Abstract
Patient‐reported outcomes (PROs) are important endpoints for clinical trials. The impact of investigational drugs on PROs of patients with advanced nonalcoholic steatohepatitis (NASH) was investigated. Patients with NASH with bridging fibrosis or compensated cirrhosis were enrolled in a phase 2, randomized, placebo‐controlled study of selonsertib, firsocostat, or cilofexor, alone or in two‐drug combinations (NCT03449446). PROs included Short Form 36 (SF‐36), Chronic Liver Disease Questionnaire (CLDQ)‐NASH, EuroQol Five Dimension (EQ‐5D), Work Productivity and Impairment (WPAI), and 5‐D Itch before and during treatment. A total of 392 patients with NASH (mean ± SD, 60 ± 9 years old; 35% men; 89% white; 72% diabetes; and 56% compensated cirrhosis) were included. Baseline Physical Functioning (PF) and Bodily Pain of SF‐36 and Fatigue and Worry of CLDQ‐NASH were significantly lower in patients with cirrhosis (total CLDQ‐NASH score mean ± SD, 4.91 ± 1.06 with cirrhosis vs. 5.16 ± 1.14 without cirrhosis; P < 0.05). Lower baseline PRO scores were independently associated with age, female sex, greater body mass index, diabetes, clinically overt fatigue, and comorbidities (all P < 0.05). After 48 weeks of treatment, patients with ≥1‐stage fibrosis improvement without worsening of NASH experienced improvement in EQ‐5D and five out of six CLDQ‐NASH domains (P < 0.05). Patients with ≥2‐point decrease in their nonalcoholic fatty liver disease activity score (NAS) also had improvements in PF and Role Physical scores and all domains of CLDQ‐NASH (P < 0.05). Progression to cirrhosis was associated with a decrease in PF scores of SF‐36 (P ≤ 0.05). Fibrosis regression was independently associated with greater improvements in PF and EQ‐5D scores, while NAS improvement was associated with improvement in fatigue and pruritus (all P < 0.05). Conclusion: Patients with advanced NASH experienced improvement in their PROs after fibrosis regression or improvement in disease activity.

As treatment for NASH advances, it is important to know the new treatments improve patients' experiences with their disease. Furthermore, the side effect profile of the new regimens should not add further impairment on PROs. This knowledge can assist healthcare practitioners counsel patients on expectations of treatment when they become available.
Abbreviations
- ATLAS
Study to Evaluate the Safety and Efficacy of Selonsertib, Firsocostat, Cilofexor, and Combinations in Participants With Bridging Fibrosis or Compensated Cirrhosis Due to Nonalcoholic Steatohepatitis (NASH)
- BMI
body mass index
- CI
confidence interval
- CLDQ
Chronic Liver Disease Questionnaire
- ELF
enhanced liver fibrosis test
- EQ5D
EuroQol Five‐Dimension
- FXR
farnesoid X receptor
- HbA1c
hemoglobin A1c
- HCC
hepatocellular carcinoma
- HRQL
health‐related quality of life
- NAFLD
nonalcoholic fatty liver disease
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- NIT
noninvasive test of fibrosis
- PF
Physical Functioning
- PRO
patient‐reported outcome
- SF‐36
Short Form 36
- SHP
Specific Health Problem
- T2DM
type 2 diabetes mellitus
- VAS
visual analog scale
- VCTE
vibration‐controlled transient elastography
- WPAI
Work Productivity and Activity Impairment
The global prevalence of nonalcoBholic fatty liver disease (NAFLD) among adults is 25% but ranges from 13% in some African countries to almost 40% in South America.( 1 ) Nonalcoholic steatohepatitis (NASH) is the progressive subtype of NAFLD that can lead to progressive fibrosis and cirrhosis as well as impairment of health‐related quality of life (HRQL). Within the United States, the prevalence of NASH in the general population is estimated to range from 1.5% to 6.5%.( 1 , 2 , 3 , 4 ) These rates are higher among at‐risk populations, including people with type 2 diabetes mellitus (T2DM) (37%) or obesity (47%).( 5 , 6 , 7 ) Also, Hispanic Americans have higher rates of NASH and fibrosis than Caucasian and African American individuals.( 6 , 7 ) Although not uniformly progressive, some patients with NASH, particularly those with histologic fibrosis, can progress to end‐stage liver disease, hepatocellular carcinoma (HCC), and ultimately, liver‐related death.( 8 ) Largely due to the growing epidemics of obesity and T2DM, NASH is becoming one of the most common causes of liver‐related death, liver transplantation, and HCC in the United States and globally.( 9 , 10 )
This report focuses on the potential new treatments for NASH as they relate to patient‐reported outcomes (PROs). Recent reports have found that NASH can lead to significant morbidity and impairment of HRQL and other PROs.( 11 , 12 , 13 ) Physical health‐related scores appear to be the most negatively affected, especially in those with NASH‐related cirrhosis.( 11 ) Indeed, studies have shown that factors independently associated with lower PRO scores in patients with NASH include presence of cirrhosis, female sex, higher body mass index (BMI), smoking, T2DM, as well as psychiatric and other comorbidities.( 14 ) While some evidence suggests that fibrosis improvement may be followed by improvement in PROs,( 15 ) some therapies may negatively impact PROs due to their adverse‐effect profile. Therefore, alongside efforts to reduce the clinical burden of NASH using preventive efforts and new treatment regimens, it is important to understand the impact of new therapies on PROs in this disease. Given this, the aim of this study was to assess PRO scores among patients with advanced fibrosis due to NASH before and after treatment with various investigational antifibrotic drugs in the context of a randomized controlled trial.
Patients and Methods
Patients and Study Design
This analysis used data collected in the Study to Evaluate the Safety and Efficacy of Selonsertib, Firsocostat, Cilofexor, and Combinations in Participants With Bridging Fibrosis or Compensated Cirrhosis Due to Nonalcoholic Steatohepatitis (NASH) (ATLAS) study, a phase 2, randomized, double‐blind, placebo‐controlled study that evaluated the apoptosis signal‐regulating kinase 1 inhibitor selonsertib, the acetyl‐coenzyme A carboxylase inhibitor firsocostat, and the farnesoid X receptor (FXR) agonist cilofexor, alone or in two‐drug combinations, in patients with advanced fibrosis due to NASH (NCT03449446). The methods and primary results of this study are reported elsewhere.( 16 ) Briefly, the ATLAS study enrolled and treated 392 patients with biopsy‐confirmed NASH (defined as the presence of at least grade 1 steatosis, hepatocellular ballooning, and lobular inflammation according to the NAFLD activity score [NAS]) with either bridging fibrosis (F3) or compensated cirrhosis (F4) based on the NASH Clinical Research Network classification.( 17 ) Subjects with grade 0 steatosis and compensated cirrhosis (F4) were also enrolled if they had at least one risk factor for NASH (diabetes, insulin resistance, overweight, obesity, dyslipidemia, hypertension). In lieu of a biopsy, approximately 20% of the cohort was enrolled based on noninvasive markers (liver stiffness by vibration‐controlled transient elastography [VCTE; FibroScan; Echosens, Paris, France] and enhanced liver fibrosis test [ELF; Siemens, Tarrytown, NY]) consistent with advanced fibrosis.
Exclusion criteria included a history of decompensated liver disease, Child‐Pugh score >6, Model of End‐Stage Liver Disease score >12, other causes of liver disease (e.g., alcoholic liver disease, hepatitis B, hepatitis C), liver transplantation, HCC, human immunodeficiency virus infection, recent excessive alcohol or illicit drug use, and any major or unstable comorbidities other than NASH and metabolic syndrome. Comorbidities were recorded using the Medical Dictionary for Regulatory Activities system and summarized by System Organ Class.( 18 )
The study was conducted at 105 sites in the United States, Canada, Hong Kong, Australia, and New Zealand. Eligible patients were randomized to one of seven treatment groups: placebo; monotherapy with selonsertib (18 mg), cilofexor (30 mg), or firsocostat (20 mg); or combination therapy with cilofexor/selonsertib, firsocostat/selonsertib, or cilofexor/firsocostat. Study drugs were administered orally once daily for 48 weeks. The selonsertib monotherapy group was discontinued following reporting of negative results of the Statin Therapies for Elevated Lipid Levels Compared Across Doses to Rosuvastatin (STELLAR) studies; their baseline data were included in this study.( 19 , 20 ) The study was approved by the institutional review boards at participating sites, and all participants provided informed consent.
Outcome Measures
Histology and Noninvasive Tests
Liver biopsies obtained at screening and week 48 were evaluated in a blinded manner by a single central pathologist. The primary efficacy endpoint of the trial was the proportion of patients with one or more stage improvement in fibrosis without worsening of NASH (defined as a ≥1‐point increase in ballooning or lobular inflammation) at week 48. Secondary endpoints included fibrosis improvement (without regard to NASH worsening), NASH resolution (defined as a reduction of lobular inflammation to 0 or 1 and hepatocellular ballooning to 0) without worsening of fibrosis, histologic progression to cirrhosis (in subjects without cirrhosis at baseline), and improvement in NASH activity defined as a ≥2‐point reduction in NAS.
Due to the limited sensitivity of conventional histologic staging for detecting fibrosis regression, we also evaluated changes in two noninvasive markers of fibrosis, ELF and liver stiffness by VCTE. Specifically, an ELF response was defined as a ≥0.5‐unit reduction and a liver stiffness by VCTE response was defined as a ≥25% relative reduction, both from baseline to week 48. Changes of this magnitude have been associated with reduced disease progression in this patient population.( 21 , 22 )
PROs
PROs were collected on the first day of treatment and every 12 weeks thereafter. Patients self‐administered PRO instruments before initiation of any treatment‐related activities at each visit. This included any discussion about liver biopsy findings. PRO instruments included Short Form 36 (SF‐36), the EuroQol Five‐Dimension (EQ‐5D), the Chronic Liver Disease Questionnaire‐NASH (CLDQ‐NASH), and the Work Productivity and Activity Impairment:Specific Health Problem (WPAI:SHP). In addition, pruritus was evaluated using the 5‐D Itch and a visual analog scale for pruritus (pruritus VAS).( 23 , 24 , 25 , 26 , 27 , 28 ) These instruments have been validated in various clinical populations and used in trials of patients with chronic liver and other disorders.
The SF‐36 is a generic HRQL instrument used to assess HRQL in eight domains: Physical Functioning (PF), Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health (all range from 0 to 100). It also includes two summary scores, physical component summary and mental component summary scores, which are linear combinations of the domain scores.( 23 ) Additionally, SF‐6D health utility scores (range, 0 to 1) were calculated from the SF‐36 responses using a described nonparametric Bayesian algorithm.( 29 )
The EQ‐5D is a generic instrument widely used for the calculation of health utility scores. In this instrument, health status is measured in terms of five dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression; the resulting 5‐digit number is then converted into a single weighted index score (range, 0 to 1) using a described crosswalk algorithm.( 30 )
The CLDQ‐NASH is a disease‐specific PRO instrument that assesses HRQL of patients with NASH by specifically addressing its most frequent manifestations. The instrument includes 36 items grouped into six domains (Abdominal, Activity/Energy, Emotional, Fatigue, Worry, and Systemic; all range from 1 to 7) and a total score that is an average of the domain scores. The CLDQ‐NASH has been validated in patients with biopsy‐proven NASH.( 24 )
The WPAI:SHP instrument evaluates impairment in daily activities and work productivity associated with a specific health problem. It includes a Work Productivity Impairment score, which is a sum of scores for Absenteeism (loss of work productivity owing to missing work hours) and Presenteeism (loss of work productivity owing to decreased productivity while working), and an activity impairment domain (all range from 0 to 1). Unlike the other instruments used in this study, WPAI:SHP returns greater scores for more impairment.( 26 )
The 5‐D Itch instrument assesses the severity of pruritus using five dimensions (degree, duration, direction, disability, and distribution; each on a scale from 1 to 5). The total score is the sum of the dimension scores (range from 5 to 25), with higher scores indicative of more pruritus.( 27 ). In addition, the pruritus VAS was used to assess the severity of itching.( 28 ) With this instrument, patients are asked to describe the severity of their itching by placing a mark on a 10‐cm‐long scale; the resulting score is the position of the mark in millimeters from the beginning of the scale (0, labeled as “no itching”).
Combined together, these instruments return a total of 25 domain and summary scores. Where stated explicitly, scores were transformed from their original scales to a universal scale ranging from 0 to 100, for presentation purposes.
Patient and Public Involvement Statement
Due to the clinical trial nature of this study, patients were not involved in the development of the study but were free to participate as they deemed reasonable for themselves.
Statistical Analyses
All clinical and demographic parameters and PRO scores were summarized as n (%) or mean ± SD. These parameters were compared between patient subgroups using the chi‐square test and Mann‐Whitney test, as appropriate; P ≤ 0.05 was considered statistically significant. Subgroups for comparison were defined by baseline cirrhosis status, treatment group, and whether or not the specific efficacy endpoint was met. Only observed PRO data were used; no imputation was performed. In addition, we calculated changes from baseline in PRO scores with reference to patients' own baseline levels. Treatment effects on the changes in PRO scores were evaluated by the treatment group for each study visit after the baseline. For this purpose, least‐square mean estimates were returned by mixed regression models that included treatment, visit, treatment‐visit interaction, baseline PRO value, and stratification factors involved in randomization (diabetes and cirrhosis) used as fixed effects and subjects used as a random effect. Changes in PRO scores were also summarized as arithmetic mean ± SEM when grouping other than by treatment group was used and were compared to zero by the matched pairs signed‐rank test and between the groups of interest by the Kruskal‐Wallis rank‐sum test.
Independent predictors of the baseline PRO scores were assessed in a series of generalized linear regression models with stepwise bidirectional selection from the list of baseline parameters, including demographics (age, sex, race, location), clinical parameters (smoking status, BMI, the presence of type 2 diabetes, and other comorbidities), presence of cirrhosis, and other histologic parameters (steatosis, hepatocellular ballooning, lobular inflammation). All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
In total, 392 patients with NASH were included, of whom the majority (56%) had cirrhosis. The mean age ± SD and BMI were 60 ± 9 years and 34.5 ± 6.9 kg/m2, respectively; 35% were men, 89% white, 6% Asian, 86% enrolled in the United States, 49% employed, and 72% had diabetes. Compared with patients without cirrhosis, those with cirrhosis were on average, 2 years older, had a higher prevalence of diabetes and gastrointestinal and vascular comorbidities, less steatosis, and substantially higher liver stiffness by VCTE and serum markers of fibrosis (all P < 0.05) (Table 1).
Table 1.
Clinicodemographic Characteristics of Patients With NASH Included in This Study
| Noncirrhotic (<F4) (n = 171) | Compensated Cirrhosis (F4) (n = 221) | P Value | Total (N = 392) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 58.1 ± 9.4 | 60.8 ± 8.5 | 0.0036 | 59.6 ± 9.0 |
| Male sex | 65 (38.0%) | 74 (33.5%) | 0.35 | 139 (35.5%) |
| White race | 150 (87.7%) | 199 (90.0%) | 0.46 | 349 (89.0%) |
| Black race | 3 (1.8%) | 4 (1.8%) | 0.97 | 7 (1.8%) |
| Asian race | 14 (8.2%) | 11 (5.0%) | 0.20 | 25 (6.4%) |
| Enrolled in the United States | 145 (84.8%) | 191 (86.4%) | 0.65 | 336 (85.7%) |
| Current smoker | 9 (5.3%) | 19 (8.6%) | 0.20 | 28 (7.1%) |
| Employed | 81 (48.5%) | 107 (49.5%) | 0.84 | 188 (49.1%) |
| BMI, kg/m2 | 34.4 ± 7.0 | 34.5 ± 6.8 | 0.95 | 34.5 ± 6.9 |
| Comorbidities | ||||
| Diabetes mellitus | 114 (66.7%) | 168 (76.0%) | 0.0410 | 282 (71.9%) |
| Blood and lymphatic system disorders | 21 (12.3%) | 42 (19.0%) | 0.07 | 63 (16.1%) |
| Cardiac disorders | 25 (14.6%) | 47 (21.3%) | 0.09 | 72 (18.4%) |
| Ear and labyrinth disorders | 23 (13.5%) | 16 (7.2%) | 0.0416 | 39 (9.9%) |
| Endocrine disorders | 39 (22.8%) | 54 (24.4%) | 0.71 | 93 (23.7%) |
| Asthenic conditions or fatigue | 27 (15.8%) | 28 (12.7%) | 0.38 | 55 (14.0%) |
| Gastrointestinal disorders | 124 (72.5%) | 184 (83.3%) | 0.0101 | 308 (78.6%) |
| Immune systemic disorders | 83 (48.5%) | 103 (46.6%) | 0.70 | 186 (47.4%) |
| Infections and infestations | 63 (36.8%) | 95 (43.0%) | 0.22 | 158 (40.3%) |
| Musculoskeletal, connective tissue disorders | 101 (59.1%) | 150 (67.9%) | 0.07 | 251 (64.0%) |
| Neoplasms, benign or malignant | 39 (22.8%) | 71 (32.1%) | 0.0417 | 110 (28.1%) |
| Nervous system disorders | 73 (42.7%) | 99 (44.8%) | 0.68 | 172 (43.9%) |
| Psychiatric disorders | 78 (45.6%) | 113 (51.1%) | 0.28 | 191 (48.7%) |
| Renal and urinary disorders | 38 (22.2%) | 57 (25.8%) | 0.41 | 95 (24.2%) |
| Respiratory disorders | 73 (42.7%) | 102 (46.2%) | 0.49 | 175 (44.6%) |
| Skin and subcutaneous tissue disorders | 43 (25.1%) | 72 (32.6%) | 0.11 | 115 (29.3%) |
| Vascular disorders | 123 (71.9%) | 180 (81.4%) | 0.0257 | 303 (77.3%) |
| Vision disorders | 57 (33.3%) | 71 (32.1%) | 0.80 | 128 (32.7%) |
| Liver histology | ||||
| Steatosis grade 0 | 2 (1.2%) | 35 (15.8%) | <0.0001 | 37 (9.4%) |
| Steatosis grade 1 | 155 (90.6%) | 181 (81.9%) | 0.0142 | 336 (85.7%) |
| Steatosis grade 2 | 14 (8.2%) | 5 (2.3%) | 0.0068 | 19 (4.8%) |
| Lobular inflammation grade 1 | 17 (9.9%) | 16 (7.2%) | 0.34 | 33 (8.4%) |
| Lobular inflammation grade 2 | 50 (29.2%) | 66 (29.9%) | 0.89 | 116 (29.6%) |
| Lobular inflammation grade 3 | 104 (60.8%) | 139 (62.9%) | 0.67 | 243 (62.0%) |
| Hepatocyte ballooning grade 0 | 2 (1.2%) | 4 (1.8%) | 0.61 | 6 (1.5%) |
| Hepatocyte ballooning grade 1 | 24 (14.0%) | 26 (11.8%) | 0.50 | 50 (12.8%) |
| Hepatocyte ballooning grade 2 | 145 (84.8%) | 191 (86.4%) | 0.65 | 336 (85.7%) |
| NAS | 5.42 ± 1.03 | 5.27 ± 1.05 | 0.13 | 5.33 ± 1.04 |
| CPA, % | 4.15 ± 2.39 | 10.4 ± 5.8 | <0.0001 | 7.63 ± 5.58 |
| NITs | ||||
| Liver stiffness by VCTE, kPa | 15.7 ± 9.9 | 22.2 ± 13.5 | <0.0001 | 19.4 ± 12.5 |
| ELF score | 9.72 ± 0.90 | 10.4 ± 1.1 | <0.0001 | 10.1 ± 1.1 |
| NFS | −0.209 ± 1.297 | 0.471 ± 1.255 | <0.0001 | 0.175 ± 1.316 |
| APRI | 0.718 ± 0.507 | 0.792 ± 0.537 | 0.0404 | 0.759 ± 0.525 |
| Fibrosis‐4 score | 1.90 ± 0.97 | 2.45 ± 1.17 | <0.0001 | 2.21 ± 1.12 |
| FibroTest score | 0.416 ± 0.229 | 0.540 ± 0.210 | <0.0001 | 0.486 ± 0.227 |
All clinical and demographic parameters and PRO scores were summarized as n (%) or mean ± SD.
Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; CPA, collagen proportionate area; NFS, NAFLD fibrosis score.
PROs at Baseline
Baseline scores indicated better PROs in patients without versus with cirrhosis; this was primarily in the physical health‐related domains (Supporting Table S1), including PF (mean ± SD, 71.1 ± 27.4 vs. 66.4 ± 26.5, respectively; P = 0.0335), Bodily Pain (67.4 ± 26.6 vs. 62.1 ± 25.5; P = 0.0480), and physical component summary (46.2 ± 10.1 vs. 44.4 ± 9.7; P = 0.0410) of the SF‐36; and Fatigue (4.72 ± 1.50 vs. 4.39 ± 1.32; P = 0.0105), Worry (5.16 ± 1.51 vs. 4.76 ± 1.61; P = 0.0113), and total score (5.16 ± 1.14 vs. 4.91 ± 1.06; P = 0.0204) of the CLDQ‐NASH. The mean pruritus VAS was lower in patients without versus with cirrhosis, indicative of less itch (12.3 ± 20.9 vs. 15.2 ± 20.9, respectively; P = 0.0136). In multivariate analysis, lower baseline PRO scores at baseline were independently associated with older age (physical health‐related scores), younger age (mental health‐related scores), female sex, higher BMI, and the presence of diabetes, clinically overt fatigue, psychiatric, musculoskeletal, nervous system, gastrointestinal, and cardiac comorbidities (all P < 0.05) (Supporting Table S2).
Changes in PROs During Treatment
During treatment, cilofexor‐containing regimens were associated with some PRO improvement, especially in scores measured by the disease‐specific CLDQ‐NASH (Fig. 1A), although the same trends in health utility scores (Fig. 1B) and physical health‐related scores measured by generic instruments (Fig. 1C) were less pronounced. On the other hand, selonsertib‐containing regimens were associated with some PRO decrease in mental health scores (Fig. 1D). No other PRO decrements were observed, while the Worry score of CLDQ‐NASH improved in all treatment groups (all P < 0.05). Finally, there was no association of any treatment regimen with changes in the two studied pruritus assessments (all P > 0.05).
Fig. 1.
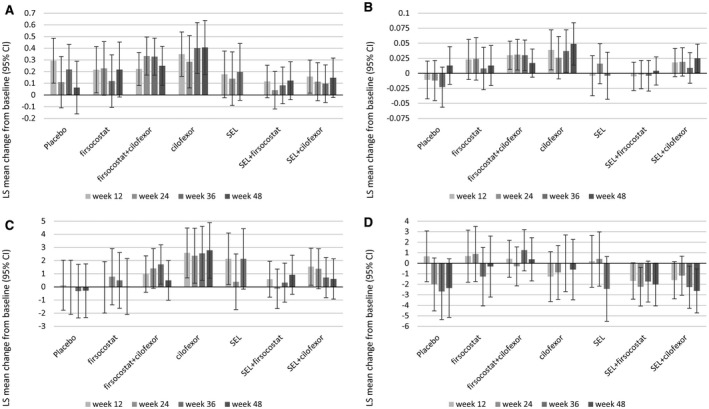
Changes in summary PRO scores by treatment regimen. (A) Total CLDQ‐NASH score; (B) EQ‐5D utility score; (C) physical component summary of SF‐36; (D) mental component summary of SF‐36. Data show LS mean ± 95% CI. Abbreviations: LS, least squares; SEL, selonsertib.
Associations Between Changes in PROs and Histologic Responses
After 48 weeks of treatment, patients who achieved the primary endpoint of the study, a ≥1‐stage improvement in fibrosis without worsening of NASH (observed in 16% of cases with paired histology overall), experienced improvement in their EQ‐5D utility score and five out of six CLDQ‐NASH domain scores (all P < 0.05) (Fig. 2A). Similar changes in PROs were observed in patients who experienced improvement in fibrosis without regard to NASH activity (19% of observed cases) (Fig. 2B). Patients who had a ≥2‐point decrease in their NAS (19% of cases) had improvements in PF and Role Physical scores and all domains of CLDQ‐NASH (all P < 0.05) (Fig. 2C). As NASH resolution without worsening of fibrosis was rarely observed in this cohort of patients with advanced fibrosis (n = 6), analyses of PROs were not conducted according to this endpoint. Patients without cirrhosis at baseline who progressed to cirrhosis at week 48 (19% of cases) experienced a decrease in their PF scores (mean ± SD, −6.0 ± 3.0; P = 0.05).
Fig. 2.
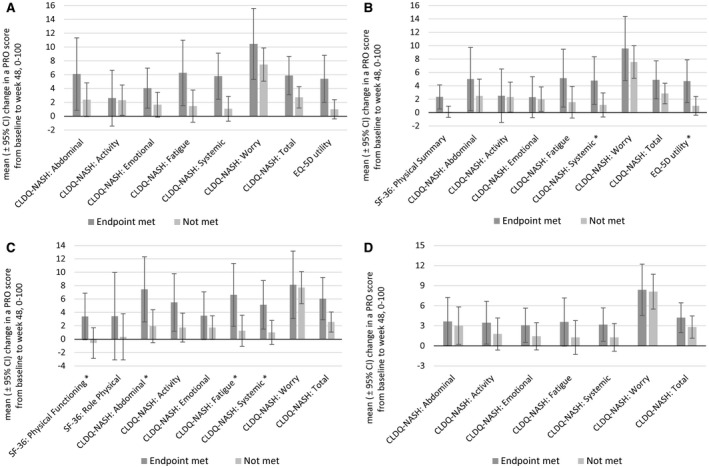
Changes in PRO scores in patients with NASH according to histologic and ELF responses. (A) Primary efficacy endpoint (≥1‐stage improvement in fibrosis without worsening of NASH); (B) ≥1‐stage improvement in fibrosis without regard to changes in NAS; (C) improvement of NAS by ≥2 points; (D) decrease in ELF score by ≥0.5 point. Additional NIT endpoints are shown in Supporting Fig. S1. Data show arithmetic mean ± 95% CI. *The difference between groups was statistically significant at P < 0.05.
In multivariate analyses with adjustment for clinicodemographic parameters (location, age, sex, race, smoking status, baseline BMI, type 2 diabetes, cirrhosis, NAS), treatment regimen, and a change in BMI and hemoglobin A1c (HbA1c) from baseline (in order to account for potential improvement in diabetes control while in the trial), improvement of fibrosis at week 48 of treatment was independently associated with a greater improvement in PF (mean ± SD, beta = +5.37 ± 2.47; P = 0.0306), physical component summary (+2.24 ± 0.96; P = 0.0199), and EQ‐5D (+0.036 ± 0.017; P = 0.0308) scores (Supporting Table S3). In addition, a ≥2‐point improvement in NAS was associated with improvement in Fatigue (mean ± SD, +0.32 ± 0.16; P = 0.0435), Systemic Symptoms (+0.25 ± 0.12; P = 0.0423), and total (+0.21 ± 0.10; P = 0.0405) scores by CLDQ‐NASH (Supporting Table S3). The improvements in CLDQ‐NASH scores seem to be driven primarily by improvement in hepatocyte ballooning (by ≥1 point mean ± SD, beta = +0.41 ± 0.14 for Activity, +0.27 ± 0.12 for Systemic Symptoms). At the same time, there was no significant association of PRO changes with improvement in lobular inflammation or steatosis (all P > 0.05). Interestingly, we also found that the only trends in PRO scores associated with changes in HbA1c were both pruritus metrics, namely, 5D‐Itch (mean ± SD, beta = +0.67 ± 0.24) and pruritus VAS (beta = +5.21 ± 1.54; both P < 0.01) (Supporting Table S3). Notably, there was no association of histologic improvement with improvement in pruritus after adjustment for improvement in HbA1c (all P > 0.05).
Associations Between Changes in PROs and Noninvasive Tests of Fibrosis Responses
Patients who had their ELF score decreased by at least 0.5 points (28%) had improvements in four out of six domains of CLDQ‐NASH (up to +8.4% of a range size, P ≤ 0.05) (Fig. 2D). However, the association with ELF improvement was found to be strongest at the cutoff of 0.3 as statistically significant improvements were detected in all domains of CLDQ‐NASH (up to +10.6% of a range size, P < 0.05) (Supporting Fig. S1A). A reduction in liver stiffness of at least 25% (37% of observed cases) was similarly associated with significant improvements in five out of six CLDQ‐NASH domains and in EQ‐5D utility scores (up to +9.6% of a range size, P < 0.05) (Supporting Fig. S1B).
Discussion
In this study, we analyzed PRO scores from patients with advanced NASH within the phase 2 ATLAS trial. The approach was to evaluate therapies targeting different pathogenic mechanisms of NASH. Serial measurement of PROs and liver histology before and during treatment with these active therapies have enabled an assessment of the impact of NASH on PROs as well as the impact of these treatment regimens on PROs and the histologic changes that may be associated with PRO improvement in NASH.
At baseline, we confirmed that patients with compensated NASH cirrhosis had lower PRO scores than patients without cirrhosis before the initiation of treatment, especially in the domains related to physical health. In addition, one of the pruritus scores (the pruritus VAS) was worse in patients with cirrhosis. Although this finding requires validation, it may be clinically relevant because some classes of medication, notably FXR agonists, may cause pruritus as a side effect.( 31 ) Given this observation, assessment of pruritus at baseline may be needed to better control the side‐effect burden of these regimens. Finally, patients with cirrhosis had worse scores related to Worry on the CLDQ‐NASH, likely due, at least in part, to uncertainty regarding their prognosis.
Consistent with reports in patients with advanced fibrosis due to NASH, predictors of lower PRO scores at baseline included older age, higher BMI, the presence of type 2 diabetes, as well as musculoskeletal, cardiovascular, and psychiatric comorbidities. Indeed, after adjustment for these factors, the presence of cirrhosis was not independently associated with PRO scores compared with bridging fibrosis (except for the Worry score of CLDQ‐NASH), likely due to the high prevalence of these comorbidities in advanced NASH. The impact of treatment of these comorbidities on PROs in patients with NASH requires further study.
In this regard, we evaluated changes in PRO scores in the context of specific therapies for NASH and did not observe statistically significant differences between treatment regimens. However, promising trends in CLDQ‐NASH scores were noted with cilofexor‐containing regimens, including the combination with firsocostat. Importantly, adverse changes in pruritus‐related PROs were not observed in patients treated with the FXR agonist cilofexor, supporting the tolerability of this therapy from an itch perspective. Indeed, only 1 of 195 patients treated with cilofexor (<1%) in the trial discontinued therapy due to pruritus.( 32 ) Interestingly, the Worry scores of the CLDQ‐NASH improved in all treatment groups, potentially attesting to the benefits of patient participation in clinical trials (e.g., due to close follow‐up and monitoring).
Of importance was the observation of improvements in PROs in patients with evidence of histologic improvement. Specifically, PRO gains were noted in patients with improvements of both NASH activity, as measured by the NAS and in particular its hepatocyte ballooning component, and fibrosis, as evaluated histologically by transient elastography or with the noninvasive serum marker ELF. In this context, the most significant gains were observed as expected in the PRO domains assessed by the disease‐specific CLDQ‐NASH, which included improvements in abdominal symptoms, physical activity, as well as NASH‐related emotional health and fatigue. Interestingly, in multivariate analysis, fibrosis regression was found to be independently associated with greater improvements in generic physical health‐related scores while a decrease of the NAS score was associated with improvement in some disease‐specific PRO scores. Conversely, patients without cirrhosis at baseline who experienced histologic progression to cirrhosis experienced a decrease in their PF scores on the SF‐36, supporting the impact of cirrhosis on PROs. All these findings are consistence with reports from clinical trials on the association of PRO improvement with achieving histologic and other treatment endpoints, such as improvement of NAS score, fibrosis stage, and noninvasive test (NITs) in patients with NASH and different stages of baseline fibrosis.( 20 , 31 , 33 ) In fact, a similar association of PRO improvement with improvement in NITs was recently shown using a large cohort of patients with advanced NASH.( 33 ) The exact mechanism(s) behind all these observations are unclear as we do not have in‐depth cytokine measurements in this study,( 34 ) but taken together they underscore the importance of reversing fibrosis and reducing the inflammatory milieu of NASH.
Assessment of PROs in the clinical trials of NASH can provide a number of valuable pieces of information. First, the assessment could shed light whether histologic or NIT improvement can lead to PRO improvement. In this context, the main findings of our study are in line with published literature.( 33 ) Second, PRO assessment could provide patient‐centric information whether the side‐effect profile of the regimen has a negative impact on patients’ experience.( 35 ) This is especially important when combination regimens, such as those combining cilofexor and firsocostat, are used. In this context, our data provide new information that the combination regimens used in this clinical trial may not have a significant negative impact on PROs.
This study has several limitations that warrant discussion. First, the patients enrolled in this clinical trial had advanced fibrosis due to NASH. The generalizability of these findings to patients with milder disease and those seen in real‐world settings requires confirmation. In addition, the PROs evaluated in this study were self‐reported and thus subject to recall or perception biases. However, the latter bias should be mitigated by the double‐blind nature of this study and the fact that patients completed the PRO instruments while unaware of their most recent clinical results. Moreover, consistency of the findings across multiple PRO domains describing similar aspects of patients’ well‐being could indirectly support their validity. Finally, the study was not powered to detect differences in PRO scores between treatment regimens. Hence, any inferences regarding differential effects of these therapies on PROs in patients with NASH should be made cautiously.
As research progresses on treatments for patients with NASH‐related fibrosis, understanding the impact of NASH therapies toward HRQL and other PROs is important. In this study of several investigational regimens, we found that patients with NASH‐related cirrhosis at baseline reported significantly lower PRO scores, especially in physical health‐related domains, compared with patients without cirrhosis. At the same time, we found that achieving clinically relevant endpoints, including fibrosis regression and improvement in NASH disease activity, was associated with improvement in some PROs captured by the disease‐specific CLDQ‐NASH. Finally, while statistically significant differences between the effects of treatment regimens on PROs were not observed, cilofexor‐containing regimens, including the combination with firsocostat, appeared to have the most positive effects on PROs. Longer term research is needed to confirm the sustainability of the reported PRO gains in patients with NASH with histologic improvement during treatment.
Supporting information
Supplementary Material
Supported by Gilead Sciences.
Potential conflict of interest: Dr. Younossi received research funding and has served as consultant to Gilead Sciences, Intercept, Bristol Myers Squibb, NovoNordisk, Viking, Terns, Siemens, Shionogi, AbbVie, Merck, and Novartis. Dr. Kohli advises Gilead Sciences, Novartis, and Intercept; she is on the speakers’ bureau of Intercept; she received grants from Gilead Sciences, Novartis, Intercept, Akero, Allergan Axcella, Bristol Myers Squibb Cirius, Conatus, CymaBay, Eli Lilly, Enyo, Galectin, Genfit, HighTide, Metacrine, NGM, NorthSea, Novo Nordisk, Madrigal, Poxel, Pfizer, Prometheus, Regeneron, Terns, and Viking. Dr. Gunn received grants from Bristol Myers Squibb, Corcept, Enyo, NorthSea, 89Bio, Poxel, Genentech, Madrigal, NGM Bio, Gilead, Novo Nordisk, Axcella, CymaBay, Genfit, and HighTide; she is on the speakers’ bureau and teaching staff of Gilead, Dova, Salix, AbbVie, and Intercept. Dr. Strasser received honoraria for advisory board participation and is on the speakers’ bureau for Gilead Sciences, Roche, Astra Zeneca, Ipsen, Eisai, Bayer Healthcare, Bristol Myers Squibb, MSD, AbbVie, Norgine, Astellas, Novartis, Pfizer, CSL‐Behring, and Dr. Falk Pharma. Dr. Wong consults for and/or is an advisory board member for 3V‐BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Hanmi Pharmaceutical, Intercept, Merck, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, and Terns; he is on the speakers’ bureau for AbbVie, Bristol Myers Squibb, Echosens, and Gilead Sciences; he received grants from Gilead Sciences. Dr. Sheikh received grants from Madrigal, Gilead, and Novartis. Dr. Noureddin advises Allergan, Gilead, and Novartis; he owns stock in Anaetos and Viking; he consults for Allergan, Gilead, Novartis, 89Bio, Intercept, Pfizer, Novo Nordisk, Blade, Echosens, Fractyl, Terns, OWL, Siemens, Roche Diagnostic, and Abbott; he received grants from Viking, Bristol Myers Squibb, Galmed, Galectin, Genfit, Conatus, Enanta, Madrigal, Shire, and Zydus. Dr. Ruane advises and is on the speakers’ bureau for Gilead and Viiv. Dr. Loomba consults for AstraZeneca, Bristol Myers Squibb, Eli Lilly, Galmed, Gilead, Intercept, Janssen, Madrigal, NGM Biopharmaceuticals, Pfizer, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, CohBar, Glympse Bio, Inipharm, Ionis, Metacrine, Novartis, Novo Nordisk, Sagimet 89Bio, and Viking Therapeutics; he received grants from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Galmed, Gilead, Intercept, Janssen, Madrigal, NGM Biopharmaceuticals, Pfizer, Allergan, Boehringer‐Ingelheim, Galectic, Genfit, Inventiva, Merck, and Siemens; he is a cofounder of Lipnexus, Inc. Dr. Kowdley consults for Altimmune, Roche, and Boeringer Inghelheim; he advises Gilead, Intercept, HighTide, Assembly, and Callidiats; he is on the speakers’ bureau for Gilead, Intercept, and AbbVie; he received grants from Gilead, Intercept, HighTide, Janssen, Allergan, Genfit, Novartis, Enanta, and CymaBay. Dr. Myers and Dr. Huss are employed by and own stock in Gilead. Dr. Caldwell received grants from Gilead, Bristol Myers Squibb, Galectin, Zydus, Madrigal, Akero, and Galmed; he receives royalties for Avanos. The other authors have nothing to report.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Golabi P, Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019;71:793‐801. [DOI] [PubMed] [Google Scholar]
- 3. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El‐Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol 2016;14:301‐308.e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non‐alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2019;4:389‐398. [DOI] [PubMed] [Google Scholar]
- 5. Pedrosa M, Balp M, Janssens N, Lopez P, Mckenna S, Chatterjee S, et al. Global prevalence of nonalcoholic steatohepatitis (NASH): findings from a targeted literature review. Value Health 2018;21(Suppl. 1):S82. [Google Scholar]
- 6. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of nonalcoholic steatohepatitis‐associated cirrhosis in the United States: an analysis of National Health And Nutrition Examination Survey data. Am J Gastroenterol 2017;112:581‐587. [DOI] [PubMed] [Google Scholar]
- 7. Bedossa P; FLIP Pathology Consortium . Utility and appropriateness of the fatty live inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565‐575. [DOI] [PubMed] [Google Scholar]
- 8. Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol 2018;68:238‐250. [DOI] [PubMed] [Google Scholar]
- 9. Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019;3:1459‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al.; Global Nonalcoholic Steatohepatitis Council . Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748‐755.e3. [DOI] [PubMed] [Google Scholar]
- 11. Younossi ZM, Stepanova M, Lawitz EJ, Reddy KR, Wai‐Sun Wong V, Mangia A, et al. Patients with nonalcoholic steatohepatitis experience severe impairment of health‐related quality of life. Am J Gastroenterol 2019;114:1636‐1641. [DOI] [PubMed] [Google Scholar]
- 12. Golabi P, Otgonsuren M, Cable R, Felix S, Koenig A, Sayiner M, et al. Non‐alcoholic fatty liver disease (NAFLD) is associated with impairment of health related quality of life (HRQOL). Health Qual Life Outcomes 2016;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinstein AA, Kallman Price J, Stepanova M, Poms LW, Fang Y, Moon J, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics 2011;52:127‐132. [DOI] [PubMed] [Google Scholar]
- 14. Younossi ZM, Stepanova M, Anstee QM, Lawitz EJ, Wai‐Sun Wong V, Romero‐Gomez M, et al. Reduced patient‐reported outcome scores associate with level of fibrosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2019;17:2552‐2560.e10. [DOI] [PubMed] [Google Scholar]
- 15. Younossi ZM, Stepanova M, Lawitz E, Charlton M, Loomba R, Myers RP, et al. Improvement of hepatic fibrosis and patient‐reported outcomes in non‐alcoholic steatohepatitis treated with selonsertib. Liver Int 2018;38:1849‐1859. [DOI] [PubMed] [Google Scholar]
- 16. Gilead Sciences . Study to evaluate the safety and efficacy of selonsertib, firsocostat, cilofexor, and combinations in participants with bridging fibrosis or compensated cirrhosis due to nonalcoholic steatohepatitis (NASH) (ATLAS). https://clinicaltrials.gov/ct2/show/NCT03449446. Published February 28, 2018. Accessed March 18, 2020.
- 17. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 18. MedDRA . Introductory Guide MedDRA Version 13.1. https://admin.new.meddra.org/sites/default/files/guidance/file/intguide_21_0_english.pdf. Accessed March 18, 2020.
- 19. Gilead Sciences . Safety and efficacy of selonsertib in adults with nonalcoholic steatohepatitis (NASH) and bridging (F3) fibrosis (STELLAR 3). https://clinicaltrials.gov/ct2/show/NCT03053050. Published February 14, 2017. Accessed March 18, 2020.
- 20. Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al.; STELLAR‐3 and STELLAR‐4 Investigators . Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol 2020;73:26‐39. [DOI] [PubMed] [Google Scholar]
- 21. Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology 2019;70:1913‐1927. [DOI] [PubMed] [Google Scholar]
- 22. Anstee QM, Lawitz EJ, Alkhouri N, Wong VW, Romero‐Gomez M, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology 2019;70:1521‐1530. [DOI] [PubMed] [Google Scholar]
- 23. Ware JE, Kosinski M. Interpreting SF‐36 summary health measures: a response. Qual Life Res 2001;10:405‐413. [DOI] [PubMed] [Google Scholar]
- 24. Younossi ZM, Stepanova M, Younossi I, Racila A. Validation of chronic liver disease questionnaire for nonalcoholic steatohepatitis in patients with biopsy‐proven nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2019;17:2093‐2100.e3. [DOI] [PubMed] [Google Scholar]
- 25. Webster K, Odom L, Peterman A, Lent L, Cella D. The functional assessment of chronic illness therapy (FACIT) measurement system: validation of version 4 of the core questionnaire [Abstract]. Qual Life Res 1999;8:604. [Google Scholar]
- 26. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353‐365. [DOI] [PubMed] [Google Scholar]
- 27. Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5‐D itch scale: a new measure of pruritus. Br J Dermatol 2010;162:587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012;92:497‐501. [DOI] [PubMed] [Google Scholar]
- 29. Brazier JE, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ‐5D and the SF‐6D across seven patient groups. Health Econ 2004;13:873‐884. [DOI] [PubMed] [Google Scholar]
- 30. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res 2011;20:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al.; REGENERATE Study Investigators . Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet 2019;394:2184‐2196.Erratum in: Lancet 2020;396:312. [DOI] [PubMed] [Google Scholar]
- 32. Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 2020;72:58‐71. [DOI] [PubMed] [Google Scholar]
- 33. Younossi ZM, Anstee QM, Wong VW, Trauner M, Lawitz EJ, Harrison SA, et al. The association of histologic and noninvasive tests with adverse clinical and patient‐reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis. Gastroenterology 2020; 10.1053/j.gastro.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 34. de Avila L, Weinstein AA, Estep JM, Curry MP, Golabi P, Escheik C, et al. Cytokine balance is restored as patient‐reported outcomes improve in patients recovering from chronic hepatitis C. Liver Int 2019;39:1631‐1640. [DOI] [PubMed] [Google Scholar]
- 35. Younossi ZM, Golabi P, Henry L. A Comprehensive review of patient‐reported outcomes in patients with chronic liver diseases. J Clin Gastroenterol 2019;53:331‐341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


