundararajan L, Smith CJ, Watson JD, Millis BA, Tyska MJ, Miller DM 3rd. Actin assembly and non-muscle myosin activity drive dendrite retraction in an UNC-6/Netrin dependent self-avoidance response. PLoS Genet. 2019;15(6):e1008228. Epub 2019/06/21. doi: 10.1371/journal.pgen.1008228 ; PubMed Central PMCID: PMC6605669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz-Balzac CA, Rahman M, Lazaro-Pena MI, Martin Hernandez LA, Salzberg Y, Aguirre-Chen C, et al. Muscle- and Skin-Derived Cues Jointly Orchestrate Patterning of Somatosensory Dendrites. Curr Biol. 2016;26(17):2379–87. doi: 10.1016/j.cub.2016.07.008 ; PubMed Central PMCID: PMC5021591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou W, Shen A, Dong X, Tugizova M, Xiang YK, Shen K. A multi-protein receptor-ligand complex underlies combinatorial dendrite guidance choices in C. elegans. Elife. 2016;5. doi: 10.7554/eLife.18345 ; PubMed Central PMCID: PMC5079751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang LT, Diaz-Balzac CA, Rahman M, Ramirez-Suarez NJ, Salzberg Y, Lazaro-Pena MI, et al. TIAM-1/GEF can shape somatosensory dendrites independently of its GEF activity by regulating F-actin localization. Elife. 2019;8. doi: 10.7554/eLife.38949 ; PubMed Central PMCID: PMC6370339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor CA, Yan J, Howell AS, Dong X, Shen K. RAB-10 Regulates Dendritic Branching by Balancing Dendritic Transport. PLoS Genet. 2015;11(12):e1005695. doi: 10.1371/journal.pgen.1005695 ; PubMed Central PMCID: PMC4669152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou W, Yadav S, DeVault L, Nung Jan Y, Sherwood DR. RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization. PLoS Genet. 2015;11(9):e1005484. doi: 10.1371/journal.pgen.1005484 ; PubMed Central PMCID: PMC4578882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whangbo J, Kenyon C. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol Cell. 1999;4(5):851–8. doi: 10.1016/s1097-2765(00)80394-9 [DOI] [PubMed] [Google Scholar]
- 44.Harterink M, Kim DH, Middelkoop TC, Doan TD, van Oudenaarden A, Korswagen HC. Neuroblast migration along the anteroposterior axis of C. elegans is controlled by opposing gradients of Wnts and a secreted Frizzled-related protein. Development. 2011;138(14):2915–24. doi: 10.1242/dev.064733 ; PubMed Central PMCID: PMC3119304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pani AM, Goldstein B. Direct visualization of a native Wnt in vivo reveals that a long-range Wnt gradient forms by extracellular dispersal. Elife. 2018;7. doi: 10.7554/eLife.38325 ; PubMed Central PMCID: PMC6143344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garriga G, Desai C, Horvitz HR. Cell interactions control the direction of outgrowth, branching and fasciculation of the HSN axons of Caenorhabditis elegans. Development. 1993;117(3):1071–87. [DOI] [PubMed] [Google Scholar]
- 47.Saied-Santiago K, Townley RA, Attonito JD, da Cunha DS, Diaz-Balzac CA, Tecle E, et al. Coordination of Heparan Sulfate Proteoglycans with Wnt Signaling To Control Cellular Migrations and Positioning in Caenorhabditis elegans. Genetics. 2017. doi: 10.1534/genetics.116.198739 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honigberg L, Kenyon C. Establishment of left/right asymmetry in neuroblast migration by UNC- 40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development. 2000;127(21):4655–68. [DOI] [PubMed] [Google Scholar]
- 49.Buettner FF, Ashikov A, Tiemann B, Lehle L, Bakker H. C. elegans DPY-19 is a C-mannosyltransferase glycosylating thrombospondin repeats. Mol Cell. 2013;50(2):295–302. Epub 2013/04/09. doi: 10.1016/j.molcel.2013.03.003 . [DOI] [PubMed] [Google Scholar]
- 50.Middelkoop TC, Williams L, Yang PT, Luchtenberg J, Betist MC, Ji N, et al. The thrombospondin repeat containing protein MIG-21 controls a left-right asymmetric Wnt signaling response in migrating C. elegans neuroblasts. Dev Biol. 2012;361(2):338–48. Epub 2011/11/15. doi: 10.1016/j.ydbio.2011.10.029 . [DOI] [PubMed] [Google Scholar]
- 51.Schmitz C, Wacker I, Hutter H. The Fat-like cadherin CDH-4 controls axon fasciculation, cell migration and hypodermis and pharynx development in Caenorhabditis elegans. Dev Biol. 2008;316(2):249–59. Epub 2008/03/11. doi: 10.1016/j.ydbio.2008.01.024 . [DOI] [PubMed] [Google Scholar]
- 52.Sundararajan L, Norris ML, Schoneich S, Ackley BD, Lundquist EA. The fat-like cadherin CDH-4 acts cell-non-autonomously in anterior-posterior neuroblast migration. Dev Biol. 2014;392(2):141–52. Epub 2014/06/24. doi: 10.1016/j.ydbio.2014.06.009 ; PubMed Central PMCID: PMC4136450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebbing A, Middelkoop TC, Betist MC, Bodewes E, Korswagen HC. Partially overlapping guidance pathways focus the activity of UNC-40/DCC along the anteroposterior axis of polarizing neuroblasts. Development. 2019;146(18). Epub 2019/09/07. doi: 10.1242/dev.180059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salser SJ, Kenyon C. Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature. 1992;355(6357):255–8. doi: 10.1038/355255a0 . [DOI] [PubMed] [Google Scholar]
- 55.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16(1):35–46. doi: 10.1016/s0896-6273(00)80021-5 [DOI] [PubMed] [Google Scholar]
- 56.Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92(2):217–27. doi: 10.1016/s0092-8674(00)80916-2 [DOI] [PubMed] [Google Scholar]
- 57.Chien J, Devkota R, Yosef N, Mörck C. Regulation of Axon Guidance by the Wnt Receptor Ror/CAM-1 in the PVT Guidepost Cell in Caenorhabditis elegans. Genetics. 2017;207(4):1533–45. Epub 2017/10/11. doi: 10.1534/genetics.117.300375 ; PubMed Central PMCID: PMC5714464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salzberg Y, Ramirez-Suarez NJ, Bülow HE. The proprotein convertase KPC-1/furin controls branching and self-avoidance of sensory dendrites in Caenorhabditis elegans. PLoS Genet. 2014;10(9):e1004657. doi: 10.1371/journal.pgen.1004657 ; PubMed Central PMCID: PMC4169376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong X, Chiu H, Park YJ, Zou W, Zou Y, Ozkan E, et al. Precise regulation of the guidance receptor DMA-1 by KPC-1/Furin instructs dendritic branching decisions. Elife. 2016;5. doi: 10.7554/eLife.11008 ; PubMed Central PMCID: PMC4811766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ochs ME, Josephson MP, Lundquist EA. The Predicted RNA-Binding Protein ETR-1/CELF1 Acts in Muscles To Regulate Neuroblast Migration in Caenorhabditis elegans. G3. 2020;10(7):2365–76. Epub 2020/05/14. doi: 10.1534/g3.120.401182 ; PubMed Central PMCID: PMC7341121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josephson MP, Miltner AM, Lundquist EA. Nonautonomous Roles of MAB-5/Hox and the Secreted Basement Membrane Molecule SPON-1/F-Spondin in Caenorhabditis elegans Neuronal Migration. Genetics. 2016;203(4):1747–62. Epub 2016/05/27. doi: 10.1534/genetics.116.188367 ; PubMed Central PMCID: PMC4981275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith CJ, O’Brien T, Chatzigeorgiou M, Spencer WC, Feingold-Link E, Husson SJ, et al. Sensory Neuron Fates Are Distinguished by a Transcriptional Switch that Regulates Dendrite Branch Stabilization. Neuron. 2013;79(2):266–80. doi: 10.1016/j.neuron.2013.05.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nance J, Frokjaer-Jensen C. The Caenorhabditis elegans Transgenic Toolbox. Genetics. 2019;212(4):959–90. Epub 2019/08/14. doi: 10.1534/genetics.119.301506 ; PubMed Central PMCID: PMC6707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bülow HE, Hobert O. Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron. 2004;41(5):723–36. doi: 10.1016/s0896-6273(04)00084-4 . [DOI] [PubMed] [Google Scholar]
- 66.Zallen JA, Kirch SA, Bargmann CI. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development. 1999;126(16):3679–92. [DOI] [PubMed] [Google Scholar]
- 67.Clark SG, Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development. 2003;130(16):3781–94. doi: 10.1242/dev.00571 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Table lists all strains that were used during this study, including strain names and genotypes.
(XLSX)
S2 Table lists all primers and oligos that were used during this study, including name, sequence and their applicationb.
(XLSX)
S3 Table lists all plasmids that were used during this study and had been previously described.
(XLSX)
S4 Table lists all plasmids that were generated and used during this study.
(XLSX)
S5 Table lists all extrachromosomal transgenes that were generated and analyzed during this study.
(XLSX)
S6 Table lists information regarding alleles, strain names and reagents that were generated and used, respectively, during this study for CRISPR/Cas9 mediated genome engineering.
(XLSX)
A. Frequency plot of N2 SNPs from whole genome sequencing results as per Hawaiian SNP mapping [26], indicating dz224 lesions reside between 6 Mbp and 13 Mbp on linkage group (LG) IV. Deficiencies covering the region are indicated below (green, complementation of dz224; red non-complementation of dz224), demonstrating that dz224 lesions lies between 10,202,993, and 12,011,007 bp, the physical positions of the last known genes inside of eDf19 and the first one outside of mDf7, respectively. The table below lists lesions detected within this range, with catp-8 containing the only stop-gain mutation. B.—E. Quantification of the number of secondary branches that reach the tertiary line where muscle and epidermis abut (B)(i.e. excluding ectopic stunted secondary branches), aggregate length of all secondary (C), tertiary (D), and quaternary (E) dendrite branches 100 μm anterior to the PVD cell body in the genotypes indicated. Data are represented as the mean ± 95% confidence interval. Statistical significance was calculated using one-sided ANOVA with Tukey’s multiple comparison test. * P ≤ 0.05, *** P ≤ 0.001, **** P ≤ 0.0001, ns not significant. F. Complementation assay tallying the percentage of animals with PVD defects. Note that there is non-complementation between dz224 and catp-8(gk860114), recessiveness of dz224 and catp-8(gk860114), and absence of maternal effects. G. Quantification of the number of quaternary and secondary branches, and the ratio of quaternary to secondary branches 100 μm anterior to the PVD cell body in catp-8(gk860114) and catp-8(gk860114)/mDf7 transheterozygous animals. No enhancement was observed in the transheterozygous genotype, showing that gk860114 behaves as a genetic null allele. Data are represented as the mean ± 95% confidence interval. ns not significant, Kruskal-Wallis test with Dunn’s multiple comparisons test. n = 14 animals per genotype. H. Body length of wildtype and catp-8(gk860114) L1 larval animals, measured and presented as mean ± 95% confidence interval. ns not significant. Statistical significance was calculated with an unpaired t-test. I. Fraction of catp-8 homozygous mutant animals presenting PQR migration defects originating from homozygous or heterozygous catp-8 mutant parents. PQR migration is completely normal in F1s originating from heterozygous animals, indicating a maternal contribution of catp-8 in PQR migration. Homozygosity of animals was determined by the PVD phenotype, which is not maternally rescued. J. Fraction of descendants from catp-8(gk860114) heterozygous animals with HSN migration defects, suggesting the absence of maternal effects. * P ≤ 0.05, Fisher exact test.
(TIFF)
A.—B. Maximum z-projection confocal images of DA/DB cholinergic motor-neurons (visualized by evIs82b (Is[unc-129p::GFP]) of wildtype (A) and catp-8 mutant animals (B). No difference between the genotypes were observed. Lateral views, ventral up, anterior to the left. C.—D. Maximum z-projection confocal images of D-type GABAergic motor-neurons (visualized by juIs76(Is[unc-25p::GFP]) of wild type (C) and catp-8 mutant animals (D). Occasional gaps caused by under-extension of neurites can be observed in catp-8 mutant animals. Lateral views, dorsal up, anterior to the left. E.—F. Maximum z-projection confocal images of AIY interneurons (visualized by mgIs32 (Is[ttx-3p::GFP]) in wildtype (E) and catp-8 mutant animals (F). No difference between the genotypes was observed. Lateral views, dorsal up, anterior to the left. G.—J. Maximum z-projection apotome images of DiI stained phasmid and amphid sensory neurons, respectively in wildtype (G,I) and catp-8 mutant animals (H,K). No difference between the genotypes was observed. Ventral views, anterior to the left. Lateral views, dorsal up, anterior to the left. K.—N. Maximum z-projection confocal images of PVQ interneurons (visualized by oyIs14 (Is[sra-6p::GFP]) (K,L) and HSN motor-neurons (visualized by dzIs75 (Is[kal-9p::GFP]) (M,N) in wildtype and catp-8 mutant animals. No defects were observed in PVQ axons at the midline, but HSN neurons showed a significant number of midline cross overs in catp-8 mutant animals (see also Table 1). Ventral views, anterior to the left.
(TIFF)
A.—E. Maximum z-projection confocal images of an embryo (A), L2 larval animal (B), L3 larval animal (C-D) and L4 larval animal (E) carrying the catp-8p promoter GFP reporter fusion array dzEx2101. dzEx2101 exhibits a high degree of mosaicism, resulting in staining of different tissues across the population, including muscle, intestine, pharynx, and some unidentified neurons.
(TIFF)
Confocal images of single copy insertion transgenes of epidermally expressed organellar reporters in catp-8(gk860114) mutant and wild type animals, for ER/endoplasmic reticulum (pwSi82 [Phyp-7::VIT2ss::oxGFP-KDEL]), cis/medial Golgi (pwSi202 [Phyp7::AMAN-2::mNG;HYG-R]), early endosomes (pwSi145 [Phyp7::mNG::RAB-5;HYG-R]), late endosomes and lysosomes pwSi140 [Phyp7::mNG::RAB-7;HYG-R]), autophagosomes (pwSi144 [Phyp7::mNG::LGG-1;HYG-R]), and lysosomes (pwSi205 [Phyp7::LMP-1::mNG;HYG-R]).
(TIFF)
A.—C. Quantification of the number of quaternary branches (A), secondary branches (B), and the ratio of quaternary to secondary branches (C) 100 μm anterior to the PVD cell body in animals of all extrachromosomal rescue lines tested. TG: transgenic animals, NTS: non-transgenic siblings. Data are represented as the mean ± 95% confidence interval. **** P < 0.0001, ns not significant, Kruskal-Wallis test with Dunn’s multiple comparisons test. n = 14 animals per genotype. D.—E. Percentage of animals with the indicated migration phenotype of PVM (D) and PQR (E) for all extrachromosomal rescue lines tested. TG: transgenic animals, NTS: non-transgenic siblings. **** P < 0.0001, ns not significant, Chi-squared test. n > 75 animals per genotype.
(TIFF)
A.—C. Quantification of the aggregate length of secondary (A), tertiary (B), and quaternary (C) dendrite branches within 100μm anterior to the PVD cell body in animals of the indicated genotypes. Note that all alleles are molecular or genetic null alleles. Data are represented as the mean ± 95% confidence interval. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 ns not significant; one-sided ANOVA with Tukey’s multiple comparison test. n = 15 animals per genotype.
(TIFF)
A. Fluorescent images of initial Q neuroblasts morphologies, when polarization is just beginning in control and catp-8(gk860114) mutant animals, imaged 1.5 hours after hatching. Left and right sides of the animals are shown as indicated. Scale bar = 5 μm. B.—C. Quantification of DMA-1::GFP puncta either in the primary (B) or on the tertiary (C) dendrite 100μm anterior of the cell body in the genotypes indicated. ns, not significant, Mann-Whitney test. n = 14 animals per genotype. D. Number of HPO-30::tagBFP puncta in the primary dendrite 100 μm anterior to the cell body in control or catp-8 mutant animals, presented as mean ± 95% confidence interval. ns not significant, Mann-Whitney test. n = 10 animals per genotype. E. Fluorescent images of animals expressing a functional fosmid based SAX-7::GFP reporter (ddIs290) in control, catp-8(gk860114) mutants, and animals overexpressing catp-8 in the epidermis (dzSi3). White arrowheads denote neuronal staining, while open arrows indicate examples of epidermal staining at the lateral epidermal ridge. F. Quantification of the percentage of animals with PVD 4° branching defects in the indicated genotypes in a ddIs290 background. Statistical comparisons were performed using the Z-test. ****, P < 0.0005, ns not significant. n = 15 animals per genotype. G. Fluorescent images of animals expressing lect-2::mNG endogenous knock-in (dz249) [38] in control and catp-8(gk860114) mutant animals. White arrowheads denote neuronal staining, while open arrows indicate examples of epidermal staining at the lateral epidermal ridge.
(TIFF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.