Abstract
Hepatocellular carcinoma has been considered to disseminate through the tumor blood drainage area. To improve curation rates, treatment should cover this area as it may contain satellite lesions. This retrospective study aimed to investigate whether radiofrequency ablation (RFA) completely covering the blood drainage area can improve the overall and disease‐free survival. We enrolled 526 patients who underwent computed tomography during hepatic arteriography following RFA from April 2001 to May 2019. Patients were categorized into a covered group in which the blood drainage area was completely covered by RFA and a noncovered group in which coverage was incomplete. The primary endpoint was the overall survival rate; secondary outcomes included disease‐free survival rate, distant intrahepatic and local recurrence rate, and changes in the Child‐Pugh score. There were no significant differences in baseline characteristics between the two groups. Cumulative overall survival rates were significantly higher in the covered group than in the noncovered group (hazard ratio, 0.63; 95% confidence interval, 0.48‐0.84; P = 0.002). On multivariate Cox proportional hazard model analysis, age <65 years, Child‐Pugh class A, and coverage of the entire drainage area were independent protective factors. Child‐Pugh worsened in 11 (4.2%) patients in the covered group compared to 18 (6.7%) patients in the noncovered group. Conclusion: RFA covering the complete drainage area improved overall survival without decreasing liver function.
Abbreviations
- ALBI
albumin–bilirubin
- CI
confidence interval
- CT
computed tomography
- CTHA
computed tomography during hepatic arteriography
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- RFA
radiofrequency ablation
Locoregional therapy, including ethanol injection, radiofrequency ablation (RFA), and microwave ablation, is widely performed in patients with early stage hepatocellular carcinoma (HCC).( 1 , 2 ) Okusaka et al.( 3 ) reported that 19% of HCC nodules ≤3.0 cm diameter had satellite lesions that were not detected during pretreatment evaluation. To improve curability after locoregional therapy, it is necessary to ensure that areas that may contain satellite lesions that cannot be delineated by imaging are covered. Thus, the safety margins should be defined considering the area at risk of containing satellite lesions rather than the distance from the main nodule. Currently, for a procedure to be considered technically successful, the tumor and at least a 5‐mm safety margin must be included in the ablation zone.( 4 , 5 ) However, it has been reported( 3 ) that satellite lesions can lie beyond this margin. Therefore, a new definition of an adequate safety margin is necessary.
HCC is considered to disseminate through the portal vein system.( 6 ) Recently, the tumor blood flow drainage area was defined using computed tomography under hepatic arteriography.( 7 , 8 , 9 ) Because microsatellite lesions metastasize in this area first, we previously proposed that safety margins should be defined using the blood drainage area rather than the distance from the primary lesion.( 10 )
Although it is expected that overall survival would be improved if locoregional therapy covered the entire blood drainage area, only cumulative recurrence rates were clarified in a previous report of our authorship.( 10 ) Our present study aimed to clarify whether coverage of the entire blood drainage area by RFA can improve overall survival and disease‐free survival rates in patients with HCC.
Patients and Methods
Study Design and Participants
This was a retrospective cohort study including patients with HCC who underwent RFA between April 2001 and May 2019. The inclusion criteria were 1) ineligibility for surgical resection/liver transplantation or refusal of surgery; 2) solitary HCC <5 cm diameter or less than three multinodular HCCs <3 cm diameter; 3) Child‐Pugh class A or B; and 4) HCC as the primary lesion. The exclusion criteria were 1) tumor not visualized on ultrasonography or not accessible percutaneously; 2) total bilirubin concentration ≥3.0 mg/dL; 3) platelet count <30 × 109/L or prothrombin activity <50%; 4) refractory ascites; 5) extrahepatic metastasis or vascular invasion; and 6) other malignancies that may affect patient prognosis. In total, 526 patients were enrolled in this study. Computed tomography (CT) during hepatic arteriography (CTHA) was performed in all patients. A 4‐french catheter was inserted through the femoral artery into the common, proper, or replaced right hepatic artery. CTHA was performed as reported.( 10 ) CT was initiated 7 seconds after the infusion of iohexol (320‐350 mg/mL iodine; Omnipaque; Daiichi, Tokyo, Japan) into the common, proper, or replaced hepatic artery at a rate of 1.8 mL/second. The infusion of contrast material was continued until 5 seconds after the completion of early phase CTHA. Scanning time varied according to the individual liver size (~20‐25 seconds). Thirty seconds after completing the contrast material infusion (~62‐67 seconds after the initiation of the infusion), late‐phase scanning was commenced. Eight patients in whom the blood drainage area was not depicted clearly were excluded. All tumors showed the typical pattern of HCC (i.e., hyperattenuation in the arterial phase of dynamic CT and CTHA and hypoattenuation in the portal venous phase of CT).
Ablation was performed until the entire tumor area was covered. Based on the comparison between CTHA performed before RFA and dynamic CT performed after the procedure, patients were categorized into two groups: 1) a covered group in which the ablated area had covered the entire blood drainage area of the tumor (n = 259) (Fig. 1); and 2) a noncovered group in which the ablated area had not covered the entire blood drainage area of the tumor (n = 267) (Fig. 2; Supporting Fig. S1). One patient in the covered group and 2 in the noncovered group had multiple lesions. In the covered group, all HCC nodules covered the entire blood drainage area, while none of the nodules covered the drainage area in the 2 patients in the noncovered group.
FIG. 1.
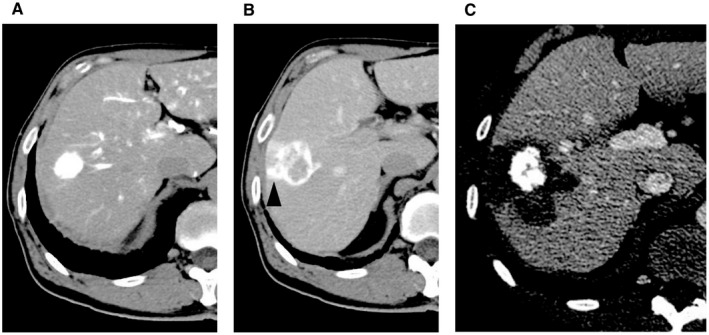
Ablation with coverage of the entire tumor blood drainage area. (A) HCC is depicted by CTHA in the early phase. (B) The tumor blood drainage area is evidenced during the delayed phase of hepatic arteriography. (C) The ablated area covers the entire tumor blood drainage area. The vertical length of these figures is 28 cm.
FIG. 2.
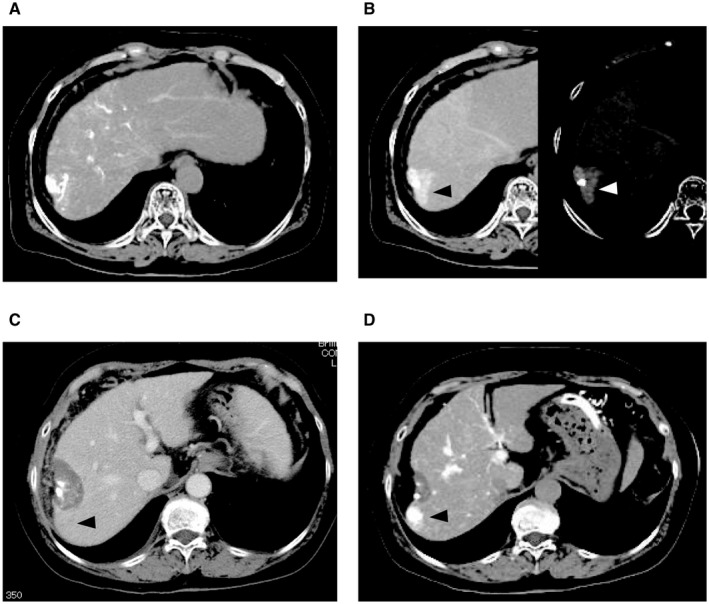
Ablation not covering the entire tumor blood drainage area. (A) HCC is depicted by CTHA in the early phase. (B) Image on the left shows the tumor blood drainage area (white arrowhead) during hepatic arteriography in the delayed phase. Image on the right side shows a highlight of the tumor blood drainage area (black arrowhead). (C) Complete necrosis is achieved. At this point, there is no recurrence lesion. (D) A recurrence lesion appeared in the drainage area (black arrowhead). The vertical length of these figures is 30 cm.
Informed consent was obtained from all patients before performing RFA. This study was conducted according to the principles of the Declaration of Helsinki; the study protocol was approved by the relevant institutional ethics committee of Ehime University Hospital.
RFA
Before RFA, 15 mg of pentazocine hydrochloride and 25 mg of hydroxyzine hydrochloride were administered intramuscularly. Local anesthesia was applied injecting 5 mL of 1% lidocaine into the peritoneum along a predetermined puncture line. When performing RFA under local anesthesia, all needle insertions were performed under ultrasound guidance. Until December 2013, RFA was performed using monopolar devices. For patients for whom treatment with multipolar devices was implausible, a single internally cooled electrode with a 200‐W generator (Cool‐tip; Radionics, Burlington, MA) was used until April 2015 and an internally cooled 17‐gauge monopolar electrode with an adjustable active tip with a generator (VIVARF system; STARmed, Seoul, Korea) was used from May 2015 onward. After January 2014, multipolar ablation was mainly performed using two or three internally cooled bipolar electrodes simultaneously and an RFA generator (CelonLabPower; Celon AG Medical Instruments, Teltow, Germany). To avoid direct puncture of the lesion, a no‐touch procedure was performed by placing all electrodes around the tumor as far as possible.( 11 , 12 ) If two or three electrodes could not be inserted because the major vessels or organs could not be avoided, monopolar ablation was selected. A total of 120 patients were treated using the no‐touch procedure by bipolar electrodes; the others were treated by direct puncture.
Follow‐Up After the Procedure and Definition of the Type of Recurrence
Postoperatively, all patients underwent daily assessments of liver function in the hospital; abdominal multiphasic CT or magnetic resonance imaging was performed until postoperative day 7. When assessing the efficacy of RFA, all frames that showed nodules were analyzed using the portal venous phase images. After discharge, all patients underwent follow‐up examinations every 3 months. Follow‐up assessments included liver function tests, tumor markers, and abdominal CT scans. Local recurrence was defined when the border of the recurrent nodule made contact with the original nodule (Fig. 3).
FIG. 3.
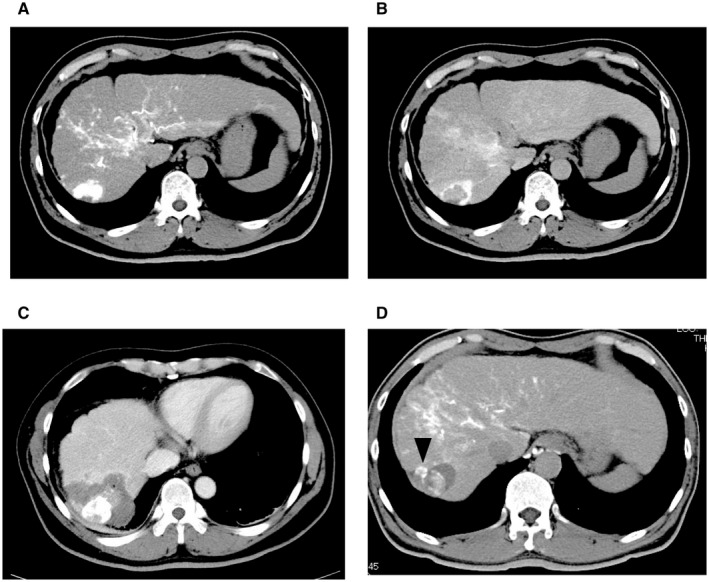
Local recurrence after ablation of the entire drainage area. (A) HCC is depicted by CTHA in the early phase. (B) The tumor blood drainage area is shown in CT during hepatic arteriography in the delayed phase. (C) Complete necrosis is achieved. (D) A local recurrence is evidenced in the tumor blood drainage area (black arrowhead). The vertical length of figure 3A, 3B and 3C is 30 cm and one of figure 3D is 30 cm.
Statistical Analysis
Quantitative variables are expressed as medians with first and third quartiles. Normally distributed continuous variables are expressed as means ± SD. To compare the clinical characteristics between two groups, the Student t test or Mann‐Whitney U test was used. Percentages were compared using the chi‐square test or Fisher’s exact test, as appropriate. To assess survival and recurrence curves, the Kaplan‐Meier method was used. Multivariate Cox proportional hazard regression was performed to predict the overall survival rate. Values of P < 0.05 were considered significant. All statistical analyses were performed using STATA version 15 (Stata Corp, College Station, TX).
Results
Baseline Characteristics
Baseline characteristics of the two RFA treatment groups are presented in Supporting Table S1. The two groups were comparable with regard to sex, age, Child‐Pugh class, albumin–bilirubin (ALBI)( 13 ) score, alpha‐fetoprotein levels, HCC etiology, maximum tumor diameter, location, and adhesion to major vessels.
Overall Survival and Disease‐Free Survival Rate
The rate of complete radiologic necrosis was 100% in both groups. Cumulative overall survival rates were significantly higher in the covered than in the noncovered group (72.6% vs. 58.9% at year 5; 40.3% vs. 24.4% at year 10; and 27.3% vs. 15.1% at year 15; P = 0.001 for all comparisons) (Fig. 4). Significant predictors of survival on univariate analysis were age <65 years, Child‐Pugh class A, use of the no‐touch procedure, safety margin >5 mm, and coverage of the entire drainage area (Table 1). On multivariate Cox proportional hazard model analysis, age <65 years, Child‐Pugh class A, and coverage of the entire drainage area remained significant predictors of survival (Table 1).
FIG. 4.
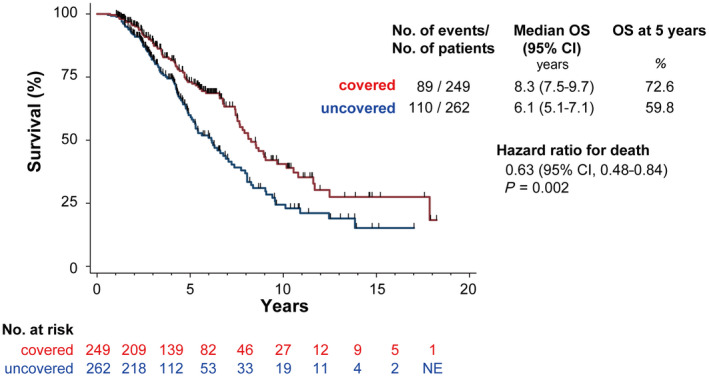
Overall survival curves. Cumulative overall survival rates were significantly higher in the group receiving ablation of the entire blood drainage area compared to the group with incomplete ablation of the blood drainage area. Abbreviations: NE, not evaluated; OS, overall survival.
Table 1.
Predictors of Survival After Radiofrequency Ablation
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | P Value | HR | P Value | |
| Male/female | 1.02 (0.74‐1.39) | 0.891 | 1.19 (0.86‐1.67) | 0.293 |
| Age <65 years | 1.40 (1.03‐1.90) | 0.029 | 1.42 (1.03‐1.98) | 0.031 |
| HCV | 1.79 (0.83‐4.48) | 0.145 | 1.24 (0.88‐1.79) | 0.218 |
| Child‐Pugh class A/B | 0.31 (0.23‐0.43) | <0.001 | 0.31 (0.22‐0.43) | <0.001 |
| ALBI grade | ||||
| 1/2 | 0.48 (0.34‐0.66) | <0.001 | ||
| 2/3 | 0.31 (0.16‐0.70) | 0.007 | ||
| AFP >100 ng/mL | 1.29 (0.88‐1.84) | 0.178 | 1.15 (0.79‐1.66) | 0.443 |
| No‐touch procedure | 0.57 (0.35‐0.88) | 0.011 | 0.74 (0.44‐1.19) | 0.219 |
| Safety margin >5 mm | 0.73 (0.55‐0.97) | 0.037 | 0.95 (0.69‐1.33) | 0.802 |
| Coverage of the entire blood drainage area | 0.63 (0.48‐0.84) | 0.002 | 0.70 (0.49‐0.98) | 0.038 |
| Tumor diameter <2 cm | 1.19 (0.88‐1.61) | 0.256 | 0.94 (0.69‐1.29) | 0.701 |
| Single tumor | 0.71 (0.16‐12.51) | 0.747 | 0.58 (0.13‐10.31) | 0.622 |
| Surface | 1.19 (0.90‐1.58) | 0.226 | 1.12 (0.83‐1.52) | 0.442 |
| Major vessel involvement | 0.81 (0.52‐1.21) | 0.316 | 0.99 (0.65‐1.57) | 0.961 |
Abbreviations: AFP, alpha‐fetoprotein; HCV, hepatitis C virus.
Cumulative disease‐free survival rates were also significantly higher for cases with ablation covering the entire blood drainage area than for those without ablation of the entire blood drainage area (40.5% vs. 21.5% at year 5; P < 0.001) (Fig. 5). Child‐Pugh class A, use of the no‐touch procedure, safety margin >5 mm, and coverage of the entire drainage area were significant predictors of disease‐free survival on univariate analysis (Table 2). On multivariate Cox proportional hazard model analysis, Child‐Pugh class A, use of the no‐touch procedure, and coverage of the entire drainage area were significant predictors of disease‐free survival (Table 2).
FIG. 5.
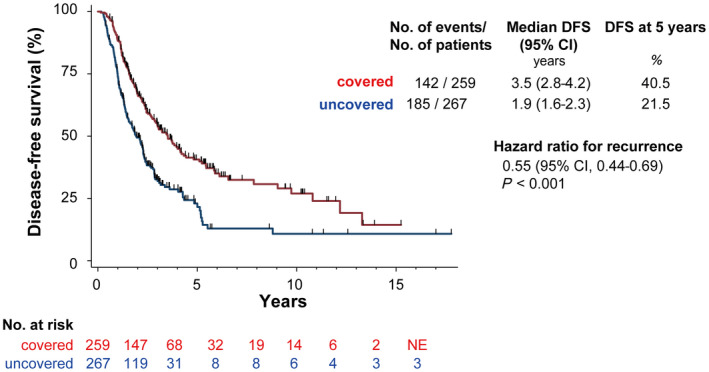
Disease‐free survival. Cumulative disease‐free survival rates were significantly higher in patients receiving ablation of the entire blood drainage area compared to patients with incomplete ablation of the blood drainage area. Abbreviations: DFS, disease‐free survival; NE, not evaluated.
Table 2.
Predictors of Disease‐Free Survival After Radiofrequency Ablation
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | P Value | HR | P Value | |
| Male/female | 1.00 (0.79‐1.28) | 0.992 | 1.08 (0.84‐1.40) | 0.524 |
| Age <65 years | 0.94 (0.75‐1.19) | 0.606 | 0.98 (0.77‐1.26) | 0.908 |
| HCV | 1.19 (0.94‐1.53) | 0.158 | 1.09 (0.85‐1.41) | 0.512 |
| Child‐Pugh class A/B | 0.57 (0.44‐0.76) | <0.001 | 0.60 (0.45‐0.80) | 0.001 |
| ALBI grade | ||||
| 1/2 | 0.54 (0.43‐0.69) | <0.001 | ||
| 2/3 | 0.81 (0.32‐1.67) | 0.597 | ||
| AFP >100 ng/mL | 1.30 (0.96‐1.75) | 0.090 | 1.15 (0.84‐1.56) | 0.360 |
| No‐touch procedure | 0.45 (0.33‐0.61) | <0.001 | 0.59 (0.42‐0.82) | 0.002 |
| Safety margin >5 mm | 0.58 (0.46‐0.73) | <0.001 | 0.76 (0.57‐1.00) | 0.053 |
| Coverage of the entire blood drainage area | 0.55 (0.44‐0.69) | <0.001 | 0.76 (0.58‐0.99) | 0.049 |
| Tumor diameter <2 cm | 1.02 (0.81‐1.31) | 0.842 | 1.02 (0.80‐1.33) | 0.834 |
| Single tumor | 0.60 (0.19‐3.66) | 0.514 | 0.29 (0.09‐1.80) | 0.154 |
| Surface | 1.24 (0.73‐2.11) | 0.432 | 1.20 (0.91‐1.59) | 0.199 |
| Major vessel involvement | 1.18 (0.86‐1.66) | 0.310 | 1.32 (0.96‐1.88) | 0.088 |
Abbreviations: AFP, alpha‐fetoprotein; HCV, hepatitis C virus.
Local and Intrahepatic Distant Recurrence
Cumulative local recurrence rates were significantly higher for cases with ablation of the entire blood drainage area than for those without ablation of the entire blood drainage area (18.1% vs. 1.6% at year 5; 21.4% vs. 1.6% at year 10; and 21.4% vs. 10.6% at year 15; P = 0.001) (Fig. 6A). The covered group showed four local recurrences, all of them occurring more than 2 years after ablation (median 1,318 days; interquartile range [IQR], 1,108‐3,755]) (Fig. 3). Fig. 1 depicts an example of a recurrence originated from the blood drainage area.
FIG. 6.
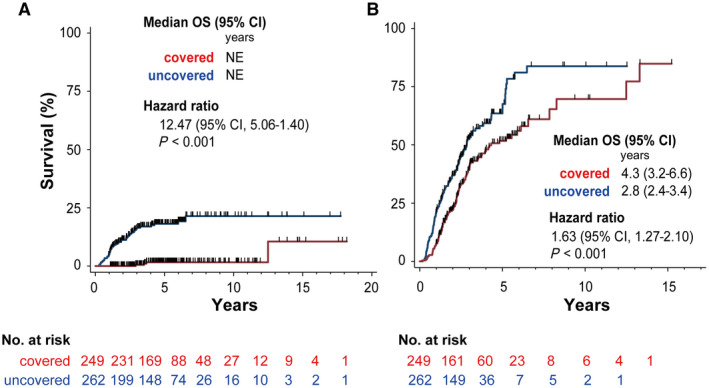
Local recurrence and distant intrahepatic metastasis. (A) Cumulative local recurrence rates in the covered and noncovered groups. (B) Cumulative intrahepatic distant recurrence rates in the covered and noncovered group. Abbreviations: NE, not evaluated; OS, overall survival.
Similarly, cumulative intrahepatic distant recurrence rates were significantly more frequent in covered than in noncovered patients (67.6% vs. 51.7% at year 5; 83.8% vs. 69.7% at year 10; P = 0.001) (Fig. 6B). In the noncovered group, recurrence lesions in nonablated blood drainage areas occurred in 11 patients (median recurrence‐free time, 147 days; IQR, 130‐174). Regarding predictive factors for distant intrahepatic recurrence, Child‐Pugh class A (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.46‐0.86; P = 0.005), ALBI grade 1 (HR, 0.52; 95% CI, 0.39‐0.68; P < 0.001), use of the no‐touch procedure (HR, 0.40; 95% CI, 0.28‐0.57; P < 0.001), safety margin >5 mm (HR, 0.70; 95% CI, 0.55‐0.90; P = 0.005), coverage of the entire drainage area (HR, 0.61; 95% CI, 0.47‐0.79; P < 0.001), and lesion on the surface of the liver (HR, 1.82; 95% CI, 1.42‐2.33; P < 0.001) were protective factors against intrahepatic distant recurrence on univariate analysis. On multivariate Cox proportional hazard model analysis, Child‐Pugh class A (HR, 0.64; 95% CI, 0.47‐0.89; P < 0.001), use of the no‐touch procedure (HR, 0.47; 95% CI, 0.31‐0.69; P < 0.001), and coverage of the drainage area (HR, 0.58; 95% CI, 0.45‐0.76; P < 0.001) remained as independent protective factors (Supporting Table S2).
Worsening of the Child‐Pugh class occurred in 11 (4.2%) patients in the covered group and 18 (6.7%) patients in the noncovered group.
Discussion
In this study, we demonstrated that ablation covering the entire blood drainage area improved overall survival in patients with HCC. Kitao et al.( 14 ) reported that HCC nodules with capillarized sinusoids are connected to the extranodular portal veins, either directly or indirectly, through portal venules within the fibrous capsule. These blood‐flow phenomena are known as “corona” enhancement in the late phase of CTHA.( 9 ) This peritumoral area is considered to contain more metastatic lesions than any other area because the tumor blood flows directly into it.( 7 , 15 ) In our previous report, local tumor progression rate was improved by ablation that covered the entire blood drainage area, irrespective of the use of a wide safety margin.( 10 ) Unfortunately, because of the short observation period, we could not draw conclusions on either overall or disease‐free survival rates.
In the present study, the high local curability by ablation covering the drainage area resulted in significant improvements in both overall and disease‐free survival rates. If treatment with a low local recurrence rate is selected, survival rate can be improved.( 1 ) In the noncovered group, most recurrent lesions occurred earlier and in the nonablated blood‐drainage area. It is likely that these lesions metastasized in the bloodstream draining from the main lesion. In contrast, local recurrences in the covered group did not occur early and might have spread hematogenously through the systemic circulation or appeared due to multicentric carcinogenesis.( 8 , 15 ) We presumed that intrahepatic distant metastases might arise from the original tumor drainage area over time; this was supported by the finding that ablation covering the entire drainage area improved the intrahepatic distant metastasis rate. In terms of both overall and disease‐free survival, ablation covering the entire drainage area and hepatic reserve function were significant predictive factors on multivariate analysis. Maintaining hepatic reserve function is important to improve prognosis. In this regard, ablation covering the entire drainage area is a safe procedure as it does not involve nontumorous areas.
Some studies have shown that the overall survival or disease‐free survival of patients with a sufficient surgical margin, such as that achieved in anatomical resections, is better than that of patients with an insufficient margin.( 16 , 17 , 18 ) Other studies could not demonstrate a prognostic difference between anatomical and nonanatomical resection.( 19 , 20 ) For this reason, locoregional therapy rather than hepatectomy is performed for patients with earlier stage HCC.
The drainage area was defined by CTHA only. To ablate the blood drainage area, the operator performed the procedure while mentally matching the drainage area on the CT to that on the ultrasound images. It is important that the entire tumor’s blood drainage is mapped in advance of the procedure. Thus, if the area is too large, surgical resection would be the preferred option. Moreover, when the ablation area does not cover the entire tumor blood drainage, additional treatment should be done.
There was significant advancement in ablation technology from 2001 to 2019. It is important to determine if using the no‐touch ablation approach would lead to different outcomes. In terms of the primary endpoint, no‐touch ablation was not a significant factor in multivariate analysis. Although the no‐touch technique tends to provide a wide ablation area, the entire blood drainage area was not always covered. The same principle applies to all RFA modalities, i.e., it is important that the ablation area is covered by the drainage area.
There were some limitations in this study. First, this was a retrospective study from a single center. Although the number of enrolled patients and duration of the study were sufficient, a prospective multicenter study would be necessary to confirm our results. It is important to determine if the same results would be achieved if the intent is to treat the entire tumoral drainage. Moreover, whether the same outcomes can be achieved with different operators should be investigated. Second, the drainage area was delineated using CTHA only; thus, it was difficult to define the drainage area using ultrasound. Although the fusion method was not used in this study, it may help to address this problem. The drainage area can be delineated using ultrasound by constructing a virtual image using the delay phase of CTHA. Third, this study was performed in Japan; its results may not be generalized to other settings.
In conclusion, we showed that RFA with coverage of the entire blood drainage area improved both overall survival and disease‐free survival without decreasing liver function in patients with HCC.
Supporting information
Fig S1
Table S1
Table S2
Supported in part by the Japan Society for the Promotion of Science (grants 18K07634 to M.H. and18K08007 to Y.H.).
Potential conflict of interest: Nothing to report.
References
- 1. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122‐130. [DOI] [PubMed] [Google Scholar]
- 2. Imajo K, Tomeno W, Kanezaki M, Honda Y, Kessoku T, Ogawa Y, et al. New microwave ablation system for unresectable liver tumors that forms large, spherical ablation zones. J Gastroenterol Hepatol 2018;33:2007‐2014. [DOI] [PubMed] [Google Scholar]
- 3. Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95:1931‐1937. [DOI] [PubMed] [Google Scholar]
- 4. Kudo M. Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol 2004;39:205‐214. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al.; Society of Interventional Radiology Technology Assessment Committee . Image‐guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005;16:765‐778. [DOI] [PubMed] [Google Scholar]
- 6. Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346‐350. [PubMed] [Google Scholar]
- 7. Sakon M, Nagano H, Shimizu J, Kondo M, Nakamori S, Dono K, et al. Hepatic resection of hepatocellular carcinomas based on tumor hemodynamics. J Surg Oncol 2000;73:179‐181. [DOI] [PubMed] [Google Scholar]
- 8. Sakon M, Nagano H, Nakamori S, Dono K, Umeshita K, Murakami T, et al. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg 2002;137:94‐99. [DOI] [PubMed] [Google Scholar]
- 9. Ueda K, Matsui O, Kawamori Y, Nakanuma Y, Kadoya M, Yoshikawa J, et al. Hypervascular hepatocellular carcinoma: evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology 1998;206:161‐166. [DOI] [PubMed] [Google Scholar]
- 10. Hirooka M, Ochi H, Koizumi Y, Tokumoto Y, Hiraoka A, Kumagi T, et al. Local recurrence of hepatocellular carcinoma in the tumor blood drainage area following radiofrequency ablation. Mol Clin Oncol 2014;2:182‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirooka M, Hiraoka A, Ochi H, Koizumi Y, Michitaka K, Joko K, et al. Prospective cohort trial to confirm the efficacy of no‐touch radio frequency ablation. J Gastroenterol Hepatol 2019;34:567‐574. [DOI] [PubMed] [Google Scholar]
- 12. Seror O, N’Kontchou G, Nault J‐C, Rabahi Y, Nahon P, Ganne‐Carrié N, et al. Hepatocellular carcinoma within Milan criteria: no‐touch multibipolar radiofrequency ablation for treatment‐long‐term results. Radiology 2016;280:611‐621.Erratum in: Radiology 2016;280:981. [DOI] [PubMed] [Google Scholar]
- 13. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach‐the ALBI grade. J Clin Oncol 2015;33:550‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography—radiologic‐pathologic correlation. Radiology 2009;252:605‐614. [DOI] [PubMed] [Google Scholar]
- 15. Sakon M, Kobayashi S, Wada H, Eguchi H, Marubashi S, Takahashi H, et al. “Logic‐based medicine” is more feasible than “evidence‐based medicine” in the local treatment for hepatocellular carcinoma. Oncology 2020;98:259‐266. [DOI] [PubMed] [Google Scholar]
- 16. Shimada K, Sakamoto Y, Esaki M, Kosuge T. Role of the width of the surgical margin in a hepatectomy for small hepatocellular carcinomas eligible for percutaneous local ablative therapy. Am J Surg 2008;195:775‐781. [DOI] [PubMed] [Google Scholar]
- 17. Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer 1998;82:10281036. [DOI] [PubMed] [Google Scholar]
- 18. Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004;101:796‐802. [DOI] [PubMed] [Google Scholar]
- 19. Nara S, Shimada K, Sakamoto Y, Esaki M, Kishi Y, Kosuge T, et al. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery 2012;151:526‐536. [DOI] [PubMed] [Google Scholar]
- 20. Yoshida Y, Kanematsu T, Matsumata T, Takenaka K, Sugimachi K. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis. Further evaluation of limited hepatic resection. Ann Surg 1989;209:297‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2


