Abstract
CXCL17 is a homeostatic chemokine in the mucosa known to chemoattract dendritic cells and macrophages but can also be expressed elsewhere under inflammatory conditions. Cxcl17−/− mice have lower numbers of macrophages or dendritic cells in mucosal tissues. CXCL17 is also able to chemoattract suppressor myeloid cells that can recruit regulatory T cells. To explore a possible role of Cxcl17 in T cells, we studied T cell populations from Cxcl17−/− or wild-type (WT) littermate mice. Cxcl17−/− mice have higher numbers of CD4+ and CD8+ T cells in spleen and lymph nodes (LNs). Upon activation, they produce higher levels of several proinflammatory cytokines and chemokines. Furthermore, a Cxcl17−/− mouse developed exacerbated disease in a T cell-dependent model of experimental autoimmune encephalomyelitis (EAE). By 18 days after immunization with myelin oligodendrocyte peptide, only 44% of Cxcl17−/− mice were still alive vs. 90% for WT mice. During EAE, Cxcl17−/− mice exhibited higher numbers of lymphoid and myeloid cells in spleen and LNs, whereas they had less myeloid cell infiltration in the CNS. Cxcl17−/− mice also had higher levels of some inflammatory cytokines in serum, suggesting that they may be involved in the poor survival of these mice. Abnormal T cell function may reflect altered myeloid cell migration, or it could be due to altered T cell development in the thymus. We conclude that CXCL17 is a novel factor regulating T cell homeostasis and function.
Keywords: CXCL17, autoimmunity, T lymphocytes, inflammation
1 |. INTRODUCTION
The chemokine superfamily includes 48 human ligands and 20 receptors.1,2 They are responsible for the control of migration of many cellular types in the body, including leukocytes and stem cells.3,4 Chemokines are involved in the development of inflammatory responses through their capacity to attract leukocytes to inflamed or damaged tissues, and to regulate homeostasis and homing of leukocytes.5,6 Their importance extends beyond immunology, as several of them, like the CCL25-CCR9 axis, are strongly involved in T cell development and homeostasis in the thymus,7 CCL19/CCL21-CCR7 play an essential role in the homing of lymphocytes and dendritic cells to secondary lymph nodes (LNs),8 or the CXCL12-CXCR4 axis, which regulates the homing of hematopoietic stem cells to the bone marrow.9
Cxcl17 was one of the last chemokines to be identified and characterized. It was originally described as dendritic cell and monocyte chemokine like protein or vascular endothelial growth factor (VEGF) correlated chemokine 1 because of its ability to induce VEGF expression.10,11 Several groups have associated the expression of CXCL17 with tumor development, because tumor cells overexpressing CXCL17 induce bigger tumors and grow faster when implanted in mice.11,12 Several groups have reported that expression of Cxcl17 is up-regulated in several cancers including esophageal, lung, breast, endometrium, liver, and pancreas, and CXCL17 expression correlates with poor survival and prognosis.13–16
We originally reported that CXCL17 is a mucosal chemokine with antimicrobial activity that is strongly expressed in the respiratory tract and digestive system,17,18 and found that it is strongly up-regulated in several diseases affecting mucosal tissues, including idiopathic pulmonary fibrosis and Sjögren’s syndrome.17,19,20 Moreover, reflecting its mucosal expression elsewhere, CXCL17 is also expressed in the female reproductive tract and in the urethra.21
We have also characterized a Cxcl17−/− mouse. This mouse exhibits a paucity of macrophages in the lung, including alveolar macrophages.19 This phenotype indicates that CXCL17 plays a role in the recruitment of certain populations of tissue macrophages. We have reported that GPR35 (G coupled protein receptor 35),22 a G protein-coupled receptor that is also associated with mucosal tissues, is a receptor for CXCL17. Accordingly, GPR35 is expressed by CXCL17-responsive cells like macrophages and dendritic cells. GPR35 is also expressed in some breast cancer cell lines that respond to CXCL17.15 However, another receptor likely exists because GPR35 does not appear to be the receptor through which CXCL17 signals in THP-1 cells.23
A recent report described that CXCL17 attenuates inflammation in a mouse psoriasis model through the recruitment of myeloid derived suppressor cells that can, in turn, recruit regulatory T cells (Tregs).24
In the present study, we have continued the characterization of a Cxcl17−/− mouse. We observed abnormalities in the numbers of several T cell populations in both spleen and LNs. Furthermore, T cells showed significant differences in their ability to produce several proinflammatory cytokines. These observations prompted us to test a Cxcl17−/− mouse in an autoimmunity model of human multiple sclerosis (experimental allergic encephalomyelitis [EAE]), because this model is known to depend on T cell responses.25 Importantly, Cxcl17−/− mice show exacerbated disease in this model. We observed that Cxcl17 is detectable in the CNS during EAE, and that there are significant differences in the leukocyte populations present in the CNS, LN, and spleen between Cxcl17−/− and wild-type (WT) mice during EAE. Furthermore, we also detected abnormalities in the thymus of Cxcl17−/− mice. We therefore conclude that, in addition to the important role that CXCL17 has in the homeostasis of myeloid cell populations,19 it is also likely to play an important role in the homeostasis and function of peripheral T lymphocytes.
2 |. MATERIALS AND METHODS
2.1 |. Mice
Cxcl17+/− mice were obtained from the Knock-out Mouse Project at University of California, Davis (Genentech ID UNQ473). Cxcl17+/− heterozygote mice were bred to generate whole Cxcl17−/− mice. WT mice for breeding purposes were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in the same facility with a 12-h dark/light cycle with autoclaved bedding and irradiated food. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California Irvine.
2.2 |. Chemokines
Recombinant mouse Cxcl17 (Cat No. 4270-DM) was purchased from R&D Systems (Minneapolis, MN).
2.3 |. Genotyping PCR
Tail tissue was collected from 3-week-old mice upon weaning. DNA was extracted using a kit (Bioland Scientific, Paramount, CA). Primers specific to the Cxcl17 were used in a 35-cycle conventional PCR to determine the genotype of each mouse. The amplified DNA products were analyzed on a 1.5% agarose gel. Primers used were as follows: CXCL17 forward: 5′-CTCTTCCGACCACAGTATCC-3′; CXCL17 reverse: 5′-CTACAGTTGCAGACATGTTGG-3′; Neo forward: 5′-GCAGCGCATCGCCTTCTATC-3′; and Neo reverse: 5′-CTACAGTTGCAGACATGTTGG-3′.
2.4 |. Flow cytometry and leukocyte counts
Spleen, LN, and thymus were collected from 2–4 months old WT or Cxcl17−/− mice.
For fetal thymus cell population analyses, 18-day gestation mouse fetuses were obtained from a C57/Bl6 mouse timed pregnant female. Fetal thymi were obtained under a dissecting microscope. Solid organs were mechanically dissociated and passed through 70-mm strainers and rinsed with 1 × PBS–5% FBS to make single-cell suspensions. A total of 1–5 × 106 cells were transferred to flow cytometry tubes. Fc receptors were blocked using blocking buffer: 1 × PBS/5% FBS/unlabeled anti-mouse CD16/32 (1 μg/mL). Screening of the lymphocyte populations was achieved by staining the cells with the following anti-mouse antibodies: CD45 (clone 30-F11), CD11b (clone M1/70), CD3 (clone 145–2C11), CD8α (clone 53–6.7), CD4 (clone GK1.5), CD25 (clone PC61), CD44 (IM7), CD69 (clone H1.2F3), CD355 (clone 11–5/CRTAM), IA/IE (clone M5/114.15.2), IFN-γ (clone XMG1.2), IL-17A (clone TC11–18H10.1), and FoxP3 (clone MF-14).
For intracellular staining, we used the BD Cytofix/Cytoperm kit (BD Biosciences, Franklin Lakes, NJ) and followed the protocol as provided by the vendor. Briefly, after cell surface Ag staining, cells were fixed using 250 μL of Cytofix/Cytoperm solution for 20 min at 4°C, followed by washing cells 2 times using 1 mL in 1 × Perm/Wash solution. Cells were incubated in Perm/Wash solution for 15 min, pellet by centrifugation and resuspended in 100 μL of Perm/Wash solution containing anti-IFN-γ, anti-IL-17A, or anti-FoxP3, at a predetermined concentration of a fluorochrome conjugated antibody or appropriate negative control. The samples were incubated at 4°C for 30 min in the dark, followed by washing 2 times with 1 mL of 1 × Perm/Wash and resuspension in staining buffer prior to flow cytometric analysis. All antibodies were obtained from BioLegend (San Diego, CA), unless otherwise noted. To determine total numbers of lymphocytes in several tissues, we labeled cells with CD45, which is a leukocyte marker. Briefly, lymphocytes were identified by being brightly labeled with CD45 monoclonal antibody and having low side-scatter properties. CD45+ low side-scatter gates for lymphocytes are assumed to contain > 95% lymphocytes, so a correction for lymphocyte subset values is not needed. The total number of lymphocytes was calculated based on the CD3+CD4+ or CD3+CD8+ subpopulation percentages obtained through flow cytometry. The samples were processed using a NovoCyte flow cytometer (ACEA Biosciences, San Diego, CA), and the data were analyzed using FlowJo software (Tree Star) and GraphPad Prism6.
2.5 |. T cell activation and proliferation
CD3+ cells were purified from 2–4 months old mouse spleens using MojoSort cell isolation kit (BioLegend). T cell activation was performed using low endotoxin, azide-free (LEAF) purified anti-mouse CD3 (Clone 145–2C11; 5 μg/mL) coated to the plate and LEAF Purified anti-mouse CD28 (clone 37.51; 2 μg/mL) in complete RPMI media for 24 or 48 h. Cytokine production was measured by LEGENDplex and confirmed by ELISA.
For T cell proliferation, CD4 T cells were isolated from spleen and LN of 10-week-old mice using negative selection (StemCell Technologies, Vancouver, Canada). CellTrace CFSE-labeled T cells were cocultured with anti-CD3/CD28-coated Dynabeads (Life Technologies, Carlsbad, CA) at 1:1 ratio according to the manufacturer’s protocol in a U bottom 96-well plate for 96 h in presence of rmIL-2 (30 units/mL).
2.6 |. ELISA and cytokine and chemokine quantification
IFN-γ and TNF-α levels in cell culture supernatants were evaluated using mouse ELISA MAX Standard kits (BioLegend). The protocol for the enzyme-linked immunosorbent assay was followed as suggested by the vendor.
The cytokine and chemokine levels in the mouse serum were measured using the Legendplex mouse inflammation panel (BioLegend). This technology allowed us to determine a panel of 13 molecules (CCL-2, GM-CSF, IFN-β, IFN-γ, IL-1α, IL-1β, IL-6, IL-10, IL-12 (p70), IL-17A, IL-23, IL-27, and TNF-α). Peripheral blood was collected through cardiac puncture following mice euthanasia at days 9 or 17 after the MOG peptide injection in the EAE experiment model. Mock-injected mice were used as controls. The protocol was followed as suggested by the vendor.
The chemokine levels in activated splenocytes were measured using the Legendplex mouse proinflammatory chemokine panel (BioLegend) that allowed us to determine the levels of CCL2, CCL3, CCL4, CCL5, CCL11, CCL17, CCL20, CCL22, CXCL1, CXCL5, CXCL9, CXCL10, and CXCL13 in the culture supernatant.
2.7 |. EAE induction model
A direct EAE model was induced in 10-week-old WT or Cxcl17−/− mice by immunization with the MOG35–55 peptide (Hooke laboratories, Lawrence, MA) in complete Freund’s adjuvant as previously reported.26 Briefly, 200 μL of emulsion was injected subcutaneously at 2 sites over the flank region. Each mouse received 200 μg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) emulsified in Complete Freund’s adjuvant. Additionally, 300 ng of pertussis toxin (List Biologic Laboratories, Campbell, CA) was injected intraperitoneally on the day of immunization and 48 h later. Mice were assessed daily in a blinded fashion by the same researcher for the development of clinical signs according to the following scoring pattern: 0—no signs, 1—tail paresis, 2—hind limb paresis, 3—hind limb paralysis, 4—tetraplegia, and 5—moribound. Groups were sacrificed at day 9 or 18, and T cell subpopulations in LN were analyzed by flow cytometry.
2.8 |. CNS tissue digestion and isolation of mononuclear cells from CNS
CNS tissues were removed from PBS-perfused mice and cut into 2-mm pieces prior to digestion with collagenase D (Sigma Aldrich, San Luis, MO; 2.5 mg/mL) and DNase I (Sigma Aldrich; 1 mg/mL) in RPMI 1640 media supplemented with 10% FCS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol for 45 min at 37°C. Tissues were then passed through a cell strainer, followed by a Percoll gradient (70%/37%) and 350 × g centrifugation. Mononuclear cells were removed from the interphase, washed twice, and resuspended in RPMI 1640 medium supplemented with 10% (v/v) FCS.
Once the brain and spinal cord were digested, they were passed through a 100-μm nylon filter to obtain a single cell suspension in RPMI, using a 5 mL syringe plunger to completely dissociate the tissues. The cells were washed with RPMI and centrifuged at 1500 rpm for 5 min, following by filtration through a 70-μm strainer and wash 1 × with PBS, followed by centrifugation at 1500 rpm for 5 min. A total of 25 mL of a 37.5% Percoll solution were added to the pellet, which was gently mixed with a pipette, following by centrifugation for 12 min at 2300 rpm at room temperature (and stopping without brake). The myelin layer was taken out with a pipette. The mononuclear cells were located in the pellet. To lyse RBC, 1 mL of ACK lysis buffer was added for 30 s, and the cells washed 3 × with PBS followed by centrifugation at 1500 rpm for 5 min. Cells were then processed for flow cytometry staining.
2.9 |. Statistics
Significance was determined using Prism Version 5.0 software (GraphPad). Statistical significance of differences was calculated using two-tailed Student’s t-tests, or one- or two-way ANOVA. P < 0.05 was considered significant. All data are presented as mean ± SEM except as noted.
2.10 |. Immgen database
Levels of expression of Cxcl17 in different subpopulations in thymus were obtained from the Immgen database (https://www.immgen.org).27
3 |. RESULTS
3.1 |. CXCL17 regulates peripheral T lymphocyte homeostasis
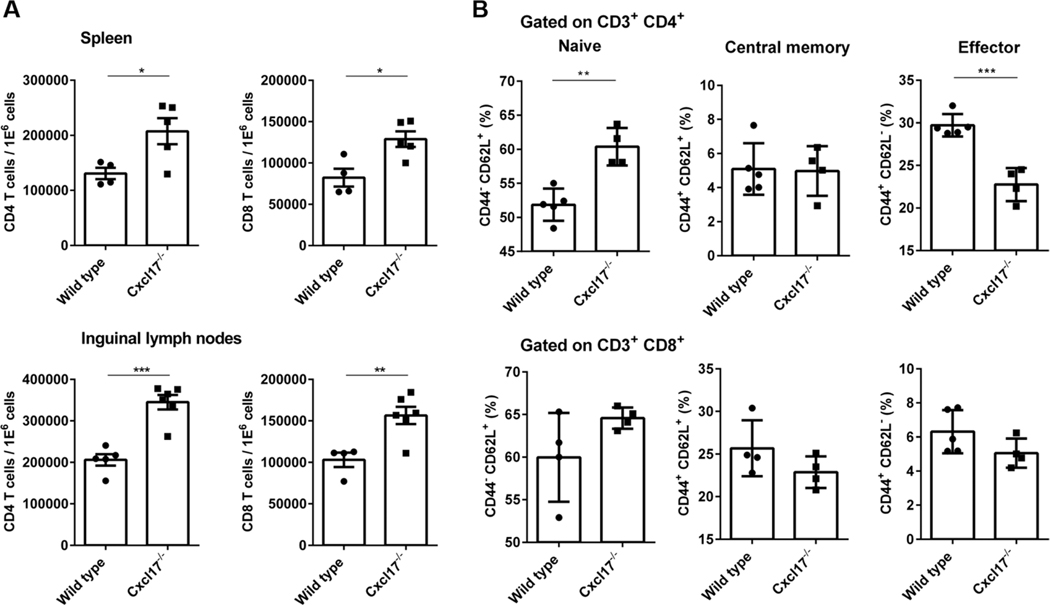
CXCL17 was the last chemokine ligand to be characterized.10 We have reported that it represents a mucosal chemokine strongly expressed in the respiratory and digestive tracts and is involved in the recruitment of myeloid cells to various mucosal sites.17 As we continued the characterization of a Cxcl17−/− mouse, we detected abnormalities in T cell populations (more CD4+ and CD8+ T cells) in both the spleen and LNs. To understand whether CXCL17 has any role in T lymphocyte biology under homeostasis, we analyzed spleen and skin draining inguinal LNs for frequencies of T cells from 2–4 months old CXCL17−/− or WT mice. CXCL17−/− mice spleen and LNs contained significantly higher number of helper (CD4) and cytotoxic (CD8) T cells (Fig. 1A). The difference in CD4 T cell numbers was more evident than CD8 T cells (50 vs. 25%). Further analyzing the different subsets of naïve (CD44–CD62L+), central memory (CD44+CD62L+), and effector memory (CD44+CD62L–) cells among T cells revealed that CXCL17−/− mice have significantly higher number of naïve CD4 T cells, coincidentally decreased number of effector memory CD4 T cells, and comparable number of central memory CD4 T cells (Fig. 1B). There were also differences among the CD8 T cells (Fig. 1A). Taken together, the absence of CXCL17 affects T cell development skewing toward CD4 population and leads to dysregulated number of T cells in the secondary lymphoid organs.
FIGURE 1. Cxcl17−/− mice show abnormalities in T cell populations in spleen and LNs.
(A) The number of CD3+ CD4+ and CD3+ CD8+ T cells is increased in spleen and inguinal LNs from Cxcl17−/− mice. (B) Naïve (CD44−CD62L+) and effector (CD44+CD62L−) T cells subpopulations are also affected in the CD4+ T cell population from Cxcl17−/− mice, with the number of naïve T cells higher, while the effector subpopulation is decreased in comparison with WT littermates. The numbers of central memory (CD44+CD62L+) T cells were not significantly different. Student’s t-test was performed. All values are means ± SEM. Means with asterisks are significantly different (*P < 0.1, **P < 0.01, ***P < 0.001). Data are representative from 3 or more individual experiments
3.2 |. CXCL17−/− leukocytes exhibit skewed cytokine and chemokine production
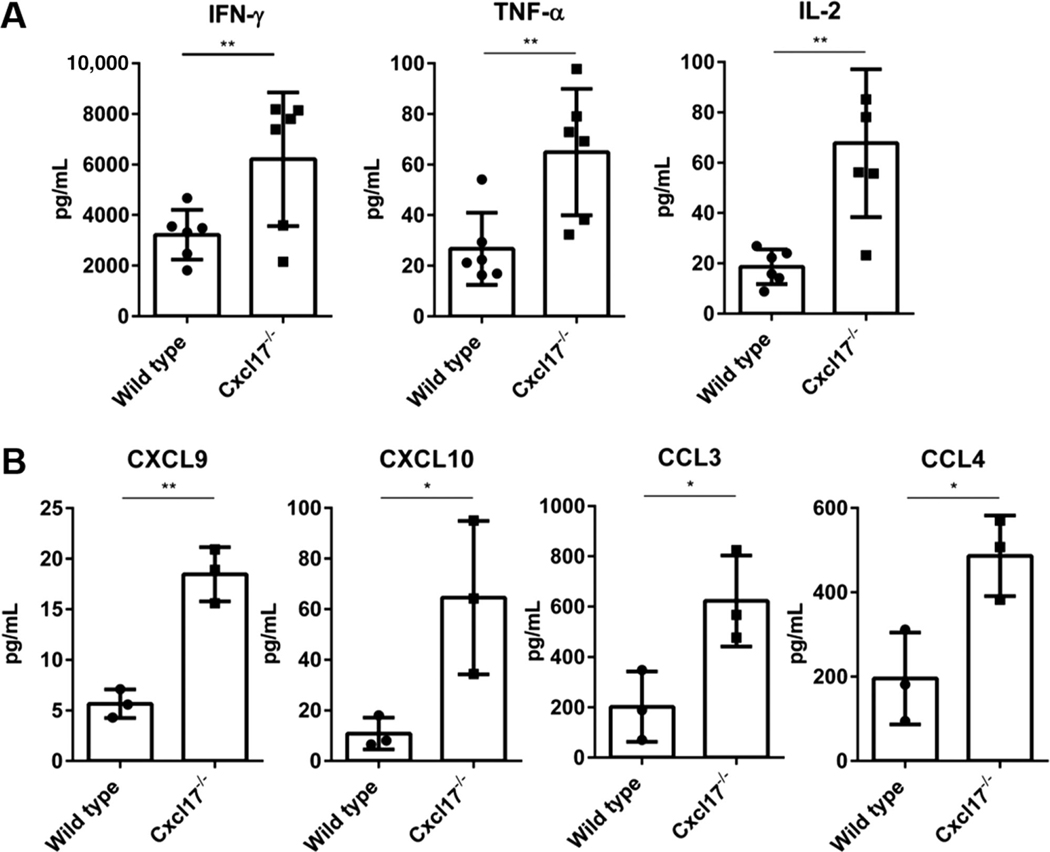
T cells from Cxcl17−/− mouse spleen produced higher levels of several proinflammatory cytokines and of chemokines upon activation with anti-CD3 and anti-CD28. Among these, were several proinflammatory cytokines (IFN-γ and TNF-α; Fig. 2A) and chemokines that favor Th1 responses (CXCL9 and CXCL10, which attract Th1 cells that express CXCR3; Fig. 2B). The higher numbers of T cells in Cxcl17−/− mice were not due to higher proliferation rates, because they showed similar proliferative capacity in a CFSE assay (Supplementary Fig. 1).
FIGURE 2. Leukocytes from Cxcl17−/− mice produce higher levels of proinflammatory cytokines.
In vitro activation of splenocytes from Cxcl17−/− mice or WT littermates revealed higher production of key activation cytokines by Cxcl17−/− leukocytes including (A) IFN-γ, TNF-α, and IL-2 and (B) CXCL9, CXCL10, CCL3, and CCL4 chemokines. Student’s t-test was performed. All values are means ± SEM. Means with asterisks are significantly different (*P < 0.1, **P < 0.01). Data are representative from 3 or more individual experiments
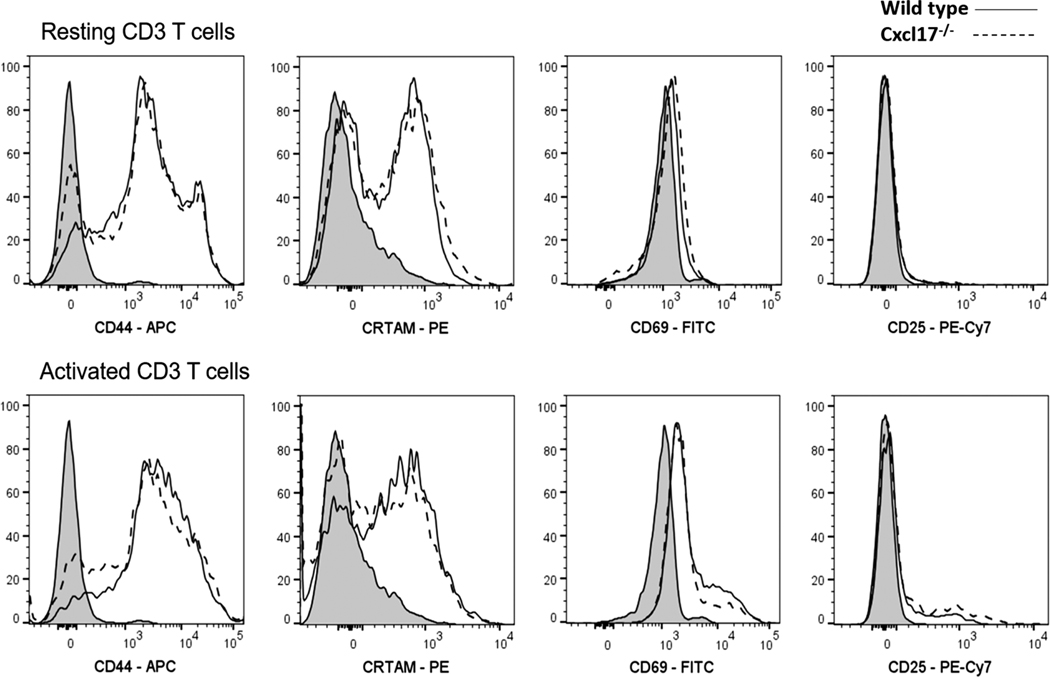
We tested the expression of several activation markers in T cells from Cxcl17−/− or WT mice either resting or upon activation. Subpopulations of activated T cells from Cxcl17−/− mice expressed less CD44 or CRTAM (an activation marker) as shown in Fig. 3.
FIGURE 3. Activated CD3 T cells from Cxcl17−/− mice express lower levels of CD44 and CRTAM activation markers.
T cell activation markers (CD44, CRTAM, CD69, and CD25) were measured in resting or activated T cells from Cxcl17−/− or WT littermate mice by flow cytometry. A subpopulation of T cells from Cxcl17−/− mice show an impairment in the expression (mean fluorescence intensity) of the activation and migration molecule CD44 in both resting and activation conditions. An impairment in the expression of CRTAM was also observed in a subpopulation of T cells from Cxcl17−/− mice. No significant changes were observed in the expression of CD69 or CD25 activation markers. Data are representative from 3 or more individual experiments
3.3 |. CXCL17−/− mice develop exaggerated EAE
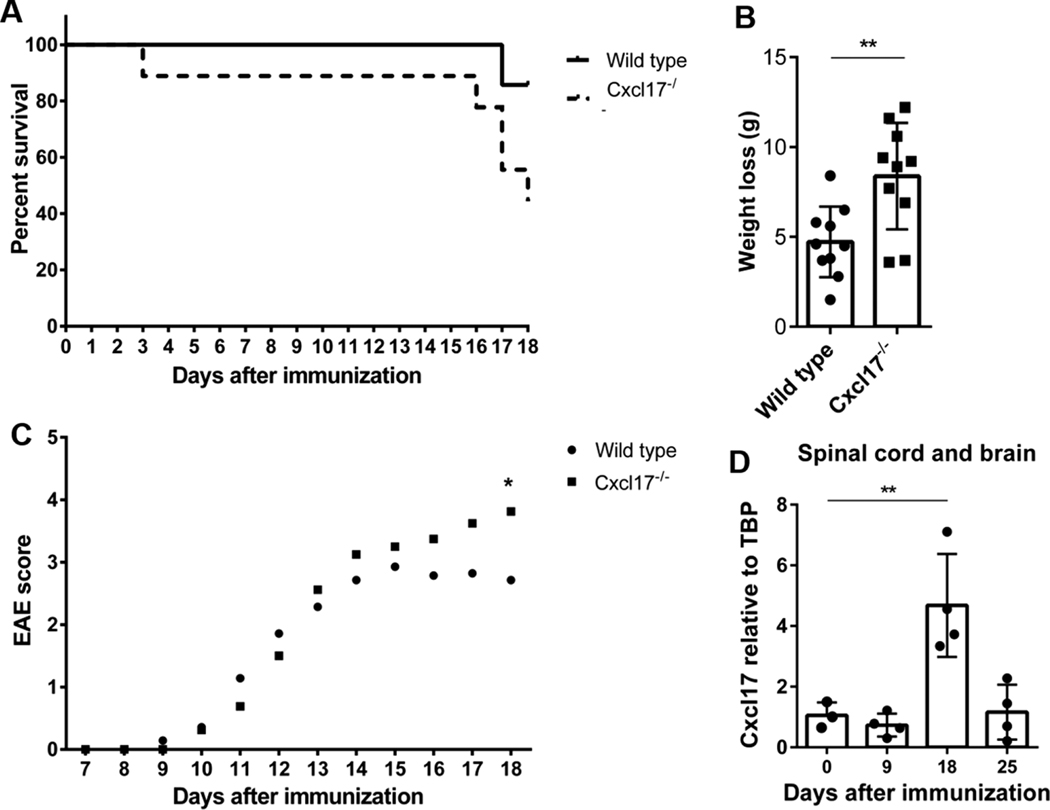
These observations indicated abnormalities in both T cell homeostasis and function in Cxcl17−/− mice, suggesting that a more proinflammatory immune microenvironment is present in these mice. We therefore decided to test the Cxcl17−/− mouse in a T cell-dependent autoimmune model. To this end, we chose a model of multiple sclerosis represented by EAE that is known to be T cell dependent.25 As shown in Fig. 4A, by day 18 following immunization with myelin oligodendrocyte glycoprotein (MOG) peptide, only 44% of Cxcl17−/− mice remained alive vs. 90% for WT littermates (Fig. 4A). Cxcl17−/− mice lost more weight than WT mice (Fig. 4B) and their clinical score of the disease was also significantly higher by day 18 following MOG immunization (Fig. 4C), correlating with poor survival. Cxcl17 mRNA was readily detectable in the CNS by day 18 after MOG immunization (Fig. 4D). The study had to be terminated at day 18 because mean maximal score of Cxcl17−/− mice was significantly higher than WT controls (Fig. 4C; Cxcl17−/−: 3.76 ± 0.16 vs. WT: 2.95 ± 0.18, Mean ± SEM). These EAE results indicate that Cxcl17−/− mice are significantly more susceptible to a T cell-mediated autoimmune disease.
FIGURE 4. Cxcl17−/− mice develop an exacerbated disease in the EAE model.
After18 days of immunization with MOG peptide, Cxcl17−/− mice show (A) lower survival (44 vs. 90%) and (B) more pronounced weight loss than WT mice. (C) EAE clinical score in Cxcl17−/− mice was significantly higher than in WT mice at day 18. (D) Cxcl17 is expressed in the CNS of WT mice at day 18 following MOG peptide immunization. Survival and Area Under the Curve analyses were performed to determine the differences between WT and Cxcl17−/− groups. Student’s t-test was performed to determine differences in weight loss. Two-way ANOVA was performed to determine differences in the expression of Cxcl17 during the EAE time course. All values are means ± SEM. Means with asterisks are significantly different (*P < 0.1, **P < 0.01). Data are representative from 2 or more individual experiments, using 6 to 10 animals per group
3.4 |. Altered homing of leukocytes to the CNS in CXCL17−/− mice during EAE
The pathophysiology of EAE is complex and heterogeneous: presentation of MOG by dendritic cells in the LN leads to priming and differentiation of Th1 and Th17 cells, which then traffic out of the LN and enter the CNS using adhesion molecules, LFA-1 and VLA-4.28,29 The presence of lymphocytic infiltrates in CNS has been well established as a clinical feature of multiple sclerosis, EAE, and many chronic inflammatory conditions.30–32 CXCL17 is known to be involved in recruiting myeloid cells to the mucosa and we have recently shown that CXCL17−/− plays a role in protection against genital herpes by recruiting effector memory CD8 T cells.21 We therefore sought to investigate whether CXCL17 plays any role in trafficking of lymphocytes to the CNS during EAE. Estimating levels in CNS at onset and peak of the EAE in WT mice revealed that Cxcl17 was readily detectable in the CNS by day 18 after MOG immunization (Fig. 4D). Interestingly, there were less myeloid cells in the CNS of Cxcl17−/− mice by day 18 (Fig. 5A), with a modest decrease in the frequency of CD4+ T cells producing IFN-γ in the CNS (Fig. 5B). Conversely, there were more T cells in LN (both CD4+ and CD8+) at day 9 after MOG immunization (Fig. 6), and increases in T cell and myeloid populations in the spleen of Cxcl17−/− mice at day 18 following MOG immunization (Fig. 7). Interestingly, the number of CD4+FoxP3+ Tregs was similar in LN from Cxcl17−/− or WT mice (Supplementary Fig. 2). However, following induction of EAE, Cxcl17−/− mice showed higher numbers of Tregs in LNs (Fig. 6). These observations suggest that Tregs may be unable to efficiently migrate into the CNS of Cxcl17−/− mice. To address whether CXCL17 regulates trafficking of leukocytes under noninflammatory conditions, we also investigated possible abnormalities in blood leukocytes in Cxcl17−/− mice. As shown in Supplementary Fig. 3, we detected significantly higher numbers of blood monocytes, eosinophils, and neutrophils in older Cxcl17−/− mice (older than 9 months). There were also less blood lymphocytes in older Cxcl17−/− mice (Supplementary Fig. 3). These observations are consistent with a role for CXCL17 in the recruitment of myeloid cells from the circulation to specific tissues, most likely mucosal.19 Homeostatic accumulation of leucocytes in circulation with age and decreased but not absent leucocytes in the EAE-CNS with concomitant increase in the spleen strongly suggests that CXCL17 plays a role in recruiting leukocytes to the CNS during ongoing autoimmune neuroinflammation.
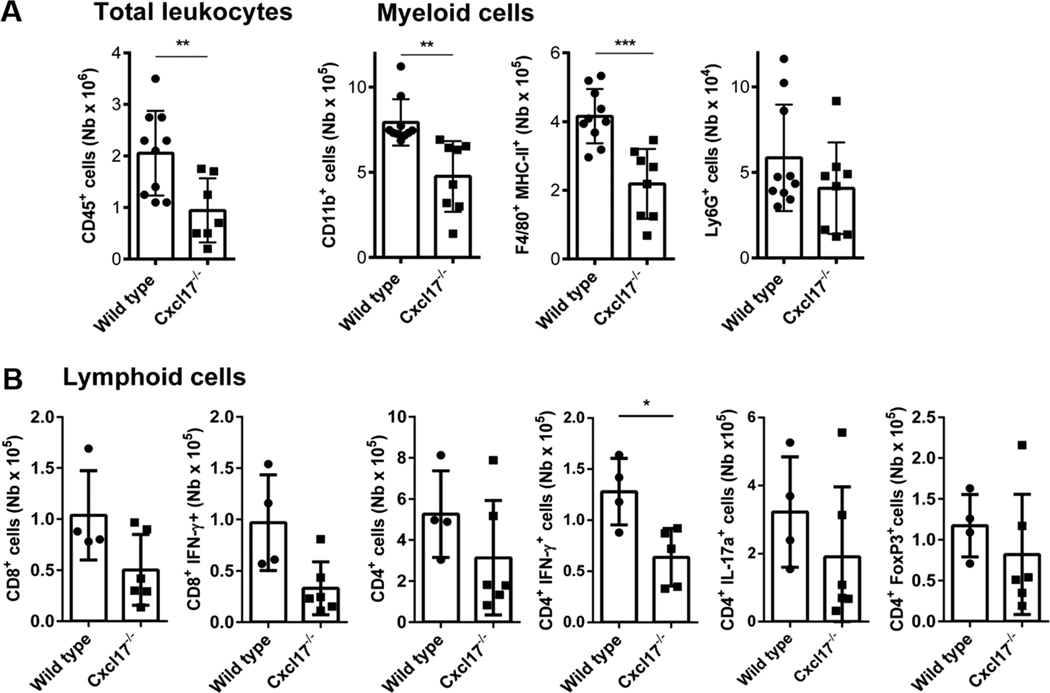
FIGURE 5. Cell subpopulations in CNS at day 18.
Leukocytes were evaluated in the CNS at day 18 after MOG immunization. (A) Total number of leukocytes shows a significant decrease in Cxcl17−/− mice compared to WT littermates. From the myeloid subpopulation evaluated, a significant reduction in CD11b+ cells and F4/80+ MHC-II+ was observed. (B) Except for the CD8+ IFN-γ+ subpopulation, similar numbers of pathogenic and regulatory FoxP3+ T cells were present in the CNS at day 18 following EAE induction. Student’s t-test was performed to determine significance. All values are means ± SEM. Asterisks indicate significant differences (*P < 0.1, **P < 0.01, ***P < 0.001). Data are representative from 2 or more individual experiments, using a minimum of 6 to 10 animal per group
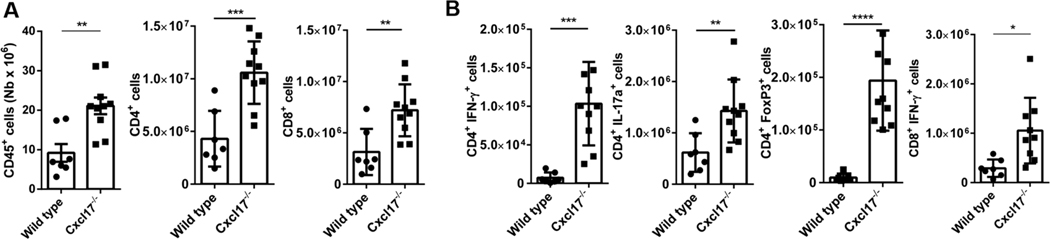
FIGURE 6. Cxcl17−/− mice exhibit higher numbers of leukocytes and pathogenic cells in LNs.
(A) The cells in inguinal LNs, the closest to the site of MOG injection, were evaluated at day 9 after EAE induction. The total number of leukocytes, represented as CD45+ cells, was significantly increased in LN from Cxcl17−/− mice, as well as total CD4+ and CD8+ T cells. (B) The total number of pathogenic T cells producing proinflammatory cytokines (CD8+ IFN-γ+, CD4+, CD4+ IFN-γ+, and CD4+ IL-17A+), as well as protector CD4+ FoxP3+ cells, was higher in LN from Cxcl17−/− mice. The differences were larger in CD4+ T cell subpopulations. All values are means ± SEM. Means with asterisks are significantly different (*P < 0.1, **P < 0.01, ***P < 0.001). Data are representative from 2 or more individual experiments
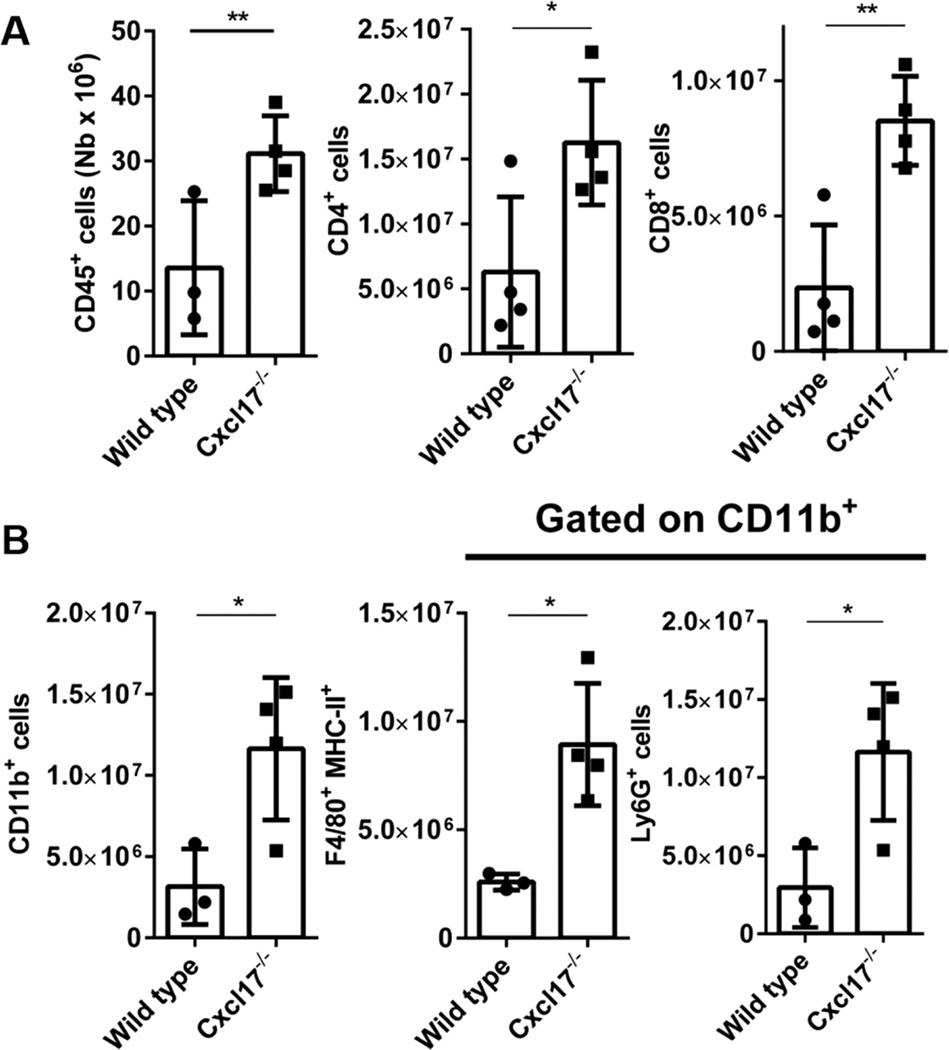
FIGURE 7. Cxcl17−/− mice exhibit higher numbers of leukocytes and pathogenic cells in spleen.
(A) At day 18 after the EAE induction, higher total numbers of CD45+, CD4+, and CD8+ lymphocytes are present in the spleen of Cxcl17−/− mice. (B) The number of cells from myeloid subpopulations CD11b+, F4/80 MHC-II+, and Ly6G+ was also significantly increased. Student’s t-test was performed. All values are means ± SEM. Means with asterisks are significantly different (*P < 0.1, **P < 0.01). Data are representative from 2 or more individual experiments
3.5 |. Cxcl17 is a regulator of systemic inflammation
During the effector phase of EAE, encephalitogenic T effector cells (Th1 and Th17) home to the CNS. Subsequently, a secondary wave of T cell activation and amplification takes place in the CNS, leading to the systemic signs of illness.33 Proposed mechanisms of demyelination and axonal damage during EAE include: deposition of complement; antibody-dependent cellular cytotoxicity; phagocytosis; attack of axons by cytotoxic T cells; secretion of proteases by neutrophils; and apoptosis of oligodendrocytes.34 The increased severity of EAE in Cxcl17−/− mice despite a decreased number of immune cells in the CNS could be due to a generalized hyper inflammatory state. We therefore sought to determine the immune status of EAE mice. To this end, we decided to test the serum at onset and peak of EAE for inflammatory signatures.
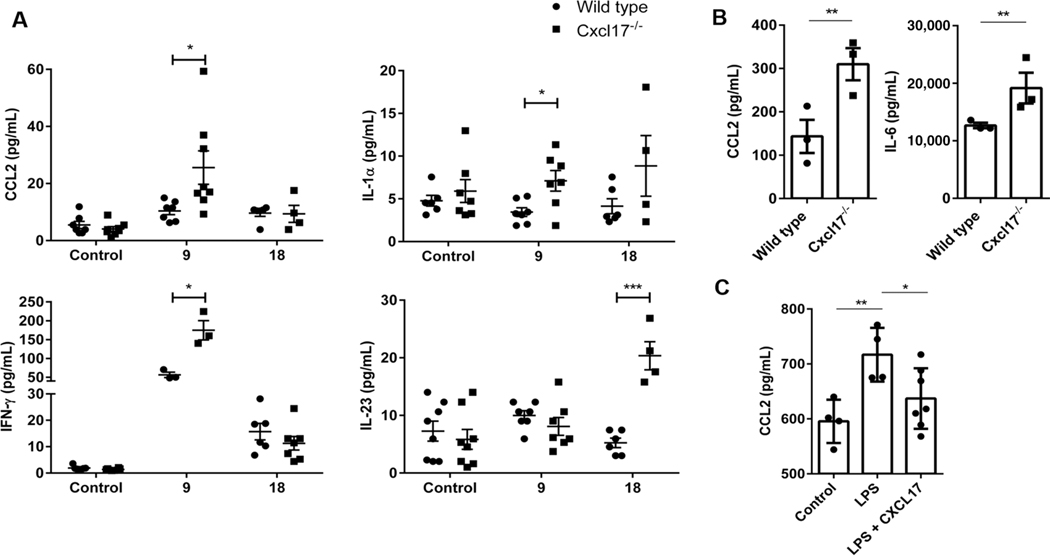
By day 9 after MOG peptide injection, and before animals developed classic EAE signs, we detected an increase in CCL2, IL-1α, and IFN-γ in sera from immunized Cxcl17−/− mice (Fig. 8A and Table 1). By day 18 following MOG injection, when signs of EAE development were evident and differences between WT and Cxcl17−/− mice became apparent, we detected higher levels of IL-23 in sera from Cxcl17−/− mice (Fig. 8A and Table 1). The levels of IFN-γ, a cytokine mainly produced by CD8+ or Th1 CD4+ cells, were also higher in sera from Cxcl17−/− mice. Alterations in CCL2, IL-1α, and IL-23 (all of which are mainly produced by myeloid populations) were evident at the systemic level in Cxcl17−/− mice and likely responsible for the development of severe EAE.
FIGURE 8. Cxcl17 is a regulator of inflammation.
(A) Measurement of a panel of inflammatory cytokines and chemokines in mouse sera revealed that several were affected during the EAE time course. Statistically significant differences between Cxcl17−/− and WT mice were observed in CCL2, IL-1α, IFN-γ, and IL-23 production, with all of these showing levels in Cxcl17−/− mice. (B) Higher concentrations of the innate proinflammatory molecules CCL2 and IL-6 were also observed in supernatants of peritoneal macrophages from Cxcl17−/− mice stimulated in vitro with LPS. (C) CXCL17 inhibits LPS-induced production of CCL2 in the macrophage cell line Raw 264.7. Two-way ANOVA was performed to determine differences in cytokines and chemokines levels during the EAE time course, and CCL2 production in macrophages stimulated with LPS in presence or absence of CXCL17. Differences in the production of innate cytokines by peritoneal macrophages were evaluated through Student’s t-test. All values are means ± SEM. Means with asterisks are significantly different (*P < 0.1, **P < 0.01, ***P < 0.001). Data are representative from 2 individual experiments, using 6 to 10 animals per group, or 3 independent experiments for in vitro results
TABLE 1.
Proinflammatory panel during EAE
| WT |
Cxcl17−/− |
|||||
|---|---|---|---|---|---|---|
| Inflammatory panel | Days after EAE | Mean (pg/mL) | SEM | Mean (pg/mL) | SEM | SS |
| IL-23 | Control | 7.27 | 1.73 | 5.84 | 1.73 | ns |
| 9 | 9.98 | 0.84 | 8.08 | 1.56 | ns | |
| 18 | 5.24 | 0.83 | 20.35 | 2.45 | *** | |
| CCL2 | Control | 5.47 | 1.26 | 4.06 | 0.84 | ns |
| 9 | 10.38 | 1.29 | 25.57 | 5.83 | * | |
| 18 | 9.63 | 1.16 | 9.35 | 2.99 | ns | |
| IL-1a | Control | 7.18 | 2.46 | 5.91 | 1.34 | ns |
| 9 | 3.45 | 0.51 | 7.11 | 1.20 | * | |
| 18 | 4.14 | 0.88 | 8.86 | 3.55 | ns | |
| IL-6 | Control | 1.85 | 0.18 | 2.33 | 0.72 | ns |
| 9 | 29.83 | 5.67 | 28.36 | 4.05 | ns | |
| 18 | 6.58 | 1.10 | 9.64 | 2.77 | ns | |
| IL-10 | Control | 3.31 | 0.69 | 2.80 | 0.89 | ns |
| 9 | 4.26 | 1.47 | 22.78 | 10.19 | ns | |
| 18 | 3.19 | 0.81 | 3.96 | 1.55 | ns | |
| TNF-α | Control | 2.43 | 0.98 | 0.86 | 0.32 | ns |
| 9 | 8.51 | 2.49 | 5.59 | 2.22 | ns | |
| 18 | 2.45 | 0.64 | 1.19 | 0.39 | ns | |
| IL-12p70 | Control | 1.15 | 0.06 | 1.02 | 0.02 | ns |
| 9 | 1.08 | 0.04 | 1.33 | 0.19 | ns | |
| 18 | 1.28 | 0.13 | 1.31 | 0.15 | ns | |
| IL-27 | Control | 20.41 | 7.91 | 17.70 | 11.50 | ns |
| 9 | 29.51 | 14.06 | 35.37 | 12.52 | ns | |
| 18 | 21.60 | 9.80 | 2.00 | 0.00 | ns | |
| IFN-γ | Control | 1.90 | 0.29 | 1.36 | 0.14 | ns |
| 9 | 56.31 | 7.17 | 174.83 | 25.53 | * | |
| 18 | 15.70 | 3.15 | 11.30 | 2.58 | ns | |
| IL-17a | Control | 1.38 | 0.07 | 1.24 | 0.05 | ns |
| 9 | 5.03 | 0.70 | 8.13 | 2.17 | ns | |
| 18 | 2.92 | 0.53 | 3.67 | 1.65 | ns | |
Several molecules are up-regulated at day 9 after the EAE induction in both WT and Cxcl17−/− mice (CCL2, IL-6, TNF-α, IL-27, IFN-γ, and IL-17A) however only CCL2, IL-1α, and IFN-γ showed significant higher production in Cxcl17−/− mice. At day 18, most of the molecules reduced its production to basal levels, except IL-23 in Cxcl17−/− mice. Student’s t-test was performed. All values are means ± SEM. Asterisks indicate statistical significance (P < 0.1,
P < 0.001). Results are representative from at least 2 individual experiments.
We then tested the ability of myeloid populations from Cxcl17−/− or WT mice to produce CCL2 or IL-6 upon stimulation with LPS. CCR2+ macrophages are believed to be proinflammatory35 through their ability to modulate CCL2 expression. As shown in Fig. 8B, peritoneal macrophages from Cxcl17−/− mice produced significantly higher levels of CCL2 or IL-6 when stimulated by LPS, suggesting that CXCL17 normally regulates the production of these mediators by macrophages. We confirmed this hypothesis by testing CCL2 production from WT peritoneal macrophages induced by LPS in the presence or absence of Cxcl17. As shown in Fig. 8C, addition of Cxcl17 to LPS-stimulated cells inhibited the ability of peritoneal macrophages to produce Ccl2. Taken together, these data indicate a strong proinflammatory immune microenvironment in Cxcl17−/− mice, which likely results in the enhanced susceptibility of the Cxcl17−/− mouse to EAE. These results provide mechanistic insight into why Cxcl17−/− mice experience severe EAE despite a decrease in the number of CNS homing leukocytes.
We also considered the possibility that the abnormal T cell phenotype observed in Cxcl17−/− mice could be due to abnormal T cell development in the thymus. To investigate this, we phenotyped the thymus of day 18 fetal Cxcl17−/− mice. As shown in Supplementary Fig. 4, Cxcl17−/− mice also exhibit abnormally high numbers of CD4+ thymocytes, suggesting that abnormal T cells in mice may arise through defects in T cell development.
These results support the conclusion that Cxcl17−/− mice experience stronger inflammatory responses than WT mice. Overall, our results indicate a novel immunoregulatory role for Cxcl17.
4 |. DISCUSSION
CXCL17 is a mucosal chemokine that exhibits homeostatic expression in mucosal tissues such as the respiratory and digestive systems, and the vagina; however, it can be expressed under inflammatory conditions elsewhere.17,20 It was the last chemokine described.10 This study also pointed out that the structure of CXCL17 is not typical of other chemokines. It is among the largest chemokines (13.8 KDa), and contains 6 cysteines instead of the typical 4 characteristic of this superfamily.1 It also contains motifs not found in other chemokines.10 These observations suggest that it may not only mediate typical chemokine functions such as chemotaxis, but may also mediate other immune functions. For example, CXCL17 displays broad antimicrobial activity17 and has been reported to have anti-inflammatory properties.36 The antimicrobial activity is not restricted to CXCL17; other chemokines including CXCL9, CXCL10, CXCL11, CXCL14, CCL25, and CCL28 have been shown to kill pathogens including gram-negative and gram-positive bacteria, parasites, and/or fungi.37–39 We should note, however, that the antimicrobial properties of mucosal homeostatic chemokines (CXCL14, CCL28, CCL25, and CXCL17) appear to be unique because they exhibit both prominent wide-spectrum antimicrobial activity and also antifungal activity (especially against Candida albicans).18 This suggests that these mucosal chemokines have mucosal-specific functions.
We have documented that CXCL17 is, along with CCL2, one of the most powerful macrophage chemoattractants of the chemokine superfamily,19 operating mainly in the mucosa. For example, administration of Cxcl17 intraperitoneally in mice causes a mean increase in the number of macrophages in the peritoneal exudate of more than 20% after 24 h (data not shown). In support of this conclusion, Cxcl17/−/− mice exhibit a paucity of macrophages in the lung.19 CXCL17 also shows the highest expression (among several mucosal chemokines) in the healthy mouse vagina and may play a role regulating the microbiome there.21 Another important observation from that study was that CXCL17 is important to control immune responses against herpesvirus in the vagina, which are depended on recruitment of GPR35+CD8+ effector memory cells and this depends on the recruitment of activated CD8+ effector T cells.21 These observations strongly suggest that one of the homeostatic functions of CXCL17 is to recruit certain myeloid cell populations to the normal mucosa.
During the characterization of a Cxcl17−/− mouse,19 we noticed significant abnormalities in T cell populations (Fig. 1), which were also able to produce higher levels of proinflammatory cytokines and chemokines upon activation than corresponding cells from WT mice (Fig. 2). The alterations in T cell function may be explained by alterations in myeloid cell populations (already documented in Cxcl17−/− mice19) and in the present study (Supplementary Fig. 3). However, an alternate explanation would be alterations in T cell development. There is precedent for important roles of chemokines in T cell development. For example, CCL25 is produced in the thymus and the expression of its receptor (CCR9) is induced in thymocytes following successful β selection of thymocytes at the DN3 stage of thymocyte differentiation.40 This allows the thymocytes that successfully rearranged the β chain of the T cell receptor to continue their differentiation and become CD4+CD8+ thymocytes that will undergo positive and negative selection.41 The role of CXCL17 in T cell development or function has not been studied. As shown in Supplementary Fig. 4, we detected higher numbers of CD4+ thymocytes in the thymus of Cxcl17−/− mice. This is potentially very interesting. In support of this, the Immgen database27 indicates that medullary epithelial cells (MECs), which are involved in positive selection in the thymus,41,42 express Cxcl17 (data not shown). This raises the possibility that positive selection of T cells in the thymus of Cxcl17−/− mouse may be abnormal, suggesting that it may be uniquely susceptible to autoimmunity.
To fully understand the role of CXCL17 in T cell biology, we set out to test the Cxcl17/−/− mouse in an autoimmune model. We used the classic EAE model (injecting the MOG35–55 peptide) that in C57BL/6 mice normally lasts 25 to 30 days and is known to be dependent on T cells.25 Importantly, in the absence of Cxcl17, this period was reduced to 18 days, at which time most Cxcl17/−/− mice exhibited either severe symptoms, had already died (Fig. 4A), or had to be sacrificed due to their very poor clinical score (66%) (Fig. 4C). No data are currently available about a role for Cxcl17 in the CNS, and its possible role in autoimmunity is poorly understood. CXCL17 is a mucosal chemokine under homeostatic conditions and is not expressed in the normal CNS, but it is expressed in the CNS during EAE (Fig. 4D). Furthermore, CXCL17 has been shown to be up-regulated in the CNS by inflammatory mediators.43 These observations indicate that CXCL17 is an inflammatory chemokine in the CNS. The markedly worse outcome of Cxcl17−/− mice in the EAE model (Fig. 4A) indicates that Cxcl17 plays a major role in controlling the pathology of the disease. As shown in Fig. 8, by day 9 following MOG peptide immunization (a day normally considered to represent the onset of the disease in WT mice), the levels of several chemokines and cytokines (CCL-2, IL-1α, and IFN-γ) were markedly higher in sera from Cxcl17−/− mice (compared to WT), and the higher levels of these cytokines and chemokines correlated with a worse outcome.
The numbers of T cells found in the CNS at day 18 after induction of EAE were slightly lower in Cxcl17−/− mice than in WT mice (Fig. 5). Conversely, we found higher numbers of T cells in spleen and LNs of Cxcl17−/− mice compared to WT mice (Figs. 6 and 7). This could be explained by decreased migration of T cells from LN and spleen to the CNS, for example, through altered expression of chemokine receptors. However, we did not find changes in the expression of GPR35, one of the proposed receptors for CXCL1722 (data not shown). Lack of CD44 expression has also been correlated with a worse outcome of mice in the EAE model,44 possibly due to cytokine production associated with Th17 cells. We detected a population of T cells with low or no expression of CD44 in Cxcl17−/− T cells upon activation. This observation may be related to a worse outcome in the EAE model, and could also account for lack of T cell migration to the CNS. Stronger T cell activation in the form of cytokine production was observed not only in vitro, but also in vivo. These results suggested intrinsic abnormalities in T cells from Cxcl17−/− mice. This observation suggests that the enhanced susceptibility of Cxcl17−/− mice in the EAE model may be due to a failure to control inflammation at the systemic level.
In the EAE model, the absence of Cxcl17 reduced but did not eliminate the number of macrophages in the CNS compared to WT littermates. The difference between outcomes in the EAE model of Ccl2−/− mice (resistance) vs. Cxcl17−/− mice (susceptibility) suggests that the function of the macrophage subpopulation(s) chemoattracted by each of these chemokines may be different. Our data strongly suggest that Cxcl17 is responsible for recruiting a population of macrophages to the mucosa, which may mediate a tolerogenic function to limit inflammation.
IL-23 is a critical cytokine that plays an important role in amplifying neuroinflammation in EAE inflammation by stabilizing pathogenic Th17 cells.45 One of the cytokines observed to change more in Cxcl17−/− mice is IL-23. The serum levels of IL-23, 18 days after MOG35–55 immunization, were almost 3 times higher than in WT littermates (Fig. 8A). At that time, more than 50% of Cxcl17−/− mice had died. IL-23 is a cytokine that drives the differentiation of a pathogenic Th17 subpopulation.46 Taken together, these data suggest that changes in the CD4 and CD8 T cell numbers, as well as IFN-γ production, could be related to the IL-23 axis. Signaling through IL-23 also supports the higher production of IL-17A observed by CD4+ T cells from Cxcl17−/− mice (Fig. 6). The EAE survival and EAE clinical score results are consistent with this observation, because the EAE mouse model is known to depend on Th1 and Th17 responses.25 We should note, however, that we did not observe significant macroscopic differences in the appearance of brains of Cxcl17−/− or WT mice (data not shown). This observation suggests that the susceptibility of Cxcl17−/− mice in the EAE model may be due to non-CNS events like a peripheral cytokine storm.
Kara et al.47 reported that during EAE development, there are 2 types of reactive Th17 cells that migrate to the CNS. The first one expresses high levels of CCR6 and is more abundant in the early phases of EAE. The second one is more “inflammatory” and may (or may not) express CCR6 but does express CCR2. The expression of the latter receptor is known to increase during the chronic phase of disease. This group also showed that the development and homing of reactive Th17 cells is controlled by IL-23 in the chronic phase following EAE induction.47 These observations support the results shown in Fig. 8B. We hypothesize that the higher production of the proinflammatory cytokines IL17A and IFN-γ by CD8 and CD4 T cells in Cxcl17−/− mice (Fig. 6) and accumulation of CD4 and CD8 lymphocytes in LN and spleen (Figs. 6 and 7) correlate with the observed higher levels of CCL2 (ligand for CCR2) and IL-23.
However, we did not observe higher levels of IL-17A in serum of Cxcl17−/− vs. WT mice with EAE (Table 1). CCL2 is known to be essential to recruit activated monocytes and bone marrow-derived macrophages to the CNS. Ccl2−/− mice show a reduction of up to 80% of migration to the CNS and marked resistance to EAE induction using MOG35–55 injection.48,49 IFN-γ serum concentrations at day 9 following MOG injection were significantly higher in WT mice compared with Ccl2−/− mice.48,50 Actually, in mice, antibodies against CCL2 have been shown to control the phenotype and relapses of EAE.48 CCL2 has also been implicated in the pathogenesis of diseases like rheumatoid arthritis, atherosclerosis, and psoriasis, therefore the Ccl2 levels in serum from Cxcl17−/− mice help explain the poor survival and worse prognosis we observed. Interestingly, we observed that Cxcl17 appears to be able to directly influence the ability of macrophages to produce Ccl2, suggesting a cross-regulation between these cytokines (Fig. 8C).
An unexpected result, however, was that the numbers of T cells found in the CNS at day 18 after induction of EAE were lower in Cxcl17−/− mice than in WT mice (Fig. 5). Conversely, we found higher numbers of T cells in spleen and LNs of Cxcl17−/− mice compared to WT mice (Figs. 6 and 7). This could be explained by decreased migration of T cells from LN and spleen to the CNS, for example, through altered expression of chemokine receptors. We have compared the expression of CCR5, CCR7, and CXCR4 in T cells from Cxcl17−/− and WT mice and found no differences (data not shown). Furthermore, we did not find changes in the expression of GPR35, one of the proposed receptors for CXCL1722 (data not shown).
Lack of CD44 expression has also been correlated with a worse outcome of mice in the EAE model,44 possibly due to cytokine production associated with Th17 cells. We detected a population of T cells with low or no expression of CD44 in Cxcl17−/− T cells upon activation (Fig. 3). This observation may be related to a worse outcome in the EAE model, and could also account for lack of T cell migration to the CNS.
Stronger T cell activation (in the form of cytokine production) was observed not only in vivo, but also in vitro (Fig. 2). These results suggested intrinsic abnormalities in T cells from Cxcl17−/− mice.
The alterations in T cell function may be explained by alterations in myeloid cell populations (already documented in Cxcl17−/− mice19) and in the present study (Supplementary Fig. 3). However, an alternate explanation would be alterations in T cell development. There is precedent for important roles of chemokines in T cell development. For example, CCL25 is produced in the thymus and the expression of its receptor (CCR9) is induced in thymocytes following successful β selection of thymocytes at the DN3 stage of thymocyte differentiation.40 The role of CXCL17 in T cell development or function has not been studied. As shown in Supplementary Fig. 4, we detected higher numbers of CD4+ thymocytes in the thymus of Cxcl17−/− mice. This is potentially very interesting. In support of this, the Immgen database27 indicates that MECs, which are involved in positive selection in the thymus,41,42 express Cxcl17. This raises the possibility that positive selection of T cells in the thymus of Cxcl17−/− mouse may be abnormal, suggesting that it may be uniquely susceptible to autoimmunity. In support of this, we have observed that older Cxcl17−/− mice (> 9 months of age) often exhibit inflammatory lesions including enlarged livers and other lesions, which are not observed in WT littermates of the same age (data not shown).
Taken together, we conclude that CXCL17 is a novel regulator of T cell homeostasis/function. We hope that our observations will stimulate future studies that will aim to determine the potential role of CXCL17 in autoimmunity, and in general, its role in T cell homeostasis/function.
Supplementary Material
ACKNOWLEDGMENTS
We want to thank the FACS facility of the Institute for Immunology at University of California Irvine for their assistance in these experiments. We thank the Immunological Genome Project (Immgen)27 for data on CXCL17 expression in thymic cells. This work was supported by The National Institute of Allergy and Infectious Diseases at the National Institutes of Health, grants (R01AI093548 and R21AI121928) to A.Z. and an UC MEXUS-CONACYT postdoctoral fellowship grant to M.H.-R., and grant R01AI121945 from the National Institutes of Health for S.O. and M.C.
Abbreviations:
- EAE
experimental autoimmune encephalomyelitis
- LEAF
low endotoxin, azide-free
- LN
lymph node
- MEC
medullary epithelial cell
- MOG
myelin oligodendrocyte glycoprotein
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
- WT
wild-type
Footnotes
SUPPORTING INFORMATION
Additional information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith H, Whittall C, Weksler B, Middleton J. Chemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cells. Stem Cells Dev. 2012;21:476–486. [DOI] [PubMed] [Google Scholar]
- 4.Bunting MD, Comerford I, McColl SR. Finding their niche: chemokines directing cell migration in the thymus. Immunol Cell Biol. 2011;89: 185–196. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. [DOI] [PubMed] [Google Scholar]
- 6.Bryant VL, Slade CA. Chemokines, their receptors and human disease: the good, the bad and the itchy. Immunol Cell Biol. 2015;93:364–371. [DOI] [PubMed] [Google Scholar]
- 7.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci USA. 2006;103:7006–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. [DOI] [PubMed] [Google Scholar]
- 9.Flomenberg N, DiPersio J, Calandra G. Role of CXCR4 chemokine receptor blockade using AMD3100 for mobilization of autologous hematopoietic progenitor cells. Acta Haematol. 2005;114:198–205. [DOI] [PubMed] [Google Scholar]
- 10.Pisabarro MT, Leung B, Kwong M, et al. Cutting edge: novel human dendritic cell- and monocyte-attracting chemokine-like protein identified by fold recognition methods. J Immunol. 2006;176:2069–2073. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein EJ, Head R, Griggs DW, et al. VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun. 2006;350: 74–81. [DOI] [PubMed] [Google Scholar]
- 12.Mu X, Chen Y, Wang S, Huang X, Pan H, Li M. Overexpression of VCC-1 gene in human hepatocellular carcinoma cells promotes cell proliferation and invasion. Acta Biochim Biophys Sin (Shanghai). 2009;41: 631–637. [DOI] [PubMed] [Google Scholar]
- 13.Saghir FS, Rose IM, Dali AZ, Shamsuddin Z, Jamal AR, Mokhtar NM. Gene expression profiling and cancer-related pathways in type I endometrial carcinoma. Int J Gynecol Cancer. 2010;20:724–731. [DOI] [PubMed] [Google Scholar]
- 14.Matsui A, Yokoo H, Negishi Y, et al. CXCL17 expression by tumor cells recruits CD11b+Gr1 high F4/80- cells and promotes tumor progression. PLoS One. 2012;7:e44080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo YJ, Zhou YJ, Yang XL, Shao ZM, Ou ZL. The role and clinical significance of the CXCL17-CXCR8 (GPR35) axis in breast cancer. Biochem Biophys Res Commun. 2017;493:1159–1167. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Yan J, Xu J, et al. CXCL17 expression predicts poor prognosis and correlates with adverse immune infiltration in hepatocellular carcinoma. PLoS One. 2014;9:e110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkhardt AM, Tai KP, Flores-Guiterrez JP, et al. CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J Immunol. 2012;188: 6399–6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Ruiz M, Zlotnik A. Mucosal Chemokines. J Interferon Cytokine Res. 2017;37:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkhardt AM, Maravillas-Montero JL, Carnevale CD, et al. CXCL17 is a major chemotactic factor for lung macrophages. J Immunol. 2014;193:1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez-Ruiz M, Zlotnik A, Llorente L, Hernandez-Molina G. Markedly high salivary and lacrimal CXCL17 levels in primary Sjogren’s syndrome. Joint Bone Spine. 2018;85:379–380. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava R, Hernandez-Ruiz M, Khan AA, et al. CXCL17 chemokine-dependent mobilization of CXCR8(+)CD8(+) effector memory and tissue-resident memory T cells in the vaginal mucosa is associated with protection against genital herpes. J Immunol. 2018;200:2915–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A. Cutting edge: gPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol. 2015;194:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SJ, Lee SJ, Nam SY, Im DS. GPR35 mediates lodoxamide-induced migration inhibitory response but not CXCL17-induced migration stimulatory response in THP-1 cells; is GPR35 a receptor for CXCL17? Br J Pharmacol. 2018;175:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oka T, Sugaya M, Takahashi N, et al. CXCL17 Attenuates Imiquimod-Induced Psoriasis-like Skin Inflammation by Recruiting MyeloidDerived Suppressor Cells and Regulatory T Cells. J Immunol. 2017;198: 3897–3908. [DOI] [PubMed] [Google Scholar]
- 25.El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Othy S, Hegde P, Topcu S, et al. Intravenous gammaglobulin inhibits encephalitogenic potential of pathogenic T cells and interferes with their trafficking to the central nervous system, implicating sphingosine-1 phosphate receptor 1-mammalian target of rapamycin axis. J Immunol. 2013;190:4535–4541. [DOI] [PubMed] [Google Scholar]
- 27.Heng TS, Painter MW, Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. [DOI] [PubMed] [Google Scholar]
- 28.Flugel A, Berkowicz T, Ritter T, et al. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14: 547–560. [DOI] [PubMed] [Google Scholar]
- 29.Odoardi F, Sie C, Streyl K, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488: 675–679. [DOI] [PubMed] [Google Scholar]
- 30.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132:3329–3341. [DOI] [PubMed] [Google Scholar]
- 32.Durelli L, Conti L, Clerico M, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65:499–509. [DOI] [PubMed] [Google Scholar]
- 33.Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. [DOI] [PubMed] [Google Scholar]
- 34.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140: 918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willenborg S, Lucas T, van Loo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. [DOI] [PubMed] [Google Scholar]
- 36.Lee WY, Wang CJ, Lin TY, Hsiao CL, Luo CW. CXCL17, an orphan chemokine, acts as a novel angiogenic and anti-inflammatory factor. Am J Physiol Endocrinol Metab. 2012;304:E32–E40. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Chatterjee M, Schmid H, Beck S, Gawaz M. CXCL14 as an emerging immune and inflammatory modulator. J Inflamm. 2016;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berri M, Virlogeux-Payant I, Chevaleyre C, et al. CCL28 involvement in mucosal tissues protection as a chemokine and as an antibacterial peptide. Dev Comp Immunol. 2014;44:286–290. [DOI] [PubMed] [Google Scholar]
- 39.Yung SC, Murphy PM. Antimicrobial chemokines. Front Immunol. 2012;3:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol. 2002;14: 311–323. [DOI] [PubMed] [Google Scholar]
- 41.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. [DOI] [PubMed] [Google Scholar]
- 42.Hamazaki Y, Sekai M, Minato N. Medullary thymic epithelial stem cells: role in thymic epithelial cell maintenance and thymic involution. Immunol Rev. 2016;271:38–55. [DOI] [PubMed] [Google Scholar]
- 43.Fil D, Borysiewicz E, Konat GW. A broad upregulation of cerebral chemokine genes by peripherally-generated inflammatory mediators. Metab Brain Dis. 2011;26:49–59. [DOI] [PubMed] [Google Scholar]
- 44.Flynn KM, Michaud M, Madri JA. CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis: a role in immune cells and vascular cells of the blood-brain barrier. Am J Pathol. 2013;182:1322–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 46.Revu S, Wu J, Henkel M, et al. IL-23 and IL-1beta drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep. 2018;22:2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kara EE, McKenzie DR, Bastow CR, et al. CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nat Commun. 2015;6:8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim RY, Hoffman AS, Itoh N, et al. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;274:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savarin C, Dutta R, Bergmann CC. Distinct gene profiles of bone marrow-derived macrophages and microglia during neurotropic coronavirus-induced demyelination. Front Immunol. 2018;9:1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.