Abstract
Hepatocellular carcinoma (HCC) can de novo develop in patients with chronic hepatitis C even after the achievement of sustained virologic response (SVR). We characterized de novo HCC after SVR, comparing it with HCC that developed in patients during persistent hepatitis C virus (HCV) infection. Characteristics, survival rates, and recurrence rates after curative treatment in 178 patients who developed initial HCC after SVR diagnosed between 2014 and 2020 were compared with those of 127 patients with initial HCC that developed during persistent HCV infection diagnosed between 2011 and 2015; HCC was detected under surveillance in both groups. HCC was less advanced and liver function worsened less in patients with SVR than in patients with persistent HCV. The survival rate after diagnosis was significantly higher for patients with SVR than for patients with persistent HCV (1‐, 3‐, and 5‐year survival rates, 98.2%, 92.5%, and 86.8% versus 89.5%, 74.7%, and 60.8%, respectively; P < 0.001). By contrast, the recurrence rate after curative treatment was similar between groups (1‐, 3‐, and 5‐year recurrence rates, 11.6%, 54.6%, and 60.4% versus 24.0%, 46.7%, and 50.4%, respectively; P = 0.7484). Liver function improved between initial HCC diagnosis and recurrence in patients with SVR (P = 0.0191), whereas it worsened in the control group (P < 0.001). In addition, patients with SVR could receive curative treatment for recurrence more frequently than patients with persistent HCV (80.4% versus 47.8%, respectively; P = 0.0008). Conclusion: Survival of patients with de novo HCC after SVR was significantly higher than that of patients in whom HCC developed during persistent HCV infection, despite similar rates of recurrence after curative treatment. A higher prevalence of curative treatment for recurrent HCC and improved liver function contributed to this result.
Abbreviations
- AFP
alpha‐fetoprotein
- ALBI
albumin–bilirubin
- DAA
direct‐acting antiviral
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IQR
interquartile range
- MELD
Model for End‐Stage Liver Disease
- PSM
propensity score matching
- RFA
radiofrequency ablation
- SVR
sustained virologic response
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers worldwide and is currently the second most common cause of cancer‐related death in the world.( 1 , 2 ) Hepatitis C virus (HCV) infection is one of the leading causes of HCC,( 3 , 4 ) which is the main complication of chronic HCV infection. The main reason to eradicate HCV is to prevent HCC and improve overall survival. The recent emergence of oral direct‐acting antiviral (DAA) regimens as anti‐HCV therapy has dramatically improved antiviral efficacy and tolerability. Consequently, the proportion of patients who achieve sustained virologic response (SVR), that is, eradication of HCV, has markedly increased. Of note, DAA regimens have high tolerability, even in elderly patients and patients with cirrhosis.( 5 ) Studies of DAA regimens have demonstrated comparable SVR rates for patients with and without compensated cirrhosis, indicating the high virologic efficacy of these drugs in patients with compensated cirrhosis.( 6 )
However, HCC can develop even in patients with chronic hepatitis C who achieve SVR,( 7 ) albeit a reportedly lower incidence of HCC after SVR.( 8 ) In particular, HCC in patients with SVR may increase in association with the increase in patients with SVR with high risk of HCC development, such as elderly patients or patients with cirrhosis by DAA regimens.( 9 ) Several studies have characterized HCC that initially developed after SVR (not recurrence), i.e., de novo HCC after SVR.( 10 , 11 , 12 , 13 ) Another study reported the improved overall survival of patients with curatively treated HCC who achieved SVR.( 14 ) However, it remains unclear whether de novo HCC after SVR has similar characteristics and prognosis as HCC that develops before SVR (during persistent HCV infection). Therefore, in this study, we attempted to further characterize de novo HCC after SVR, compare it with HCC that developed in patients with persistent HCV infection, and analyze the patients’ prognosis.
Patients and Methods
Patients, Treatment, and Follow‐Up
Patients with chronic hepatitis C who underwent DAA‐based anti‐HCV therapy in one of the participating institutions between October 2014 and December 2018 were considered for enrollment. Among these patients, patients who had a history of HCC before the start of DAA therapy and patients who failed to achieve SVR were excluded. For the remaining patients, patients with de novo HCC that was detected under surveillance after SVR were enrolled in the study, excluding patients with HCC who had not been surveyed for HCC after SVR. All patients who achieved SVR were advised to continue regular biannual visits and undergo surveillance for HCC in Japan, including ultrasonography and laboratory testing.
Between January 2011 and December 2015, 273 patients developed HCC during persistent HCV infection at Ogaki Municipal Hospital or Ehime Prefectural Central Hospital. It means that patients with Child‐Pugh class B or C cirrhosis were not allowed to undergo DAA regimens by the Japanese government during the study period, we excluded 58 patients with Child‐Pugh class B or C liver function. In addition, we excluded 88 patients who were not surveyed for HCC at diagnosis. We ultimately analyzed 127 patients with HCC during persistent HCV infection as a control group (Supporting Fig. S1).
The presence of cirrhosis, including the presence of esophageal or gastric varices, collateral veins due to portal hypertension, or splenomegaly, was defined clinically by imaging and endoscopic study findings, by liver stiffness measurement with ultrasonography or magnetic resonance imaging, and by laboratory liver fibrosis indices, including the fibrosis‐4 index or aspartate aminotransferase‐to‐platelet ratio index. The diagnosis of HCC was based on radiographic appearance (arterial enhancement and delayed washout) or compatible histology. Decisions regarding treatment and follow‐up after diagnosis for each patient were principally based on Japanese guidelines for HCC.( 15 ) Surgical resection or local ablative therapy, including radio frequency ablation (RFA) or the combination of transarterial chemoembolization (TACE) followed by RFA for the purpose of enhancing the effect of RFA,( 16 ) was considered curative treatment. No patients underwent liver transplantation due to the difficulty of cadaveric transplantation in Japan and living donor transplantation. Other therapies, including TACE, hepatic arterial infusion chemotherapy, systemic chemotherapy, molecular‐targeted drugs, and best supportive care, were considered noncurative. In both groups, all patients were followed every 3 months after the initial diagnosis of HCC and underwent surveillance for recurrence.
The entire protocol of this study was in compliance with the Declaration of Helsinki and was approved by the institutional review board of each participating institution. Written informed consent was waived due to the retrospective nature of this study.
Analysis of Characteristics of Patients and Outcomes
Data on demographics, degree of liver dysfunction, tumor burden (including maximum diameter, number of lesions, presence of portal vein invasion, and extrahepatic metastases), HCC tumor marker levels, HCC‐directed treatments, recurrence, and overall survival were collected from medical records. For the assessment of liver function, we used the albumin–bilirubin (ALBI) score( 17 ) and Model for End‐Stage Liver Disease (MELD) score( 18 ) as indicators of liver function. The ALBI score was calculated using the following formula: ALBI = (log10 total bilirubin [TBIL] [μmol/L] × 0.66) + (albumin [ALB] [g/L] × 0.085), where 1 mg/dL = 17.1 μmol/L for TBIL and 1 g/dL = 10 g/L for ALB. Because all study patients had Child‐Pugh class A liver function, it was difficult to analyze changes in liver function by Child‐Pugh class or score. By contrast, ALBI score can assess mild liver dysfunction in more details and is well correlated with results from an indocyanine green test.( 19 ) Lower ALBI scores indicate better liver function. All study patients were followed after the initial diagnosis of HCC to assess for recurrences and survival outcomes.
Statistical Analysis
Differences in percentages between groups were analyzed using the chi‐square test. Differences in continuous variables were analyzed using the Mann‐Whitney U test. The degree of liver fibrosis in resected HCC specimens was compared using the Cochran‐Armitage test. Changes in ALBI score between the initial diagnosis of HCC and recurrence were compared using the paired t test. The date of initial HCC diagnosis was defined as time 0 for calculations of survival and recurrence rates. The date of diagnosis of first recurrence was defined as time 0 for calculations of survival rates after first recurrence. In the survival analysis, we censored patients who were alive at the end of the follow‐up period. Patients with persistent HCV infection were censored at the date of SVR if they achieved SVR after the initial diagnosis of HCC. In the recurrence analysis, we censored patients without recurrence until the end of the follow‐up period. Patients with persistent HCV in whom HCC recurred after achieving SVR were excluded from recurrence. The Kaplan‐Meier method was used to calculate survival and recurrence rates, and the log‐rank test was used to analyze differences.
Cox proportional hazards models were used for univariate and multivariate analyses of factors associated with survival. Variables analyzed included patient age, sex, platelet counts, ALBI score, MELD score, maximum tumor size, number of tumors, portal vein invasion, alpha‐fetoprotein (AFP) levels, and SVR before the development of initial HCC. Variables that reached marginal significance (P < 0.10) in univariate analysis were subsequently included in multivariate analysis. Data analysis was performed using JMP statistical software, version 11.0.0 (Macintosh version; SAS Institute, Cary, NC).
Propensity score matching (PSM) with one‐to‐one pairing of patients was performed using R (The R Foundation for Statistical Computing, Vienna, Austria), with propensity scores matched to two decimal places. PSM between groups was conducted based on patient age, sex, platelet counts, ALBI score, maximum tumor size, number of tumors, and portal vein invasion. The discriminative ability of the propensity score model was assessed using the area under the receiver operating characteristic curve. Calibration was fixed at 0.2. All P values were derived from two‐tailed tests, with P < 0.05 accepted as statistically significant.
Results
Characteristics of HCC That Developed in Patients After SVR Versus Patients With Persistent HCV Infection
During the study period, 6,179 patients with chronic hepatitis C underwent DAA‐based anti‐HCV therapy in one of the participating institutions. Among these patients, 702 patients who had a history of HCC before the start of DAA therapy were excluded. In the remaining 5,477 patients with no history of HCC, 5,248 patients (95.8%) achieved SVR. HCC developed after SVR in 181 of these 5,248 patients (3.4%), with a median interval between SVR and HCC development of 2.59 years (interquartile range [IQR], 1.35‐3.25 years) during a median observation period of 2.51 years (IQR, 1.17‐3.26 years) after SVR. Of these 181 patients, 3 patients who had not been surveyed for HCC after SVR were excluded and the remaining 178 patients in whom de novo HCC after SVR was detected under surveillance were ultimately analyzed (Fig. 1).
FIG. 1.

Schematic representation of patients with de novo HCC after SVR. HCC developed in 181 of 5,248 patients with no history of HCC who achieved SVR by DAA therapy. HCC was detected under surveillance in 178 of 181 patients who enrolled in the study.
The characteristics of patients with HCC that developed after SVR and those in whom HCC developed during persistent HCV infection are compared in Table 1. In patients with persistent HCV infection, 43 patients (33.9%) underwent DAA‐based anti‐HCV therapy at a median of 2.74 years (IQR, 1.68‐3.70 years) after the initial diagnosis of HCC; 40 patients achieved SVR. HCC was more advanced in control patients, with larger tumors and a higher percentage of multinodular HCC tumors. Tumor marker levels at diagnosis were also higher in patients with persistent HCV. However, the percentage of patients who could undergo curative treatment was similar.
TABLE 1.
Characteristics of Patients With Initial HCC That Developed After SVR to HCV Infection and Patients With HCC That Developed During Persistent HCV Infection
| HCC After SVR (n = 178) | HCC With Persistent HCV (n = 127) | P Value | |
|---|---|---|---|
| Age (years, median) | 72 (66, 78) | 74 (69, 79) | 0.0555 |
| Sex (male/female) | 120 (67.4)/58 (32.6) | 81 (63.8)/46 (36.2) | 0.5412 |
| HBsAg (negative/positive) | 173 (97.2)/5 (2.8) | 123 (96.9)/4 (3.2) | 1.0000 |
| Habitual alcohol intake (no/yes) | 126 (70.8)/52 (29.2) | 97 (76.4)/30 (23.6) | 0.2970 |
| Diabetes (no/yes) | 122 (68.5)/56 (31.5) | 91 (71.7)/36 (28.4) | 0.6134 |
| Cirrhosis (no/yes) | 64 (36.0)/114 (64.0) | 41 (32.3)/86 (67.7) | 0.5423 |
| Platelet count (×1,000/μL) | 123 (88, 166) | 115 (85, 152) | 0.2459 |
| ALBI score | −2.818 (−3.063, −2.546) | −2.574 (−2.870, −2.256) | <0.001 |
| MELD score | 7.0 (6.0, 8.3) | 7.0 (6.0, 9.0) | 0.2324 |
| Maximal tumor size (cm) | 1.6 (1.2, 2.0) | 1.9 (1.4, 2.6) | 0.0002 |
| Number of tumors (single/multiple) | 158 (88.8)/20 (11.2) | 94 (74.0)/33 (26.0) | 0.0008 |
| Portal vein invasion (no/yes) | 171 (96.1)/7 (3.9) | 126 (99.2)/1 (0.8) | 0.1546 |
| Extrahepatic metastasis (no/yes) | 178 (100)/0 | 127 (100)/0 | 1.0000 |
| AFP (ng/mL) | 6.3 (4.0, 15.3) | 17.5 (5.3, 69.1) | <0.001 |
| Lens culinaris agglutinin A‐reactive fraction of AFP (%) | 0.5 (0.5, 5.5) | 5.3 (0.5, 9.1) | <0.001 |
| Des‐gamma‐carboxy prothrombin (mAU/mL) | 23 (18, 40) | 32 (17, 76) | 0.0630 |
| Treatment (curative/noncurative) | 148 (83.2)/30 (16.9) | 103 (81.1)/24 (18.9) | 0.6512 |
HCC was detected under surveillance in all patients.
IQRs or percentages are given in parentheses.
Abbreviation: AU, arbitrary unit; HBsAg, hepatitis B virus surface antigen.
Surgical hepatic resection was performed as curative treatment for HCC in 50 patients with SVR and 46 patients with persistent HCV. On pathologic examination (Table 2), the distribution of HCC differentiation was similar between the groups (P = 0.8467). In addition, the prevalence of microscopic vascular invasion or satellite lesions was comparable. The degree of fibrosis of the liver tissue adjacent to the HCC tumor did not differ between patients with SVR and patients with persistent HCV (P = 0.4958).
TABLE 2.
Pathologic Features of Resected HCC That Developed After SVR to HCV Infection and Resected HCC That Developed During Persistent HCV Infection
| HCC After SVR (n = 50) | HCC With Persistent HCV (n = 46) | P Value | |
|---|---|---|---|
| Differentiation | 0.8467 | ||
| Well differentiated | 19 (38.8) | 17 (37.0) | |
| Moderately differentiated | 28 (57.1) | 26 (56.5) | |
| Poorly differentiated | 2 (4.1) | 3 (6.5) | |
| Microvascular invasion | 0.4898 | ||
| Absent | 44 (88.0) | 43 (93.5) | |
| Present | 6 (12.0) | 3 (6.5) | |
| Microscopic satellite lesion | 0.0825 | ||
| Absent | 48 (96.0) | 39 (84.8) | |
| Present | 2 (4.0) | 7 (15.2) | |
| Fibrosis of adjacent liver tissue* | 0.4958 | ||
| F0, F1 | 9 (18.0) | 4 (8.7) | |
| F2 | 5 (10.0) | 3 (6.5) | |
| F3 | 8 (16.0) | 9 (19.6) | |
| F4 | 28 (56.0) | 30 (65.2) |
Data show number (percentage).
Assessed based on METAVIR score.( 24 )
Survival Rates After HCC Diagnosis
Patients were followed up after the initial diagnosis of HCC for a median of 2.36 years (IQR, 1.49‐3.27 years) for patients with SVR and 4.24 years (IQR, 2.34‐5.41 years) for patients with persistent HCV. A comparison of survival rates between patients who developed initial HCC after SVR and patients who developed initial HCC during persistent HCV infection is shown in Fig. 2A. The overall survival rate was significantly higher for patients after SVR than for patients with persistent HCV (P < 0.001). The 1‐, 3‐, and 5‐year survival rates were 98.2%, 92.5%, and 86.8%, respectively, for patients with SVR and 89.5%, 74.7%, and 60.8%, respectively, for patients with persistent HCV.
FIG. 2.

Survival rates of patients with HCC after diagnosis. (A) All study patients. (B) After adjustment for patient age, sex, platelet count, ALBI score, maximum tumor size, number of tumors, and portal vein invasion with PSM. Blue line shows patients who developed HCC after SVR; red line indicates patients who developed HCC during persistent HCV infection. HCC was detected under surveillance in all patients.
Univariate analysis identified patient sex, ALBI score, maximum tumor size, portal vein invasion, AFP, and achievement of SVR before the development of HCC as factors significantly associated with survival. MELD score was marginally associated with survival (Table 3). Mortality was significantly lower for patients with SVR than for patients with persistent HCV in direct comparison (Supporting Table S1). In the multivariate analysis, HCC development after SVR was independently associated with survival after HCC diagnosis in addition to patient sex, ALBI score, maximum tumor size, and AFP. The risk ratio for survival decreased to 26% when HCV was eradicated before the development of initial HCC (Table 3).
TABLE 3.
Univariate and Multivariate Analyses of Factors Associated With Mortality in Patients With HCC
| Factors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| P Value | Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | ||
| Age | Per 1.0 year | 0.1415 | 1.023 (0.993‐1.057) | ||
| Sex | Male | Reference | Reference | ||
| Female | 0.0099 | 0.471 (0.245‐0.841) | 0.0010 | 0.374 (0.192‐0.681) | |
| Platelet count | Per 1,000/μL | 0.9646 | 0.999 (0.952‐1.045) | ||
| ALBI score | Per 1.0 | 0.0008 | 2.298 (1.429‐3.606) | 0.0030 | 2.179 (1.311‐3.563) |
| MELD score | Per 1.0 | 0.0708 | 1.069 (0.994‐1.137) | 0.1635 | 1.061 (0.974‐1.138) |
| Maximum tumor size | Per 1.0 cm | 0.0008 | 1.529 (1.204‐1.892) | 0.0198 | 1.411 (1.058‐1.848) |
| Number of tumors | Single | Reference | |||
| Multiple | 0.1203 | 1.572 (0.883‐2.682) | |||
| Portal vein invasion | Absent | Reference | Reference | ||
| Present | 0.0387 | 4.469 (1.100‐12.686) | 0.4490 | 2.096 (0.257‐11.346) | |
| AFP | Per 1.0 ng/mL | 0.0149 | 1.000 (1.000‐1.000) | 0.0108 | 1.000 (1.000‐1.000) |
| SVR | Persistent HCV | Reference | Reference | ||
| SVR | <0.0001 | 0.277 (0.134‐0.525) | <0.0001 | 0.263 (0.118‐0.530) | |
Abbreviation: CI, confidence interval.
In a sensitivity analysis, the higher survival rate of patients with SVR was still observed for patients with SVR from only Ogaki Municipal Hospital or Ehime Prefectural Central Hospital, the institutions with control patients (Supporting Fig. S2; P = 0.0490). Univariate and multivariate analyses also showed that HCC development after SVR was associated with survival in this sensitivity analysis (Supporting Table S2).
After PSM of patients with SVR and controls for patient age, sex, platelet counts, ALBI score, tumor size, number of tumors, and portal vein invasion (Supporting Table S3), the difference in survival between patients with SVR and patients with persistent HCV remained significant (P = 0.0174; Fig. 2B). The 1‐, 3‐, and 5‐year survival rates were 98.9%, 93.1%, and 82.4%, respectively, for patients with SVR and 90.2%, 80.0%, and 66.5%, respectively, for patients with persistent HCV.
Recurrence Rates in Patients Who Underwent Curative Treatment for Initial HCC and Treatment for Recurrence
HCC recurred after curative treatment for initial HCC in 56 of 148 patients (37.8%) with SVR and 46 of 103 patients (44.7%) with persistent HCV. Recurrence rates after curative treatment for initial HCC between patients with SVR and patients with persistent HCV are compared in Fig. 3. There were no differences in recurrence rates after curative treatment between the two groups (P = 0.7484). The 1‐, 3‐, and 5‐year recurrence rates were 11.6%, 54.6%, and 60.4%, respectively, for patients with SVR and 24.0%, 46.7%, and 50.4%, respectively, for patients with persistent HCV. The ALBI score was significantly lower at the time of recurrence than at the time of initial diagnosis of HCC for patients whose HCC developed after SVR (−2.603 [IQR, −3.096 to −2.603] versus −2.870 [IQR, −3.096 to −2.530]; P = 0.0191), showing improved liver function (Fig. 4A). In patients whose HCC developed during persistent HCV infection, the ALBI score was significantly higher at the time of recurrence (−2.616 [IQR, −2.871 to −2.275] versus −2.176 [IQR, −2.733 to −1.882]; P < 0.001), showing further deterioration of liver function (Fig. 4B). Curative treatment was offered for recurrent HCC to 45 of 56 patients (80.4%) with SVR and 22 of 46 patients (47.8%) with persistent HCV (P = 0.0008).
FIG. 3.
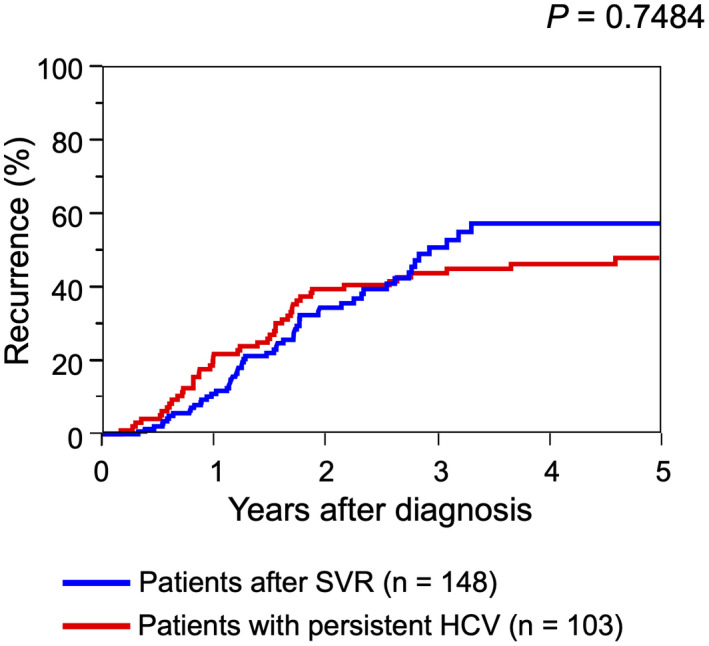
Recurrence rates of patients with HCC who underwent curative treatment for initial HCC. Blue line shows patients who developed HCC after SVR; red line indicates patients who developed HCC during persistent HCV infection.
FIG. 4.
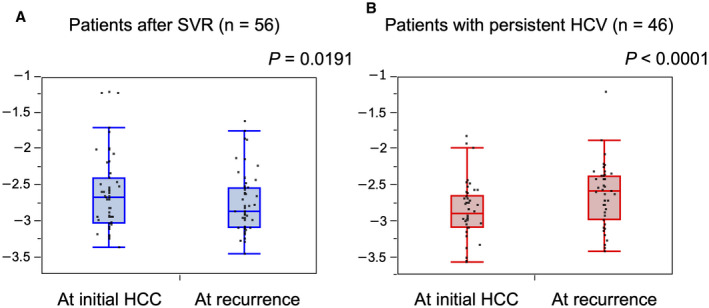
ALBI score at the time of initial diagnosis of HCC and at recurrence in patients who underwent curative treatment for initial HCC. (A) Patients who developed HCC after SVR. (B) Patients who developed HCC during persistent HCV infection. Lower ALBI scores indicate better liver function. Graphs show interquartile range (box), median (horizontal line), and outliers (whiskers).
Survival rates after treatment of recurrent HCC between patients with SVR and patients with persistent HCV are compared in Supporting Fig. S3. The survival rate after recurrence was significantly higher for patients with SVR than for patients with persistent HCV (P = 0.0087). The 1‐, 3‐, and 5‐year survival rates were 94.6%, 75.5%, and 75.5%, respectively, for patients with SVR and 78.8%, 46.5%, and 30.7%, respectively, for patients with persistent HCV.
Discussion
This study adds information on characteristics and prognosis of de novo HCC after SVR to that of previous studies( 10 , 11 , 12 , 13 ) by comparing them with HCC in patients with persistent HCV. Our results showed that the development of de novo HCC after SVR is not unusual; HCC developed in 3.4% of patients with SVR during a median observation period of 2.59 years. This indicates the importance of characterizing HCC after SVR as a new entity.
In Japan, patients who achieve SVR are usually advised to continue surveillance for HCC regardless of the degree of liver fibrosis at SVR. The risk of de novo HCC after SVR was reportedly low in patients with moderate or mild fibrosis at SVR.( 12 , 20 ) However, in the analysis of resected specimens in this study, nearly 20% of patients with SVR had mild or moderate fibrosis (F0, F1, or F2). D’Ambrosio et al.( 21 ) reported that HCC surveillance is warranted in all patients with pretreatment cirrhosis, despite the achievement of SVR, even if they only have mild to moderate fibrosis at post‐SVR HCC diagnosis. The necessity of HCC surveillance for patients with SVR if they only have mild or moderate fibrosis before DAA therapy or after SVR should be investigated further.
When comparing the characteristics of HCC at diagnosis among all study patients, HCC that developed in patients with SVR was less advanced than in patients with persistent HCV. The reasons for this observation are unclear. In both groups, all study patients maintained their biannual visits for surveillance of HCC before diagnosis and regularly underwent ultrasonography and laboratory tests, including AFP. In addition, the surveillance, diagnosis, and treatment strategy for HCC and their quality was constant throughout the study period in Japan. Further, all institutions that participated in this study were high‐volume academic centers with a similar quality of surveillance, diagnosis, and treatment. Therefore, the difference in the time period or institution of HCC diagnosis could not influence the progression of HCC at diagnosis. Elevation of HCC tumor markers, a potential trigger for HCC detection, was less frequent in HCC that developed after SVR relative to HCC that developed during persistent HCV infection. Therefore, tumor markers did not contribute to earlier detection of HCC in patients with SVR. The resolution of liver inflammation in patients with SVR may have facilitated the detection of liver tumors with ultrasonography. Further studies will be necessary to elucidate whether and how SVR contributes to the early detection of HCC under surveillance.
In pathologic analysis of resected HCC specimens, differentiation, prevalence of microvascular invasion, and prevalence of microscopic satellite lesions did not differ among groups. Although a study reported that de novo HCC that develops after SVR achieved with DAA is aggressive,( 22 ) this study did not yield similar results.
The survival rate after diagnosis was significantly higher for patients with SVR. This finding was supported by sensitivity analyses focusing on patients with SVR from institutions where patients with persistent HCV had originated. A less advanced HCC tumor burden in patients with SVR under surveillance might have contributed to higher survival, but the percentage of patients who underwent curative treatment did not differ. By contrast, no difference in recurrence rates was found between subgroups of patients who underwent curative treatment. This result clearly indicates that the eradication of HCV does not suppress the recurrence of HCC once it develops, even when there is no history of HCC before SVR. However, there were marked differences in the change in liver function during the interval between initial HCC diagnosis and recurrence. Whereas liver function was further impaired during this interval in patients with persistent HCV infection, it was improved in patients with SVR. The absence of HCV resulted in maintenance or even improvement of liver function after HCC treatment. This could have contributed to a higher probability of curative treatment for recurrence, resulting in higher survival rates for patients with SVR than in controls.
There were several limitations to this study. The number of patients with HCC developing after SVR was not large even though there were more than 6,000 patients who achieved SVR. Further studies are necessary to confirm the findings of this study. The patients with persistent HCV as control patients were from only two participating institutions based on the availability of sufficient information about HCC, including outcome, in patients with persistent HCV infection. Although the management of HCC, including treatment and follow‐up, is standardized throughout Japan based on guidelines,( 15 , 23 ) the management of patients with HCC might not have been balanced perfectly between the two groups. However, the sensitivity analysis restricted to patients from two institutions with patients in both groups led to similar results.
In conclusion, de novo HCC after SVR is associated with higher survival rates than HCC that develops during persistent HCV infection. The recurrence rate after curative treatment did not differ between the groups, but patients with SVR were more likely to undergo curative treatment for recurrence, resulting in higher overall survival. Although HCC can develop even after SVR in some patients, SVR before the development of initial HCC has an important favorable impact on survival.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1‐S3
Potential conflict of interest: Dr. Atsukawa is on the speakers’ bureau for and received grants from AbbVie. Dr. Toyoda is on the speakers’ bureau for AbbVie, MSD, and Bayer. Dr. Hiraoka is on the speakers’ bureau for Eisai, Bayer, and Otsuka. Dr. Takaguchi is on the speakers’ bureau for AbbVie. The other authors have nothing to report.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends‐‐an update. Cancer Epidemiol Biomarkers Prev 2016;25:16‐27. [DOI] [PubMed] [Google Scholar]
- 3. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology 2013;57:1333‐1342. [DOI] [PubMed] [Google Scholar]
- 4. Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(Suppl. 1):S45‐S57. [DOI] [PubMed] [Google Scholar]
- 5. Toyoda H, Kumada T, Tada T, Shimada N, Takaguchi K, Senoh T, et al. Efficacy and tolerability of an IFN‐free regimen with DCV/ASV for elderly patients infected with HCV genotype 1B. J Hepatol 2017;66:521‐527. [DOI] [PubMed] [Google Scholar]
- 6. Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct‐acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther 2016;43:1276‐1292. [DOI] [PubMed] [Google Scholar]
- 7. Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, et al. A risk for hepatocellular carcinoma persists long‐term after sustained virologic response in patients with hepatitis C‐associated liver cirrhosis. Clin Infect Dis 2013;57:230‐236. [DOI] [PubMed] [Google Scholar]
- 8. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El‐Serag HB. Risk of hepatocellular carcinoma in HCV patients treated with direct‐acting antiviral agents. Gastroenterology 2017;153:996‐1005.e1. [DOI] [PubMed] [Google Scholar]
- 9. Toyoda H, Kumada T, Tada T. Changes in patient backgrounds may increase the incidence of HCC after SVR in the era of IFN‐free therapy for HCV. Hepatology 2016;64:1818‐1819. [DOI] [PubMed] [Google Scholar]
- 10. Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV‐related cirrhosis treated with direct‐acting antiviral. J Hepatol 2016;65:727‐733. [DOI] [PubMed] [Google Scholar]
- 11. Ravi S, Axley P, Jones D, Kodali S, Simpson H, McGuire BM, et al. Unusually high rates of hepatocellular carcinoma after treatment with direct‐acting antiviral therapy for hepatitis C related cirrhosis. Gastroenterology 2017;152:911‐912. [DOI] [PubMed] [Google Scholar]
- 12. Degasperi E, D’Ambrosio R, Iavarone M, Sangiovanni A, Aghemo A, Soffredini R, et al. Factors associated with increased risk of de novo or recurrent hepatocellular carcinoma in patients with cirrhosis treated with direct‐acting antivirals for HCV infection. Clin Gastroenterol Hepatol 2019;17:1183‐1191.e7. [DOI] [PubMed] [Google Scholar]
- 13. Sangiovanni A, Alimenti E, Gattai R, Filomia R, Parente E, Valenti L, et al. Undefined/non‐malignant hepatic nodules are associated with early occurrence of HCC in DAA‐treated patients with HCV‐related cirrhosis. J Hepatol 2020;73:593‐602. [DOI] [PubMed] [Google Scholar]
- 14. Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al.; HCV (RESIST‐HCV) and Italian Liver Cancer (ITA.LI.CA.) Group . Direct‐acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV‐cirrhotic patients. J Hepatol 2019;71:265‐273. [DOI] [PubMed] [Google Scholar]
- 15. Kokudo N, Makuuchi M. Evidence‐based clinical practice guidelines for hepatocellular carcinoma in Japan: the J‐HCC guidelines. J Gastroenterol 2009;44(Suppl. 19):119‐121. [DOI] [PubMed] [Google Scholar]
- 16. Kitamoto M, Imagawa M, Yamada H, Watanabe C, Sumioka M, Satoh O, et al. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentogenol 2003;181:997‐1003. [DOI] [PubMed] [Google Scholar]
- 17. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach‐the ALBI grade. J Clin Oncol 2015;33:550‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 19. Toyoda H, Lai PBS, O'Beirne J, Chong CC, Berhane S, Reeves H, et al. Long‐term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB‐4 scores. Gastroenterology 2019;157:1264‐1278.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Ambrosio R, Aghemo A, Rumi MG, Degasperi E, Sangiovanni A, Maggioni M, et al. Persistence of hepatocellular carcinoma risk in hepatitis C patients with a response to IFN and cirrhosis regression. Liver Int 2018;38:1459‐1467. [DOI] [PubMed] [Google Scholar]
- 22. Nakao Y, Hashimoto S, Abiru S, Komori A, Yamasaki K, Nagaoka S, et al. Rapidly growing, moderately differentiated HCC: a clinicopathological characteristic of HCC occurrence after IFN‐free DAA therapy? J Hepatol 2018;68:854‐855. [DOI] [PubMed] [Google Scholar]
- 23. Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, et al. Evidence‐based clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH‐HCC guidelines). Hepatol Res 2015;45. [DOI] [PubMed] [Google Scholar]
- 24. No authors listed . Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15‐20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1‐S3


