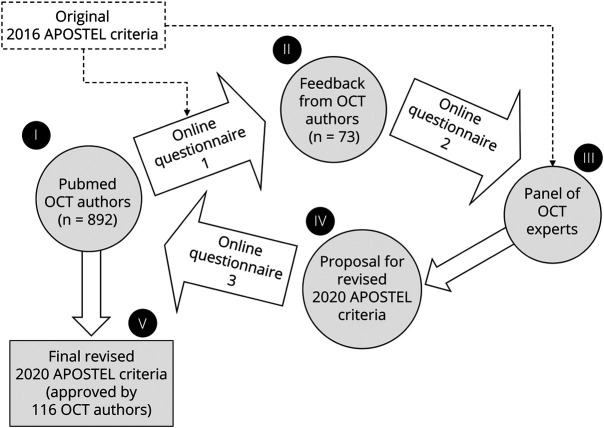
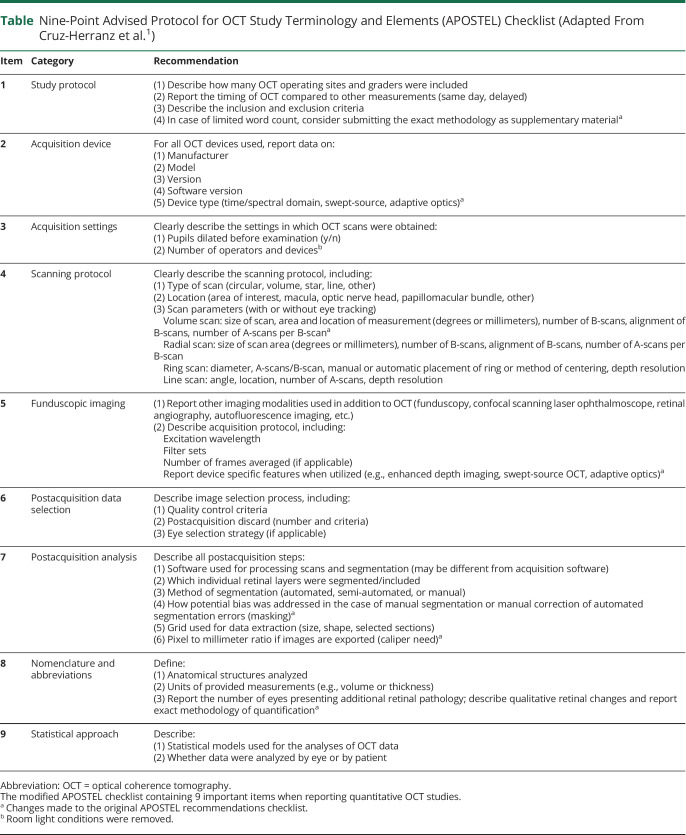
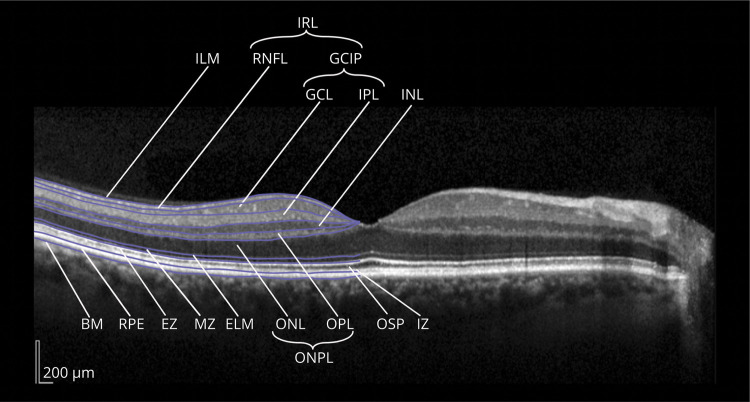
Aykut Aytulun
Aykut Aytulun, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,*,
Andrés Cruz-Herranz
Andrés Cruz-Herranz, MD, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,*,
Orhan Aktas
Orhan Aktas, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Laura J Balcer
Laura J Balcer, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Lisanne Balk
Lisanne Balk, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Piero Barboni
Piero Barboni, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Augusto Azuara Blanco
Augusto Azuara Blanco, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Peter A Calabresi
Peter A Calabresi, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Fiona Costello
Fiona Costello, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Bernardo Sanchez-Dalmau
Bernardo Sanchez-Dalmau, MD, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Delia Cabrera DeBuc
Delia Cabrera DeBuc, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Nicolas Feltgen
Nicolas Feltgen, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Robert P Finger
Robert P Finger, MD, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Jette Lautrup Frederiksen
Jette Lautrup Frederiksen, MD, DMSci
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Elliot Frohman
Elliot Frohman, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Teresa Frohman
Teresa Frohman, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
David Garway-Heath
David Garway-Heath, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Iñigo Gabilondo
Iñigo Gabilondo, MD, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Jennifer S Graves
Jennifer S Graves, MD, PHD, MAS
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Ari J Green
Ari J Green, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Hans-Peter Hartung
Hans-Peter Hartung, MD, FRCP
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Joachim Havla
Joachim Havla, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Frank G Holz
Frank G Holz, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Jaime Imitola
Jaime Imitola, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Rachel Kenney
Rachel Kenney, MPhil
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Alexander Klistorner
Alexander Klistorner, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Benjamin Knier
Benjamin Knier, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Thomas Korn
Thomas Korn, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Scott Kolbe
Scott Kolbe, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Julia Krämer
Julia Krämer, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Wolf A Lagrèze
Wolf A Lagrèze, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Letizia Leocani
Letizia Leocani, MD, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Oliver Maier
Oliver Maier, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Elena H Martínez-Lapiscina
Elena H Martínez-Lapiscina, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Sven Meuth
Sven Meuth, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Olivier Outteryck
Olivier Outteryck, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Friedemann Paul
Friedemann Paul, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Axel Petzold
Axel Petzold, MD, PhD, FRCP
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Gorm Pihl-Jensen
Gorm Pihl-Jensen, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Jana Lizrova Preiningerova
Jana Lizrova Preiningerova, MD, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Gema Rebolleda
Gema Rebolleda, MD, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Marius Ringelstein
Marius Ringelstein, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Shiv Saidha
Shiv Saidha, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Sven Schippling
Sven Schippling, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Joel S Schuman
Joel S Schuman, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Robert C Sergott
Robert C Sergott, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Ahmed Toosy
Ahmed Toosy, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Pablo Villoslada
Pablo Villoslada, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Sebastian Wolf
Sebastian Wolf, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
E Ann Yeh
E Ann Yeh, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Patrick Yu-Wai-Man
Patrick Yu-Wai-Man, PhD, FRCOphth, FRCPath
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Hanna G Zimmermann
Hanna G Zimmermann, PhD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,
Alexander U Brandt
Alexander U Brandt, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,†,
Philipp Albrecht
Philipp Albrecht, MD
1From the Department of Neurology, Medical Faculty (A.A., O.A., H.-P.H., O.M., S.M., M.R., P.A.), Heinrich-Heine University Düsseldorf, Germany; Department of Neurology (A.C.-H., A.J.G.), University of California San Francisco; Departments of Neurology, Population Health, and Ophthalmology (L.J.B., R.K.), NYU Grossman School of Medicine, New York, NY; Mulier Institute (L.B.), Centre for Research on Sports in Society, Utrecht, the Netherlands; Scientific Institute San Raffaele (P.B.), Milan, Italy; Centre for Public Health (A.A.B.), Queen's University Belfast, Northern Ireland, UK; Division of Neuroimmunology (P.A.C., S. Saidha), Johns Hopkins University, Baltimore, MD; Departments of Clinical Neurosciences and Surgery (F.C.), University of Calgary, Alberta, Canada; Institut d’Investigacións Biomediques August Pi iSunyer (IDIBAPS) and Hospital Clinic (B.S.-D., E.H.M.-L., P.V.), University of Barcelona, Spain; Bascom Palmer Eye Institute (D.C.D.), University of Miami Miller School of Medicine, FL; Department of Ophthalmology (N.F.), University Medical Center, Göttingen; Department of Ophthalmology (R.P.F., F.G.H.), University of Bonn, Germany; Department of Neurology (J.L.F., G.P.-J.), Rigshospitalet Glostrup and University of Copenhagen, Denmark; Laboratory of Neuroimmunology (E.F., T.F.), Stanford University School of Medicine, CA; Institute of Ophthalmology (D.G.-H.), National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (D.G.-H.), London, UK; Biocruces Bizkaia Health Research Institute (I.G.), Barakaldo, Spain; Department of Neurosciences (J.S.G.), University of California, San Diego; Brain and Mind Centre (H.-P.H.), University of Sydney, Australia; Department of Neurology (H.-P.H.), Medical University of Vienna, Austria; Institute of Clinical Neuroimmunology (J.H.), LMU Hospital, Ludwig-Maximilians Universität München, Germany; UConn Health Comprehensive MS Center, Division of Multiple Sclerosis and Neuroimmunology, Department of Neurology (J.I.), University of Connecticut School of Medicine, Farmington; Faculty of Medicine and Health Sciences (A.K.), Macquarie University, Sydney, Australia; Department of Neurology (B.K., T.K.), Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany; Department of Medicine and Radiology (S.K.), University of Melbourne, Australia; Department of Neurology with Institute of Translational Neurology (J.K.), University of Münster; Eye Center, Medical Center, Faculty of Medicine (W.A.L.), University of Freiburg, Germany; Experimental Neurophysiology Unit (L.L.), Institute of Experimental Neurology (INSPE), IRCCS San Raffaele, University Vita-Salute San Raffaele, Milan, Italy; Lille Neurosciences & Cognition (O.O.), Univ Lille, Inserm, CHU Lille, U1172-LilNCog (JPARC), France; Experimental and Clinical Research Center (F.P., H.G.Z., A.U.B.), Max Delbrück Center for Molecular Medicine and Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany; Moorfields Eye Hospital (A.P.), The National Hospital for Neurology and Neurosurgery, Queen Square, UCL Institute of Neurology, London, UK; Neuro-ophthalmology Expert Center (A.P.), Amsterdam UMC, the Netherlands; Department of Neurology, First Faculty of Medicine (J.L.P.), Charles University and General University Hospital in Prague, Czech Republic; Department of Ophthalmology (G.R.), Ramon y Cajal Hospital, Medicine University of Alcalá, Madrid, Spain; Department of Neurology (M.R.), Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Germany; Department of Neurology (S. Schippling), University Hospital Zurich, Switzerland; Departments of Ophthalmology, Neuroscience, and Physiology (J.S.S.), NYU Langone Health, NYU Grossman School of Medicine, New York; Departments of Biomedical Engineering, Electrical and Computer Engineering (J.S.S.), NYU Tandon School of Engineering, Brooklyn, NY; Thomas Jefferson University Medical College (R.C.S.), Philadelphia, PA; Queen Square MS Centre, Department of Neuroinflammation (A.T.), UCL Institute of Neurology, University College London, UK; Departments of Ophthalmology and Clinical Research (S.W.), Bern University Hospital, University of Bern, Switzerland; Division of Neurology, Department of Pediatrics (E.A.Y.), Hospital for Sick Children, Division of Neurosciences and Mental Health SickKids Research Institute, University of Toronto, Canada; Department of Clinical Neurosciences (P.Y.-W.-M.), University of Cambridge; Moorfields Eye Hospital (P.Y.-W.-M.), London, UK; University of California (A.U.B.), Irvine; and IMSVISUAL (A.A., A.C.-H., O.A., L.J.B., L.B., P.A.C., F.C., J.L.F., E.F., T.F., I.G., J.S.G., A.J.G., H.-P.H., J.H., J.I., R.K., A.K., B.K., T.K., J.K., L.L., E.H.M.-L., S.M., O.O., F.P., A.P., G.P.-J., J.L.P., M.R., S. Saidha, S. Schippling, R.C.S., P.V., E.A.Y., H.G.Z., A.U.B., P.A.), International Multiple Sclerosis Visual System Consortium, Middleton, WI.
1,†,✉