Abstract
PURPOSE:
To quantitatively analyze clinically relevant features on longitudinal multimodal imaging of L-ORD (late-onset retinal degeneration) to characterize disease progression.
METHODS:
Fundus autofluorescence (FAF), infrared reflectance (IR), and optical coherence tomography (OCT) imaging of 4 L-ORD patients were acquired over 3–15 years (20 visits total). Corresponding regions-of-interest were analyzed on FAF (reticular pseudodrusen (RPD), “speckled FAF”, and chorioretinal atrophy) and IR (hyporeflective RPD and target RPD) using quantitative measurements including contour area, distance to fovea, contour overlap, retinal thickness, and texture features.
RESULTS:
Cross-sectional analysis revealed moderate correlation (RPD FAF ∩ RPD IR = 63%) between contour area across modalities. Quantification of retina thickness and texture analysis of areas contoured on FAF objectively differentiated the contour types. Longitudinal analysis of aligned images demonstrates that the contoured region of atrophy both encroaches toward the fovea and grows monotonically with a rate of 0.531–1.969 mm/year (square root of area, n=5 eyes). A retrospective analysis of precursor lesions of atrophy reveals quantifiable progression from RPD to speckled FAF to atrophy.
CONCLUSIONS:
Image analysis of timepoints prior to the development of atrophy reveals consistent patterns over time and space in L-ORD that may provide useful outcomes for L-ORD and other degenerative retinal diseases.
Keywords: late onset retinal degeneration, autofluorescence, reticular pseudodrusen, subretinal drusenoid deposits, outcome measures
Precis/Summary:
Quantitative analysis of longitudinal multimodal imaging in Late-onset retinal degeneration (LORD) reveal changes in retina thickness and texture metrics in areas that progress from reticular pseudodrusen to atrophy. Analysis of aligned images demonstrate consistent quantifiable patterns prior to the development of atrophy that may be useful as outcome measures.
Introduction:
Late-onset retinal degeneration (L-ORD) is a rare autosomal dominant retinal disease that presents usually in patients between 40 and 50 years of age with primary symptoms of difficulty adjusting to different lighting conditions 1. The disease is progressive and evolves to chorioretinal atrophy and the development of choroidal neovascularization1–3 leading to central vision loss typically by the seventh decade.
LORD is caused by a point mutation (S163R) in the gene that encodes C1q and tumor necrosis factor related protein 5 (C1QTNF5), previously known as CTRP54 which is expressed almost exclusively in the RPE. Evidence of RPE dysfunction is observed functionally in dramatic delays in dark adaptation5, 6. Structural repercussions of RPE dysfunction are observable in fundus autofluorescence imaging, which captures the relative quantities of lipofuscin7 and on optical coherence tomography (OCT) where deposits accumulate on both the apical and basal lateral side of the RPE.
Observations of longitudinal retinal imaging of patients with LORD have qualitatively described the evolution of structural retinal changes that initiate with reticular pseudodrusen (RPD) and later progress to atrophy8,9. This study builds on our previous observations and aims to develop quantification of lesions that provide insights to both understand the structural and temporal nature of these changes and identify parameters that could have use in clinical trials. First, we identify and contour characteristic lesion patterns in L-ORD prior to the development of atrophy in FAF and IR images. We use longitudinal and multimodal image registration9 to be able to correlate the findings across the modalities, monitor disease progression, and quantify the rate of structural changes.
Methods:
Subjects and ocular assessments
The study was approved by the Institutional Review Board and adheres to the tenets of the Declaration of Helsinki. This study includes four related patients from a previously described pedigree1, 4: three females and one male. Patients had previously been described and demonstrated the S163R mutation in C1QTNF510 and some had been qualitatively described8. Patients 1 and 2 are siblings; they are also first cousins to Patients 3 and 4, who are identical twins. All subjects exhibit typical clinical presentation of L-ORD, such as long anterior lens zonules and delayed dark adaptation.
All patients underwent a complete ophthalmoscopic examination including best-corrected visual acuity testing (BCVA), slit lamp examination, and dilated fundus exam on multiple visits. Fundus autofluorescence (FAF), infrared reflectance (IR), and spectral domain optical coherence tomography (SD-OCT) scans were acquired with the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany)
Feature definition and contouring
FAF images were contoured and reviewed by two independent graders (DN, CC) for the 3 main lesions of interest (Figure 1 and 2): reticular pseudodrusen (RPD) 11, 12, chorioretinal atrophy (atrophy) 11, and areas of abnormal speckled autofluorescence (speckled FAF). On IR images, two sub-phenotypes of RPD were contoured: areas of hyporeflective RPD and target- RPD lesions13. Areas fitting these definitions were manually contoured using 3D Slicer (v 4.10.2)14, separately on each modality (Figures 1 and 2).
Figure 1: Multimodal imaging of Patient 1.
(A) 30-degree fundus autofluorescence image. Contoured areas of RPD (green) and speckled FAF (yellow) are overlaid on the image in (C). (B) 30-degree infrared reflectance image with (D) contoured areas of hyporeflective RPD (blue) and target RPD (pink) overlaid. (E) OCT image with colored bars indicating various contoured sections from both 2D imaging modalities. The colors of the bars correspond to the colors of the contoured areas on FAF and IR. The yellow line in (C) and (D) indicates the location of the OCT B-scan.
Figure 2: Multimodal imaging of Patient 2.
Imaging was taken at the first visit date with atrophy. (A) 30-degree fundus autofluorescence image with contoured areas of RPD (green), speckled FAF (yellow) and atrophy(red). (B) 30-degree infrared reflectance image with overlays of contoured areas of hyporeflective RPD (blue) and target RPD (pink). (C) 2D projection of volumetric box generated from outer retinal thickness measurements in microns. (D) OCT image with colored bars indicating various contoured sections from both 2D imaging modalities. The colors of the bars correspond to the colors of the contoured areas on FAF and IR. The yellow line in (A) and (B) indicates the location of the OCT slice. The area contoured as atrophy on FAF corresponds with the area of choroidal hypertransmission on OCT, which is in line with the classical definition of atrophy on OCT. The solid vertical yellow line in (A) indicates the transition from contoured speckled FAF (left of the line) to contoured RPD on FAF (right of the line). To the left of the solid line on OCT, the RPE is significantly elevated, and the EZ effectively disappears; to the right of the line, classic reticular pseudodrusen are seen in the subretinal space.
OCT images collected as 121 B scans (30° × 20°) were automatically segmented15–17 and manually adjusted as needed18, 19. The thickness of the outer retina, defined as the distance between the inner nuclear layer-outer plexiform layer (INL-OPL) and the outer boundary of the RPE (OB-RPE) was quantified. A 2D projection of outer retinal thickness was generated for each OCT volume and then superimposed on 2D contour maps of the corresponding registered FAF/IR images to calculate the average outer retinal thickness in the FAF/IR-defined contour region.
Image texture analysis
Characteristic patterns of image-texture were qualitatively interpreted during manual delineation of contours with different phenotypes of interest (e.g., RPD, speckled FAF, and atrophy). Several texture-based features representing multiple categories were extracted from contoured areas on FAF images. The categories included first order statistics that captured image intensity variations within the contoured region) and features extracted from multiple matrices [Gray Level Cooccurrence Matrix (GLCM), Gray Level Run Length Matrix (GLRLM), Gray Level Size Zone Matrix (GLSZM), Neighbouring Gray Tone Difference Matrix (NGTDM), and Gray Level Dependence Matrix (GLDM)] that captured different texture properties. We used the definitions and implementation in Pyradiomics (v 2.2.0) 20 and a total of 94 texture features within contoured areas on FAF images were computed for each phenotype of interest (RPD, speckled FAF, and atrophy) and a distribution of texture-based values was generated. The distinguishability among different phenotypic distributions were assessed using ROC analysis with a univariate logistic regression classifier.
Registration and quantitative overlap analysis
FAF, IR, and OCT images were spatially registered in time and across modalities using automatic custom algorithms9. This enabled the comparison of contours across time and modalities after achieving anatomical correspondence in images. Overlap areas of multimodal contours were thus calculated after superimposing registered multimodal images (of the same patient, eye, and visit).
Longitudinal analysis
Contoured areas of longitudinally registered FAF images for each study eye were quantified. Furthermore, areas of longitudinal overlap were calculated by superimposing an aligned contour from a later timepoint onto the contours from an earlier timepoint.
Results:
The average age at initial examination was 50.9 years (range 45.3 −59.0 years) and all eyes had visual acuities 20/25 or better at the initial visit. Table 1 lists the patient characteristics including age, visual acuity, the number of visits, and time between visits.
Table 1:
Demographics of participants
| # visits | Age at 1st visit | Years spanned | VA OD (first) Letters (Snellen equivalent) |
VA OS (first) Letters (Snellen equivalent) |
VA OD (last) Letters (Snellen equivalent) |
VA OS (last) Letters (Snellen equivalent) |
|
|---|---|---|---|---|---|---|---|
| Patient 1 | 6 | 45.3 | 13 | 96 letters (20/12.5) | 93 letters (20/16) | 90 letters (20/16) | 91 letters (20/16) |
| Patient 2 | 8 | 50.3 | 15 | 90 letters (20/16) | 95 letters (20/12.5) | 85 letters (20/20) | 84 letters (20/20) |
| Patient 3 | 3 | 59.0 | 3 | 85 letters (20/20) | 85 letters (20/20) | 81 letters (20/25) | 80 letters (20/25) |
| Patient 4 | 3 | 48.9 | 13 | 78 letters (20/32) | 88 letters (20/20) | 9 letters (20/640) | 53 letters (20/100) |
VA= visual acuity
Qualitative cross-modality analysis
Figure 1 demonstrates the two main lesion types contoured for Patient 1 : RPD lesions, contoured in green, representing areas of “hypoautofluorescent pattern on a background of increased autofluorescence”, and speckled FAF lesions, contoured in yellow, having a mottled “salt-and-pepper” hyperFAF appearance. On the corresponding IR image, contoured independently from the FAF image, two sub-phenotypes of RPD were contoured: areas of hyporeflective RPD, contoured in blue and target -like RPD lesions contoured in pink (Figure 1D).
While the contouring on the 2D FAF and IR imaging modalities was performed completely independently of OCT imaging, the B-scan (Figure 1E) corresponding to a horizontal section shows the cross-sectional appearance of the 2D contoured regions. In Figure 1E, the areas of RPD (green) on FAF can be seen to correspond with areas of hyporeflective RPD on IR (blue) and to regions fitting the classic definitions of subretinal deposits (stage 2) on OCT21. Areas contoured as speckled FAF (yellow) generally corresponded to areas characterized as target RPD regions contoured on IR (pink) and in the OCT B-scan to areas of stage 3 RPD demonstrating more degenerative changes with elevated and hyperreflective RPE, a disruption in the EZ, and thinning of the overlying outer nuclear layer (ONL).
Similar assessments of contours in FAF and IR relative to a B scan can be observed in Patient 2 whose retina is at a more advanced degenerative stage (Figure 2). Similar comparisons can be seen with the addition of atrophy which is contoured on FAF (red), Figure 2A and appears on OCT as RPE loss and hypertransmission of signal into the choroid. Overall, the 2D retinal thickness map (Figure 2C) demonstrates a spatially similar pattern across the macula resembling autofluorescence contours with thinner areas corresponding to speckled FAF compared to RPD thickness.
Objective metrics of contoured areas
We investigated the ability of objective structural metrics of OCT and image-intensity-based metrics from FAF to distinguish between the contour types.
For retina thickness analysis, OCT outer retina thickness maps which were registered to the corresponding contoured FAF and IR images (patient 2, n = 6 with corresponding OCT, FAF and IR). In Figure 3, the average outer retinal thickness of RPD on FAF has a mean outer retinal thickness of 131 (± 12) microns and is similar in thickness to areas defined as hyporeflective RPD and target RPD on IR (132 ± 7 and 137 ± 10 microns, respectively). In comparison, areas contoured as speckled FAF or atrophy were found to have significantly thinner OCT measures of 109 (± 10) microns and 100 (± 12) microns, respectively (p< 0.05).
Figure 3: Mean outer retinal thickness of regions contoured on FAF and IR.
RPD, speckled FAF and atrophy, were contour areas from FAF images. HypoRPD and Target RPD were contoured areas from IR images. Starred bars indicate a statistically significant difference (p<0.05) between groups.
For FAF intensity analysis, 94 texture metrics were applied to the contoured regions of all (40 images) FAF images. The separability of contours using texture metrics was investigated via ROC analysis and computing the area under the curves (AUCs). The separability of atrophy and speckled FAF was measured to be perfect on this data set, with the best 10 AUC’s averaging 1.0 (± 0) (supplementary tables 1–3). The separability of atrophy and RPD was also very high with the average AUC of the best 10 texture features is 0.94 (± 0.02). The ability to differentiate RPD and speckled FAF contoured regions was more challenging, yet 10 best features still exhibited an average AUC = 0.80 (± 0.03).
Quantitative cross-modality analysis
The 2D contoured regions for all images in the study (4 patients, 20 visits) were assessed in three ways: to compare the areas in each modality between the eyes of a given patient, to understand the correspondence of the contoured areas within an eye across modalities and to compare the areas in each modalities in each eye over time. Area sizes of contoured regions had moderate to good interocular agreement (RPD on FAF r=0.40; speckled FAF r=0.85); target RPD lesions on IR r=0.67; hyporeflective RPD on IR r=0.37). These correlations appeared to be independent of the general size of the contours
The correspondence between the phenotyped areas within the same eye was investigated across modalities. RPD on FAF has a greater average overlap with IR-defined hyporeflective RPD (42%) than with target RPD (21%). Further, we observed that 63% of the area defined as RPD on FAF is also defined as RPD (either hyporeflective or target) on IR.
Longitudinal analysis
The longitudinal FAF images were used to investigate the spatial progression of the defined lesion types on FAF in two ways: first, to measure the total increase in atrophic area and, second, to assess how the leading “front” of the area of atrophy approaches the fovea. Figure 4 illustrates the progression of atrophy over 3 timepoints. In Figure 4A, the atrophy first appears as a small area in the temporal macula, which expands in a temporal to foveal direction to involve areas that had previously been defined as speckled FAF (yellow) (Figure 4B–C). Across the 5 eyes with more than one timepoint with atrophy, we find the range of the square root of atrophy area growth rates to be 0.531–1.969 mm/year (0.55 mm/year for patient with the most timepoints) (Figure 5A). The contoured areas were also used to quantitate the distance of the closest point of the contour to the fovea. For both atrophy and speckled FAF, the contours monotonically approach the fovea over time (Figure 5B–C).
Figure 4: Longitudinal AF images of Patient 2 that follow 8 years of atrophy progression.
(A) AF image on the timepoint first demonstrating an area of atrophy (red) with contoured areas of RPD (green) and speckled FAF (yellow). (B) and (C) demonstrate the same lesion phenotypes contoured 4 (B) and 8 (C) years later where the atrophy area (red) is shown to increase and move closer towards the fovea.
Figure 5: Longitudinal atrophy growth for all eyes that have developed atrophy (n = 6 eyes).
(A) Square root of atrophy area (mm) is plotted against the number of years after atrophy was first detected. Year 0 indicates the measurement of atrophy in the image that first contained an atrophy contour. (B) The rate of foveal encroachment by atrophy, expressed in distance (mm) from the center of the fovea. (C) The rate of foveal encroachment by speckled FAF, expressed in distance (mm) from the center of the fovea for all 8 eyes.
While the area of the atrophy (the final pathologic stage) increases monotonically over time, other contour areas do not increase monotonically as the local retina changes evolve and transition to different lesion-types. To understand the path of the evolution of a given area of retina over time, descriptors of that area were analyzed retrospectively. Contours of newly evolved areas of atrophy were aligned with images, with their respective contours, taken from previous timepoints. Figure 6 illustrates the method we used to assess longitudinal overlap percentages for a single instance of “newly-appearing” atrophy (A). By superimposing a contour of atrophy that had not been detected in previous timepoints (i.e., “new atrophy”) onto a previous timepoint’s image with contours of areas of RPD and speckled FAF, the status of the retinal area can be described. Figure 6B illustrates how newly evolved atrophy (red) superimposed on a contoured image taken 4 years prior, seems to be almost entirely contained within an area of speckled FAF.
Figure 6: Illustration of the methodology in retrospective longitudinal analysis.
(A) Contour of “newly evolved” atrophy (red) defined as atrophy that had not been present in previous images (B) Same atrophy contour, superimposed on contoured FAF image (RPD = green, speckled FAF = yellow) from 4 years prior. (C) Same atrophy contour (red) superimposed on a contoured AF image from 11 years prior to image in (A).
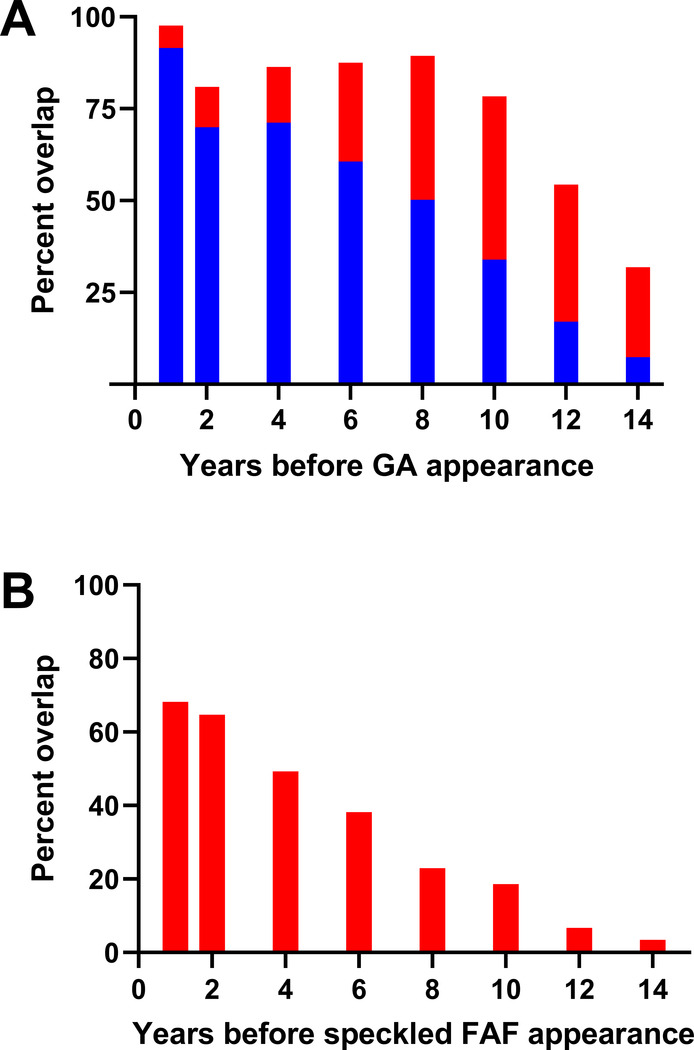
Expanding the process demonstrated in Figure 6, we overlaid all possible instances of areas with “newly-formed” atrophy with previous images containing contours of RPD and speckled FAF contours at previous timepoints. By pooling all these data together and calculating the areas of overlap, we quantify the composition of the areas that later correspond to atrophy at timepoints before their development. Figure 7 demonstrates that 1 year prior to detection, 92% of the area was defined as speckled FAF and 6% was defined as RPD. 6 years before the onset of atrophy, 61% is defined as speckled FAF and 27% is defined as RPD and 10 years prior, 34% is speckled FAF and 44% is RPD(Figure 7A). A similar analysis was performed retrospectively analyzing areas that evolve to speckled FAF (Figure 7B) finding that one year prior to the appearance of new regions of speckled FAF, 68% is occupied by FAF RPD. This contribution from RPD decreases monotonically at more remote timepoints (Figure 7B).
Figure 7: Analysis of overlap areas in retrospective longitudinal study.
(A) Pooled, averaged longitudinal overlap percentages for “newly-formed” atrophy. Percent overlap with other phenotypes (RPD, red and speckled FAF, blue) is provided as time walks backward from the appearance of a given region of “newly formed” atrophy. (B) A similar plot, which walks back from newly formed speckled FAF to find how much of the region was previously defined as RPD, red.
Discussion:
This study quantitatively examines the characterization of the structural features observed in L-ORD across multiple imaging modalities and describes the longitudinal progression of the disease. This analysis corroborates previous work with the disease, and demonstrates an overall sequence of events in L-ORD involving three distinct stages: 1) RPD, meeting the classic definitions on FAF and IR with evidence of subretinal deposits on OCT and thinning of the outer neural retina 2) speckled FAF, with OCT B-scans revealing patchy EZ loss and an uneven RPE layer with sub-RPE accumulations and dramatic thinning of the outer neural retina 3) atrophy, with hypoautofluorescence on FAF and confluent RPE loss and hypertransmission on OCT with thinning but only incremental further thinning of the outer neural retina.
While the RPD lesion types were contoured independently on FAF and IR, quantitative analysis of the neural retina thickness using OCT reveals that RPD contoured areas on FAF and hyporeflective and target RPD contoured areas on IR have very similar outer retinal thicknesses. However, the intersection of the FAF RPD contours with hyporeflective IR RPD contours was higher than the intersection of FAF RPD contours with target IR RPD contours (42% vs 21%) suggesting that these IR subtypes may be revealing different aspects of FAF-defined RPD. In the future, analyzing additional patients will facilitate the quantitative investigation of heterogeneous RPD subtypes in IR (target and hyporeflective) which may provide additional insights in the genesis of these lesions..
Paavo et al.22 demonstrate the use of autofluorescence and enface OCT to assess intraretinal correlates to the traditional two-dimensional signals in the setting of RPD associated with AMD, which demonstrates how correlating modalities can support the importance of a multimodal approach to imaging of retinal disease. We find that the mean outer retinal thickness of areas classified as atrophy are significantly lower than areas classified as RPD but only marginally thinner than areas of speckled FAF demonstrating that significant loss of retinal cells and neural degeneration that is present even before the evolution of chorioretinal atrophy. The thickness measurements in this study were derived from automated OCT segmentations combined with manual adjustments when needed. The accuracy of thickness measurements is thus limited by any segmentation errors and the operator variability in manual adjustments. Although such variability induce uncertainty (accuracy of the algorithm to be 5.7 ± 2.4 μm and interobserver variability in manual contouring to be 5.7 ± 2.0 μm23), it is relatively small compared to the thickness measurements reported in this paper. Overall, the OCT quantitative data supports that the lesion-types identified on FAF reflect different stages of neural degeneration and further underscores the need for intervention prior to the onset of atrophy.
This study also demonstrated that texture-based metrics can provide excellent discriminability of different L-ORD phenotypes on autofluorescence imaging. Such performance indicates the potential of texture features to automatically identify and define clinically relevant phenotypes and provide an objective, numerical method to quantify the longitudinal changes in a continuous scale. This study was limited to univariate models using individual texture metrics. In this small-scale data set (N=40), we achieved good AUC values even with a univariate model. These findings warrant further investigation and technical development to provide superior multivariate classifiers and automatic segmentation methods that combine multiple features/metrics. Top AUCs in univariate analysis came from different texture feature groups suggesting less collinearity. Future investigations involving larger datasets will facilitate the development of classification tools powered by multivariate analysis and/or decision trees to combine disparate texture metrics, creating versatile classifier models with high AUC. Automatic methods for delineating lesion subtypes provides a more desirable alternative to the manual definition of contours adopted in this work.
With our longitudinal overlap method, we gain a quantitative understanding and a time course of the retinal changes that occur before atrophy, both immediately before onset and at more distant timepoints. Approximately 8–10 years before a specific retinal area evolves into atrophy, we see an area shift from predominantly RPD to predominantly speckled FAF. This transition point is significant—though our sample size is small, this represents the potential for using quantitative longitudinal study to determine L-ORD prognosis and predict progression. In the same way that atrophy is preceded by speckled FAF, we also show through quantitation, that areas of speckled FAF are preceded by RPD. Once formed, atrophic areas increase in size at a fast rate with Patient 2, demonstrating a growth rate with a range of 0.53–0.56 mm/year (Figure 5A). While this study comprised of a limited number of patients – there are numerous timepoints that demonstrate a growth rate much higher than that of GA growth reported in AMD patients typically ranging 0.3–0.4 mm/year24–26.
One limitation to this study is the small patient sample size, which certainly is affected by the rarity of L-ORD. However, the development of analytic methods and approaches that can help quantify changes in the retina both spatially and longitudinally provide additional levels of insight and use in clinical trial settings. The work in this study is highly translatable to other diseases that also share a path of progression involving RPD and evolution to atrophy, such as AMD.
Application of our methodology to AMD datasets would allow for more robust conclusions about the antecedents of GA, as well as better quantitative metrics (e.g. texture-based metrics) to characterize retinal disease features. In both diseases, identifying, and even automating the detection of, quantifiable features prior to the final neurodegenerative stage would aid in assessing patients appropriate for certain clinical trials, as well as the possibility of using these earlier biomarkers as surrogate endpoints in clinical trials.
Supplementary Material
Acknowledgements:
Supported by funds from the National Eye Institute Intramural Research Program, National Institutes of Health. We also acknowledge support from the Distinguished Scholars Program, NIH. We thank the participants and their families for their time and commitment to research.
Grant information: This study is supported by funds from the National Eye Institute Intramural Research Program, National Institutes of Health (NIH).
Footnotes
Competing Interests: The authors declare that they have no competing interests.
References:
- 1.Ayyagari R, Griesinger IB, Bingham E, et al. Autosomal dominant hemorrhagic macular dystrophy not associated with the TIMP3 gene. Arch Ophthalmol 2000;118(1):85–92. [DOI] [PubMed] [Google Scholar]
- 2.Kuntz CA, Jacobson SG, Cideciyan AV, et al. Sub-retinal pigment epithelial deposits in a dominant late-onset retinal degeneration. Invest Ophthalmol Vis Sci 1996;37(9):1772–82. [PubMed] [Google Scholar]
- 3.Vincent A, Munier FL, Vandenhoven CC, et al. The characterization of retinal phenotype in a family with C1QTNF5-related late-onset retinal degeneration. Retina 2012;32(8):1643–51. [DOI] [PubMed] [Google Scholar]
- 4.Ayyagari R, Mandal MN, Karoukis AJ, et al. Late-onset macular degeneration and long anterior lens zonules result from a CTRP5 gene mutation. Invest Ophthalmol Vis Sci 2005;46(9):3363–71. [DOI] [PubMed] [Google Scholar]
- 5.Borooah S, Collins C, Wright A, Dhillon B. Late-onset Retinal Macular Degeneration: Clinical Insights Into an Inherited Retinal Degeneration. The British journal of ophthalmology 2009;93(3). [DOI] [PubMed] [Google Scholar]
- 6.Jacobson S, Cideciyan A, Wright E, Wright AF. Phenotypic marker for early disease detection in dominant late-onset retinal degeneration. Investigative ophthalmology & visual science 2001;42:1882–90. [PubMed] [Google Scholar]
- 7.Delori FC, Dorey CK, Staurenghi G, et al. In Vivo Fluorescence of the Ocular Fundus Exhibits Retinal Pigment Epithelium Lipofuscin Characteristics. Investigative Ophthalmology & Visual Science 1995;36(3). [PubMed] [Google Scholar]
- 8.Cukras C, Flamendorf J, Wong WT, et al. Longitudinal Structural Changes in Late-Onset Retinal Degeneration. Retina 2016;36(12):2348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Silva T, Hotaling N, Chew E, Cukras C. Feature-based retinal image registration for longitudinal analysis of patients with age-related macular degeneration. Medical Imaging 2020: Image Processing: International Society for Optics and Photonics, 2020; v. 11313. [Google Scholar]
- 10.Chavali VR, Khan NW, Cukras CA, et al. A CTRP5 gene S163R mutation knock-in mouse model for late-onset retinal degeneration. Hum Mol Genet 2011;20(10):2000–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lois N, Owens SL, Coco R, et al. Fundus Autofluorescence in Patients With Age-Related Macular Degeneration and High Risk of Visual Loss. Am J Ophthalmol 2002;133(3). [DOI] [PubMed] [Google Scholar]
- 12.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52(9):5009–15. [DOI] [PubMed] [Google Scholar]
- 13.Ooto S, Ellabban AA, Ueda-Arakawa N, et al. Reduction of Retinal Sensitivity in Eyes With Reticular Pseudodrusen. American Journal of Ophthalmology 2013;156(6):1184–91.e2. [DOI] [PubMed] [Google Scholar]
- 14.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn Reson Imaging 2012;30(9):1323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abràmoff MD, Garvin MK, Sonka M. Retinal Imaging and Image Analysis. IEEE Reviews in Biomedical Engineering 2010;3:169–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garvin MK, Abramoff MD, Wu X, et al. Automated 3-D Intraretinal Layer Segmentation of Macular Spectral-Domain Optical Coherence Tomography Images. IEEE Transactions on Medical Imaging 2009;28(9):1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang L, Xiaodong W, Chen DZ, Sonka M. Optimal Surface Segmentation in Volumetric Images-A Graph-Theoretic Approach. IEEE Transactions on Pattern Analysis and Machine Intelligence 2006;28(1):119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai CX, Locke KG, Ramachandran R, et al. A comparison of progressive loss of the ellipsoid zone (EZ) band in autosomal dominant and x-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci 2014;55(11):7417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birch DG, Locke KG, Wen Y, et al. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol 2013;131(9):1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 2017;77(21):e104–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina 2011;31(8):1609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paavo M, Lee W, Merriam J, et al. Intraretinal Correlates of Reticular Pseudodrusen Revealed by Autofluorescence and En Face OCT. Invest Ophthalmol Vis Sci 2017;58(11):4769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garvin MK, Abramoff MD, Wu X, et al. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. IEEE Trans Med Imaging 2009;28(9):1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domalpally A, Danis R, Agrón E, et al. Evaluation of Geographic Atrophy From Color Photographs and Fundus Autofluorescence Images: Age-Related Eye Disease Study 2 Report Number 11. Ophthalmology 2016;123(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caire J, Recalde S, Velazquez-Villoria A, et al. Growth of Geographic Atrophy on Fundus Autofluorescence and Polymorphisms of CFH, CFB, C3, FHR1–3, and ARMS2 in Age-Related Macular Degeneration. JAMA Ophthalmol 2014;132(5). [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Cruickshanks KJ, Nash SD, et al. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol 2010;128(6):750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.