Abstract
Introduction
Hypogonadotropic hypogonadism (HH) is an almost universal, yet underappreciated, endocrinological complication of traumatic brain injury (TBI). The goal of this study was to determine whether the developmental hormone human chorionic gonadotropin (hCG) treatment could reverse HH induced by a TBI.
Methods
Plasma samples were collected at post‐surgery/post‐injury (PSD/PID) days ‐10, 1, 11, 19 and 29 from male Sprague‐Dawley rats (5‐ to 6‐month‐old) that had undergone a Sham surgery (craniectomy alone) or CCI injury (craniectomy + bilateral moderate‐to‐severe CCI injury) and treatment with saline or hCG (400 IU/kg; i.m.) every other day.
Results
Both Sham and CCI injury significantly decreased circulating testosterone (T), 11‐deoxycorticosterone (11‐DOC) and corticosterone concentrations to a similar extent (79.1% vs. 80.0%; 46.6% vs. 48.4%; 56.2% vs. 32.5%; respectively) by PSD/PID 1. hCG treatment returned circulating T to baseline concentrations by PSD/PID 1 (8.9 ± 1.5 ng/ml and 8.3 ± 1.9 ng/ml; respectively) and was maintained through PSD/PID 29. hCG treatment significantly, but transiently, increased circulating progesterone (P4) ~3‐fold (30.2 ± 10.5 ng/ml and 24.2 ± 5.8 ng/ml) above that of baseline concentrations on PSD 1 and PID 1, respectively. hCG treatment did not reverse hypoadrenalism following either procedure.
Conclusions
Together, these data indicate that (1) craniectomy is sufficient to induce persistent hypogonadism and hypoadrenalism, (2) hCG can reverse hypogonadism induced by a craniectomy or craniectomy +CCI injury, suggesting that (3) craniectomy and CCI injury induce a persistent hypogonadism by decreasing hypothalamic and/or pituitary function rather than testicular function in male rats. The potential role of hCG as a cheap, safe and readily available treatment for reversing surgery or TBI‐induced hypogonadism is discussed.
Keywords: human chorionic gonadotropin, hypoadrenalism, hypogonadism, RU‐486, testosterone, traumatic brain injury
Traumatic brain injury (TBI) is a major public health problem that induces hypogonadism and exacerbates the length of recovery. We find that craniectomy or TBI in male rats induces a persistent hypogonadism that can be reversed using the developmental hormone human chorionic gonadotropin (hCG). hCG is a cheap and readily available FDA‐approved drug that could be a safe treatment for reversing hypogonadism and improving recovery from brain trauma.
1. INTRODUCTION
Traumatic brain injury (TBI) is a major public health problem 1 due to the relatively high incidence rate (106 per 100,000 globally), 2 and the lack of effective treatments. The incidence of TBI in males is 3 times that of females, but normalizes to 1:1 by age 65. 3 The consequences of a TBI can include functional (eg decreased cognitive performance), psychopathological (eg post‐traumatic stress disorder), neuroanatomical (eg cystic infarcts, neurodegeneration) and biochemical (eg inflammation) changes.
An underappreciated endocrinological complication of TBI is hypogonadotropic hypogonadism (HH). TBI can markedly suppress pituitary gonadotropin secretion and gonadal sex steroid production. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Such hypothalamic‐pituitary‐gonadal (HPG) axis hormones have well‐described roles in the formation and maintenance of brain structure and cognitive function (reviewed in Hara et al. 17 ). Our early studies utilizing human embryonic stem cells (hESC) as a model of early embryogenesis 18 identified human chorionic gonadotropin (hCG), a trophoblastic gonadotropin hormone, as modulating the expression and processing of the amyloid‐β precursor protein (AβPP 19 , 20 ). Although this protein has well‐defined neurogenic properties, 21 , 22 , 23 , 24 , 25 , 26 our data suggested AβPP also had important developmental functions during early human embryogenesis (prior to the formation of neural precursor cells). We subsequently determined that hCG signals via the luteinizing hormone/chorionic gonadotropin receptor (LHCGR) on hESC to promote the growth and development of the pre‐implantation embryo, including the formation of the 3 germ layers in the morula and its development into a blastocyst. 27 , 28 hCG mediated these effects via the upregulation of steroidogenesis (P4 synthesis); P4 signalling was found to be obligatory for both embryoid body (aka morula) and neuroectodermal rosette (aka primitive neural tube) formation.
In addition to hCG's role in early embryonic and brain development, 27 hCG and its adult homolog luteinizing hormone (LH) promote neuronal proliferation, 29 while hCG and sex steroids regulate adult neuronal differentiation (ie neuritogenesis, spine density, synaptogenesis). 30 , 31 Conversely, age‐related or age‐induced reproductive endocrine dyscrasia has a negative impact on cognitive function, 32 an effect that can be slowed or halted by the partial rebalancing of the HPG axis with sex steroid supplementation 33 , 34 or gonadotropin‐releasing hormone (GnRH) superagonist and antagonist treatment. 35 HH could therefore greatly compromise cognitive performance and neuroregeneration following a TBI. Importantly, hCG has been shown to improve functional recovery following spinal cord injury in rats, 30 , 36 , 37 and decrease ischaemic brain injury in adult and neonatal rodent models of stroke. 38 , 39 , 40 Aside from these neuroprotectant properties, hCG also promotes neurite sprouting and neuron survival. 40
TBI‐induced HH is thought to result from damage to the hypothalamus or pituitary 41 , 42 and/or stress‐induced cortisol‐mediated suppression of the HPG axis. 43 , 44 While prevalence rates for HH vary widely, likely due to the severity of the injury, location and type of injury, time of screening and design of the study, there is increasing consensus that even mild TBIs can induce HH and that severe TBIs induce persistent HH. 6 , 14 , 45 , 46 , 47 This silent condition goes mostly undiagnosed and therefore untreated. Based on the pleiotropic properties of hCG, in this study we tested whether hCG treatment could reverse HH induced by a commonly used TBI model (controlled cortical impact; CCI). We find that hCG treatment reverses HH induced by either a craniectomy alone, or a craniectomy and penetrating CCI injury, in adult male rats.
2. MATERIALS AND METHODS
2.1. Subjects
Male Sprague‐Dawley rats (n = 58, 5 to 6 months old) were acquired from Harlan Laboratories Inc. (Madison, WI) and acclimated to the environment over 2 days. Rats were then weighed and handled for no less than 5 min. each for 5 consecutive days and daily thereafter while being housed, fed and maintained on a 12‐h reverse light/dark cycle. The Institutional Animal Care and Use Committee (Animal Component of Research Protocol) at the William S. Middleton Veterans Administration Hospital approved the procedures used in this study, and the research was conducted in an AAALAC‐approved facility. Experimenters were blinded as to the identity of the animals throughout injections, blood collections, and body weight and hormone data analyses.
2.2. Surgeries
All surgical procedures were carried out under isoflurane gas anaesthesia (5% for induction; 1.5%–3.0% for maintenance, craniectomy ~15‐ to 30‐min duration; craniectomy +CCI injury ~25‐ to 45‐min duration). An anaesthesia chamber was used for induction, and a nose cone was used for maintenance.
2.2.1. Controlled cortical impact and sham surgeries
Anaesthetized rats were mounted in a Kopf stereotaxic device (Model 900), where the animal's head was held in place by non‐traumatic ear bars and a bite bar. Anaesthesia was maintained by nose cone while the head was shaved and sterilized with 70% ethanol and Betadine™ (Purdue Products L.P.) antiseptic solution. Throughout surgery, anaesthesia levels were monitored closely and were frequently adjusted as needed, based on heart rate, respiration rate and oxygen saturation. A homeothermic blanket control unit (Harvard Apparatus, Holliston, MA) was used to monitor body temperature and to prevent hypothermia throughout surgery.
Under aseptic conditions, the cranium and its bony landmarks including bregma (β) and lambda (λ) were exposed by making a midline incision along the scalp into the skin and fascia covering the skull. A 6‐mm‐diameter craniectomy was centred on the midline at 2.5 mm anterior to β. The cortical impact was made at 2.5 mm anterior to β over the midline of the medial frontal cortex with an Impact One™ Stereotaxic CCI instrument (Leica), using a 5 mm impactor (bit size), travelling at 2.25 m/s (velocity), extending 3 mm below the cortical surface (impact depth) for 100 ms (dwell time). Sham‐injured groups received the same surgical procedures up to and including craniectomy but no CCI injury. After surgery, the rats were placed on a heating pad, monitored closely and upon awakening were tested 30 min later for righting reflex to assess any immediate effects of craniectomy or CCI injury on righting ability, and then returned to their home cages.
2.3. Experimental design
Once out of quarantine all rats were weighed and handled for one week. The final body weights at the end of this week were (1) ranked from highest to lowest (as a function of age) and then (2) used to assign each rat to a surgery/treatment group in a counterbalanced manner (ie using the ABBA method).
2.3.1. Experiment 1
Rats were assigned to the following groups: Sham + saline (n = 5), Sham + hCG (n = 5), CCI + saline (n = 8), CCI + hCG (n = 8). Baseline blood draws were collected from all animals. Beginning 1 hour after craniectomy or CCI, Pregnyl® (hCG, 400 IU/kg; Merck & Co., Inc.) or saline (0.9% NaCl in deionized H2O; equivalent volume to that injected for hCG) was injected intramuscularly every other day for 29 days. Pregnyl® is a highly purified pyrogen‐free preparation obtained from the urine of pregnant females. Each vial contains 10,000 USP units of sterile dried powder with 5 mg monobasic sodium phosphate and 4.4 mg dibasic sodium phosphate that is diluted in solvent containing water, 0.56% sodium chloride and 0.9% benzyl alcohol (https://www.drugs.com/pro/pregnyl.html).
2.3.2. Experiment 2
Rats were assigned to the following groups: Sham + saline + RU‐486 (n = 5), Sham + hCG + RU‐486 (n = 5), CCI + saline + RU‐486 (n = 5), or CCI + hCG + RU‐486 (n = 5). RU‐486 (Mifepristone, 100 mg/ml solution, CAS Number 84371‐65‐3; 40 mg/kg in 100% ethanol; Sigma‐Aldrich Corp.) was injected intraperitoneally 15–20 min before every hCG or saline control injection.
2.4. Blood collection and hormone analyses
Rats were anaesthetized (between 9:00 a.m.–12:00 noon) and their tails placed in a 200‐ml beaker filled with warm water (≤44°C) for 5 min. The tail was cleaned with 70% alcohol, the minimal amount of the tail tip snipped with a blade, and/or the wound reopened by removal of the scab for subsequent bleeds, and ~1 ml of whole blood was collected directly into EDTA tubes at baseline (post‐injury day (PID)−10) and at PID, 1, 11, 19 and 29. Blood collected did not exceed 1% of body weight every 2‐week period. Animals were injected with Lactate Ringers solution (5 ml) for fluid resuscitation. At the terminal bleed (day 29), blood also was collected via heart puncture. Collected blood was immediately centrifuged at 4000 g for 10–20 min. and the plasma aliquoted into Eppendorf tubes for storage at −80°C. Plasma samples were analysed at the Assay Services Laboratory in the Wisconsin National Primate Research Center of the UW‐Madison Institute for Clinical and Translational Research for progesterone (P4), testosterone (T), 11‐deoxycorticosterone (11‐DOC) and corticosterone adapted from a method previously described. 48 , 49 Briefly, to plasma samples (400 μl) internal standard (200 pg d9‐progesterone and d5‐testosterone and 1 ng d4‐cortisol) was added and the samples were extracted with methyl tert butyl ether. The organic phase was transferred to a clean vial and evaporated to dryness, and then, a second dichloromethane extraction was performed. The organic phase was transferred into a clean test tube and evaporated to dryness and reconstituted in mobile phase. Samples were analysed on a QTRAP 5500 quadruple linear ion trap mass spectrometer (AB Sciex) equipped with an atmospheric pressure chemical ionization source. The system includes two Shimadzu LC20ADXR pumps and a Shimadzu SIL20ACXR autosampler. A sample of 30 μl was injected onto a Phenomenex Kinetex 2.6u C18 100A, 100 × 2.1 mm column (Torrance, CA) for separation using a mobile phase: water with 1% formic acid (Solution A) and acetonitrile with 1% formic acid (Solution B), at a flow rate of 200 μl/min. Quantitative results were recorded as multiple reaction monitoring (MRM) area counts after determination for the response factor for each compound and internal standard. Each steroid had a MRM used for quantitation and 1 or 2 additional MRMs as qualifiers. The linearity was r > .818, and the curve fit was linear with 1/x weighting. None of the compounds of interest were detected in blank or double blank samples. Inter‐assay coefficients of variation were determined from a pool of rat plasma: T—2.1%, P4—11.2%, 11‐DOC—5.3%, corticosterone—8.0%, androstenedione—13.3%.
2.5. Statistical analysis
A mixed factorial analysis of variance (ANOVA) for repeated measures was performed on the weight, behavioural, hormonal and gross lesion data (GraphPad Prism, v.7; GraphPad Software, Inc.). Post hoc analyses were performed using the Tukey multiple comparison test. Independent paired t tests were also used to compare the differences between baseline (pre‐injury) and post‐injury data when data were normally distributed. Hormone data collected on post‐surgery (PSD) or post‐injury (PID) days were analysed using the R statistical program, V.3.4.1 (R: A language and environment for statistical computing (program), Vienna, Austria: R Foundation for Statistical Computing, 2008), with package ‘rmcorr’ (rmcorr: repeated‐measures correlation (program), R package version 0.2.0, 2017), based on Bland and Altman's 50 , 51 statistical technique. Non‐repeated baseline data were analysed using Pearson's correlation with the R program. Statistical significance was established at p ≤ .05.
3. RESULTS
3.1. Craniectomy and controlled cortical impact injury induce hypogonadism and hypoadrenalism
ANOVA indicated a significant main effect of treatment for T (F(3,82) = 14.35, p < .0001), P4 (F(3,80) = 11.34, p < .0001), 11‐DOC (F(3,88) = 9.27, p < .0001) and corticosterone (F(3,88) = 24.98, p < .0001). A main effect of day was found for P4 (F(3,80) = 5.26, p = .0023), but not for T (F(3,82) = 1.02, p = .386), 11‐DOC (F(3,88) = 1.31, p = .277) and corticosterone (F(3,88) = 0.91, p = .440). No main effects of treatment × day interaction were found for T (F(9,82) = 0.33, p = .964), P4 (F(9,80) = 1.74, p = .095), 11‐DOC (F(9,88) = 1.07, p = .396) or corticosterone (F(9,88) = 0.94, p = .498).
Sham surgery (craniectomy + saline group) in male adult rats induced a decline from baseline in the circulating concentrations of T (79.1%; 7.5 ± 1.5 ng/ml to 1.6 ± 0.3 ng/ml; p < .05), P4 (61.6%; 9.0 ± 3.8 ng/ml to 3.5 ± 0.7 ng/ml; p = .061), 11‐DOC (46.6%; 338.3 ± 55.8 ng/ml to 180.7 ± 3.3 ng/ml, p < .05) and corticosterone (56.2%; 218.7 ± 24.5 ng/ml to 95.9 ± 2.2 ng/ml, p < .05) by PSD1 (Figure 1). 52 Similar declines in circulating concentrations of T (80.0%, 1.5 ± 0.4 ng/ml, p < .01), P4 (56.8%, 3.9 ± 1.4 ng/ml; p = .065), 11‐DOC (48.4%, 174.5 ± 19.4 ng/ml; p < .05) and corticosterone (32.5%, 147.7 ± 17.4 ng/ml; p < .05) were observed by PID 1 for CCI‐injured animals (ie craniectomy + CCI + saline group), indicating that Sham surgery alone was sufficient to induce hypogonadotropic hypogonadism (Figure 1A,B) and hypoadrenalism (Figure 1C,D). 52 Circulating concentrations for all hormones in both Sham surgery and CCI‐injured animals remained at these lower concentrations through PSD/PID 29 (except corticosterone in the CCI group on PID 29, which rose to 190.0 ± 33.0 ng/ml, Figure 1D). Circulating concentrations of androstenedione did not significantly change from baseline (0.58 ± 0.10 ng/ml) in Sham surgery or CCI injury groups (data not shown). These results suggest that sham surgery, and sham surgery plus a bilateral moderate‐to‐severe CCI injury, induces hypogonadism in rats.
FIGURE 1.
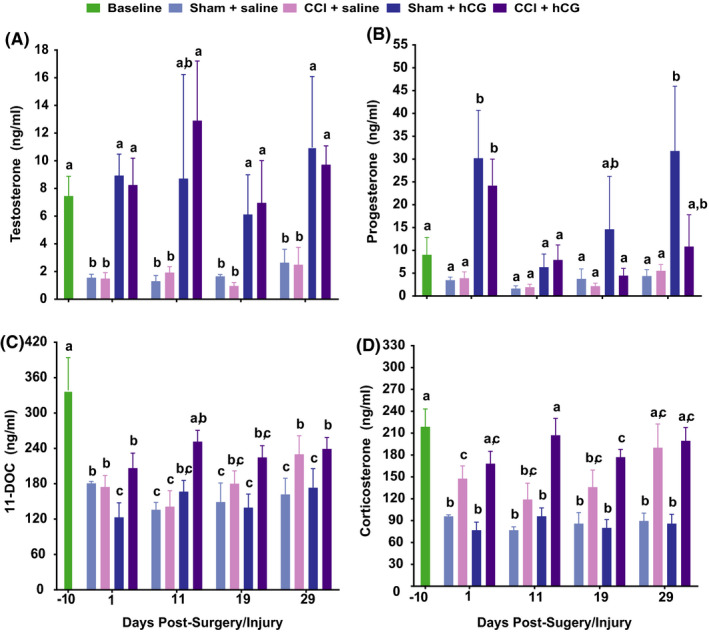
hCG reverses CCI‐induced decreases in circulating testosterone and progesterone. Plasma concentrations (mean ± SEM) of T (A), P4 (B), 11‐DOC (C) and corticosterone (D) in ng/ml on PID ‐10, 1, 11, 19 and 29 for the following groups: Sham +vehicle (n = 5), Sham +hCG (n = 5), CCI +saline (n = 8) and CCI +hCG (n = 8). Data were analysed using 2‐way repeated‐measures ANOVA; post hoc analyses were performed using the Tukey multiple comparison test (p < .05; letters indicate differences between treatment groups and pre‐ and post‐injury days)
3.2. hCG reverses craniectomy and CCI‐induced hypogonadism and attenuates hypoadrenalism
hCG treatment of animals that underwent a craniectomy (Sham surgery) or craniectomy plus CCI injury (CCI group) significantly increased circulating concentrations of T and P4 back to baseline concentrations by PSD/PID 1 (Figure 1A,B). Unlike P4, elevations in T were maintained through PSD/PID 29. While a significant main effect of treatment group for androstenedione concentration also was identified, post hoc analyses determined that androstenedione concentration was only elevated on PID 11 in the CCI +hCG group (4.5 ± 2.1 ng/ml) compared with the CCI +saline group (0.51 ± 0.2 ng/ml, p < .03). hCG treatment transiently reduced circulating 11‐DOC in Sham surgery animals (123.1 ± 24.6 ng/ml) when compared to Sham +saline animals (180.7 ± 3.3 ng/ml; p < .05) on PID 1, but not on PID 11, 19 and 29 (Figure 1C). hCG treatment had no effect on increasing corticosterone concentrations in Sham animals at any time point, but did increase circulating corticosterone in the CCI animals on PID 1 and 11 (Figure 1D). Together, these results demonstrate that hCG can reverse hypogonadism induced by a craniectomy or a craniectomy +CCI injury, but has lesser effect on reversing hypoadrenalism.
3.3. RU‐486 diminishes craniectomy + CCI‐induced hypoadrenalism
ANOVA indicated a significant main effect of treatment for T (F(3,71) = 3.76, p = .0145), P4 (F(3,61) = 5.82, p = .0011), 11‐DOC (F(3,61) = 3.66, p = .0171), and corticosterone (F(3,61) = 5.41, p = .0023). A main effect of day was found for P4 (F(3,61) = 5.82, p = .0015) and corticosterone (F(3,61) = 3.38, p = .0238), but not for T (F(3,71) = 1.86, p = .145) or 11‐DOC (F(3,61) = 1.80, p = .158). No main effects of treatment × day interaction were found for T (F(9,71) = 0.33, p = .965), P4 (F(9,61) = 1.09, p = .3838), 11‐DOC (F(9,61) = 0.54, p = .836) or corticosterone (F(9,61) = 0.65, p = .750).
Pretreatment of animals with RU‐486, a P4 receptor and glucocorticoid receptor antagonist, had little effect on Sham +hCG animals suppressing only P4 concentration on PID 29 (8.5 ± 4.5 ng/ml vs. 31.8 ± 14.2 ng/ml, p < .05; compare Figures 1B and 2B). In CCI‐injured animals, RU‐486 pretreatment increased P4 concentrations on PID 1 (40.9 ± 10.0 ng/ml vs. 24.2 ± 5.8 ng/ml, p < .05), and suppressed T concentrations on PID 11 (4.5 ± 1.5 ng/ml vs. 12.9 ± 4.3 ng/ml, p < .05) and PID 19 (3.1 ± 1.1 ng/ml vs. 9.7 ± 1.4 ng/ml, p < .05; Figure 2A,B). RU‐486 pretreatment had more significant effects on 11‐DOC and corticosterone, preventing the Sham + saline treatment‐induced decrease in 11‐DOC through PID 29 (Figure 2C) and preventing in Sham +hCG rats the decrease in 11‐DOC at PID 1 (282.4 ± 28.0 ng/ml vs. 123.1 ± 24.6 ng/ml, p < .05). RU486 pretreatment prevented Sham surgery‐induced decreases in corticosterone through PID 29 in both saline‐ and hCG‐treated animals (except on PID 19 in the Sham + hCG group; Figure 2D). RU‐486 pretreatment had no significant effects on circulating 11‐DOC and corticosterone in CCI‐injured animals (Figure 2C,D).
FIGURE 2.

RU‐486 treatment attenuates hCG‐induced reversal of circulating testosterone plasma concentrations. Plasma concentrations (mean ± SEM) of T (A), P4 (B), 11‐DOC (C) and corticosterone (D) in ng/ml on PID −10, 1, 11, 19 and 29 for the following groups: RU‐486: Sham +vehicle (n = 5), RU‐486: Sham +hCG (n = 5), RU‐486: CCI +saline (n = 5) and RU‐486: CCI +hCG (n = 5). Data were analysed using 2‐way repeated‐measures ANOVA; post hoc analyses were performed using the Tukey multiple comparison test (p < .05; letters indicate differences between treatment groups and pre‐ and post‐injury days). Differences between RU‐486‐induced changes in plasma hormones between treatment groups in Figures 1 and 2 are illustrated by (1) vertical lines represent an increase in plasma hormone concentration in RU‐486‐treated animals, and (2) horizontal lines represent a decrease in plasma hormone concentration in RU‐486‐treated animals
3.4. Relationships between circulating steroid concentrations before and after sham surgery, CCI injury and hCG treatment
Correlation analyses demonstrated strong positive correlations in baseline plasma samples between P4 with androstenedione (r = .84, p < .01), androstenedione with its metabolite T (r = .82, p < .01) and with corticosterone and its precursor 11‐DOC (r = .89, p < .001; Table 1). Sham injury obviated the significant correlations between sex steroids, but not corticosterone and its precursor 11‐DOC (r = 0.98, p < .001, Table 2). hCG treatment of Sham animals was sufficient in restoring the strong positive relationship between T and androstenedione (r = .76, p < .05), but not between P4 with androstenedione. hCG treatment induced two additional positive correlations between androstenedione with 11‐DOC (r = .84, p < .01) and androstenedione with corticosterone (r = .72, p < .05; Table 2). These results suggest that Sham surgery alone is sufficient to disrupt the relationship between sex steroid metabolism, a relationship that is partially reversed with hCG treatment.
TABLE 1.
The relationship between the concentrations of plasma steroids in rats
|
All groups and time points (PSD/PID 1, 11, 19 and 29) |
||||||
|---|---|---|---|---|---|---|
| T | P4 | Androstenedione | 11‐DOC | Corticosterone | ||
| Baseline samples (PSD/PID ‐10) | T |
0.13 104 |
0.68*** 92 |
0.22 104 |
0.15 104 |
|
| P4 |
0.41 10 |
0.28* 92 |
0.29* 104 |
0.25* 104 |
||
| Androstenedione |
0.82** 10 |
0.84** 10 |
0.33** 92 |
0.19 108 |
||
| 11‐DOC |
0.40 10 |
−0.15 10 |
0.06 10 |
0.90*** 104 |
||
| Corticosterone |
0.27 10 |
−0.28 10 |
−0.11 10 |
0.89*** 10 |
||
The top figure in each square is the coefficient of determination (r 2), and the bottom figure is the number of pairs that were analysed. The results comprising the bottom left triangle are for rats at baseline and the top right triangle are for rats from all time points: post‐surgery days (PSD)/post‐injury days (PID) 1, 11, 19 and 29 for all treatment groups (*p < .05, **p < .01, ***p < .001).
TABLE 2.
The relationship between the concentrations of plasma steroids in Sham surgery rats treated with or without hCG
| Sham + Saline (PSD 1, 11, 19 and 29) | ||||||
|---|---|---|---|---|---|---|
| T | P4 | Androstenedione | 11‐DOC | Corticosterone | ||
| Sham + hCG (PSD 1, 11, 19 and 29) | T |
0.29 20 |
0.04 20 |
<0.01 20 |
0.08 20 |
|
| P4 |
0.05 20 |
0.11 20 |
0.84*** 20 |
0.87*** 20 |
||
| Androstenedione |
0.76* 20 |
0.29 14 |
0.34 20 |
0.34 20 |
||
| 11‐DOC |
0.44 20 |
0. 27 20 |
0.84** 14 |
0.98*** 20 |
||
| Corticosterone |
0.25 14 |
0.24 20 |
0.72* 14 |
0.94*** 20 |
||
The top figure in each square is the coefficient of determination (r 2), and the bottom figure is the number of pairs that were analysed. The results comprising the bottom left triangle are for Sham surgery rats treated with hCG and the top right triangle are for Sham surgery rats treated with saline, from post‐surgery days (PSD) 1, 11, 19 and 29 (*p < .05, **p < .01, ***p < .001).
CCI injury, like sham injury, resulted in positive relationships between P4 with corticosterone (r = .58, p < .01) and 11‐DOC (r = .68, p < .001), between corticosterone and its precursor 11‐DOC (r = .91, p < .001; Table 3), as well as the loss of significant correlations between sex steroids. Unlike sham injury, CCI injury resulted in a strong correlation between androstenedione with its metabolite T (r = .97, p < .001). Like Sham animals, hCG treatment of CCI animals restored the positive relationship between T and androstenedione (r = .64, p < .001), while the positive correlation between corticosterone and 11‐DOC (r = .93, p < .001) was maintained. Together, these results suggest that like Sham surgery, CCI injury disrupts sex steroid metabolism, while hCG treatment partially restores sex steroid metabolism.
TABLE 3.
The relationship between the concentrations of plasma steroids in CCI‐injured rats treated with or without hCG
| CCI + Saline (PID 1, 11, 19 and 29) | ||||||
|---|---|---|---|---|---|---|
| T | P4 | Androstenedione | 11‐DOC | Corticosterone | ||
|
CCI + hCG (PID 1, 11, 19 and 29) |
T |
0.04 32 |
0.97*** 32 |
−0.06 32 |
−0.10 32 |
|
| P4 |
0.27 32 |
0.08 32 |
0.67*** 32 |
0.58** 32 |
||
| Androstenedione |
0.64*** 32 |
0.30 32 |
−0.03 32 |
−0. 08 32 |
||
| 11‐DOC |
0.26 32 |
0.30 32 |
0.20 32 |
0.91*** 32 |
||
| Corticosterone |
0.32 32 |
0.31 32 |
0.20 32 |
0.93*** 32 |
||
The top figure in each square is the coefficient of determination (r 2), and the bottom figure is the number of pairs that were analysed. The results comprising the bottom left triangle are for CCI injury rats treated with hCG and the top right triangle are for CCI injury rats treated with saline, from post‐injury days (PID) 1, 11, 19 and 29 (*p < .05, **p < .01, ***p < .001).
4. DISCUSSION
We demonstrate for the first time that intraperitoneal injection of hCG is effective in reversing hypogonadism (Figure 1A,B) and attenuating hypoadrenalism (Figure 1C,D) following a craniectomy or craniectomy +CCI injury in young adult male rats. Both craniectomy and craniectomy +CCI injury promoted corticosteroid production in favour of sex steroid (P4) production, a relationship that was reversed with hCG treatment (Figure 1; Tables 1, 2, 3). hCG's ability to increase sex steroid plasma concentrations following a craniectomy and following a moderate‐to‐severe brain injury supports its potential as a treatment for TBI‐induced hypogonadism.
It is important to note that while hCG has potential to reverse hypogonadism and promote neurogenesis and cognitive recovery, an increase in circulating hCG/LH concentrations as a result of ovariectomy 53 , 54 , 55 , 56 , 57 , 58 or treatment 59 , 60 has been shown to impair cognition in rodents, while lowering LH or blocking LHCGR signalling is protective of memory in rodents 53 , 54 , 56 , 57 , 58 , 60 , 61 , 62 , 63 and humans. 35 Conversely, one study has demonstrated that intracerebroventricular hCG delivery after OVX rescued dendritic spine density and spatial memory. 64 The general negative impact of LH/hCG on cognitive performance appears to be dependent upon the ratio of gonadotropins to sex steroids since situations where gonadotropins and sex steroids are in balance such as during the adult reproductive period 65 are periods of normal cognitive performance and do not involve dyotic signalling. 32 , 33 , 34 This is illustrated by the findings that interventions that reverse dyotic signalling such as sex steroid supplementation of ovariectomized animals (see references above), GnRH agonist suppression of gonadotropins in post‐menopausal women 35 and caloric restriction (eg Ref. 66), either reverse or halt cognitive decline. Therefore, one might predict that functioning gonads are essential for hCG to promote cognitive recovery from a TBI, as we have found in intact male rats 67 (unpublished data). Thus, hCG treatment might be expected to be most beneficial in pre‐menopausal and pre‐andropausal individuals, while those further along the post‐reproduction spectrum might benefit most from a combination therapy of hCG supplemented with appropriate sex steroids.
4.1. Causes of craniectomy and CCI injury induced hypogonadism and hypoadrenalism
The induction of hypogonadism and hypoadrenalism in young male rats following a craniectomy and a craniectomy +CCI injury (reduction in plasma concentrations of P4, T, 11‐DOC and corticosterone; Figure 1) is consistent with previous reports in rats. 53 , 68 Since hCG reversed hypogonadism and diminished hypoadrenalism in Sprague‐Dawley rats (Figures 1 and 2, Tables 1, 2, 3), our results suggest isoflurane and/or surgical trauma/stress to the HP are impacting the long‐term hypothalamic release of GnRH or the pituitary release of gonadotropins rather than the production of steroids by the testes (or adrenals). Circulating cortisol concentrations are elevated following neurosurgical procedures in humans, 69 , 70 , 71 but not during anaesthesia (nitrous oxide and halothane, after thiopentone induction). Alternatively, or in conjunction, isoflurane anaesthesia administered during the craniectomy may have inhibited hypothalamic and/or pituitary function since it has been reported that isoflurane anaesthesia can dose‐dependently suppress circulating follicle‐stimulating hormone and T concentrations, post‐natal neurogenesis and cognitive performance in adult male Sprague‐Dawley rats. 72 , 73 , 74 , 75 Anaesthesia‐induced hypogonadism and hypoadrenalism represents another complication of anaesthesia that could impact the recovery from and quality of life for those undergoing anaesthesia for a surgical procedure. Further research is required to delineate whether this effect is attributed to isoflurane on the functioning of the hypothalamus and/or pituitary, a combination of isoflurane and surgical stress or an effect of isoflurane early and of surgical stress later in the maintenance of the hypogonadism over 29 days.
Our data are consistent with early studies in humans demonstrating that TBI could alter hypothalamic morphology and induce hypogonadism and hypothyroidism. 76 , 77 However, while our study implicates isoflurane in the induction of hypogonadism and hypopituitarism via suppression of HP function, human studies suggest that TBI‐induced hypogonadism and hypopituitarism is mediated via suppression of HP function by elevated circulating cortisol. 78 , 79 Ranganathan et al. 79 demonstrated that the stress of TBI results in anovulation and central hypothalamic‐pituitary‐ovarian axis suppression, with menstruation resuming among pre‐menopausal women when serum cortisol normalized to luteal phase control levels. It is apparent that TBI‐induced HH, even when limited to the anterior hypothalamus, 13 , 80 is a system problem commonly involving both the HPG axis and the HPA axis. 81 From our study, it is not possible to determine whether the CCI injury had an impact beyond that of craniectomy on promoting hypogonadism or hypoadrenalism, as has been reported for human TBI. 6 , 7 , 8 , 10 , 14 , 76 , 77 , 78 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 These results demonstrate that future studies need to take into account the effects of isoflurane alone in any model of TBI‐induced hypogonadism.
4.2. hCG treatment for reversing hypopituitarism
Our results in craniectomized and craniectomized plus CCI‐injured rats demonstrate that post‐surgery and post‐injury male rats retain the capacity to synthesize and secrete T (Figure 1). The reversal of hypothalamic/pituitary function in animals induced by a TBI, craniectomy and/or isoflurane anaesthesia indicates the utility of hCG for reversing hypogonadism and hypoadrenalism in these conditions. hCG treatment comes with the advantage of not only increasing neurotropic hCG/LH, but also increasing the dozens of gonadal sex steroid and protein hormones that regulate normal brain structure and function. 32
hCG has been shown to increase T production in aged male rats. 97 , 98 hCG is a safe, cheap, FDA‐approved treatment for hypogonadism in men (chronically), infertility in men and women, and to promote the descent of testicles in young boys with cryptorchidism. 99 In this context, hCG treatment has recently been shown to be effective in (1) raising plasma T concentrations in healthy men with chronic spinal cord injury, and this was not significantly different from hCG’s elevation of plasma T in able‐bodied male control subjects, 100 (2) protecting the rodent adult 37 , 38 and neonatal brain from hypoxic‐ischaemic cellular degeneration in vivo and inhibiting glutamate‐dependent excitotoxic or necrotic neuronal cell death in vitro 40 ; and (3) increasing ERK phosphorylation, neurite outgrowth and rescuing ovariectomy‐induced spatial memory deficits in C57Bl/6J mice. 64 In addition, hCG also partially attenuated hypoadrenalism in male rats. Although there are few studies that have assessed the impact of hCG on regulating adrenal steroid production, hCG has been demonstrated to increase follicular fluid concentrations of 11‐DOC, but not corticosterone, 101 while LHβ overexpressing female mice have enlarged adrenals, increased LHCGR expression and a 14‐fold elevation in serum corticosterone. 102 In this latter study, the authors proposed that enhanced ovarian oestrogen synthesis causes increased secretion of prolactin, which elevates LHCGR expression in the mouse adrenal cortex, leading to elevated, LH‐dependent, corticosterone production. 102 Continuous exposure to hCG is, however, known to suppress the expression of LHCGR via the down‐regulation of mRNA (eg Ref. 103, 104). To circumvent the down‐regulation of the receptor, in our study hCG was administered in the form of Pregnyl every other day, as is used clinically. 105 Since initial phase half‐life of urinary‐derived Pregnyl is between 5.6 and 11 h (https://www.merck.ca/), the 48 h between doses appears sufficient to maintain LHCGR expression, as circulating concentrations of sex steroids (Figure 1A,B) were sustained over the 29‐day experiment.
4.3. RU‐486 impact on plasma steroid concentrations
Elevations in circulating corticosterone observed in our study following RU‐486 treatment are consistent with elevations in corticosterone in the male rats 106 and cortisol, corticotropin or adrenocorticotropic hormone that is observed in human men, 107 , 108 women, 108 , 109 and non‐human primates (Macaca fascicularis 110 ). Blocking glucocorticoid and P4 signalling using RU‐486 had little effect on sex steroid changes induced by craniectomy (uninjured) or CCI injury, but significantly diminished the decline in 11‐DOC and corticosterone concentrations in craniectomy (uninjured) but not CCI‐injured animals (Figure 2A–D), indicating that blocking glucocorticoid (and perhaps P4) receptor signalling partially prevents the suppression of 11‐DOC and corticosterone (either by limiting stress‐induced suppression of 11‐DOC/corticosterone or by elevating their synthesis).
5. LIMITATIONS OF THE STUDY
The suppression of circulating sex steroids by the Sham surgery procedures was unexpected, and the “Sham CCI” group cannot be considered a normal “Control” group in the usual sense of the term. Nonetheless, the fact that stress‐induced HH can be reversed with hCG administration is important from the perspective of a potential treatment option for veterans returning from combat with stress‐ or TBI‐induced HH. 6 , 14 , 45 , 46 , 47
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
RIG, KH, RR, MW, QB, ADJ, IMA, GF and CSA performed surgeries, controlled cortical impact injuries and collected blood. AM and TEZ performed blood hormone analyses. RIG, SVM and CSA performed data and statistical analyses. CSA, SVM and RIG conceived the study.
ACKNOWLEDGEMENTS
This study was funded by VA Merit Review grant #1I21RX001371 (CSA). RIG was funded by the National Institute on Aging T32 training grant #5T32AG00213. This material is the result of work supported with resources at the William S. Middleton Memorial Veterans Hospital, Madison, WI. The opinions expressed herein are those of the authors. The contents do not represent the views of the Department of Veterans Affairs or the U.S. government. This article is Geriatrics Research, Education and Clinical Center VA paper 005‐2021. The Institute for Clinical and Translational Research, UW‐Madison, NIH UL1T000427, provided support for the steroid assays.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Centers for Disease Control . What is traumatic brain injury. 2009. http://www.cdc.gov/ncipc/tbi/TBI.htm
- 2. Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Safety Res. 2012;43(4):299‐307. [DOI] [PubMed] [Google Scholar]
- 3. Mushkudiani NA, Engel DC, Steyerberg EW, et al. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):259‐269. [DOI] [PubMed] [Google Scholar]
- 4. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60(5):584‐591. [DOI] [PubMed] [Google Scholar]
- 5. Altman R, Pruzanski W. Post‐traumatic hypopituitarism. Anterior pituitary insufficiency following skull fracture. Ann Intern Med. 1961;55:149‐154. [DOI] [PubMed] [Google Scholar]
- 6. Barton DJ, Kumar RG, McCullough EH, et al. Persistent hypogonadotropic hypogonadism in men after severe traumatic brain injury: temporal hormone profiles and outcome prediction. J Head Trauma Rehabil. 2016a;31(4):277‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlson NE, Brenner LA, Wierman ME, et al. Hypogonadism on admission to acute rehabilitation is correlated with lower functional status at admission and discharge. Brain Inj. 2009;23(4):336‐344. [DOI] [PubMed] [Google Scholar]
- 8. Gallagher S, Carlson N, Cusick C, et al. Poster 20: the correlation between testosterone levels and function following traumatic brain injury. Arch Phys Med Rehabil. 2008;89(11):e26. [Google Scholar]
- 9. Guerrero AF, Alfonso A. Traumatic brain injury‐related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175(8):574‐580. [DOI] [PubMed] [Google Scholar]
- 10. Klose M, Juul A, Struck J, Morgenthaler NG, Kosteljanetz M, Feldt‐Rasmussen U. Acute and long‐term pituitary insufficiency in traumatic brain injury: a prospective single‐centre study. Clin Endocrinol (Oxf). 2007;67(4):598‐606. [DOI] [PubMed] [Google Scholar]
- 11. Scranton RA, Baskin DS. Impaired pituitary axes following traumatic brain injury. J Clin Med. 2015;4(7):1463‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanriverdi F, Kelestimur F. Pituitary dysfunction following traumatic brain injury: clinical perspectives. Neuropsychiatr Dis Treat. 2015;11:1835‐1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urban RJ, Harris P, Masel B. Anterior hypopituitarism following traumatic brain injury. Brain Injury. 2005;19(5):349‐358. [DOI] [PubMed] [Google Scholar]
- 14. Wagner AK, Brett CA, McCullough EH, et al. Persistent hypogonadism influences estradiol synthesis, cognition and outcome in males after severe TBI. Brain Inj. 2012a;26(10):1226‐1242. [DOI] [PubMed] [Google Scholar]
- 15. Wagner AK, McCullough EH, Niyonkuru C, et al. Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J Neurotrauma. 2011;28(6):871‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target‐organ hormone abnormalities after blast‐related mild traumatic brain injury. Front Neurol. 2012;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hara Y, Waters EM, McEwen BS, Morrison JH. estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev. 2015;95(3):785‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atwood CS, Vadakkadath Meethal S. Human embryonic stem cells as a model system for understanding early human embryogenesis and age‐related diseases. In: Atwood CS, ed. Embryonic Stem Cells: The Hormonal Regulation of Pluripotency and Embryogenesis. Rijeka, Croatia: InTech; 2011:251‐270. [Google Scholar]
- 19. Porayette P, Gallego MJ, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. Differential processing of amyloid‐beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J Biol Chem. 2009;284(35):23806‐23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porayette P, Gallego MJ, Kaltcheva MM, Meethal SV, Atwood CS. Amyloid‐beta precursor protein expression and modulation in human embryonic stem cells: a novel role for human chorionic gonadotropin. Biochem Biophys Res Commun. 2007;364(3):522‐527. [DOI] [PubMed] [Google Scholar]
- 21. Allinquant B, Hantraye P, Mailleux P, Moya K, Bouillot C, Prochiantz A. Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J Cell Biol. 1995;128(5):919‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurogibol. 2007;82(1):11‐32. [DOI] [PubMed] [Google Scholar]
- 23. Milward EA, Papadopoulos R, Fuller SJ, et al. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9(1):129‐137. [DOI] [PubMed] [Google Scholar]
- 24. Rossjohn J, Cappai R, Feil SC, et al. Crystal structure of the N‐terminal, growth factor‐like domain of Alzheimer amyloid precursor protein. Nat Struct Biol. 1999;6(4):327‐331. [DOI] [PubMed] [Google Scholar]
- 25. Small DH, Nurcombe V, Reed G, et al. A heparin‐binding domain in the amyloid protein precursor of Alzheimer's disease is involved in the regulation of neurite outgrowth. J Neurosci. 1994;14(4):2117‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trapp BD, Hauer PE. Amyloid precursor protein is enriched in radial glia: implications for neuronal development. J Neurosci Res. 1994;37(4):538‐550. [DOI] [PubMed] [Google Scholar]
- 27. Gallego MJ, Porayette P, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. The pregnancy hormones human chorionic gonadotropin and progesterone induce human embryonic stem cell proliferation and differentiation into neuroectodermal rosettes. Stem Cell Res Ther. 2010;1(4):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallego MJ, Porayette P, Kaltcheva MM, Meethal SV, Atwood CS. Opioid and progesterone signaling is obligatory for early human embryogenesis. Stem Cells Dev. 2009;18(5):737‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mak GK, Enwere EK, Gregg C, et al. Male pheromone‐stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10(8):1003‐1011. [DOI] [PubMed] [Google Scholar]
- 30. Patil A, Fillmore K, Valentine J, Hill D. The study of the effect of human chorionic gonadotrophic (HCG) hormone on the survival of adrenal medulla transplant in brain. Preliminary study. Acta Neurochir (Wien). 1987;87(1‐2):76‐78. [DOI] [PubMed] [Google Scholar]
- 31. Reddy RC, Amodei R, Estill CT, Stormshak F, Meaker M, Roselli CE. Effect of testosterone on neuronal morphology and neuritic growth of fetal lamb hypothalamus‐preoptic area and cerebral cortex in primary culture. PLoS One. 2015;10(6):e0129521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atwood CS, Bowen RL. The endocrine dyscrasia that accompanies menopause and andropause induces aberrant cell cycle signaling that triggers re‐entry of post‐mitotic neurons into the cell cycle, neurodysfunction, neurodegeneration and cognitive disease. Horm Behav. 2015;76:63‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Atwood CS, Bowen RL. The reproductive‐cell cycle theory of aging: an update. Exp Gerontol. 2011;46(2–3):100‐107. [DOI] [PubMed] [Google Scholar]
- 34. Atwood CS, Bowen RL. A unified hypothesis of early‐ and late‐onset Alzheimer's disease pathogenesis. J Alzheimers Dis. 2015;47(1):33‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A clinical study of lupron depot in the treatment of women with Alzheimer's disease: preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose lupron over 48 weeks. J Alzheimers Dis. 2015;44(2):549‐560. [DOI] [PubMed] [Google Scholar]
- 36. Patil AA, Filmore K, Hill D. The effect of human chorionic gonadotropin (HCG) on restoration of physiological continuity of the spinal cord. A preliminary report. Int Surg. 1990;75(1):54‐57. [PubMed] [Google Scholar]
- 37. Patil AA, Nagaraj MP. The effect of human chorionic gonadotropin (HCG) on functional recovery of spinal cord sectioned rats. Acta Neurochir (Wien). 1983;69(3‐4):205‐218. [DOI] [PubMed] [Google Scholar]
- 38. Babahajian A, Sarveazad A, Golab F, et al. Neuroprotective effects of Trolox, human chorionic gonadotropin, and carnosic acid on hippocampal neurodegeneration after ischemia reperfusion Injury. Curr Stem Cell Res Ther. 2019;14(2):177‐183. [DOI] [PubMed] [Google Scholar]
- 39. Belayev L, Khoutorova L, Zhao KL, Davidoff AW, Moore AF, Cramer SC. A novel neurotrophic therapeutic strategy for experimental stroke. Brain Res. 2009;1280:117‐123. [DOI] [PubMed] [Google Scholar]
- 40. Movsas TZ, Weiner RL, Greenberg MB, Holtzman DM, Galindo R. Pretreatment with human chorionic gonadotropin protects the neonatal brain against the effects of hypoxic‐ischemic injury. Front Pediatr. 2017;5:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Javed Z, Qamar U, Sathyapalan T. Pituitary and/or hypothalamic dysfunction following moderate to severe traumatic brain injury: Current perspectives. Indian J Endocrinol Metab. 2015;19(6):753‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Renner CI. Interrelation between neuroendocrine disturbances and medical complications encountered during rehabilitation after TBI. J Clin Med. 2015;4(9):1815‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Huang W, Constantini S. Concepts and strategies for clinical management of blast‐induced traumatic brain injury and posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2013;25(2):103‐110. [DOI] [PubMed] [Google Scholar]
- 44. Mirzaie B, Mohajeri‐Tehrani MR, Annabestani Z, et al. Traumatic brain injury and adrenal insufficiency: morning cortisol and cosyntropin stimulation tests. Arch Med Sci. 2013;9(1):68‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21(6):685‐696. [DOI] [PubMed] [Google Scholar]
- 46. Kleindienst A, Brabant G, Bock C, Maser‐Gluth C, Buchfelder M. Neuroendocrine function following traumatic brain injury and subsequent intensive care treatment: a prospective longitudinal evaluation. J Neurotrauma. 2009;26(9):1435‐1446. [DOI] [PubMed] [Google Scholar]
- 47. Schneider HJ, Samann PG, Schneider M, et al. Pituitary imaging abnormalities in patients with and without hypopituitarism after traumatic brain injury. J Endocrinol Invest. 2007;30(4):RC9‐RC12. [DOI] [PubMed] [Google Scholar]
- 48. Kenealy BP, Kapoor A, Guerriero KA, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33(49):19051‐19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kenealy BP, Keen KL, Kapoor A, Terasawa E. Neuroestradiol in the stalk median eminence of female rhesus macaques decreases in association with puberty onset. Endocrinology. 2016;157(1):70‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1–Correlation within subjects. BMJ. 1995;310(6977):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Geddes RI, Hayashi K, Bongers Q, et al. Conjugated linoleic acid administration induces amnesia in male Sprague Dawley rats and exacerbates recovery from functional deficits induced by a controlled cortical impact injury. PLoS One. 2017;12(1):e0169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bohm‐Levine N, Goldberg AR, Mariani M, Frankfurt M, Thornton J. Reducing luteinizing hormone levels after ovariectomy improves spatial memory: possible role of brain‐derived neurotrophic factor. Horm Behav. 2020;118:104590. [DOI] [PubMed] [Google Scholar]
- 54. Bryan KJ, Mudd JC, Richardson SL, et al. Down‐regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause‐associated cognitive deficits. J Neurochem. 2010;112(4):870‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Casadesus G, Milliken EL, Webber KM, et al. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269(1‐2):107‐111. [DOI] [PubMed] [Google Scholar]
- 56. McConnell SE, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm Behav. 2012a;61(4):479‐486. [DOI] [PubMed] [Google Scholar]
- 57. Palm R, Chang J, Blair J, et al. Down‐regulation of serum gonadotropins but not estrogen replacement improves cognition in aged‐ovariectomized 3xTg AD female mice. J Neurochem. 2014;130(1):115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Riordan AJ, Schaler AW, Fried J, Paine TA, Thornton JE. Estradiol and luteinizing hormone regulate recognition memory following subchronic phencyclidine: Evidence for hippocampal GABA action. Psychoneuroendocrinology. 2018;91:86‐94. [DOI] [PubMed] [Google Scholar]
- 59. Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid‐beta levels in female rats. Horm Behav. 2008;54(1):143‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Burnham V, Sundby C, Laman‐Maharg A, Thornton J. Luteinizing hormone acts at the hippocampus to dampen spatial memory. Horm Behav. 2017;89:55‐63. [DOI] [PubMed] [Google Scholar]
- 61. Blair JA, Palm R, Chang J, et al. Luteinizing hormone downregulation but not estrogen replacement improves ovariectomy‐associated cognition and spine density loss independently of treatment onset timing. Horm Behav. 2016;78:60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mariani MM, Mojziszek K, Curley E, Thornton JE. Lowering luteinizing hormone (LH) reverses spatial memory deficits associated with neurotoxin infusion into the hippocampus of ovx rats. Horm Behav. 2020;119:104631. [DOI] [PubMed] [Google Scholar]
- 63. Ziegler SG, Thornton JE. Low luteinizing hormone enhances spatial memory and has protective effects on memory loss in rats. Horm Behav. 2010;58(5):705‐713. [DOI] [PubMed] [Google Scholar]
- 64. Blair JA, Bhatta S, Casadesus G. CNS luteinizing hormone receptor activation rescues ovariectomy‐related loss of spatial memory and neuronal plasticity. Neurobiol Aging. 2019;78:111‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Atwood CS, Hayashi K, Meethal SV, Gonzales T, Bowen RL. Does the degree of endocrine dyscrasia post‐reproduction dictate post‐reproductive lifespan? Lessons from semelparous and iteroparous species. GeroScience. 2017;39(1):103‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kuhla A, Lange S, Holzmann C, et al. Lifelong caloric restriction increases working memory in mice. PLoS One. 2013;8(7):e68778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Geddes R, Hayashi K, Wehber M, Rauh R, Meethal S, Atwood C. Human chorionic gonadotropin treatment improves cognitive and motor performance following focal penetrating traumatic brain injury in adult male rats. Soc Neurosci. 2015;J38:688.26. [Google Scholar]
- 68. Meffre D, Pianos A, Liere P, et al. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148(5):2505‐2517. [DOI] [PubMed] [Google Scholar]
- 69. Lines JG, Loder RE, Millar RA. Plasma cortisol responses during neurosurgical and abdominal operations. Br J Anaesth. 1971;43(12):1136‐1144. [DOI] [PubMed] [Google Scholar]
- 70. O'Brien D, Opi PT, Stodulski G, Saibaba P, Hau J. Stress perception of surgical anaesthesia in rats. Paper presented at the Welfare and science: proceedings of the Fifth Symposium of the Federation of European Laboratory Animal Science Associations, 8‐11 June 1993, Brighton, UK.
- 71. Woodman DD. Laboratory Animal Endocrinology: Hormonal Action, Control Mechanisms, and Interactions with Drugs. Chichester, UK; New York: John Wiley and Sons; 1997. [Google Scholar]
- 72. Dong Y, Wu X, Xu Z, Zhang Y, Xie Z. Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Abeta generation. PLoS One. 2012;7(6):e39386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Erasso DM, Camporesi EM, Mangar D, Saporta S. Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats. Brain Res. 2013;1530:1‐12. [DOI] [PubMed] [Google Scholar]
- 74. Wang H, Xu Z, Feng C, et al. Changes of learning and memory in aged rats after isoflurane inhalational anaesthesia correlated with hippocampal acetylcholine level. Ann Fr Anesth Reanim. 2012;31(3):e61‐e66. [DOI] [PubMed] [Google Scholar]
- 75. Xu XL, Pan C, Hu JX, et al. Effects of isoflurane inhalation on the male reproductive system in rats. Environ Toxicol Pharmacol. 2012;34(3):688‐693. [DOI] [PubMed] [Google Scholar]
- 76. Fleischer AS, Rudman DR, Payne NS, Tindall GT. Hypothalamic hypothyroidism and hypogonadism in prolonged traumatic coma. J Neurosurg. 1978;49(5):650‐657. [DOI] [PubMed] [Google Scholar]
- 77. Miyasaki K, Miyachi Y, Arimitsu K, Kita E, Yoshida M. Post‐traumatic hypothalamic obesity–an autopsy case. Acta Pathol Jpn. 1972;22(4):779‐802. [DOI] [PubMed] [Google Scholar]
- 78. Marina D, Klose M, Nordenbo A, Liebach A, Feldt‐Rasmussen U. Early endocrine alterations reflect prolonged stress and relate to 1‐year functional outcome in patients with severe brain injury. Eur J Endocrinol. 2015;172(6):813‐822. [DOI] [PubMed] [Google Scholar]
- 79. Ranganathan P, Kumar RG, Davis K, McCullough EH, Berga SL, Wagner AK. Longitudinal sex and stress hormone profiles among reproductive age and post‐menopausal women after severe TBI: A case series analysis. Brain Inj. 2016;30(4):452‐461. [DOI] [PubMed] [Google Scholar]
- 80. Tanriverdi F, De Bellis A, Ulutabanca H, et al. Five years prospective investigation of anterior pituitary function after traumatic brain injury: is hypopituitarism long‐term after head trauma associated with autoimmunity? J Neurotrauma. 2013;30(16):1426‐1433. [DOI] [PubMed] [Google Scholar]
- 81. Kalyani RR, Gavini S, Dobs AS. Male hypogonadism in systemic disease. Endocrinol Metab Clin North Am. 2007;36(2):333‐348. [DOI] [PubMed] [Google Scholar]
- 82. Aimaretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury‐induced hypopituitarism: a prospective 12‐month study. J Clin Endocrinol Metabol. 2005;90(11):6085‐6092. [DOI] [PubMed] [Google Scholar]
- 83. Auer M, Stalla GK, Athanasoulia AP. Isolated gonadotropic deficiency after multiple concussions in a professional soccer player. Dtsch Med Wochenschr. 2013;138(16):831‐833. [DOI] [PubMed] [Google Scholar]
- 84. Berg C, Oeffner A, Schumm‐Draeger PM, et al. Prevalence of anterior pituitary dysfunction in patients following traumatic brain injury in a German multi‐centre screening program. Exp Clin Endocrinol Diabetes. 2010;118(2):139‐144. [DOI] [PubMed] [Google Scholar]
- 85. Cepicky P, Cizkova J, Roth Z, Stroufova A. Changes in hypophyseal‐ovarian axis hormone levels in the first days after central nervous system surgery. Cesk Gynekol. 1993;58(6):288‐290. [PubMed] [Google Scholar]
- 86. Einaudi S, Matarazzo P, Peretta P, et al. Hypothalamo‐hypophysial dysfunction after traumatic brain injury in children and adolescents: a preliminary retrospective and prospective study. J Pediatr Endocrinol Metab. 2006;19(5):691‐703. [DOI] [PubMed] [Google Scholar]
- 87. Fernandez‐Castaner M, Martinez de Osaba MJ, Vilardell E. Posttraumatic pituitary insufficiency. Value of pretreatment with LHRH in the differentiation between pituitary and hypothalamic deficits. Ann Endocrinol (Paris). 1982;43(3):213‐218. [PubMed] [Google Scholar]
- 88. Hohl A, Marques MO, Coral MH, Walz R. Evaluation of late‐onset hypogonadism (andropause) treatment using three different formulations of injectable testosterone. Arq Bras Endocrinol Metabol. 2009;53(8):989‐995. [DOI] [PubMed] [Google Scholar]
- 89. Hohl A, Ronsoni MF, Debona R, et al. Role of hormonal levels on hospital mortality for male patients with severe traumatic brain injury. Brain Inj. 2014;28(10):1262‐1269. [DOI] [PubMed] [Google Scholar]
- 90. Kelly B, Maguire‐Herring V, Rose CM, et al. Short‐term testosterone manipulations do not affect cognition or motor function but differentially modulate emotions in young and older male rhesus monkeys. Horm Behav. 2014;66(5):731‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kelly DF, McArthur DL, Levin H, et al. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J Neurotrauma. 2006;23(6):928‐942. [DOI] [PubMed] [Google Scholar]
- 92. Lee SC, Zasler ND, Kreutzer JS. Male pituitary‐gonadal dysfunction following severe traumatic brain injury. Brain Inj. 1994;8(6):571‐577. [DOI] [PubMed] [Google Scholar]
- 93. Lieberman SA, Oberoi AL, Gilkison CR, Masel BE, Urban RJ. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J Clin Endocrinol Metab. 2001;86(6):2752‐2756. [DOI] [PubMed] [Google Scholar]
- 94. Ruggeri RM, Smedile G, Granata F, et al. Spontaneous recovery from isolated post‐traumatic central hypogonadism in a woman. Hormones (Athens). 2010;9(4):332‐337. [DOI] [PubMed] [Google Scholar]
- 95. Wagner J, Dusick JR, McArthur DL, et al. Acute gonadotroph and somatotroph hormonal suppression after traumatic brain injury. J Neurotrauma. 2010;27(6):1007‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M. Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab. 1985;60(3):444‐450. [DOI] [PubMed] [Google Scholar]
- 97. Chasalow F, Marr H, Haour F, Saez JM. Testicular steroidogenesis after human chorionic gonadotropin desensitization in rats. J Biol Chem. 1979;254(13):5613‐5617. [PubMed] [Google Scholar]
- 98. Miller AE, Riegle GD. Serum testosterone and testicular response to HCG in young and aged male rats. J Gerontol. 1978;33(2):197‐203. [DOI] [PubMed] [Google Scholar]
- 99. Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril. 2000;74(2):234‐238. [DOI] [PubMed] [Google Scholar]
- 100. Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, Spungen AM. Provocative stimulation of the hypothalamic‐pituitary‐testicular axis in men with spinal cord injury. Spinal Cord. 2016;54(11):961‐966. [DOI] [PubMed] [Google Scholar]
- 101. Fru KN, VandeVoort CA, Chaffin CL. Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod. 2006;75(4):568‐574. [DOI] [PubMed] [Google Scholar]
- 102. Kero J, Poutanen M, Zhang FP, et al. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J Clin Investig. 2000;105(5):633‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KM. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down‐regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128(1):388‐393. [DOI] [PubMed] [Google Scholar]
- 104. Menon B, Franzo‐Romain M, Damanpour S, Menon KM. Luteinizing hormone receptor mRNA down‐regulation is mediated through ERK‐dependent induction of RNA binding protein. Mol Endocrinol. 2011;25(2):282‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Surampudi P, Swerdloff RS, Wang C. An update on male hypogonadism therapy. Expert Opin Pharmacother. 2014;15(9):1247‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wu HB, Niyomchai T, Festa E, et al. Effects of RU 486 and tamoxifen on cocaine‐induced behavioral and endocrinologic activations in male and female Fischer rats. Ethn Dis. 2008;18(2 Suppl 2):S2‐81‐S2‐86. [PMC free article] [PubMed] [Google Scholar]
- 107. Bertagna X. Pituitary‐adrenal response to RU 486 in man. Psychoneuroendocrinology. 1997;22(Suppl 1):S51‐S55. [DOI] [PubMed] [Google Scholar]
- 108. Gaillard RC, Riondel A, Muller AF, Herrmann W, Baulieu EE. RU 486: a steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary‐adrenal system at a specific time of day. Proc Natl Acad Sci USA. 1984;81(12):3879‐3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang JD, Shi WL, Zhang GQ, Bai XM. Tissue and serum levels of steroid hormones and RU 486 after administration of mifepristone. Contraception. 1994;49(3):245‐253. [DOI] [PubMed] [Google Scholar]
- 110. Healy DL, Chrousos GP, Schulte HM, et al. Pituitary and adrenal responses to the anti‐progesterone and anti‐glucocorticoid steroid RU 486 in primates. J Clin Endocrinol Metab. 1983;57(4):863‐865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


