Abstract
Background:
Prostate cancer (PCa) is the second leading cause of cancer death in American population. In this manner, novel therapeutic approaches for identification of therapeutic targets for PCa has significant clinical implications. Quercetin is a potent cancer therapeutic agent and dietary antioxidant present in fruit and vegetables.
Methods:
To investigate the underlying mechanism by which the PCa was regulated, nanoparticles of quercetin were administrated to cells. For in vitro experiments, human PCa cell line LNCaP were involved. Cell viability assay and quantitative RT-PCR (qRT-PCR) for hedgehog signaling pathway genes were used to determine the key signaling pathway regulated for PCa progression.
Results:
The cell viability gradually decreased with increased concentration of quercetin nanoparticles. At 48 h, 40 mM concentration of quercetin treatment showed near 50% of viable cells. Quercetin nanoparticles upregulates Su(Fu) mRNA expressions and downregulates gli mRNA expressions in the LNCaP cells.
Conclusion:
The results showed that the hedgehog signaling targeted inhibition may have important implications of PCa therapeutics. Additionally, the outcomes provided new mechanistic basis for further examination of quercetin nanoparticles to discover potential treatment strategies and new targets for PCa inhibition.
Key Words: Hedgehog, Prostate cancer, Proliferation, Quercetin nanoparticles, Signaling pathway
Introduction
Hedgehog (Hh) signal transduction pathway has a main role in the homeostasis, growth, survival, and malignancy (1, 2). Secreted hedgehog molecules attach to the PTC-PTCH1, PTCH2, therefore alleviating PTC-mediated suppression of smoothened (SMO), a putative seven-transmembrane protein. In addition, SMO signaling activates a cascade of intracellular events, provoking activation of the pathway via Gli family transcription factors (3). The PTCH1 hedgehog receptor is also a pathway target gene, which forms a negative feedback loops to maintain the pathway activity at a proper level. Hedgehog signaling activation via PTCH1 loss-of-function somatic mutations in human basal cell carcinomas (BCCs) disrupts this feedback regulation, inciting uncontrolled SMO signaling (4). Activation of the Hh pathway is frequently shown by raised levels of HIP and PTCH1. In addition to PTCH1 mutation, Hh overexpression and SMO activation can result in activation of the Hh pathway (5). Su(Fu) as a negative regulator of the Hh pathway prompting inactivation of this pathway by inhibiting the function of Gli molecules.
Prostate development need Hh signaling. Although the initial formation of prostate buds does not need sonic hedgehog signaling (shh), shh is vital for keeping up suitable prostate development, proliferation and tissue polarity (6, 7). The hedgehog pathway activity is low in the normal prostate, while activation of Hh pathway show greatly increased among prostate cancer (PCa) development. Therefore, inhibition of Gli function might be a promising prevention and therapeutic target in specific tumors (8).
Quercetin is a principal flavanoid compound that can be found in apples, onions, tea, and red wine, which possesses a wide spectrum of pharmacological properties (9). It has been showed that this compound has growth inhibitory effects on a wide range of cancer cell lines in vitro and in vivo (10). Quercetin nanoparticles (NQ) are ubiquitous in the environment, which their effectiveness in malignancy treatment has been reported (11). The NQ can cross many barriers, for instance the blood brain barrier, due to their special physicochemical features. And exposing cells to NQ can prompt negative effects such as inducing cell death, particularly in cancer cells (12). Howbeit, quercetins action molecular mechanism on cancer prevention and treatment are not totally described, but the their anti-cancer effects have been shown in different type of cancers including prostate, cervical, pancreatic cancers, and breast (13, 14). In this assessment, utilizing PCa cell lines with wild-type androgen receptor (AR) expression (LNCaP), we have characterized the change in molecular profile and hence the anti-cancer effect induced by quercetin in LNCaP. We recommend that the prostate cancer–preventative effects of quercetin may result from inhibition of the Hh pathway and that they possibly represent an inexpensive, safe, and effective decision for cancer prevention and treatment.
Materials and Methods
Quercetin nanoparticle preparation by microfluidic reactor
High performance liquid chromatography (HPLC)-grade quercetin was purchased from Behansar Pharmaceutical Company (Iran). Polysorbate 40 (Tween 40) and acetonitrile (HPLC grade) were provided from Sigma-Aldrich (Germany). The preparation of NQ by microfluidic reactor was performed according to Azarian et al. (15). Briefly, at predetermined temperatures and certain flow rates of solvent/anti-solvent, quercetin saturated solutions in acetonitrile were injected into the reactor. Quercetin nanosuspension was produced by a microfluidic reactor of polylactide (polylacetic acid) with an interior diameter of 1 mm and inlet angles of 30 °, 60 °, 90 °, 120 °, and 180 °. Four different input variables were considered in this survey (Tween 40 concentrations, anti-solvent flow rate, the angle between two entries (inlet angle), and viz. longitude output arm), which these parameters can affect the precipitation of nanoparticles prepared, possibly. At a constant room temperature of 22±2 °C, different concentrations of Tween 40 were used as the anti-solvent system. Fluid minor volumes were injected by hydrodynamic micro pumps. As an indicator of physical stability of the nanosuspension in order to ascertain parameters impacting the prepared nanosuspension stability, the time of sedimentation was taken. Finally, the produced samples were tightly closed and stored at 22±2 °C along with daily observations to monitor the phase separation of nano dispersions formed.
Size measurement of nanoparticles
To determine the particle size and morphology of prepared nanosuspension after examining the input variables affecting the output, the optimum formulation was studied.
Cell cultures and treatments
The human PCa cell line LNCaP was obtained from the Cell Bank of Institute Pasteur of Iran, Tehran. The cells were propagated in 100 mm culture dishes at the desired density in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin and 100 mg/ml streptomycin (Sigma-Aldrich) at 37 °C and 5% CO2 until reaching 50–70% confluence.
Cell viability assay
The cell viability assay was conducted by MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide). Briefly, in a 96-well culture plate, 5× 103 cells/ml of LNCaP cells were seeded and incubated overnight at 37 °C and 5% CO2. Cells were washed twice with PBS after attachment and starved for 6-12 h with serum-free medium (SFM). After starvation, cells were treated with different concentrations of quercetin nanoparticles (10, 20, 40, 80, and 100 μM) for 24, 48 and 72 h. Then, the wells were washed twice with PBS, added 100 ml of MTT (0.5 mg/1 ml) to each well and cells were incubated for 4 h at 37 °C. Then supernatant was removed and 100 μL of DMSO was added to dissolve formazan crystals formed by viable cells. By a spectrometer reader (SCO diagnostic, Germany), optical density (O.D.) was measured at 570 nm. The cell viability was assessed as the percent cell viability compared to the vehicle-treated control cells without quercetin nanoparticles administration, which were determined arbitrarily as 100% viability.
Pyrogenicity test and general safety
Taking advantage of the previous procedure, the pyrogenicity and toxicity of the antigens were checked (19). The amount of endotoxin in the prepared antigens was measured by a commercial Limulus amebocyte lysate kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s recommendations.
Real-time quantitative PCR
Total RNA was isolated from the LNCaP cell line using the RNA extraction kit (GeneAll Biotechnology, South Korea), based on the manufacturer's guidelines. RNA concentration was determined using the NanoDrop 1000 Spectrophotometer (Wilmington, USA). Then the cDNA synthesis was performed from RNA, using the cDNA synthesis kit (Takara Bio, Shiga, Japan), based on the manufacturer's guidelines.
Real-time TaqMan qPCR amplification was conducted with a Rotor-Gene 6000 real-time PCR cycler (Qiagen Corbett, Hilden, Germany) with 5 ng cDNA and 10 pM related primers of Su(Fu) and gli genes. The data were normalized to the housekeeping β-actin gene (F: 5'- TGGGCATCCACGAAACTAC -3' and R: 5'- GATCTCCTTCTGCATCCTGT -3') and calculated as 2-ΔΔCT expression. The primers and probes were manufactured by Pishgam (Tehran, Iran) are shown in Table 1.
Table 1.
Gene-targeted specific primers used in this study.
| Target Gene | Primer | Oligonucleotide sequence (5'-3') | Product size (bp) | Ref. |
|---|---|---|---|---|
| SUFU | F | CCAATCAACCCTCAGCGGCAGAATG | 159 | This study |
| R | GTAGGTGAGAAAGAGGGCTGTC | |||
| GLI | F | CAGGACCTAAGGACATATCTGGA | 77 | This study |
| R | CTCGGTACCAGAGTGTAACAACC |
Statistical analysis
Data were expressed as mean±standard error of the mean (SEM) and analyzed by SPSS software (version 22.0, SPSS Inc, Chicago, IL, USA). Groups were compared with Student’s t-test or one-way analysis of variance (ANOVA) followed by Newman-Keuls post-hoc analysis. A p value of< 0.05 was considered statistically significant.
Results
To determine the effect of quercetin-induced cell viability, human PCa cell line LNCaP were treated with different concentrations of quercetin. NQ decreased the cell viability gradually with increased concentration of quercetin. At 48 h, 40 mM concentration of quercetin treatment showed near 50% of viable cells (p< 0.05), (Fig. 1).
Fig. 1.
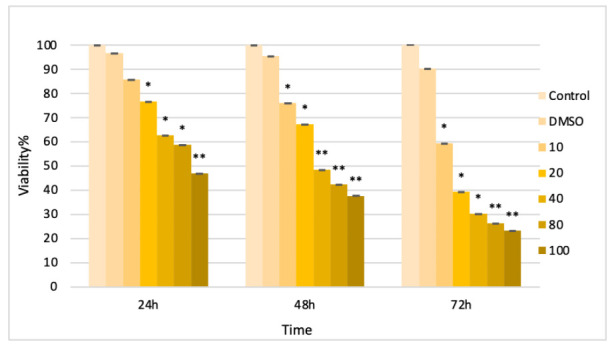
Effects of quercetin nanoparticles on LNCaP cell viability. The cell viability of LNCaP cells was measured by MTT assay for 24, 48 and 72 h.
In the present study, we determined the levels Su(Fu) and gli mRNA expression in cancer cell line LNCaP. Detectable levels of Su(Fu) and gli mRNA was observed in the LNCaP cells. NQ downregulates gli mRNA expressions and upregulates Su(Fu) mRNA expressions in the LNCaP cells (Fig. 2). Therefore, quercetin inhibits proliferation and cell survival of PCa cells.
Fig. 2.
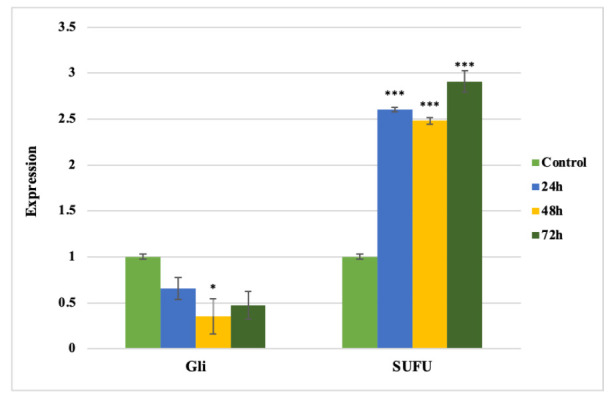
Quercetin nanoparticle downregulates gli mRNA expression. Each bar represents the mean±SEM of three independent observations.
Discussion
Chemotherapy is regularly utilized alone or in combination with therapeutic modalities to treat advance PCa. In any case, patients frequently develop resistance that can prompt poor therapeutic reaction and disease relapse. Thus, new agents with fewer side effects are needed to improve therapeutic outcome of PCa. Phytochemicals are used extensively for their properties in cancer therapeutic (16). In this manner, natural agents such as flavonoids clinically have been used in many countries to determine their cancer-preventive properties. Quercetin is a compound researched for its antiproliferative and anti-cancerous properties (1, 17, 18). Also, quercetin as a nutritional supplement utilized for prostatitis treatment, which can be an marker for development of PCa (19, 20).
Recently, liposomes and lipid based nanocarriers have shown efficacy in medication and gene therapy (21, 22). The effective and successful utilization of liposome nanocarriers has been catalyzed via targeted delivery and subsequent preferential intracellular uptake, enhancing permeability and retention effect with improved efficacy, selectivity, and overall safety (23). In the current study, quercetin nanoparticles were used to accomplish the promoting effects on PCa suppression through Hh signaling pathway. Hedgehog signaling pathway controls tissue polarity, cell proliferation and cell differentiation within normal development. Abnormal signaling of this pathway has been described in different human cancers, including small cell lung cancer, medulloblastomas, basal cell carcinomas, GI and prostate cancers (4, 24, 25). Our findings in this report indicate that NQ downregulates gli mRNA expressions and upregulates Su(Fu) mRNA expressions in the LNCaP cells. Therefore, quercetin inhibits proliferation and cell survival of PCa cells by influencing on the Hh signaling pathway. All the results here might serve as a basis for providing the possible treatment of natural anticancer compounds in developing new therapy for PCa in future.
It has been proved that androgens play an important role in PCa progression (10, 26). Hence, the main treatment given to the patients with androgen-dependent disease is androgen deprivation therapy (27). Unfortunately, recurrence of the disease usually happens within 12 to 18 months, leaving patients with castration-resistant PCa (28). Different investigations have reported the anti-proliferative and anti-inflammatory effects of quercetin on human cancer cell lines (29, 30). Apoptosis is one way is to establish tissue homeostasis; however, cancer cells develop mechanisms to elude cell death (31). In spite of primary prostate epithelial cells, PCa cells exposed to quercetin as time and dose dependent manner demonstrated more accumulation of dead cells. The data supports the conditions that the treatment regime of quercetin will be associated by fewer side effects. Quercetin induces intrinsic and extrinsic pathway-mediated apoptosis and modulates the components of insulin-like growth factor signaling in androgen-independent conditions (32). Further, quercetin induces c-jun/sp1-mediated downregulation of AR expression and activity in PCa cells (33). Moreover, by expression repressing in androgen-responsive PCa cells, quercetin attenuated the transcriptional yield of AR (10). These outcomes usually concentrate on utilizing LNCaP and need more data in cell lines that has mutated (DU-145) and lac AR (PC-3) to grasp mechanistic perspective. Therefore, quercetin resensitizes the resistant PCa to anti-androgen treatment. This inhibitory impact of quercetin on AR signaling involves its promising role as a chemopreventive agent or as an adjunct to existing treatment for PCa. Yue et al. (34) revealed that Su(Fu) is degraded quickly in certain cancer cells and indicated that Shh signaling promotes ubiquitination of Su(Fu), which results in its destruction in the proteasomes. These outcomes show that Shh signaling controls Su(Fu) activity by inducing its turnover by means of the ubiquitin–proteasome system (34). Their information demonstrated that the Shh control of Su(Fu) stability is physiological, and propose that Shh may overcome the negative inhibition of Su(Fu) on Gli-mediated target gene expression at least in part by inducing its degradation (34).
To sum up, nanoparticles of quercetin improved the inhibitory role in progression of PCa on cell line LNCaP via Hh signaling pathway. Accordingly, the results recommend that NQ is a promising candidate in PCa therapeutics in future. There could also be a couple of mechanisms by which the Hh pathway is activated in advanced prostate cancers, including over-expression of sonic hedgehog, loss of Su(Fu) protein expression, or other alterations. Our investigations predict that targeted inhibition of the Hh pathway could also be an efficient way to stop PCa progression. However, the apoptotic effects and anti-proliferative molecular mechanism of quercetin nanoparticles remains to be determined.
Acknowledgements
This study is performed in Cancer Biomedical Center. We are thankful to the staffs of Cancer Biomedical Center for their technical assistance. The authors declare no conflicts of interest.
References
- 1.Ward AB, Mir H, Kapur N, Gales DN, Carriere PP, Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. . World J Surg Oncol. . 2018;16(1):108. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseini MS, Hosseini F, Ahmadi A, Mozafari M, Amjadi I. Antiproliferative Activity of Hypericum perforatum, Achillea millefolium, and Aloe vera in Interaction with the Prostatic Activity of CD82. . Rep Biochem Mol Biol. . 2019;8(3):260, 268. [PMC free article] [PubMed] [Google Scholar]
- 3.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. . Nature. 2001;411(6835):349, 54. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 4.Sheng T, Li C, Zhang X, Chi S, He N, Chen K, et al. Activation of the hedgehog pathway in advanced prostate cancer. . Mol Cancer. . 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leube B, Drechsler M, Muhlmann K, Schafer R, Schulz WA, Santourlidis S. Refined mapping of allele loss at chromosome 10q23-26 in prostate cancer. . Prostate. 2002;50(3):135–44. doi: 10.1002/pros.10038. [DOI] [PubMed] [Google Scholar]
- 6.Berman DM, Desai N, Wang X, Karhadkar SS, Reynon M, Abate-Shen C, et al. Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. . Dev Biol. . 2004;267(2):387–98. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Wang BE, Shou J, Ross S, Koeppen H, De Sauvage FJ, Gao WQ. Inhibition of epithelial ductal branching in the prostate by sonic hedgehog is indirectly mediated by stromal cells. . J Biol Chem. . 2003;278(20):18506–13. doi: 10.1074/jbc.M300968200. [DOI] [PubMed] [Google Scholar]
- 8.Ślusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. . Cancer Res. . 2010;70(8):3382–90. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 9.Senthilkumar K, Arunkumar R, Elumalai P, Sharmila G, Gunadharini DN, Banudevi S, et al. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). . Cell Biochem Funct. . 2011;29(2):87–95. doi: 10.1002/cbf.1725. [DOI] [PubMed] [Google Scholar]
- 10.Xing N, Chen Y, Mitchell SH, Young CY. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. . Carcinogenesis. 2001;22(3):409–14. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharyya SS, Paul S, De A, Das D, Samadder A, Boujedaini N, et al. Poly (lactide-co-glycolide) acid nanoencapsulation of a synthetic coumarin: cytotoxicity and bio-distribution in mice, in cancer cell line and interaction with calf thymus DNA as target. . Toxicol Appl Pharmacol. . 2011;253(3):270–81. doi: 10.1016/j.taap.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Maitland ML, Schilsky RL. Clinical trials in the era of personalized oncology. CA Cancer J Clin. 2011;61(6):365–81. doi: 10.3322/caac.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep. . 2017;38(2):819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandyopadhyay S, Romero JR, Chattopadhyay N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol Cell Endocrinol. . 2008;287(1-2):57–64. doi: 10.1016/j.mce.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Azarian M, Amani A, Faramarzi MA, Divsalar A, Eidi A. Design and optimization of noscapine nanosuspensions and study of its cytotoxic effect. . J Biomol Struct Dyn. 2019;37(1):147–55. doi: 10.1080/07391102.2017.1420490. [DOI] [PubMed] [Google Scholar]
- 16.Tsao R. Chemistry and biochemistry of dietary polyphenols. . Nutrients. . 2010;2(12):1231–46. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Ys, Wang Jl, Feng Dy, Qin Hz, Wen H, Yin Zm, et al. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. . Int J Med Sci. 2014;11(3):282–90. doi: 10.7150/ijms.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobori M, Takahashi Y, Sakurai M, Akimoto Y, Tsushida T, Oike H, et al. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet‐induced obese mice. . Mol Nutr Food Res. 2016;60(2):300–12. doi: 10.1002/mnfr.201500595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoskes DA, Manickam K, editors. Herbal and complementary medicine in chronic prostatitis. . World J Urol. . 2003;21(2):109–13. doi: 10.1007/s00345-003-0332-5. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu JS. Prostate cancer and chronic prostatitis. Curr Urol Rep. 2008;9(4):328–32. doi: 10.1007/s11934-008-0056-6. [DOI] [PubMed] [Google Scholar]
- 21.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. . Drug discovery today. . 2006;11(17-18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Cho HJ, Yoon HY, Koo H, Ko SH, Shim JS, Lee JH, et al. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials. . 2011;32(29):7181–90. doi: 10.1016/j.biomaterials.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Sharma Vijay K, Mishra D, Sharma A, Srivastava B. Liposomes: present prospective and future challenges. . International Journal of Current Pharmaceutical Review & Research. . 2010;1(2):6–16. [Google Scholar]
- 24.Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. . Proc Natl Acad Sci U S A. . 2004;101(34):12561–6. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. . Nature. 2004;431(7009):707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 26.Ross RK, Pike MC, Coetzee GA, Reichardt JK, Mimi CY, Feigelson H, et al. Androgen metabolism and prostate cancer: establishing a model of genetic susceptibility. . Cancer Res. 1998;58(20):4497–504. [PubMed] [Google Scholar]
- 27.Huang Y, Jiang X, Liang X, Jiang G. Molecular and cellular mechanisms of castration resistant prostate cancer. . Oncol Lett. 2018;15(5):6063–6076. doi: 10.3892/ol.2018.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Semin Surg Oncol. . 2000;18(1):52, 74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Priyadarsini RV, Murugan RS, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. . Eur J Pharmacol. . 2010;649(1-3):84, 91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Chan ST, Yang NC, Huang CS, Liao JW, Yeh SL. Quercetin enhances the antitumor activity of trichostatin A through upregulation of p53 protein expression in vitro and in vivo. . PLoS One. 2013;8(1):e54255. doi: 10.1371/journal.pone.0054255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. . cell. . 2011 ;144(5):646, 74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Senthilkumar K, Elumalai P, Arunkumar R, Banudevi S, Gunadharini ND, Sharmila G, et al. Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3). . Mol Cell Biochem. 2010;344(1-2):173, 84. doi: 10.1007/s11010-010-0540-4. [DOI] [PubMed] [Google Scholar]
- 33.Yuan H, Young CY, Tian Y, Liu Z, Zhang M, Lou H. Suppression of the androgen receptor function by quercetin through protein–protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. . Mol Cell Biochem. . 2010;339(1-2):253, 62. doi: 10.1007/s11010-010-0388-7. [DOI] [PubMed] [Google Scholar]
- 34.Yue S, Chen Y, Cheng S. Hedgehog signaling promotes the degradation of tumor suppressor Sufu through the ubiquitin–proteasome pathway. . Oncogene. . 2009;28(4):492, 9. doi: 10.1038/onc.2008.403. [DOI] [PubMed] [Google Scholar]


