Abstract
The benefit of colonoscopy and/or polypectomy for colorectal cancer (CRC) prevention in patients with nonalcoholic fatty liver disease (NAFLD) remains unclear. We aimed to estimate the incidence rate of CRC in patients with NAFLD who had and had not undergone colonoscopy. We conducted a retrospective territory‐wide cohort study for patients aged over 40 years with NAFLD identified with the International Classification of Diseases, Ninth Revision, Clinical Modification codes between January 1, 2000, and December 31, 2014. Patients were followed until CRC diagnosis, death, or December 31, 2017. We estimated CRC incidence and standardized incidence ratio (SIR) using the general population of Hong Kong as reference. We included 8,351 patients with NAFLD in the final analysis (median age, 56.2 years; interquartile ratio [IQR], 49.2‐65.3 years; 45.4% male; median follow‐up, 7.4 years; IQR, 5.4‐9.6 years). Compared with the general population, patients with NAFLD who had not undergone colonoscopy had a higher incidence of CRC (SIR, 2.20; 95% confidence interval [CI], 1.64‐2.88; P < 0.001). Patients with NAFLD who had undergone colonoscopy had a lower incidence of CRC (SIR, 0.54; 95% CI, 0.37‐0.75; P < 0.001), especially among those aged above 50 years or with diabetes mellitus (DM). Patients with NAFLD with a high fibrosis‐4 (FIB‐4) score (>2.67) had a significantly higher risk of CRC after adjusting for demographic and metabolic factors. Conclusion: Patients with NAFLD who had undergone colonoscopy had a lower incidence of CRC than the general population, especially among those aged ≥50 years or with DM. A high FIB‐4 index was associated with a higher risk of CRC.

Abbreviations
- AST
aspartate aminotransferase
- CDARS
Clinical Data Analysis and Reporting System
- CI
confidence interval
- CRC
colorectal cancer
- DM
diabetes mellitus
- FIB‐4
fibrosis‐4
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency virus
- HR
hazard ratio
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- NAFLD
nonalcoholic fatty liver disease
- SIR
standardized incidence ratio
Colorectal cancer (CRC) is one of the most common cancers worldwide and a leading cause of cancer mortality.( 1 ) Colonoscopy is a screening method for reducing CRC incidence and mortality through detection of early stage cancers and removal of adenomas.( 2 ) As a result, current guidelines recommend regular colonoscopy screening in adults aged above 50 years.( 3 ) However, only a minority of the population at risk undergoes colonoscopy because of health, psychological reasons, and access barriers.( 4 )
Nonalcoholic fatty liver disease (NAFLD) is currently the most common chronic liver disease and has emerged as one of the main causes of advanced liver disease and hepatocellular carcinoma (HCC) worldwide.( 5 ) NAFLD is strongly associated with obesity, insulin resistance, and metabolic syndrome.( 6 , 7 ) In epidemiologic studies, diabetes and obesity also have been associated with an increased risk of CRC.( 8 , 9 ) Because NAFLD and CRC share some similar risk factors, previous studies have shown that patients with NAFLD have an increased risk of CRC.( 10 , 11 ) However, the impact of colonoscopy in the prevention of CRC in patients with NAFLD remains to be determined.
Liver fibrosis in patients with NAFLD leads to several serious problems and is a key driver of liver disease‐related morbidity and mortality.( 12 ) Recent studies suggest that it is not NAFLD but its severe form that is associated with various adverse conditions, e.g., cardiovascular events, chronic kidney disease, and extrahepatic cancers.( 13 , 14 ) However, the association between liver fibrosis in patients with NAFLD and CRC has not been well investigated.
In this study, we estimated the incidence of CRC in patients with NAFLD who had and had not undergone colonoscopy compared with the general population and explore the association between liver fibrosis in patients with NAFLD and CRC.
Patients and Methods
Study Design and Data Source
This is a retrospective territory‐wide cohort study based on data retrieved from the Clinical Data Analysis and Reporting System (CDARS) under the Hospital Authority, Hong Kong.( 15 ) CDARS is an electronic health care database that covers patients’ demographics, death, diagnoses, procedures, drug prescription and dispensing history, and laboratory results from all public hospitals and clinics in Hong Kong; this covers approximately 80% of the local population.( 16 ) Several territory‐wide studies have been conducted based on CDARS.( 17 , 18 ) The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) coding was used in CDARS and was validated to be 99% accurate by chart review on clinical, laboratory, imaging, and endoscopy results.( 19 ) The available statistics on CRC until year 2017, stratified by age and sex of the general Hong Kong population, were extracted from the Hong Kong Cancer Registry, Hospital Authority.( 20 ) The total Hong Kong population over the years stratified by age and sex was obtained from the Hong Kong Census and Statistics Department.( 21 )
Subjects
All subjects aged 40 years or above with NAFLD first diagnosed between January 1, 2000, and December 31, 2014, in Hong Kong were identified. Subjects who had and had not undergone prior colonoscopy were classified based on ICD‐9‐CM procedure codes (Supporting Table S1), and subjects who had CRC diagnosed within 3 months of the index colonoscopy were classified as no prior colonoscopy because CRC would likely have been present at the time of this colonoscopy. The common indications of colonoscopy in a public hospital include rectal bleeding, anemia, positive fecal occult blood test, altered bowel habits, diarrhea, constipation, abdominal pain, and unexplained weight loss.
Patients were excluded if they were younger than 40 years old at the time of NAFLD diagnosis; were infected with hepatitis B virus and/or hepatitis C virus based on ICD‐9‐CM diagnosis codes, viral and serologic markers, and/or use of antiviral therapy for hepatitis B and/or hepatitis C; did not perform hepatitis B virus serology tests; were infected with human immunodeficiency virus (HIV) infection based on ICD‐9‐CM diagnosis codes and/or use of antiviral therapy for HIV; had excessive use of alcohol based on the nursing assessment form or ICD‐9‐CM diagnosis codes; had a history of inflammatory bowel disease (IBD) or other hepatobiliary diseases based on ICD‐9‐CM diagnosis codes; had a history of CRC, HCC, other cancers, and liver failure before baseline or within 6 months from baseline based on ICD‐9‐CM diagnosis codes; or had registered death within 6 months from baseline (Fig. 1; Supporting Table S1). Patients were followed until diagnosis of CRC, censored at death, or last follow‐up date (December 31, 2017), whichever came first. The study protocol was approved by the Joint Chinese University of Hong Kong‐New Territories East Cluster Clinical Research Ethics Committee.
FIG. 1.
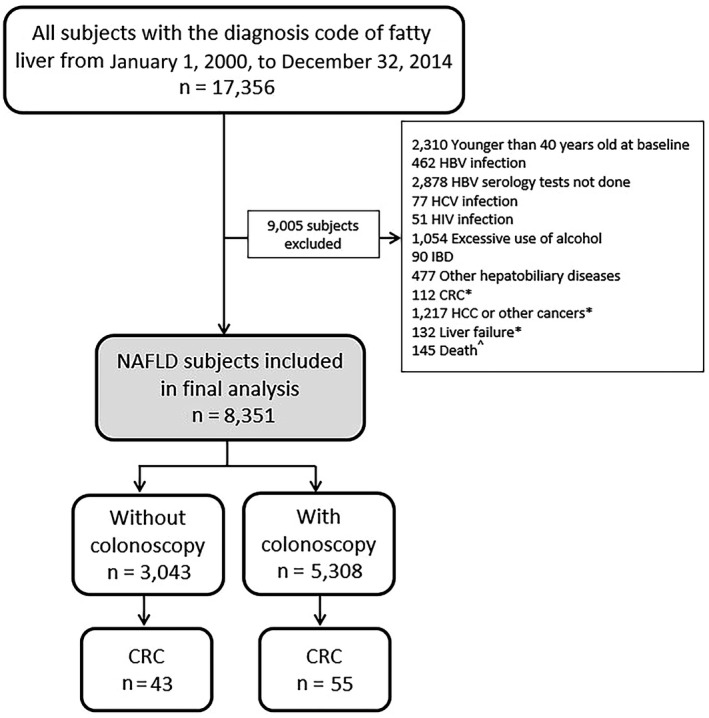
Patient flowchart. *Before baseline and up to 6 months; ^ within 6 months from baseline. Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; IBD, inflammatory bowel disease.
Data Collection
Data were obtained from the CDARS in January 2020. We defined the baseline date as the date of first diagnosis of NAFLD. Demographic data, including sex and date of birth, were captured. Liver and renal biochemistries, hematologic parameters, relevant diagnoses and procedures, concomitant drugs, and other laboratory parameters were also collected at baseline and during follow‐up.
Definitions
The primary outcome was CRC. CRC was identified based on ICD‐9‐CM diagnosis codes or procedure codes for CRC treatment (Supporting Table S1). Hypertension was identified by a resting blood pressure of ≥140/90 mm Hg and/or ICD‐9‐CM diagnosis codes. Diabetes mellitus (DM) was defined by any or all of the following: exposure to any antidiabetic agents, hemoglobin A1c ≥6.5%, fasting plasma glucose ≥7.0 mmol/L in two measurements 1 month apart, and/or the ICD‐9‐CM diagnosis codes. Dyslipidemia was defined as having any or all of the following: any antilipidemic drug prescribed, triglyceride ≥1.7 mmol/L, low high‐density lipoprotein <1.03 mmol/L in men or <1.29 mmol/L in women, high low‐density lipoprotein ≥4.1 mmol/L, and/or the ICD‐9‐CM diagnosis codes for hyperlipidemia. Obesity was defined as body mass index ≥25 kg/m2 and/or the ICD‐9‐CM diagnosis codes.( 22 , 23 )
Statistical Analysis
All statistical analyses were performed using Statistical Product and Service Solutions (SPSS) version 25.0 (SPSS, Inc., Chicago, IL), SAS version 9.4 (SAS Institute Inc., Cary, NC), and R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as mean ± SD or median (interquartile range [IQR]), while categorical variables were presented as number (percentage). Qualitative and quantitative differences between subgroups were analyzed by chi‐square or Fisher’s exact tests for categorical parameters and the Student t test or Mann‐Whitney test for continuous parameters. Expected cumulative incidence of CRC in the general population was estimated by the Ederer II method and compared with cumulative incidence of CRC in patients with NAFLD who had and had not undergone prior colonoscopy, estimated by the Kaplan‐Meier method. Age‐ and sex‐standardized incidence ratio (SIR) of CRC in patients with NAFLD who had and had not undergone prior colonoscopy to the general population was estimated by the Poisson model.
Cox regression was used to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for CRC among patients with NAFLD stratified by different fibrosis‐4 index (FIB‐4) levels and aspirin use during follow‐up. Three models were used to progressively reduce confounding associations for patients with NAFLD stratified by different FIB‐4 levels. The multivariate model 1 was adjusted for sex. The multivariate model 2 was adjusted for sex, type 2 diabetes, and obesity. The multivariate model 3 was adjusted for sex, type 2 diabetes, obesity, and colonoscopy/polypectomy. The three multivariate models were not adjusted for age because age was one of the components of FIB‐4.( 24 ) The Kaplan‐Meier method was used to estimate the cumulative incidence of CRC, whereas a log‐rank test was used to compare the cumulative incidence between groups. All statistical tests were two‐sided. P < 0.05 was taken as statistical significance. SIRs were calculated for CRC in several prespecified subgroups of patients with NAFLD who had and had not undergone prior colonoscopy, including subgroups defined by age, sex, and DM (yes/no). Sensitivity analysis was performed to redefine that patients with NAFLD who had CRC diagnosed within 6 months of the index colonoscopy were considered not to have had prior colonoscopy.
Results
Patient Characteristics
We identified 17,356 subjects with the diagnosis code of fatty liver from January 1, 2000, to December 31, 2014; 9,005 subjects were excluded according to the inclusion and exclusion criteria (Fig. 1). Finally, 8,351 patients with NAFLD were included for analysis. Among these patients, 3,043 had not undergone prior colonoscopy and 5,308 had undergone prior colonoscopy. Among patients who had undergone prior colonoscopy, 804 (15.1%) had undergone colonoscopy once, 690 (13.0%) had undergone colonoscopy twice, and 3,814 (71.9%) had undergone colonoscopy 3 times or more. The median interval between two colonoscopic examinations was 2.6 (IQR, 0.7‐4.4) years among patients who had undergone colonoscopy twice or more. At a median follow‐up of 7.4 (IQR, 5.4‐9.6) years, 55 (56.1%) patients who had not undergone prior colonoscopy and 43 (43.9%) patients who had undergone prior colonoscopy developed CRC. The median interval between colonoscopy to CRC discovery in patients in the endoscopy group was 3.5 (IQR, 1.2‐6.8) years; 18 (41.9%) patients had CRC diagnosed within 3 years from the index colonoscopy. The median age was 56.2 (IQR, 49.2‐65.3) years; 45.4% were men (Table 1), and 59.5%, 67.3%, 82.7%, and 25.9% had type 2 diabetes, hypertension, dyslipidemia, and obesity, respectively. In the overall cohort, 3,272 (39.2%) patients were on aspirin; 759 (24.9%) patients who had not undergone prior colonoscopy and 2,513 (47.3%) patients who had undergone prior colonoscopy had aspirin use.
TABLE 1.
Baseline Clinical Characteristics of NAFLD Patients With and Without Prior Colonoscopy
| Characteristics | Overall cohort | Without Colonoscopy | With Colonoscopy | P Value |
|---|---|---|---|---|
| Number (%) | 8,351 | 3,043 (36.4) | 5,308 (63.6) | |
| Age (years) | 56.2 (49.2‐65.3) | 54.2 (47.9‐61.4) | 57.4 (50.0‐67.4) | <0.001 |
| Male sex, n (%) | 3,791 (45.4) | 1,483 (48.7) | 2,308 (43.5) | <0.001 |
| Ever smoker, n (%) ‡ | 449 (14.0) | 85 (13.0) | 364 (14.3) | 0.385 |
| BMI (kg/m2) ‡ | 26.2 ± 4.8 | 26.1 ± 4.5 | 26.2 ± 4.9 | 0.965 |
| Fasting glucose (mmol/L)* | 5.8 (5.2‐7.0) | 5.7 (5.2‐6.8) | 5.9 (5.2‐7.2) | <0.001 |
| HbA1c (%) † | 6.4 (5.9‐7.4) | 6.4 (5.9‐7.2) | 6.5 (5.9‐7.5) | <0.001 |
| Total cholesterol (mmol/L) † | 5.1 ± 1.1 | 5.0 ± 1.1 | 5.1 ± 1.2 | <0.001 |
| HDL cholesterol (mmol/L) † | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.881 |
| LDL cholesterol (mmol/L) † | 3.0 ± 1.0 | 2.9 ± 1.0 | 3.0 ± 1.0 | <0.001 |
| Triglycerides (mmol/L) † | 1.7 (1.2‐2.4) | 1.6 (1.2‐2.3) | 1.7 (1.2‐2.4) | <0.001 |
| Albumin (g/L) | 42 (39‐45) | 43 (39‐45) | 42 (38‐45) | <0.001 |
| Total bilirubin (μmol/L) | 11 (8‐15) | 12 (8‐15) | 11 (8‐15) | <0.001 |
| ALT (IU/L) | 41 (24‐67) | 43 (26‐72) | 39 (23‐65) | <0.001 |
| AST (IU/L) † | 30 (22‐46) | 31 (22‐47) | 30 (22‐46) | 0.413 |
| GGT (IU/L) † | 57 (32‐119) | 55 (32‐111) | 58 (33‐122) | 0.069 |
| Hemoglobin (g/dL)* | 13.7 ± 1.7 | 13.9 ± 1.6 | 13.6 ± 1.8 | <0.001 |
| Platelet count (×109/L)* | 244 (202‐293) | 246 (205‐294) | 243 (201‐292) | 0.048 |
| Creatinine (μmol/L)* | 77 (64‐92) | 75 (62‐89) | 78 (64‐94) | <0.001 |
| Uric acid (mmol/L) † | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.978 |
| Diabetes, n (%) | 4,968 (59.5) | 1,612 (53.0) | 3,356 (63.2) | <0.001 |
| Hypertension, n (%) | 5,623 (67.3) | 1,839 (60.4) | 3,784 (71.3) | <0.001 |
| Dyslipidemia, n (%) | 6,907 (82.7) | 2,331 (76.6) | 4,576 (86.2) | <0.001 |
| Obesity, n (%) ‡ | 2,159 (25.9) | 691 (22.7) | 1,468 (27.7) | <0.001 |
| Aspirin, n (%) | 3,272 (39.2) | 759 (24.9) | 2,513 (47.3) | <0.001 |
| FIB‐4 † | 1.13 (0.77‐1.78) | 1.06 (0.75‐1.60) | 1.16 (0.78‐1.90) | <0.001 |
| APRI † | 0.32 (0.21‐0.51) | 0.32 (0.22‐0.51) | 0.32 (0.21‐0.52) | 0.849 |
| Follow‐up duration (years) | 7.4 (5.4‐9.6) | 6.6 (4.7‐8.4) | 7.9 (6.0‐10.7) | <0.001 |
Continuous variables are expressed as mean ± SD or median (IQR).
Missing values <10%.
Missing values 20%‐50%.
Missing values >50%.
Abbreviations: ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; BMI, body mass index; GGT, gamma‐glutamyl transferase; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Compared with patients who had not undergone prior colonoscopy, patients who had undergone prior colonoscopy were older; less likely to be men; more likely to have type 2 diabetes, hypertension, dyslipidemia, and obesity; had lower albumin, total bilirubin, alanine aminotransferase, aspartate aminotransferase (AST), and platelet counts; and had higher creatinine and FIB‐4 values at baseline.
Cumulative Incidence of CRC Compared to the General Population
In the overall NAFLD cohort, the 5‐ and 10‐year cumulative incidence (95% CI) of CRC was 0.5% (0.4%‐0.7%) and 1.4% (1.1%‐1.8%), respectively (Supporting Fig. S1). Compared to the general population, patients with NAFLD in the overall cohort had a similar age‐ and sex‐standardized incidence of CRC (SIR, 1.00; 95% CI, 0.80‐1.24; P = 0.978).
In patients who had not undergone prior colonoscopy in the overall cohort, the 5‐ and 10‐year cumulative incidences of CRC were 0.9% (95% CI, 0.6%‐1.3%) and 2.5% (95% CI, 1.9%‐3.5%), respectively (Fig. 2A). In patients who had undergone prior colonoscopy in the overall cohort, the 5‐ and 10‐year cumulative incidences of CRC were 0.2% (95% CI, 0.1%‐0.4%) and 0.9% (95% CI, 0.6%‐1.3%), respectively. Compared to the general population, patients who had not undergone prior colonoscopy had a higher age‐ and sex‐standardized incidence of CRC (SIR, 2.20; 95% CI, 1.64‐2.88; P < 0.001), whereas patients who had undergone prior colonoscopy had a lower age‐ and sex‐standardized incidence of CRC (SIR, 0.54; 95% CI, 0.37‐0.75; P < 0.001).
FIG. 2.
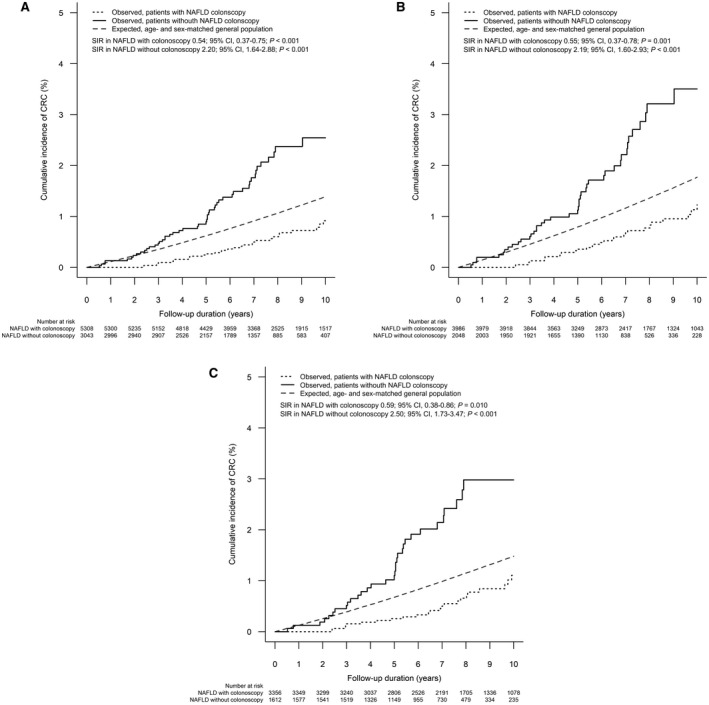
Cumulative incidence of CRC in patients with NAFLD with and without prior colonoscopy. (A) Overall cohort; (B) ≥50‐year‐old subgroup; (C) DM subgroup.
Subgroup and Sensitivity Analysis
Age 50 Years or Above
In this subgroup analysis for patients aged ≥50 years who had not undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 1.1% (95% CI, 0.7%‐1.7%) and 3.5% (95% CI, 2.5%‐4.9%), respectively (Fig. 2B). Among patients aged ≥50 years who had undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 0.3% (95% CI, 0.2%‐0.6%) and 1.1% (95% CI, 0.8%‐1.7%), respectively. Compared with the general population, patients aged ≥50 years who had not undergone prior colonoscopy had a higher incidence of CRC (SIR, 2.19; 95% CI, 1.60‐2.93; P < 0.001), whereas patients aged ≥50 years who had undergone colonoscopy had a lower incidence of CRC (SIR, 0.55; 95% CI, 0.37‐0.78; P = 0.001) (Fig. 3).
FIG. 3.
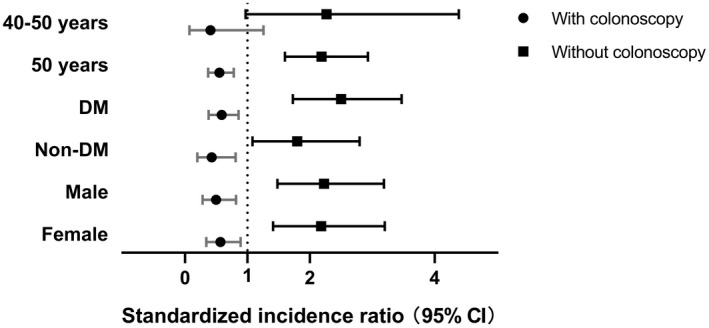
Incidence of CRC in different subgroups of patients with NAFLD with and without prior colonoscopy. Subgroups are 40‐50 years, n = 2,317; 50 years, n = 6,034; DM, n = 4,968; non‐DM, n = 3,383; men, n = 3,791; women, n = 4,560. The box and horizontal lines represent median and IQR, respectively.
Patients With DM
In the subgroup of patients with DM who had not undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 1.1% (95% CI, 0.7%‐1.8%) and 3.0% (95% CI, 2.1%‐4.3%), respectively (Fig. 2C). In patients with DM who had undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 0.3% (95% CI, 0.1%‐0.5%) and 1.1% (95% CI, 0.7%‐1.7%), respectively. Compared with the general population, patients with DM who had not undergone prior colonoscopy had a higher incidence of CRC (SIR, 2.50; 95% CI, 1.73‐3.47; P < 0.001), whereas patients with DM who had undergone prior colonoscopy had a lower incidence of CRC (SIR, 0.59; 95% CI, 0.38‐0.86; P = 0.01).
Sex of Patients
In the subgroup of male patients who had not undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 1.0% (95% CI, 0.6%‐1.7%) and 2.7% (95% CI, 1.8%‐4.1%), respectively. On the other hand, for male patients who had undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 0.2% (95% CI, 0.1%‐0.5%) and 1.0% (95% CI, 0.6%‐1.8%), respectively. Compared with the general population, male patients who had not undergone prior colonoscopy had a higher incidence of CRC (SIR, 2.23; 95% CI, 1.48‐3.19; P < 0.001), whereas male patients who had undergone prior colonoscopy had a lower incidence of CRC (SIR, 0.50; 95% CI, 0.28‐0.82; P = 0.01).
In the subgroup of female patients who had not undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 0.8% (95% CI, 0.5%‐1.5%) and 2.4% (95% CI, 1.5%‐3.8%), respectively. For female patients who had undergone prior colonoscopy, the 5‐ and 10‐year cumulative incidences of CRC were 0.3% (95% CI, 0.1%‐0.6%) and 0.8% (95% CI, 0.5%‐1.4%), respectively. Compared with the general population, female patients who had not undergone prior colonoscopy had a higher incidence of CRC (SIR, 2.18; 95% CI, 1.41‐3.20; P < 0.001), whereas female patients who had undergone prior colonoscopy had a lower incidence of CRC (SIR, 0.57; 95% CI, 0.34‐0.89; P = 0.02).
Interval Between Colonoscopy and CRC
Sensitivity analysis was performed to reclassify patients with NAFLD who had CRC diagnosed within 6 months of the index colonoscopy as no prior colonoscopy. All the findings showed a similar trend to those in the main analysis (Supporting Figs. S2 and S3).
Risk of CRC and Liver Fibrosis
On Kaplan‐Meier analysis, the 5‐ and 10‐year cumulative incidences of CRC were 0.2% (95% CI, 0.1%‐0.4%) and 0.9% (95% CI, 0.6%‐1.4%) in patients with a low FIB‐4 level (<1.3); 0.9% (95% CI, 0.5%‐1.5%) and 1.5% (95% CI, 1.0%‐2.5%) in patients with an intermediate FIB‐4 level (1.3‐2.67); 0.9% (95% CI, 0.4%‐2.1%) and 3.9% (95% CI, 2.1%‐7.3%) in patients with a high FIB‐4 level (>2.67), respectively (log rank, P < 0.001) (Fig. 4).
FIG. 4.
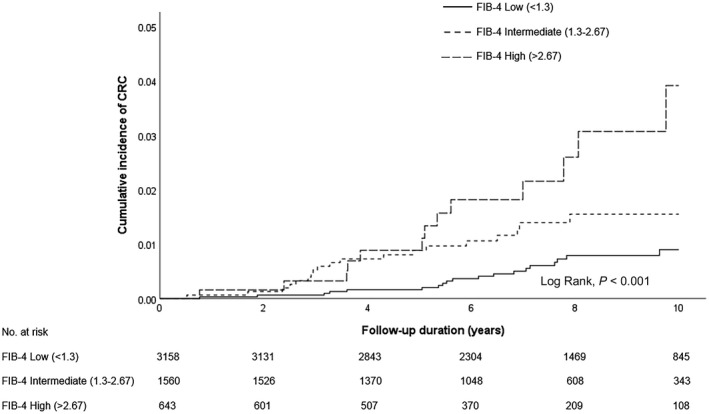
Cumulative incidence of CRC among patients with NAFLD stratified by different FIB‐4 levels.
By univariate analysis, taking patients with a low FIB‐4 level as the reference, patients with increased FIB‐4 had a significantly higher risk of CRC (high FIB‐4 HR, 4.08; 95% CI, 2.01‐8.27; P < 0.001; intermediate FIB‐4 HR, 2.19; 95% CI, 1.16‐4.13; P = 0.016) (Table 2). The association between increased FIB‐4 and CRC remained significant in three multivariable models adjusting for sex, type 2 diabetes, obesity, and prior colonoscopy or polypectomy.
TABLE 2.
HRs of CRC Among Patients With NAFLD Stratified by Different FIB‐4 Levels During Follow‐Up
| Characteristics | Cases | Univariate | Multivariate Model 1 | Multivariate Model 2 | Multivariate Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | ||
| FIB‐4 | |||||||||
| Low (<1.3) | 3,158 | reference | reference | reference | reference | ||||
| Intermediate (1.3‐2.67) | 1,560 | 2.19 (1.16‐4.13) | 0.016 | 2.24 (1.18‐4.23) | 0.013 | 2.16 (1.14‐4.10) | 0.018 | 2.26 (1.19‐4.30) | 0.012 |
| High (>2.67) | 643 | 4.08 (2.01‐8.27) | <0.001 | 4.22 (2.08‐8.58) | <0.001 | 4.04 (1.98‐8.22) | <0.001 | 4.88 (2.38‐10.02) | <0.001 |
Analysis was by Cox regression. Multivariate model 1 was adjusted for sex. Multivariate model 2 was adjusted for sex, type 2 diabetes, and obesity. Multivariate model 3 was adjusted for sex, type 2 diabetes, obesity, and colonoscopy/polypectomy. Multivariate models were not adjusted for age because age was one of the components of FIB‐4.
Risk of CRC and Aspirin Use
On Kaplan‐Meier analysis, the 5‐ and 10‐year cumulative incidences of CRC were 0.4% (95% CI, 0.2%‐0.6%) and 1.2% (95% CI, 0.9%‐1.7%) in patients without aspirin use and 0.7% (95% CI, 0.4%‐1.0%) and 1.8% (95% CI, 1.3%‐2.5%) in patients with aspirin use, respectively (log rank, P = 0.085) (Supporting Fig. S4).
By univariate analysis, taking patients without aspirin use as the reference, patients with aspirin use had no association with risk of CRC (HR, 1.47; 95% CI, 0.95‐2.28; P = 0.087) (Supporting Table S2). After adjusting for age, sex, type 2 diabetes, hypertension, obesity, and colonoscopy/polypectomy, there was still no significant association between CRC and patients with aspirin use (HR, 0.79; 95% CI, 0.49‐1.27; P = 0.336).
Discussion
In this retrospective territory‐wide cohort study, we demonstrated that patients with NAFLD who had not undergone prior colonoscopy had a higher incidence of CRC than the general population and that patients with NAFLD who had undergone prior colonoscopy had a lower incidence of CRC than the general population, especially for those aged ≥50 years or with DM. The severity of NAFLD was also linked with CRC risk; patients with NAFLD with advanced fibrosis, as reflected by a high FIB‐4 score, had a significantly higher risk of CRC.
To the best of our knowledge, this is the first territory‐wide cohort study to report the impact of prior colonoscopy on the incidence of CRC in patients with NAFLD compared with the general population. NAFLD is a well‐established important risk factor for CRC.( 10 , 25 ) Nevertheless, we found that there was no difference in the incidence of CRC between patients with NAFLD for this overall cohort and the general population. This may be because NAFLD prevalence is around 25% in the general population in Asia.( 22 ) Colonoscopy is proven to reduce CRC incidence and mortality.( 2 , 26 ) Our study links these two pieces of important facts by demonstrating that colonoscopy could reduce the incidence of CRC in patients with NAFLD.
Age is an important risk factor for CRC. CRC incidence and mortality are dramatically increased in people aged 50 years or older.( 27 ) The vast majority (about 90%) of new cases of CRC occur in people who are 50 years old or more. Most American and European guidelines therefore recommend regular CRC screening starting from 50 years of age.( 28 , 29 ) Consistently, our study showed that colonoscopy significantly reduced the incidence of CRC in patients with NAFLD aged ≥50 years. However, the incidence of early onset CRC in adults younger than 50 has been increasing worldwide.( 30 ) This has prompted the American Cancer Society to recommend initiating CRC screening at age 45 years instead of age 50 years.( 3 ) However, in our study, we could not demonstrate any difference in the incidence of CRC for patients with NAFLD aged 40‐50 years undergoing colonoscopy or not compared to the general population. One possible reason would be the relatively small number of CRC events (n = 10; 10.2%) in our patients with NAFLD aged 40‐50 years (n = 2,317; 27.7%).
Epidemiologic studies have suggested that DM, especially type 2 DM, is associated with increased risk of cancer, including CRC.( 31 ) It is estimated that approximately 9.5% of CRC in men and women is attributable to DM.( 32 ) The American Diabetes Association and the American Cancer Society suggest that the association between DM and CRC may be partly due to shared risk factors (e.g., insulin resistance, obesity) between the two diseases.( 33 ) In this study, we observed that patients with NAFLD with DM who had undergone prior colonoscopy had a lower incidence of CRC than the general population. However, current guidelines do not specifically recommend patients with DM as a high‐risk group for regular colonoscopy screening. Collectively, our finding highlights the importance of colonoscopy in patients with NAFLD with DM to prevent CRC. Mechanisms linking diabetes and CRC risk remain to be fully illustrated. Insulin resistance has been indicated as one of the most important mediators in which insulin and insulin‐like growth factors may promote cancer development through their proliferative and anti‐apoptotic effects.( 34 ) Inflammation is also a critical component of diabetes‐induced CRC initiation and progression.( 35 ) In addition, diabetes could serve as a surrogate for other established lifestyle factors for CRC, such as sedentary behavior( 36 ) and a western diet.( 37 )
The latest edition of Colorectal Cancer Statistics published by the American Cancer Society reports that the numbers for colon cancer are fairly equal in men and women, and rectal cancer is more common in men.( 38 ) Colorectal cancer screening guidelines in general do not apply sex‐specific recommendations. Similarly, our study observed that there was not much difference in the impact of colonoscopy between male and female patients with NAFLD, providing evidence that both men and women equally need colonoscopy to prevent CRC. The findings were consistent in the sensitivity analysis, which did not classify colonoscopies within 6 months of CRC diagnosis as prior colonoscopies.
NAFLD, especially nonalcoholic steatohepatitis (NASH), may progress to fibrosis, leading to cirrhosis and HCC.( 39 ) Ahn et al.( 25 ) reported that the risk of any colorectal neoplasia or advanced colorectal neoplasia was higher in patients with NAFLD with increased FIB‐4 and NAFLD fibrosis scores. In a systematic review and meta‐analysis, patients with cirrhosis showed a statistically significant increased risk of CRC compared with the general population.( 40 ) However, the association between liver fibrosis in patients with NAFLD and CRC has not been fully elucidated. In our study, we calculated the FIB‐4 score to define liver disease severity and found a stepwise increase in the risk of CRC with increasing FIB‐4 score. After adjusting for traditional risk factors, the association between CRC and patients with NAFLD with advanced fibrosis remained significant. One possible mechanism is that insulin resistance and hyperinsulinemia are particularly severe in patients with advanced liver fibrosis.( 41 ) Some studies have suggested that proinflammatory cytokines play a role in the development of colorectal neoplasia and CRC, which can be altered in NASH as well.( 42 , 43 )
Studies have shown that regular aspirin use is associated with a lower risk of CRC development, cancer‐related mortality, and adenoma prevalence rate.( 44 , 45 ) Nonetheless, it is important to note that prior studies showed that the effect of aspirin on CRC prevention was only apparent after 10 years.( 46 ) In our study, we showed there was no significant association between CRC and patients with aspirin use. This negative finding may be explained by the duration of aspirin use in most of patients was not long enough to show the protective effect or the small number of CRC events did not show a drug effect in a power analysis.
The strengths of our study include a large sample size, long follow‐up duration, and the use of territory‐wide data representative of the local population. Nonetheless, it also has a few limitations. First, we have missing data as in other retrospective studies. In particular, AST is not a routine test, and anthropometric measurements and smoking are not often captured in the electronic health record. Second, the presence of NAFLD was defined by ICD‐9‐CM codes. Previous studies have shown that undercoding of NAFLD is a ubiquitous issue worldwide as this condition is often dismissed as unimportant or not diagnosed.( 47 ) While other studies have used prediction scores based on liver biochemistry and metabolic risk factors, this approach is not ideal in this study as the findings might be driven by other metabolic conditions.( 48 ) Third, other unmeasured factors might have confounded the results. For example, we did not have information on the patients’ family history of CRC and the types and prevalence of adenomas in this cohort. However, we performed multivariable analyses to adjust for some confounding. Fourth, studies have shown that unhealthy diets, namely high intake of red meat, processed meat, sugar‐sweetened beverages, refined grains, desserts, and potatoes are associated with higher CRC risk.( 5 , 49 ) Accordingly, an unhealthy diet is an important risk factor for CRC. Unfortunately, such important dietary data were not captured in our electronic health record system. Fifth, the number of CRC deaths was too small to support further analysis; however, several studies showed that CRC mortality could also be reduced after removal of adenomas during colonoscopies.( 26 , 50 ) Lastly, this was not a screening cohort. Most patients in this study had indications for colonoscopy, such as rectal bleeding and anemia.
In conclusion, this retrospective territory‐wide cohort study demonstrated that patients with NAFLD who had undergone colonoscopy had a lower incidence of CRC than the general population, especially among those aged ≥50 years or with DM. A high FIB‐4 index was associated with a significantly higher risk of CRC.
Supporting information
Supplementary Material
Potential conflict of interest: Dr. Wong advises 3V‐BIO, AbbVie, Allergan, Echosens, Gilead Sciences, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, and Terns; he is on the speakers’ bureau for Bristol‐Myers Squibb, Echosens, Gilead Sciences, and Merck; he received a research grant from Gilead Sciences. Dr. Yip advises and is on the speakers’ bureau for Gilead Sciences. Dr. Chan is a member of the board of directors for Shanghai Henlius Biotech Inc.; he advises AbbVie, Aligos, Aptorum, Arbutus, Hepion, Intellia, Janssen, Gilead, Medimmune, Roche, Vaccitech, VenetoRx, Vir Biotechnology, and GRAIL; he is on the speakers’ bureau for Mylan, Gilead, and Roche. Dr. Wong advises Gilead Sciences and Janssen and is on the speakers’ bureau for Abbott, AbbVie, Ascletis, Bristol‐Myers Squibb, Echosens, Gilead Sciences, Janssen, and Roche; she received a research grant from Gilead Sciences.
References
Author names in bold designate shared co‐first authorship.
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394‐424.Erratum in: CA Cancer J Clin 2020;70:313. [DOI] [PubMed] [Google Scholar]
- 2. Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;315:2576‐2594.Erratum in: JAMA 2016;316:545. [DOI] [PubMed] [Google Scholar]
- 3. Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250‐281. [DOI] [PubMed] [Google Scholar]
- 4. Sung JJ, Choi SY, Chan FK, Ching JY, Lau JT, Griffiths S. Obstacles to colorectal cancer screening in Chinese: a study based on the health belief model. Am J Gastroenterol 2008;103:974‐981. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Goh GB, Chan W‐K, Wong GL, Fan J‐G, Seto W‐K, et al. Unhealthy lifestyle habits and physical inactivity among Asian patients with non‐alcoholic fatty liver disease. Liver Int 2020;40:2719‐2731. [DOI] [PubMed] [Google Scholar]
- 6. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non‐alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta‐analysis of 501 022 adult individuals. Gut 2020. 10.1136/gutjnl-2020-322572. [DOI] [PubMed] [Google Scholar]
- 7. Wong VW, Wong GL, Yeung DK, Lau TK, Chan CK, Chim AM, et al. Incidence of non‐alcoholic fatty liver disease in Hong Kong: a population study with paired proton‐magnetic resonance spectroscopy. J Hepatol 2015;62:182‐189. [DOI] [PubMed] [Google Scholar]
- 8. Bailly L, Fabre R, Pradier C, Iannelli A. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surg 2020;155:395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H, Zheng X, Zong X, Li Z, Li NA, Hur J, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early‐onset colorectal cancer. Gut 2020. 10.1136/gutjnl-2020-321661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z, Zhao X, Chen S, Wang Y, Cao L, Liao W, et al. Associations between nonalcoholic fatty liver disease and cancers in a large cohort in China. Clin Gastroenterol Hepatol 2020. 10.1016/j.cgh.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 11. Wong VW, Wong GL, Tsang SW, Fan T, Chu WC, Woo J, et al. High prevalence of colorectal neoplasm in patients with non‐alcoholic steatohepatitis. Gut 2011;60:829‐836. [DOI] [PubMed] [Google Scholar]
- 12. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611‐1625.e1612. [DOI] [PubMed] [Google Scholar]
- 13. Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N, et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol 2020;18:2324‐2331.e2324. [DOI] [PubMed] [Google Scholar]
- 14. Yeung M‐W, Wong GL, Choi KC, Luk AO, Kwok R, Shu SS, et al. Advanced liver fibrosis but not steatosis is independently associated with albuminuria in Chinese patients with type 2 diabetes. J Hepatol 2017. 10.1016/j.jhep.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 15. Sing CW, Woo YC, Lee ACH, Lam JKY, Chu JKP, Wong ICK, et al. Validity of major osteoporotic fracture diagnosis codes in the Clinical Data Analysis and Reporting System in Hong Kong. Pharmacoepidemiol Drug Saf 2017;26:973‐976. [DOI] [PubMed] [Google Scholar]
- 16. Cheung NT, Fung V, Kong JH. The Hong Kong Hospital Authority's information architecture. Stud Health Technol Inform 2004;107:1183‐1186. [PubMed] [Google Scholar]
- 17. Wong GL, Chan HL, Tse Y‐K, Yip TC, Lam KL, Lui GC, et al. Chronic kidney disease progression in patients with chronic hepatitis B on tenofovir, entecavir, or no treatment. Aliment Pharmacol Ther 2018;48:984‐992. [DOI] [PubMed] [Google Scholar]
- 18. Yip TC, Wong GL, Wong VW, Tse YK, Lui GC, Lam KL, et al. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue‐treated patients. J Hepatol 2017. 10.1016/j.jhep.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 19. Wong JC, Chan HL, Tse YK, Yip TC, Wong VW, Wong GL. Statins reduce the risk of liver decompensation and death in chronic viral hepatitis: a propensity score weighted landmark analysis. Aliment Pharmacol Ther 2017;46:1001‐1010. [DOI] [PubMed] [Google Scholar]
- 20. Lam TH, Wong KH, Chan KK, Chan MC, Chao DV, Cheung AN, et al. Recommendations on prevention and screening for colorectal cancer in Hong Kong. Hong Kong Med J 2018;24:521‐526. [DOI] [PubMed] [Google Scholar]
- 21. Yip TF, Wong GH, Tse YK, Yuen BY, Luk HS, Lam MB, et al. High incidence of hepatocellular carcinoma and cirrhotic complications in patients with psychiatric illness: a territory‐wide cohort study. BMC Gastroenterol 2020;20:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol 2017;67:862‐873. [DOI] [PubMed] [Google Scholar]
- 23. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol 2017;67:1265‐1273. [DOI] [PubMed] [Google Scholar]
- 24. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 25. Ahn JS, Sinn DH, Min YW, Hong SN, Kim HS, Jung S‐H, et al. Non‐alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther 2017;45:345‐353. [DOI] [PubMed] [Google Scholar]
- 26. Lee JK, Jensen CD, Levin TR, Doubeni CA, Zauber AG, Chubak J, et al. Long‐term risk of colorectal cancer and related death after adenoma removal in a large, community‐based population. Gastroenterology 2020;158:884‐894.e885.Erratum in: Gastroenterology 2020. 10.1053/j.gastro.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490‐1502. [DOI] [PubMed] [Google Scholar]
- 28. US Preventive Services Task Force ; Bibbins‐Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FAR, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016;315:2564‐2575.Erratum in: JAMA 2017;317:2239. [DOI] [PubMed] [Google Scholar]
- 29. Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, et al.; Hereditary CRC guidelines eDelphi consensus group . Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020;69:411‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68:2179‐2185. [DOI] [PubMed] [Google Scholar]
- 31. Suh S, Kim KW. Diabetes and cancer: is diabetes causally related to cancer? Diabetes Metab J 2011;35:193‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pearson‐Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body‐mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018;6:e6‐e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pollak M. Insulin and insulin‐like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915‐928.Erratum in: Nat Rev Cancer 2009;9:224. [DOI] [PubMed] [Google Scholar]
- 35. Josse C, Bours V. MicroRNAs and inflammation in colorectal cancer. Adv Exp Med Biol 2016;937:53‐69. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen LH, Liu PH, Zheng X, Keum N, Zong X, Li X, et al. Sedentary behaviors, TV viewing time, and risk of young‐onset colorectal cancer. JNCI Cancer Spectr 2018;2:pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farinetti A, Zurlo V, Manenti A, Coppi F, Mattioli AV. Mediterranean diet and colorectal cancer: a systematic review. Nutrition 2017;43‐44:83‐88. [DOI] [PubMed] [Google Scholar]
- 38. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 39. Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, Castellanos M, Aller‐de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology 2018;155:443‐457.e417. [DOI] [PubMed] [Google Scholar]
- 40. Komaki Y, Komaki F, Micic D, Ido A, Sakuraba A. Risk of colorectal cancer in chronic liver diseases: a systematic review and meta‐analysis. Gastrointest Endosc 2017;86:93‐104.e105. [DOI] [PubMed] [Google Scholar]
- 41. Wong VW, Hui AY, Tsang SW, Chan JL, Tse AM, Chan K, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006;4:1154‐1161. [DOI] [PubMed] [Google Scholar]
- 42. Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res 2008;68:323‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song M, Sasazuki S, Camargo MC, Shimazu T, Charvat H, Yamaji T, et al. Circulating inflammatory markers and colorectal cancer risk: a prospective case‐cohort study in Japan. Int J Cancer 2018;143:2767‐2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. U.S. Preventive Services Task Force . Routine aspirin or nonsteroidal anti‐inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2007;146:361‐364. [PubMed] [Google Scholar]
- 45. Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016;16:173‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long‐term use of aspirin and nonsteroidal anti‐inflammatory drugs and risk of colorectal cancer. JAMA 2005;294:914‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pang Y, Kartsonaki C, Turnbull I, Guo YU, Clarke R, Chen Y, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology 2018;68:1308‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yip TC, Ma AJ, Wong VW, Tse YK, Chan HL, Yuen PC, et al. Laboratory parameter‐based machine learning model for excluding non‐alcoholic fatty liver disease (NAFLD) in the general population. Aliment Pharmacol Ther 2017;46:447‐456. [DOI] [PubMed] [Google Scholar]
- 49. Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR, et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology 2017;152:1944‐1953.e1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wieszczy P, Kaminski MF, Franczyk R, Loberg M, Kobiela J, Rupinska M, et al. Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology 2020;158:875‐883.e875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


