Snake venom–inspired visible light–triggered bioadhesives can be used to treat tissue injuries by accelerating blood clot forming.
Abstract
Bioadhesives reduce operation time and surgical complications. However, in the presence of blood, adhesion strength is often compromised. Inspired by the blood clotting activity of snake venom, we report a visible light–induced blood-resistant hemostatic adhesive (HAD) containing gelatin methacryloyl and reptilase, which is a hemocoagulase (HC) extracted from Bothrops atrox. HAD leads to the activation and aggregation of platelets and efficiently transforms fibrinogen into fibrin to achieve rapid hemostasis and seal the tissue. Blood clotting time with HAD was about 45 s compared with 5 to 6 min without HAD. HAD instantaneously achieved hemostasis on liver incision (~45 s) and cut rat tail (~34 s) and reduced blood loss by 79 and 78%, respectively. HAD is also efficient in sealing severely injured liver and abdominal aorta. HAD has great potential to bridge injured tissues by combing hemostasis with adhesives.
INTRODUCTION
There has been considerable interest in bioadhesives for tissue repair and sealing (1–5). Compared with sutures, tissue adhesives reduce operation time and alleviate surgical complications (6–8). Bioadhesives can be categorized into two classes: (i) synthetic adhesives, such as cyanoacrylates and polyethylene glycol–based adhesives; and (ii) naturally derived adhesives based on fibrin, polysaccharides, albumin, and gelatin (9). Synthetic adhesives can be optimized to the desired properties, but there are concerns about their biocompatibility and toxicity. For example, cyanoacrylates, once initiated by water, will undergo rapid polymerization to form a bonding network with the tissue surface to achieve a rapid and strong adhesive interface. However, potential toxicity limits their use because the degradation of cyanoacrylates would release histotoxic components (cyanoacetate and formaldehyde) and causes inflammatory responses (10, 11). Natural bioadhesives have excellent biocompatibility, but they often have low mechanical integrity and adhesion. Prepared from a number of components produced from pooled human plasma, fibrin glue has excellent properties such as supporting cell growth and biocompatibility, but its poor mechanical strength is still a limitation (7). Typically, the bioadhesion between adhesives and tissues is accomplished via chemical bonds (covalent bonds and ionic bonds) or physical interactions (including hydrogen bonding, hydrophobic interaction, metal complexation, and π-π stacking). Bioinspired adhesive architectures are also designed by simulating animals such as gecko lizards, beetles, endoparasites, octopi, and slugs. For example, a tissue adhesive in the form of dry double-sided tape that can form a tough and strong adhesive in seconds by removing interfacial water from the surface, followed by covalent cross-linking with tissue is reported (2). A photocrosslinked biodegradable elastomer having immobilized type I collagen enhanced in vitro cellular attachment and proliferation (12). A bioinspired adhesive by electrostatic interactions, covalent bonds, and physical interpenetration and amplified energy dissipation achieved tough adhering to the substrate (1). Although progress has been made toward improved bioadhesives, bonding failure at the tissue interface during bleeding is detrimental because bleeding weakens the adhesive interaction with adjacent tissues (13–15). Bleeding due to injury, trauma, and surgical procedures is a primary issue leading to morbidities and mortality (16–18). Compressible hemorrhage happens in accessible sites such as the extremities, where physical pressure or stress can be applied to alleviate severe bleeding scenarios. In general, death from compressible hemorrhage can be avoidable by rapid hemostasis. However, a noncompressible hemorrhage is found in nonaccessible sites where surgical intervention is needed for hemostasis. Noncompressive hemorrhage is the main cause of death on battlefields and in civilian traumatic injuries (19). Therefore, wound closure and hemostasis become a key bioadhesive design goal to avoid blood leakage to maintain adhesive strength and save lives (20, 21).
In a natural hemostasis process, platelet aggregation and fibrin mesh formation make a temporary sealant to control bleeding (22, 23). Fibrin plays a significant role in hemostasis and wound healing (24, 25) because the activated platelets can generate contractile forces through fibrin fibers to shrink the clot (26). The venom of the snake Bothrops atrox is reported to have blood procoagulant activity by rapidly converting fibrinogen to fibrin (27–29). This procoagulant enzyme, known as reptilase, cleaves fibrinogen at Arg16-Gly17 releasing fibrinopeptide A and generating fibrin and is used in functional clotting assays. Hartgerink and co-workers (30) showed that batroxobin-loaded hydrogels have the potential to enhance clotting and rapidly stop bleeding in both normal and heparin-treated rats in a lateral liver incision model. Extending the use of B. atrox reptilase beyond functional clotting assay, we designed a previously unidentified bioadhesive for hemostasis by incorporating it into methacrylated gelatin. Gelatin is a derivative of collagen with low immunogenicity (31, 32) and can be modified to have pendant functional groups such as methacrylates [gelatin methacryloyl (gelatin-MA, hereafter referred to as GelMA)] (33). Recently, light-induced photocrosslinking adhesives have attracted great interest for their controllable gelation time and tunable physical properties (34). Although ultraviolet (UV) light–cross-linkable tissue sealants have been widely reported for repairing wounds (33, 35), in situ UV cross-linking may cause oxidative DNA damage to tissues (36). Visible light with a longer wavelength could reduce tissue damages and improve cell viability (37, 38). Furthermore, visible light can penetrate tissues deeper with lower energy (39). For instance, Eosin Y, a U.S. Food and Drug Administration–approved visible light photosensitizer, has proven to be a safe photocrosslinking system for biomedical applications (34). During polymerization, a triplet state of Eosin Y can accept hydrogen atoms from coinitiator triethanolamine (TEA) (40), while the comonomer N-vinylcaprolactam (VC) can accelerate the gelation of GelMA (10, 41). These characteristics make visible light suitable for in situ injectable and cross-linkable materials. Nevertheless, GelMA has limitations in severe bleeding cases because of its relatively low hemostatic performance, thus weakening the adhesion efficacy (7, 9).
To address the concerns of tissue adhesive failure, we propose a hemostatic bioadhesive (HAD) composed of hemocoagulase (HC, the same as reptilase) and GelMA inspired by the coagulation function of snake venom. Visible light (430 to 530 nm) photocrosslinking of HAD in the bleeding tissue can create a physical barrier and accelerate fibrin formation to stop bleeding. In vitro clotting time assay was conducted for coagulation efficiency. Rat tail and rat liver wound models and closure of abdominal aorta defects with HAD were performed to test hemostasis and adhesive efficacy. Fibrin formation and the morphological changes of blood cells were studied.
RESULTS
Preparation and characterizations of HAD
The visible light–cross-linked adhesive was formed by the photochemical reaction of MA side groups on GelMA activated by the photosensitizer Eosin Y. The reaction mechanism is shown in Fig. 1A. When exposed to visible light, the cross-linking of HAD can be achieved by free radical polymerization with the help of Eosin Y, TEA, and VC. Figure 1B shows digital images of HAD gel formation in response to visible light exposure for 60 s. Figure 1C shows the UV-visible (UV-vis) absorption spectra of all components. It is noted that GelMA, VC, TEA, and HC rarely absorb light when the wavelength exceeds 400 nm. However, Eosin Y and HAD have a broad absorption from 450 to 550 nm with a peak around 516 nm, which indicates that Eosin Y was present in HAD (40). According to a previous report (10), Eosin Y as a photoinitiator can be excited from the ground state to a triplet state by visible light (450 to 550 nm) and extracts hydrogen atoms from TEA coinitiator. The deprotonated TEA radical then initiated the formation of a radical center on the MA groups of GelMA and cross-linked through free radical reaction. With the reaction going on, the red Eosin Y became almost colorless, and the absorption ability decreased at 450 to 550 nm. As shown in Fig. 1D, with the illumination time increased, the absorption from 430 to 550 nm gradually declined. All the above results demonstrated that HAD had been cross-linked by visible light. To verify the visible light cross-linking, gelatin, GelMA, and HAD (photocrosslinked for 100 s) were partially dissolved in dimethyl sulfoxide (DMSO)–d6 for 1H nuclear magnetic resonance (NMR) analysis (10). As shown in fig. S1A, compared with gelatin, the peaks at 5.52 and 5.28 parts per million indicated that the MA groups were successfully incorporated into the gelatin, thus forming GelMA (42, 43). After a 60-s treatment, the peaks of MA (─C═CH2) functional groups in the gelatin disappeared, which demonstrated that the hydrogel was cross-linked (10). Figure S1B shows the Fourier transform infrared (FTIR) of gelatin, GelMA, and HAD. In the spectrum of gelation and GelMA, the absorption peak at 3294 cm−1 belongs to the N─H bands stretching vibration (amide A), and the characteristic band at 1534 cm−1 is attributed to the stretching vibration of N─H bands (amide B) (44). The peak at 3076 cm−1 of GelMA belongs to the stretching vibration of C═C─H (45). It can be seen that the methacrylate reaction did not change the basic chemical structure of gelatin (46). After visible light exposure, the peak at 3076 cm−1 almost disappeared, which is consistent with the 1H NMR that the hydrogel has cross-linked through a free radical polymerization. Scanning electron microscopy (SEM) (Fig. 1, E and F) showed that the microstructures of GelMA and HAD were well defined with smooth, porous walls, and the average pore size was about 50 μm, which indicated that the presence of HC did not affect the original GelMA structure. In addition, the pores sizes in the hydrogels range from 20 to 100 μm.
Fig. 1. Preparation and characterizations of HAD.

(A) Schematic representation of a visible light–induced photopolymerization system. (B) Digital photograph of the HAD gelling transition before (top), during (middle), and after visible light illumination (bottom). (C) UV-vis absorption spectra of TEA, VC, HC, GelMA, Eosin Y, GelMA prepolymer, and HAD prepolymer. Concentration of TEA, VC, Eosin Y, HC, and GelMA was 1.88, 1.25% (w/v), 0.5 mM, and 1 and 20% (w/v), respectively. (D) UV-vis absorption spectra of HAD after illumination for 0, 20, 40, and 60 s. (E and F) SEM images of GelMA and HAD, respectively. GelMA and HAD were freeze-dried and used for SEM observation. The average pore size was about 50 μm for both HAD and GelMA. Scale bar, 30 μm. Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
Mechanical properties of HAD
Rheology tests were performed on HAD to monitor the gelling process. From dynamic time sweep (Fig. 2A), the storage modulus G′ was lower than loss modulus G″ before light exposure, but as the illumination time increased, G′ values exceeded G″ at 30 s, confirming irreversible gelation (33). As shown in Fig. 2B, when the strain was larger than 274%, G″ was higher than G′, indicating gel collapsing. Oscillation frequency tests showed that the adhesive was stable under the 0.1 to 100 rad/s condition (Fig. 2C).
Fig. 2. Mechanical properties of HAD.
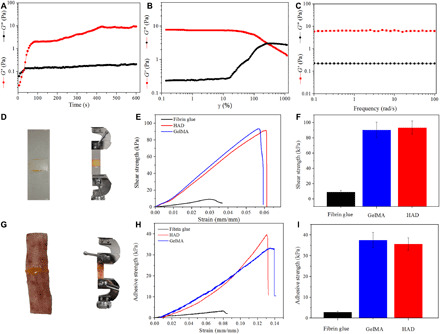
(A) Dynamic time-sweep rheological analysis showing the gelation kinetics of HAD. (B) G′ and G″ of HAD on strain amplitude sweep (γ = 0.1 to 1000%) at a fixed angular frequency (10 rad s−1). (C) Rheological performance of HAD under oscillation frequency test at 37°C. (D) Schematic of the lap shear test to determine the shear strength of HAD, GelMA, and fibrin glue on glass slides (n = 3). (E) Shear strength (stress) versus strain curves of lap shear tests. (F) Lap shear strength of HAD, GelMA, and fibrin glue (n = 3). (G) Porcine skin as a substrate for end-to-end adhesion strength of HAD, GelMA, and fibrin glue sealant. (H) Adhesive strength (stress) versus strain curves on a porcine skin test. (I) The end-to-end adhesive strength of HAD, GelMA, and fibrin glue (n = 3). For the dynamic time-sweep rheological analysis, the gel was cross-linked in situ during the course of the experiment (A). For other tests (B to I), the gel was precross-linked. Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
The adhesion strength of the hydrogel is one of the most important properties to evaluate the interfacial interaction of adhesives and tissue (1). In this study, lap shear strength and the end-to-end adhesive strength of HAD were evaluated. Fibrin glue, a commercially available medical adhesive, was used as a control. Figure 2D displays the lap shear test. As presented in Fig. 2 (E and F), there was no significant difference between the shear strength of GelMA (90.55 ± 10.26 kPa) and HAD (93.52 ± 8.61 kPa). However, the shear strength of the fibrin glue (9.08 ± 2.35 kPa) was significantly lower than that of HAD. An end-to-end adhesive test was conducted on pig skin (Fig. 2G), and the adhesive strength of GelMA and HAD were 37.56 ± 3.60 kPa and 35.64 ± 2.96 kPa, respectively (Fig. 2, H and I). A much lower adhesive strength of 2.93 ± 0.41 kPa was observed for fibrin glue. Overall, the hemostatic adhesive demonstrated superior mechanical properties in both glass slide (lap shear) and porcine skin (end to end).
In vitro hemostatic performance of HAD
The amount of HC to be incorporated in HAD was chosen by monitoring fibrin formation (at 405 nm) in platelet-poor plasma (PPP) over time (Fig. 3, A and B). Ca2+ was used as a control because it plays an important role in blood clotting (47). The relative absorption of the clotting plasma curve shows that with the concentration of HC increased from 0.25 to 2.0 mg/ml, the corresponding relative slope also increased. Because the slope did not change above 1.0 mg/ml HC concentration (Fig. 3B), 1.0 mg/ml was chosen as the optimum HC dose incorporated into HAD. In Fig. 3C, we also studied the HC release from HAD by the potency relationship. In the first 3 min, 0.25 mg of HC was released from 1 ml of HAD, and by 30 min, the released HC reached 0.65 mg. The released HC was also bioactive to convert fibrinogen to fibrin. The hemostatic ability of HAD was evaluated by monitoring the clotting time of blood in contact with the hydrogel surface in a 96-well plate (Fig. 3D and movie S1). Under normal conditions without any intervention, blood will clot in 5 to 6 min (48, 49). As expected, the control group clotting time was 5.61 ± 0.51 min (Fig. 3, D and E). The HC and GelMA groups significantly decreased the clotting times, with 3.74 ± 0.32 min and 2.13 ± 0.23 min, respectively. As a positive control, the clotting time with fibrin glue was 1.29 ± 0.13 min. HC accelerated clotting by converting fibrinogen into fibrin, and the HAD group showed an unexpected decrease of the clotting time to 0.76 ± 0.05 min. We further investigated the morphology of blood with different contact times by SEM. For the GelMA group, the red blood cells (RBCs) started to aggregate on the surface of the adhesive, but there was no sign of fibrin formation at 1 min (Fig. 3F). Some fibrin meshwork was sparsely observed after 2 and 3 min of contacts on the GelMA (Fig. 3, G and H). Compared with the GelMA group, RBC aggregation on HAD at 1 min was accompanied by a loose fibrin meshwork (Fig. 3I). After 2- and 3-min contact with HAD, the fibrin mesh density covering the RBCs increased (Fig. 3, J and K), indicating that HAD accelerated fibrin meshwork formation. We also quantified the fibrin morphology via SEM image analysis. Ca2+, GelMA, and HC were used as controls. As shown in fig. S2, the average fibrin fiber size in Ca2+, HC, GelMA, and HAD groups was 107.59, 142.50, 103.77, and 196.91 nm, respectively. The average fibrin meshwork pore size for Ca2+, HC, GelMA, and HAD group was 266.52, 316.53, 392.45, and 223.88 nm, respectively. The fibrin incubated with Ca2+ has a similar fiber size of around 100 nm to GelMA, but the GelMA group generated less fibrin. The fibrin incubated with HAD was significantly thicker, which indicated that the combination of the HC and GelMA was a plus for fibrin generation. In the clotting SEM images (Fig. 3, F to K), there was no clear sign of whole-blood platelet aggregation both in the GelMA group and the HAD group. Therefore, platelet-rich plasma (PRP) was prepared to investigate platelet interactions with HAD. Platelet adsorption to HAD is shown in fig. S3A, which demonstrated the high adhesion of platelets on HAD. Activated platelets adhered to the surface of HAD, and some fibrin was found (fig. S3B). These results demonstrated that the quick clot formation was attributed to the synergy between GelMA and HC, where activated platelets and RBCs adhered to the adhesive, and the HC in HAD converted fibrinogen into fibrin. Collectively, these in vitro clot data showed that HC in GelMA could significantly reduce blood clotting time.
Fig. 3. In vitro hemostatic performance of HAD.

(A) Selection of the amount of HC to be incorporated in the GelMA by plasma clotting kinetics. (B) Slope (clotting rate) in the linear region of plasma clotting kinetics curves (n = 3). (C) The release of HC incorporated in HAD (n = 3). (D) Clot formation as a function of time for control, HC, GelMA, HAD, and fibrin glue. (E) Quantitative clot times (n = 3). (F to H) SEM images of whole-blood contact with GelMA for 1, 2, and 3 min, respectively. (I to K) SEM images of whole-blood contact with HAD for 1, 2, and 3 min, respectively. Scale bars, 3 μm. (*P < 0.05, **P < 0.01). Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
In vivo biodegradation and biocompatibility of HAD
As shown in fig. S4A, the implanted HAD hydrogel showed gradual degradation. The degradation rate was evaluated by volumes (fig. S4B). After 28 days of degradation, only 17.47% of the gel volume remained. Hematoxylin and eosin (H&E) staining revealed a small amount of mononuclear inflammatory cell recruitment at days 7 and 28, indicating a minor local host inflammatory response (fig. S4, C and D). Through immunofluorescence staining, macrophages (F4/80) and lymphocytes (CD3) were used to further characterize the local immune response. CD3+ lymphocyte invasion at the interface between HAD gel and the subcutaneous tissue was observed on day 7 but reduced at day 28. Furthermore, the F4/80+ macrophage infiltration observed on day 7 almost disappeared at day 28 (fig. S4, E and F). These degradation results demonstrated that HAD maintains dimensional stability to allow tissue healing while eliciting only minimal inflammatory responses by the host. L929 fibroblastic cells were used to evaluate the cytocompatibility of HAD. As shown in fig. S5, over 98% of cell viability was observed after 5 days of culture, suggesting that HAD did not have toxicity effects.
In vivo HAD hemostasis evaluation
The in vivo hemostatic performance was first examined by the rat tail amputation model (Fig. 4A). Tail cutting will cause injury to multiple types of tissues, including skin, bone, connective tissue, and three large blood vessels (a central artery and two lateral veins) (50). Immediately after tail cutting, 20 μl of HAD pregel solution was applied to the wound under visible light illumination for 60 s (Fig. 4B). Clearly, there was only very little blood loss during the light-induced HAD gelling. Shown in Fig. 4C are the qualitative bleeding wounds for the different treatment conditions over 5 min. The blood loss in the control groups (untreated group) was significantly more than other groups, reaching 178.66 ± 31.45 mg of blood, and 2.54 ± 0.26 min was needed to achieve hemostasis (Fig. 4, D and E, and movie S2). For HC and GelMA groups, less blood loss and shorter hemostasis times were found (Fig. 4, D and E). HC took 1.72 ± 0.31 min to achieve hemostasis and lost 121.30 ± 10.53 mg of blood. For GelMA, hemostasis time was 1.12 ± 0.24 min with 88.12 ± 10.79 mg of blood loss. Fibrin glue reduced hemostasis time to 0.86 ± 0.11 min with 78.65 ± 7.58 mg of blood loss. However, it took only 0.57 ± 0.11 min and 39.62 ± 7.13 mg of blood loss for HAD (movie S3). The significant reduction in both hemostasis time and blood loss from the current hemostatic adhesive suggested that the combination of GelMA and HC could efficiently achieve hemorrhage control and thus maintain the adhesive mechanics.
Fig. 4. Hemostatic performance of HAD in rat tail amputation model.

(A) Schematic illustration of the establishment of rat tail bleeding and hemostatic model. (B) The hemostatic test process in the rat tail model. (C) Qualitative bleeding images for different treatments. (D and E) Blood loss and hemostasis time, respectively (n = 3). (*P < 0.05, **P < 0.01, and ***P < 0.001). Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
Skin incision adhesion and wound healing in normal rats
The in vivo adhesion of HAD was evaluated by the rat skin incision model (Fig. 5A). A 2-cm incision was made using a surgical knife, and 20 μl of HAD pregel solution was added to the injury site, followed by pressing the wound edge in contact and light illumination for 1 min. As a comparison, sutures, GelMA, and fibrin glue were also used to treat the incision. It was observed that in GelMA- and HAD-treated groups, the skin was well bridged, and the adhering effect of the skin incision was better than the fibrin glue group (Fig. 5B). All wound sites were examined daily to observe the healing. On day 3, no wound split was observed on the GelMA- and HAD-treated end-to-end adhesion skin, while wound split was still observed on fibrin glue–treated skin. On day 5, the HAD- and suture-treated wound was completely closed. However, for the control, GelMA, and fibrin glue groups, the wound was not completely closed. On day 20, the wound in all groups was completely closed. Histological analysis was conducted to evaluate wound healing on days 5 and 20 (Fig. 5C). In the HAD group, the epithelium fused with the basement membrane continuously, and no deep opening in the tissue was found on day 5. GelMA and suture groups showed some tissue granulation filled with nuclear-stained neonate cells, which means that the wound healed well. In the fibrin glue group, wound healing was limited by poor adhesive strength, so it was better than the untreated group but worse than the HAD group. By day 20, all the groups were epithelialized, and the HAD- and suture-treated wound grew hair follicles. We also quantified the healing response by measuring the wound contraction area on day 5. According to fig. S6, the HAD group wound areas were significantly reduced compared to the untreated and fibrin glue groups. Therefore, HAD exhibited the highest adhesive strength to the epidermis and accelerated wound healing.
Fig. 5. Skin wound closure with different treatments.

(A) Schematic illustration of the skin incision. (B) Photographs of the incision at days 0, 3, 5, and 20 (D0, D3, D5, and D20). (C) H&E staining of the wound at days 5 and 20. Wound profile is shown by the dotted yellow lines. Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
Hemostasis evaluation of HAD
The hemostatic adhesive was further tested in the rat liver. Figure 6A shows the schematic illustration of the liver bleeding model, and the hemostatic process is presented in Fig. 6B and movie S4. At about 40 s, bleeding was slowed and completely stopped at 60 s. However, when only a medical gauze was used instead of HAD, the gauze quickly saturated with blood (Fig. 6C). As shown in Fig. 6D, filter paper was placed under the liver to assess blood loss for the different treatment groups. The control group had the most blood loss, reaching 382.31 ± 31.63 mg (Fig. 6E). The amount of blood loss for the GelMA and HC groups was 150.65 ± 20.67 mg and 219.32 ± 13.82 mg, respectively. HAD reduced blood loss from the liver to 78.67 ± 10.07 mg, down about 79% compared with the control group. The bleeding cessation time was 3.23 ± 0.29 min for the control group. For the GelMA, HC, and fibrin glue groups, the hemostasis time was 1.32 ± 0.12 min, 2.26 ± 0.12 min, and 1.02 ± 0.08 min, respectively. HAD has the shortest hemostasis time of 0.75 ± 0.11 min (Fig. 6F). These data strongly indicate that HAD significantly reduced blood loss and shortened hemostasis time and suggest its potential utility. Figure 6G also presents the histological analysis at day 5. For the untreated and HC groups, the wound had a considerable granulation tissue. In contrast, GelMA, fibrin glue, and HAD had minimal granulation tissue, suggesting effective wound healing. These results demonstrated that HAD adhesive not only achieves rapid hemostasis but also promotes wound healing.
Fig. 6. Hemostatic properties of HAD in liver injury.

(A) Schematic illustration of the establishment of rat liver bleeding and hemostasis. (B) Testing process in liver injury. (C) Liver injury treated with gauze. (D) Time-course bleeding images of the liver. (E and F) Hemostasis time and blood loss (n = 3). (G) H&E staining of the wound at day 5 (*P < 0.05, **P < 0.01, and ***P < 0.001). Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
To further evaluate the hemostatic ability of HAD, a severe liver injury was created in rats. In Fig. 7A, a part of the rat liver was cut off, and then HAD was applied to the injury site (Fig. 7B and movie S5). When HAD was applied onto the wound and cross-linked by visible light, hemostasis was rapidly achieved with minimal blood loss. However, as shown in Fig. 7C, the GelMA-treated group showed extensive bleeding from the injured liver (movie S6), suggesting that GelMA could only achieve hemostasis under severe trauma conditions compared with the nontreatment group (Fig. 7D) but not when compared with HAD. Overall, these results demonstrated that HAD could effectively control extensive blood loss in severe trauma cases.
Fig. 7. Hemostatic effect on severe injury liver.

(A) Schematic illustration of the hemostatic process. (B) Hemostatic test process with HAD in severe liver wound model. (C) Hemostatic test process with GelMA in severe liver wound model. (D) Untreated wound as a control group. Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
Closure of abdominal aorta defects with HAD
A 2-to3-mm-incision model was established in the abdominal aorta of Sprague-Dawley rats to compare the hemostatic properties of HAD and GelMA (Fig. 8A). First, the abdominal aorta was separated and clipped in the two sides by vascular clamps. Then, an incision was created, and HAD was applied and illuminated for 3 min. Last, the distal and proximal vascular clamps were removed, and the abdominal aorta was sealed. While HAD displayed effective hemostasis and sealing properties (Fig. 8B and movie S7), the injured aorta treated with GelMA showed bleeding after the vascular clamps were removed (Fig. 8C and movie S8). As shown in Fig. 8D, HAD firmly adhered to and sealed the abdominal aorta. There was no obvious embolism (Fig. 8E). Even upon being twisted and bent, HAD maintained its adhesion to the surface of the abdominal aorta (Fig. 8, F and G). SEM images also confirmed the sealed interfaces between HAD and the abdominal aorta. The H&E staining of the sealed abdominal aorta has been conducted (fig. S7). The H&E staining showed that the interface between HAD and the artery was tight to indicate a strong bonding between the sealant and the tissue.
Fig. 8. Hemostatic effect of HAD on rat abdominal aorta injury model.

(A) Schematic illustration of the establishment of the rat abdominal aorta injury model and sealing. (B) Hemostasis and sealing test in rat abdominal aorta injury model of HAD. (C) Hemostasis and sealing test in rat abdominal aorta injury model of GelMA. (D) The outer surface image of the abdominal aorta sealed by HAD. (E) The inner surface image of the abdominal aorta sealed by HAD. (F and G) HAD sealed abdominal aorta subjected to large bending and twisting. (H) SEM of the interface between HAD and the abdominal aorta. Scale bars, 100 μm. Photo credit: Y. C. Guo and Y. Wang, Army Medical University.
HAD mechanism of action in rat liver
In vivo hemostasis performance and possible underlying mechanisms were further investigated by SEM. As shown in Fig. 9A, we put three SEM observation areas: In area I, it is the surface of the sealed wound site (Fig. 9, A1 and A2); in area II, it is the inner view of the cross section about the cross section of the sealed wound site (Fig. 9, B1 and B2); and in area III, it is the internal view about the cross section of the sealed wound site on the severe liver wound model (Fig. 9C1). For the GelMA group, the fibrin fibers formed were thin, and the clots were loosely distributed after complete hemostasis for 5 min (Fig. 9, A1 and A3). Furthermore, the interface between the wound and GelMA was loosely packed (arrowhead in Fig. 9B1). In contrast, the blood clots in the HAD-treated liver showed a thick fibrin mesh wrapping and covering the cellular components of blood (Fig. 9, A2 and A4). The fibrin fibers also appeared sturdy, and the clots were densely packed. It suggests that the HC in the adhesive contributed a critical role in fibrinogen conversion to fibrin. The interface between HAD and the bleeding tissue was also well bonded (Fig. 9B2). Compared with the typical biconcave disk-shaped RBCs in the GelMA group, RBCs in the HAD group have become irregular. Figures 9C2-C5 show the SEM images of the liver cross section after hemostasis. The RBCs were seen deposited closer to HAD. RBCs already lost their normal disk shape and deformed to a close-packed polyhedron. These irregular RBCs mainly appeared inside, with very few seen at the periphery. In contrast to RBCs, platelets were aggregated on the outer edge but rarely inside. It is noted that the aggregated platelets around the edge were more activated than platelets on the inside. By comparing the two groups, we made an inference that the polyhedral shape of RBCs could help build a tight interface between the adhesive and the bleeding tissues.
Fig. 9. SEM investigation of hemostasis surface and interface in vivo.

(A-C) Illustration of the observation area about SEM. SEM observation area I is shown in (A1) and (A2); SEM observation area II is shown in (B1) and (B2); SEM observation area III is shown in (C). (A1 and A3) SEM of the exterior blood clot in GelMA group after cessation of bleeding. (A2 and A4) SEM of the exterior blood clot in HAD group after cessation of bleeding. (B1) SEM of the interior interface between bleeding liver and the GelMA (white arrow). (B2) SEM of the interior interface between bleeding liver and HAD (white arrow). (C1) SEM of the interior wound. Arbitrary locations from the cut site to the edge were marked as (C2) to (C5), and their corresponding magnified images in (C2), (C3), (C4), and (C5), respectively. Scale bars, 3 μm (A1 to A4), 2 μm (B1 and B2), 10 μm (C1), and 2 μm (C2 to C5).
To understand the relationship of HC and the polyhedral RBC morphology formation, we further investigated the blood clot inside the rat liver. In Fig. 10A, according to the observation area, we also classify the SEM observation area into three different views, namely, SEM observation area I, the surface of the blood clot on the liver (Fig. 10B); SEM observation area II, the inner fibrin mesh view of the blood clot (Fig. 10, C and D); and SEM observation area III, the inner platelet and RBC view of the blood clot (Fig. 10, E to J). As shown in Fig. 10B, the typical biconcave disk-shaped RBCs were covered with a thick fibrin meshwork and platelets. Because of the contractile stress generated by platelets to pull and bend the fibrin fiber, the trapped RBCs will be forced to move closer to each other and deform (Fig. 10C). In Fig. 10D, in the interior of the hemostasis surface, RBCs were seen deformed and acquired a polyhedral shape. The time-dependent (30 s after the start of bleeding) morphology change of RBCs in the process of hemostasis revealed the transition from their biconcave disk shape to a significantly deformed shape (Fig. 10, E and F). After 2 min, RBCs have totally contracted into polyhedra and became more compact (Fig. 10F). A similar phenomenon was also observed in the areas of platelet aggregation (Fig. 10, H to J). It can be seen that platelets mechanically acted on the fibrin fibers, which generated contractile stress to elicit clot contraction. Another interesting point is that most of the platelets were observed on the outside, which was consistent with previous reports where RBCs tend to segregate within a fibrin clot in the process of hemostasis. In contrast, platelet contraction forces adjacent RBCs to come together, forming larger clusters (25, 51).
Fig. 10. SEM of the blood clots in rat liver with HAD treatment.

(A) Illustration of the SEM observation area. SEM observation area I shown in (B); SEM observation area II shown in (C) and (D); SEM observation area III shown in (E) to (J). (B) SEM of the interaction of fibrin and RBCs. (C and D) SEM image of an inside clot. (E to G) SEM of RBCs at 30 s and 1 and 2 min in a bleeding liver. (H) Platelets pulling and bending fibrin fibers to generate contractile stress and causing clot contraction. (I) Platelets deformed to form a compact structure. (J) The internally contracted platelets developed a close-packed, tessellated array of compressed polyhedra, forming a dense barrier important for hemostasis. (K) The process of clot contraction in the shrinkage of clot volume over time. Scale bars, 1 μm.
From our study, we propose a potential hemostasis mechanism of adhesion in Fig. 10K. This process can be separated into three phases: (i) initiation of hemostasis, (ii) platelet aggregation and fibrin formation, and (iii) clot contraction and total hemostasis. For the first phase, the HAD prepolymer solution diffuses and penetrates the tissue interface, and after polymerization, the polymer chains entangle within the tissue to form an interlock. Then, GelMA in HAD promotes aggregation and activation of blood cells such as platelets and RBCs. Meanwhile, the HC rapidly triggers the conversion of fibrinogen into fibrin, which is beneficial for capturing more platelets. It will then amplify the native thrombin to convert more fibrinogen into fibrin. After that, the filopodia of platelets pull and bend fibrin fibers, which generate stress and cause clot contraction to form a mechanical stabilization structure to reach complete hemostasis. Therefore, the synergy of GelMA and HC achieved efficient hemostasis and strong adhesion.
DISCUSSION
Existing clinical bioadhesives such as fibrin glue can easily be washed out because of the continuous tissue contractions and the presence of blood, which reduces the adhesion strength (14). Gelatin has hemostatic properties like collagen, which can accelerate clot formation (52). However, it cannot handle heavy bleeding, such as arterial injury (53). Inspired by the procoagulant activities of B. atrox venom, we engineered a snake-inspired HAD by loading HC to GelMA as a blood-resistant adhesive. In our system, photoreactive MA side groups were grafted to gelatin to form cross-linked gels induced by visible light. When the gels were loaded with HC, they displayed strong hemostatic bioadhesive properties, rapidly triggering fibrinogen conversion to fibrin. Owing to the synergistic effect of the two components, HAD has excellent functions of blood resistance as well as superior adhesive properties, which can significantly promote blood coagulation, rapid hemostasis in vivo, and thus potential wound healing.
Upon visible light–induced cross-linking, HAD gels were formed rapidly. The fast-gelling property benefited them to quickly and effectively seal injured tissues. The lap shear test indicated that the shear strength of HAD was 93.52 kPa, which is 10 times higher than commercial fibrin glue. The tensile strength of fibrin glue was 2.93 kPa, while that of HAD was 35.64 kPa. The adhesive strength of HAD is superior to fibrin glue. HAD also presented excellent blood resistance by an exceptional blood clotting ability in vitro. It completely coagulated whole blood in ~0.76 min. Recently, a visible light–cross-linkable GelMA bioadhesive was reported for corneal reconstruction, but because of the avascular nature of the cornea, blood is absent, and hemostasis was not a material design goal (10). Two other studies (14, 34) investigated UV–cross-linkable poly(glycerol sebacate acrylate) and MA-substituted tropoelastin bioadhesives for cardiac wall defect and lung tissue repairs. However, in situ UV exposure is not desirable because UV light causes mutagenic and cytotoxic DNA lesions (36). Furthermore, the rapid sealing of heavily bleeding tissues is critical in acute care and emergency situations (e.g., trauma and war wounds). The significant advance made in our study is twofold: (i) HAD considerably accelerated skin wound closure compared with several comparators, including GelMA (Fig. 5), and (ii) HAD displayed significantly fast blood clot time with a reduction of about 50% in both the amount of blood loss and hemostasis in highly vascularized tissues (rat tail, liver, and abdominal artery) compared with GelMA (Figs. 6 to 8).
It has been well established that when platelets interact with fibrin polymers within blood clots, clot-associated contraction of activated platelets facilitates the compaction and stiffening of clots (54). Previous research has proved that during clot maturation, filopodia from platelets would pull and bend fibrin fibers, generate stress, and cause clot contraction (55, 56). Furthermore, platelet-driven blood clot contraction is thought to promote wound closure and secure hemostasis while preventing vascular occlusion (57). In the current study, a proposed three-step mechanism for HAD action is provided. When HAD was applied to the bleeding site, the GelMA component was in situ cross-linked rapidly by visible light through free radical polymerization and aggregated blood cells. Because HAD was used in the form of a prepolymer solution, it was able to diffuse and penetrate through the tissue interface. After photocrosslinking, the polymer chains in HAD entangled with the tissue, which leads to the interface interlock between the adhesive material and the tissue (14). Meanwhile, HC can convert fibrinogen into fibrin mesh, followed by platelet-driven blood clot contraction to form a solid physical barrier.
Overall, our results strongly suggest that HAD can be an efficient hemostatic tissue adhesive/sealant, especially in noncompressible bleeding tissues, such as diffuse traumatic heavy hemorrhage and artery bleeding that cause considerable mortality. From the perspective of tissue penetration, the visible light–induced cross-linking system could reach a thickness of 2.5 mm. This is beneficial for treating accessible thick wounds as the controlled release of HC could even diffuse further and induce hemostasis, which has been verified in our severely injured liver model. In addition, the visible light–cross-linkable HAD system may also be a valuable candidate for direct application to even deeper tissue injury models with extensive bleeding and should be considered in future studies. The controllable degradation rate of HAD in different tissues and organs is also worthy of future studies.
MATERIALS AND METHODS
Materials
Type A gelatin from porcine skin, TEA, VC, Eosin Y, and methacrylic anhydride were obtained from Sigma-Aldrich. HC was purchased from Penglai Nuokang Pharmaceutical Co. Ltd. (Shandong, China). Dulbecco’s phosphate-buffered saline (DPBS) was obtained from Macklin Co. (Beijing, China). Fibrin glue was obtained from Shanghai RAAS Blood Products Co. Ltd. (Shanghai, China).
Preparation of HAD
GelMA was synthesized by following the previously published protocol (58). Briefly, 1 g of gelatin was dissolved in 10 ml of DPBS and heated to 60°C for 1 hour. Methacrylic anhydride (0.8 ml) was then added to the solution dropwise and stirred for another 3 hours at 50°C. The reaction was stopped by adding 40 ml of DPBS, and the product was dialyzed (molecular weight cutoff, 12 to 14 kDa) in a deionized water bath at 55°C for 5 days to remove the unreacted methacrylic anhydride. Last, the solution was filtered and lyophilized for 12 hours.
Preparation of HAD adhesive
HAD precursor solutions were prepared by dissolving TEA [1.88% (w/v)], VC [1.25% (w/v)], Eosin Y (0.5 mM), and HC [1% (w/v)] in GelMA solution [20% (w/v)].
Characterization
1H NMR analyses of gelatin, GelMA, and HAD were obtained from a 500-MHz proton NMR in DMSO-d6. The functional groups of gelatin, GelMA, and HAD were determined by FTIR spectroscopy (Nicolet 6700). The freeze-dried gelatin, GelMA, and HAD powders were pressed with potassium bromide and analyzed in the spectral region 4000 to 400 cm−1. SEM analysis was performed to evaluate the porosity of the cross-linked gels. Samples were frozen and then dried under vacuum at −40°C overnight. Samples were sputter-coated by gold before analysis, and SEM images were obtained by using an FEI/Phillips XL30 Field Emission Gun scanning electron microscope (15 kV).
Mechanical characterization of HAD
Lap shear test
The lap shear test of tissue adhesives (HAD, GelMA, and control fibrin glue) was performed according to American Society of Testing Materials (ASTM) F2255-05 standard with minor modification. Briefly, 20-μl samples were injected between the surfaces of two pieces of glass slides (20 mm by 25 mm) and then photocrosslinked by visible light for 4 min. After that, an Instron mechanical tester was used to measure the samples’ shear strengths at a strain rate of 1 mm/min (n = 3).
End-to-end adhesive strength test
The end-to-end adhesive strengths of the GelMA, HAD, and fibrin glue were tested using the modified ASTM F2458-05 standards. Porcine skin was obtained from a local supermarket and cut into 30 mm by 10 mm, followed by excess fat removal. Two pieces of porcine skin were glued by 50 μl of as-prepared glue and cross-linked using visible light for 4 min. Adhesion strength was conducted with a strain rate of 1 mm/min (n = 3).
Rheological analysis
Rheology measurements of the adhesive were carried out with a TA DHR-2 rheometer. HAD samples were prepared for rheological tests to evaluate rheological properties. The oscillation strain dependence of the storage modulus G′ and loss modulus G″ was determined at a fixed strain of 0.5%, which ensured enough sensitivity but still in the region linear viscoelasticity. All tests were carried out at a changing angular frequency ranging from 0.1 to 100 rad/s.
UV-vis spectra
UV-vis spectroscopy (UV-3600, Shimadzu, Japan) was used for the optical absorption of GelMA, TEA, VC, Eosin Y, HC, GelMA precursor solution, and HAD precursor solution after different cross-linking times (0, 20, 40, and 60 s).
Clotting time assay
Clotting time assay was performed according to a previously reported protocol (48). Briefly, a volume of citrated blood was collected into an Eppendorf tube. Then, 0.1 M calcium chloride was added to the tube and mixed with blood for 10 s. Then, 50 μl of the mixture was deposited into a 96-well plate. At predetermined time intervals, each well was washed with saline solution, and the liquid was immediately removed. The clotting time was marked in the well to form a uniform clot. For testing HAD, 20 μl of HAD adhesive was injected into the wells and distributed to the bottom of the wells. Then, 50 μl of blood was added to the wells. Visible light was used immediately. At selected times, each well was washed with saline solution (9 g/liter) to halt clotting. The liquid was immediately aspirated and repeatedly washed until the solution was clear, indicating the removal of all soluble blood components. For SEM, samples were fixed in 2% glutaraldehyde for 12 hours, gradient-dehydrated in ethanol and tert-butanol, sputter-coated with gold, and then examined by SEM.
Cytocompatibility
The cytotoxicity of the adhesive was evaluated by following the previously reported protocol with minor modification (59). L929 fibroblast cells were used to evaluate the cytotoxicity of HAD by direct contact. Briefly, the HAD precursor was cross-linked by visible light for 3 min and disinfected with 75% alcohol. Then, HAD was placed at the bottom of the well. L929 cells were seeded into 48-well plates with a density of 2000 cells per well and cultured at 37°C and 5% CO2 for 1, 3, and 5 days. At selected times, the culture solution was replaced by Cell Counting Kit-8 (CCK-8) reagent for 2 hours at 37°C, and then the absorbance at 450 nm was measured (n = 3).
Plasma clotting kinetics
Plasma clotting kinetics was conducted following a previous report (47). Briefly, PPP was obtained by centrifugation of whole blood at 2000g for 10 min. Forty microliters of HAD and 60 μl of PPP solutions were added to the 96-well plate and then illuminated for 1 min. The absorbance of plasma was recorded by an enzyme-labeled instrument (UV-3600, Shimadzu, Japan) every 60 s at 405 nm. Experiments with and without CaCl2 served as positive and negative controls, respectively. Furthermore, the slope of the linear range and maximum halftime was calculated to reflect the rate of plasma clotting.
The bioactivity of released HC from HAD was measured by the potency relationship. First, 60 μl of solution containing 60, 40, 20, 10, and 5 μg of HC was added to the 96-well plate and added 60 μl of PPP and incubated at 37°C for 5 min. Then, the absorbance at 405 nm was recorded. HAD with HC (1.0 mg/ml) was cross-linked by visible light and placed in 60 μl of deionized water. After soaking for 5, 10, and 30 min, the hydrogel was removed. Last, 60 μl of PPP was added to the 96-well plate and reacted at 37°C for 5 min. The absorbance at 405 nm was measured by comparing it with the standard curve and calculating the released HC.
Platelet adsorption and morphology
Platelet adhesion was analyzed by a lactate dehydrogenase (LDH) assay (60). Whole blood was centrifuged at 200g for 15 min to obtain PRP, and then 20 μl of PRP was added to a 48-well plate that contains 100 μl of HAD solutions. After 1-min illumination, the well was washed by PBS four times. One milliliter of 1% Triton X-100 was used to lyse platelets for 1 hour. Last, the adhesive efficiency was determined by an LDH kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
In vivo liver hemostatic ability
All animal studies were approved by the Institutional Animal Care and Use Committee of the Third Military Medical University (AMUWEC2020861). All experimental animals were purchased from the Experimental Animal Center of the Third Military Medical University. A mouse hemorrhaging liver model (male Sprague-Dawley rats, 180 to 250 g) to investigate the in vivo hemostatic ability of HAD adhesive was used. First, mice were anesthetized by 1% pentobarbital. Then, the liver was dissected and punctured to bleed with a needle. Preweighed filter paper was placed beneath the liver. Immediately, 50 μl of HAD adhesive was prepared in situ on the surface of the bleeding site as a hemostatic agent and then photocrosslinked with visible light. The weight of the filter paper with blood was measured, and the mass of blood was calculated for 60 s until the equilibrium state is reached. The hemostatic time of fibrin glue–treated and blank groups were measured as controls (n = 3).
For the liver severe-injury model, one-quarter of the liver was cut off, and then 100 μl of HAD was injected into the wound immediately and then photocrosslinked with visible light. The nontreatment wound served as the negative control.
In vivo degradation of HAD
First, mice were anesthetized by 1% pentobarbital, and a 1-cm-long incision was created. The sterilized HAD (n = 6, 6 mm by 2 mm) was implanted. On days 7, 14, 21, and 28, the mice were euthanized, and the remaining HAD volume was recorded. The inflammatory response was analyzed by H&E staining and immunofluorescence staining.
In vivo rat tail hemostatic ability
A mouse rat tail model (male C57 rats, 16 to 20 g) was used to investigate the in vivo hemostatic ability of HAD adhesive. First, mice were anesthetized by 1% pentobarbital. Next, preweighed filter paper was placed beneath the tail, and the tail was cut 1.5 cm away from the base. Immediately, 20 μl of HAD prepolymer was injected into the bleeding site and photocrosslinked with a dental curing lamp (430 to 530 nm) for a certain time. The mass of blood was measured for 5 min until the equilibrium state is reached. The hemostatic time of fibrin glue–treated and blank groups was measured as controls (n = 3).
Closure of abdominal aorta defects with HAD in vivo
For closure of abdominal aorta defects with HAD in vivo, animals (n = 4) were anesthetized by 1% pentobarbital. After that, the abdominal aorta was separated and clipped in the two sides by vascular clamps, and an incision was created. Next, HAD was applied and illuminated for 3 min. Last, the distal and proximal vascular clamps were removed, and the abdominal aorta was inspected for up to 5 min to detect bleeding. After the animals were euthanized, the abdominal aorta was fixed with 4% paraformaldehyde. H&E staining was performed on the cross sections of the defect site.
Adhesion assessment in vivo
Male Sprague-Dawley rats (6 to 8 weeks, 200 ± 20 g) were used to assess the wound adhesive effect of HAD in vivo. After anesthesia with 1% pentobarbital, the backs of the rats were shaved and disinfected by 75% ethanol, and then four incisions (2 cm) were made on each rat. The following five treatments were used: suture closure (4-0 unabsorbable suture), fibrin glue, GelMA, HAD, and untreated (negative control). Twenty microliters of fibrin glue, GelMA, and HAD was applied to each incision followed by pressing the two wound edges into contact for approximately 20 s. The rats were euthanized at days 5 and 20 after operation. Skin sizes measuring 3 cm by 3 cm were used for histological analysis and to quantify the healing response by measuring the wound contraction area on day 5.
Statistical analysis
For each experiment, at least three samples were tested, and data were presented as means ± SD (*P < 0.05, **P < 0.01, and ***P < 0.001). One-way or two-way analysis of variance (ANOVA) test was performed, followed by Tukey’s test for statistical analysis (GraphPad Prism 6.0).
Acknowledgments
Funding: This work was financially supported by the National Natural Science Foundation of China (grant number 81920108022) and Natural Science Foundation Innovation Group Science Foundation of Chongqing (grant number cstc2019jcyj-cxttX0001). M.X. and K.M. would like to acknowledge NSERC Discovery grants and NSERC Discovery Accelerator Supplements (DAS) Awards. Author contributions: G.L., M.X., and R.Z. supervised the project. Y.G. and Y.W. conducted the synthesis and chemical characterization of the materials. Y.G. and Y.W. performed the animal experiments with the help of X.Z. and X.L. Y.W., Y.G., R.Z., M.X., G.L., and X.L. analyzed the in vitro experiments. K.M. contributed to data interpretation, discussion, and critical comments on shaping the manuscript. All authors wrote, revised, and corrected the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/29/eabf9635/DC1
REFERENCES AND NOTES
- 1.Li J., Celiz A. D., Yang J., Yang Q., Wamala I., Whyte W., Seo B. R., Vasilyev N. V., Vlassak J. J., Suo Z., Mooney D. J., Tough adhesives for diverse wet surfaces. Science 357, 378–381 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuk H., Varela C. E., Nabzdyk C. S., Mao X., Padera R. F., Roche E. T., Zhao X., Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ghobril C., Charoen K., Rodriguez E. K., Nazarian A., Grinstaff M. W., A dendritic thioester hydrogel based on thiol-thioester exchange as a dissolvable sealant system for wound closure. Angew. Chem. Int. Ed. 52, 14070–14074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma B., Fermanian S., Gibson M., Unterman S., Herzka D. A., Cascio B., Coburn J., Hui A. Y., Marcus N., Gold G. E., Elisseeff J. H., Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci. Transl. Med. 5, 167ra166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X., Xia X., Zhang K., Rai A., Li Z., Zhao P., Wei K., Zou L., Yang B., Wong W.-K., Chiu P. W.-Y., Bian L., Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci. Transl. Med. 12, eaba8014 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Taboada G. M., Yang K., Pereira M. J. N., Sophie S. L., Hu Y. S., Karp J. M., Artzi N., Lee Y., Overcoming the translational barriers of tissue adhesives. Nat. Rev. Mater. 5, 310–329 (2020). [Google Scholar]
- 7.Duarte A. P., Coelho J. F., Bordado J. C., Cidade M. T., Gil M. H., Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 37, 1031–1050 (2012). [Google Scholar]
- 8.Blacklow S. O., Li J., Freedman B. R., Zeidi M., Chen C., Mooney D. J., Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 5, eaaw3963 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickman D. A., Pawlowski C. L., Sekhon U. D. S., Marks J., Gupta A. S., Biomaterials and advanced technologies for hemostatic management of bleeding. Adv. Mater. 30, 1700859 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sani E. S., Kheirkhah A., Rana D., Sun Z., Foulsham W., Sheikhi A., Khademhosseini A., Dana R., Annabi N., Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci. Adv. 5, eaav1281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.M Y., Yao J., Liu Q., Han T., Zhao J., Ma X., Tong Y., Jin G., Qu K., Li B., Xu F., Liquid bandage harvests robust adhesive, hemostatic, and antibacterial performances as a first-aid tissue adhesive. Adv. Funct. Mater. 30, 2001820 (2020). [Google Scholar]
- 12.Gyawali D., Tran R. T., Guleserian K. J., Tang L., Yang J., Citric-acid-derived photo-cross-linked biodegradable elastomers. J. Biomater. Sci. Polym. Ed. 21, 1761–1782 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artzi N., Shazly T., Baker A., Bon A., Edelman E., Aldehyde-amine chemistry enables modulated biosealants with tissue-specific adhesion. Adv. Mater. 21, 3399–3403 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang N., Pereira M. J., Lee Y., Friehs I., Vasilyev N. V., Feins E. N., Ablasser K., O’Cearbhaill E. D., Xu C., Fabozzo A., Padera R., Wasserman S., Freudenthal F., Ferreira L. S., Langer R., Karp J. M., del Nido P. J., A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects. Sci. Transl. Med. 6, 218ra216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q., Lee D. W., Ahn B. K., Seo S., Kaufman Y., Israelachvili J. N., Waite J. H., Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange. Nat. Mater. 15, 407–412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D. Berwick, A. Downey, E. Cornett, A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury (National Academies Press, 2016). [PubMed] [Google Scholar]
- 17.Hsu B. B., Conway W., Tschabrunn C. M., Mehta M., Perez-Cuevas M. B., Zhang S. G., Hammond P. T., Clotting mimicry from robust hemostatic bandages based on self-assembling peptides. ACS Nano 9, 9394–9406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonhardt E. E., Kang N., Hamad M. A., Wooley K. L., Elsabahy M., Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 10, 2307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein M. K., Kassam H. A., Lee R. H., Bergmeier W., Peters E. B., Gillis D. C., Dandurand B. R., Rouan J. R., Karver M. R., Struble M. D., Clemons T. D., Palmer L. C., Gavitt B., Pritts T. A., Tsihlis N. D., Stupp S. I., Kibbe M. R., Development of optimized tissue-factor-targeted peptide amphiphile nanofibers to slow noncompressible torso hemorrhage. ACS Nano 14, 6649–6662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King D. R., Initial care of the severely injured patient. N. Engl. J. Med. 380, 763–770 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Gao Y., Sarode A., Kokoroskos N., Ukidve A., Zhao Z., Guo S., Flaumenhaft R., Gupta A. S., Saillant N., Mitragotri S., A polymer-based systemic hemostatic agent. Sci. Adv. 6, eaba0588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Undas A., Ariëns R. A. S., Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 31, e88–e99 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Brown A. E. X., Litvinov R. I., Discher D. E., Purohit P. K., Weisel J. W., Multiscale mechanics of fibrin polymer: Gel stretching with protein unfolding and loss of water. Science 325, 741–744 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macrae F. L., Duval C., Papareddy P., Baker S. R., Yuldasheva N., Kearney K. J., McPherson H. R., Asquith N., Konings J., Casini A., Degen J. L., Connell S. D., Philippou H., Wolberg A. S., Herwald H., Ariëns R. A., A fibrin biofilm covers blood clots and protects from microbial invasion. J. Clin. Invest. 128, 3356–3368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cines D. B., Lebedeva T., Nagaswami C., Hayes V., Massefski W., Litvinov R. I., Rauova L., Lowery T. J., Weisel J. W., Clot contraction: Compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 123, 1596–1603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stalker T. J., Welsh J. D., Tomaiuolo M., Wu J., Colace T. V., Diamond S. L., Brass L. F., A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood 124, 1824–1831 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janszky B., Action of the venom of Bothrops atrox on fibrillogen. Science 110, 307 (1949). [DOI] [PubMed] [Google Scholar]
- 28.Hofmann H., Bon C., Blood coagulation induced by the venom of Bothrops atrox. 1. Identification, purification, and properties of a prothrombin activator. Biochemistry 26, 772–780 (1987). [DOI] [PubMed] [Google Scholar]
- 29.Hofmann H., Bon C., Blood coagulation induced by the venom of Bothrops atrox. 2. Identification, purification, and properties of two factor X activators. Biochemistry 26, 780–787 (1987). [DOI] [PubMed] [Google Scholar]
- 30.Kumar V. A., Wickremasinghe N. C., Shi S. Y., Hartgerink J. D., Nanofibrous snake venom hemostat. ACS Biomater Sci. Eng. 1, 1300–1305 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouten P. J. M., Zonjee M., Bender J., Yauw S. T. K., Goor H. V., van Hesta J. C. M., Hoogenboom R., The chemistry of tissue adhesive materials. Prog. Polym. Sci. 39, 1375–1405 (2014). [Google Scholar]
- 32.Xuan C., Hao L., Liu X., Zhu Y., Yan H., Ren Y., Wang L., Toshinori F., Wui H., Chen Y., Shi X., Mao C., Wet-adhesive, haemostatic and antimicrobial bilayered composite nanosheets for sealing and healing soft-tissue bleeding wounds. Biomaterials 252, 120018 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Hong Y., Zhou F., Hua Y., Zhang X., Ni C., Pan D., Zhang Y., Jiang D., Yang L., Lin Q., Zou Y., Yu D., Arnot D. E., Zou X., Zhu L., Zhang S., Ouyang H., A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 10, 2060 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annabi N., Zhang Y.-N., Assmann A., Sani E. S., Cheng G., Lassaletta A. D., Vegh A., Dehghani B., Esparza G. U. R., Wang X., Gangadharan S., Weiss A. S., Khademhosseini A., Engineering a highly elastic human protein–based sealant for surgical applications. Sci. Transl. Med. 9, eaai7466 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assmann A., Vegh A., Ghasemi-Rad M., Bagherifard S., Cheng G., Sani E. S., Ruiz-Esparza G. U., Noshadi I., Lassaletta A. D., Gangadharan S., Tamayol A., Khademhosseini A., Annabi N., A highly adhesive and naturally derived sealant. Biomaterials 140, 115–127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadet J., Wagner J. R., DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 5, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryant S. J., Nuttelman C. R., Anseth K. S., Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 11, 439–457 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Soucy J. R., Shirzaei Sani E., Portillo Lara R., Diaz D., Dias F., Dias F., Weiss A. S., Koppes A. N., Koppes R. A., Annabi N., Photocrosslinkable gelatin/tropoelastin hydrogel adhesives for peripheral nerve repair. Tissue Eng. Part A 24, 1393–1405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.L. Fodor, Y. Ullmann, M. Elman, Aesthetic Applications of Intense Pulsed Light (Springer, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noshadi I., Hong S., Sullivan K. E., Shirzaei Sani E., Portillo-Lara R., Tamayol A., Shin S. R., Gao A. E., Stoppel W. L., Black L. D. III, Khademhosseini A., Annabi N., In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 5, 2093–2105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sani E. S., Lara R. P., Aldawood Z., Bassir S. H., Nguyen D., Kantarci A., Intini G., Annabi N., An antimicrobial dental light curable bioadhesive hydrogel for treatment of peri-implant diseases. Matter 1, 926–944 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gan D., Xu T., Xing W., Wang M., Fang J., Wang K., Ge X., Chan C. W., Ren F., Tan H., Lu X., Mussel-inspired dopamine oligomer intercalated tough and resilient gelatin methacryloyl (GelMA) hydrogels for cartilage regeneration. J. Mater. Chem. B 7, 1716–1725 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Nichol J. W., Koshy S. T., Bae H., Hwang C. M., Yamanlar S., Khademhosseini A., Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao F., Xu Z., Liang Q., Li H., Peng L., Wu M., Zhao X., Cui X., Ruan C., Liu W., Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv. Sci. 6, 1900867 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q., Qian C., Xiao W., Zhu H., Guo J., Ge Z., Cui W., Development of a visible light, cross-linked GelMA hydrogel containing decellularized human amniotic particles as a soft tissue replacement for oral mucosa repair. RSC Adv. 9, 18344–18352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Q., Gao F., Zeng Z. W., Yang J. R., Wu M., Gao C., Cheng D., Pan H., Liu W., Ruan C., Coaxial scale-up printing of diameter-tunable biohybrid hydrogel microtubes with high strength, perfusability, and endothelialization. Adv. Funct. Mater. 30, 2001485 (2020). [Google Scholar]
- 47.Liu C., Liu X., Liu C., Wang N., Chen H., Yao W., Sun G., Song Q., Qiao W., A highly efficient, in situ wet-adhesive dextran derivative sponge for rapid hemostasis. Biomaterials 205, 23–37 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Gaharwar A. K., Avery R. K., Assmann A., Paul A., McKinley G. H., Khademhosseini A., Olsen B. D., Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano 8, 9833–9842 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davie E. W., Ratnoff O. D., Waterfall sequence for intrinsic blood clotting. Science 145, 1310–1312 (1964). [DOI] [PubMed] [Google Scholar]
- 50.Mohammed B. M., Monroe D. M., Gailani D., Mouse models of hemostasis. Platelets 31, 417–422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gersh K. C., Nagaswami C., Weisel J. W., Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb. Haemost. 102, 1169–1175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z., Kuang G., Zong S., Liu S., Xiao H., Chen X., Zhou D., Huang Y., Sandwich-like fibers/sponge composite combining chemotherapy and hemostasis for efficient postoperative prevention of tumor recurrence and metastasis. Adv. Mater. 30, e1803217 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Huang Y., Zhao X., Zhang Z., Liang Y., Yin Z., Chen B., Bai L., Han Y., Guo B., Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem. Mater. 32, 6595–6610 (2020). [Google Scholar]
- 54.Rahmany M. B., Hantgan R. R., Dyke M. V., A mechanistic investigation of the effect of keratin-based hemostatic agents on coagulation. Biomaterials 34, 2492–2500 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Kim O. V., Litvinov R. I., Alber M. S., Weisel J. W., Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nat. Commun. 8, 1274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z., Milionis A., Zheng Y., Yee M., Codispoti L., Tan F., Poulikakos D., Yap C. H., Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 10, 5562 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen C. E., Qiu Y., McCarty O. J. T., Lam W. A., Platelet mechanotransduction. Annu. Rev. Biomed. Eng. 20, 253–275 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Loessner D., Meinert C., Kaemmerer E., Martine L. C., Yue K., Levett P. A., Klein T. J., Melchels F. P., Khademhosseini A., Hutmacher D. W., Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 11, 727–746 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Deng Z., Hu T., Lei Q., He J., Ma P. X., Guo B., Stimuli-Responsive conductive nanocomposite hydrogels with high stretchability, self-healing, adhesiveness, and 3D printability for human motion sensing. ACS Appl. Mater. Interfaces 11, 6796–6808 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Liu C., Yao W., Tian M., Wei J., Song Q., Qiao W., Mussel-inspired degradable antibacterial polydopamine/silica nanoparticle for rapid hemostasis. Biomaterials 179, 83–95 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/29/eabf9635/DC1


