Abstract
The GLP‐1 receptor (GLP‐1R) in the kidney is expressed exclusively in vascular smooth muscle cells in arteries and arterioles. Downstream effects of the activation of the renal vascular GLP‐1R are elusive but may involve regulation of the renin‐angiotensin‐aldosterone system (RAAS). The expression of Ren1 in the mouse renal vasculature was investigated by in situ hybridization after a single subcutaneous dose of liraglutide, semaglutide and after repeated injections of liraglutide. Single and repeated exposure to GLP‐1R agonists induced expression of Ren1 in the renal vascular smooth muscle cell compartment compared with vehicle injected controls (p < .0001) for both semaglutide and liraglutide. The present data show a robust induction of Ren1 expression in the vascular smooth muscle cells of the kidney after single and repeated GLP‐1R activation and this renin recruitment may be involved in the effects of GLP‐1R agonist treatment on kidney disease.
Keywords: GLP‐1, GLP‐1 receptor, GLP‐1R agonists, in situ hybridization, renal vasculature, renin recruitment
Single and repeated injections with long acting GLP‐1R agonists leads to induction of Ren1 transcription from VSMC in renal vasculature in mice.
1. INTRODUCTION
GLP‐1 is a postprandial hormone released upon a carbohydrate rich meal to stimulate glucose dependent insulin secretion, decrease glucagon secretion, and gastric motility, and increase satiety. 1 In patients, long‐acting GLP‐1R analogues such as liraglutide and semaglutide have been used for treatment of type 2 diabetes mellitus (T2DM), and obesity and are known to decrease cardiovascular disease and chronic kidney disease in people with T2DM. 2 , 3 , 4 , 5 The mechanism behind this has been studied in animal models and has been suggested to involve anti‐inflammatory properties, 6 local and systemic haemodynamic effects, 7 and natriuretic effects. 8 It has also been suggested that the effect could involve regulation of RAAS. 9 Interestingly, in humans the systemic blood levels of renin, angiotensin and aldosterone are only slightly or not affected by treatment with GLP‐1R agonists (GLP‐1RA). 10 , 11
In the kidney, the GLP‐1R is expressed exclusively in vascular smooth muscle cells (VSMC) of arteries and arterioles, and in the juxtaglomerular apparatus (JGA), including in specialized renin‐producing SMC cells located at the glomerular poles. 12 Upon decrease in blood pressure, sympathetic stimulation or decrease in distal tubule sodium levels, the renin producing cells of the JGA secrete renin to the systemic circulation. Renin is an aspartyl protease with an active domain that binds angiotensinogen and converts it to angiotensin I, which is further cleaved by angiotensin converting enzyme to angiotensin II. Angiotensin II receptors are expressed in endothelial cells, VSMC, neuronal cells, adrenal glomerulosa and renal tubular cells. Stimulation of the angiotensin II receptor type‐1 (AT1) leads to sodium reabsorption, vasoconstriction, aldosterone secretion and sympathetic nerve activation, ultimately restoring blood pressure and kidney perfusion. 13 A second angiotensin II receptor is present in renal endothelial cells leading to a nitric oxide dependent vasodilation opposing the effects of AT1 receptor stimulation. 14
In humans, renin is coded for by REN, whereas in some mouse strains two genes code for Renin: Ren1 and Ren2. 15 The C57BL/6 strain only have one gene for renin: Ren1, which holds 76% homology to human REN. 16 The Ren1 gene codes for the renin precursor, the pro‐renin complex, which is cleaved into active renin by association with a soluble or membrane bound pro‐renin receptor. 17 Besides activation of the pro‐renin complex, the membranous pro‐renin receptor is involved in intracellular signalling including regulation of cell survival, cell cycle, growth and intracellular acidosis. 18 In rodents, transgenic manipulation of renin expression levels can lead to renal disease, hypertension and inflammation 19 indicating that tight regulation of renin transcription and bioactivation could play an important role as a physiological regulator of kidney function. This study describes the effect of GLP‐1RAs on the expression of Ren1 mRNA in the mouse kidney after acute and chronic exposure.
2. MATERIALS AND METHODS
2.1. In vivo
The studies were approved by the Danish Animal Experimentation Inspectorate under the Ministry of Environment and Food and carried out by trained and licensed personnel. All animals were housed in cages of eight animals per cage under 12‐h light dark cycle at 22 ± 2°C and 50 ± 20% humidity with ad libitum access to food and water. The mice were acclimatized for two weeks prior to study initiation. Animal caretakers inspected animals daily.
2.2. Experimental protocol—four hours exposure to semaglutide or liraglutide
Thirty C57BL/6J male mice, 8–10 weeks (Taconic, Lille Skensved, Denmark), were injected once subcutaneously with vehicle (pH = 7.4; 50 mmol/L phosphate; 70 mmol/L sodium chloride; 0.05% polysorbate 80, n = 10), semaglutide (15 nmol/kg, n = 10) or liraglutide (270 nmol/kg, n = 10), all from Novo Nordisk A/S, Denmark. Four hours after injection, animals were anaesthetized by inhalation of isoflurane, euthanized by cervical dislocation, and kidneys were collected.
2.3. Experimental protocol—26 days exposure to liraglutide in NTN mice
Eighteen C57BL/6J male mice, 8–10 weeks (Taconic, Lille Skensved Denmark), were divided into three groups. Two groups were anaesthetized in isoflurane and pre‐sensitized by two subcutaneous injections of 100 µg sheep IgG (P130‐100, lot#1128‐7, Bethyl Laboratories Inc, Denmark) in Complete Freund's adjuvant (5 mg/mL mycobacterium butyricum in mineral oil, MP Biomedicals, Eschwege, Germany) each injection. Four days later, 100µL nephrotoxic serum (NTS) from sheep was given intravenously in the tail vein (Probetex, San Antonio, USA), Batch PTX‐001S). One group received once daily subcutaneous injection with vehicle (pH = 7.4; 50 mmol/L phosphate; 70 mmol/L sodium chloride; 0.05% polysorbate 80) or liraglutide (270 nmol/kg, Novo Nordisk, Måløv, Denmark) after a dose titration scheme of 0.3, 0.6 and 1 mg/mL each day such that the animals received 1 mg/mL on the day of the NTS injection. The last group (n = 6) did not get NTS and was left untreated, thus serving as a naïve control. Following 26 days of treatment, animals were euthanized approximately 24 h after last dosing and the right kidney was collected.
2.4. In situ hybridization of REN1 and immunohistochemical staining of GLP1R
All kidneys were dissected free of surrounding tissue and placed in 10% neutral buffered formalin (NBF, 16004‐130, VWR), processed in the Leica ASP 300 S tissue processor (Leica Biosystems, Buffalo Grove, IL, USA) and embedded in paraffin for sectioning.
Kidneys were cut (4.5 µm) and mounted onto Superfrost Plus slides (Termo Fisher Scientific, Waltham, MA, USA) and placed in the Ventata Discovery XT automation system (Ventana Inc., Roche, Tucson, USA). The RNAscope technology was used to perform in situ hybridization using the VS 2,5 RED reagent kit (ref: 322220) and probe Mm‐Ren1 (ref: 322220). A section was hybridized with a probe for bacterial DapB (ref: 312039) and for PPIB (313919) serving as negative and positive control, respectively (all from Advanced Cell Diagnostics, Newark, CA). The slides were pre‐treated with protease and target retrieval. Some slides were double stained with Rb‐GLP‐1R antibody (2.7 µg/mL, ab218532 Abcam, Cambridge, UK) enhanced using the Rb‐HQ and a‐HQ‐HRP kits (760–4818 and 760–4820, respectively, Roche, Basel, Switzerland) and labelled using FITC in TSA (Roche, Basel, Switzerland). All slides were counterstained with DAPI for 8 minutes (10−4 µg/mL). Cover slides were mounted using DAKO fluorescent mounting media (DAKO, Glostrup, Denmark).
2.5. Quantification of histological staining
Slides were scanned using the Olympus VS120 Virtual Slide Microscope scanner (Olympus Corporation, Tokyo, Japan) and dots were quantified using the HALO software (Indica Labs, Albuquerque, USA) FISH algorithm version 2.1.7. A subset of arteries (3–10 per kidney dependent on availability on specific section) were selected. The muscularized artery should lie adjacent to a vein and free of perivascular adipose tissue. Results are shown as H‐score calculated, as recommended by manufacturer, from the equation: H = [1 × (cell fraction with 1–3 dots/cell) + 2 × (cell fraction with 4–9 dots/cell) + 3 × (cell fraction with 10–15 dots/cell) + 4 × (cell fraction with >15 dots/cell)].
2.6. Statistics
Statistical analyses were performed using GraphPad Prism version 8 (Graph Pad, San Diego, California). Differences with p < .05 were considered as significant. Individual values represent the mean of 3–9 arteries from the same mouse. All data are presented as individual values, mean and standard error of the mean (SEM).
3. RESULTS
3.1. Effect of 4 h of GLP‐1RA injection on Ren1 expression in naïve mice
Naïve mice injected with saline (Figure 1A–D) showed expression of Ren1 mRNA in proximity to glomeruli, with no positive stain found in the large and small blood vessels. Immunostaining using a specific anti‐GLP‐1R antibody detected GLP‐1R in arteries and arterioles only. Four hours after injection of semaglutide (Figure 1E–H) or liraglutide (Figure 1I–L), Ren1 expression was detected in the vasculature where it co‐localized with the immunoreactivity for GLP‐1R. The levels of Ren1 mRNA were significantly upregulated in both semaglutide and liraglutide treated animals as compared with the vehicle treated animals (p < .0001 for both compounds, Figure 1M).
FIGURE 1.
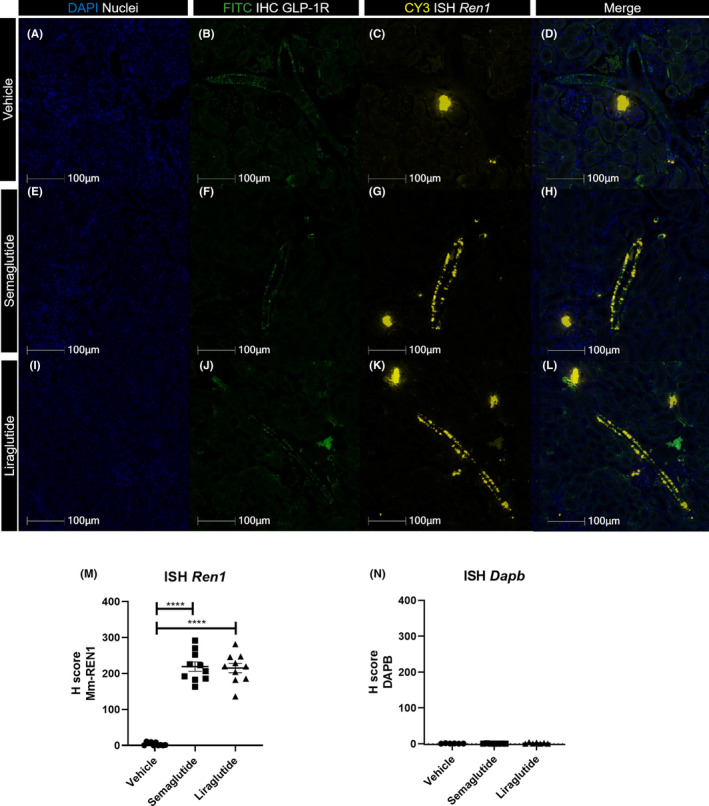
Expression of Ren1 four hours post single injection in mouse kidney. (A‐L) Panel showing representative photos from Ren1 in situ hybridization and GLP‐1R IHC double stain, from C57BL/6 J mice injected with a single dose of vehicle (top row), semaglutide (second row) or liraglutide (third row). DAPI nuclear stain (blue, first column), GLP‐1R IHC stain labelled with FITC (green, second column), Ren1 ISH labelled with CY3 (yellow, column three), and merge of all three stains (Last column). Scale bars 100 µm, 40× magnification. (M) Quantification of Mm‐Ren1 detection in renal vasculature from mice injected with vehicle (●), semaglutide (■) or liraglutide (▲). Individual values represent mean of 6–9 arteries. Data shown as individual values, mean and SEM. Analysed by Brown‐Forsyth and Welch ANOVA followed by Games‐Howell's test for multiple comparisons, ****p < .0001. (N) Dapb stain as negative control from vehicle injected (●), semaglutide injected (■) and liraglutide injected (▲) mice. Individual values represent mean of 6–9 arteries. Data shown as individual values, mean and SEM
3.2. Ren1 expression in kidneys from 26 days liraglutide treated NTN mice
ISH for Ren1 after 26 days dosing with liraglutide showed a significant increase of Ren1 mRNA expression as compared with naïve mice (p = .0004) and NTN vehicle treated mice (p = .0005, Figure 2A). Comparison of arteries from vehicle treated NTN mice and naïve mice showed that NTN induction per se did not affect Ren1 mRNA expression in arteries (Figure 2A, C, D). A clear signal of positive Ren1 hybridization was detected in the arteries of liraglutide treated animals (Figure 2E).
FIGURE 2.
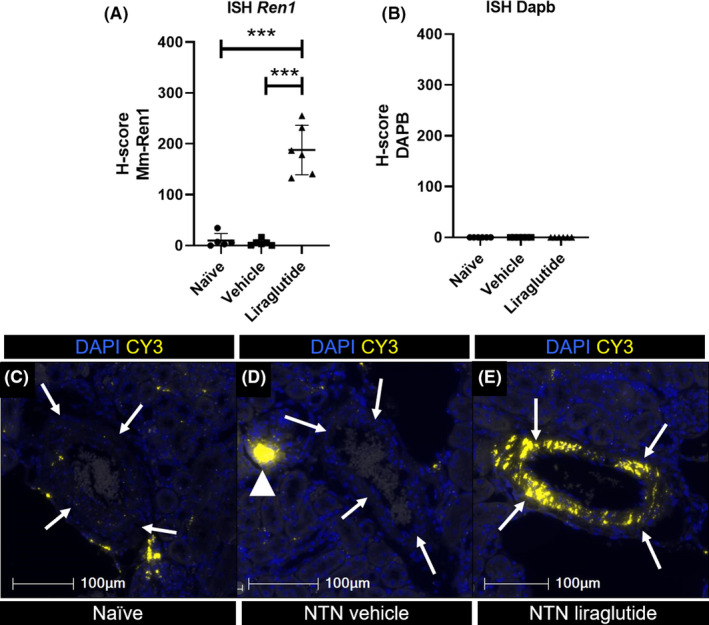
Ren1 ISH from repeatedly injected mice. (A) Quantification of ISH of Ren1 from Naïve mice with no treatment injection (●), vehicle treated NTN mice (■) and NTN mice treated with liraglutide for 26 days (▲). Individual values represent the mean of 5–6 selected arteries. Data shown as individual values, mean and SEM ***p ≤ .005. Brown‐Forsythe and Welch ANOVA test with Holm‐Sidak's multiple comparisons test. (B) Negative control of ISH using DAPB from naïve (●), vehicle treated NTN mice (■) and NTN mice treated with liraglutide for 26 days (▲). (C–E) Representative images of artery (white arrows) and Ren1 in macula densa (white arrow‐head) from naïve (C), NTN dosed vehicle treated animals (D) and NTN dosed liraglutide treated animals (E). ISH of Ren1 in CY3 (yellow) merged with nuclear stain DAPI (blue). 40× magnification, scale bar: 100 µm. NTN, nephrotoxic nephritis
4. DISCUSSION
In the present study, we show that GLP‐1R activation by liraglutide or semaglutide leads to expression of the renin gene in vascular smooth muscle cells in the arteriolar and arterial part of the renal vasculature. The switch of VSMC in the renal vasculature from contractile to renin producing cells is a well‐described phenomenon known as renin recruitment. 20
The Ren1 gene (in humans the REN gene) codes for the precursor pro‐renin. The level of active renin in plasma is dependent on bioactivation by proteolytic and non‐proteolytic cleavage of the pro‐part from renin by binding to a soluble or membranous pro‐renin receptor. 21 , 22 The expression of renin is regulated by the levels of angiotensin II and renal perfusion pressure 23 and may be affected by physiological and pathological conditions including gender and diabetes mellitus. 24 Consequently, the levels of renin mRNA expression cannot be directly translated into the amount of active renin in plasma. 25 Published results from human studies of plasma renin levels after treatment with either native GLP‐1 or GLP‐1RA are inconsistent. In randomized placebo controlled blinded cross‐over studies of native GLP‐1 infusion in healthy men, one study showed that there was no effect of native GLP‐1 infusion on renin plasma levels in healthy volunteers, 10 whereas another showed significantly decreased renin plasma levels. 26 In a similar setup, there were no acute effects on renin levels in men with T2DM, 11 , 27 however, in a placebo controlled experimental setup in healthy subjects with high sodium intake, native GLP‐1 infusion resulted in unchanged renin plasma levels, but showed significantly reduced angiotensin II levels by 20%. 28 These studies suggest that renin gene expression and plasma renin activity are both regulated via complex mechanisms, and the present data warrant further investigation of the transcriptional and post‐translational mechanisms that determine local and systemic renin activity following GLP‐1RA exposure.
The molecular pathway leading to renin recruitment by GLP‐1R activation may include cAMP as a downstream mediator. The GLP‐1R typically activates cAMP by coupling with adenylate cyclase 29 and forskolin, a cAMP activator, is known to induce renin recruitment. 30 , 31 , 32 Other downstream mediators of the renin recruitment effect may be factors belonging to the RAAS system. From preclinical and clinical studies RAAS inhibitors are known to decrease fibrosis, inflammation and albuminuria, 33 , 34 , 35 but they also lead to renin recruitment. 36 , 37 In this context, GLP‐1RA’s have previously shown effects on the regulation of components of RAAS independently of haemodynamic effects. Skov et al. and Asmar et al. found that liraglutide decreased angiotensin II levels acutely in men. 10 , 11 , 28 Liraglutide treatment in the NTN mouse model significantly increased Mas1 expression in glomeruli. The Mas1 gene codes for the receptor of angiotensin 1‐7, the peptide arising from angiotensin II following proteolytic processing by angiotensin converting enzyme 2. Hence, it could be speculated that liraglutide treatment can act locally in the kidney to induce angiotensin II cleavage and hence upregulation of the angiotensin 1‐7/mas1 pathway. 33 This explanation is supported by an increase in angiotensin 1‐7 levels found in diabetic rats following liraglutide treatment. 38 Angiotensin 1‐7 activation of the g‐protein coupled receptor Mas1 mediate tissue protective processes including anti‐inflammatory signalling, reduction in fibrosis, and increased endothelial signalling. 10 , 39 In people with diabetes, angiotensin 1‐7 levels were positively correlated with increased left ventricular ejection fraction 40 pointing to a positive cardiovascular effect of activation of the angiotensin 1‐7 axis (Figure 3).
FIGURE 3.
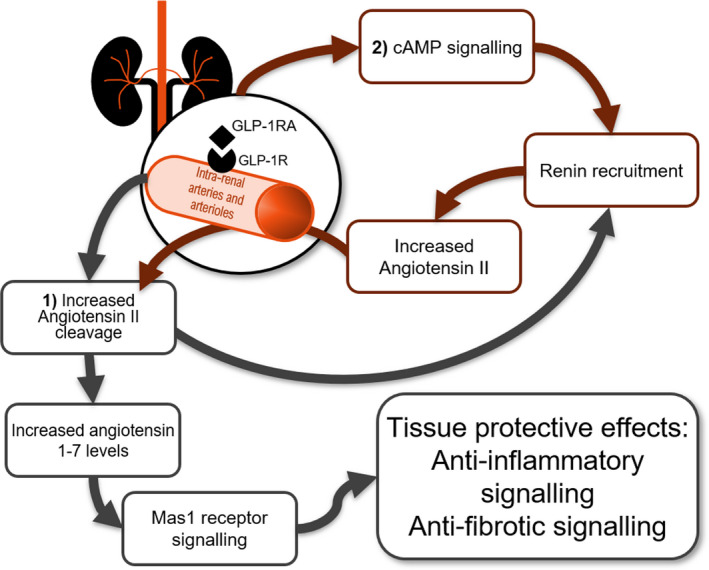
Proposed link between renal GLP‐1R activation and renin recruitment. Expression of Ren1 after GLP‐1RA exposure may be initiated by two mechanisms, both starting with GLP‐1R activation in renal arteries: (1) Activation leads to increased angiotensin II cleavage, which in turn leads to renin recruitment by lack of negative feedback mechanism. The product of the cleavage of angiotensin II, angiotensin 1‐7, activates the Mas1 receptor resulting in anti‐inflammatory and antifibrotic effects. (2) Activation leads to an increase in cAMP levels resulting in increased expression of Ren1 in the VSMC of the arteries. This would lead to an increase in local RAAS components including angiotensin II, which when cleaved as proposed above would further contribute to increased angiotensin 1‐7 levels
Renin production leads to an increase in mean arterial pressure, 41 and thus it is expected that an induction of renin production would lead to hypertension. Increased blood pressure is associated with increased risk of kidney damage and cardiovascular disease, which challenges the explanation for a positive effect of renin production following GLP‐1RA treatment. Nonetheless, chronic GLP‐RA treatment results in a slight decrease in systolic blood pressure and lowers the risks of cardiovascular disease and chronic kidney disease. 2 , 3 , 5 This suggests that renin gene expression can stimulate biological events beyond RAAS, potentially including angiogenesis and glomerulus repair, 42 which are beneficial for maintaining a healthy kidney.
The present study was limited to healthy mice and mice with nephrotoxic serum nephritis independent of the pathophysiological consequences of diabetic nephropathy, that includes hyperglycemia and hypoinsulinemia, conditions that are known to affect renal vascular injury. 43 Studies in the hypercholesterolemic and prediabetic Ldlr −/− mouse on western diet and the NTN mouse model of chronic kidney disease showed a decrease in GLP‐1R expression in the renal vasculature in both models. 44 This suggests that the renal vascular GLP‐1 system is affected by prediabetes and atherosclerosis as well as direct chronic kidney damage. In humans, diabetic nephropathy occurs predominantly in T2DM patients and is coincident with cardiovascular disease including atherosclerosis and hypertension. 45 Mann et al 5 show that liraglutide treatment significantly decreased the risk of renal disease in T2DM patients with an effect greater than the composite effect of alleviating T2DM and obesity, and the authors suggest a multifactorial mechanism including anti‐inflammatory effects. The present results suggest an involvement of renin gene expression in the NTN mouse independent of T2DM, chronic hypertension, and atherosclerosis, pointing to potential beneficial effects of GLP‐1R agonist treatment relevant for several forms of nephropathy.
In conclusion, we show that GLP‐1R activation leads to significant and robust renin gene expression in the renal vascular tree in mice and suggest that effects of renin independent of the blood pressure regulating effects of RAAS is important for the physiological and pharmacological effects of GLP‐1 on renal vascular health.
AUTHOR CONTRIBUTION
CP and LBK conceived the presented idea and designed and oversaw the execution of experiments. KDB analyzed the results and drafted the manuscript. GFS and MEO aided in writing the manuscript and in the interpretation of data. The final paper was prepared with input from all authors.
CONFLICT OF INTEREST
MEO, LBK and CP are employed by Novo Nordisk A/S, which markets liraglutide and semaglutide for the treatment of diabetes and weight management. LBK and CP hold minor stock portions as part of an employee‐offering programme.
ACKNOWLEDGEMENTS
This work was funded my Novo Nordisk A/S and the LIFEPHARM In Vivo Pharmacology program, University of Copenhagen. Bidda Rolin and Constanze Hess are thanked for executing the in vivo work. A special thanks to Bettina Brandrup for excellent technical assistance with histological preparations.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev. 2007;87(4):1409‐1439. [DOI] [PubMed] [Google Scholar]
- 2. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 3. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. Lancet. 2009;374(9701):1606‐1616. [DOI] [PubMed] [Google Scholar]
- 5. Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839‐848. [DOI] [PubMed] [Google Scholar]
- 6. Moschovaki Filippidou F, Kirsch AH, Thelen M, et al. Glucagon like peptide‐1 receptor agonism improves nephrotoxic serum nephritis by inhibiting T cell proliferation. Am J Pathol. 2020;190(2):400‐411. [DOI] [PubMed] [Google Scholar]
- 7. Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon‐like peptide 1 in Dahl salt‐sensitive rats. J Hypertens. 2003;21(6):1125‐1135. [DOI] [PubMed] [Google Scholar]
- 8. Kim M, Platt MJ, Shibasaki T, et al. GLP‐1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19(5):567‐575. [DOI] [PubMed] [Google Scholar]
- 9. Sedman T, Heinla K, Vasar E, Volke V. Liraglutide treatment may affect renin and aldosterone release. Horm Metab Res. 2017;49(1):5‐9. [DOI] [PubMed] [Google Scholar]
- 10. Skov J, Dejgaard A, Frøkiær J, et al. Glucagon‐like peptide‐1 (GLP‐1): effect on kidney hemodynamics and renin‐angiotensin‐aldosterone system in healthy men. J Clin Endocrinol Metabol. 2013;98(4):E664‐E671. [DOI] [PubMed] [Google Scholar]
- 11. Skov J, Pedersen M, Holst JJ, et al. Short‐term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab. 2016;18(6):581‐589. [DOI] [PubMed] [Google Scholar]
- 12. Pyke C, Heller RS, Kirk RK, et al. GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280‐1290. [DOI] [PubMed] [Google Scholar]
- 13. Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32(2):81. [PubMed] [Google Scholar]
- 14. Kaschina E, Namsolleck P, Unger T. AT2 receptors in cardiovascular and renal diseases. Pharmacol Res. 2017;125(Pt A):39‐47. [DOI] [PubMed] [Google Scholar]
- 15. Sigmund CD, Fabian JR, Gross KW. Expression and regulation of the renin gene. Trends Cardiovasc Med. 1992;2(6):237‐245. [DOI] [PubMed] [Google Scholar]
- 16. Imai T, Miyazaki H, Hirose S, et al. Cloning and sequence analysis of cDNA for human renin precursor. Proc Natl Acad Sci USA. 1983;80(24):7405‐7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dzau VJ, Burt DW, Pratt RE. Molecular biology of the renin‐angiotensin system. Am J Physiol‐Renal Physiol. 1988;255(4):F563‐F573. [DOI] [PubMed] [Google Scholar]
- 18. Ichihara A, Yatabe MS. The (pro)renin receptor in health and disease. Nat Rev Nephrol. 2019;15(11):693‐712. [DOI] [PubMed] [Google Scholar]
- 19. Chen L, Faulhaber‐Walter R, Wen Y, et al. Renal failure in mice with Gsalpha deletion in juxtaglomerular cells. Am J Nephrol. 2010;32(1):83‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hickmann L, Steglich A, Gerlach M, et al. Persistent and inducible neogenesis repopulates progenitor renin lineage cells in the kidney. Kidney Int. 2017;92(6):1419‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sequeira‐Lopez ML, Nagalakshmi VK, Li M, Sigmund CD, Gomez RA. Vascular versus tubular renin: role in kidney development. Am J Physiol Regul Integr Comp Physiol. 2015;309(6):R650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Catanzaro DF. Chapter 21 ‐ Molecular Biology of Renin and Regulation of Its Gene. In: Singh AK, Williams GH, eds. Textbook of Nephro‐Endocrinology (Second Edition). Academic Press; 2018:389‐400. [Google Scholar]
- 23. Tufro‐McReddie A, Chevalier RL, Everett AD, Gomez RA. Decreased perfusion pressure modulates renin and ANG II type 1 receptor gene expression in the rat kidney. Am J Physiol‐Regulat Integrat Compar Physiol. 1993;264(4):R696‐R702. [DOI] [PubMed] [Google Scholar]
- 24. Tang J, Wysocki J, Ye M, et al. Urinary renin in patients and mice with diabetic kidney disease. Hypertension. 2019;74(1):83‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura N, Soubrier F, Menard J, Panthier JJ, Rougeon F, Corvol P. Nonproportional changes in plasma renin concentration, renal renin content, and rat renin messenger RNA. Hypertension. 1985;7(6_pt_1):855‐859. [DOI] [PubMed] [Google Scholar]
- 26. Asmar A, Simonsen L, Asmar M, et al. Renal extraction and acute effects of glucagon‐like peptide‐1 on central and renal hemodynamics in healthy men. Am J Physiol Endocrinol Metab. 2015;308(8):E641‐649. [DOI] [PubMed] [Google Scholar]
- 27. Asmar A, Simonsen L, Asmar M, et al. Glucagon‐like peptide‐1 does not have acute effects on central or renal hemodynamics in patients with type 2 diabetes without nephropathy. Am J Physiol ‐ Endocrinol Metabol. 2016;310(9):E744‐E753. [DOI] [PubMed] [Google Scholar]
- 28. Asmar A, Cramon PK, Simonsen L, et al. Extracellular fluid volume expansion uncovers a natriuretic action of GLP‐1: a functional GLP‐1‐renal axis in man. J Clin Endocrinol Metab. 2019;104(7):2509‐2519. [DOI] [PubMed] [Google Scholar]
- 29. Widmann C, Bürki E, Dolci W, Thorens B. Signal transduction by the cloned glucagon‐like peptide‐1 receptor: comparison with signaling by the endogenous receptors of beta cell lines. Mol Pharmacol. 1994;45(5):1029‐1035. [PubMed] [Google Scholar]
- 30. Everett AD, Carey RM, Chevalier RL, Peach MJ, Gomez RA. Renin release and gene expression in intact rat kidney microvessels and single cells. J Clin Invest. 1990;86(1):169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pentz ES, Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol. 2008;294(2):H699‐707. [DOI] [PubMed] [Google Scholar]
- 32. Klar J, Sandner P, Müller MW, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflügers Archiv. 2002;444(3):335‐344. [DOI] [PubMed] [Google Scholar]
- 33. Ougaard ME, Sembach FE, Jensen HE, Pyke C, Knudsen LB, Kvist PH. Liraglutide improves the kidney function in a murine model of chronic kidney disease. Nephron. 2020;144(11):595‐606. [DOI] [PubMed] [Google Scholar]
- 34. Ougaard ME, Jensen HE, Thuen ID, Petersen EG, Kvist PH. Inhibitors of the renin‐angiotensin system ameliorates clinical and pathological aspects of experimentally induced nephrotoxic serum nephritis. Ren Fail. 2018;40(1):640‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hartono SP, Knudsen BE, Lerman LO, Textor SC, Grande JP. Combined effect of hyperfiltration and renin angiotensin system activation on development of chronic kidney disease in diabetic db/db mice. BMC Nephrol. 2014;15:58‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gomez RA, Chevalier RL, Everett AD, et al. Recruitment of renin gene‐expressing cells in adult rat kidneys. Am J Physiol‐Renal Physiol. 1990;259(4):F660‐F665. [DOI] [PubMed] [Google Scholar]
- 37. Gomez RA, Lynch KR, Chevalier RL, et al. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol‐Renal Physiol. 1988;254(6):F900‐F906. [DOI] [PubMed] [Google Scholar]
- 38. Romaní‐Pérez M, Outeiriño‐Iglesias V, Moya CM, et al. Activation of the GLP‐1 receptor by liraglutide increases ACE2 Expression, reversing right ventricle hypertrophy, and improving the production of SP‐A and SP‐B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559‐3569. [DOI] [PubMed] [Google Scholar]
- 39. Fraga‐Silva RA, Costa‐Fraga FP, Murça TM, et al. Angiotensin‐converting enzyme 2 activation improves endothelial function. Hypertension. 2013;61(6):1233‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hao P‐P, Chen Y‐G, Liu Y‐P, et al. Association of plasma angiotensin‐(1–7) level and left ventricular function in patients with type 2 diabetes mellitus. PLoS One. 2013;8(5):e62788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taugner R, Hackenthal E, Waldherr R. The Juxtaglomerular Apparatus: Structure and Function. Berlin, Germany: Springer‐Verlag; 1989. [Google Scholar]
- 42. Gomez RA, Sequeira‐Lopez MLS. Renin cells in homeostasis, regeneration and immune defence mechanisms. Nat Rev Nephrol. 2018;14(4):231‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arildsen L, Andersen JV, Waagepetersen HS, Nissen JBD, Sheykhzade M. Hypermetabolism and impaired endothelium‐dependent vasodilation in mesenteric arteries of type 2 diabetes mellitus db/db mice. Diab Vasc Dis Res. 2019;16(6):539‐548. [DOI] [PubMed] [Google Scholar]
- 44. Bjørnholm KD, Povlsen GK, Ougaard ME, et al. Decreased expression of the GLP‐1 receptor after segmental artery injury in mice. J Endocrinol. 2021. 10.1530/JOE-20-0608. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45. Chen Y, Lee K, Ni Z, He JC. Diabetic kidney disease: challenges, advances, and opportunities. Kidney Dis. 2020;6(4):215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


