Abstract
Background:
Hearing loss due to noise can cause the disturbances toward the quality of life and cause mechanical damage and metabolic decompensation. Distortion Product Otoacoustic Emission (DPOAE) is an examination to assess the sensory function of outer hair cells. Superoxide Dismutase (SOD) and Malondialdehyde (MDA) are markers of oxidative stress. The aim of this study was to identify the correlation between DPOAE examination and SOD and MDA expression in rats exposed to noise.
Methods:
This research was conducted on 27 rats which were divided into 3 groups, group 1 (control), group 2, and group 3 were groups with 100 dB and 110 dB noise exposure respectively.
Results:
Our findings show a decrease in SOD expression and DPOAE values as well as an increase in MDA expression in rats exposed to noise and there is a positive correlation between Signal to Noise Ratio (SNR) value with SOD expression (r= 0.733, p= 0.025) and a negative correlation between SNR value with MDA expression (r= -0.678, p= 0.045).
Conclusion:
our study find the correlation of oxidant and antioxidant status values in the organ of corti and changes in the function of outer hair cells in noise-exposed rat models.
Key Words: Malondialdehyde, Noise Induced Hearing Loss, Otoacoustic Emission, Superoxide Dismutase
Introduction
Noise can cause hearing loss both from work and the environment (not from the work) (1,2,3). In addition to hearing loss, noise can also cause quality of life disturbances such as sleep disorders, fatigue, disruption of social life, ability to work and can even cause hypertension and other cardiovascular diseases (1,2,4,5). It is estimated that around 1.3 billion world population suffer from hearing loss and 10% of the entire world population is at risk of suffering from Noise Induced Hearing Loss (NIHL) (2,6).
Cochlear hair cell damage and damage to synapses are the main causes of NIHL (7). In general, cochlear damage in NIHL is caused by two mechanisms, they are mechanical damage and metabolic disorders (8,9). Otoacoustic Emission (OAE) is an acoustic emission produced by active electro-motile vibrations from outer hair cells in the organ of corti (10). The resulting emission is a response from stimuli originating from various frequencies, so this can be an indicator of sensory function of outer hair cells in the cochlea (11). Distortion products Otoaccoustic Emissions (DPOAEs) are the result of an active intermodulation process in the cochlea mediated by outer hair cells and obtained simultaneously which indicates the stimulation of two pure tones (12).
Cochlear hair cell damage is reversible is called a temporary threshold shift (TTS) and permanent hair cell damage or loss of outer hair cells and synapses are called Permanent threshold shift (PTS). When the hair cell structure has been completely damaged there will be a buildup of Reactive Oxygen Species (ROS) and cause active stimulation of intracellular stress pathways so that the process of apoptosis and cell necrosis (7). Reactive Oxygen Species (ROS) works through 2 pathways, namely cellular stress pathways and defense mechanisms. When there is an imbalance in production between ROS and antioxidant mechanisms, oxidative stress will occur (13). Malondialdehyde (MDA) is a substance produced when ROS acts on the lipoprotein membrane and Polyunsaturated Fatty Acid (PUFA) at the cellular and subcellular levels, so that MDA measurements can give an idea of the extent of damage. Superoxide Dismutase (SOD) is an enzyme catalyst that converts superoxide radicals into harmless hydrogen peroxide molecules and oxygen molecules. Then the next antioxidant enzyme in the cellular pathway, Glutathione peroxidase (GSH-Px) and catalase (CAT), catalyzes the reaction of hydrogen peroxide breakdown into water so that the measurement of these three enzymes can determine the antioxidant status in workers exposed to noise (14).
This study is different from previous studies, in this study we wanted to find out how cochlear outer hair cell damage was measured by assessing changes in oxidant and antioxidant status in organ of corti, does it have a correlation with changes in outer hair cell function as measured by DPOAE in rats exposed to noise.
Materials and Methods
Design of study
This study used a randomized posttest only control group laboratory experimental design. This study was approved by the research ethics institute of the Faculty of Medicine, Universitas Sumatera Utara (No.509 / TGL / KEPK FK USU-RSUP HAM / 2018).
Procedures of animal care
This study conducted on 27 male rattus norvegicus pure strain rats weighing 200-300 grams and declared healthy by veterinarians. The environment was maintained at the cage temperature of 20 °C-26 °C and the humidity is 30-70% and was ensured to get adequate sources of light, food and drink (15).
The groups of the study
The experimental group was divided into 3 groups consisting of 9 rats in each group. The first group was a control group that does not get noisy treatment; group 2 and group 3 were groups of rats that received 100 dB and 110 dB noise, respectively.
Procedures of noise exposure
Noise treatment was done by placing mice on a box with a size (64.5 x 45 x 40) cm made of cork coated with foam, speakers are placed attached to the roof of the box cover and the base of the box is made a hole to measure the intensity, measurement of noise intensity using a sound level tool. The meter was carried out at eight points where the mouse cage will be placed with a noise difference not exceeding 1 dB which is measured using a sound level meter. The recorded noise was given at frequencies 1-10.000 Hz and the amplifier was used to adjust the intensity (dB) according to the volume and sound level meter buttons. Noise was given for 2 hours every day for 2 days.
Procedures of DPOAE examination
Distortion products Otoaccoustic Emissions (DPOAEs) examination was carried out twice, where the value of the SNR (Signal to Noise Ratio) calculations was done before the treatment was carried out and after the noisy treatment was finished. The DPOAE tool used was the Elios Elito Otodia (Echodia Ltd., London, UK). Before examination, rats were first anesthetized using ketamine at a dose of 90 mg/kgBB and Xylazine at a dose of 10 mg /kgBB which were injected intraperitonially (16, 17).
Procedures of hematoxylin-eosin and immunohistochemical staining
After DPOAE examination, all rats were terminated, and temporal bone necropsy was performed. Temporal bone taken was fixed with 10% formalin buffered solution, then by using EDTA for 4 weeks it was expected that decalcification would occur. Next, each tissue sample was prepared in a paraffin block and sliced into 4 μm thick sections and placed in a slide and then stained with hematoxylin-eosin and immunohistochemical staining, namely SOD with primary antibodies (SOD-2 (A-2) (Santa Cruz Biotechnology, Inc. cat # sc-133134)) and MDA with anti-malondialdehyde antibody (abcam cat # ab6463) to assess the expression of both proteins in cochlear organ of corti. Calculation of SOD and MDA expression using XC 10 Olympus microscope using 40x magnification performed by 2 examiners separately (researcher and anatomic pathologist) by double-blind method (18). SOD and MDA expressions were assessed by broad (P) and Intensity (I) scores of brown colors on the cytoplasm. Intensity score: 1-3, broad score 0%; 1: <10%; 2: 11%–50%; 3: 51-80%; 4: >80% and immune-reactive scores obtained from P and I multiplications, resulting in a score of 0–12 (19).
Statistical analysis
All collected data was analyzed statistically using the ANNOVA test to assess differences in each treatment and bivariate analysis was also performed using the Pearson test to assess the correlation between DPOAE examination with SOD and MDA expressions.
Results
Before immunohistochemical staining was done, Hematoxylin Eosin (HE) staining was used to obtain cochlear pieces more precisely. The HE colors can be seen in Figure 1.
Fig. 1.
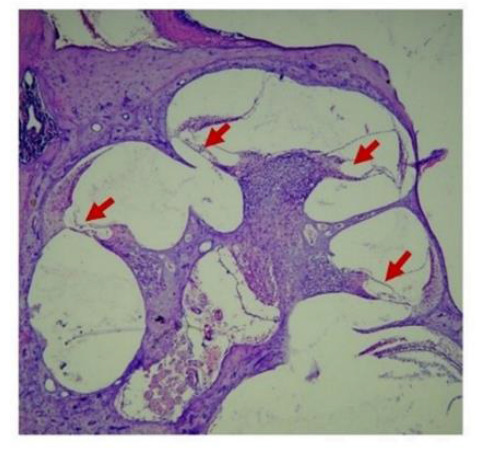
Overview of cochlear of Rattus novergicus. Red arrows show organ of corti with hematoxylin-eosin staining under 40x magnification.
The values of SOD expression
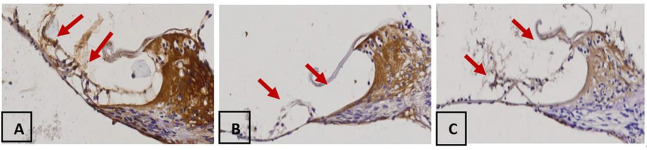
The decrease was found in SOD expression in groups of rats that received noise exposure both in groups with 100 dB noise exposure (group 2) and 110 dB (group 3) compared to the control group (group 1). In the Figure 2, the brown color on the cytoplasm shows SOD expression, in which a decrease in the intensity of brown color in cochlear organ of corti group 3 compared with group 2 and group 1. The higher noise intensity given, the average value of SOD expression decreases. In the Post Hoc test in Table 1, a significant difference was found in the value of SOD expression between groups 1 and groups 2 and 3 (p< 0.05), but no significant differences were found between group 2 and group 3 on the SOD value (p> 0.05).
Fig. 2.
SOD expression with magnification of 400 in each group, namely (A) Group 1, (B) Groups 2 and (C) Group 3, arrows show SOD expression in cochlear organ of corti marked in brown on the cytoplasm.
Table 1.
Post Hoc Test Results on SOD Expression in Each Group.
| Group | Mean difference | p value | |
|---|---|---|---|
| group 1 | group 2 | 2.667 | 0.047* |
| group 3 | 5.000 | 0.000* | |
| group 2 | group 3 | 2.333 | 0.096 |
Statistically significant (p< 0.05).
The values of MDA expression
The result of the study found the average value of MDA expression increased significantly in group 2 and group 3 compared to group 1. In the Figure 3, the brown color in the cytoplasm shows MDA expression where there is an increase in the intensity of brown color in the cochlear organ of corti in group 3 compared to group 2 and group 1. The higher noise intensity is given, the higher the MDA expression that is shown. The Post Hoc test in Table 2 showed a significant difference in the MDA expression values in groups 1 with groups 2 and 3 (p< 0.05), but significant differences were not found between group 2 and group 3 on the MDA value (p> 0.05).
Fig. 3.
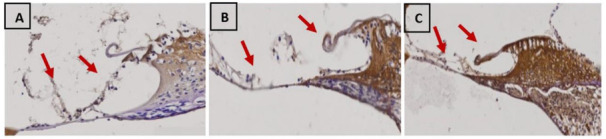
MDA expression with magnification of 400 in each group, namely (A) Group 1, (B) Groups 2 and (C) Group 3, the arrows show MDA expression in the cochlear organ of corti which are marked in brown on the cytoplasm.
Table 2.
Post Hoc Test Results on MDA Expression in Each Group.
| Group | Mean difference | p value | |
|---|---|---|---|
| group 1 | group 2 | 3.222 | 0.037* |
| group 3 | 4.667 | 0.002* | |
| group 2 | group 3 | 1.444 | 0.711 |
Statistically significant (p< 0.05).
The values of SNR
The average value of DPOAE was found to decrease in the group of rats that received noise exposure in both group 2 and group 3 compared to group 1. In the Post Hoc test in Table 3, significant differences were found in the DPOAE values between groups 1 and groups 2 and 3 (p< 0.05), but no significant differences were found between group 2 and group 3 (p> 0.05).
Table 3.
Post Hoc Test Results on SNR scores in each group.
| Group | Mean difference | p value | |
|---|---|---|---|
| group 1 | group 2 | 5.1778 | 0.000* |
| group 3 | 5.0889 | 0.000* | |
| group 2 | group 3 | 0.0889 | 0.995 |
Statistically significant (p< 0.05).
The correlation between SOD expressions and MDA expressions with SNR values
In this study as shown in Table 4, a positive correlation was found between SOD expression and SNR values. Instead, a negative correlation was found between MDA expression and SNR values in group 3, but no significant correlation was found between SOD expression and MDA expression with SNR values in the group 2.
Table 4.
Correlation between SOD expression and MDA expression with SNR values of group 3.
| Group 3 | Mean±SD | r | p value |
|---|---|---|---|
| SOD | 4,22±1,641 | 0.733 | 0.025* |
| MDA | 7,11±3,333 | -0.678 | 0.045* |
| SNR | 4,600±2,0785 |
Discussion
Most biological research on sensorineural hearing loss has been done in cats, chinchillas, gerbils, guinea pigs, mouse, and rats. Along with the rapid development in molecular biology and genomics, research has shifted from the use of animals mentioned above to the use of rats and mice, in particular, innate strains where the effects of genetic variability are greatly reduced (20). In a study conducted by Millon et al in 2018, found that female mice were significantly more protected against NIHL compared to male mice (21).
This study uses a noise frequency of 1-10 KHz because the research conducted by Heffner shows that the hearing frequency of mice ranges from 1-72 KHz, and the best hearing sensitivity of mice at a frequency of 8KHz (22). The intensity given is 100 dB and 110 dB for 2 hours, following the previous research, with the use of the noise with an intensity of 100 dB for 2 hours, there have been significant changes in the expression of calcineurin protein, NFAT1c, and apoptosis index (23).
In this study there was a decrease in SOD expression and an increase in MDA expression in the group with noise exposure and there were significant differences in SOD expression and MDA expression in rats exposed to noise compared to the control group. The higher the intensity of noise given the higher the MDA expression and the lower the SOD expression obtained when compared to the group without noise exposure. Noise exposure causes increased energy requirements. This causes an increase in ROS. Cochlear damage occurs due to excessive production of ROS thereby activating endogenous antioxidant mechanisms such as SOD. Increased production of Superoxide Anion Radicals (O2 • -) can directly cause damage to hair cells and / or increase the production of more dangerous ROS, including hydrogen peroxide (via a catalyzed reaction by SOD) and Hydroxil Radical (through the Fenton and Haber-Weiss reaction) (24). Hydroxyl Radical will initiate the process of lipid peroxidation directly on the structure of polyunsaturated fatty acids (PUFA) contained in cell membrane walls and mitochondria. Lipid peroxidation end products such as MDA and 4-hydroxyalkenal (HAE) are proven indicators of oxidative stress found in hair cells, support cells, spiral ganglion neurons and stria vascularis after noisy exposure (25).
The results obtained in this study are also in accordance with the results of research conducted on chinchillas given a 4 kHz noise exposure with an intensity of 150 dB SPL for 2 hours that found an increase in MDA levels in outer hair cell lines of cochlear (26). Research Demirel et al in 2009 found an increase in MDA in noisy exposure which meant the presence of oxidative stress through lipid peroxidation pathways (27).
Research on guinea pig cochlear tissue found a significant increase in MDA concentration due to noisy exposure of 176 dB SPL with a frequency of 1.05 - 20.3 kHz for 72 hours compared to the control group. After analysis with an electron microscope, outer hair cells and stereosilia suffered more damage in the basal turn and second turn areas of the cochlea. This indicates that these areas are more vulnerable than the apex areas of the cochlea (28).
Dehghani et al. Found significantly elevated levels of MDA in serum and liver tissue in Wistar albino rats after noisy exposure (100 dB, 700 - 5700 Hz, 8 hours / day for 8 days and 14 days compared to the control group that was not exposed to noise) (29).
Various types of cochlear damage mainly occur in outer hair cells can be caused by exposure to noise resulting in hearing loss. Noise exposure causes oxidative stress which results in apoptosis and cell necrosis. Cochlear cell death, caused by noise, ototoxic drugs, and aging occurs either through increased production of ROS or depletion of antioxidant defenses. An imbalance in the ROS / antioxidant ratio can cause cell death due to damage to proteins, DNA, and cell membranes. In addition, ROS is thought to act as a trigger for apoptosis. Apoptotic cell death has been implicated as a major cell death process in the expansion of hair cell damage after noise exposure (24). Nasezadeh et al. research conducted on rats with 100 dB of noise for 14 days found that there had been severe damage to cochlear outer hair cells and mild damage to inner hair cells and supporting cells and increased activity of antioxidant enzymes such as SOD, CAT and GPX in rats exposed to noise. They also found that there was an increase in MDA levels in groups of rats with noisy exposure (30).
ROS is one of the mechanisms underlying hearing loss caused by noise (NIHL). Research conducted by Tuerdi investigated the role of Mn-SOD in NIHL by examining the level of hearing loss and hair cell damage after noise exposure with an intensity of 120 dB for 4 hours for 14 days in C57BL / 6 wild type (WT) mice and Mn- mice HOD heterozygous knockout (HET). They found the mean ABR threshold was the same at all frequencies in both groups 1 hour after exposure to noise, but significantly worse, especially at 4 kHz, on days 7 and 14 after noise exposure in HET mice compared with WT mice. Collectively, these findings indicate that Mn-SOD plays an important role in protecting cochlea from damage caused by noise (31).
In this study, the cause of NIHL was oxidative stress, it was found that changes in oxidative stress markers were seen with a decrease in SOD expression and an increase in MDA expression in the group of rats exposed to noise both 100 dB and 110 dB compared to the control group. This result corresponds with the research conducted by Abdel-Tawab et al which was shown by the occurrence of oxidative stress, namely a decrease in SOD levels and an increase in MDA levels in the group of rats given 20 mg / kg BW indomethacin to induce gastric ulcer (32). Another study conducted by Cheraghi et al also showed an increase in MDA serum in patients with Coronary Heart Disease (CHD) compared to controls. An increase in the number of free radicals and a decrease in antioxidant defense activity led to an increase in oxidative stress (33).
NIHL is a permanent hearing loss that occurs gradually after exposure to high-intensity noise that causes outer hair cell damage and stereosilia (34). Distortion product Otoaccoustic Emissions (DPOAEs) are the result of an active intermodulation process in the cochlea mediated by outer hair cells and obtained simultaneously which indicates the stimulation of two pure tones (12), so the function of outer hair cells can be assessed by DPOAE examination.
In this study found that the higher the intensity of noise given, the lower the value of the SNR obtained, there was a significant difference in the value of the SNR between group 1 with group 2 and group 3, this is in accordance with previous studies conducted by Nassiri et al. with noise intensity of 65 dB, 85 dB, 95 dB and 105 dB for 3 hours and 8 hours per day where they find a negative correlation of SNR values with given noise intensity, the higher the intensity of noise given the lower the SNR value obtained with the significant difference (35).
In this study found a significant positive correlation between SNR values with SOD expression and a significant negative correlation between SNR values with MDA expression in group 3 but did not find a significant correlation between SNR values with SOD and MDA expression between groups 1 and 2. This can be interpreted if there has been a decrease in the value of SNR, it is found that the value of SOD expression is lower, and the value of MDA expression is higher. Every occurrence of damage to the function of outer hair cells has been found to change the value of oxidants and antioxidants. Increased MDA levels in rabbits who were given 100 dB of noise exposure for 1 hour showed a strong relationship between hearing loss due to noise and the antioxidant system (36). The process of lipid peroxidation in guinea pig’s outer hair cell membrane was found to increase after noisy exposure by 4 kHz with an intensity of 115 dB SPL, which in turn caused death of outer hair cells (37).
Research conducted by Yildirim, et al. in 2007 found the textile workers exposed to noise showed significantly higher MDA levels than controls. The study also showed that noise exposure caused hearing loss and increased oxidative stress and the two had a correlation (38).
Noise exposure can cause changes in the value of SOD expressions, MDA expressions and SNR values. The higher noise given, the value of the SOD expression and the SNR value will decrease, while the value of the MDA expression produced will increase. This proves that hearing loss due to noise occurs from 2 mechanisms namely mechanical and metabolic decompensation resulting in oxidative stress. This research has been able to prove the correlation between SNR values with SOD expression and MDA expression at 110 dB noise (group 3).
Acknowledgements
The authors thanked to the Research Institute of the Universitas Sumatera Utara for funding support under the TALENTA Research Implementation Contract of the Universitas Sumatera Utara 2018 fiscal year No: 2590 / UN.5.1.R / PPM / 2018 on March 16, 2018.
References
- 1.Hong O, Kerr MJ, Poling GL, Dhar S. Understanding and preventing noise induced hearing loss. Dis Mon. 2013;59(4):110, 8. doi: 10.1016/j.disamonth.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, et al. Auditory and non-auditory effects of noise on health. . Lancet. 2014;383(9925):1325, 1332. doi: 10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathur NN. Noise induced hearing loss emedicine background, pathophysiology, epidemiology. 2020. Available from: https://emedicine.medscape.com/article/857813-overview.
- 4.Kowalska M, Davis A. Noise-induced hearing loss. Noise & Health. 2012;14(61):274–80. doi: 10.4103/1463-1741.104893. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Determination of risk of noise-induced hearing loss due to recreational sound: review [web page on the internet]. 2017. Available from: from:https://www.who.int/pbd/deafness/Monograph_on_determination_of_risk_of_HL_due_to_exposure_to_recreational_sounds.pdf. [cited 2020 Nov 25]
- 6.Stucken EZ, Hong RS. Noise-induced hearing loss: an occupational medicine perspective. . Curr Opin Otolaryngol Head Neck Surg. 2014;22(5):388–93. doi: 10.1097/MOO.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 7.Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong ACY. Cellular mechanisms of noise-induced hearing loss. Hear Res. 2017;349:129–137. doi: 10.1016/j.heares.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi CH. Mechanisms of noise-induced hearing loss and treatment. . Audiology Speech Res. 2011;7:124–32. [Google Scholar]
- 9.Hearing loss due to recreational exposure to loud sounds: A Review. 2015. Available from: https://apps.who.int/iris/bitstream/handle/10665/154589/9789241508513_eng.pdf?sequence=1&isAllowed=y. World Health Organization.
- 10.Zimatore G, Stanzial D, Orlando MP. Otoacoustic Emissions. In: Research and Applications. In-Tech, London. 2013:204–23. [Google Scholar]
- 11.Kemp DT. Otoacoustic emissions. In Encyclopedia of neuroscience. . Centre for Auditory Research Elsevier. . 2009:317–36. [Google Scholar]
- 12.Saurini P, Nola G, Lendvai D. Otoacoustic emissions: a new method for newborn hearing screening. . Eur Rev Med Pharma Sci. 2004;8:129–33. [PubMed] [Google Scholar]
- 13.Yuan H, Wang X, Hill K, Chen J, Lemasters J, Yang SM, et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal. . 2015;22(15):1308–24. doi: 10.1089/ars.2014.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinar T, Atli AK, Alacam H, Karabulu I, Soguksulu I, Atas A, et al. The effects of noise on oxidative and antioxidative balance in human erythrocytes. . Int J Hem Onc. . 2011;(21):10–18. [Google Scholar]
- 15.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 16.Animal Care and Use Program. Rat and Mouse anesthesia and analgesia: Formulary and General Drug Information. The University of British Columbia. 2016. https://animalcare.ubc.ca/sites/default/files/documents/Guideline%20-%20Rodent%20Anesthesia%20Analgesia%20Formulary%20%282016%29.pdf.
- 17.Toydemir T, Kanter M, Erboga M, Oguz S, Erenoglu C. Antioxidative, antiapoptotic and proliferative effect of curcumin on liver regeneration after partial hepatectomy in rats. . Toxicol Ind Health. 2015;31(2):162–72. doi: 10.1177/0748233712469658. [DOI] [PubMed] [Google Scholar]
- 18.Haryuna TSH, Purba AH. W, Farhat F, Putra ST. The effect of Curcumin as an antioxidant on cochlea fibroblasts in ototoxic rat models. Journal of Chinese Pharmaceutical Sciences . 2018;27(12):847–854. [Google Scholar]
- 19.Czogalla B, Kahaly M, Mayr D, Schmoekel E, Niesler B, Kolben T, et al., editors. Interaction of ERα and NRF2 impacts survival in ovarian cancer Patients. Int J Mol Sci. 2019;20(1):112. doi: 10.3390/ijms20010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvi R, Boettcher FA. Animal Models of Noise-Induced Hearing Loss. . Sourcebook of Models for Biomedical Research. . 2008:289–301. [Google Scholar]
- 21.Millon B, Mitra S, Song Y, Marguilles Z, Casserly R, Drake V, et al. The impact of biological sex on the response to nois and of otoprotective therapies against acoustic injury in mice. Biol Sex Differ. 2018;9(1):12. doi: 10.1186/s13293-018-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heffner H. Hearing in glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. . Journal of the Acoustical Society of America. . 1980;68(6):1584–99. [Google Scholar]
- 23.Haryuna TSH, Riawan W, Nasution A, Ma’at S, Harahap J, Adriztina I. Curcumin reduces the noise-exposed cochlear fibroblasts apoptosis. . Int Arch Otorhinolaryngol. . 2016;20(4):370–376. doi: 10.1055/s-0036-1579742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bielefeld EC, Hu BH, Harris KC, Henderson D. Damage and threshold shift resulting from cochlear exposure to Paraquat-generated superoxide. Hear Res. 2005;207(1-2):35–42. doi: 10.1016/j.heares.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B. Oxidative stress in the cochlea: An update. . Curr Med Chem. 2010;17(30):3591–604. doi: 10.2174/092986710792927895. [DOI] [PubMed] [Google Scholar]
- 26.Henderson D, Hu B, Bielefeld E. Patterns and mechanisms of noise-induced cochlear pathology. . Springer. . 2008;31:195–217. [Google Scholar]
- 27.Demirel R, Mollaoğlu H, Yeşilyurt H, Üçok K, Ayçiçek A, Akkaya M, et al. Noise induces oxidative stress in rat. . Eur J Gen Med. 2009;6(1):20–4. [Google Scholar]
- 28.Xiong M, Lai H, Yang C, Huang W, Wang J, Fu X, et al. Comparison of the protective effects of radix astragali, α-lipolic acid, and vitamin E on accute acouctic trauma. . Clin Med Insights Ear Nose Throat. . 2012;5:25, 31. doi: 10.4137/CMENT.S10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deghani A, Ranjbarian M, Khavanin A, Rezazade-Azari M, Vosooghi S. Expossure to noise pollution and its effect on oxidants and antioxidant parameter in blood and liver tissue of rat. . Zahedan Journal Research in Medical Sciences. . 2013;15(5):13, 17. [Google Scholar]
- 30.Nasezadeh P, Shahi F, Fridoni M, Seydi E, Izadi M, Salimi A. Moderate O3/O2 therapy enhances enzymatic andnnon-enzymatic antioxidant in brain and cochlear that protects noise-induced hearing loss. Free Radic Res. . 2017;51(9-10):828, 837. doi: 10.1080/10715762.2017.1381695. [DOI] [PubMed] [Google Scholar]
- 31.Tuerdi A, Kinoshitaa M, Kamogashiraa T, Fujimotoa C, Iwasakia S, Shimizub T, et al. Manganese superoxide dismutase influences the extent of noise-induced hearing loss in mice. . Neurosci Lett. . 2017;642:123, 128. doi: 10.1016/j.neulet.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Tawab MS, Tork OM, Mostafa-Hedeab G, Ewaiss-Hassan M. Protective Effects of Quercetin and Melatonin on Indomethacin Induced Gastric Ulcers in Rats. Rep Biochem Mol Biol. 2020;9(3):278, 90. doi: 10.29252/rbmb.9.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheraghi M, Ahmadvand H, Maleki A, Babaeenezhad E, Shakiba S, Hassanzadeh F. Oxidative Stress Status and Liver Markers in Coronary Heart Disease. Rep Biochem Mol Biol. . 2019;8(1):49, 55. [PMC free article] [PubMed] [Google Scholar]
- 34.Nandi SS, Dhatrak SV. Occupational noise-induced hearing loss in India. . Indian J Occup Environ Med. 2008;12(2):53, 56. doi: 10.4103/0019-5278.43260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nassiri P, Zare S, EsmaeelPour MRM, Pourbakht A, Azam K, Golmohammadi T. Assessment of the Effects of Different Sound Pressure Levels on Distortion Product Otoacoustic Emissions (DPOAEs) in Rats. . International Journal of Occupational Hygiene. . 2016;8(2):93, 9. [Google Scholar]
- 36.Derekoy FS, Dundar Y, Aslan R, Cangal A. Influence of noise exposure on antioxidant system and TEOAEs in rabbits. . Eur Arch Otorhinolaryngol. . 2001;258(10):518, 22. doi: 10.1007/s004050100388. [DOI] [PubMed] [Google Scholar]
- 37.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. . Ear Hear. . 2006;27(1):1, 19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 38.Yildirim I, Kilinc M, Okur E, Inanc TF, Kilic MA, Kurutas EB, et al. The effects of noise on hearing and oxidative stress in textile workers. Ind Health. 2007;45(6):743, 9. doi: 10.2486/indhealth.45.743. [DOI] [PubMed] [Google Scholar]