Abstract
Purpose
We investigated the association of effector memory (EM) CD8+ T cell and CD4+ T cell immunity with metabolic syndrome (MS).
Methods
Surface and intracellular staining of peripheral blood mononuclear cells was performed. Anti-interleukin-7 receptor-alpha (IL-7Rα) and CX3CR1 antibodies were used to stain the subsets of EM CD8+ T cells, while anti-interferon-gamma (IFN-γ), interleukin-17 (IL-17), and forkhead box P3 (FOXP3) antibodies were used for CD4+ T cell subsets.
Results
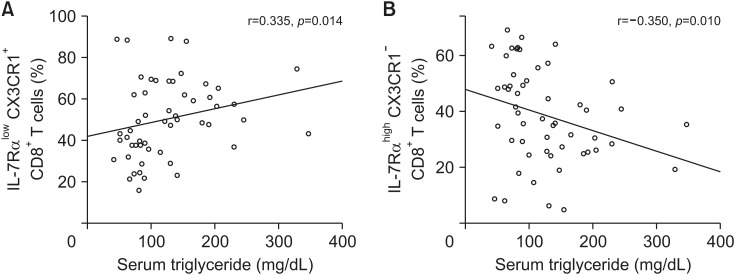
Of the 47 obese children, 11 were female. Children with MS had significantly higher levels of serum insulin (34.8±13.8 vs. 16.4±6.3 μU/mL, p<0.001) and homeostasis model assessment of insulin resistance (8.9±4.1 vs. 3.9±1.5, p<0.001) than children without MS. Children with MS revealed significantly higher frequencies of IL-7Rαlow CD8+ T cells (60.1 ±19.1% vs. 48.4±11.5%, p=0.047) and IL-7RαlowCX3CR1+ CD8+ T cells (53.8±20.1% vs. 41.5 ±11.9%, p=0.036) than children without MS. As the serum triglyceride levels increased, the frequency of IL-7RαlowCX3CR1+ and IL-7RαhighCX3CR1– CD8+ T cells increased and decreased, respectively (r=0.335, p=0.014 and r=−0.350, p=0.010, respectively), in 47 children. However, no CD4+ T cell subset parameters were significantly different between children with and without MS.
Conclusion
In obese children with MS, the changes in immunity due to changes in EM CD8+ T cells might be related to the morbidity of obesity.
Keywords: Child, Obesity, Metabolic syndrome, Hypertriglyceridemia, CD4, CD8, T-Lymphocytes
INTRODUCTION
According to global health observatory data from the World Health Organization, 18% of children and adolescents aged 5–19 years were overweight and obese in 2016 [1]. Among school-age children aged 7–18 years in South Korea, the prevalence of obesity has increased gradually from 8.4% in 2008 to 14.4% in 2018 [2]. According to a systematic review of the worldwide literature, the median prevalence of metabolic syndrome (MS) in children was 3.3% in the whole population, 11.9% in overweight children, and 29.2% in obese children [3].
There are many criteria for defining MS in children [4]. To define childhood MS, the criteria, which include obesity, central obesity, hypertension, elevated glucose levels, hypertriglyceridemia, and decreased high-density lipoprotein (HDL)-cholesterol levels in children, established by the International Diabetes Federation (IDF), are commonly used [5].
T cell immunity, a type of adaptive immunity, has been recently postulated to be related to changes in immunity in obesity and the pathogenesis of obesity-related comorbidities, especially diabetes mellitus (DM) type 2 [6,7,8]. However, there are no reports on the changes in adaptive immunity in obese children. Thus, in this study we aimed to investigate the differences in T cell immunity between children with and without MS.
MATERIALS AND METHODS
Human subjects
From January 2016 to December 2018, a total of 89 obese children who were managed at the obesity clinic for children and adolescents at Jeju National University Hospital were enrolled in the study. Children with underlying diseases and/or under 10 years of age were excluded from the study. Eventually, clinical and laboratory data were collected from 47 obese children.
The IDF criteria were applied to identify MS in the included children [5]. According to the IDF criteria, childhood MS was defined as central obesity (waist circumflex greater than the 90th percentile) with at least two of the following four criteria: 1) fasting glucose level >100 mg/dL, 2) triglyceride level ≥150 mg/dL, 3) HDL-cholesterol level ≤40 mg/dL, and 4) systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg. Hypertension was defined as a systolic or diastolic blood pressure greater than the 95th percentile for sex and age.
Flow cytometric analysis
To evaluate CD4+ and CD8+ T cell immunity in obese children, whole blood samples were collected in heparinized tubes. Peripheral blood mononuclear cells (PBMCs) were extracted from the whole blood samples using Ficol-Paque Premium (GE Healthcare, Chicago, IL, USA) gradients and stained with fluorescent antibodies against surface and/or intracellular markers. For the staining of surface and intracellular CD4+ T cell subset markers, the PBMCs were stimulated by a combined cocktail of phorbol myristate acetate (50 ng/mL; Sigma-Aldrich, St. Louis, MO, USA), ionomycin (1 μg/mL; Sigma-Aldrich), and GolgiPlug (BD Biosciences, San Jose, CA, USA) for 4 hours. Control PBMCs were incubated with phosphate-buffered saline and GolgiPlug (BD Biosciences) for 4 hours. Anti-APC-Cy7-CD3 and anti-Alexa Fluor 700-CD4 (BD Biosciences) antibodies were used for surface staining of the stimulated cells. The fixation and permeabilization of cells were performed using forkhead box P3 (FOXP3) Fix/Perm Buffer (BioLegend, San Diego, CA, USA). For the intracellular staining of CD4+ cell subsets, cells were incubated for 30 minutes in the dark with a cocktail of anti-PE-FOXP3 (BioLegend), anti-Alexa Fluor 488-IL-17A (eBioscience, San Diego, CA, USA), and anti-PE-Cy7-IFN-r (BD Biosciences) antibodies. For surface staining of CD8+ T cell subsets, the PBMCs were stained with antibodies against APC-Cy7-CD3, Pacific Blue-CD8, PE-Cy-7-CCR7, PE-Cy5-CD45RA (BD Biosciences), FITC-interleukin-7 receptor-alpha (IL-7Rα) (R&D Systems, Minneapolis, MN, USA), and PE-CX3CR1 (BioLegend). Finally, the cells were assayed using an LSRFortessa® flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analyses
SPSS version 24.0 (IBM Co., Armonk, NY, USA) was used for the statistical analyses. The results are presented as the mean±standard deviation (SD) for each group. The two groups were statistically compared using the Mann–Whitney U-test, Fisher's exact test, and Spearman's correlation analysis. Statistical significance was set at p<0.05.
Ethics statement
This study was approved by the Institutional Review Board of the Jeju National University Hospital (JNUH 2014-07-005). Informed consent was obtained from all children and their parents.
RESULTS
Clinical and laboratory characteristics
Of the 47 children, 11 were female. The mean age (mean±SD) was 12.9±2.1 years for 15 children without MS and 14.0±2.4 years for 32 children with MS (p=0.119). The group with MS had a significantly higher body mass index (BMI) (31.4±4.4 vs. 27.9±5.2, p=0.032), BMI Z-score (3.2±1.0 vs. 2.4±1.2, p=0.026), hypertension percentage (63.3% vs. 0%, p<0.001), insulin levels (34.8±13.8 μU/mL vs. 16.4±6.3 μU/mL, p<0.001), homeostasis model assessment of insulin resistance (8.9±4.1 vs. 3.9±1.5, p<0.001), and triglyceride levels (142.4±71.2 mg/dL vs. 88.8±40.2 mg/dL, p=0.002) than the group without MS (Table 1). Between the two groups, there were no significant differences in the aspartate aminotransferase, alanine aminotransferase, glucose, hemoglobin A1c, total cholesterol, HDL-cholesterol, and low-density lipoprotein-cholesterol values.
Table 1. Demographic and clinical characteristics of 47 obese children with or without metabolic syndrome based on the criteria established by the International Diabetes Federation.
| Variable | Metabolic syndrome | ||
|---|---|---|---|
| Negative (n=15) | Positive (n=32) | p-value* | |
| Sex (n=47), male/female | 13/2 | 23/9 | |
| Age (yr) | 12.9±2.1 | 14.0±2.4 | 0.119 |
| BMI (kg/m2) | 27.9±5.2 | 31.4±4.4 | 0.032 |
| BMI Z-score | 2.4±1.2 | 3.2±1.0 | 0.026 |
| Hypertension (%)† | 0 | 63.3 | <0.001 |
| AST (IU/L) | 42.4±17.4 | 65.4±64.8 | 0.185 |
| ALT (IU/L) | 76.9±45.7 | 129.1±137.5 | 0.161 |
| Glucose (mg/dL) | 94.5±6.2 | 103.4±32.0 | 0.136 |
| Insulin (μU/mL) | 16.4±6.3 | 34.8±13.8 | <0.001 |
| HOMA-IR | 3.9±1.5 | 8.9±4.1 | <0.001 |
| HbA1c (%) | 5.3±0.2 | 5.8±0.9 | 0.019 |
| Total cholesterol (mg/dL) | 173.1±12.4 | 178.5±35.2 | 0.565 |
| Triglyceride (mg/dL) | 88.8±40.2 | 142.4±71.2 | 0.002 |
| HDL-cholesterol (mg/dL) | 47.1±5.9 | 43.5±9.0 | 0.116 |
| LDL-cholesterol (mg/dL) | 107.8±12.6 | 113.5±31.6 | 0.509 |
Values are presented as number only or mean±standard deviation.
Metabolic syndrome was defined according to criteria established by the International Diabetes Federation [5].
BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, HOMA-IR: homeostasis model assessment of insulin resistance, HbA1c: hemoglobin A1c, HDL: high-density lipoprotein, LDL: low-density lipoprotein.
*Mann–Whitney U-test was used for all parameters except hypertension. †Fisher's exact test.
Surface and/or intracellular staining for CD8+ and CD4+ T cell subsets
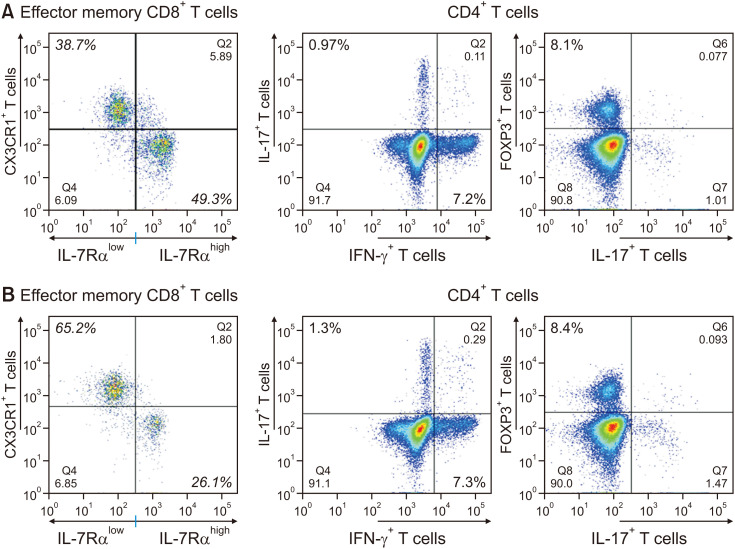
Children with MS revealed significantly higher frequencies of IL-7Rαlow CD8+ T cells (60.1±19.1% vs. 48.4±11.5%, p=0.047) and IL-7RαlowCX3CR1+ CD8+ T cells (53.8±20.1% vs. 41.5±11.9%, p=0.036) than children without MS (Table 2, Fig. 1). Children with MS demonstrated a significantly lower frequency of IL-7RαhighCX3CR1– effector memory (EM) CD8+ T cells (35.3±17.8% vs. 46.5±12.3%, p=0.028) than children without MS (Table 2, Fig. 1). As the serum triglyceride levels increased, the frequency of IL-7RαlowCX3CR1+ CD8+ T cells and IL-7RαhighCX3CR1− CD8+ T cells increased and decreased, respectively (r=0.335, p=0.014 and r=−0.350, p=0.010, respectively), in 47 children (Fig. 2). However, the CD4+ T cell subset parameter was not significantly different between children with and without MS (Table 3, Fig. 1).
Table 2. Frequency of CD8+ T lymphocytes in obese children with or without metabolic syndrome.
| EM CD8+ T cell (n=47) | Metabolic syndrome | ||
|---|---|---|---|
| Negative (n=15) | Positive (n=32) | p-value* | |
| IL-7Rαlow | 48.4±11.5% | 60.1±19.1% | 0.047 |
| IL-7Rαlow CX3CR1+ | 41.5±11.9% | 53.8±20.1% | 0.036 |
| IL-7Rαhigh CX3CR1− | 46.5±12.3% | 35.3±17.8% | 0.028 |
Values are presented as mean±standard deviation.
CD8+ T cells are cytotoxic T cells that include IL-7Rαlow/CX3CR1+ and IL-7Rαhigh/CX3CR1– effector memory (EM) T cells.
IL-7Rα: interleukin-7 receptor-alpha.
*Mann–Whitney U-test.
Fig. 1. Frequency of CD4+ T cells (helper T cells) and effector memory CD8+ T cells (cytotoxic T cells) in two obese children without (A) or with (B) metabolic syndrome. As shown in Tables 2 and 3, the frequency of IL-7Rαlow CX3CR1+ CD8+ T cells and IL-7Rαhigh CX3CR1– CD8+ T cells in obese children with metabolic syndrome was significantly higher and lower, respectively (p=0.036 and p=0.028), than those in obese children without metabolic syndrome. The parameters related to CD4+ T cells did not show a significant difference between the two groups.
IL-7Rα: interleukin-7 receptor-alpha, IFN-γ: interferon-gamma, IL-17: interleukin-17, FOXP3: forkhead box P3.
Fig. 2. Correlation between the levels of serum triglyceride and the frequency of (A) IL-7Rαlow CX3CR1+ CD8+ T cells or (B) IL-7RαhighCX3CR1– CD8+ T cells. As the serum triglyceride level increased, the frequency of IL-7RαlowCX3CR1+ CD8+ T cells and IL-7RαhighCX3CR1– CD8+ T cells increased and decreased, respectively (r=0.335, p=0.014 and r=–0.350, p=0.010, Spearman's correlation analysis).
IL-7Rα: interleukin-7 receptor-alpha.
Table 3. Frequency of CD4+ T lymphocytes in obese children with or without metabolic syndrome.
| CD4+ T cell (n=32) | Metabolic syndrome | ||
|---|---|---|---|
| Negative (n=7) | Positive (n=25) | p-value* | |
| IFN-γ+ | 8.9±7.1% | 10.1±4.7% | 0.635 |
| IL-17+ | 1.4±1.1% | 1.8±1.1% | 0.338 |
| FOXP3+ | 8.7±2.7% | 7.7±2.5% | 0.410 |
Values are presented as mean±standard deviation.
The subsets of CD4+ T cells include IFN-γ+ CD4+ T cells (Th1 cells), IL-17+CD4+ T cells (Th17 cells), and FOXP3+ regulatory T cells.
IFN-γ: interferon-gamma, IL-17: interleukin-17, FOXP3: forkhead box P3.
*Mann–Whitney U-test.
DISCUSSION
Some studies have reported a connection between adaptive immunity and insulin resistance such as in type 2 DM in animal models and humans [9]. Several studies employing animal models of type 2 DM induced by a high-fat diet have shown that CD4+ and CD8+ T cells might affect phenotypic changes in innate immunity such as M2 macrophage dominance and M1 macrophage infiltration into adipose tissues or visceral fat depots [10,11,12]. M1 and M2 macrophages have proinflammatory and anti-inflammatory effects, respectively. However, Sultan et al. [13] have reported that inflammation of adipose tissue mediated by T cells did not cause insulin resistance.
MS is greatly associated with insulin resistance. Based on studies reporting the relationship between adaptive immunity and insulin resistance, we hypothesized that there might be significant differences in CD8+ and CD4+ T cell immunity in the blood between children with and without MS. The CD8+ T cell subsets included IL-7Rαlow EM CD8+ T cells. CD8+ T cells in human peripheral blood are expressed as two subsets, IL-7Rαhigh and IL-7Rαlow, which have different responses to IL-7 for cell survival [14]. DNA methylation plays a major role in controlling IL-7Rα expression in T cells, which is mediated by the promoter activity of the IL-7Rα gene [15]. IL-7Rαlow EM CD8+ T cells increase CX3CR1 expression on CD8+ T cell membranes for binding to CX3C-chemokine ligand 1 (CX3CL1), a CXC3CR1 ligand fractalkine on endothelial cells near inflamed tissues [16]. CX3CR1 is a receptor for CX3CL1, which is known to function as an adhesive and chemoattractant molecule [17]. The present study demonstrated a significantly higher frequency of IL-7Rαlow and IL-7RαlowCX3CR1+ EM CD8+ T cells in children with MS than in those without MS (Table 2, Fig. 1).
As the serum triglyceride level increased, the frequency of IL-7RαlowCX3CR1+ and IL-7RαhighCX3CR1− CD8+ T cells increased and decreased, respectively. Serum triglyceride levels were positively correlated with the frequency of IL-7RαlowCX3CR1+ CD8+ T cells (Fig. 2). Hypertriglyceridemia is highly associated with metabolic abnormalities such as insulin resistance, nonalcoholic fatty liver disease, and advanced bone age in obese children [18,19]. MS can be associated with inflammatory status. Unfortunately, we did not evaluate serum inflammatory markers such as C-reactive protein or erythrocyte sedimentation rate. Thus, the differences in serum inflammatory markers between the two groups could not be assessed.
CD4+ T cell subsets include proinflammatory IFN-γ+CD4+ (Th1 cells) and IL-17+CD4+ T cells (Th17 cells) and anti-inflammatory FOXP3+ regulatory T cells. No CD4+ T cell subset parameters were significantly different between children with and without MS (Table 3). Surendar et al. [20] reported that blood concentrations of both TH1-produced cytokines (IL-2, IL-12, and IFN-γ) and TH2-secreted cytokines (IL-4, IL-5, and IL-13) were higher in the sera of patients with MS than in those without MS.
This study has some limitations. First, cytokine analysis could not be performed for all obese children because all sera samples were inadvertently thawed following freezing due to an accidental shutdown of electric current to the deep freezer. Second, we did not analyze the tissues of obese children. Collecting tissues from children is difficult because of ethical issues.
In conclusion, obese children with MS showed a change in immunity due to changes in EM CD8+ T cells, which may be related to the morbidity of obesity. Serum triglyceride levels were positively correlated with the frequency of IL-7RαlowCX3CR1+ CD8+ T cells. Such correlation may also play a role in the pathogenesis of obesity morbidities, which are associated with hypertriglyceridemia.
Footnotes
Funding: This research was supported by research grants from Jeju National University Hospital (JNUH-16-03) in 2016.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.WHO global health observatory data. Prevalence of obesity among children and adolescents [Internet] Geneva: WHO; 2020. [cited 2020 Aug 4]. Available from: https://www.who.int/data/gho/data/themes/theme-details/GHO/body-mass-index-(bmi) [Google Scholar]
- 2.Kang KS. Nutritional counseling for obese children with obesity-related metabolic abnormalities in Korea. Pediatr Gastroenterol Hepatol Nutr. 2017;20:71–78. doi: 10.5223/pghn.2017.20.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friend A, Craig L, Turner S. The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab Syndr Relat Disord. 2013;11:71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- 4.Wang HH, Lee DK, Liu M, Portincasa P, Wang DQ. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr. 2020;23:189–230. doi: 10.5223/pghn.2020.23.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 6.Guzmán-Flores JM, Ramírez-Emiliano J, Pérez-Vázquez V, López-Briones S. Th17 and regulatory T cells in patients with different time of progression of type 2 diabetes mellitus. Cent Eur J Immunol. 2020;45:29–36. doi: 10.5114/ceji.2020.94670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Chen F, Wang J, Zeng Z, Yang Q, Shao S. Th17 and Treg lymphocytes in obesity and Type 2 diabetic patients. Clin Immunol. 2018;197:77–85. doi: 10.1016/j.clim.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Jung C, Lichtenauer M, Strodthoff D, Winkels H, Wernly B, Bürger C, et al. Alterations in systemic levels of Th1, Th2, and Th17 cytokines in overweight adolescents and obese mice. Pediatr Diabetes. 2017;18:714–721. doi: 10.1111/pedi.12435. [DOI] [PubMed] [Google Scholar]
- 9.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 10.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 13.Sultan A, Strodthoff D, Robertson AK, Paulsson-Berne G, Fauconnier J, Parini P, et al. T cell-mediated inflammation in adipose tissue does not cause insulin resistance in hyperlipidemic mice. Circ Res. 2009;104:961–968. doi: 10.1161/CIRCRESAHA.108.190280. [DOI] [PubMed] [Google Scholar]
- 14.Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7Ralpha expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HR, Hwang KA, Kim KC, Kang I. Down-regulation of IL-7Ralpha expression in human T cells via DNA methylation. J Immunol. 2007;178:5473–5479. doi: 10.4049/jimmunol.178.9.5473. [DOI] [PubMed] [Google Scholar]
- 16.Shin MS, You S, Kang Y, Lee N, Yoo SA, Park K, et al. DNA methylation regulates the differential expression of CX3CR1 on human IL-7Rαlow and IL-7Rαhigh effector memory CD8+ T cells with distinct migratory capacities to the fractalkine. J Immunol. 2015;195:2861–2869. doi: 10.4049/jimmunol.1500877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Jiang D. Fractalkine/CX3CR1 and atherosclerosis. Clin Chim Acta. 2011;412:1180–1186. doi: 10.1016/j.cca.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Oh MS, Kim S, Jang JH, Park JY, Kang HS, Lee MS, et al. Associations among the degree of nonalcoholic fatty liver disease, metabolic syndrome, degree of obesity in children, and parental obesity. Pediatr Gastroenterol Hepatol Nutr. 2016;19:199–206. doi: 10.5223/pghn.2016.19.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh MS, Kim S, Lee J, Lee MS, Kim YJ, Kang KS. Factors associated with advanced bone age in overweight and obese children. Pediatr Gastroenterol Hepatol Nutr. 2020;23:89–97. doi: 10.5223/pghn.2020.23.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surendar J, Mohan V, Rao MM, Babu S, Aravindhan V. Increased levels of both Th1 and Th2 cytokines in subjects with metabolic syndrome (CURES-103) Diabetes Technol Ther. 2011;13:477–482. doi: 10.1089/dia.2010.0178. [DOI] [PubMed] [Google Scholar]