Abstract
Objective
To determine the influence of patent foramen ovale (PFO) closure on circulatory biomarkers.
Methods
Consecutive patients with PFO-related stroke were prospectively enrolled and followed with serial sampling of cardiac atrial and venous blood pre- and post-PFO closure over time. Candidate biomarkers were identified by mass spectrometry in a discovery cohort first, and lead candidates were validated in an independent cohort.
Results
Patients with PFO-related stroke (n = 254) were recruited and followed up to 4 years (median 2.01; interquartile range 0.77–2.54). Metabolite profiling in the discovery cohort (n = 12) identified homocysteine as the most significantly decreased factor in intracardiac plasma after PFO closure (false discovery rate 0.001). This was confirmed in a validation cohort (n = 181), where intracardiac total homocysteine (tHcy) was immediately reduced in patients with complete closure, but not in those with residual shunting, suggesting association of PFO shunting with tHcy elevation (β 0.115; 95% confidence interval [CI] 0.047–0.183; p = 0.001). tHcy reduction was more dramatic in left atrium than right (p < 0.001), suggesting clearance through pulmonary circulation. Long-term effect of PFO closure was also monitored and compared to medical treatment alone (n = 61). Complete PFO closure resulted in long-term tHcy reduction in peripheral blood, whereas medical therapy alone showed no effect (β −0.208; 95% CI −0.375∼-0.058; p = 0.007). Residual shunting was again independently associated with persistently elevated tHcy (β 0.184; 95% CI 0.051–0.316; p = 0.007).
Conclusions
PFO shunting may contribute to circulatory tHcy elevation, which is renormalized by PFO closure. PFO is not just a door for clots, but may itself enhance clot formation and injure neurovasculature by clot-independent mechanisms. Biomarkers such as tHcy can potentially serve as cost-effective measures of residual shunting and neurovascular risk for PFO stroke.
Patent foramen ovale (PFO), a common congenital variant cardiac anatomy characterized by right-to-left interatrial blood shunting, is associated with more than 150,000 strokes per year. PFO-related stroke is thought to be the result of venous thrombi that enter the arterial circulation and travel directly to the brain,1-5 which can be prevented by PFO closure.6-12 However, only a small portion of patients with PFO-related stroke have a known tendency to form blood clots, and patient selection for appropriate treatment remains challenging despite successful clinical trials.6-8 For patients with thrombophilic conditions, the risk of stroke recurrence is significantly increased.13 Especially in light of current increased incidence of venous clotting and cryptogenic stroke in the COVID-19 pandemic, the molecular landscape of PFO shunting becomes even more relevant to explore.
In addition to facilitating the passage of thrombi, we hypothesize that PFO-related venous–arterial mixing may enable other harmful factors to bypass pulmonary filtration, accumulate in circulation, and ultimately increase the risk of neurovascular injury and ischemic stroke,3,14,15 similar to speculated migraine physiology.16-18 Thus, understanding the blood molecular signature of PFO shunting, critical in heart–brain signaling, may benefit future patient selection for treatment and prevention of PFO-related stroke.3,14
Studies have suggested an important role of small molecular metabolites in the pathophysiologic processes of cardiovascular and cerebrovascular disease.19,20 We thus used mass spectrometry–based methods to investigate the influence of PFO on circulatory profile in patients undergoing endovascular PFO closure, with initial intracardiac exploratory metabolomic discovery followed by target validation in a larger cohort over time. The effect of PFO closure in peripheral circulation was also examined during long-term follow-up and compared with medical therapy alone. We aim to map circulatory metabolites as novel biomarkers for shunting physiology and therapeutic targets for PFO-related neurovascular disorders.
Methods
Study Population
From December 2007 to July 2014, 254 patients with PFO-related stroke eligible for PFO closure were prospectively recruited from the Cardio-Neurology Division at Massachusetts General Hospital (MGH). All enrolled patients underwent extensive cardiac, neurologic, hematologic, and imaging evaluations, including brain imaging with MRI or CT, evaluation of intracranial and extracranial vascular diseases with magnetic resonance angiography or ultrasonographic imaging, prolonged outpatient cardiac telemetry, hypercoagulability workup, and May-Thurner anatomy screening. All patients were diagnosed with cryptogenic stroke attributable to PFO after other identifiable causes of stroke were ruled out, and were unanimously deemed reasonable candidates for transcatheter PFO closure by a multidisciplinary MGH PFO Committee, which includes several external neurologists, cardiologists, hematologists, and peripheral vascular disease experts to review patients' medical records and studies independently (12 members, rotating to avoid bias), as mandated by institutional review board. Of these patients, 193 opted for PFO closure, and 61 opted to stay on medical therapy with antithrombotic agents alone, since positive results of PFO closure trials were not yet available. Antithrombotic treatment options, which included antiplatelet (aspirin, clopidogrel) and anticoagulant (warfarin) agents, were the same for patients with PFO closure and for patients receiving medical therapy only. Patient information on vitamin B supplementation was also obtained.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Massachusetts General Hospital institutional review board. Written informed consent was obtained from all participants.
PFO Closure and Residual Shunting Monitoring
PFO closure was performed under echocardiographic guidance. All patients were given the same weight-based dosing of fentanyl and midazolam for conscious sedation and IV heparin for anticoagulation throughout their stay in the catheterization laboratory (preprocedure, during procedure, and postprocedure). Echocardiography with bubble study was performed postclosure to evaluate device position and monitor residual shunting. Follow-up echoes were performed at 1, 6, and 12 months, and then annually up to 5 years postclosure. PFO shunting size was reviewed by a trained cardiologist blinded to the study, and was defined by the number of bubbles present in the left atrium (LA) within 3 cardiac cycles at rest or at the release of the Valsalva maneuver, with 0 bubbles as complete closure, 1–9 bubbles as small shunting, 10–30 bubbles as moderate shunting, and >30 bubbles as large shunting.6,9,21
Blood Sampling
To investigate the immediate effect of PFO closure, proximal (i.e., intra-atrial) venous and arterial blood was collected from right atrium (RA) and LA pre PFO closure (pre-RA, pre-LA). Post PFO closure, RA and LA blood (post-RA, post-LA) was sampled from the atria or from pulmonary vein or femoral artery in accordance with clinical procedure to avoid additional risk. Coronary angiogram with right and left heart catheterization was also performed to evaluate heart function and cardiovascular condition as per routine hospital protocol. All atrial samples were consistently obtained after anesthesia and systemic anticoagulants were administered, at similar time intervals (both pre and post closure) during each procedure, and were immediately processed in real time to avoid bias. To understand the long-term effect of PFO closure, regular peripheral venous blood was also sampled (from antecubital vein) before procedures (baseline) and at follow-up visits. For patients receiving medical therapy only, venous blood was drawn at baseline and follow-ups. Blood was collected in EDTA-treated tubes. After centrifuging at 1,900 g for 15 minutes, supernatant plasma was aliquoted and immediately frozen at −80°C for further analysis in accordance with previously published and validated standard operating procedure (SOP).14
Mass Spectrometry Profiling
Discovery metabolite profiling was performed by Michigan Regional Comprehensive Metabolomics Resource Core (MCR2). Plasma metabolites extracted from pre-RA, pre-LA, post-RA, and post-LA blood were analyzed by high-performance liquid chromatography (HPLC)/quadrupole time-of-flight (qTOF) (Agilent) operated in both positive and negative mode. Mass spectral features that represent metabolites were extracted and aligned between samples, and a total of 6,320 and 5,738 features were obtained in positive and negative modes, respectively. To identify the metabolites, the features were searched against an in-house library of MRC2, comprising the spectral peaks, accurate mass, fragmentation pattern, and retention time for ∼800 known metabolite standards previously analyzed under the same experimental condition as the samples.22-24 Peak heights were used to estimate relative concentration of each metabolite. All features were used for data normalization and statistical analysis, while only known metabolites were considered for further validation.
Total Homocysteine Measurement
Candidate validation was performed using stable-isotope dilution HPLC–mass spectrometry (MS) with selected reaction monitoring (SRM) in accordance with well-established protocol.25 Briefly, plasma samples were mixed with dl-homocysteine-3,3,3′,3′,4,4,4′,4′-d8 stock solution, followed by complete reduction of disulfides with dithiothreitol (DTT). Metabolite mixture was then extracted by acidified acetonitrile, injected into Thermo Syncronis HILIC column (50 × 2.1 mm [id]; 5 μm bead size) by Surveyor Autosampler combined with MS pump (Thermo Fisher Scientific), and analyzed by TSQ Quantum mass spectrometer (Thermo Fisher Scientific) equipped with heated electrospray ionization and operated in positive mode. Homocysteine (Hcy) was detected under SRM mode, with ion transitions m/z 136.0→90.0 for Hcy and 140.0→94.0 for Hcy-d4. Raw data were processed by Pinpoint software 1.1 (Thermo Fisher Scientific), in which the peak area for Hcy and Hcy-d4 was extracted and their ratio was calculated.14 The plasma levels of total homocysteine (tHcy) were determined using external standard curve with tHcy concentration ranging from 0 to 50 μmol/L.
Statistical Analysis
Patient characteristics were compared between the discovery and validation cohorts as well as between the follow-up cohorts of PFO closure and medical therapy, using Student t test for continuous variables and χ2 test for categorical variables. MS profiling data were interquartile log-ratio transformed and analyzed by orthogonal projections to latent structures-discriminant analysis (OPLS-DA) (SIMCA-P, v11.5, Umetrics) as well as by 2-way repeated-measures analysis of variance (ANOVA) followed by multiple testing correction via false discovery rate (FDR) method. tHcy levels in the validation cohort were also analyzed using 2-way repeated measures to determine the main effect of time (pre vs post), location (RA vs LA), and time-by-location interaction, followed by post hoc pairwise comparisons. To evaluate the effect of residual shunting or different PFO treatment (PFO closure vs medical therapy only) on the changes in tHcy level and control for potential clinical confounders (age, sex, hyperlipidemia, hypertension, diabetes, migraine, prior stroke/TIA, atrial septal aneurysm, May-Thurner anatomy, deep vein thrombosis, chronic obstructive pulmonary disease (COPD), asthma and renal disease, vitamin B intake, lipid-lowering agents, and antihypertensive agents), mixed effects model repeated measures analysis was used, in which study subject was included as random effect; pre- and post-tHcy levels (or baseline and follow-up levels) were included as dependent variables; and time, residual shunting (or PFO treatment), and other confounders were included as covariates. The interactions between time and covariates were used to evaluate the effect of PFO shunting (or PFO treatment) and potential confounders on tHcy changes. Data were log-transformed to meet the normal assumption as appropriate. Assumptions of homoscedasticity, linearity, and absence of multicollinearity were assessed and no violations were observed. Ordinal logistic regression with cumulative logits was used to evaluate tHcy changes in predicting the residual shunting after PFO closure in the long-term follow-up cohort, in which residual shunting size in ordinal categories (no, small, and moderate/large) was included as dependent variable, and the changes in tHcy from baseline to follow-up and other potential confounders were included as covariates. Proportional odds assumptions were tested for ordinal logistic regression models and no violations were detected. Receiver operator characteristic (ROC) curve was used to evaluate tHcy changes in discriminating residual shunting (no vs small + moderate/large) and moderate/large residual shunting (no + small vs moderate/large) (SPSS v25.0, IBM Corporation). Continuous variables are expressed as mean ± SD in tables and text, and as mean with 95% confidence intervals (CIs) in figures; categorical variables are presented as numbers and ratios (%).
Data Availability
Data supporting the findings of this study are available from the corresponding author on reasonable request.
Results
Patient Demographics
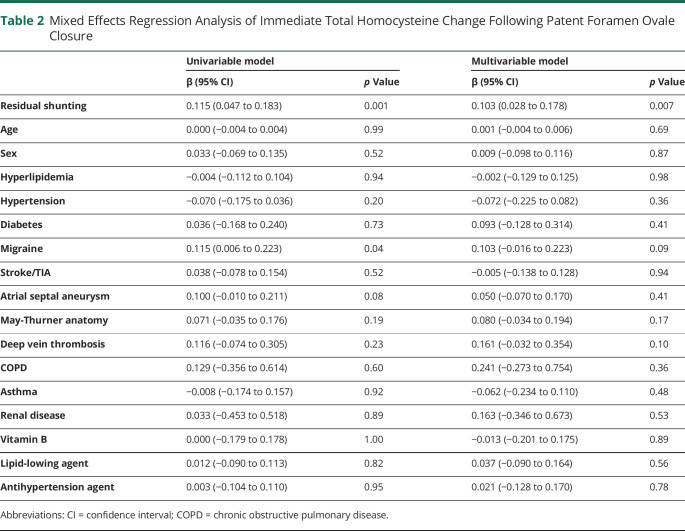
A total of 254 patients with PFO-attributable cryptogenic stroke eligible for PFO closure were consecutively enrolled, of whom 193 received PFO closure and 61 stayed on medical therapy alone. To evaluate the immediate effect of PFO closure, RA and LA blood collected from the first 12 patients pre and post closure was used for discovery metabolite profiling, and the samples from the remaining 181 patients were used for validation. Of the patients undergoing PFO closure, 115 were followed clinically and were compared to medical treatment alone. Baseline patient characteristics are summarized in table 1, including cardiovascular and cerebrovascular risk factors, history of stroke, TIA, and migraine, as well as medication status. No significant difference was observed between the discovery and validation groups or between PFO closure and medical therapy groups.
Table 1.
Clinical Features of Patients With Patent Foramen Ovale (PFO)–Related Stroke in Each Study Group
Intracardiac Metabolite Profiling Identified Hcy Reduction Following PFO Closure in the Discovery Cohort
Through mass spectrometry profiling, 341 metabolites were quantified in RA and LA plasma sampled from patients with PFO-related stroke pre and post PFO closure (n = 12) (figure 1A).
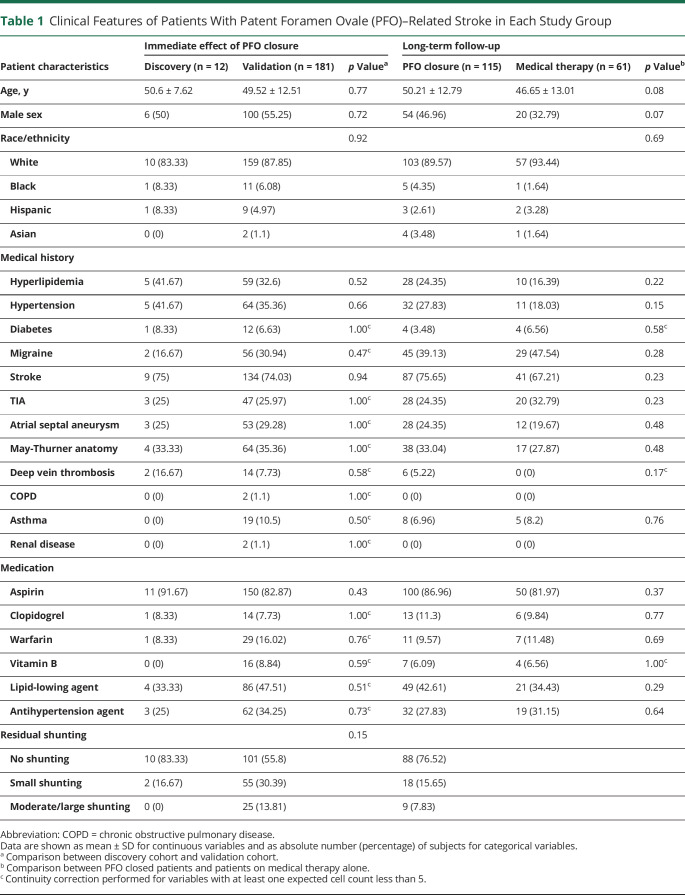
Figure 1. Metabolite Profiling in Discovery Patent Foramen Ovale (PFO) Closure Patient Cohort (n = 12).
(A) Diagram of blood sampling during PFO closure. (B) Scatterplot of orthogonal projections to latent structures-discriminant analysis (OPLS-DA) score for the metabolites identified in pre–right atrium (RA), pre–left atrium (LA), post-RA, and post-LA. (C) Scatterplot of variable importance for projection (VIP) values from OPLS-DA vs log-transformed false discovery rate (FDR) from 2-way repeated-measures analysis of variance. (D) Peak intensity of homocysteine with pairwise comparisons. *p < 0.05; **p < 0.01; ***p < 0.001.
To identify the metabolites associated with PFO closure, OPLS-DA was employed for satisfactory discrimination of the pre-RA, pre-LA, post-RA, and post-LA groups (figure 1B), and a variable importance for projection (VIP) score was assigned to each metabolite in the OPLS-DA model for group discrimination. The differences in individual metabolite abundance between sample groups were further analyzed using 2-way repeated-measures ANOVA. Coupling the classification found by OPLS-DA and the differential expression obtained by 2-way repeated-measures ANOVA (time-by-location interaction), Hcy was identified as the top differential metabolite in this discovery cohort (figure 1C; VIP = 2.39, time-by-location interaction: FDR = 0.001). Specifically, although Hcy level was similar between RA and LA pre closure (figure 1D; p = 0.55), post PFO closure, Hcy level was immediately reduced in LA but not in RA (p < 0.001), suggesting a critical role of PFO in regulating Hcy level.
Validation of tHcy Reduction Following PFO Closure in an Independent Cohort
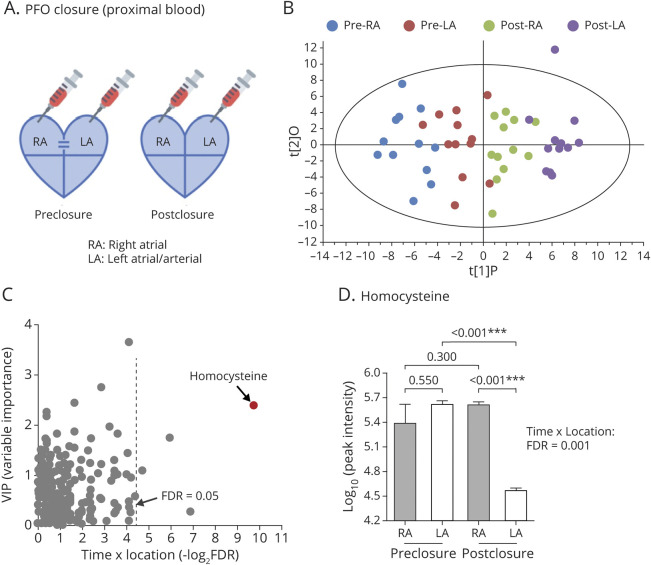
We further investigated the change of tHcy in a larger validation cohort (n = 181). Consistent with the discovery cohort, PFO closure resulted in a significant reduction of tHcy in the validation cohort (figure 2A; time-by-location interaction: p = 0.030; main effect of time: p < 0.001; main effect of location: p < 0.001). Before closure, RA and LA showed similar tHcy levels (p = 0.70), as would be expected in a context of PFO-enabled venous–arterial mixing. Immediately after PFO closure, tHcy reduction was seen in both RA (p = 0.001) and LA (p < 0.001). A more dramatic tHcy reduction was seen in LA than in RA (p < 0.001), implying a potential tHcy clearance mechanism through pulmonary circulation.
Figure 2. Immediate Effect of Patent Foramen Ovale (PFO) Closure on Intracardiac Total Homocysteine (tHcy) Levels (Validation Cohort).
(A) tHcy levels in pre–right atrium (RA), pre–left atrium (LA), post-RA, and post-LA were plotted for all patients receiving PFO closure (n = 181). (B–D) tHcy levels in patients with no residual shunting (n = 101), small residual shunting (n = 55), and moderate/large residual shunting (n = 25) post closure. Values are expressed as mean ± 95% confidence interval. Two-way repeated-measures analysis of variance was used to compare tHcy levels in each cardiac atrium followed by pairwise comparisons. *p < 0.05; **p < 0.01; ***p < 0.001.
Association of tHcy With Residual Shunting Post-PFO Closure
We have recently shown that residual shunting post PFO closure increases the risk of stroke recurrence.26 In the present study, PFOs were completely closed (no shunting) in 101 patients immediately (55.80%), while residual shunting was observed in 80 patients, with small shunting detected in 55 patients (30.39%) and moderate/large in 25 patients (13.81%).
Significant 3-way interaction was observed for the degree of residual shunting, time (pre vs post), and location (RA vs LA) (p = 0.038), suggesting a potential influence of shunting size on tHcy. As shown in figure 2B, tHcy levels were significantly reduced in patients with complete closure (time-by-location interaction: p = 0.010; main effect of time: p < 0.001; main effect of location: p < 0.001). Differential tHcy level between LA and RA was only observed post closure (p < 0.001) but not pre closure (p = 0.87). Patients with small residual shunting were also seen to have a slight tHcy reduction (figure 2C; time-by-location interaction: p = 0.23; main effect of time: p = 0.019; main effect of location: p = 0.16). tHcy level was significantly reduced in LA (p = 0.020); however, no changes in RA (p = 0.26) and no arteriovenous difference (p = 0.077) were observed. No significant changes were found in patients with moderate/large residual shunting (figure 2D; time-by-location interaction: p = 0.067; main effect of time: p = 0.69; main effect of location: p = 0.74). Taken together, these findings suggest a dose–response effect of PFO shunting on tHcy reduction.
Mixed effects model repeated measures analysis confirmed the association of residual shunting with the changes of tHcy (table 2; β: 0.115; 95% CI 0.047–0.183; p = 0.001). The result remained robust after controlling for other potential confounders in multivariate model (β: 0.103; 95% CI 0.028–0.178; p = 0.007), establishing that residual shunting was an independent determinant of tHcy reduction immediately following PFO closure.
Table 2.
Mixed Effects Regression Analysis of Immediate Total Homocysteine Change Following Patent Foramen Ovale Closure
Long-Term Effect of PFO Closure on tHcy Level in Peripheral Venous Blood
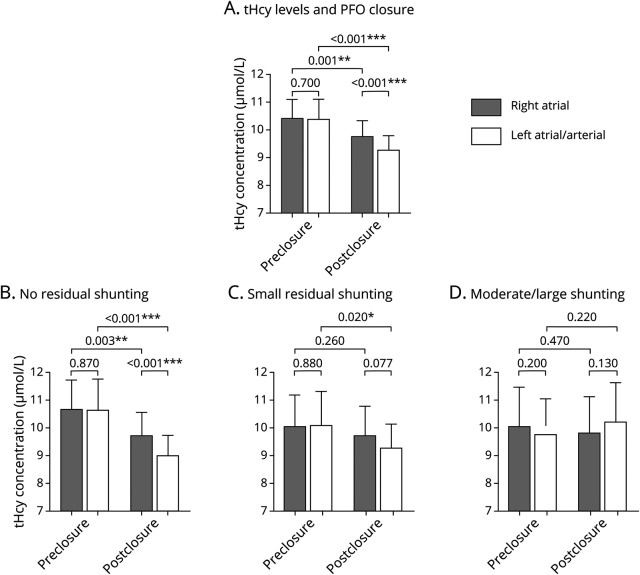
To determine the long-term effect of PFO closure on tHcy level, 115 patients were followed up to 4.29 years (median: 2.01 years; interquartile range: 0.68–2.53 years) post PFO closure (table 1). Peripheral venous tHcy was measured at baseline (pre closure) and at follow-ups post PFO closure (figure 3A). Our results revealed a long-term reduction of tHcy levels by PFO closure (baseline: 10.23 ± 4.31 μmol/L; follow-up: 9.56 ± 3.91 μmol/L; p = 0.039; figure 3D).
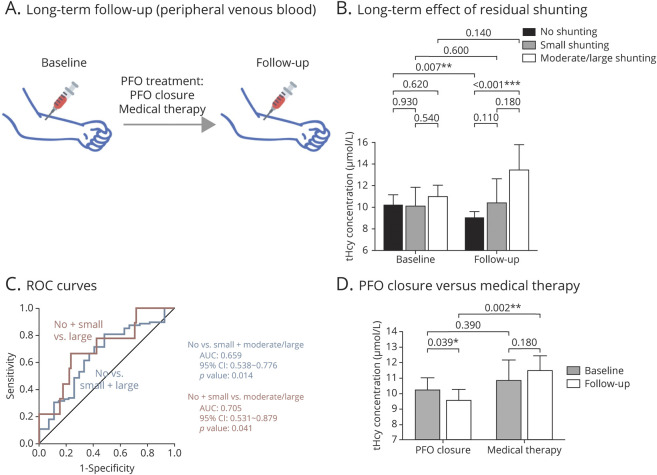
Figure 3. Long-Term Effect of Patent Foramen Ovale (PFO) Closure and Medical Therapy on Circulatory Venous Total Homocysteine (tHcy) Levels.
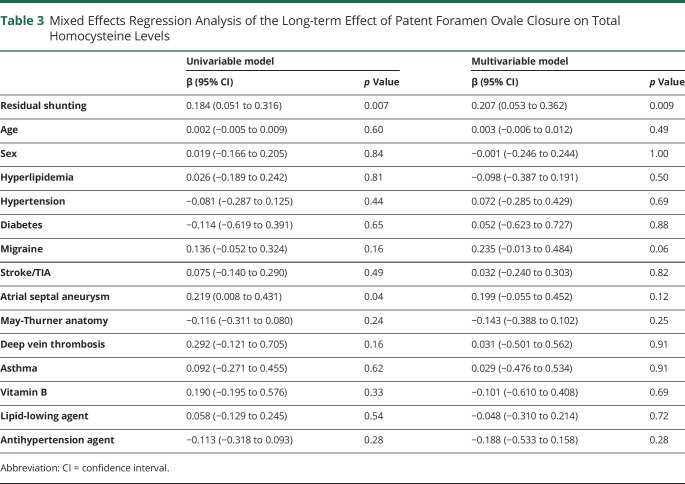
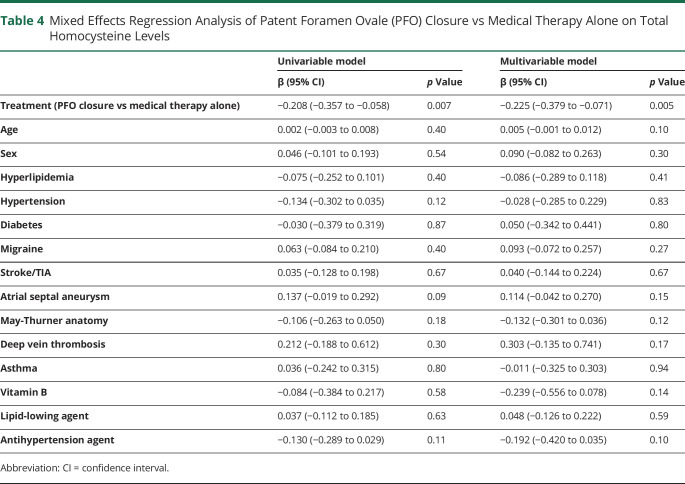
(A) Diagram of blood sampling during long-term follow-up. (B) Baseline and follow-up tHcy levels in patients with no residual shunting (n = 88), small residual shunting (n = 18), and moderate/large residual shunting (n = 9) post PFO closure. (C) Receiver operator characteristic (ROC) curves for tHcy changes in detecting the presence of residual shunting (no vs small + large) and large residual shunting (no + small vs large). (D) Baseline and follow-up tHcy levels in patients treated with PFO closure (n = 115) and medical therapy alone (n = 61). Values are expressed as mean ± 95% confidence interval (CI). Intergroup comparisons at each time point (baseline and follow-up) were performed using Student t test (C) or 1-way analysis of variance followed by pairwise comparisons (B). *p < 0.05; **p < 0.01; ***p < 0.001. AUC = area under the curve.
Of these patients, 88 (76.52%) showed complete PFO closure with no residual shunting, 18 patients (15.65%) had small residual shunting, and 9 patients (7.83%) had moderate/large residual shunting. As shown in figure 3B, patients with complete closure exhibited a significantly decreased tHcy level during follow-up as compared to baseline (10.18 ± 4.56 vs 8.98 ± 3.05 μmol/L; p = 0.007), whereas no significant change was observed in patients with either small or large residual shunting (small: 10.08 ± 3.55 vs 10.38 ± 4.58 μmol/L; p = 0.60; moderate/large: 10.96 ± 3.27 vs 13.47 ± 7.06 μmol/L; p = 0.14). The dose–response effect of residual PFO shunting was again observed in peripheral circulation over the long term (table 3; β: 0.184; 95% CI 0.051–0.316; p = 0.007). After adjusting for potential confounders, residual PFO shunting remained as an independent factor associated with tHcy changes (β: 0.207; 95% CI 0.053–0.362; p = 0.009).
Table 3.
Mixed Effects Regression Analysis of the Long-term Effect of Patent Foramen Ovale Closure on Total Homocysteine Levels
Residual PFO shunting can likewise be predicted by changes in tHcy level at follow-up (β: 0.222; 95% CI 0.072–0.372; p = 0.004). This result remained robust after covariate adjustment (β: 0.309; 95% CI 0.112–0.505; p = 0.002), suggesting tHcy as an independent predictor of residual shunting post PFO closure. As shown by ROC curves (figure 3C), tHcy changes exhibited discriminatory ability to predict the presence of residual shunting (no vs small + moderate/large: area under the curve [AUC]: 0.659; 95% CI 0.538–0.776; p = 0.014) and the presence of high-risk moderate/large residual shunting (no + small vs moderate/large: AUC: 0.705; 95% CI 0.531–0.879; p = 0.041).
Long-term Effect of PFO Closure vs Medical Therapy Alone (From Peripheral Venous Blood)
We compared the effects of PFO closure vs medical therapy alone. A total of 61 consecutive patients with cryptogenic PFO-related stroke, who were eligible for PFO closure but opted to stay on medical therapy alone, were recruited and followed for up to 4 years (median: 2.06 years; interquartile range: 0.87–2.56 years) (table 1). Peripheral venous tHcy was measured at baseline and at follow-ups. In contrast to PFO closure, medical therapy alone had no significant effects on plasma tHcy levels (figure 3D; 10.86 ± 5.14 vs 11.49 ± 3.74 μmol/L; p = 0.18).
A comparison between PFO closure and medical therapy revealed that different treatment choice was associated with tHcy levels at long term (table 4; β: −0.208; 95% CI −0.375∼–0.058; p = 0.007). Although no significant difference was observed between the 2 groups at baseline (p = 0.39), PFO closure showed more efficient tHcy clearance compared to medical therapy alone (p = 0.002) (figure 3D). After adjusting for all potential major confounders, the choice of treatment remained as an independent predictor of tHcy changes (table 4; β: −0.225; 95% CI −0.379∼−0.071; p = 0.005).
Table 4.
Mixed Effects Regression Analysis of Patent Foramen Ovale (PFO) Closure vs Medical Therapy Alone on Total Homocysteine Levels
Discussion
Our study provides proof-of-concept that successful PFO closure can independently reduce elevated levels of tHcy in patients with stroke. These findings suggest that PFO itself may participate in clot formation, beyond its role as a conduit for clots. In our study, by sampling blood directly from cardiac atrium during PFO closure procedure, we found that PFO-related right-to-left interatrial blood shunting contributes to elevated tHcy level with a dose–response effect such that the degree of shunting correlates directly to the degree of tHcy elevation. Furthermore, tHcy reduction is sustained over the long term after PFO closure in venous blood, whereas medical therapy does not alter tHcy level.
Growing evidence from clinical trials and system reviews identified preclosure moderate/large PFO shunting as a significant predictor of the benefit from PFO closure.6-8,27-29 Our recent study also revealed that moderate/large residual shunting post PFO closure increases the risk of stroke recurrence and is crucial for treatment decisions.26 However, the underlying mechanism remains to be understood. The results from the present study lend molecular evidence to our key clinical findings that the presence of PFO shunting, particularly large shunting,26 can enable the accumulation and persistence of Hcy as well as other vasoactive substances in circulation.
Hcy has long been recognized as a stroke risk factor, especially for small vessel stroke.30,31 Among patients with stroke, it has been found that tHcy level is higher in patients with a PFO than those without a PFO,32 implying a potential relation between PFO shunting and tHcy. Here, our study provides a proof-of-concept that PFO-related right-to-left interatrial blood shunting contributes to the elevation of harmful circulatory factor tHcy, while complete PFO closure results in immediate and persistent tHcy reduction in circulation. In contrast, medical therapy alone showed no influence on tHcy level. These findings support our hypothesis that PFO is not just a door to allow clots through, but may itself enhance clot formation and injure neurovasculature by clot-independent mechanisms. Moreover, long-term tHcy level in peripheral circulation represents a potential marker for quantifying the size of residual shunting post PFO closure, which may facilitate accurate shunt monitoring in clinic.
In our study, patients had an average tHcy level of 10.45 μmol/L at baseline. For high-risk populations, a tHcy level <10 µmol/L has been recommended as a reasonable therapeutic goal.33 Although our level is only borderline abnormal, there are several lines of evidence suggesting that tHcy at 10 μmol/L is a risk factor for cerebrovascular dysfunction and stroke. For example, patients with tHcy >10.2 μmol/L are at 2 times greater risk of stroke,34 and tHcy level >10.3 μmol/L is predictive of early neurologic deterioration after ischemic stroke.35 In the healthy population, tHcy level >9.6 µmol/L is associated with enlarged perivascular space.36 Even 1 μmol/L tHcy elevation in circulation is capable of promoting white matter lesions and lacunar infarcts, increasing the risk of future strokes.37 We thus suggest that high tHcy levels as a result of PFO-related shunting may continue to promote a hypercoagulable status important in paradoxical embolism.5
Whereas kidney has also been considered as an important organ responsible for Hcy clearance, the enzymes involved in Hcy metabolism, such as methylene tetrahydrofolate reductase, 5-methyltetrahydrofolate-Hcy methyltransferase, cystathionine-β-synthase, and cystathionine γ-lyase, are also expressed in appreciable levels in the lung and are crucial for normal pulmonary functions.38,39 Other Hcy uptake mechanisms, alanine-serine-cysteine transporter system, aspartate and glutamate transporter system, and large branched-chain neutral amino acids transporter system also actively function in pulmonary artery endothelium and alveoli.40,41 Moreover, elevated tHcy has frequently been identified in a variety of pulmonary disorders, such as COPD and pulmonary hypertension.42,43 This evidence all suggests a role of lung in Hcy reabsorbance and metabolism.44
Although the exact causal relationship requires further investigation, our finding of the differential tHcy levels between LA and RA following complete PFO closure (figure 2, A and B) implies that venous Hcy may be metabolized via pulmonary circulation. While no PFO stroke animal model exists, the study of the connection between the heart and brain is an emerging field highlighting the importance of organ–organ interaction.4 Our attempt to understand the molecular landscape of PFO physiology directly at the bedside offers a glimpse of how PFO-related stroke is a multiorgan systemic disease that will require a multidisciplinary approach from experts in heart, brain, blood, and lung.3
Whereas tHcy stands out as a rare, easily modifiable risk factor, which can be corrected with vitamin supplementation, the presence of PFO shunting, as evidenced by our metabolite profiling (figure 1), may cause circulatory imbalance of many other vasoactive substances. These procoagulable substances can team up to contribute to various clinical symptoms and lead to different responses to therapy. These findings may also relate to migraine physiology, such that PFO closure affects migraine frequency based on this circulatory signaling relationship of PFO anatomy.3 As PFO shunting is far more complicated than previously thought, an in-depth understanding of the molecular landscape associated with PFO-related circulation will not only expand our knowledge of the pathology of PFO-related disorders, such as stroke, migraine, and white matter disease, but also guide treatment and medication usage,45,46 which may be of particular importance for patients with PFO-related stroke on medical therapy alone.
Compared to current gold standard of cardiac echo with bubble counting for PFO sizing, small molecules, such as Hcy, have the potential to give more sensitive and physiologic quantification of PFO shunting. Bubble study is an anatomical estimate of PFO size; developing molecular markers to monitor not only shunting, but also the consequence of PFO shunting in promoting a hypercoagulable state, may offer additional value to guide therapy.
Due to the complexity of obtaining proximal intra-atrial blood and peripheral blood comparison, there are several limitations to our study. Blood sampling during PFO closure can be challenging due to clinical complexity and to ensure patient safety. However, every effort was made to compare pre- and postclosure level within the same individual at the same location with temporal profiling as internal control. We also found similar tHcy level between venous blood collected from proximal and peripheral sites (pre-RA vs baseline: 10.40 ± 4.79 vs 10.23 ± 4.31 μmol/L, p = 0.75); the potential technique noise did not cause a significant deviation in tHcy measurement. The medications used during PFO closure and in long-term treatment may also have potential influence on tHcy levels. However, except for vitamin B, which was adjusted during analysis to have no difference between groups, there is no reported interaction for any of the antiplatelets (aspirin, P2Y12 inhibitors), anticoagulants (heparin, warfarin), or sedatives (fentanyl and midazolam) on Hcy metabolism. For the acute medications used during PFO closure, all patients were given the same anesthesia and systemic anticoagulants throughout the procedure (see Methods), and we did not see any acute influence of these medications on tHcy concentrations, as tHcy levels were similar between baseline (venous) and pre-RA (both venous blood), which were collected respectively before and after administration of conscious sedation medication. The short half-lives of these acute medications (∼1 hour for heparin, 2–4 hours for fentanyl, and 1.5–2.5 hours for midazolam) further suggest that they may have little, if any, long-term influence. For the medications used for chronic treatment, including antiplatelets (aspirin, clopidogrel), anticoagulants (warfarin), vitamin B, lipid-lowing agents, and antihypertension agents, we found no significant difference in their use between groups (table 1) and no significant influence was observed on tHcy levels in either the PFO closure group or the medical therapy group (tables 2–4). Our exploratory profiling had a modest sample size due to limited resources at the time, but we made every effort to eliminate confounders with rigorous SOP during sample acquisition, analyzed all samples in a single batch to avoid variability, and validated our finding in a much larger cohort. We had other potential candidates, but due to limited resources, we selected tHcy because it was the most significantly changed candidate and is biologically plausible (due to pulmonary inactivation) in addition to being clinically relevant and easily modifiable. tHcy alone showed some ability to discriminate residual shunting, but exploring circulatory alterations in a larger patient cohort and developing a multi-biomarker panel will not only expand our understanding of the complex PFO physiology, but also improve performance in predicting residual shunting.
PFO-related stroke requires an in-depth understanding of the molecular landscape associated with right-to-left shunting, and novel biomarkers and therapeutic targets are needed for better risk stratification and effective treatment. Future studies are warranted to more rigorously map the proteomic, metabolomic, genetic, and functional correlates of PFO pathophysiology and their effects on neurovascular injury in stroke and other disease such as migraine.
The present study demonstrates that PFO closure may serve to renormalize circulating tHcy in patients with PFO-related stroke. Accordingly, differential tHcy levels pre and post closure may be a useful inexpensive biomarker (compared to echocardiogram) to monitor PFO closure efficacy (i.e., the degree of residual shunting). Our results further suggest a means by which PFO shunting itself may contribute to clot formation, setting the stage for PFO-related stroke.
Acknowledgment
The authors thank Dr. Charles F. Burant (U24 DK097153) for metabolomic profiling analysis.
Glossary
- ANOVA
analysis of variance
- AUC
area under the curve
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- FDR
false discovery rate
- Hcy
homocysteine
- HPLC
high-performance liquid chromatography
- LA
left atrium/arterial
- MCR2
Michigan Regional Comprehensive Metabolomics Resource Core
- MGH
Massachusetts General Hospital
- MS
mass spectrometry
- OPLS-DA
orthogonal projections to latent structures-discriminant analysis
- PFO
patent foramen ovale
- qTOF
quadrupole time-of-flight
- RA
right atrium
- ROC
receiver operator characteristic
- SOP
standard operating procedure
- SRM
selected reaction monitoring
- tHcy
total homocysteine
- VIP
variable importance for projection
Appendix. Authors
Footnotes
Editorial, page 55
Study Funding
NIH (R01NS067139, R01NS093415, and U24 DK097153).
Disclosure
The authors report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Saver JL. Clinical Practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. [DOI] [PubMed] [Google Scholar]
- 2.Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318(18):1148-1152. [DOI] [PubMed] [Google Scholar]
- 3.Ning M, Lo EH, Ning PC, et al. The brain's heart: therapeutic opportunities for patent foramen ovale (PFO) and neurovascular disease. Pharmacol Ther. 2013;139(2):111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-heart interaction: cardiac complications after stroke. Circ Res. 2017;121(4):451-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345(24):1740-1746. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-1032. [DOI] [PubMed] [Google Scholar]
- 7.Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-1042. [DOI] [PubMed] [Google Scholar]
- 8.Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021. [DOI] [PubMed] [Google Scholar]
- 9.Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368(12:1092-1100. [DOI] [PubMed] [Google Scholar]
- 10.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366(11):991-999. [DOI] [PubMed] [Google Scholar]
- 11.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368(12):1083-1091. [DOI] [PubMed] [Google Scholar]
- 12.Mojadidi MK, Elgendy AY, Elgendy IY, et al. Transcatheter patent foramen ovale closure after cryptogenic stroke: an updated meta-analysis of randomized trials. JACC Cardiovasc Interv. 2017;10(21):2228-2230. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Song B, Palacios IF, et al. Patent foramen ovale attributable cryptogenic embolism with thrombophilia has higher risk for recurrence and responds to closure. JACC Cardiovasc Interv. 2020;13(23):2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez MF, Krastins B, Sarracino DA, et al. Proteomic signatures of serum albumin-bound proteins from stroke patients with and without endovascular closure of PFO are significantly different and suggest a novel mechanism for cholesterol efflux. Clin Proteomics. 2015;12(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning M, Gonzalez RG. Case records of the Massachusetts General Hospital: case 34-2013: a 69-year-old man with dizziness and vomiting. N Engl J Med. 2013;369(18):1736-1748. [DOI] [PubMed] [Google Scholar]
- 16.Anzola GP. Patent foramen ovale and migraine: an example of heart-brain interaction. Nat Clin Pract Neurol. 2009;5(1):20-21. [DOI] [PubMed] [Google Scholar]
- 17.Diener HC, Kurth T, Dodick D. Patent foramen ovale, stroke, and cardiovascular disease in migraine. Curr Opin Neurol. 2007;20(3):310-319. [DOI] [PubMed] [Google Scholar]
- 18.Tobis JM, Charles A, Silberstein SD, et al. Percutaneous closure of patent foramen ovale in patients with migraine: the PREMIUM trial. J Am Coll Cardiol. 2017;70(22):2766-2774. [DOI] [PubMed] [Google Scholar]
- 19.Rhee EP, Gerszten RE. Metabolomics and cardiovascular biomarker discovery. Clin Chem. 2012;58(1):139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wurtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inglessis I, Elmariah S, Rengifo-Moreno PA, et al. Long-term experience and outcomes with transcatheter closure of patent foramen ovale. JACC Cardiovasc Interv. 2013;6(11):1176-1183. [DOI] [PubMed] [Google Scholar]
- 22.Evans CR, Karnovsky A, Kovach MA, Standiford TJ, Burant CF, Stringer KA. Untargeted LC-MS metabolomics of bronchoalveolar lavage fluid differentiates acute respiratory distress syndrome from health. J Proteome Res. 2014;13(2):640-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perng W, Hector EC, Song PXK, et al. Metabolomic determinants of metabolic risk in Mexican adolescents. Obesity. 2017;25(9):1594-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S, Duren W, Evans CR, Burant CF, Michailidis G, Karnovsky A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics. 2017;33(10):1545-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persichilli S, Gervasoni J, Iavarone F, Zuppi C, Zappacosta B. A simplified method for the determination of total homocysteine in plasma by electrospray tandem mass spectrometry. J Sep Sci. 2010;33(20):3119-3124. [DOI] [PubMed] [Google Scholar]
- 26.Deng W, Yin S, McMullin D, et al. Residual shunt after patent foramen ovale closure and long-term stroke recurrence. Ann Intern Med. 2020;172(11):717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smer A, Salih M, Mahfood Haddad T, et al. Meta-analysis of randomized controlled trials on patent foramen ovale closure versus medical therapy for secondary prevention of cryptogenic stroke. Am J Cardiol. 2018;121(4):1393-1399. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad Y, Howard JP, Arnold A, et al. Patent foramen ovale closure vs. medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J. 2018;39(18):1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lattanzi S, Brigo F, Cagnetti C, Di Napoli M, Silvestrini M. Patent foramen ovale and cryptogenic stroke or transient ischemic attack: to close or not to close? A systematic review and meta-analysis. Cerebrovasc Dis. 2018;45(5-6):193-203. [DOI] [PubMed] [Google Scholar]
- 30.Larsson SC, Traylor M, Markus HS. Homocysteine and small vessel stroke: a mendelian randomization analysis. Ann Neurol. 2019;85(4):495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoamanesh A, Preis SR, Beiser AS, et al. Circulating biomarkers and incident ischemic stroke in the Framingham Offspring Study. Neurology. 2016;87(12):1206-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozdemir AO, Tamayo A, Munoz C, Dias B, Spence JD. Cryptogenic stroke and patent foramen ovale: clinical clues to paradoxical embolism. J Neurol Sci. 2008;275(1-2):121-127. [DOI] [PubMed] [Google Scholar]
- 33.Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1999;99(1):178-182. [DOI] [PubMed] [Google Scholar]
- 34.Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. JAMA. 1997;277(22):1775-1781. [DOI] [PubMed] [Google Scholar]
- 35.Kwon HM, Lee YS, Bae HJ, Kang DW. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke. 2014;45(3):871-873. [DOI] [PubMed] [Google Scholar]
- 36.Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology. 2019;92(4):e317-e325. [DOI] [PubMed] [Google Scholar]
- 37.Kloppenborg RP, Geerlings MI, Visseren FL, et al. Homocysteine and progression of generalized small-vessel disease: the SMART-MR study. Neurology. 2014;82(9):777-783. [DOI] [PubMed] [Google Scholar]
- 38.Madurga A, Golec A, Pozarska A, et al. The H2S-generating enzymes cystathionine beta-synthase and cystathionine gamma-lyase play a role in vascular development during normal lung alveolarization. Am J Physiol Lung Cell Mol Physiol. 2015;309(7):L710-L724. [DOI] [PubMed] [Google Scholar]
- 39.Chen NC, Yang F, Capecci LM, et al. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J. 2010;24(8):2804-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deneke SM, Susanto I, Vogel KA, Williams CE, Lawrence RA. Mechanisms of use of extracellular glutathione by lung epithelial cells and pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1995;12(6):662-668. [DOI] [PubMed] [Google Scholar]
- 41.Jiang X, Yang F, Brailoiu E, et al. Differential regulation of homocysteine transport in vascular endothelial and smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27(9):1976-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seemungal TA, Lun JC, Davis G, et al. Plasma homocysteine is elevated in COPD patients and is related to COPD severity. Int J Chron Obstruct Pulm Dis. 2007;2(3):313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anwar A, Ruffenach G, Mahajan A, Eghbali M, Umar S. Novel biomarkers for pulmonary arterial hypertension. Respir Res. 2016;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12(10):2181-2189. [DOI] [PubMed] [Google Scholar]
- 45.Arab S, Gramolini AO, Ping P, et al. Cardiovascular proteomics: tools to develop novel biomarkers and potential applications. J Am Coll Cardiol. 2006;48(9):1733-1741. [DOI] [PubMed] [Google Scholar]
- 46.Lau E, Watson KE, Ping P. Connecting the dots: from big data to healthy heart. Circulation. 2016;134(5):362-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on reasonable request.