Abstract
Historically, the field of regenerative medicine has aimed to heal damaged tissue through the use of biomaterials scaffolds or delivery of foreign progenitor cells. Despite 30 years of research, however, translation and commercialization of these techniques has been limited. To enable mammalian regeneration, a more practical approach may instead be to develop therapies that evoke endogenous processes reminiscent of those seen in innate regenerators. Recently, investigations into tadpole tail regrowth, zebrafish limb restoration, and the super- healing Murphy Roths Large (MRL) mouse strain, have identified ancient oxygen-sensing pathways as a possible target to achieve this goal. Specifically, upregulation of the transcription factor, hypoxia-inducible factor one alpha (HIF-1α) has been shown to modulate cell metabolism and plasticity, as well as inflammation and tissue remodeling, possibly priming injuries for regeneration. Since HIF-1α signaling is conserved across species, environmental or pharmacological manipulation of oxygen-dependent pathways may elicit a regenerative response in non-healing mammals. In this review, we will explore the emerging role of HIF-1α in mammalian healing and regeneration, as well as attempts to modulate protein stability through hyperbaric oxygen treatment, intermittent hypoxia therapy, and pharmacological targeting. We believe that these therapies could breathe new life into the field of regenerative medicine.
Keywords: Drug delivery, Tissue regeneration, Hypoxia-inducible factor, Oxygen signaling
1. Introduction
The ability of non-mammalian vertebrates to regenerate lost tissue has captivated the imagination of biomedical researchers for some time [1–3]. In the early 18th century, observations on the regrowth of appendages in insects, frogs, and salamanders fueled scientific discussions into the competing developmental theories of epigenesis and pre-formationism [1]. Although modern advancements in molecular biology and genetics have solidified our understanding of organismal development, the scientific community’s fascination with regenerative species remains. Today, detailed studies into the molecular origins of zebrafish heart regeneration, newt tail regrowth, and annelid morphallaxis, are motivated by the desire to recapitulate these processes in adult mammals [4–7]. While human fetuses can achieve complete restoration of skin of wounds, this capability is lost after week 24 of gestation [8,9]. At this stage, regeneration is replaced by a reparative healing process, which fails to reproduce healthy tissue and instead achieves wound closure through non-specific scarring [10]. Compared to the original tissue, the resulting dense, fibrotic matrix lacks elasticity and differs in both composition and organization of extracellular matrix (ECM) components and basement membrane proteins [11–13]. As a result, the accumulation of scar tissue can lead to impairments in organ function, reduced range of motion, and severe physical disfigurement [14–16]. Since the mammalian healing cascade is highly conserved across organ systems and occurs regardless of injury stimuli, fibrosis plays a significant role in almost every pathology and contributes to all instances of organ failure [10]. Thus, the development of therapeutics capable of manipulating healing to mimic regeneration rather than repair, will have far-reaching clinical implications.
To achieve this goal, clinicians and bioengineers have primarily focused on the development of cell-based therapies and biomaterials interventions [17]. In the former approach, autologous or allogenic progenitor cells are delivered to injury sites to promote regrowth. Currently, it is unclear whether these cells directly contribute to new tissue formation or catalyze resident cell proliferation and recruitment through paracrine signaling activities [18,19]. Regardless of the mechanism of action, some tissue damage, such as that which is isolated to the eye, responds well to this intervention [20,21]. However, these injuries require just a few cells to regenerate and may benefit from tissue-specific advantages, such as those associated with ocular immune privilege [22, 23]. In other models representing cardiac failure, brain injuries, and tissue ischemia, the long-term efficacy of cell therapies and subsequent functional improvements, have been less straightforward [24–29]. Thus, these inconsistencies in pre-clinical and clinical data combined with high costs associated with scale-up and limited cell availability, have prevented translation of cell-based regenerative therapies [22].
Given the challenges associated with progenitor cell delivery, acellular biomaterials have also been created to aid in the restoration of lost tissue [30–32]. These constructs are engineered to recapitulate the chemical, mechanical, and physical properties of the tissue microenvironment they aim to replace. In addition to providing structural support, through the delivery of bioactive compounds, tissue engineering scaffolds are also now designed to modulate the local immune environment [33–35], facilitate cell infiltration [36–39], and direct differentiation of resident progenitor cells [40,41]. While the therapeutic potential of acellular biomaterials may be clear, their clinical translation has faced challenges akin to those afflicting cell-based therapies [30,41], and their use may even exacerbate tissue damage by evoking a foreign body reaction [42–44].
Given the complications associated with translation of these current strategies in regenerative medicine, a more practical approach may instead be to develop therapies that evoke endogenous tissue repair, reminiscent of what is seen in innate regenerators [45]. Such innate regenerations, like amphibians, are capable of restoring critical injuries through a process known as epimorphosis [46–48]. In this response, tissue regrowth is achieved through the development and reprogramming of a mass of undifferentiated cells known as a blastema [46,47]. Preceding blastemal development, the early stages of epimorphosis mirror those seen in mammalian healing (hemostasis, immune cell infiltration, and re-epithelization) [10,46]. Why the two processes diverge at later stages, is not fully understood although it has been postulated that fibrosis emerged as a mechanism to prevent infection and contain tissue damage [10,49]. Given the similarities in molecular machinery between amphibians and mammals, however, it is not unreasonable to believe that mammalian regeneration may be reawakened.
Recently, the discovery of a new model for mammalian regeneration has provided evidence for this claim, and identified ancient, cellular oxygen-sensing pathways as possible targets to achieve regeneration in non-healers. This model known as the Murphy Roths Large (MRL) mouse is an inbred strain originally developed for the study of the autoimmune disease systemic lupus erythematosus [50]. In 1996, however, a serendipitous observation revealed that the MRL mouse was capable of healing critical size defects in ear tissue through a process reminiscent of salamander limb regrowth [51,52]. Seminal work performed by Dr. Ellen Heber-Katz, later identified the transcription factor hypoxia-inducible factor 1 alpha (HIF-1α) as a central mediator of this regenerative response [52]. Although constitutively expressed in normal mammalian cells, HIF-1α is broken down under normoxic conditions and only stabilized in hypoxia [52–55]. In the MRL mouse, however, abnormally high basal expression of HIF-1α is further upregulated after injury and likely allows for the retention of a fetal-like metabolism, and possibly a progenitor cell population that contribute to non-fibrotic healing [52,54,55].
As described by Heber-Katz [56], it is not surprising that the molecular pathways governing oxygen-sensing and regeneration are coupled. Naturally regenerating organisms including sea cucumbers, starfish, and hydra live in shallow water with fluctuating oxygen concentrations. Here, turbulent conditions lead to acute injuries requiring immediate attention and robust regeneration [56]. In other organisms found on both land and sea, loss of the epithelial barrier during wounding results in a sudden influx of oxygen and burst of reactive oxygen species (ROS) [57]. These factors have been shown to contribute to local tissue hypoxia and serve as signaling molecules in blastemal development and tissue regrowth [58–63]. Since these oxygen-sensing pathways have been evolutionarily conserved from lower organisms, it is possible that their manipulation in mammals may be utilized to unlock a lost regenerative phenotype.
To develop new therapeutics capable of manipulating oxygen- sensing pathways for regeneration, we must first understand oxygen’s natural role in healing. This review will explore this idea, by presenting an overview of the HIF-1α signaling pathway and its role in normal and pathogenic mammalian wounding healing, as well as regeneration. Inspired by these ideas, current strategies aimed at eliciting regeneration through environmental and pharmacological manipulation of these oxygen-dependent pathways in non-healing organisms will then be discussed.
2. Overview of the oxygen sensing pathway in cells
The ability to respond to local changes in oxygen concentration became an essential characteristic of life when early eukaryotes evolved to use oxygen as a substrate for energy production 2.5 billion years ago [64]. Through a process known as oxidative phosphorylation, the reduction of molecular oxygen to water generates free energy to be used in the production ATP. This is in contrast to glycolysis, an oxygen-independent pathway, which instead generates free energy through the degradation of glucose. Compared to glycolysis, oxidative phosphorylation affords an 18-fold increase in energy production [64, 65]. This gain, however, is not without risk. Premature reduction of O2 can generate superoxide anions, resulting in the production of destructive reactive oxygen species (ROS). Therefore, to maintain oxygen hemostasis, primitive multicellular organisms such as C. elegans as well as complex mammalian cells, possess specialized oxygen-sensing pathways to regulate cell behavior. A central factor in these pathways is the ubiquitously expressed transcription factor hypoxia inducible factor one (HIF-1).
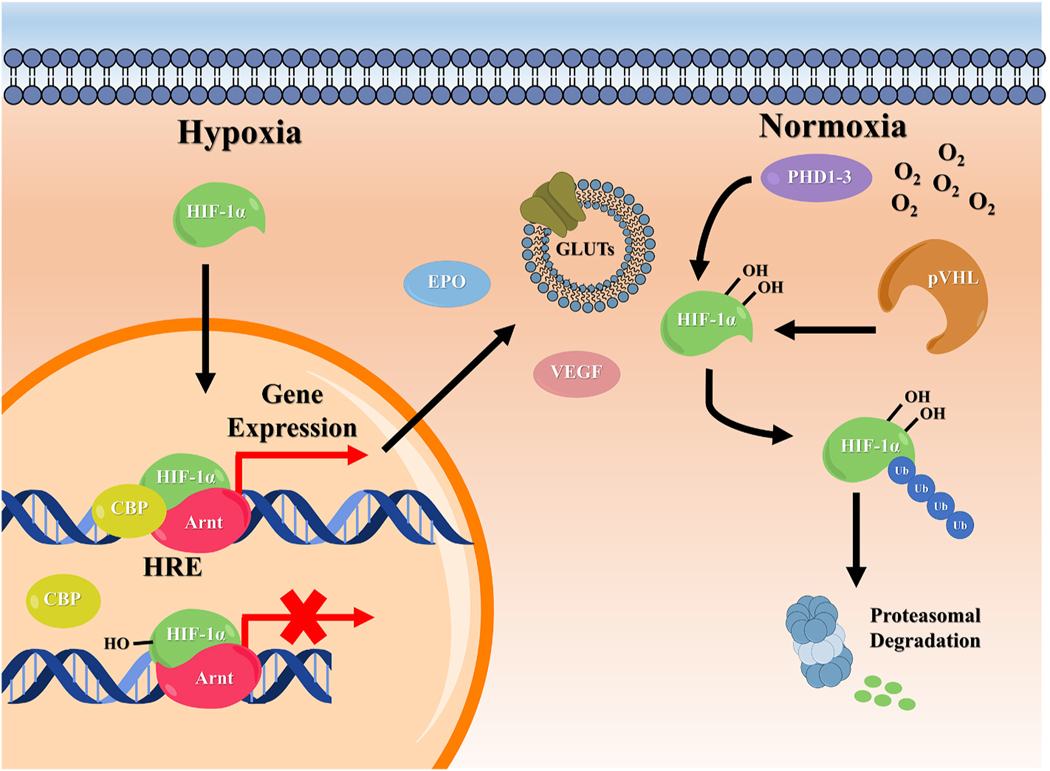
Seminal work in the field of oxygen regulation first identified HIF-1 in hepatocytes, where the protein serves as a key regulator of erythropoietin production in hypoxic conditions [66,67]. Since this discovery, however, the function of HIF-1 has been found to be far more diverse. As an essential factor in all cell types, HIF-1 is now known to orchestrate a variety of processes such as angiogenesis [68–71], cell proliferation [72–74], energy metabolism [75–77], stem cell maintenance [78–82], early development [83–86], cancer aggression [87–89], wound healing [90,91], and tissue regeneration [51,55,60]. In each of these instances, HIF-1 activity is coupled to local oxygen concentrations, through regulation of two Per-Arnt-Sim (PAS), basic helix-loop-helix (bHLH) family subunits referred to as HIF-1α and HIF-1β [92]. Although both of these subunits are constitutively expressed, in normoxia the HIF-1α subunit is rapidly degraded and exhibits a half-life on the order of minutes [53]. Only in hypoxia, where this degradation is impeded, is the alpha subunit able to accumulate and translocate to the nucleus, where it dimerizes with the oxygen-insensitive HIF-1β subunit (also referred to as aryl hydrocarbon receptor nuclear translocator or Arnt). This heterodimer then binds to hypoxia response elements (HREs) containing conserved RCGTG sequences. For transcription of target genes to occur, coactivators such as p300/CBP [93,94], SWI/SNF [95,96], Pontin [97], CDK8 [98], or SRC-1 [99] are recruited. Thus, through this process, the activation of HRE-linked genes such as those essential for vascular endothelial growth factor (VEGF) and glucose transporter (GLUTs) production is only achieved under hypoxic conditions.
Local oxygen concentration is tied to HIF-1α regulation through the action of enzymes known as prolyl hydroxylase domain-containing enzymes (PHDs). Currently, three PHD isoforms, PHD1, PHD2, and PHD3 have been identified and are thought to exhibit variability in their expression and target affinity [100–103]. All forms, however, contain a non-heme iron(II) capable of interacting with molecular oxygen and α-ketoglutarate (2-oxoglutaric acid, 2OG). As seen in Fig. 1, when oxygen is in high abundance (normoxia), PHDs bind HIF-1α and split molecular oxygen. One atom is reacted with α-ketoglutarate to form succinate, while the other is transferred to the substrate, resulting in hydroxylation of HIF-1α [104]. Hydroxylation of two proline residues in the oxygen dependent degradation domain (ODDD) of the subunit allows it to interact with the von Hippel–Lindau tumor suppressor protein (pVHL). As a member of the multi-component ubiquitin ligase (pVHL–elonginB–elonginC–Cul2–Rbx), recognition by pVHL will direct the ubiquitination and subsequent proteasomal degradation of HIF-1α [105–110]. In the absence of oxygen, PHDs are unable to hydroxylate HIF-1α, allowing it to escape pVHL association [111]. A second enzyme known as factor inhibiting HIF-1 (FIH) has also been shown to regulate HIF-1α and may retain activity at lower oxygen levels than PHDs [112]. Like PHDs, FIH is an iron(II)-dependent enzyme that uses molecular oxygen to hydroxylate the alpha subunit of HIF-1. Unlike PHDs, FIH-mediated hydroxylation occurs at asparagine residues within the C-terminal transactivation domain (C-TAD) and prevents HIF-1/p300 binding in the nucleus [113]. Due to the implication of HIF-1 in the expression of over 100 target genes [114,115], both PHDs and FIH have been explored as pharmacological targets for a wide range of applications [104,116].
Fig. 1.
Overview of HIF-1α signaling pathways in cells. Local changes in oxygen availability govern the regulation of HIF-1α. During normoxia, PHD enzymes hydroxylate the transcription factor at two proline residues found within the ODDD, leading to ubiquitination and proteasomal degradation. In low oxygen environments (hypoxia), hydroxylation cannot be performed and HIF-1α accumulates and translocates to the nucleus. Here, it dimerizes with the HIF-β unit (Arnt) and binds to the HRE of the target gene promoter. The HIF-1 complex then recruits transcriptional cofactors, such as CBP, resulting in the transcription of target genes such as those governing, EPO, VEGF, and GLUTs production. At moderate oxygen levels, the inhibitory enzyme FIH is also able to hydroxylate HIF-1α at an Asn residue within the C- TAD. This does not affect HIF-1α stability, but prevents the HIF-1 complex from binding to transcriptional coactivators.
In addition to HIF-1, there exists two alternative isoforms of the alpha subunit, known as HIF-2α and HIF-3α. Of the two, HIF-2α is the most well-characterized, and can be found in select cell types including hepatocytes [117,118], astrocytes [119–121], cardiomyocytes [122, 123], adipocytes [124], pneumocytes [125,126], and renal interstitial fibroblast-like cells [127], as well as several carcinomas [128–132], tumor-associated cell types [133,134], and all transformed cell lines [135]. Despite sharing 48% sequence identity with the HIF-1α isoform, HIF-2α regulation and function shows several key differences [136]. For example, the accumulation of HIF-2α is known to occur at higher oxygen concentrations than HIF-1α, perhaps due to variations in affinity for PHD1–3 [137]. Notably, silencing of PHD2 alone is sufficient to stabilize HIF-1α in human cells during normoxia, while silencing of PHD1 or PHD3 shows negligible effects on protein expression [138]. In contrast, siRNA knockdown of PHD2 does not increase HIF-2α stability in MCF7 cells, and significant accumulation is only achieved through silencing of PHD1 and/or PHD3. These differential responses likely stem from sequence variations at the N-terminal ODDD of HIF-1α and HIF-2α [100]. Apart from this domain, however, HIF-2α and HIF-1α exhibit substantial similarities in sequence identity within their bHLH (85%), PAS-A (68%), and PAS-B (73%) regions [139]. Therefore, it is not surprising that like HIF-1α, HIF-2α is also able to dimerize with HIF-1β, at HREs of shared target genes [140]. Due to differences in the N-terminal transactivation domain (N-TAD) of HIF-2α, HIF-2α/β complexes may also interact with different transcriptional cofactors than HIF-1α/β dimers, resulting in unique transcriptional targets as well [141]. Overall, like HIF-1α, HIF-2α is a master regulator of the hypoxic response and a critical factor in pathologies such as cancer [140]. Given its differences from HIF-1α, HIF-2α-specific inhibitors hold much clinical promise, but development of such pharmacological agents has proved a significant challenge [104,116].
Unlike HIF-1α and HIF-2α, the role of HIF-3α in the hypoxic response is poorly understood. As the least homologous of the three factors, the N- terminal bHLH-PAS domain of HIF-3α shares only 57% and 53% sequence identity to HIF-1α and HIF-2α, respectively. Even greater differences are seen at the C-terminus of the protein which contains a leucine zipper domain in place of the C-TAD and only one hydroxylatable proline in the ODDD [142]. As a result, HIF-3α is still susceptible to oxygen-dependent regulation by PHDs and proteasomal degradation by the pVHL-E3 ubiquitin ligase complex [143]. However, this regulation may be attenuated by the presence of the single proline residue within the ODDD, resulting in higher levels of cytoplasmic and nuclear HIF-3α during normoxia in some cell types [144]. Numerous studies have shown that HIF-3α is capable of interacting with Arnt, yet the function of this heterodimer remains unclear. While it was speculated that HIF-3α/Arnt was able to bind HREs and promote the transcription of HIF-1α-controlled genes [142], recent work suggests that HIF-3α/Arnt is incapable of binding DNA. In these instances, it is presumed that HIF-3α serves as a negative regulator of the hypoxic response by blocking binding of HIF-1α and HIF-2α to Arnt. This hypothesis is supported by the observation that HIF-3α silencing results in increased VEGF production in HEK293A cells [145]. This disagreement in the literature concerning the role of HIF-3α likely arises from the large number of existing variants. To date, up to 10 variants have been predicted, and likely exhibit tissue-specific expression patterns and differing functions [146,147]. Due to its ambiguity, the pathological consequences of HIF-3α dysregulation are poorly understood, yet thought to play a role in obesity and gestational diabetes [148–150]. Considering the importance of the other HIF factors in cell homeostasis and disease, the function and regulation of HIF-3α necessitates further research.
The ability to respond to local changes in oxygen is one of life’s most essential processes. Elucidating the molecular mechanisms that enable this capability represented a monumental achievement worthy of the 2019 Nobel Prize in Physiology and Medicine. With the function and regulation of HIFs, now largely understood, their involvement in homeostasis, development, and disease is beginning to be elucidated [83, 124,131,151]. In all organisms, injury is known to drive fluctuations in oxygen concentration within the wound bed [60,152,153]. Therefore, it is not surprising that these hypoxic signaling pathways play an important role in healing and repair.
3. Oxygen regulation in regenerating animals and mammalian wound healing
3.1. The role of oxygen in mammalian wound healing
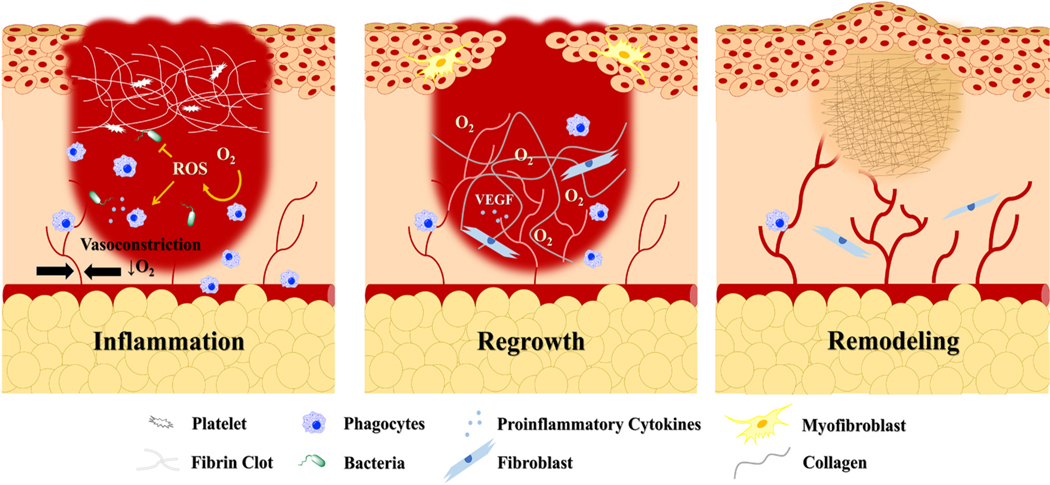
The normal mammalian wound healing process (acute wounding) can be divided into three overlapping phases: inflammation, new tissue formation, and remodeling summarized in Fig. 2 [10]. In each of these phases, oxygen plays an important role in cell proliferation, collagen production, tissue reorganization, and infection prevention. In the early stages of injury, however, disruptions in blood flow due to vessel damage and vasoconstriction, synergistically decrease the transport of oxygen to the wound bed [152,153]. Although initial loss of the epidermal barrier can result in a sudden influx of extracellular oxygen [60], it is quickly consumed by metabolically active cells [154] or converted to ROS [60]. Therefore, immediately after injury, the wound bed can be considered a hypoxic environment. As a result, HIF-1α is stabilized in various cell types and activates the transcription of proinflammatory cytokines [91,155,156]. In addition to mediating the inflammatory phase of healing, some of these cytokines also contribute to further HIF-1α stabilization [157–160].
Fig. 2.
The three phase of the normal mammalian wound healing cascade. In the inflammatory phase, hypoxia caused by vasoconstriction and increased cellular oxygen consumption is essential for orchestrating early wound healing events such as proinflammatory cytokine production and cell migration. In the tissue regrowth phase, oxygen is supplied by new vasculature and consumed by proliferating cells to begin to restore the epidermal barrier. Oxygen is also used to produce collagen, which serves as a precursor for the mature scar developed during the remodeling phase. Compared to the original tissue, this fibrotic matrix differs in both mechanical and biochemical properties and can impair normal function and motion.
In the first stage of wound healing, the release of proinflammatory cytokines drives the migration of circulating neutrophils, and later monocytes to the site of injury [10]. Once there, these phagocytes engulf dying cells and foreign pathogens within phagosomes. To destroy the contents of these phagosomes, large amounts of molecular oxygen are consumed and converted to ROS [161]. These molecules, which include hydrogen peroxide (H2O2), superoxide free radicals () and hydroxyl radicals (), can disrupt metabolic pathways through protein oxidation and cause lethal damage to DNA [162]. Since H2O2 can freely diffuse through lipid bilayers, a high concentration of ROS is also released into the extracellular space, in an event known as a “respiratory burst” [161,163]. In addition to preventing the growth of invading microbes, these species serve as an another chemoattractant and activator for inflammatory cells, and can facilitate the proliferation of fibroblasts and vascular progenitor cells in the next stage of healing [161,164,165].
The tissue regrowth phase of healing commences 2–10 days post- injury, and is marked by the migration of keratinocytes to the wound margins. Numerous reports have shown that the motility of these cells is increased in hypoxia through induction of urokinase plasminogen activator and mTORC1 signaling [166–169]. However, their proliferation and maturation require a sufficient supply of oxygen to allow for ATP production. To meet this demand, release of the HIF-1α-target, VEGF, mediates the recruitment of endothelial cells to initiate the growth of new vasculature [170]. To ensure proper tube formation, developing vessels require a collagen-rich matrix [171,172]. In a process reminiscent of HIF-1α regulation, the production of this collagen relies on molecular oxygen to enable hydroxylation of proline and lysine residues within precursor procollagen polypeptides. Only after hydroxylation and processing can tropocollagen molecules be assembled into mature collagen fibrils by lysyl oxidase [173]. This oxygen-dependent process also becomes important in later stages of the proliferative phase, which are initiated after the arrival of myofibroblasts and fibroblasts. To achieve closure, myofibroblasts generate force to contract the wound margins, while simultaneously producing collagen [10,174]. During the final stage of healing, this acellular matrix will be remodeled through the release of matrix metalloproteins (MMPs) by ROS-stimulated macrophages, keratinocytes, endothelial cells and fibroblasts to produce the mature scar [175]. Although this begins just 2–3 weeks after injury, the remodeling process can persist for several years [10]. The timely progression of the wound healing cascade through each of these phases, is required to control damage and is heavily influenced by changing oxygen concentrations.
Throughout the normal mammalian wound healing cascade, HIF-1α signaling allows cells to adapt to local fluctuations in oxygen. Therefore, dysregulation of this pathway, as well as abnormal microenvironments can greatly impede healing. For example, although local hypoxia is required to initiate the early stages of healing, its persistence can result in the formation of a chronic wound [90]. A chronic wound is defined as a wound that does not follow the normal timeline of healing, and is often considered to be trapped in the inflammatory phase [176]. Common causes of chronic wounds include venous insufficiency, diabetes mellitus, and hypertension, which all result in alterations to vasculature that contribute to tissue hypoxia [90]. Fibroblasts, keratinocytes, and endothelial progenitor cells from aged patients are especially susceptible to these low oxygen conditions and show reduced migration and proliferation compared to cells derived from young adults [177–180]. Some studies have suggested that this sensitivity in part arises from a functional loss of HIF-1α, which prevents the expression of key cytokines needed to mobilize proangiogenic cells [181]. Aging has also been associated with increased PHD activity, resulting in the rapid degradation of HIF-1α and impairments to the hypoxic response [182]. If cells are able to adapt to hypoxia within chronic wounds, they will undergo a metabolic switch to glycolysis, resulting in reduced production of ATP. In addition to there now being an insufficient amount of energy to fuel the proliferative phase of healing, ATP-dependent processes necessary to maintain viability such as ion transport may also be impeded. This dysregulation, combined with the robust accumulation of intracellular lactic acid, can result in tissue necrosis independent from that caused by injury [172,183]. In conjunction with the cytokines released during normal healing, inflammatory initiators now freed from intracellular compartments will recruit excess neutrophils and macrophages to the wound site [90,184,185]. Homing of these cells into the afflicted tissue is enabled by endothelial adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, both of which have been shown to be increased in hypoxia [186]. Although inflammation plays an important role in acute healing, in chronic wounds its persistence and overstimulation accelerate tissue damage. Due to the lack of molecular oxygen needed for collagen synthesis, new ECM cannot be produced to replace this loss tissue [187]. As wound margins grow, the chronic inflammatory state contributes to more tissue necrosis that is further aggregated by the continuing hypoxia.
While the development of many chronic wounds is exacerbated by the inability of cells to adapt to hypoxia, excessive upregulation of HIF- 1α can also impede normal healing. This effect is seen in keloid and hypertrophic scars which are characterized by excessive ECM accumulation and fibroblast proliferation. Unlike normal scars, keloids can grow to extend beyond the original wound margins, creating a dense fibrotic environment that is hypoxic in nature [188]. As a result, HIF-1α is highly expressed in keloid tissue and recent studies have suggested that the transcription factor may drive fibrogenesis by inducing epithelial-to-mesenchymal transition in keratinocytes and fibroblasts [189,190]. Hypoxia is also likely to contribute to the abnormal abundance of growth factors found within the keloid environment. Of these cytokines, the HIF-1α-target, VEGF has been shown to be directly involved in the upregulation of plasminogen activator inhibitor-1 (PAI-1) [191–193]. In keloid fibroblasts, high levels of PAI-1 prevent fibrin degradation, resulting in increased ECM density within the scar.
Since the mammalian wound healing cascade is highly conserved, to eliminate the clinical burden of fibrosis successful therapeutics must be easily adapted to address injuries afflicting different tissues. Such non-specific treatments will likely be required to simultaneously alter immune response, progenitor cell populations, and structural components. Universal oxygen signaling pathways, already utilized in mammalian repair, represent a promising target since they exist in all mammalian cell types. To inspire the development of such therapies, however, it is necessary to first understand their role in natural regeneration.
3.2. Oxygen signaling pathways in regenerating organisms
Although regeneration is generally considered to be reserved for amphibians and lower vertebrates, select mammals including deer [194], African spiny mice [195], and rabbits [196] exhibit some regenerative capacity in adulthood. In these models, tissue regrowth is often preceded by the development of what is described as a “blastema-like” structure [197]. For example, in cervid antler regeneration, this structure is thought to be composed of progenitor mesenchymal cells. In contrast to blastemal cells mediating urodele limb regrowth, it is unlikely that these cells arise from dedifferentiation. Rather, they originate from a normally quiescent population residing in the periosteum of the distal pedicle. Unlike the amphibian blastema, the cervid analog is also vascularized, and develops independent of nerve stimulation or epidermal signaling [194]. A true example of mammalian epimorphosis is thus lacking.
In 1998, however, Clark, et al. [51]. discovered that an inbred mouse strain known as the Murphy Roths Large (MRL) mouse was capable of regenerating critical size wounds. Originally selected for its large size, the MRL mouse had become a standard model of autoimmunity due to a mutation in the structural gene encoding the Fas antigen. Since this cell surface protein mediates apoptosis of developing T cells in the thymus, mice possessing the lymphoproliferation (lpr) mutation exhibited clinical symptoms reminiscent of those seen in human systemic lupus erythematosus [198,199]. When numbering mice using the conventional ear-hole punch method for this use, Clark, et al. [51]. discovered that MRL mice were closing the wounds after several weeks. Compared to the C57Bl/6 strain which achieved only 30% reduction in wound size after two weeks, MRL mice exhibited a remarkable 85% closure. This regenerative response was preceded by the rapid breakdown of the basement membrane layer and re-epithelialization of the wound site, as well as the generation of a blastema-like structure resembling that present during salamander limb regrowth. In the MRL, new tissue closely resembled pre-injury architecture including the presence of hair follicles and sebaceous glands. By day 81, cartilage ingrowth into the wound area not seen in C57Bl/6 mice was also observed. Later work demonstrated that the MRL regenerative response was not limited to ear tissue. Since publication of Clark et al. MRL have been shown to completely regenerate or substantially heal amputated digits [200], full thickness articular cartilage wounds [201,202], intraarticular fractures [203], cardiac cryoinjuries [204], peripheral nerve damage [205], and alkali-burned corneas [206].
Given the genetic similarities between mice and humans, considerable interest emerged to establish the MRL mouse as the standard model for mammalian regeneration and elucidate the mechanism of this response. A potentional cause of this peculiar regenerative ability can likely be tied to the altered metabolic function of the MRL mouse. Compared to the non-healing C57Bl/6 strain, MRL mice rely more heavily on glycolysis than oxidative phosphorylation for ATP synthesis. This is evident by a 2-fold increase in lactate production by MRL fibroblasts in culture compared to C57Bl/6-derived cells [54]. Differences in oxygen consumption were not observed between the two cell types, demonstrating a phenomenon reminiscent of the Warburg effect often seen in cancer, development, and wound healing [207,208]. Unlike cancer cells, however, MRL fibroblasts exhibit reduced mitochondrial transmembrane potentials and ROS, making them more analogous to pluripotent [209] and hematopoietic stem cells [210]. In fact, stem cell markers Nanog, Islet-1, and Sox2 were observed in adult MRL hearts both pre- and post-injury, suggesting that the retention of a fetal-like metabolism may allow for the existence of undifferentiated cells in adult tissue [54]. Other regenerative organisms including newts, axolotls, and zebrafish may also display a similar reliance upon glycolysis over oxidative phosphorylation [54,211].
It has been postulated that the autoimmune phenotype of the MRL mouse may also contribute to tissue regeneration. However, genetic mapping of MRL/lpr-derived F2 and backcross progeny did not identify any link between healing capabilities and the fas gene or any other gene associated with autoimmunity [52]. Additionally, MRL/+ mice demonstrate the same regenerative response seen in MRL/lpr strains and no correlation is observed between lymph node cell number and wound closure [51,52]. While adaptive immune responses are unlikely to contribute to regeneration, numerous reports have identified differences in innate immunity which may mediate MRL healing. Following cornea alkali burning, MRL mice achieve rapid re-epithelialization which ultimately contributes to improved healing and reduced corneal opacity compared to C57Bl/6 mice. It is possible that differences in re-epithelialization rates arise from disparate neutrophil infiltration into the wound area, which appears to be markedly reduced in the MRL along with the transcription of several pro-inflammatory genes [206]. In contrast, other injury models including the well-characterized ear hole wound, demonstrate increased infiltration of inflammatory cells at day 1 postinjury. Cell populations include neutrophils and macrophages, as well as a unique, glycolytic mast cell population expressing both mature and immature cell markers [212]. Migratory immune cells including monocytes and neutrophils are also likely to secrete increased levels of MMPs and tissue inhibitors of matrix metalloproteinases (TIMPs) which contribute to the rapid breakdown of the basement membrane in wound areas [213,214]. This pro-inflammatory phenotype likely contributes to the generation of a microenvironment that supports cell dedifferentiation and blastema formation. This claim is supported by findings that show that treatment with the anti-inflammatory agent, meloxicam, attenuates MRL healing [212].
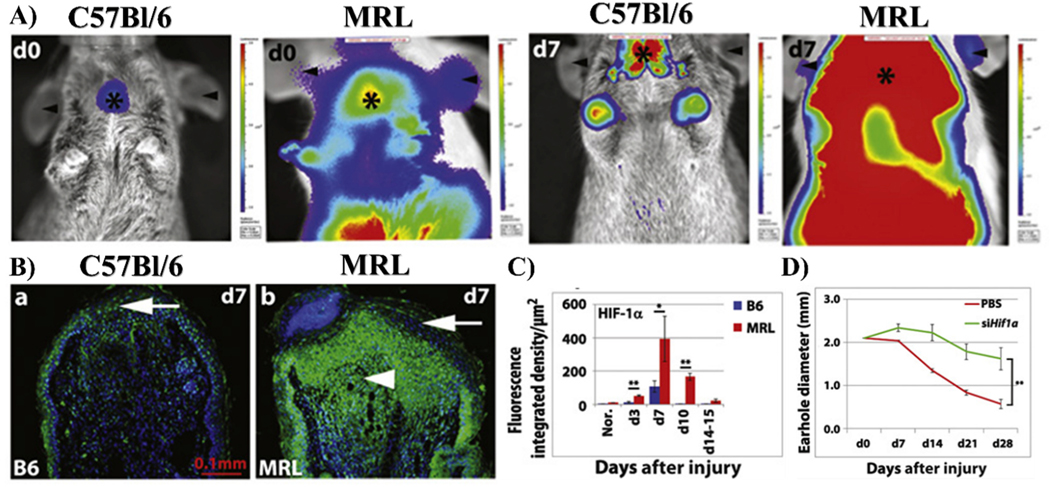
Key differences between MRL and C57Bl/6 mice concerning metabolism and inflammation may both be mediated by HIF-1α and the classical cellular oxygen-sensing pathway. Evidence for this claim was first established following genetic mapping studies of MRL and non- regenerating mice which identified differential expression of HIF-1α regulators Rnf7, Psmd8, and Psmb9 [212]. All of these proteins are involved in the ubiquitination and/or degradation of HIF-1α and are downregulated in the MRL, with Rnf7, a ROS scavenger and E3 ubiquitin ligase component [215], demonstrating the most significant change. As a result, these mice exhibit incredibly high basal expression of HIF-1α and HIF-1α-target genes Ldha, Vegfc, Hmox1, Met, Nt5e, Pkm2, and Gadph [55,212]. Following injury, HIF-1α levels follow a biphasic pattern characterized by a transient increase (Fig. 3A–C), then decrease in expression [55]. Inhibition of HIF-1α completely inhibits regeneration of ear hole wounds (Fig. 3D) and reduces expression of the stem cell marker, Nanog, in MRL fibroblasts in vitro. HIF-1α has long been known to govern the transcriptional activation of glucose transporters and glycolytic enzymes HK, GLUT1, PFKL, ALDOC, GAPDH, PGK2, ENO1, LDH, and PDK1, making it a master regulator of glycolysis [56,77]. Thus, the fetal-like, glycolytic characteristics of MRL cells are likely mediated by enhanced HIF-1α stabilization. As previously discussed, this metabolic state is essential for MRL regeneration with treatment of the PDK1-inhibitor and pro-oxidative phosphorylation drug, dichloroacetate, inhibiting ear hole closure [56]. HIF-1α stabilization is also likely to contribute to the glycolytic metabolism of MRL-specific mast cells as well as the curious upregulation of PKM2, LDHA, VEGF, and HMOX1 genes in this population [212].
Fig. 3.
Abnormalities in HIF-1α expression in the MRL mouse. (A) HIF-1α expression in MRL and C57Bl/6 mice backcrossed to HIF-1α peptide-luciferase reporter mice is shown. High bioluminescent activity (red) can be seen in MRL mice 7 days after injury to ears (d7). Only slight upregulation is seen in non-healing C57Bl/6 mice. (B) Immunofluorescent assay in ear hole tissue confirms that HIF-1α is upregulated at the site of injury in MRL mice. (C) HIF-1α appears to peak at d7 post- injury and returns to baseline levels by d15 post-injury (n = 3 to 7 samples, N = 2 experiments, *P < 0.05 and **P < 0.01) (D) This corresponds to ear hole closure, which begins on d7, but fails to occur when HIF-1α is blocked by siRNA (**P < 0.001, n = 4 ears per group, N = 2 experiments). Copyright © 2015, Copyright © 2015, American Association for the Advancement of Science. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The same oxygen-sensing pathways that govern MRL healing, have also been implemented in non-mammalian regeneration occurring in zebrafish [58,59], tadpoles [60,61], and geckos [62,63]. Although these events seem to be species and injury dependent, in all cases, a sudden increase in ROS production at the site of injury appears to be essential in orchestrating the early stages of regeneration by catalyzing growth factor production [59,216], initiating immune cell recruitment [58], and increasing cell proliferation [63,217]. In addition to the direct signaling activity of ROS, their production is also known to deplete oxygen from the wound bed, creating a highly hypoxic environment, which may also affect tissue regeneration. By inhibiting HIF-1α, the master regulator of the hypoxic response, Ferreira, et al. [57]. demonstrated that this low oxygen environment was essential for regeneration in tadpoles during the first 3 h after tail amputation. The pro-regenerative effects of HIF-1α during this time period stem from its ability to upregulate HSP90, a stress responsive chaperone protein known to mediate wound healing in other organisms [218]. In the regenerating tadpole tail, HSP90 as well as its secreted, extracellular form eHSP90α, are likely to contribute to wound re-epithelialization and regenerating bud development by promoting cell migration. Additional work has shown that HIF-1α in conjunction with hydrogen peroxide, can also influence cell migration by orchestrating changes to transepithelial potential and electric current densities during regeneration [60,61]. Similar effects may also be seen in the house gecko, where high expression of HIF-1α and HIF-2α characterize the early stages of tissue regeneration [62].
Given the role of HIF-1α in both MRL and non-mammalian regeneration, cellular oxygen-sensing pathways have emerged as possible therapeutic target for mammalian regeneration. Compared to other targets, these pathways represent promising therapeutic targets because they are ubiquitously expressed, and can be easily modulated by altering exposure to environmental oxygen or through the use of small molecules. Thus, a solution to mammalian fibrosis may be close to clinical translation.
4. Clinical implications
4.1. Hyperbaric oxygen treatment for chronic wound healing
Given the pathologies associated with chronic hypoxia, hyperbaric oxygen therapy (HBOT) may be used to decrease instances of infection and facilitate healing in chronic or severe wounds [219,220]. The foundation of HBOT is built on the belief that intermittent exposure to oxygen will initiate wound repair by increasing cell proliferation, collagen deposition, angiogenesis, and host defense [219,220]. Currently, two methods are used to expose patients to 100% oxygen for this treatment. The first is known as “topical oxygen therapy” (TOT). The objective of TOT is to apply oxygen directly to the wound surface by surrounding the injury with a plastic bag or chamber which is then filled with 100% oxygen [221–223]. Despite its simplicity and low cost, this technique has not been shown to improve healing in a clinical setting likely due to shallow penetration of O2 into the wound bed [224,225]. Therefore, to facilitate robust tissue oxygenation, systemic exposure can be achieved by placing a patient in a pressurized chamber and administering 100% oxygen via a mask, head hood, or endotracheal tube. Alternatively, a patient can also be placed in a pressurized chamber filled with 100% oxygen [220]. For most wound healing applications, it is recommended to perform HBOT for 90–120 min at 2–2.5 atm twice daily for 30–40 days. In other applications of HBOT including its use for the treatment of acute thermal burns, compromised grafts, radiation injuries, and tissue infections, however, treatment duration may vary (see review [226]).
Some of the effects of HBOT may be orchestrated by induction of HIF-1α. Surprisingly, Sunkari, et al. [227]. found that HIF-1α expression was upregulated in human dermal fibroblasts derived from diabetic foot ulcers (DFUs) at later time points following HBOT. Initially, HIF-1α levels were undetectable due to the abundance of molecular oxygen. Four hours after treatment, however, HIF-1α levels begin to increase relative to normoxia controls. HIF-1α target genes such as VEGF and SDF-1α were also upregulated. This facilitated the migration of endothelial progenitor cells to the wound bed and subsequent neovascularization. Additional cytokines including transforming growth factor-β1 [228], angiopoietin-2 [229], and platelet-derived growth factor receptor [230], as well as the remodeling enzyme, MMP-9 [231], have also been shown to increase following HBOT in distinct cell populations. Together, these factors further increase angiogenesis, as well as cell proliferation and growth factor signaling.
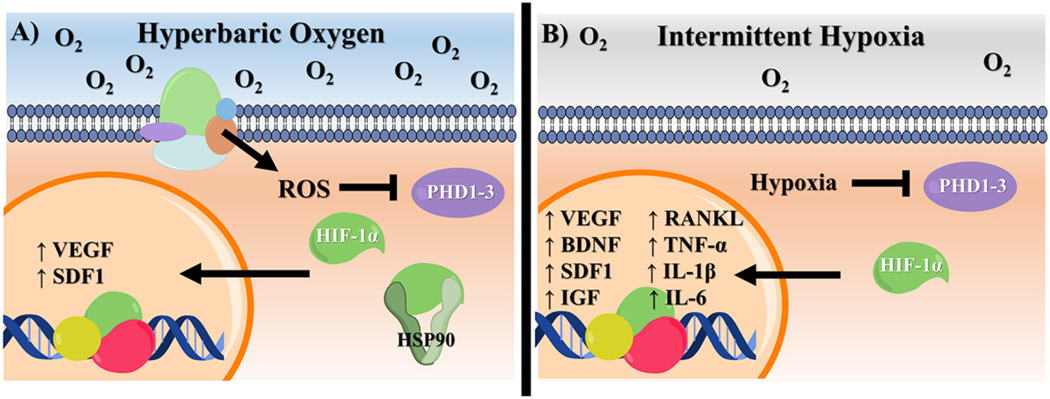
While it seems contradictory that hyperoxia would be able to stabilize HIF-1α, this phenomenon likely occurs through a pVHL-independent manner. One possible pathway involves HIF-1α complexation with the chaperone protein, HSP90. This has been shown to prevent HIF-1α ubiquitination and degradation in both normoxia and hypoxia [232, 233]. Hyperbaric and hyperoxic conditions can lead to slight increases in HSP90 expression, as well as its association with other factors such as the NOS3 protein [234–236]. An alternative explanation for hyperoxia stabilization of HIF-1α is through ROS production, which is increased by HBOT due to the abundance of molecular oxygen within the wound bed [237]. In addition to fighting infection, ROS may inactive PHDs and FIH by oxidizing their iron active sites to stabilize HIF-1α [238–240]. However, the direct relationship between ROS and HIF-1α remains controversial. Other reports have suggested that ROS accumulation indirectly influences HIF-1α stabilization by regulating cellular oxygen availability and can actually promote its degradation [241–244]. The effects of HBOT treatment and the following role of HIF-1α in these responses are summarized in Fig. 4A.
Fig. 4.
Possible mechanisms of HIF-1α stabilization following (A) HBOT or (B) IH. While HIF-1α is stabilization is generally achieved through conventional PHD inhibitor in IH, in HBOT, ROS serve to directly or indirectly deactivate the enzyme. In high oxygen settings, upregulation of HSP90 may also play a role in HIF-1α stabilization.
Although HBOT has demonstrated unquestionable, pro-healing benefits in preclinical studies, its clinical utility is less clear. Compared to conventional wound care alone, adjunctive HBOT is time- consuming and comes at a substantial cost, with treatments ranging from $50,000 to $200,000 in the US [245]. As a result, many physicians have been reluctant to accept the therapy without definitive proof of its efficacy. Currently, there is little consensus on such efficacy despite the completion of many clinical trials designed to explore the use of HBOT for chronic wounds such as DFUs. Advocates of HBOT claim that the treatment can expediate healing and re-epithelialization of the wound bed, reducing a patient’s risk of amputation and improving their overall quality of life [246–254]. While the treatment is expensive, some reports have suggested that HBOT can actually decrease overall wound care-related costs by reducing the number of hospital visits in the long-term [247,255]. Many of these claims, however, are supported by open-label or observational studies, which have been heavily criticized due to biases related to poor patient compliance, lack of blinding, selective reporting, improper patient allocation, and insufficient patient numbers [254,256].
To elucidate the true effects of HBOT, randomized, double-blinded studies have been attempted. One such trial completed by Londhal et al. [250]. in 2010, showed that after 1 year, 61% of patients that underwent >35 90-min sessions of HBOT achieved complete DFU closure. This was compared to only 27% of placebo-control patients, and was accompanied by improvements in health-related quality of life (HRQoL) [249]. Similar benefits were also seen by Abidia et al. [247]. who reported that HBOT led to a median decrease in wound area of 100% versus only 52% in the control-arm. Although significant improvements were observed, it has been suggested that healing rates in control groups may have been inadvertently influenced by the employed sham therapy. In both reports, this sham therapy involved subjecting placebo-arm patients to high pressures used in HBOT (2–3 ATA) while receiving air through a mask. Many argue that such high pressures can have detrimental effects on vascular function and inflammation, leading to compromised healing [257]. Thus, it is likely that abnormal healing in control groups bolstered the apparent significance of HBOT, making the results of these studies unclear.
Due to concerns surrounding pressurized treatments, more recent double-blind, controlled HBOT trials now conduct sham therapies at reduced pressures. At approximately half the pressure used in HBOT, the patient is still able to experience the sensation of compression and decompression, without alterations to vascular function. Fedorko et al. [257]. subjected 54 patients with DFU to this sham therapy at these conditions, and then compared their risk of amputation to 49 patients receiving 30 daily sessions of 90 min HBOT. In the treatment group, 22.9% of patients who received at least 27 HBOT treatments met the criteria for major amputation as assessed by a vascular surgeon. This was not significantly different from rates seen in the placebo-arm, where 20.5% of patients met the amputation criteria. Significant reductions in wound surface area and width were also not observed following HBOT. As a result, HBOT lead to minimal improvements in health-related quality of life [258]. Compared to other adjunctive therapies such as extracorporeal shock wave therapy, HBOT appears to offer no advantages in the treatment of DFUs [259].
While many trials have demonstrated the ineffectiveness of HBOT, few have also suggested that its use can actually have negative effects. In a longitudinal, observational cohort study, Margolis, et al. [260]. found that patients undergoing HBOT were actually 1.5 to 3 times more likely to require amputation and 1.2 to 3 times less likely to heal DFUs. Additional studies have also reported instances of adverse events including oxygen-induced seizures [261], barotraumatic injuries [257, 261], and changes in vision [257].
Given the inconsistencies in clinical data (summarized in Table 1), a 2012 Cochrane review [256] concluded that larger, more rigorous, randomized-controlled trials were required to assess the true efficacy of HBOT. Within the past 8 years, however, few of these trials have been completed and the therapeutic potentional of HBOT still remains largely unknown. Given the importance of oxygen in regulating the normal mammalian wound healing cascade, as well as its role in orchestrating natural regeneration, it is likely that HBOT could provide some benefits in the management of chronic wounds.
Table 1.
Summary of clinical trials investigating the use of HBOT. CWM refers to convention wound care management and involves surgical debridement, antibiotic treatment, off-loading and wound care dressings. In all instances, HBOT was performed as an adjunctive therapy to CWM.
| Citation | Trial Type | Patient Population | Treatment | Conclusion | Limitations |
|---|---|---|---|---|---|
| Doctor, 1991 | Prospective RCT | 30 patients with chronic DFUs | Control – CWM Treatment – 4 sessions of 45 min HBOT at 3 ATA over 2 weeks |
HBOT reduced incidence of infection, rate of amputation, and need for skin grafts, but did not significantly reduce length of hospital stay | Lack of blinding |
| Faglia, 1996 | RCT | 70 patients with chronic DFUs | Control – CWM Treatment – 38 ± 8 daily, 90 min sessions of HBOT at 2.2 to 2.5 ATA |
Amputation rate in HBOT group decreased to 8.6% vs. 33.3% in control. TcPO2 increased and remained elevated for several days | Lack of blinding |
| Abidia, 2003 | Double-blind, Placebo-controlled RCT |
18 patients with 1–10 cm DFUs that did not show healing after 6+ weeks | Control – hyperbaric air at conditions of treatment Treatment – 90 min HBOT at 2.4 AT5 days per week for 30 sessions |
At 6 weeks, complete healing was seen in 5/8 patients in HBOT and 1/8 control patients. At 1 year, healing was consistence in HBOT group, but decreased to 0/8 for control. No significance difference between overall QOL | Control treatment may decrease healing |
| Kessler, 2003 | Prospective RCT | 28 patients with DFUs with Wagner grades I- III |
Control – CWM Treatment - two 90-min daily sessions of HBOT at 2.5 ATA 5 days/week for 2 weeks |
TcPO2 significantly increased during HBOT, leading to improved healing at day 15. At day 30, no difference was observed | Lack of blinding |
| Duzgun, 2008 | Prospective RCT | 100 patients with persistent DFU for 4+ weeks | Control – CWM Treatment – 90-min daily sessions of HBOT at 2 to 3 ATA alternating 1 and 2/day for 20–30 days |
0% patients healed without surgical intervention in control groups compared to 66% in HBOT. HBOT decreased the need for debridement, as well as amputation rate and severity | Statistically significant differences existed in baseline conditions between HBOT and controls |
| Londahl, 2010 | Double-blinded, Placebo-controlled RCT |
94 patients with DFUs Wagner grade II-IV persisting for 3+ months | Control – hyperbaric air at conditions of treatment Treatment – daily 90-min HBOT sessions at 2.5 ATA 5 days/wk for 8 wks |
61% of patients completing 35+ HBOT sessions had complete healing compared to only 27% in control | Control treatment may decrease healing |
| Londahl, 2010 | Double-blinded, Placebo-controlled RCT |
75 patients with DFUs Wagner grade II-IV persisting for 3+ months | Control – hyperbaric air at conditions of treatment Treatment – daily 90-min HBOT sessions at 2.5 ATA 5 days/wk for 8 wks |
Healing was correlated to TcPO2 values following HBOT | Control treatment may decrease healing |
| Londahl, 2010 | Double-blinded, Placebo-controlled RCT |
75 patients with DFUs Wagner grade II-IV persisting for 3+ months | Control – hyperbaric air at conditions of treatment Treatment – daily 90-min HBOT sessions at 2.5 ATA 5 days/wk for 8 wks |
At one year follow up, HBOT patients reported improvements to HRQOL, likely related to ulcer healing | Did not take into account benefits due to social interactions |
| Wang, 2011 | Prospective, open- label RCT | 86 patients with 93 chronic DFUs | Treatment 1–6 treatments of EWST completed over 3 weeks Treatment 2–20 treatments of HBOT at 2.5 ATA for 25 min increments with 5 min break for 90 min performed daily 5 days/ wk |
EWST led to greater improvements in blood perfusion, cell proliferation, and rate of apoptosis, resulting in improved healing overall | Lack of blinding, long term follow-up not conducted |
| Margolis, 2013 | Longitudinal observational cohort study | 6259 individuals with DFUs accounting for 767,060 person-days of wound care |
HBOT was administered to 12.7% of subjects, often on a 5 day/wk, regimen with 90 min sessions at 2 atm for a median of 29 sessions | HBOT patients were more likely to undergo lower limb amputations, but amputation time was postponed 3 wks compared to control | Lack of blinding, effect of patient compliance not accounted for, did not differentiate between treatment alternatives |
| Fedorko, 2016 | Double-blind, placebo-controlled RCT |
118 patients with DFUs Wagner grade II-IV |
Control – hyperbaric air at 1.25 ATA Treatment - 30 treatments of HBOT at 2.5 ATA for 90 min performed daily 5 days/wk |
No significant difference was found in major amputation rate or wound size | Short-term follow-up, actual amputation rates were not reported |
| Li. 2017 | Prospective, placebo-controlled, double-blind RCT | 103 patients with DFUs Wagner grade II-IV persisting for 4+ weeks | Control – hyperbaric air at 1.25 ATA Treatment - 30 treatments of HBOT at 2.5 ATA for 90 min performed daily 5 days/wk |
HBOT did not result in significant improvements to HFQOL, but participants reported fewer problems associated with mobility and pain discomfort compared to sham-treated | Small sample size, insufficient power to assess significance |
| Santema, 2018 | multi-center randomized parallel group superiority trial |
120 patients with DFUs Wagner grade II-IV persisting for 4+ weeks and limb ischemia | Control – CWM Treatment - 40 treatments of HBOT at 2.5 ATA for 25 min increments with 5 min break for 90 min performed daily 5 days/ wk |
HBOT did not significantly improve healing compared to control and resulted in one patient experiencing an oxygen- induced seizure and one had barotrauma | Significant reduction in sample size during trial, no sham treatment |
4.2. Hypoxia treatment
While HBOT aims to oxygenate damaged tissue, some injuries have been shown to respond more favorably to low doses of intermittent hypoxia, as shown in Fig. 4B. This is especially true in the case of neuronal injuries, where intermittent hypoxia (IH) has been shown to improve motor neuron plasticity and function following chronic spinal injury, as well as axonal outgrowth and regeneration after sensory nerve damage [262–264]. In these instances, it is likely that IH invokes a cellular stress response, that leads to chromatin remodeling and the transcription of pro-regenerative genes [262]. A similar process naturally occurs following injury to the peripheral axon branch of sensory neurons, which exhibit innate regenerative abilities [262,265]. Remarkably, initial injuries to this region also have the potential to enhance axon regrowth in centrally projecting axons, which normally display minimal regenerative potential. Comparative studies between regenerating and non-regenerating neurons, have identified HIF-1α as a possible mediator of this effect [266]. In vitro studies [262] have shown that injury induces a 1.2-fold increase in HIF-1α protein levels in regenerating sensory neurons, accompanied by 2 or more-fold increase in expression of 63.7% of known HIF-1α-target genes. Knockdown of HIF-1α both in vivo and in vitro, attenuates axonal regrowth and abolishes the preconditioning effect of initial injuries. Thus, to enhance the pro-regenerative potential of HIF-1α, adult mice with sciatic nerve damage were subjected to IH, which was found to stimulate long-range axon regeneration and reinnervation in motor neurons, as well as increased axonal growth. It has long been known that the HIF-1α-target, VEGFA, can promote neuronal signaling and axon guidance. Direct administration of the growth factor to HIF-1α knock out mice, however, was insufficient in restoring regeneration to levels seen in controls. This suggests that VEGFA is only one of many targets involved in the pro-regenerative function of HIF-1α signaling.
Other potential mechanisms of IH-induced neuronal plasticity involve upregulation of brain-derived neurotrophic factor (BDNF) [263, 267]. As a member of the neurotrophin family, BDNF has been shown to facilitate axonal growth and neurogenesis upon binding to the tyrosine kinase receptor, trkB. Subsequent phosphorylation of trkB triggers three possible signaling cascades involving activation of phospholipase C gamma (PLCɤ), phosphotidyl-inositol-3 kinase (PI3K), or mitogen activated protein kinase/extracellular receptor kinase (MAPK/ERK) pathways which all eventually converge to regulate the transcription of pro-regenerative genes (see review [268]). One such target includes the transcription of β-actin, which is then transported down the axon to the growth cone to facilitate its enlargement, branching, and extension [269]. Other targets include the production of cyclic AMP (cAMP) and regulation of mTOR which serve as important mediators of cell growth and survival [268,270,271]. Although normally produced by motoneurons and Schwann cells in response to peripheral nerve injury, IH treatments have been shown to further increase BDNF production and trkB phosphorylation [263,267]. This likely occurs as a result of local tissue hypoxia in addition to subsequent serotonin receptor activation, which drives the production of BDNF [267]. In animal models of chronic spinal cord injuries, IH-induced BDNF signaling strengthens synaptic input and motor output of respiratory and somatic motor nuclei, facilitating improvements to forelimb and respiratory motor function [263].
Recently, human studies have suggested that the benefits of IH may be translated to the clinic. In a study by Trumbower et al. a single IH exposure was shown to increase ankle strength in patients with SCI, enabling an 82 ± 33% increase in plantar flexion torque [264]. However, these results were only recorded up to 4 h after treatment. To achieve long-term benefits, repetitive, daily sessions of IH are likely required. When patients with chronic incomplete SCI were subjected to this treatment, walking speeds during 10 m walk tests significantly increased compared to patients receiving normoxic, sham therapy. While daily IH alone was capable of soliciting improvements to walking endurance as well, these benefits only became statistically significant when IH treatment was combined with overground walking. After 5 days of this combination therapy, walking distance increased over 30% and continued to surpass baseline levels at 2-weeks follow-up [272]. IH-induced improvements to respiratory function observed in mice and rat models, have also been shown to occur in human patients. Tester et al. reported that SCI patients exposed to eight 2-min intervals of 8% oxygen for 10 days demonstrated improvements in minute ventilation. Repeated exposure did not result in cumulative increases; however, improvements were maintained over the 10-day period [273]. IH-induced improvements in ventilatory load compensation have also been reported in cervical SCI patients [274].
In addition to neuronal injuries, IH may be beneficial in the treatment of bone injuries and defects. As seen in many other tissue injuries, the early stages of bone fracturing are marked by a disruption in oxygen and nutrient supply, which initiates HIF-1α signaling [275]. Recent studies have shown that the accumulation of HIF-1α in bone and endothelial progenitor cells serves as a key mediator of angiogenesis and osteogenesis [151,276–278]. This is primarily orchestrated through VEGF signaling which in addition to promoting the vascularization of developing endochondral bone [276], drives mesenchymal stem cell differentiation towards osteogenic linages [279,280]. Recruitment of these progenitor cell is itself mediated by HIF-1α which stimulates stem cell chemotaxis through release of stromal cell-derived factor-1 (SDF-1) from periosteal cells [281]. Hypoxia has also been shown to increase the generation and resorption activity of osteoclast cells stimulated by M-CSF and RANKL in vitro [282]. Importantly, re-oxygenation following exposure to 2% oxygen was required to achieve this effect, as constant hypoxia was later shown to decrease osteoclast activity [283]. In vivo, hypoxia is likely to further increase resorption by stimulating the release of pro-osteoclastogenic cytokines including RANKL, VEGF, insulin-like growth factor, and growth differentiation factor 15 [277].
Given the importance of HIF-1α in normal bone development and growth, IH therapy has the potential to significantly enhance remodeling following injury. Improvements to bone mineral density have been observed in rats exposed to long-term, chronic IH treatments. This regimen consisted of 5 h daily sessions of hypobaric hypoxia performed 5 days per week for 5 weeks. Throughout the treatment, high levels of pro-inflammatory cytokines including IL-1 B, IL-6, and TNF-a were observed, in conjunction with increased production of nitric oxide. Surprisingly, these two effects appeared to work in opposition of each other. While IL-1 B, IL-6, and TNF-α are known to contribute to RANKL production and bone loss, NO accumulation appeared to have an inhibitory effect on osteoclast activity, possibly through inactivation of the protease, cathepsin K. As a result, bone turnover was significantly decreased in IH-treated rats, leading to an average increase in bone mineral density of 30% [284]. A similar mechanism may also explain why patients with obstructive sleep apnea, a condition characterized by episodes of recurrent intermittent hypoxia, are less likely to experience age-related bone loss and are at a decreased risk of osteoporosis [285].
The potential benefits of IH, however, are not without risk. In extreme cases, repetitive hypoxia-reoxygenation cycles can lead to oxidative stress and systemic inflammation, mimicking damage often seen in ischemia-reperfusion injuries [286]. This effect is likely due to increased accumulation of ROS, which promotes NF-κB activation and the subsequent production of pro-inflammatory cytokines such as TNF-α and IL-6 [287,288]. Therefore, to avoid these effects, the extent of IH treatment must be closely monitored. In a recent review, Navarrete-Opazo and Mitchell [289] identified six characteristics which generally differentiate IH protocols. These include 1) the severity of hypoxia, 2) the duration of exposure, 3) the number of hypoxic episodes, 4) the schedule of treatment, 5) the duration of total treatment, and 6) the regulation of secondary parameters, such as arterial carbon dioxide. While all of these parameters varied greatly throughout published reports, the severity of hypoxia and number of cycles per day, appeared to be the most indicative of treatment success. In many studies, it was found that low dose hypoxia (9–16% inspired oxygen) and moderate cycle numbers (3–15 episodes/day) resulted in beneficial effects, without serious damage. When dose or duration were increased, deleterious side effects such as increased blood pressure [290,291], inflammation [292,293], and cognitive impairment [294–296] were observed.
4.3. Pharmacological inhibition
While modulation of the oxygen-sensing pathway has been shown to affect healing in a variety of injuries, clinical translation of HBOT and hypoxia treatment has been hindered by their associated infrastructure challenges, high costs, and inconsistencies in efficacy. An alternative approach to simulating these effects in patients is to utilize small molecule drugs capable of manipulating oxygen-sensing pathways. To achieve “pseudohypoxia,” two classes of small molecules can be used to inhibit PHD enzymes and increase HIF-1α levels. Notable compounds are shown in Fig. 5. Given that many of these compounds have been granted or are in the process of gaining clinical approval for other disorders, such as the treatment of anemia, they may easily be translated to regenerative medicine applications in the near future.
Fig. 5.
Common PHD inhibitors in preclinical and clinical testing. Most compounds such as Roxadustat, Daprodustat, DPCA, GSK60A, and DMOG, serve as 2OG competitive inhibitors. IOX4 and Molidustat may also compete with the ODDD of HIF-1α. Iron chelators such as DFO can also be used to inhibit PHDs by binging to the iron active site.
4.3.1. Active site inhibitors
The activity of PHD enzymes is reliant upon binding of the co- activator, α-ketoglutarate or 2-OG. When bound to the iron active site, 2-OG forms a remarkably stable complex with each of the PHD isoforms. This allows the enzymes to have higher oxygen Km values than other members of the 2OG oxygenase family, increasing their sensitivity to hypoxia [297,298]. The oxidative decarboxylation of 2OG to yield a ferryl intermediate is also required to drive substrate oxidation [104]. Thus, inhibition of 2OG binding can greatly impede PHD activity and subsequent HIF-1α degradation. Other pharmacological agents have also been developed to compete with the ODDD of HIF-1α to block substrate binding to the PHD active site [299]. Although this activity has been supported by crystallographic analysis, NMR studies have suggested alternative binding modes in the solution state [300].
Interest in developing 2OG competitive inhibitors first arose for the treatment of renal anemia associated with chronic kidney disease (CKD). This complication results from an erythropoietin (EPO) deficiency, caused by disease-associated damage to the peritubular cells of the kidney [301]. While recombinant EPO therapies have been shown to stabilize hemoglobin levels and reduce the need for red blood cell transfusion in these patients [302,303], high doses of EPO can raise plasma concentrations to supra-physiological levels, resulting in increased risk of stroke and myocardial infarction [116,304,305].
As natural mediators of cellular EPO production, manipulation of HIF-1α and HIF-2α signaling through PHD inhibition has emerged as a safer alternative to recombinant EPO therapy. Often, binding of these molecules to the 2OG pocket is achieved through a glycinamide side chain, as seen in Fibrogen’s FG-4592 (Roxadustat). This drug is perhaps the most well-studied PHD inhibitor and is currently undergoing Phase III clinical trials. In Phase II trials, when administered at 1.1–1.75 mg/kg, FG-4592 was shown to raise hemoglobin levels ≥1 g/dL from baseline in 80.0% of patients with CKD. Increasing the dose of FG-4592 to 1.50–2.25 mg/kg led to increases in 87.1% of patients, without the occurrence of adverse events [306]. Similar improvements have been noted in Phase III trials, which have demonstrated that FG-4592 is just as effective as recombinant EPO treatment [307]. Other PHD inhibitors with glycinamide side chains include GSK’s, GSK1278863 (Daprodustat) and GSK360A. While the latter has yet to enter clinical trials, Phase II studies of Daprodustat have demonstrated its effectiveness in managing anemia and improving iron metabolism in dialysis-dependent and non-dialysis-dependent CKD patients [308]. Triazole-based drugs such as IOX4 and Bayer’s BAY 85–3924 (Molidustat), are also capable of inactivating PHD enzymes by binding to their iron-active site via the nitrogen atoms of their pyridine and pyrazolone rings [299,309]. Pre-clinical studies on these compounds have shown their potential to upregulate HIF-1α and plasma EPO in mice, rats, and non-human primates [300,310]. Ongoing clinical studies with Molidustat suggest that it is effective at restoring hemoglobin levels in CKD patients for up to 36 months, demonstrating the first long-term analysis of PHD inhibition efficacy and saftey [311–313]. Recently, Roxadustat was approved for CKD treatment in China, and with ongoing trials nearing completion in the US, FDA approval of novel PHD inhibitors is likely to occur in the near future.
4.3.2. Iron chelators and transition metal ions
The active site of PHD enzymes contains a relatively labile, Fe2+ bound to the two-histidine, one-carboxylate motif that is required to bind substrates, oxygen, and cofactors [111]. Treatments with iron chelators such as Deferoxamine (DFO), can thus impede PHD activity and permit HIF-1α accumulation. For years, DFO has been used as a treatment for acute iron poisoning, which may occur as a result of multiple blood transfusions. Its role in HIF-1α stabilization was not realized until the mid-1990s when Wang et al. and Gleadle et al. demonstrated that iron-chelating agents such as DFO and hydroxypyr-idinones could be used to increase the production of EPO and angiogenic growth factors in Hep3B cells [314,315]. Despite these benefits, however, the use of DFO and iron chelators has been marked by adverse effects including hypotension during infusion, ophthalmic and auditory toxicity, infections, allergic and skin reactions, and pulmonary, renal, and neurological effects [316].
The labile Fe2+ within the PHD active site can also be substituted with other divalent transition metals such as Co2+, Cu2+, Zn2+ and Mn2+ [111]. In these instances, loss of Fe2+ abolishes the catalytic activity of these enzymes, although other mechanisms of inhibition have been proposed. For example, it is possible that transition metals can facilitate the degradation of ascorbate/redox-sensitive PHD cofactors, induce oxidative damage to the enzymes, or interact with other metal binding sites away from the active site [317]. Cobalt compounds in particular, have been shown to bind directly to HIF-1α at the ODDD, inhibiting its ability to interact with pVHL even after hydroxylation [318]. This behavior explains why cobalt-based compounds were some of the first medications used for treatment of anemia in the early 1950s, although the mechanism of action was yet to be understood [319,320].
Unlike most of the small molecule active site inhibitors described above, iron chelators and transition metal compounds are not specific to PHD enzymes. Their ability to interact with other 2OG oxygenases, as well as iron-containing proteins, results in many off-target effects. Some studies have shown that their use in regenerative medicine applications can result in progenitor cell death and antiproliferative effects [321–323]. The lack of specificity of these compounds counteracts the benefits of HIF-1α stabilization. Therefore, recent research has mainly focused on the use of PHD-specific small molecule inhibitors for regenerative medicine. In the following sections, notable studies illustrating how systemic and local delivery of these drugs can be used to catalyze endogenous healing and regeneration will be discussed. The role of PHD inhibitors in cell-based therapies will also highlighted.
4.4. In vivo delivery of PHD inhibitors for tissue regeneration
The role of HIF-1α and the oxygen sensing pathway in regeneration, suggests that these small molecule PHD inhibitors may have additional uses in wound care. For this application, possible advantages associated with pharmacological inhibition of HIF-1α are summarized in Fig. 6. Inspired by these effects as well as the robust accumulation of HIF-1α following injury in the MRL mouse, our group has attempted to use the PHD inhibitor, 1,4-dihydrophenonthrolin-4-one-3-Carboxylic acid (DPCA), to recapitulate the characteristic, biphasic expression pattern and induce regeneration in non-healing strains. Like many PHD inhibitors, DPCA was first developed for the treatment of anemia and exerts its inhibitory effects on PHDs and FIH by displacing 2OG from their iron active site [324]. We developed an injectable, PEG-based hydrogel that physically encapsulated polymer-stabilized DPCA microcrystals [55] as a consequence of rapid chemical cross-linking under physiological conditions [55,325]. Multiple doses given to non-regenerative Swiss Webster (SW) mice caused HIF-1α upregulation and tissue regeneration. Compared to control mice receiving drug-free PEG hydrogels, SW mice receiving DPCA demonstrated accelerated and increased closure of critical size ear hole wounds. Within the treatment group, even instances of complete closure were observed. This regenerative response was also accompanied by increased MMP expression and upregulation of a diverse panel of stem cell markers including NANOG, SOX2, OCT3/4, CD34, CD133, NESTIN, PAX7, and PREF1, closely mimicking the naturally regenerative phenotype of the MRL mouse [55]. Given these positive results, the DPCA-loaded hydrogel was then used to treat bone and ligament damage brought on by a ligature-induced model of periodontitis. Again, the gel was delivered at sites peripheral to the injury, but resulted in robust expression of HIF-1α in gingival tissue. After just 5 days, DPCA-treated groups showed alveolar bone regrowth to levels comparable to unligated controls, as well as periodontal ligament reattachment [326].
Fig. 6.
Possible roles of HIF-1α in regeneration. Recent studies into the use of small molecule PHD inhibitors have shown that pharmacological stabilization of HIF-1α may be used to unlock regeneration and accelerate wound healing in mammals. In many cases, the biological mechanism of this effect is not fully understood. However, the expression of various gene targets governing tissue remodeling, angiogenesis, inflammation, differentiation, and metabolism, likely play a role.
More recently, we developed a second-generation delivery platform that consisted of DPCA conjugated to a polymer via a multivalent hydrolyzable ester [327]. In our design, three molecules of DPCA were conjugated at one (P7D3) or both (P80D6) ends of a linear PEG using a trivalent linker (Fig. 7). Spontaneous self-assembly of P7D3 into well-defined nanofibers was observed in the presence of water, and the rheological properties of the prodrug solution was adjustable through P7D3:P80D6 ratio. Treatment of earhole wounds in SW mice with the polymer prodrug facilitated wound closure that was reminiscent of that seen in the MRL mouse [327]. Given the ability of the MRL mouse to regenerate defects in cardiac tissue, cartilage, and peripheral nerves, it is likely that the DPCA-based carriers outlined here can be used to treat a variety of injuries.
Fig. 7.
Novel prodrug vehicle used to facilitate tissue regeneration. To deliver the poorly soluble and hydrophobic drug, DPCA, PEG-based prodrugs were developed. Monofunctional, amphiphiles containing 3 DPCA molecules self- assembled in aqueous solutions to form nanofibers with a DPCA-rich core and PEG corona (P7D3). Fibers could be crossed linked with the introduction of a telechelic prodrug made from a higher molecular weight PEG and 6 DPCA molecules (P80D6). Hydrolysis of the ester linkage (red) facilitates release of free drug, bioactive drug (blue). Adapted with permission from (J. Cheng et al. ACS Nano 2019, 13, 5, 5493–5501). Copyright 2019 American Chemical Society. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Although the exact mechanism of DPCA-induced tissue regeneration is not understood, it is hypothesized that the transient upregulation of HIF-1α at wound sites causes cells to adopt a progenitor phenotype, enabling blastemal-like regeneration to occur. Similar effects have been observed after exposing cells from human exfoliated deciduous teeth to cobalt chloride. Robust induction of stem cell markers OCT4, NANOG, SOX2, and c-Myc was observed, as well as increases in cell migratory behavior [328]. Pharmacological inhibition of PHD may activate other pro-regenerative pathways such as the expression of remodeling enzymes, lysyl oxidase and lysyl hydroxylases which are normally upregulated during hypoxia [55]. Given the role of HIF-1α in the induction of glycolysis, it’s also possible that treatment with DPCA induces a metabolic switch in cells. This would closely mirror the fetal-like metabolism observed in the MRL mouse, as well as other natural epimorphic regenerators such as zebrafish and planarias [54,329,330]. While the effects of DPCA on cell metabolism have yet to be investigated, treatment with other small molecules such as Roxadustat and DMOG, have verified metabolic reprogramming as a result of pharmacological stabilization of HIF-1α [331].
In addition to DPCA’s role in progenitor cell recruitment and/or development, it has also been shown to modulate the local immune environment at the site of injury. Specifically, treatment with DPCA was accompanied by an increase in mast cell migration to mouse ear wounds [55]. As previously mentioned, this event is also seen in the MRL mouse, and thus may prime the region for regeneration [212]. It is likely that mast cell recruitment is directly linked to HIF-1α stabilization, since the protein is known to regulate a metabolic switch, essential for myeloid survival and function [332]. In addition to regulating immune cell metabolism, HIF-1α stabilization can also modulate cell homing through production of the C-X-C motif chemokine receptor 4 (CXCR4). In a murine model of periodontitis, this effect was observed following treatment with DPCA, and resulted in an increase in T regulatory (Treg) cell numbers in gingival tissue. Blocking of CXCR4 using a small molecule antagonist attenuated Treg recruitment, as well as bone regrowth, suggesting that the immunomodulatory activity of DPCA plays a direct role in regeneration [326]. Similar augmentations to cell homing have been observed in macrophages and neutrophils as a result of HIF-1α stabilization [332,333]. Therefore, it is likely that treatment with DPCA results in substantial changes in inflammation that need to be further elucidated.
Alternative approaches to HIF-1α upregulation following injury have also been attempted using FG-4592. As a pro-regenerative agent, FG- 4592 may contribute to cutaneous wound healing by inducing epidermal stem cell proliferation and motility. This effect was seen in BALB/c mice receiving daily intraperitoneal injections of FG-4592 solution at 10 mg/kg. Compared to controls, FG-4592-treated mice exhibited higher expression of cell proliferation marker, PCNA, and thicker neoepidermis. Full wound closure was also accelerated, occurring on day 8.13 for treatment groups and day 9.88 for control animals. In vitro assays demonstrated that blocking of HIF-1α abolished improvements to epidermal stem cell proliferation and motility following FG-4592 treatment, verifying that HIF-1α likely orchestrates these effects [334]. Although the exact signaling pathway that elicited this response was not identified, other reports have shown that the HIF-1α/SCAP/SREBP1 pathway can influence stem cell migration and proliferation via upregulation of FASN and subsequent mTORC1 activation [335].
The use of FG-4592 has not been limited to cutaneous wound healing, and may also be beneficial for the treatment of SCI. Given the regenerative response of SCI to IH therapy, it is not surprising that improvements to neural survival following FG-4592 treatment are primarily mediated by HIF-1α stabilization. While the exact neuroprotective mechanism is largely unknown, studies have postulated that HIF-1α upregulation can decrease apoptosis, hence containing lesion damage. Recovery is then augmented by VEGF production and the induction of autophagy through BNIP3 signaling, both of which prime axonal regrowth [336–338]. However, other studies have shown that the neuroprotective effects of PHD inhibition occur through HIF-1α-independent pathways [339,340]. In one model, it was suggested that inhibition of PHD1 induces regeneration through stabilization of Rbp1, a subunit of RNA polymerase II complex. Like HIF-1α, Rbp1 can be hydroxylated by PHDs, and this step may be important in regulating Rbp1-DNA binding. Therefore, inhibition of PHD1 and subsequent hydroxylation, may prevent the synthesis of pro-apoptotic mRNA during neuronal injuries or oxidative stress [340]. Regardless of the mechanism of action, PHD inhibition using active site inhibitors can lead to robust improvements in regrowth and functional recovery following neuronal injuries.
VEGF, perhaps the most well-characterized HIF-1α target, plays a crucial role in regenerative medicine since the viability of new tissue is dependent upon revascularization. By incorporating FG-4592 into tissue engineering scaffolds, the development and maturation of such neovasculature can be encouraged at the host-material interface [341]. Similar effects can also be achieved through delivery of dimethyl-oxalylglycine (DMOG), a N-oxalylglycine (NOG) derivative that serves as an analog for 2OG [342,343]. As a proof of concept study, the effects of systemic DMOG delivery on vascularization from an Arteriovenous Loop (AV Loop) model was recently investigated [344]. In tissue engineering, the AV Loop has served as an important strategy for facilitating microcirculatory development and is created by connecting a vein and artery via a vein graft. This loop is then embedded into an implantation chamber in vivo, to promote vascularization of newly grown tissue [345,346]. Intraperitoneal delivery of DMOG at low doses one week after AV loop generation increased vessel density, as well as proliferation of infiltrating cells within the chamber microenvironment. These effects correspond to increases in HIF-1α nuclear localization, as well as the production of angiogenic cytokines including VEGF and vWF [344].
When coupled with the osteogenic potential of hypoxia, the angiogenic effects of small molecule PHD inhibition can be used to facilitate bone regeneration in addition to the vascularization of scaffolds. This can be achieved through in vivo delivery of PHD inhibitors directly into fracture sites via tissue engineering scaffolds [347–349]. For bone regeneration applications, mesoporous bioactive glass (MBG) has emerged as a promising material for scaffold generation. Like most bioactive glass materials, a calcium-deficient, carbonated phosphate surface layer on MBG enables it to chemically bind to bone, while its mesoporous structure affords higher surface area and pore volume, allowing for improved apatite mineralization [350,351]. However, the limited pore size prevents the delivery of large growth factors and only allows for small molecule transport. Therefore, to increase VEGF and osteogenic cytokine expression, DMOG was incorporated into MBG constructs seeded with MSCs. Compared to unloaded scaffolds, MSCs exposed to DMOG had higher expression of osteogenic markers ALP, OCN and OPN, and VEGF secretion [352]. Similar effects were observed when DMOG was loaded into β-tricalcium phosphate-based scaffolds used to repair critical-sized calvarial defects in rats. In this study, bone regrowth increased to 30% with DMOG treatment, compared to just 22% with cell-based therapy alone [353]. Scaffold delivery strategies are not always required for PHD inhibitor treatment and improved bone regrowth has also been observed following direct injection of DMOG solutions into fracture sites [347].
Many PHD inhibitors designed for the treatment of CKD-associated anemia are taken orally and result in HIF-1α stabilization primarily localized to the liver and kidneys [306,307,309,331]. For regenerative medicine applications, where a precise timeline of HIF-1α stabilization is required at the site of injury, this pharmacokinetic profile will likely not be ideal. As an alternative to oral delivery, many of the studies outlined above have instead administered PHD inhibitors through intraperitoneal injections [335]. However, we note that this approach results in systemic upregulation of HIF-1α, which may contribute to unknown off-target effects due to the protein’s vast bioactivity [90,181,189,190, 193]. By relying on IP injection, these studies also have limited control over the timescale of HIF-1α stabilization, which appears to be essential for proper regeneration [354]. To overcome these challenges, biomaterials capable of reversibly storing and releasing small molecule PHD inhibitors have the potential to greatly increase the safety and efficacy of HIF-1α-induced regeneration. For example, carrier degradation and subsequent drug release could be tuned to achieve the biphasic expression pattern of HIF-1α stabilization in the MRL mouse [55]. Carriers could also be designed to administer PHD inhibitors, locally at the site of injury, likely reducing the required therapeutic dose and potential for deleterious side effects. As with all instances involving the in vivo implantation of biomaterials, if local delivery of drug carriers is to be investigated, careful engineering of the material will be required to ensure that foreign body reactions do not impede tissue regrowth [355]. Any construct implanted at the site of injury should replicate the biochemical and biomechanical characteristics of the existing tissue to provide an appropriate environment for cell survival and proliferation. However, even if local delivery is not desired, we hypothesize that the use of biomaterials to achieve systemic upregulation of HIF-1α will also be advantageous, since such systems have the potential to limit dose frequency and alter drug biodistribution.
4.5. In vitro preconditioning with PHD inhibitors in cell-based therapies
The activity of PHD inhibitors, beyond their interactions with HIF-specific PHDs, is not completely understood. Prolonged use of these inhibitors may result in severe side effects. These beliefs are rooted in the fact that hypoxia may result in decreased mitochondrial function and cardiomyopathy, while long-term HIF-1α stabilization can potentially improve the microenvironment for tumor growth and metastasis [356,357]. Favier et al. [356] observed a significant reduction in the heart function of rats who were administered DMOG intraperitoneally for one week; this was attributed to HIF-1α dependent cardiac remodeling. Therefore, in-vitro preconditioning of cells using PHD inhibitors may be a better, safer, alternative to in vivo delivery. This type of preconditioning would also be more practical for larger scale cell culture and hence be better suited for clinical applications [358].
Preconditioning has been an attractive strategy for enhancing the viability of stem cells prior to transplantation. The average physiological oxygen concentration ranges from 2 to 9%, however, cells are standardly incubated in ~21% oxygen. This does not accurately capture the hypoxic stem cell niche and therefore hinders cellular characteristics such as proliferation ability, developmental potential, and karyotype stability which results in a low survival rate of transplanted cells [358]. Pre-treatment is predicted to condition cells to survive the harsh micro-environment that immediately follows implantation [359]. Thus far, this has mainly been accomplished using hypoxic preconditioning. Liu et al. [359] detected increased cell survival, angiogenesis, and improved functional recovery after transplantation of hypoxic preconditioned bone marrow mesenchymal stem cells (BMSCs) in myocardial and cerebral ischemia rat models. Deveza et al. has also shown that overexpression of the hypoxia-related genes CXCR4 and VEGF within adipose-derived stem cells, can improve cell survival, modulate inflammation, and accelerate blood reperfusion upon transplantation into a mouse hindlimb ischemia model [360]. In-vitro preconditioning with PHD inhibitors can be used to mimic these effects while addressing a major challenge of hypoxic preconditioning, the steep decrease in HIF-1α levels upon exposure to ambient air, owing to the protein’s short half-life [361].
Several studies have demonstrated the utility of PHD inhibitor preconditioning for cell-based therapies. Chu et al. observed a protective effect of PHD inhibitors, credited to the drugs’ induction of HIF-1α accumulation and subsequent VEGF upregulation. After exposure to hydrogen peroxide, which mimics the oxidative stress related to brain disease, pre-treated cells with DFO or ethyl-3,4-dihydroxybenzoate (EDHB) retained practically complete viability compared to control groups, whereas non-preconditioned astrocytes had a fifty percent reduction in cell count [362]. Similarly, Liu et al. detected a time-dependent protective effect of DMOG preconditioning to hydrogen peroxide while control groups experienced more than fifty percent cell death.
Myocardial infarction (MI) treatment can be greatly impacted by in-vitro pre-treatment of PHD inhibitors. Transplantation of 1 mM DMOG pre-treated BMSCs in MI rat models significantly reduced heart infarct size and increased the expression of HIF-1α, VEGF, Glut-1, and phospho-Akt. This led to increased vessel formation and better overall recovery of the heart in comparison to control groups [359]. Cardiosphere-derived cells (CDCs) transplantation can also be used to treat MI and preconditioning with PHD inhibitors would greatly enhance the efficiency of this therapy. DMOG treated CDCs were shown to have increased mRNA levels of EPO, VEGF, c-Kit and CXCR-4. addition, these CDCs are thought to have decreased oxygen consumption and increased glycolytic metabolism, improving the therapeutic potential of CDCs [358].
In vitro pre-treatment of human neural stem cells (NSCs) with DFO was accompanied by production of neuroprotective and neurogenic factors, VEGF and BDNF, as well as expression of CXCR-4 [363]. Usually highly expressed in hematopoietic stem cells, the CXCR-4 receptor plays an important role in cell homing by binding to the chemokine SDF-1α [364]. Increased expression in NSCs, is therefore likely to improve NSC migration and retention within injury sites following in vivo implantation [363]. Similar improvements in cell homing and retention have been associated with increased expression of CXCR-4/CCR-2 and proteolytic enzymes in MSCs induced by exposure to DFO as well [365]. As previously discussed, use of these nonspecific PHD inhibitors such as DFO and cobalt chloride, can prevent cell proliferation. When these compounds are used synergistically with Rho kinase (ROCK) inhibitor, Y-27632, however, changes to cell cycle progression can induce MSC differentiation towards dopaminergic neuron-like cells. This response is likely mediated through HIF-1α induced upregulation of VEGF and EPO, as well as cyclin-dependent kinase inhibitor 1as (p21) [323].
Preconditioning with PDH inhibitors may also be beneficial for cerebral ischemia where reduced blood flow to the brain decreases available oxygen levels, greatly impacting synaptic transition. Preconditioning of rat hippocampal slices with DMOG resulted in an increased recovery rate of field excitatory postsynaptic potentials in the CA1 region following acute hypoxic treatments. DMOG was shown to increase HIF-1α levels and reduce PO2 concentrations. This was thought to condition the cells by decreasing the oxidative stress related with deoxygenation from acute hypoxia and hence allow the neurons to return to maximum functions at a faster rate. However, it is possible that this effect was independent of HIF-1α expression. Hypoxia can regulate glutamate receptors without the need for HIF-1α and therefore have a different process for reducing oxidative stress [366]. Further research is needed to elucidate the exact mechanism. Preconditioning of progenitor cells with PHD inhibitors can also increase their angiogenic potential, leading to improvements in vascularization of ischemic and diabetic limb injuries. The most substantial benefits were achieved when DMOG treatment was combined with HIF-1α gene therapy in bone marrow-derived angiogenic cells (BMDAC). While gene therapy alone was sufficient to induce mobilization of BMDAC and increase homing to ischemic muscle, it did not induce significant recovery in older mice, likely due to HIF-1α age-related loss of function [181,367]. When combined with DMOG pre-treatment, however, increased HIF-1α and HIF-2α activity led to the transcription of pro-angiogenic and glycolytic genes, as well as β2 integrin expression. β2 integrin allowed cells to strongly adhere to endothelial cells, facilitating their retention within ischemic tissues [367].
In addition to direct injection, cells preconditioned with hypoxia-mimicking agents, may also be delivered within biomaterial scaffolds. Given the role of HIF-1α target genes in vascularization, this approach may overcome one of the major challenges in biomaterials interventions – the development of a necrotic core within tissue engineering scaffolds. When cells are seeded into a 3D matrix, they are initially retained at the surface, resulting in tissue formation around the exterior. This limits the infiltration of cells, as well as diffusion of oxygen and nutrients into the center of the implant [368]. For cells to survive within this core, scaffolds with high porosity or vascular-like architecture must be developed. Within native tissue, almost every cell is within 50–100 μm of a blood vessel [369]. Despite advancements in 3D printing and materials fabrication, the intricate network of capillaries that is required to meet this demand is difficult to engineer into synthetic scaffolds. Therefore, a practical alternative may be to instead precondition cells to survive in the hypoxic core with PHD inhibitors. Recently, Singh, et al. reported that pre-treating neuron-like PC12 cells with 100 μM of FG-4592, FG-2216, GSK1278863, or Bay85–3934, made the cells significantly less vulnerable to oxygen-glucose deprivation [370]. Although these cells were intended to model tissue ischemia, these conditions also mirror a scaffold’s nutrient-deficient core. Considering the studies outlined above, the protective action of pseudohypoxia, may allow cells to survive the lag between in vivo implantation and vascularization. Recently, An, et al. used HIF-1α-mutated muscle-derived stem cells seeded within heparin-coated 3D-printed hydrogel scaffolds to accelerate neovascularization of damaged corpus cavernosa tissue, suggesting that upregulation of HIF-1α may even be able to decrease this lag period [371].
The development of a necrotic core similar to that seen within scaffolds, can also be found within in vitro tissues or organoids. To overcome this challenge, biomanufacturing techniques such as 3D printing and stereolithography aim to develop tissue constructs with complex vascular networks to allow for nutrient transport [372–376]. Without such architecture, engineered tissues are limited to only a few hundred microns in thickness [376]. Despite advancements in these fabrication techniques, however, most approaches still lack the resolution to create capillaries at the same size and density seen in native tissue [375]. Thus, preconditioning of cells through pseudohypoxia may be a useful strategy to overcome current technical limitations in the field of organoids as well.
Different PHD inhibitors have varying effects on HIF-1α and HIF-2α expression levels which in turn have divergent overall effects on cells. PHD inhibitors may also interact with other molecules in the cell to have adverse consequences. The development of tolerance to injury may be generated by HIF-1α independent pathways, where HIF-1α up regulation may happen indirectly as a secondary event [362]. Therefore, although in-vitro preconditioning resolves some concerns with preconditioning using these small molecules, further research must be done prior to incorporating this method for clinical applications.
5. Conclusion
There is now growing evidence to suggest that ancient oxygen-sensing pathways may be manipulated to unlock regeneration in adult mammals. If true, the universality of molecular machinery governing this response, make oxygen-signaling proteins ideal therapeutic target. Although oxygen can be converted into ROS, which serve as important signaling molecules during both wound repair and regeneration, control over the master regulator of hypoxia, HIF-1α, has been the main objective of research. Insights gained from mammalian models of regeneration, as well as lower vertebrates have shown that HIF-1α upregulation after injury can increase cell migration, initiate angiogenesis, and perhaps contribute to progenitor cell maintenance or generation. To recapitulate HIF-1α signaling in non-healing mammals, it is possible to tune local oxygen concentrations through hyperbaric oxygen or intermittent hypoxia treatment. Clinical acceptance of these therapies, however, has been impeded by logistical challenges, as well as questionable efficacy. To precisely control HIF-1α, small molecule PHD inhibitors, originally designed for the treatment of anemia, can be utilized. By delivering these molecules locally or systemically, “pseudohypoxia” can be achieved to promote regeneration. While the exact mechanism of this effect is not known, HIF-1α upregulation likely contributes to cell migration and angiogenesis, and may even allow cells to adopt a more “stem cell-like” phenotype to guide blastemal regrowth. Hypoxic or pseudohypoxia preconditioning of progenitor cells may also be used to recapitulate the stem cell niche in vitro, resulting in improved cell survival and proliferation. Although little clinical success has been seen for cell-based regenerative therapies, implementation of these practices may increase cell engraftment and the overall feasibility of the approach.
Acknowledgements
This work was supported by the National Science Foundation Graduate Research Fellowship under Grant No. [DGE 1752814]; the National Institutes of Health Grant No. [RO1 DE021104]; and US Department of Defense Grant No. W81XWH1910468.
No datasets were generated or analyzed during the current study.
Abbreviations and definitions
- Extracellular Matrix (ECM)
three-dimensional network of bio- macromolecules, that provides structure and support for surrounding cells, and contributes to cell adhesion, migration, differentiation, and signaling
- Polyethylene glycol (PEG)
synthetic polymer composed of repeating units of ethylene oxide commonly used in biological applications due to its hydrophilicity and low protein binding affinity
- Hypoxia-inducible Factor 1 alpha (HIF-1α)
oxygen-sensitive subunit of the HIF-1 transcription factor complex. Although HIF-1α is constitutively expressed, hydroxylation of the protein during normoxia results in degradation. In hypoxia, however, HIF-1α accumulates and translocates to the nucleus where it combines with HIF-1β and transcriptional cofactors, leading to gene expression relating to angiogenesis, metabolism, survival, migration, and proliferation. Although HIF-1α is the most well-characterized form of the HIF-α subunit, two additional isoforms (HIF-2α and HIF-3α) have been discovered
- Hypoxia-inducible Factor 1 beta (HIF-1β) or oxygen-insensitive aryl hydrocarbon receptor nuclear translocator (Arnt)
the oxygen- insensitive subunit of the HIF-1 complex. In the nucleus, Arnt binds HIF-1α as well as cofactors such as CBP/p300 at HIF-responsive element promoters. In addition to HIF-1α, Arnt has been shown to interact with HIF-2α and HIF-3α, as well as other transcription factors unrelated to hypoxia
- HIF Prolyl-hydroxylase (PHD)
an enzyme responsible for hydroxylating proline residues within the HIF-α subunit, facilitating ubiquitination and subsequent degradation of the protein in normoxia. Three PHD isoforms have been identified (PHD1, PHD2, and PHD3) and appear to differ in target affinity and expression
- Factor Inhibiting HIF (FIH)
an asparaginal hydroxylase shown to hydroxylate HIF-α subunits. This modification blocks binding of HIF-α to CBP/p300 transcriptional co-activators and prevents gene expression. Because FIH require molecular oxygen, this process serves as an alternative means of regulating HIF-α in response to environmental changes
- Von Hippel–Lindau tumor suppressor (pVHL)
component of the E3 ubiquitin ligase complex that interacts with hydroxylated HIF-α and directs its polyubiquitylation. After this step, HIF-α is targeted for degradation proteasomal degradation
- Murphy Roths Large Mouse (MRL/MpJ)
an inbred mouse strain originally developed as a model for autoimmunity. Recent work has identified the MRL as a new model of mammalian regeneration, since the strain is also able to heal critical size defects in a variety of tissues without scar formation. bone, cartilage, nerve, and skin tissues through a process emulating salamander limb regrowth
- Hyperbaric Oxygen Therapy (HBOT)
potential treatment for common wounds, as well as other pathologies, that involves exposing patients to pure oxygen at elevated pressures
- Intermittent Hypoxia (IH)
therapy used to treat spinal cord injuries and improve respiratory function, that involves exposing patients to short episodes of hypoxia (9–16% oxygen), followed by re- oxygenation
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declare the following competing financial interest(s): PBM and EHK are co-founders of MRL Bio, Inc. All other authors declare no conflicts of interest.
References
- [1].Tsonis PA, Fox TP, Regeneration according to spallanzani, Dev. Dynam 238 (9) (2009) 2357–2363. [DOI] [PubMed] [Google Scholar]
- [2].Okada TS, A brief history of regeneration research—for admiring Professor Niazi’s discovery of the effect of vitamin A on regeneration, J. Biosci 21 (3) (1996) 261–271. [Google Scholar]
- [3].Dinsmore CE, A History of Regeneration Research: Milestones in the Evolution of a Science, Cambridge University Press; 1991. [Google Scholar]
- [4].Major RJ, Poss KD, Zebrafish heart regeneration as a model for cardiac tissue repair, Drug Discov. Today Dis. Model 4 (4) (2007) 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beffagna G, Zebrafish as a smart model to understand regeneration after heart injury: how fish could help humans, Frontiers in Cardiovascular Medicine 6 (107) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stocum DL, Mechanisms of urodele limb regeneration, Regeneration (Oxf) 4 (4) 2017) 159–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ribeiro RP, Bleidorn C, Aguado MT, Regeneration mechanisms in syllidae (Annelida), Regeneration (Oxf) 5 (1) (2018) 26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Colwell AS, Longaker MT, Lorenz HP, Mammalian fetal organ regeneration, in: Yannas IV (Ed.), Regenerative Medicine I: Theories, Models and Methods, Springer Berlin Heidelberg, Berlin, Heidelberg, 2005, pp. 83–100. [DOI] [PubMed] [Google Scholar]
- [9].Larson BJ, Longaker MT, Lorenz HP, Scarless fetal wound healing: a basic science review, Plast. Reconstr. Surg 126 (4) (2010) 1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gurtner GC, Werner S, Barrandon Y, Longaker MT, Wound repair and regeneration, Nature 453 (7193) (2008) 314–321. [DOI] [PubMed] [Google Scholar]
- [11].Yang S.-w., Geng Z.-j., Ma K, Sun X.-y., Fu X.-b., Comparison of the histological morphology between normal skin and scar tissue, J. Huazhong Univ. Sci. Technol. - Med. Sci 36 (2) (2016) 265–269. [DOI] [PubMed] [Google Scholar]
- [12].Corr DT, Hart DA, Biomechanics of scar tissue and uninjured skin, Adv. Wound Care 2 (2) (2013) 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kordestani SS, Chapter 5 - wound care management, in: Kordestani SS (Ed.), Atlas of Wound Healing, Elsevier, 2019, pp. 31–47. [Google Scholar]
- [14].Thannickal VJ, Zhou Y, Gaggar A, Duncan SR, Fibrosis: ultimate and proximate causes, J. Clin. Invest 124 (11) (2014) 4673–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diamantopoulos A, Wright E, Vlahopoulou K, Cornic L, Schoof N, Maher TM, The burden of illness of idiopathic pulmonary fibrosis: a comprehensive evidence review, Pharmacoeconomics 36 (7) (2018) 779–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eming SA, Martin P, Tomic-Canic M, Wound repair and regeneration: mechanisms, signaling, and translation, Sci. Transl. Med 6 (265) (2014), 265sr6–265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xia H, Li X, Gao W, Fu X, Fang RH, Zhang L, Zhang K, Tissue repair and regeneration with endogenous stem cells, Nature Reviews Materials 3 (7) (2018) 174–193. [Google Scholar]
- [18].Li H, Fu X, Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration, Cell Tissue Res. 348 (3) (2012) 371–377. [DOI] [PubMed] [Google Scholar]
- [19].Gnecchi M, Zhang Z, Ni A, Dzau VJ, Paracrine mechanisms in adult stem cell signaling and therapy, Circ. Res 103 (11) (2008) 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sharma R, Khristov V, Rising A, Jha BS, Dejene R, Hotaling N, Li Y, Stoddard J, Stankewicz C, Wan Q, Zhang C, Campos MM, Miyagishima KJ, McGaughey D, Villasmil R, Mattapallil M, Stanzel B, Qian H, Wong W, Chase L, Charles S, McGill T, Miller S, Maminishkis A, Amaral J, Bharti K, Clinical-grade stem cell–derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs, Sci. Transl. Med 11 (475) (2019) eaat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bharti K, Rao M, Hull SC, Stroncek D, Brooks BP, Feigal E, van Meurs JC, Huang CA, Miller SS, Developing cellular therapies for retinal degenerative diseases, Invest. Ophthalmol. Vis. Sci 55 (2) (2014) 1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dimmeler S, Ding S, Rando TA, Trounson A, Translational strategies and challenges in regenerative medicine, Nat. Med 20 (8) (2014) 814–821. [DOI] [PubMed] [Google Scholar]
- [23].Trounson A, McDonald C, Stem cell therapies in clinical trials: progress and challenges, Cell stem cell 17 (1) (2015) 11–22. [DOI] [PubMed] [Google Scholar]
- [24].Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E, Schachinger V, Dimmeler S, Zeiher AM, Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry, Circ. Res 100 (8) (2007) 1234–1241. [DOI] [PubMed] [Google Scholar]
- [25].Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM, Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial, J. Am. Coll. Cardiol 44 (8) (2004) 1690–1699. [DOI] [PubMed] [Google Scholar]
- [26].Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E, Cardiomyocyte proliferation and progenitor celĺ recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart, EMBO Mol. Med 5 (2) (2013) 191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Y, Xu F, Ma J, Shi J, Chen S, Liu Z, Liu J, Effect of stem cell transplantation on patients with ischemic heart failure: a systematic review and meta-analysis of randomized controlled trials, Stem Cell Res. Ther 10 (1) (2019) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cox CS Jr., J. Juranek, S. Bedi, Clinical trials in traumatic brain injury: cellular therapy and outcome measures, Transfusion 59 (S1) (2019) 858–868. [DOI] [PubMed] [Google Scholar]
- [29].Phillips AW, Johnston MV, Fatemi A, The potential for cell-based therapy in perinatal brain injuries, Translational Stroke Research 4 (2) (2013) 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Burdick JA, Mauck RL, Gorman JH 3rd, Gorman RC, Acellular biomaterials: an evolving alternative to cell-based therapies, Sci. Transl. Med 5 (176) (2013), 176ps4–176ps4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee E, Kim HJ, Shaker MR, Ryu JR, Ham MS, Seo SH, Kim DH, Lee K, Jung N, Choe Y, Son GH, Rhyu IJ, Kim H, Sun W, High-performance acellular tissue scaffold combined with hydrogel polymers for regenerative medicine, ACS Biomater. Sci. Eng 5 (7) (2019) 3462–3474. [DOI] [PubMed] [Google Scholar]
- [32].Medina-Fernandez I, Celiz AD, Acellular biomaterial strategies for endodontic regeneration, Biomaterials science 7 (2) (2019) 506–519. [DOI] [PubMed] [Google Scholar]
- [33].Liang H, Jin C, Ma L, Feng X, Deng X, Wu S, Liu X, Yang C, Accelerated bone regeneration by gold-nanoparticle-loaded mesoporous silica through stimulating immunomodulation, ACS Appl. Mater. Interfaces 11 (44) (2019) 41758–41769. [DOI] [PubMed] [Google Scholar]
- [34].Ogle ME, Krieger JR, Tellier LE, McFaline-Figueroa J, Temenoff JS, Botchwey EA, Dual affinity heparin-based hydrogels achieve pro-regenerative immunomodulation and microvascular remodeling, ACS Biomater. Sci. Eng 4 (4) (2018) 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dellacherie MO, Seo BR, Mooney DJ, Macroscale biomaterials strategies for local immunomodulation, Nature Reviews Materials 4 (6) (2019) 379–397. [Google Scholar]
- [36].Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ, Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study, Lancet 376 (9739) (2010) 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA, Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium, Biomaterials 33 (31) (2012) 7849–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu R-X, Xu X-Y, Wang J, He X-T, Sun H-H, Chen F-M, Biomaterials for endogenous regenerative medicine: coaxing stem cell homing and beyond, Applied Materials Today 11 (2018) 144–165. [Google Scholar]
- [39].Choi JS, Choi SH, Yoo HS, Coaxial electrospun nanofibers for treatment of diabetic ulcers with binary release of multiple growth factors, J. Mater. Chem 21 (14) (2011) 5258–5267. [Google Scholar]
- [40].Ma T, Acellular biomaterials in mesenchymal stem cell-mediated endogenous tissue regeneration, J. Mater. Chem B 2 (1) (2014) 31–35. [DOI] [PubMed] [Google Scholar]
- [41].Qi C, Yan X, Huang C, Melerzanov A, Du Y, Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine, Protein Cell 6 (9) (2015) 638–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gurevich DB, French KE, Collin JD, Cross SJ, Martin P, Live imaging the foreign body response in zebrafish reveals how dampening inflammation reduces fibrosis, J. Cell Sci 133 (5) (2020) jcs236075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin. Immunol 20 (2) (2008) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW, Friedl P, Examination of the foreign body response to biomaterials by nonlinear intravital microscopy, Nature Biomedical Engineering 1 (1) (2016), 0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zeng Y, Shih Y-RV, Baht GS, Varghese S, In vivo sequestration of innate small molecules to promote bone healing, Adv. Mater 32 (8) (2020) 1906022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Londono R, Sun AX, Tuan RS, Lozito TP, Tissue repair and epimorphic regeneration: an overview, Current Pathobiology Reports 6 (1) (2018) 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yokoyama H, Initiation of limb regeneration: the critical steps for regenerative capacity, Development, Growth & Differentiation 50 (1) (2008) 13–22. [DOI] [PubMed] [Google Scholar]
- [48].Sandoval-Guzman T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, Tazaki A, Joven A, Tanaka Elly M., Simon A, Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species, Cell stem cell 14 (2) (2014) 174–187. [DOI] [PubMed] [Google Scholar]
- [49].Goss RJ, The evolution of regeneration: adaptive or inherent? J. Theor. Biol 159 (2) (1992) 241–260. [DOI] [PubMed] [Google Scholar]
- [50].Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ, Spontaneous murine lupus- like syndromes. Clinical and immunopathological manifestations in several strains, J. Exp. Med 148 (5) (1978) 1198–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Clark LD, Clark RK, Heber-Katz E, A new murine model for mammalian wound repair and regeneration, Clin. Immunol. Immunopathol 88 (1) (1998) 35–45. [DOI] [PubMed] [Google Scholar]
- [52].McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E, Genetic analysis of a mammalian wound-healing trait, Proc. Natl. Acad. Sci. U. S. A 95 (20) (1998) 11792–11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Huang LE, Gu J, Schau M, Bunn HF, Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin- proteasome pathway, Proc. Natl. Acad. Sci. U. S. A 95 (14) (1998) 7987–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Naviaux RK, Le TP, Bedelbaeva K, Leferovich J, Gourevitch D, Sachadyn P, Zhang X-M, Clark L, Heber-Katz E, Retained features of embryonic metabolism in the adult MRL mouse, Mol. Genet. Metabol 96 (3) (2009) 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang Y, Strehin I, Bedelbaeva K, Gourevitch D, Clark L, Leferovich J, Messersmith PB, Heber-Katz E, Drug-induced regeneration in adult mice, Sci. Transl. Med 7 (290) (2015), 290ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Heber-Katz E, Oxygen, metabolism, and regeneration: lessons from mice, Trends Mol. Med 23 (11) (2017) 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hameed LS, Berg DA, Belnoue L, Jensen LD, Cao Y, Simon A, Environmental changes in oxygen tension reveal ROS-dependent neurogenesis and regeneration in the adult newt brain, Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Niethammer P, Grabher C, Look AT, Mitchison TJ, A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish, Nature 459 (7249) (2009) 996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Han P, Zhou XH, Chang N, Xiao CL, Yan S, Ren H, Yang XZ, Zhang ML, Wu Q, Tang B, Diao JP, Zhu X, Zhang C, Li CY, Cheng H, Xiong JW, Hydrogen peroxide primes heart regeneration with a derepression mechanism, Cell Res. 24 (9) (2014) 1091–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ferreira F, Raghunathan V, Luxardi G, Zhu K, Zhao M, Early redox activities modulate Xenopus tail regeneration, Nat. Commun 9 (1) (2018) 4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ferreira F, Luxardi G, Reid B, Zhao M, Early bioelectric activities mediate redox-modulated regeneration, Development 143 (24) (2016) 4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Novianti T, Juniantito V, Jusuf AA, Arida EA, Jusman SWA, Sadikin M, Expression and role of HIF-1α and HIF-2α in tissue regeneration: a study of hypoxia in house gecko tail regeneration, Organogenesis 15 (3) (2019) 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang Q, Wang Y, Man L, Zhu Z, Bai X, Wei S, Liu Y, Liu M, Wang X, Gu X, Wang Y, Reactive oxygen species generated from skeletal muscles are required for gecko tail regeneration, Sci. Rep 6 (2016) 20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Semenza GL, Life with oxygen, Science 318 (5847) (2007) 62–64. [DOI] [PubMed] [Google Scholar]
- [65].Zheng J, Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (Review), Oncol Lett 4 (6) (2012) 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Semenza GL, Wang GL, A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation, Mol. Cell Biol 12 (12) (1992) 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang GL, Jiang BH, Rue EA, Semenza GL, Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension, Proc. Natl. Acad. Sci. U. S. A 92 (12) (1995) 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu Y, Cox SR, Morita T, Kourembanas S, Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer, Circ. Res 77 (3) (1995) 638–643. [DOI] [PubMed] [Google Scholar]
- [69].Levy AP, Levy NS, Wegner S, Goldberg MA, Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia, J. Biol. Chem 270 (22) (1995) 13333–13340. [DOI] [PubMed] [Google Scholar]
- [70].Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL, Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1, Mol. Cell Biol 16 (9) (1996) 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Befani C, Liakos P, The role of hypoxia-inducible factor-2 alpha in angiogenesis, J. Cell. Physiol 233 (12) (2018) 9087–9098. [DOI] [PubMed] [Google Scholar]
- [72].Hubbi ME, Semenza GL, Regulation of cell proliferation by hypoxia-inducible factors, American journal of physiology, Cell physiology 309 (12) (2015) C775–C782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE, HIF- 1alpha induces cell cycle arrest by functionally counteracting Myc, EMBO J. 23 (9) (2004) 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hubbi ME, Kshitiz, Gilkes DM, Rey S, Wong CC, Luo W, Kim D-H, Dang CV, Levchenko A, Semenza GL, A non-transcriptional role for HIF-1α as a direct inhibitor of DNA replication, Sci. Signal 6 (262) (2013) ra10-ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Del Rey MJ, Valín Á, Usategui A, García-Herrero CM, Sánchez-Aragó M, Cuezva JM, Galindo M, Bravo B, Cañete JD, Blanco FJ, Criado G, Pablos JL, Hif-1α knockdown reduces glycolytic metabolism and induces cell death of human synovial fibroblasts under normoxic conditions, Sci. Rep 7 (1) (2017), 3644–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ, Rodriguez-Enriquez S, Moreno-Sanchez R, HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms, Mini Rev. Med. Chem 9 (9) (2009) 1084–1101. [DOI] [PubMed] [Google Scholar]
- [77].Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H, HIF-1-Dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance, Int. J. Mol. Sci 20 (2) (2019) 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hu C-J, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC, Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells, Mol. Cell Biol 26 (9) (2006) 3514–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H, Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency, Cell stem cell 14 (5) (2014) 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Palomäki S, Pietilä M, Laitinen S, Pesälä J, Sormunen R, Lehenkari P, Koivunen P, HIF-1α is upregulated in human mesenchymal stem cells, Stem cells (Dayton, Ohio) 31 (9) (2013) 1902–1909. [DOI] [PubMed] [Google Scholar]
- [81].Li L, Candelario KM, Thomas K, Wang R, Wright K, Messier A, Cunningham LA, Hypoxia inducible factor-1α (HIF-1α) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ, J. Neurosci 34 (50) (2014) 16713–16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M, Hypoxia requires notch signaling to maintain the undifferentiated cell state, Dev. Cell 9 (5) (2005) 617–628. [DOI] [PubMed] [Google Scholar]
- [83].Bohuslavova R, Cerychova R, Papousek F, Olejnickova V, Bartos M, Görlach A, Kolar F, Sedmera D, Semenza GL, Pavlinkova G, HIF-1α is required for development of the sympathetic nervous system, Proc. Natl. Acad. Sci. Unit. States Am 116 (27) (2019) 13414–13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kotch LE, Iyer NV, Laughner E, Semenza GL, Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death, Dev. Biol 209 (2) (1999) 254–267. [DOI] [PubMed] [Google Scholar]
- [85].Cerychova R, Pavlinkova G, HIF-1, metabolism, and diabetes in the embryonic and adult heart, Front. Endocrinol 9 (2018), 460–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Merceron C, Mangiavini L, Robling A, Wilson TL, Giaccia AJ, Shapiro IM, Schipani E, Risbud MV, Loss of HIF-1 alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus, PloS One 9 (10) (2014) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Calzada MJ, del Peso L, Hypoxia-inducible factors and cancer, Clin. Transl. Oncol 9 (5) (2007) 278–289. [DOI] [PubMed] [Google Scholar]
- [88].Balamurugan K, HIF-1 at the crossroads of hypoxia, inflammation, and cancer, Int. J. Canc 138 (5) (2016) 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Airley RE, Mobasheri A, Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics, Chemotherapy 53 (4) (2007) 233–256. [DOI] [PubMed] [Google Scholar]
- [90].Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P, Oxygen in acute and chronic wound healing, Br. J. Dermatol 163 (2) (2010) 257–268. [DOI] [PubMed] [Google Scholar]
- [91].Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM, Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing, Canc. Res 60 (21) (2000) 6189–6195. [PubMed] [Google Scholar]
- [92].Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang B-H, Leung S, Roe R, Wiener C, Yu A, Structural and functional analysis of hypoxia-inducible factor 1, Kidney Int. 51 (2) (1997) 553–555. [DOI] [PubMed] [Google Scholar]
- [93].Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM, An essential role for p300/CBP in the cellular response to hypoxia, Proc. Natl. Acad. Sci. U. S. A 93 (23) (1996) 12969–12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ebert BL, Bunn HF, Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein, Mol. Cell Biol 18 (7) (1998) 4089–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang F, Zhang R, Beischlag TV, Muchardt C, Yaniv M, Hankinson O, Roles of Brahma and Brahma/SWI2-related gene 1 in hypoxic induction of the erythropoietin gene, J. Biol. Chem 279 (45) (2004) 46733–46741. [DOI] [PubMed] [Google Scholar]
- [96].Kenneth NS, Mudie S, van Uden P, Rocha S, SWI/SNF regulates the cellular response to hypoxia, J. Biol. Chem 284 (7) (2009) 4123–4131. [DOI] [PubMed] [Google Scholar]
- [97].Lee JS, Kim Y, Bhin J, Shin HJ, Nam HJ, Lee SH, Yoon JB, Binda O, Gozani O, Hwang D, Baek SH, Hypoxia-induced methylation of a pontin chromatin remodeling factor, Proc. Natl. Acad. Sci. U. S. A 108 (33) (2011) 13510–13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Galbraith MD, Allen MA, Bensard CL, Wang X, Schwinn MK, Qin B, Long HW, Daniels DL, Hahn WC, Dowell RD, Espinosa JM, HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia, Cell 153 (6) (2013) 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Freedman SJ, Sun Z-YJ, Poy F, Kung AL, Livingston DM, Wagner G, J Eck M, Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor- 1 alpha, Proc. Natl. Acad. Sci. U. S. A 99 (8) (2002) 5367–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM, Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor, J. Biol. Chem 279 (37) (2004) 38458–38465. [DOI] [PubMed] [Google Scholar]
- [101].Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouyssègur J, HIF prolyl- hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia, EMBO J. 22 (16) (2003) 4082–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Soilleux EJ, Turley H, Tian YM, Pugh CW, Gatter KC, Harris AL, Use of novel monoclonal antibodies to determine the expression and distribution of the hypoxia regulatory factors PHD-1, PHD-2, PHD-3 and FIH in normal and neoplastic human tissues, Histopathology 47 (6) (2005) 602–610. [DOI] [PubMed] [Google Scholar]
- [103].Lieb ME, Menzies K, Moschella MC, Ni R, Taubman MB, Mammalian EGLN genes have distinct patterns of mRNA expression and regulation, Biochemistry and cell biology = Biochimie et biologie cellulaire 80 (4) (2002) 421–426. [DOI] [PubMed] [Google Scholar]
- [104].Chan MC, Holt-Martyn JP, Schofield CJ, Ratcliffe PJ, Pharmacological targeting of the HIF hydroxylases–A new field in medicine development, Mol. Aspect. Med 47–48 (2016) 54–75. [DOI] [PubMed] [Google Scholar]
- [105].Huang LE, Gu J, Schau M, Bunn HF, Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin- proteasome pathway, Proc. Natl. Acad. Sci. Unit. States Am 95 (14) (1998) 7987–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pugh CW, O’Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ, Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit, J. Biol. Chem 272 (17) (1997) 11205–11214. [DOI] [PubMed] [Google Scholar]
- [107].Maxwell PH, Wiesener MS, Chang G-W, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ, The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis, Nature 399 (6733) (1999) 271–275. [DOI] [PubMed] [Google Scholar]
- [108].Tanimoto K, Makino Y, Pereira T, Poellinger L, Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein, EMBO J. 19 (16) (2000) 4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ohh M, Park CW, Ivan M, Hoffman MA, Kim T-Y, Huang LE, Pavletich N, Chau V, Kaelin WG, Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel–Lindau protein, Nat. Cell Biol 2 (7) (2000) 423–427. [DOI] [PubMed] [Google Scholar]
- [110].Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH, Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein, J. Biol. Chem 275 (33) (2000) 25733–25741. [DOI] [PubMed] [Google Scholar]
- [111].Schofield CJ, Ratcliffe PJ, Oxygen sensing by HIF hydroxylases, Nat. Rev. Mol. Cell Biol 5 (5) (2004) 343–354. [DOI] [PubMed] [Google Scholar]
- [112].Tarhonskaya H, Hardy AP, Howe EA, Loik ND, Kramer HB, McCullagh JS, Schofield CJ, Flashman E, Kinetic investigations of the role of factor inhibiting hypoxia-inducible factor (FIH) as an oxygen sensor, J. Biol. Chem 290 (32) (2015) 19726–19742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK, FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor, Genes Dev. 16 (12) (2002) 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wenger RH, Stiehl DP, Camenisch G, Integration of oxygen signaling at the consensus HRE, Sci. STKE 2005 (306) (2005) re12-re12. [DOI] [PubMed] [Google Scholar]
- [115].Liu W, Shen S-M, Zhao X-Y, Chen G-Q, Targeted genes and interacting proteins of hypoxia inducible factor-1, Int J Biochem Mol Biol 3 (2) (2012) 165–178. [PMC free article] [PubMed] [Google Scholar]
- [116].Haase VH, Therapeutic targeting of the HIF oxygen-sensing pathway: lessons learned from clinical studies, Exp. Cell Res 356 (2) (2017) 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mastrogiannaki M, Matak P, Mathieu JR, Delga S, Mayeux P, Vaulont S, Peyssonnaux C, Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis, Haematologica 97 (6) (2012) 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Rankin EB, Biju MP, Liu QD, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH, Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo, J. Clin. Invest 117 (4) (2007) 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chavez JC, Baranova O, Lin J, Pichiule P, The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes, J. Neurosci 26 (37) (2006) 9471–9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Duan LJ, Takeda K, Fong GH, Hypoxia inducible factor-2 alpha regulates the development of retinal astrocytic network by maintaining adequate supply of astrocyte progenitors, PloS One 9 (1) (2014) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Nagaya Y, Aoyama M, Tamura T, Kakita H, Kato S, Hida H, Saitoh S, Asai K, Inflammatory cytokine tumor necrosis factor a suppresses neuroprotective endogenous erythropoietin from astrocytes mediated by hypoxia-inducible factor- 2 alpha, Eur. J. Neurosci 40 (11) (2014) 3620–3626. [DOI] [PubMed] [Google Scholar]
- [122].Bautista L, Castro MJ, Lopez-Barneo J, Castellano A, Hypoxia inducible factor- 2 alpha stabilization and maxi-K+ channel beta(1)-subunit gene repression by hypoxia in cardiac myocytes role in preconditioning, Circ. Res 104 (12) (2009) 1364–1372. [DOI] [PubMed] [Google Scholar]
- [123].Koeppen M, Lee JW, Seo S-W, Brodsky KS, Kreth S, Yang IV, Buttrick PM, Eckle T, Eltzschig HK, Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury, Nat. Commun 9 (1) (2018) 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lin Q, Huang Y, Booth CJ, Haase VH, Johnson RS, Celeste Simon M, J Giordano F, Yun Z, Activation of hypoxia-inducible factor-2 in adipocytes results in pathological cardiac hypertrophy, J Am Heart Assoc 2 (6) (2013) e000548- e000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P, Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice, Nat. Med 8 (7) (2002) 702–710. [DOI] [PubMed] [Google Scholar]
- [126].Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU, Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs, Faseb. J. : official publication of the Federation of American Societies for Experimental Biology 17 (2) (2003) 271–273. [DOI] [PubMed] [Google Scholar]
- [127].Shu S, Wang Y, Zheng M, Liu Z, Cai J, Tang C, Dong Z, Hypoxia and hypoxia- inducible factors in kidney injury and repair, Cells 8 (3) (2019) 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jarman EJ, Ward C, Turnbull AK, Martinez-Perez C, Meehan J, Xintaropoulou C, Sims AH, Langdon SP, HER2 regulates HIF-2α and drives an increased hypoxic response in breast cancer, Breast Cancer Res. 21 (1) (2019), 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH, Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice, Oncogene 27 (40) (2008) 5354–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cuvillier O, The therapeutic potential of HIF-2 antagonism in renal cell carcinoma, Transl. Androl. Urol 6 (1) (2017) 131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Keith B, Johnson RS, Simon MC, HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression, Nat. Rev. Canc 12 (1) (2011) 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Moreno Roig E, Yaromina A, Houben R, Groot AJ, Dubois L, Vooijs M, Prognostic role of hypoxia-inducible factor-2α tumor cell expression in cancer patients: a meta-analysis, Front Oncol 8 (2018), 224–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL, The expression and distribution of the hypoxia-inducible factors HIF- 1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages, Am. J. Pathol 157 (2) (2000) 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan L-J, Hammond R, Gimotty PA, Keith B, Simon MC, Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation, J. Clin. Invest 120 (8) (2010) 2699–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hu C-J, Wang L-Y, Chodosh LA, Keith B, Simon MC, Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation, Mol. Cell Biol 23 (24) (2003) 9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC, Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation, Mol. Cell Biol 23 (24) (2003) 9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C, Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha, J. Biol. Chem 279 (15) (2004) 14871–14878. [DOI] [PubMed] [Google Scholar]
- [138].Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J, HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia, EMBO J. 22 (16) (2003) 4082–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Tian H, McKnight SL, Russell DW, Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells, Genes Dev. 11 (1) (1997) 72–82. [DOI] [PubMed] [Google Scholar]
- [140].Keith B, Johnson RS, Simon MC, HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression, Nat. Rev. Canc 12 (1) (2011) 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hu C-J, Sataur A, Wang L, Chen H, Simon MC, The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha, Mol. Biol. Cell 18 (11) (2007) 4528–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA, Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3 alpha, Gene Expr. 7 (3) (1998) 205–213. [PMC free article] [PubMed] [Google Scholar]
- [143].Maynard MA, Qi H, Chung J, Lee EH, Kondo Y, Hara S, Conaway RC, Conaway JW, Ohh M, Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex, J. Biol. Chem 278 (13) (2003) 11032–11040. [DOI] [PubMed] [Google Scholar]
- [144].Ravenna L, Cardillo I, Curzio G, Baldi A, Mattioni M, Vincenzi B, Russo M, Soddu S, Verdina A, Mesothelioma and hypoxia: modulation of the inflammation-related phenotype and identification of prognostic markers, J. Canc. Sci. Ther 6 (2014) 378–387. [Google Scholar]
- [145].Maynard MA, Evans AJ, Hosomi T, Hara S, Jewett MA, Ohh M, Human HIF- 3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma, Faseb. J. : official publication of the Federation of American Societies for Experimental Biology 19 (11) (2005) 1396–1406. [DOI] [PubMed] [Google Scholar]
- [146].Pasanen A, Heikkilä M, Rautavuoma K, Hirsilä M, Kivirikko KI, Myllyharju J, Hypoxia-inducible factor (HIF)-3α is subject to extensive alternative splicing in human tissues and cancer cells and is regulated by HIF-1 but not HIF-2, Int. J. Biochem. Cell Biol 42 (7) (2010) 1189–1200. [DOI] [PubMed] [Google Scholar]
- [147].Ravenna L, Salvatori L, Russo MA, HIF3alpha: the little we know, FEBS J. 283 (6) (2016) 993–1003. [DOI] [PubMed] [Google Scholar]
- [148].Zhang Y, Chen Y, Qu H, Wang Y, Methylation of HIF3A promoter CpG islands contributes to insulin resistance in gestational diabetes mellitus, Molecular Genetics & Genomic Medicine 7 (4) (2019), e00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Mansell T, Ponsonby A-L, Januar V, Novakovic B, Collier F, Burgner D, Vuillermin P, Ryan J, Saffery R, Barwon T. Infant Study Investigator, Early-life determinants of hypoxia-inducible factor 3A gene (HIF3A) methylation: a birth cohort study, Clin. Epigenet 11 (1) (2019), 96–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pfeiffer S, Krüger J, Maierhofer A, Böttcher Y, Köloting N, El Hajj N, Schleinitz D, Schon MR, Dietrich A, Fasshauer M, Lohmann T, Dreßler M, Stumvoll M, Haaf T, Blüher M, Kovacs P, Hypoxia-inducible factor 3A gene expression and methylation in adipose tissue is related to adipose tissue dysfunction, Sci. Rep 6 (2016), 27969–27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, Schipani E, Clemens TL, Role of HIF-1alpha in skeletal development, Ann. N. Y. Acad. Sci 1192 (2010) 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Knighton D, Silver I, Hunt T, Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration, Surgery 90 (2) (1981) 262–270. [PubMed] [Google Scholar]
- [153].Niinikoski J, Heughan C, Hunt TK, Oxygen tensions in human wounds, J. Surg. Res 12 (2) (1972) 77–82. [DOI] [PubMed] [Google Scholar]
- [154].Lokmic Z, Musyoka J, Hewitson TD, Darby IA, Chapter three - hypoxia and hypoxia signaling in tissue repair and fibrosis, in: Jeon KW (Ed.), International Review of Cell and Molecular Biology, Academic Press; 2012, pp. 139–185. [DOI] [PubMed] [Google Scholar]
- [155].Baatar D, Jones MK, Tsugawa K, Pai R, Moon WS, Koh GY, Kim I, Kitano S, Tarnawski AS, Esophageal ulceration triggers expression of hypoxia-inducible factor-1 alpha and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing, Am. J. Pathol 161 (4) (2002) 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Mingyuan X, Qianqian P, Shengquan X, Chenyi Y, Rui L, Yichen S, Jinghong X, Hypoxia-inducible factor-1α activates transforming growth factor- β1/Smad signaling and increases collagen deposition in dermal fibroblasts, Oncotarget 9 (3) (2017) 3188–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW, Interdependence of HIF-1alpha and TGF-beta/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression, Am. J. Physiol. Ren. Physiol 300 (4) (2011) F898–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM, Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression, J. Biol. Chem 281 (34) (2006) 24171–24181. [DOI] [PubMed] [Google Scholar]
- [159].Tsapournioti S, Mylonis I, Hatziefthimiou A, Ioannou MG, Stamatiou R, Koukoulis GK, Simos G, Molyvdas PA, Paraskeva E, TNFalpha induces expression of HIF-1alpha mRNA and protein but inhibits hypoxic stimulation of HIF-1 transcriptional activity in airway smooth muscle cells, J. Cell. Physiol 228 (8) (2013) 1745–1753. [DOI] [PubMed] [Google Scholar]
- [160].Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L, Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor- interacting protein-dependent nuclear factor kappa B activation, Biochem. J 370 (Pt 3) (2003) 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, Georgopoulos N, Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process, Int. Wound J 14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Fang FC, Antimicrobial actions of reactive oxygen species, mBio 2 (5) (2011) e00141–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yang P, Huang S, Xu A, Chapter 8 - the oxidative burst system in amphioxus, in: Xu A. (Ed.), Amphioxus Immunity, Academic Press; 2016, pp. 153–165. [Google Scholar]
- [164].Stone JR, Collins T, The role of hydrogen peroxide in endothelial proliferative responses, Endothelium 9 (4) (2002) 231–238. [DOI] [PubMed] [Google Scholar]
- [165].Murrell GA, Francis MJ, Bromley L, Modulation of fibroblast proliferation by oxygen free radicals, Biochem. J 265 (3) (1990) 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Daniel RJ, Groves RW, Increased migration of murine keratinocytes under hypoxia is mediated by induction of urokinase plasminogen activator, J. Invest. Dermatol 119 (6) (2002) 1304–1309. [DOI] [PubMed] [Google Scholar]
- [167].Yan T, Zhang J, Tang D, Zhang X, Jiang X, Zhao L, Zhang Q, Zhang D, Huang Y, Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway, PloS One 12 (1) (2017) e0169155-e0169155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].O’Toole EA, Marinkovich MP, Peavey CL, Amieva MR, Furthmayr H, Mustoe TA, Woodley DT, Hypoxia increases human keratinocyte motility on connective tissue, J. Clin. Invest 100 (11) (1997) 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Vogler M, Vogel S, Krull S, Farhat K, Leisering P, Lutz S, Wuertz CM, Katschinski DM, Zieseniss A, Hypoxia modulates fibroblastic architecture, adhesion and migration: a role for HIF-1α in cofilin regulation and cytoplasmic actin distribution, PloS One 8 (7) (2013) e69128-e69128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Shweiki D, Itin A, Soffer D, Keshet E, Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis, Nature 359 (6398) (1992) 843–845. [DOI] [PubMed] [Google Scholar]
- [171].Berthod F, Germain L, Tremblay N, Auger F, Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro, J. Cell. Physiol 207 (2006) 491–498. [DOI] [PubMed] [Google Scholar]
- [172].Sen CK, Wound healing essentials: let there be oxygen, Wound repair and regeneration, official publication of the Wound Healing Society [and] the European Tissue Repair Society 17 (1) (2009) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Pinnell SR, Regulation of collagen synthesis, J. Invest. Dermatol 79 (Suppl 1) (1982) 73s–76s. [DOI] [PubMed] [Google Scholar]
- [174].Darby IA, Laverdet B, Bonté F, Desmoulière A, Fibroblasts and myofibroblasts in wound healing, Clin. Cosmet. Invest. Dermatol 7 (2014) 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Svineng G, Ravuri C, Rikardsen O, Huseby N-E, Winberg J-O, The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function, Connect. Tissue Res 49 (3–4) (2008) 197–202. [DOI] [PubMed] [Google Scholar]
- [176].Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P, Oxygen in acute and chronic wound healing, Br. J. Dermatol 163 (2) (2010) 257–268. [DOI] [PubMed] [Google Scholar]
- [177].Xia Y-P, Zhao Y, Tyrone JW, Chen A, Mustoe TA, Differential activation of migration by hypoxia in keratinocytes isolated from donors of increasing age: implication for chronic wounds in the elderly, J. Invest. Dermatol 116 (1) (2001) 50–56. [DOI] [PubMed] [Google Scholar]
- [178].Wu T-W, Liu C-C, Hung C-L, Yen C-H, Wu Y-J, Wang L-Y, Yeh H-I, Genetic profiling of young and aged endothelial progenitor cells in hypoxia, PloS One 13 (4) (2018) e0196572-e0196572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wu L, Xia YP, Roth SI, Gruskin E, Mustoe TA, Transforming growth factor- beta1 fails to stimulate wound healing and impairs its signal transduction in an aged ischemic ulcer model: importance of oxygen and age, Am. J. Pathol 154 (1) (1999) 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Mogford JE, Sisco M, Bonomo SR, Robinson AM, Mustoe TA, Impact of aging on gene expression in a rat model of ischemic cutaneous wound healing, J. Surg. Res 118 (2) (2004) 190–196. [DOI] [PubMed] [Google Scholar]
- [181].Bosch-Marce M, Okuyama H, Wesley Jacob B, Sarkar K, Kimura H, Liu Ye V, Zhang H, Strazza M, Rey S, Savino L, Zhou Yi F, McDonald Karin R, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, LaVallee T, Semenza Gregg L, Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia, Circ. Res 101 (12) (2007) 1310–1318. [DOI] [PubMed] [Google Scholar]
- [182].Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC, Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia, Circulation 116 (24) (2007) 2818–2829. [DOI] [PubMed] [Google Scholar]
- [183].King TC, in: King TC (Ed.), 1 - Cell Injury, Cellular Responses to Injury, and Cell Death, Elsevier’s Integrated Pathology, Mosby, Philadelphia, 2007, pp. 1–20. [Google Scholar]
- [184].Caley MP, Martins VLC, O’Toole EA, Metalloproteinases and wound healing, Adv. Wound Care 4 (4) (2015) 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Wlaschek M, Scharffetter-Kochanek K, Oxidative stress in chronic venous leg ulcers, Wound repair and regeneration, official publication of the Wound Healing Society [and] the European Tissue Repair Society 13 (5) (2005) 452–461. [DOI] [PubMed] [Google Scholar]
- [186].Peschen M, Lahaye T, Hennig B, Weyl A, Simon JC, Vanscheidt W, Expression of the adhesion molecules ICAM-1, VCAM-1, LFA-1 and VLA-4 in the skin is modulated in progressing stages of chronic venous insufficiency, Acta Derm. Venereol 79 (1) (1999) 27–32. [DOI] [PubMed] [Google Scholar]
- [187].Alizadeh N, Pepper MS, Modarressi A, Alfo K, Schlaudraff K, Montandon D, Gabbiani G, Bochaton-Piallat ML, Pittet B, Persistent ischemia impairs myofibroblast development in wound granulation tissue: a new model of delayed wound healing, Wound repair and regeneration, official publication of the Wound Healing Society [and] the European Tissue Repair Society 15 (6) (2007) 809–816. [DOI] [PubMed] [Google Scholar]
- [188].Alster TS, Tanzi EL, Hypertrophic scars and keloids, Am. J. Clin. Dermatol 4 (4) (2003) 235–243. [DOI] [PubMed] [Google Scholar]
- [189].Kim J, Kim B, Kim SM, Yang CE, Song SY, Lee WJ, Lee JH, Hypoxia- induced epithelial-to-mesenchymal transition mediates fibroblast Abnormalities via ERK activation in cutaneous wound healing, Int. J. Mol. Sci 20 (10) (2019) 2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Ma X, Chen J, Xu B, Long X, Qin H, Zhao RC, Wang X, Keloid-derived keratinocytes acquire a fibroblast-like appearance and an enhanced invasive capacity in a hypoxic microenvironment in vitro, Int. J. Mol. Med 35 (5) (2015) 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Zhang Q, Wu Y, Chau CH, Ann DK, Bertolami CN, Le AD, Crosstalk of hypoxia-mediated signaling pathways in upregulating plasminogen activator inhibitor-1 expression in keloid fibroblasts, J. Cell. Physiol 199 (1) (2004) 89–97. [DOI] [PubMed] [Google Scholar]
- [192].Wu Y, Zhang Q, Ann DK, Akhondzadeh A, Duong HS, Messadi DV, Le AD, Increased vascular endothelial growth factor may account for elevated level of plasminogen activator inhibitor-1 via activating ERK1/2 in keloid fibroblasts, American journal of physiology, Cell physiology 286 (4) (2004) C905–C912. [DOI] [PubMed] [Google Scholar]
- [193].Zhang Q, Wu Y, Ann DK, Messadi DV, Tuan TL, Kelly AP, Bertolami CN, Le AD, Mechanisms of hypoxic regulation of plasminogen activator inhibitor-1 gene expression in keloid fibroblasts, J. Invest. Dermatol 121 (5) (2003) 1005–1012. [DOI] [PubMed] [Google Scholar]
- [194].Kierdorf U, Kierdorf H, Szuwart T, Deer antler regeneration: cells, concepts, and controversies, J. Morphol 268 (8) (2007) 726–738. [DOI] [PubMed] [Google Scholar]
- [195].Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M, Skin shedding and tissue regeneration in African spiny mice (Acomys), Nature 489 (7417) (2012) 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Williams-Boyce PK, Daniel JC Jr., Regeneration of rabbit ear tissue, J. Exp. Zool 212 (2) (1980) 243–253. [DOI] [PubMed] [Google Scholar]
- [197].Muneoka K, Allan CH, Yang X, Lee J, Han M, Mammalian regeneration and regenerative medicine, Birth defects research, Part C, Embryo today : reviews 84 (4) (2008) 265–280. [DOI] [PubMed] [Google Scholar]
- [198].Murphy E, Roths J, Autoimmunity, lymphoproliferation, Induction by mutant gene ppr, and acceleration by a male-associated factor in strain bxsb mice, in: Rose NR, et al. (Eds.), Genetic Control of Autoimmune, 2020, pp. 207–221. [Google Scholar]
- [199].Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S, Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis, Nature 356 (6367) (1992) 314–317. [DOI] [PubMed] [Google Scholar]
- [200].Chadwick RB, Bu L, Yu H, Hu Y, Wergedal JE, Mohan S, Baylink DJ, Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice, Wound repair and regeneration, official publication of the Wound Healing Society [and] the European Tissue Repair Society 15 (2) (2007) 275–284. [DOI] [PubMed] [Google Scholar]
- [201].Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB, Evidence for articular cartilage regeneration in MRL/MpJ mice, Osteoarthritis Cartilage 16 (11) (2008) 1319–1326. [DOI] [PubMed] [Google Scholar]
- [202].Rai MF, Hashimoto S, Johnson EE, Janiszak KL, Fitzgerald J, Heber-Katz E, Cheverud JM, Sandell LJ, Heritability of articular cartilage regeneration and its association with ear wound healing in mice, Arthritis Rheum. 64 (7) (2012) 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA, Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse, Arthritis Rheum. 58 (3) (2008) 744–753. [DOI] [PubMed] [Google Scholar]
- [204].Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang X-M, Zwas D, Lankford EB, Heber-Katz E, Heart regeneration in adult MRL mice, Proc. Natl. Acad. Sci. Unit. States Am 98 (17) (2001) 9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Buckley G, Metcalfe AD, Ferguson MWJ, Peripheral nerve regeneration in the MRL/MpJ ear wound model, J. Anat 218 (2) (2011) 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ueno M, Lyons BL, Burzenski LM, Gott B, Shaffer DJ, Roopenian DC, Shultz LD, Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature, Invest. Ophthalmol. Vis. Sci 46 (11) (2005) 4097–4106. [DOI] [PubMed] [Google Scholar]
- [207].Foster DS, Jones RE, Ransom RC, Longaker MT, Norton JA, The evolving relationship of wound healing and tumor stroma, JCI Insight 3 (18) (2018), e99911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Arnold KM, Opdenaker LM, Flynn D, Sims-Mourtada J, Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer, Canc. Growth Metastasis 8 (2015) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Van Blerkom J, Cox H, Davis P, Regulatory roles for mitochondria in the peri- implantation mouse blastocyst: possible origins and developmental significance of differential DeltaPsim, Reproduction 131 (5) (2006) 961–976. [DOI] [PubMed] [Google Scholar]
- [210].Jang Y-Y, Sharkis SJ, A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche, Blood 110 (8) (2007) 3056–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Nye HL, Cameron JA, Chernoff EA, Stocum DL, Extending the table of stages of normal development of the axolotl: limb development, Dev. Dynam. : an official publication of the American Association of Anatomists 226 (3) (2003) 555–560. [DOI] [PubMed] [Google Scholar]
- [212].Gourevitch D, Kossenkov AV, Zhang Y, Clark L, Chang C, Showe LC, Heber-Katz E, Inflammation and its correlates in regenerative wound healing: an alternate perspective, Adv. Wound Care 3 (9) (2014) 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Heber-Katz E, Gourevitch D, The relationship between inflammation and regeneration in the MRL mouse, Ann. N. Y. Acad. Sci 1172 (1) (2009) 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Gourevitch D, Clark L, Chen P, Seitz A, Samulewicz SJ, Heber-Katz E, Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model, Dev. Dynam. : an official publication of the American Association of Anatomists 226 (2) (2003) 377–387. [DOI] [PubMed] [Google Scholar]
- [215].Sun Y, Li H, Functional characterization of SAG/RBX2/ROC2/RNF7, an antioxidant protein and an E3 ubiquitin ligase, Protein Cell 4 (2) (2013) 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Romero MMG, McCathie G, Jankun P, Roehl HH, Damage-induced reactive oxygen species enable zebrafish tail regeneration by repositioning of Hedgehog expressing cells, Nat. Commun 9 (1) (2018) 4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vriz S, Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed, Sci. Rep 3 (2013) 2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Li W, Sahu D, Tsen F, Secreted heat shock protein-90 (Hsp90) in wound healing and cancer, Biochim. Biophys. Acta 1823 (3) (2012) 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Smart DR, Bennett MH, Mitchell SJ, Transcutaneous oximetry, problem wounds and hyperbaric oxygen therapy, DIVING AND HYPERBARIC MEDICINE- SOUTH PACIFIC UNDERWATER MEDICINE SOCIETY 36 (2) (2006) 72. [Google Scholar]
- [220].Thackham JA, McElwain DL, Long RJ, The use of hyperbaric oxygen therapy to treat chronic wounds: a review, Wound repair and regeneration, official publication of the Wound Healing Society [and] the European Tissue Repair Society 16 (3) (2008) 321–330. [DOI] [PubMed] [Google Scholar]
- [221].Gordillo GM, Roy S, Khanna S, Schlanger R, Khandelwal S, Phillips G, Sen CK, Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure OF clinically presented chronic wounds, Clin. Exp. Pharmacol. Physiol 35 (8) (2008) 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Kalliainen LK, Gordillo GM, Schlanger R, Sen CK, Topical oxygen as an adjunct to wound healing: a clinical case series, Pathophysiology 9 (2) (2003) 81–87. [DOI] [PubMed] [Google Scholar]
- [223].Yu J, Lu S, McLaren A-M, Perry JA, Cross KM, Topical oxygen therapy results in complete wound healing in diabetic foot ulcers, Wound Repair Regen. 24 (6) (2016) 1066–1072. [DOI] [PubMed] [Google Scholar]
- [224].Feldmeier JJ, Hopf HW, Warriner RA 3rd, Fife CE, Gesell LB, Bennett M, UHMS position statement: topical oxygen for chronic wounds, Undersea & hyperbaric medicine, journal of the Undersea and Hyperbaric Medical Society, Inc 32 (3) (2005) 157–168. [PubMed] [Google Scholar]
- [225].Gordillo GM, Sen CK, Revisiting the essential role of oxygen in wound healing, Am. J. Surg 186 (3) (2003) 259–263. [DOI] [PubMed] [Google Scholar]
- [226].Shah J, Hyperbaric oxygen therapy, J Am Col Certif Wound Spec 2 (1) (2010) 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Sunkari VG, Lind F, Botusan IR, Kashif A, Liu ZJ, Yla-Herttuala S, Brismar K, Velazquez O, Catrina SB, Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice, Wound repair and regeneration, official publication of the Wound Healing Society [and] the European Tissue Repair Society 23 (1) (2015) 98–103. [DOI] [PubMed] [Google Scholar]
- [228].Kang TS, Gorti GK, Quan SY, Ho M, Koch RJ, Effect of hyperbaric oxygen on the growth factor profile of fibroblasts, Arch. Facial Plast. Surg 6 (1) (2004) 31–35. [DOI] [PubMed] [Google Scholar]
- [229].Lin S, Shyu KG, Lee CC, Wang BW, Chang CC, Liu YC, Huang FY, Chang H, Hyperbaric oxygen selectively induces angiopoietin-2 in human umbilical vein endothelial cells, Biochem. Biophys. Res. Commun 296 (3) (2002) 710–715. [DOI] [PubMed] [Google Scholar]
- [230].Bonomo SR, Davidson JD, Yu Y, Xia Y, Lin X, Mustoe TA, Hyperbaric oxygen as a signal transducer: upregulation of platelet derived growth factor-beta receptor in the presence of HBO2 and PDGF, Undersea & hyperbaric medicine, journal of the Undersea and Hyperbaric Medical Society, Inc 25 (4) (1998) 211–216. [PubMed] [Google Scholar]
- [231].Nguyen TT, Jones JI, Wolter WR, Perez RL, Schroeder VA, Champion MM, Hesek D, Lee M, Suckow MA, Mobashery S, Chang M, Hyperbaric Oxygen Therapy Accelerates Wound Healing in Diabetic Mice by Decreasing Active Matrix Metalloproteinase-9, Wound Repair and Regeneration : Official Publication of the Wound Healing Society [and] the European Tissue Repair Society, 2019. [DOI] [PubMed] [Google Scholar]
- [232].Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, Michiels C, Hypoxia-induced activation of HIF-1: role of HIF-1α-Hsp90 interaction, FEBS (Fed. Eur. Biochem. Soc.) Lett 460 (2) (1999) 251–256. [DOI] [PubMed] [Google Scholar]
- [233].Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM, Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway, J. Biol. Chem 277 (33) (2002) 29936–29944. [DOI] [PubMed] [Google Scholar]
- [234].Ni XX, Ni M, Fan DF, Sun Q, Kang ZM, Cai ZY, Liu Y, Liu K, Li RP, Xu WG, Heat-shock protein 70 is involved in hyperbaric oxygen preconditioning on decompression sickness in rats, Experimental biology and medicine, (Maywood, N.J.) 238 (1) (2013) 12–22. [DOI] [PubMed] [Google Scholar]
- [235].Cabigas BP, Su J, Hutchins W, Shi Y, Schaefer RB, Recinos RF, Nilakantan V, Kindwall E, Niezgoda JA, Baker JE, Hyperoxic and hyperbaric- induced cardioprotection: role of nitric oxide synthase 3, Cardiovasc. Res 72 (1) (2006) 143–151. [DOI] [PubMed] [Google Scholar]
- [236].Huang G, Xu J, Xu L, Wang S, Li R, Liu K, Zheng J, Cai Z, Zhang K, Luo Y, Xu W, Hyperbaric oxygen preconditioning induces tolerance against oxidative injury and oxygen-glucose deprivation by up-regulating heat shock protein 32 in rat spinal neurons, PloS One 9 (1) (2014), e85967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Sureda A, Batle JM, Martorell M, Capó X, Tejada S, Tur JA, Pons A, Antioxidant response of chronic wounds to hyperbaric oxygen therapy, PloS One 11 (9) (2016), e0163371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Masson N, Singleton RS, Sekirnik R, Trudgian DC, Ambrose LJ, Miranda MX, Tian Y-M, Kessler BM, Schofield CJ, Ratcliffe PJ, The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity, EMBO Rep. 13 (3) (2012) 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC, Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation, Cell Metabol. 1 (6) (2005) 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT, Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing, Cell Metabol. 1 (6) (2005) 401–408. [DOI] [PubMed] [Google Scholar]
- [241].Wenger RH, Mitochondria: oxygen sinks rather than sensors? Med. Hypotheses 66 (2) (2006) 380–383. [DOI] [PubMed] [Google Scholar]
- [242].Chua YL, Dufour E, Dassa EP, Rustin P, Jacobs HT, Taylor CT, Hagen T, Stabilization of hypoxia-inducible factor-1alpha protein in hypoxia occurs independently of mitochondrial reactive oxygen species production, J. Biol. Chem 285 (41) (2010) 31277–31284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Doege K, Heine S, Jensen I, Jelkmann W, Metzen E, Inhibition of mitochondrial respiration elevates oxygen concentration but leaves regulation of hypoxia-inducible factor (HIF) intact, Blood 106 (7) (2005) 2311–2317. [DOI] [PubMed] [Google Scholar]
- [244].Hagen T, Taylor CT, Lam F, Moncada S, Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α, Science 302 (5652) (2003) 1975–1978. [DOI] [PubMed] [Google Scholar]
- [245].Lipsky BA, Berendt AR, Hyperbaric oxygen therapy for diabetic foot wounds: has hope hurdled hype? Diabetes Care 33 (5) (2010) 1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Londahl M, Katzman P, Hammarlund C, Nilsson A, Landin-Olsson M, Relationship between ulcer healing after hyperbaric oxygen therapy and transcutaneous oximetry, toe blood pressure and ankle-brachial index in patients with diabetes and chronic foot ulcers, Diabetologia 54 (1) (2011) 65–68. [DOI] [PubMed] [Google Scholar]
- [247].Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, Masson EA, McCollum PT, The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial, Eur. J Vasc. Endovasc. Surg. : the official journal of the European Society for VascularSurgery 25 (6) (2003) 513–518. [DOI] [PubMed] [Google Scholar]
- [248].Kessler L, Bilbault P, Ortega F, Grasso C, Passemard R, Stephan D, Pinget M, Schneider F, Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study, Diabetes Care 26 (8) (2003) 2378–2382. [DOI] [PubMed] [Google Scholar]
- [249].Londahl M, Landin-Olsson M, Katzman P, Hyperbaric oxygen therapy improves health-related quality of life in patients with diabetes and chronic foot ulcer, Diabetic medicine, a journal of the British Diabetic Association 28 (2) (2011) 186–190. [DOI] [PubMed] [Google Scholar]
- [250].Londahl M, Katzman P, Nilsson A, Hammarlund C, Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes, Diabetes Care 33 (5) (2010) 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Faglia E, Favales F, Aldeghi A, Calia P, Quarantiello A, Oriani G, Michael M, Campagnoli P, Morabito A, Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study, Diabetes Care 19 (12) (1996) 1338–1343. [DOI] [PubMed] [Google Scholar]
- [252].Duzgun AP, Satir HZ, Ozozan O, Saylam B, Kulah B, Coskun F, Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers, J. Foot Ankle Surg. : official publication of the American College of Foot and Ankle Surgeons 47 (6) (2008) 515–519. [DOI] [PubMed] [Google Scholar]
- [253].Doctor N, Pandya S, Supe A, Hyperbaric oxygen therapy in diabetic foot, J. Postgrad. Med 38 (3) (1992) 112–114, 111. [PubMed] [Google Scholar]
- [254].Londahl M, Boulton AJM, Hyperbaric Oxygen Therapy: Useless or Useful? A Battle, Diabetes/metabolism research and reviews, 2020, e3233. [DOI] [PubMed] [Google Scholar]
- [255].Chuck AW, Hailey D, Jacobs P, Perry DC, Cost-effectiveness and budget impact of adjunctive hyperbaric oxygen therapy for diabetic foot ulcers, Int. J. Technol. Assess. Health Care 24 (2) (2008) 178–183. [DOI] [PubMed] [Google Scholar]
- [256].Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S, Hyperbaric oxygen therapy for chronic wounds, Cochrane Database Syst. Rev 6 (2015), Cd004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Fedorko L, Bowen JM, Jones W, Oreopoulos G, Goeree R, Hopkins RB, O’Reilly DJ, Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind, randomized controlled clinical trial, Diabetes Care 39 (3) (2016) 392–399. [DOI] [PubMed] [Google Scholar]
- [258].Li G, Hopkins RB, Levine MAH, Jin X, Bowen JM, Thabane L, Goeree R, Fedorko L, O’Reilly DJ, Relationship between hyperbaric oxygen therapy and quality of life in participants with chronic diabetic foot ulcers: data from a randomized controlled trial, Acta Diabetol. 54 (9) (2017) 823–831. [DOI] [PubMed] [Google Scholar]
- [259].Wang C-J, Re-Wen W, Yang Y-J, Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy, Diabetes Res. Clin. Pract 92 (2011) 187–193. [DOI] [PubMed] [Google Scholar]
- [260].Margolis DJ, Gupta J, Hoffstad O, Papdopoulos M, Glick HA, Thom SR, Mitra N, Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study, Diabetes Care 36 (7) (2013) 1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Santema KTB, Stoekenbroek RM, Koelemay MJW, Reekers JA, van Dortmont LMC, Oomen A, Smeets L, Wever JJ, Legemate DA, Ubbink DT, Hyperbaric oxygen therapy in the treatment of ischemic lower- extremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial, Diabetes Care 41 (1) (2018) 112–119. [DOI] [PubMed] [Google Scholar]
- [262].Cho Y, Shin JE, Ewan EE, Oh YM, Pita-Thomas W, Cavalli V, Activating injury-responsive genes with hypoxia enhances axon regeneration through neuronal HIF-1alpha, Neuron 88 (4) (2015) 720–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS, Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury, J. Neurosci. : the official journal of the Society for Neuroscience 32 (11) (2012) 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ, Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury, Neurorehabilitation Neural Repair 26 (2) (2012) 163–172. [DOI] [PubMed] [Google Scholar]
- [265].Wood MD, Mackinnon SE, Pathways regulating modality-specific axonal regeneration in peripheral nerve, Exp. Neurol 265 (2015) 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Blackmore MG, Chapter three - molecular control of axon growth: insights from comparative gene profiling and high-throughput screening, in: Goldberg JL, Trakhtenberg EF (Eds.), International Review of Neurobiology, Academic Press; 2012, pp. 39–70. [DOI] [PubMed] [Google Scholar]
- [267].Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia, Nat. Neurosci 7 (1) (2004) 48–55. [DOI] [PubMed] [Google Scholar]
- [268].McGregor CE, English AW, The role of BDNF in peripheral nerve regeneration: activity-dependent treatments and Val66Met, Front. Cell. Neurosci 12 (2019), 522–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Difato F, Tsushima H, Pesce M, Benfenati F, Blau A, Chieregatti E, The formation of actin waves during regeneration after axonal lesion is enhanced by BDNF, Sci. Rep 1 (1) (2011) 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Gao Y, Nikulina E, Mellado W, Filbin MT, Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase, J. Neurosci 23 (37) (2003) 11770–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V, Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves, J. Biol. Chem 285 (36) (2010) 28034–28043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial, Neurology 82 (2) (2014) 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH, Long-term facilitation of ventilation in humans with chronic spinal cord injury, Am. J. Respir. Crit. Care Med 189 (1) (2014) 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Jaiswal PB, Tester NJ, Davenport PW, Effect of acute intermittent hypoxia treatment on ventilatory load compensation and magnitude estimation of inspiratory resistive loads in an individual with chronic incomplete cervical spinal cord injury, J Spinal Cord Med 39 (1) (2016) 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Liu T, Zou W, Shi G, Xu J, Zhang F, Xiao J, Wang Y, Hypoxia-induced MTA1 promotes MC3T3 osteoblast growth but suppresses MC3T3 osteoblast differentiation, Eur. J. Med. Res 20 (1) (2015) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Maes C, Carmeliet G, Schipani E, Hypoxia-driven pathways in bone development, regeneration and disease, Nat. Rev. Rheumatol 8 (6) (2012) 358–366. [DOI] [PubMed] [Google Scholar]
- [277].Knowles HJ, Hypoxic regulation of osteoclast differentiation and bone resorption activity, Hypoxia 3 (2015) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Drager J, Harvey EJ, Barralet J, Hypoxia signalling manipulation for bone regeneration, Expet Rev. Mol. Med 17 (2015) e6. [DOI] [PubMed] [Google Scholar]
- [279].Yu X, Wan Q, Ye X, Cheng Y, Pathak JL, Li Z, Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling, Cell. Mol. Biol. Lett 24 (1) (2019) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR, Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation, J. Clin. Invest 122 (9) (2012) 3101–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC, Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1, Nat. Med 10 (8) (2004) 858–864. [DOI] [PubMed] [Google Scholar]
- [282].Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S, Hypoxia is a major stimulator of osteoclast formation and bone resorption, J. Cell. Physiol 196 (1) (2003) 2–8. [DOI] [PubMed] [Google Scholar]
- [283].Knowles HJ, Athanasou NA, Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis, J. Pathol 218 (2) (2009) 256–264. [DOI] [PubMed] [Google Scholar]
- [284].Guner I, Uzun DD, Yaman MO, Genc H, Gelisgen R, Korkmaz GG, Hallac M, Yelmen N, Sahin G, Karter Y, Simsek G, The effect of chronic long-term intermittent hypobaric hypoxia on bone mineral density in rats: role of nitric oxide, Biol. Trace Elem. Res 154 (2) (2013) 262–267. [DOI] [PubMed] [Google Scholar]
- [285].Sforza E, Thomas T, Barthélémy J-C, Collet P, Roche F, Obstructive sleep apnea is associated with preserved bone mineral density in healthy elderly subjects, Sleep 36 (10) (2013) 1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Wu J, Stefaniak J, Hafner C, Schramel JP, Kaun C, Wojta J, Ullrich R, Tretter VE, Markstaller K, Klein KU, Intermittent hypoxia causes inflammation and injury to human adult cardiac myocytes, Anesth. Analg 122 (2) (2016) 373–380. [DOI] [PubMed] [Google Scholar]
- [287].Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR, Transcriptional responses to intermittent hypoxia, Respir. Physiol. Neurobiol 164 (1–2) (2008) 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Lavie L, Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach, Neurol. Clin 23 (4) (2005) 1059–1075. [DOI] [PubMed] [Google Scholar]
- [289].Navarrete-Opazo A, Mitchell GS, Therapeutic potential of intermittent hypoxia: a matter of dose, Am. J. Physiol. Regul. Integr. Comp. Physiol 307 (10) (2014) R1181–R1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA, Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model, J. Clin. Invest 99 (1) (1997) 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Fletcher EC, Lesske J, Culman J, Miller CC, Unger T, Sympathetic denervation blocks blood pressure elevation in episodic hypoxia, Hypertension (Dallas, Tex. : 1979) 20 (5) (1992) 612–619. [DOI] [PubMed] [Google Scholar]
- [292].Xu XM, Yao D, Cai XD, Ding C, Lin QD, Wang LX, Huang XY, Effect of chronic continual- and intermittent hypoxia-induced systemic inflammation on the cardiovascular system in rats, Sleep & breathing = Schlaf & Atmung 19 (2) (2015) 677–684. [DOI] [PubMed] [Google Scholar]
- [293].Oyarce MP, Iturriaga R, Contribution of oxidative stress and inflammation to the neurogenic hypertension induced by intermittent hypoxia, Front. Physiol 9 (893) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D, Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep, Behav. Brain Res 177 (2) (2007) 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Champod AS, Eskes GA, Foster GE, Hanly PJ, Pialoux V, Beaudin AE, Poulin MJ, Effects of acute intermittent hypoxia on working memory in young healthy adults, Am. J. Respir. Crit. Care Med 187 (10) (2013) 1148–1150. [DOI] [PubMed] [Google Scholar]
- [296].Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP, Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus, Am. J. Physiol. Regul. Integr. Comp. Physiol 292 (3) (2007) R1254–R1259. [DOI] [PubMed] [Google Scholar]
- [297].Flashman E, Hoffart LM, Hamed RB, Bollinger JM Jr., Krebs C, Schofield CJ, Evidence for the slow reaction of hypoxia-inducible factor prolyl hydroxylase 2 with oxygen, FEBS J. 277 (19) (2010) 4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Wilkins SE, Flashman E, Scotti JS, Hopkinson RJ, Chowdhury R, Schofield CJ, CHAPTER 6 the Role of 2-oxoglutarate-dependent Oxygenases in Hypoxia Sensing, 2-oxoglutarate-dependent Oxygenases, The Royal Society of Chemistry 2015, pp. 169–209. [Google Scholar]
- [299].Yeh T-L, Leissing Thomas M., Abboud MI, Thinnes CC, Atasoylu O, Holt- Martyn JP, Zhang D, Tumber A, Lippl K, Lohans CT, Leung IKH, Morcrette H, Clifton IJ, Claridge TDW, Kawamura A, Flashman E, Lu X, Ratcliffe PJ, Chowdhury R, Pugh CW, Schofield CJ, Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials, Chem. Sci 8 (11) (2017) 7651–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Chan MC, Atasoylu O, Hodson E, Tumber A, Leung IKH, Chowdhury R, Gómez-Pérez V, Demetriades M, Rydzik AM, Holt-Martyn J, Tian Y-M, Bishop T, Claridge TDW, Kawamura A, Pugh CW, Ratcliffe PJ, Schofield CJ, Potent and selective triazole-based inhibitors of the hypoxia- inducible factor prolyl-hydroxylases with activity in the murine brain, PloS One 10 (7) (2015) e0132004-e0132004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Jensen J, Madsen J, Jensen L, Pedersen EB, Reduced production, absorption, and elimination of erythropoietin in uremia compared with healthy volunteers, J. Am. Soc. Nephrol 5 (2) (1994) 177–185. [DOI] [PubMed] [Google Scholar]
- [302].Winearls C, Pippard M, Downing M, Oliver D, Reid C, Cotes PM, Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis, Lancet 328 (8517) (1986) 1175–1178. [DOI] [PubMed] [Google Scholar]
- [303].Cody JD, Hodson EM, Recombinant human erythropoietin versus placebo or no treatment for the anaemia of chronic kidney disease in people not requiring dialysis, Cochrane Database Syst. Rev 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Singh A, Szczech L, Tang K, Barnhart H, Sapp S, Wolfson M, Reddan D, Correction of anemia with epoetin alfa in chronic kidney disease, N. Engl. J. Med 355 (2006) 2085–2098. [DOI] [PubMed] [Google Scholar]
- [305].Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK, Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes, Kidney Int. 74 (6) (2008) 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, Jiang G, Lin H, Zhang X, Zuo L, He Q, Fu P, Li X, Ni D, Hemmerich S, Liu C, Szczech L, Besarab A, Neff TB, Peony Yu K-H, Valone FH, Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China, Nephrol. Dial. Transplant 32 (8) (2017) 1373–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Liu Z, Cai GY, Hao L, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP, Roxadustat treatment for anemia in patients undergoing long-term dialysis, N. Engl. J. Med 381 (11) (2019) 1011–1022. [DOI] [PubMed] [Google Scholar]
- [308].Sanghani NS, Haase VH, Hypoxia-inducible factor Activators in renal anemia: current clinical experience, Adv. Chron. Kidney Dis 26 (4) (2019) 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Beck H, Jeske M, Thede K, Stoll F, Flamme I, Akbaba M, Ergüden J-K, Karig G, Keldenich J, Oehme F, Militzer H-C, Hartung I, Thuss U, Discovery of Molidustat (BAY 85–3934): a small-molecule oral HIF-prolyl hydroxylase (HIF- PH) inhibitor for the treatment of renal anemia, ChemMedChem 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Flamme I, Oehme F, Ellinghaus P, Jeske M, Keldenich J, Thuss U, Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85–3934 (Molidustat) stimulates erythropoietin production without hypertensive effects, PloS One 9 (11) (2014) e111838-e111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Yamamoto H, Taguchi M, Matsuda Y, Iekushi K, Yamada T, Akizawa T, Molidustat for the treatment of renal anaemia in patients with non-dialysis- dependent chronic kidney disease: design and rationale of two phase III studies, BMJ Open 9 (6) (2019), e026704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Akizawa T, Taguchi M, Matsuda Y, Iekushi K, Yamada T, Yamamoto H, Molidustat for the treatment of renal anaemia in patients with dialysis-dependent chronic kidney disease: design and rationale of three phase III studies, BMJ Open 9 (6) (2019), e026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Akizawa T, Macdougall IC, Berns JS, Bernhardt T, Staedtler G, Taguchi M, Iekushi K, Krueger T, Long-term efficacy and safety of Molidustat for anemia in chronic kidney disease: DIALOGUE extension studies, Am. J. Nephrol 49 (4) (2019) 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ, Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents, Am. J. Physiol. Cell Physiol 268 (6) (1995) C1362–C1368. [DOI] [PubMed] [Google Scholar]
- [315].Wang GL, Semenza GL, Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction, Blood 82 (12) (1993) 3610–3615. [PubMed] [Google Scholar]
- [316].Chen C-H, Shu K-H, Yang Y, Long-term effects of an oral iron chelator, deferasirox, in hemodialysis patients with iron overload, Hematology 20 (5) (2015) 304–310. [DOI] [PubMed] [Google Scholar]
- [317].Sekirnik R, Rose NR, Mecinović J, Schofield CJ, 2-Oxoglutarate oxygenases are inhibited by a range of transition metals, Metall 2 (6) (2010) 397–399. [DOI] [PubMed] [Google Scholar]
- [318].Yuan Y, Hilliard G, Ferguson T, Millhorn DE, Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha, J. Biol. Chem 278 (18) (2003) 15911–15916. [DOI] [PubMed] [Google Scholar]
- [319].Berk L, Burchenal JH, Castle WB, Erythropoietic effect of cobalt in patients with or without anemia, N. Engl. J. Med 240 (19) (1949) 754–761. [DOI] [PubMed] [Google Scholar]
- [320].Wolf J, Levy IJ, Treatment OF sickle-cell anemia with cobalt chloride, A.M.A. Archives of Internal Medicine 93 (3) (1954) 387–396. [DOI] [PubMed] [Google Scholar]
- [321].Milosevic J, Adler I, Manaenko A, Schwarz SC, Walkinshaw G, Arend M, Flippin LA, Storch A, Schwarz J, Non-hypoxic stabilization of hypoxia-inducible factor alpha (HIF-alpha): relevance in neural progenitor/stem cells, Neurotox. Res 15 (4) (2009) 367–380. [DOI] [PubMed] [Google Scholar]
- [322].Kim B-S, Yoon K-H, Oh H-M, Choi E-Y, Kim S-W, Han W-C, Kim E-A, Choi S-C, Kim T-H, Yun K-J, Kim E-C, Lyou J-H, Nah Y-H, Chung H-T, Cha YN, Jun C-D, Involvement of p38 MAP kinase during iron chelator-mediated apoptotic cell death, Cell. Immunol 220 (2) (2002) 96–106. [DOI] [PubMed] [Google Scholar]
- [323].Pacary E, Legros H, Valable S, Duchatelle P, Lecocq M, Petit E, Nicole O, Bernaudin M, Synergistic effects of CoCl(2) and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells, J. Cell Sci 119 (Pt 13) (2006) 2667–2678. [DOI] [PubMed] [Google Scholar]
- [324].Banerji B, Conejo-Garcia A, McNeill LA, McDonough MA, Buck MR, Hewitson KS, Oldham NJ, Schofield CJ, The inhibition of factor inhibiting hypoxia-inducible factor (FIH) by beta-oxocarboxylic acids, Chem. Commun (43) (2005) 5438–5440. [DOI] [PubMed] [Google Scholar]
- [325].Strehin I, Gourevitch D, Zhang Y, Heber-Katz E, Messersmith PB, Hydrogels formed by oxo-ester mediated native chemical ligation, Biomaterials science 1 (6) (2013) 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Nagai K, Ideguchi H, Kajikawa T, Li X, Chavakis T, Cheng J, Messersmith PB, Heber-Katz E, Hajishengallis G, An injectable hydrogel-formulated inhibitor of prolyl-4-hydroxylase promotes T regulatory cell recruitment and enhances alveolar bone regeneration during resolution of experimental periodontitis, Faseb. J 34 (10) (2020) 13726–13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Cheng J, Amin D, Latona J, Heber-Katz E, Messersmith PB, Supramolecular polymer hydrogels for drug-induced tissue regeneration, ACS Nano 13 (5) (2019) 5493–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Chen Y, Zhao Q, Yang X, Yu X, Yu D, Zhao W, Effects of cobalt chloride on the stem cell marker expression and osteogenic differentiation of stem cells from human exfoliated deciduous teeth, Cell Stress Chaperones 24 (3) (2019) 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Sinclair J, Hoying D, Bresciani E, Dalle Nogare D, Needle C, Wu W, Bishop K, Elkahloun A, Chitnis A, Liu P, Burgess S, A metabolic shift to glycolysis promotes zebrafish tail regeneration through TGF-β dependent dedifferentiation of notochord cells to form the blastema, SSRN Electronic Journal (2020). [Google Scholar]
- [330].Osuma EA, Riggs DW, Gibb AA, Hill BG, High throughput measurement of metabolism in planarians reveals activation of glycolysis during regeneration, Regeneration (Oxf) 5 (1) (2018) 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Hoppe G, Yoon S, Gopalan B, Savage AR, Brown R, Case K, Vasanji A, Chan ER, Silver RB, Sears JE, Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity, Proc. Natl. Acad. Sci. U. S. A 113 (18) (2016) E2516–E2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS, HIF-1alpha is essential for myeloid cell-mediated inflammation, Cell 112 (5) (2003) 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Lin N, Simon MC, Hypoxia-inducible factors: key regulators of myeloid cells during inflammation, J. Clin. Invest 126 (10) (2016) 3661–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Tang D, Zhang J, Yan T, Wei J, Jiang X, Zhang D, Zhang Q, Jia J, Huang Y, FG-4592 accelerates cutaneous wound healing by epidermal stem cell activation via HIF-1alpha stabilization, Cell. Physiol. Biochem. : international journal of experimental cellular physiology, biochemistry, and pharmacology 46 (6) (2018) 2460–2470. [DOI] [PubMed] [Google Scholar]
- [335].Lee HJ, Ryu JM, Jung YH, Oh SY, Lee SJ, Han HJ, Novel pathway for hypoxia-induced proliferation and migration in human mesenchymal stem cells: involvement of HIF-1alpha, FASN, and mTORC1, Stem cells (Dayton, Ohio) 33 (7) (2015) 2182–2195. [DOI] [PubMed] [Google Scholar]
- [336].Ogle ME, Gu X, Espinera AR, Wei L, Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1α, Neurobiol. Dis 45 (2) (2012) 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Wu K, Zhou K, Wang Y, Zhou Y, Tian N, Wu Y, Chen D, Zhang D, Wang X, Xu H, Zhang X, Stabilization of HIF-1alpha by FG-4592 promotes functional recovery and neural protection in experimental spinal cord injury, Brain Res. 1632 (2016) 19–26. [DOI] [PubMed] [Google Scholar]
- [338].Li Y, Han W, Wu Y, Zhou K, Zheng Z, Wang H, Xie L, Li R, Xu K, Liu Y, Wang X, Xiao J, Stabilization of hypoxia inducible factor-1alpha by dimethyloxalylglycine promotes recovery from acute spinal cord injury by inhibiting neural apoptosis and enhancing axon regeneration, J. Neurotrauma 36 (24) (2019) 3394–3409. [DOI] [PubMed] [Google Scholar]
- [339].Karuppagounder SS, Alim I, Khim SJ, Bourassa MW, Sleiman SF, John R, Thinnes CC, Yeh TL, Demetriades M, Neitemeier S, Cruz D, Gazaryan I, Killilea DW, Morgenstern L, Xi G, Keep RF, Schallert T, Tappero RV, Zhong J, Cho S, Maxfield FR, Holman TR, Culmsee C, Fong GH, Su Y, Ming GL, Song H, Cave JW, Schofield CJ, Colbourne F, Coppola G, Ratan RR, Therapeutic targeting of oxygen-sensing prolyl hydroxylases abrogates ATF4- dependent neuronal death and improves outcomes after brain hemorrhage in several rodent models, Sci. Transl. Med 8 (328) (2016), 328ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Siddiq A, Aminova LR, Troy CM, Suh K, Messer Z, Semenza GL, Ratan RR, Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB- independent pathways, J. Neurosci 29 (27) (2009) 8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Zhou M, Hou J, Li Y, Mou S, Wang Z, Horch RE, Sun J, Yuan Q, The pro-angiogenic role of hypoxia inducible factor stabilizer FG-4592 and its application in an in vivo tissue engineering chamber model, Sci. Rep 9 (1) (2019) 6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Cunliffe CJ, Franklin TJ, Hales NJ, Hill GB, Novel inhibitors of prolyl 4-hydroxylase. 3. Inhibition by the substrate analog N-oxaloglycine and its derivatives, J. Med. Chem 35 (14) (1992) 2652–2658. [DOI] [PubMed] [Google Scholar]
- [343].Baader E, Tschank G, Baringhaus KH, Burghard H, Günzler V, Inhibition of prolyl 4-hydroxylase by oxalyl amino acid derivatives in vitro, in isolated microsomes and in embryonic chicken tissues, Biochem. J 300 (2) (1994) 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Yuan Q, Bleiziffer O, Boos AM, Sun J, Brandl A, Beier JP, Arkudas A, Schmitz M, Kneser U, Horch RE, PHDs inhibitor DMOG promotes the vascularization process in the AV loop by HIF-1a up-regulation and the preliminary discussion on its kinetics in rat, BMC Biotechnol. 14 (1) (2014) 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Weigand A, Horch RE, Boos AM, Beier JP, Arkudas A, The arteriovenous loop: engineering of axially vascularized tissue, European surgical research. Europaische chirurgische forschung, Recherches chirurgicales europeennes 59 (3–4) (2018) 286–299. [DOI] [PubMed] [Google Scholar]
- [346].Weigand A, Beier JP, Arkudas A, Al-Abboodi M, Polykandriotis E, Horch RE, Boos AM, The arteriovenous (AV) loop in a small animal model to study angiogenesis and vascularized tissue engineering, JoVE : JoVE 117 (2016) 54676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Shen X, Wan C, Ramaswamy G, Mavalli M, Wang Y, Duvall CL, Deng LF, Guldberg RE, Eberhart A, Clemens TL, Gilbert SR, Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice, J. Orthop. Res 27 (10) (2009) 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Agis H, Hueber L, Pour Sadeghian N, Pensch M, Gruber R, In vitro release of dimethyloxaloylglycine and l-mimosine from bovine bone mineral, Arch. Oral Biol 59 (10) (2014) 1024–1031. [DOI] [PubMed] [Google Scholar]
- [349].Vinzenz P, Schröckmair S, Gruber R, Agis H, Bone substitute materials supplemented with prolyl hydroxylase inhibitors decrease osteoclastogenesis in vitro, J. Biomed. Mater. Res. B Appl. Biomater 103 (6) (2015) 1198–1203. [DOI] [PubMed] [Google Scholar]
- [350].Lalzawmliana V, Anand A, Roy M, Kundu B, Nandi SK, Mesoporous bioactive glasses for bone healing and biomolecules delivery, Mater. Sci. Eng. C 106 (2020) 110180. [DOI] [PubMed] [Google Scholar]
- [351].Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR, Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering, Biomaterials 27 (18) (2006) 3413–3431. [DOI] [PubMed] [Google Scholar]
- [352].Wu C, Zhou Y, Chang J, Xiao Y, Delivery of dimethyloxallyl glycine in mesoporous bioactive glass scaffolds to improve angiogenesis and osteogenesis of human bone marrow stromal cells, Acta Biomater. 9 (11) (2013) 9159–9168. [DOI] [PubMed] [Google Scholar]
- [353].Ding H, Chen S, Song W-Q, Gao Y-S, Guan J-J, Wang Y, Sun Y, Zhang C-Q, Dimethyloxaloylglycine improves angiogenic activity of bone marrow stromal cells in the tissue-engineered bone, Int. J. Biol. Sci 10 (7) (2014) 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Zhu S, Segura T, Cell-demanded VEGF release via nanocapsules elicits different receptor activation dynamics and enhanced angiogenesis, Ann. Biomed. Eng 44 (6) (2016) 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin. Immunol. 20 (2) (2008) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Favier FB, Britto FA, Ponçon B, Begue G, Chabi B, Reboul C, Meyer G, Py G, Endurance training prevents negative effects of the hypoxia mimetic dimethyloxalylglycine on cardiac and skeletal muscle function, J. Appl. Physiol 120 (4) (2015) 455–463. [DOI] [PubMed] [Google Scholar]
- [357].Esfahani M, Karimi F, Afshar S, niknazar S, Sohrabi S, Najafi R, Prolyl hydroxylase inhibitors act as agents to enhance the efficiency of cell therapy, Expet Opin. Biol. Ther 15 (12) (2015) 1739–1755. [DOI] [PubMed] [Google Scholar]
- [358].Tan SC, Gomes RSM, Yeoh KK, Perbellini F, Malandraki-Miller S, Ambrose L, Heather LC, Faggian G, Schofield CJ, Davies KE, Clarke K, Carr CA, Preconditioning of cardiosphere-derived cells with hypoxia or prolyl-4- hydroxylase inhibitors increases stemness and decreases reliance on oxidative metabolism, Cell Transplant. 25 (1) (2016) 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Liu X-B, Wang J-A, Ji X-Y, Yu SP, Wei L, Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats, Stem Cell Res. Ther 5 (5) (2014), 111–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Deveza L, Choi J, Lee J, Huang N, Cooke J, Yang F, Polymer-DNA nanoparticle-induced CXCR4 overexpression improves stem cell engraftment and tissue regeneration in a mouse hindlimb ischemia model, Theranostics 6 (8) (2016) 1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Wu L-Y, He Y-L, Zhu L-L, Possible role of PHD inhibitors as hypoxia- mimicking agents in the maintenance of neural stem cells’ self-renewal properties, Front Cell Dev Biol 6 (2018), 169–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Chu PWY, Beart PM, Jones NM, Preconditioning protects against oxidative injury involving hypoxia-inducible factor-1 and vascular endothelial growth factor in cultured astrocytes, Eur. J. Pharmacol 633 (1) (2010) 24–32. [DOI] [PubMed] [Google Scholar]
- [363].Chu K, Jung K-H, Kim S-J, Lee S-T, Kim J, Park H-K, Song E-C, Kim SU, Kim M, Lee SK, Roh J-K, Transplantation of human neural stem cells protect against ischemia in a preventive mode via hypoxia-inducible factor-1α stabilization in the host brain, Brain Res. 1207 (2008) 182–192. [DOI] [PubMed] [Google Scholar]
- [364].Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T, Stromal cell- derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization, Circulation 107 (9) (2003) 1322–1328. [DOI] [PubMed] [Google Scholar]
- [365].Najafi R, Sharifi AM, Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats, Expet Opin. Biol. Ther 13 (7) (2013) 959–972. [DOI] [PubMed] [Google Scholar]
- [366].Lanigan S, Corcoran AE, Wall A, Mukandala G, O’Connor JJ, Acute hypoxic exposure and prolyl-hydroxylase inhibition improves synaptic transmission recovery time from a subsequent hypoxic insult in rat hippocampus, Brain Res. 1701 (2018) 212–218. [DOI] [PubMed] [Google Scholar]
- [367].Rey S, Lee K, Wang CJ, Gupta K, Chen S, McMillan A, Bhise N, Levchenko A, Semenza GL, Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia, Proc. Natl. Acad. Sci. U. S. A 106 (48) (2009) 20399–20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Patel ZS, Mikos AG, Angiogenesis with biomaterial-based drug- and cell-delivery systems, Journal of Biomaterials Science, Polymer Edition 15 (6) (2004) 701–726. [DOI] [PubMed] [Google Scholar]
- [369].Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, in: Science G. (Ed.), Molecular Biology of the Cell, 2002. New York. [Google Scholar]
- [370].Singh A, Wilson JW, Schofield CJ, Chen R, Hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors induce autophagy and have a protective effect in an in-vitro ischaemia model, Sci. Rep 10 (1) (2020) 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].An G, Guo F, Liu X, Wang Z, Zhu Y, Fan Y, Xuan C, Li Y, Wu H, Shi X, Mao C, Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1α-expressing stem cells, Nat. Commun 11 (1) (2020) 2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Three-dimensional bioprinting of thick vascularized tissues, Proc. Natl. Acad. Sci. Unit. States Am 113 (12) (2016) 3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Han X, Courseaus J, Khamassi J, Nottrodt N, Engelhardt S, Jacobsen F, Bierwisch C, Meyer W, Walter T, Weisser J, Jaeger R, Bibb R, Harris R, Optimized vascular network by stereolithography for tissue engineered skin, Int J Bioprint 4 (2) (2018), 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Sarker MD, Naghieh S, Sharma NK, Chen X, 3D biofabrication of vascular networks for tissue regeneration: a report on recent advances, Journal of Pharmaceutical Analysis 8 (5) (2018) 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Hann SY, Cui H, Esworthy T, Miao S, Zhou X, Lee S.-j., Fisher JP, Zhang LG, Recent advances in 3D printing: vascular network for tissue and organ regeneration, Transl. Res 211 (2019) 46–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, Lewis JA, Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels, Science Advances 5 (9) (2019), eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]