Abstract
Deep brain stimulation (DBS) has emerged as a promising therapy for neuropsychiatric illnesses, including depression and obsessive-compulsive disorder, but has shown inconsistent results in prior clinical trials. We propose a shift away from the empirical paradigm for developing new DBS applications, traditionally based on testing brain targets with conventional stimulation paradigms. Instead, we propose a multimodal approach centered on an individualized intracranial investigation adapted from the epilepsy monitoring experience, which integrates comprehensive behavioral assessment, such as the Research Domain Criteria proposed by the National Institutes of Mental Health. In this paradigm-shifting approach, we combine readouts obtained from neurophysiology, behavioral assessments, and self-report during broad exploration of stimulation parameters and behavioral tasks to inform the selection of ideal DBS parameters. Such an approach not only provides a foundational understanding of dysfunctional circuits underlying symptom domains in neuropsychiatric conditions but also aims to identify generalizable principles that can ultimately enable individualization and optimization of therapy without intracranial monitoring.
Keywords: deep brain stimulation, neuromodulation, depression, stereoelectroencephalography, neuropsychiatry
Surgical neuromodulation is increasingly utilized for refractory neurological and psychiatric disorders, and deep brain stimulation (DBS) has played an important role in this endeavor. Despite the numerous studies using DBS for various conditions,1 there remain only 3 fully approved indications for DBS (essential tremor, Parkinson disease [PD], epilepsy), and 2 indications with limited approval in the form of a Humanitarian Device Exemption (HDE) (dystonia, obsessive-compulsive disorder [OCD]) in the United States.
This discrepancy between the number of investigational applications relative to the few approved indications deserves a reappraisal of historical approaches for DBS therapy development. While some conditions have only few published cases reported,2,3 others have a reference base of hundreds of patients, studied in extensive and costly trials, such as pain4-6 and treatment-resistant depression (TRD).7 These latter examples share a history of initially promising open-label studies, followed by controlled trials failing to meet outcome measures sufficient for obtaining regulatory approval (for a review of this trend in psychiatric neurosurgery, see Bari et al8).
We propose that a key reason for the limited success of previous DBS trials is an insufficient understanding of network physiology underlying each disorder and its response to DBS (which is exacerbated by a lack of mechanistic understanding of DBS). This knowledge gap exists at the population level and is exemplified at the individual patient level partially due to clinical symptom heterogeneity across patients. While factors such as patient selection, trial design, placebo effects, among other biases may contribute to unsuccessful trial outcomes, the limited understanding of the symptomatic network will either lead to failure or produce outcomes difficult to reproduce or generalize.
We propose an alternative approach to developing surgical neuromodulation therapies for novel neurological and psychiatric indications. This approach focuses on deriving a detailed understanding of the involved brain networks using intracranial recording and stimulation in the target population. Intracranial monitoring
is commonly utilized in medication-refractory epilepsy: patients are admitted to an epilepsy monitoring unit (EMU) to identify patient-specific epileptogenic networks. While seizures are inherently robust electrographic markers, validated biomarkers to guide effective individualized therapy for psychiatric disorders still do not exist. One goal of our suggested approach is the development of such biomarkers to enable a better understanding of the brain networks underlying the target disorder and their response to stimulation prior to embarking on large clinical trials. Here, we focus on developing this concept and illustrating it in the context of mental health disorders, using TRD as an exemplar.
CHARACTERIZING BRAIN NETWORDS UNDERLYING DISORDERS OF MENTAL HEALTH
Research in mental illness has undergone a large-scale reorganization in its approach to categorizing disorders. Aimed at resolving impediments to research and therapeutic progress due to patient symptom profile heterogeneity, this new approach uses Research Domain Criteria (RDoC) to formalize and measure behavior and neurobiological indices across domains.9 Utilizing this approach with major depressive disorder (MDD) serves as a useful example. MDD is a major public health concern, affecting at least 4.4% of the global population,10 with low remission rates,11 and demonstrated lack of success in past approaches for treatment optimization.12 Using traditional symptom-based classification, a patient may be diagnosed with MDD by displaying 5 of 9 diagnostic criteria. Thus, 2 patients may carry the same diagnosis despite overlapping in only 1 criterion. In contrast, a transdiagnostic approach focuses on dysfunctional domain systems rather than symptom clusters. For example, patients with OCD and MDD may both experience dysfunction in reward sensitivity, as well as abnormalities of the orbitofrontal cortex, a region involved in processing rewarding stimuli. In MDD, such an approach would aim to understand the dysfunctional networks underlying reward sensitivity, recognizing that individuals may have varying combinations of dysfunction across different domains. Rather than trying to treat “MDD” as a monolithic entity, it may be treated as a combination of relevant symptomatic domains (eg, “negative valence,” “positive valence,” “cognitive control” systems and constructs) using the RDoC framework. Each of these domains may be studied and targeted individually with experimental tasks to identify candidate brain areas and patient-specific biomarkers. Using this concept of orthogonalizing symptoms onto RDoC-style axes may improve individualized network targeting and hopefully produce better treatment outcomes.
INTRACRANIAL RECORDINGS FOR TREATMENT INDIVIDUALIZATION
Initial work seeking neural biomarkers of depression using neuroimaging tools has had some success. Differential network connectivity has been found in individuals with MDD, such as the cognitive control network13,14 and the reward learning network.15,16 Scalp electroencephalography (EEG) has also been conducted in patients with depression with the goal of finding biomarkers and predictors of antidepressant treatment response.17 Resulting putative biomarkers included asymmetric regional changes in frontal alpha18-20 and changes in theta power,21 but they have not been consistent or replicable enough to be integrated in a clinical capacity.20,22 This outcome can be partially attributed to limitations in spatial and temporal resolution from noninvasive tools. Intracranial recordings, however, would enable spatiotemporally precise sampling across putative networks, including deep structures previously inaccessible using scalp EEG. Applied to TRD, precise neurophysiological intracranial monitoring can shed fundamental insight on dysfunctional networks and provide unique opportunities to execute behavioral tasks in relevant RDoC domains to measure network involvement. If the diversity of phenotypes observed in TRD is a manifestation of varying levels of disruption in different networks, the ability to precisely measure from these networks and relate them to observed behaviors and subject report would enable understanding of the neurophysiological basis of depression biotypes,23,24 driving personalized and effective therapy. Data streams such as synchronized audio/video recordings of the patient and physiological responses indicative of autonomic response can be collected simultaneously to provide an extensive, multimodal dataset to build a comprehensive understanding of the brain-behavior relationship.
DBS IN THE MONITORING UNIT
There exists a critical knowledge gap with respect to mechanisms of therapeutic stimulation and biomarkers that can optimize our therapeutic interventions. Little is known about the effects of stimulation parameters on the therapeutic efficacy of DBS for TRD, restricted by an underlying assumption that parameters relevant to DBS for movement disorders can be extrapolated to other psychiatric diseases. Given anatomic and neurophysiological differences in targeted networks, defining dose-response relationships seems imperative to advancing DBS therapies for new indications. The use of intracranial recording and stimulation in the Neurophysiology Monitoring Unit or NMU (broadening on the concept of EMU) can serve as a therapy development platform to address this gap. The value of intracranial recordings for understanding therapeutic mechanisms of DBS is currently exemplified in its application for movement disorders, eg, PD, where such recordings have yielded putative biomarkers and insight on physiological changes induced by DBS.25-27
Here, we focus on stereo-EEG28-30 as the intracranial technique of choice, given its ability to sample subcortical regions implicated in TRD. Performing DBS in the NMU enables assessment of the network-wide impact of stimulation on (pathological) oscillations and allows correlation of neurophysiological changes with behavior. Importantly, one can address whether therapeutic stimulation is mediated by normalization of “aberrant” activity or by modifying activity in other networks to compensate for abnormal activity in parallel circuits. Extended testing in the NMU also enables evaluation of stimulation dose-response relationships (behavioral and neurophysiological) with large parameter sweeps in an automated fashion, overcoming the significant limitations of prior trials largely assuming equivalence of therapeutic mechanisms despite differences in networks and likely key spectral frequencies. This sEEG-based platform enables characterization of behavioral and neural responses to stimulation at other nodes in physiologically defined disease-related networks, thereby providing a pathway to defining new potential targets for DBS. In light of the numerous stimulation parameter combinations and number of contacts available for stimulation, investigators must be cognizant of time limitations and proceed in a hypothesis-driven manner to maximize the value of NMU-based investigations.
Stimulation in the NMU also offers an important venue to validate 2 major tools used to advance the field of DBS: magnetic resonance (MR) tractography and stimulation field models (SFM, also known as volume of tissue activated). MR tractography can estimate structural brain connectivity of disease-related brain networks and of effective vs ineffective stimulation sites. SFMs can estimate spatial reach and influence of neuromodulation,31-33 and DBS in the NMU can investigate the intersection of MR tractography and SFMs, providing both physiologically based and imaging-based biomarkers for therapy. These biomarkers are more apparent and meaningful in the context of having characterized the pathophysiological network basis of disease prior to stimulation, in addition to being important for development of future closed-loop therapies.
IMPLEMENTATION AND VARIATIONS OF THE INTRACRANIAL PLATFORM
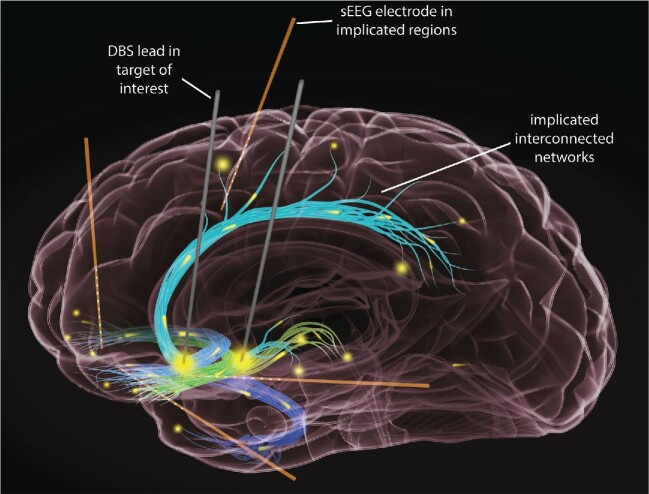
In our vision of “sEEG-informed DBS” (Figure), patients with the target disorder (eg, TRD) meeting criteria for neurosurgery undergo 2 surgical procedures book-ending a stay in the NMU. The first consists of implantation of a therapeutic stimulation system and a separate recording system. The stimulation system consists of DBS leads in historically promising targets. Extensions connected to the other end of the leads are externalized and connected to the research neurophysiology system using standard surgical methods.34-36 The recording system consists of sEEG electrodes placed in brain regions implicated in TRD, based on findings from noninvasive methods23,24 (Figure). In the NMU, the patient undergoes planned testing to build an understanding of the network and its response to stimulation. Following the monitoring period, the patient returns to the odds ratio (OR) for a second procedure for removal of sEEG electrodes and tunneling of DBS leads to an implantable pulse generator.
FIGURE.
Intracranial recordings from sEEG electrodes in brain regions implicated in TRD, such as the dorsomedial, temporal and ventromedial cortex, as well as the orbitofrontal cortex, to name a few. Ideally, recordings from the aforementioned brain regions would be obtained in gray matter structures at the termini of white matter tracts, as we conceive of the DBS targets as white matter targets. The goal of these electrophysiological recordings is to help understand the pathophysiological network dynamics at the individual patient level. This NMU-based platform also allows appreciation of the network response to stimulation across a broad range of parameters. These 2 pieces of information can be combined to optimize DBS parameter selection.
An alternative “staged approach” decouples the stimulation and recording phases, where first only sEEG electrodes are implanted, covering areas of interest for stimulation and recording in the NMU. Following the NMU phase, the patient undergoes removal of sEEG electrodes. After several weeks or months, the patient is implanted with a permanent DBS system in brain targets informed by analysis of acquired data. This approach has been used previously in pediatric dystonia.37 Variations of either approach could also use electrocorticography (EcoG) strips38,39 rather than sEEG, and responsive neurostimulation (RNS)40 rather than DBS.
The sEEG-informed DBS approach and staged approach differ significantly in their strategy for permanent stimulation electrodes placement. An advantage to placement during the initial surgery is delivery of stimulation through the same electrode configuration during chronic, long-term management as was delivered in the NMU, as DBS and sEEG leads have different geometries. A potential advantage to the staged approach is the availability of stimulation testing results in the NMU prior to implantation of permanent DBS leads. This information could lead to novel and highly individualized lead placement but is limited by the extent to which acute stimulation effects predict long-term effects. In movement disorders, such as the dystonia example above, the predictive power may be higher than in psychiatric disorders where this relationship is unclear.
The differences in approaches highlight a major goal of the sEEG-informed DBS approach: identifying generalizable principles and electrophysiological biomarkers to guide future implants without the future use of sEEG. This intracranial platform for therapy development is time- and resource-intensive per patient, more invasive, and therefore less appealing to some patients than typical DBS procedures and unsustainable as a permanent approach. We envision this intracranial platform as a bridge spanning the gap of incomplete network characterization of a disorder, only necessary until enough is known to be able to perform future implants successfully without this intracranial intermediate, eg, intracranial data may correlate well with tractography data for a disorder, such that future implants can be planned and individualized based purely on preoperative imaging. Alternatively, intracranial data may provide electrophysiological biomarkers to enable future closed-loop neurostimulation.
The strategy behind the staged approach is to find patient-specific physiologically informed targets and therapies. It is designed for maximum flexibility, allowing placement of the permanent neurostimulation system almost anywhere in the putative network, at the cost of reduced generalizability. Finding generalizable principles that can later obviate the need for invasive monitoring in each patient with this approach is challenging. The staged approach may therefore be more appropriate for earlier stage and more exploratory, investigations. The sEEG-informed approach may be more appropriate for disorders with more pre-existing evidence, those closer to bridging toward a large, randomized trial.
Finally, the risks in this approach must be considered. sEEG has been used for decades, and recent advances in imaging and stereotactic precision have made this procedure quite safe.41,42 Balanced against this small but nonzero risk is the risk of inaction: neuropsychiatric disorders are the leading cause of disability in the US,43 and concomitant with increased risk of suicide, especially mood disorders. For severe refractory disorders where noninvasive treatment options have been exhausted, intervention with DBS therapy is a promising consideration. However, to leverage the potential of this therapy, more comprehensive evaluation and multifaceted efforts are needed,44-46 as trials of DBS for neuropsychiatric disorders, such as TRD have not produced consistent results,7,47,48 yielding little new information about the disorder or treatment approaches.8 We must avoid the temptation of quickly taking results from a small number of uncontrolled studies into expensive randomized trials with all our proverbial eggs in the basket of a single brain target and set of stimulation parameters. Our overall neuroscientific understanding of neuropsychiatric disease and signal analyses has significantly improved, providing a better foundation and therefore greater likelihood that using these invasive techniques will advance our understanding of the underlying mechanisms of disease and therapy. Smaller studies utilizing an intracranial platform may help us derive a more complete understanding of the disorder, its variability across patients, and strategies for delivering stimulation to treat it. We hope that judicious use of this approach will usher in an era of a scientific, reasoned approach to surgical modulation for severe neurological and psychiatric disorders.
Funding
This work was supported by the National Institutes of Health award UH3 NS103549 (S.S., N.P., W.G., D.B., J.C., K.B.), the McNair Foundation (S.S.), the Dana Foundation (S.S.), and the National Science Foundation Graduate Research Fellowship Program (A.A.).
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Bijanki has funding from NIH K01-MH116364 and R21-NS104953. Dr Viswanathan has received funds for teaching engagements and consulting services from Boston Scientific Corporation and Abbott Laboratories. Dr Sheth is a consultant for Boston Scientific, Zimmer Biomet, Neuropace, and Abbott. Dr Goodman receives research support from NIH, McNair Medical Foundation, the IOCDF, and Biohaven Pharmaceuticals; he is a consultant to Biohaven; research devices for Dr Goodman's NIH-funded study were donated by Medtronic.
Contributor Information
Anusha Allawala, School of Engineering, Brown University, Providence, Rhode Island, USA.
Kelly R Bijanki, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA.
Wayne Goodman, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, Texas, USA.
Jeffrey F Cohn, Department of Psychology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Ashwin Viswanathan, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA.
Daniel Yoshor, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA; Department of Neurosurgery, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
David A Borton, School of Engineering, Brown University, Providence, Rhode Island, USA; Carney Institute for Brain Science, Brown University, Providence, Rhode Island, USA; Department of Veterans Affairs, Providence VA Medical Center for Neurorestoration and Neurotechnology, Providence, Rhode Island, USA.
Nader Pouratian, Department of Neurological Surgery, UT Southwestern Medical Center, Dallas, Texas, USA.
Sameer A Sheth, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA.
COMMENT
The authors of this concept paper suggest a different paradigm in determination of deep brain stimulation (DBS) targets and stimulation parameters that is based on physiological information from the patient's brain – which does indeed make perfect sense taking into consideration individual variability of brain connectivity and significant difference in clinical presentation (and therefore underlying dysfunction) of common psychiatric conditions.
Although the exact details are somewhat novel in nature, the actual concept reminds me of the original experience of pioneers in surgical management of psychiatric conditions from the 1950s, 1960s, and 1970s when implantation of long-term recording electrodes was used as both a research tool and a guide for choosing individualized lesioning targets.
I strongly support the authors’ desire to be more scientific in choice of surgical intervention for medically refractory psychiatric conditions and share their optimism about eventual replacement of invasive diagnostic interventions with imaging and electrophysiological surrogates.
Konstantin Slavin
Chicago, Illinois, USA
REFERENCES
- 1. Youngerman BE, Chan AK, Mikell CB, McKhann GM, Sheth SA.. A decade of emerging indications: deep brain stimulation in the United States. J Neurosurg. 2016;125(2):461-471. [DOI] [PubMed] [Google Scholar]
- 2. Reese R, Gruber D, Schoenecker Tet al. Long-term clinical outcome in Meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord. 2011;26(4):691-698. [DOI] [PubMed] [Google Scholar]
- 3. Ostrem JL, Marks WJ Jr, Volz MM, Heath SL, Starr PA.. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome). Mov Disord. 2007;22(13):1885-1891. [DOI] [PubMed] [Google Scholar]
- 4. Boccard SGJ, Prangnell SJ, Pycroft Let al. Long-term results of deep brain stimulation of the anterior cingulate cortex for neuropathic pain. World Neurosurg. 2017;106:625-637. [DOI] [PubMed] [Google Scholar]
- 5. Lempka SF, Malone DA Jr, Hu Bet al. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol. 2017;81(5):653-663. [DOI] [PubMed] [Google Scholar]
- 6. Keifer OP Jr, Riley JP, Boulis NM.. Deep brain stimulation for chronic pain: intracranial targets, clinical outcomes, and trial design considerations. Neurosurg Clin N Am. 2014;25(4):671-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dougherty DD, Rezai AR, Carpenter LLet al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248. [DOI] [PubMed] [Google Scholar]
- 8. Bari AA, Mikell CB, Abosch Aet al. Charting the road forward in psychiatric neurosurgery: proceedings of the 2016 American society for stereotactic and functional neurosurgery workshop on neuromodulation for psychiatric disorders. J Neurol Neurosurg Psychiatry. 2018;89(8):886-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Insel T, Cuthbert B, Garvey Met al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. [DOI] [PubMed] [Google Scholar]
- 10. WHO . Depression and other common mental disorders [published online ahead of print: February 23, 2017]. https://www.who.int/mental_health/management/depression/prevalence_global_health_estimates/en/. Accessed April 7, 2020. [Google Scholar]
- 11. Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ.. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445. [DOI] [PubMed] [Google Scholar]
- 12. Rush AJ, Trivedi MH, Wisniewski SRet al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. [DOI] [PubMed] [Google Scholar]
- 13. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA.. Large-Scale network dysfunction in major depressive disorder: a Meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hwang JW, Egorova N, Yang XQet al. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl Psychiatry. 2015;5(11):e683-e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pujara M, Koenigs M.. Mechanisms of reward circuit dysfunction in psychiatric illness: prefrontal-striatal interactions. Neuroscientist. 2014;20(1):82-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ng TH, Alloy LB, Smith DV.. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry. 2019;9(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLoughlin G, Makeig S, Tsuang MT.. In search of biomarkers in psychiatry: EEG-based measures of brain function. Am J Med Genet. 2014;165(2):111-121. [DOI] [PubMed] [Google Scholar]
- 18. Tenke CE, Kayser J, Manna CGet al. Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biol Psychiatry. 2011;70(4):388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Webb CA, Dillon DG, Pechtel Pet al. Neural correlates of three promising endophenotypes of depression: evidence from the EMBARC study. Neuropsychopharmacology. 2016;41(2):454-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wade EC, Iosifescu DV.. Using electroencephalography for treatment guidance in major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(5):411-422. [DOI] [PubMed] [Google Scholar]
- 21. Broadway JM, Holtzheimer PE, Hilimire MRet al. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology. 2012;37(7):1764-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Widge AS, Taha Bilge M, Montana Ret al. Electroencephalographic biomarkers for treatment response prediction in major depressive illness: a meta-analysis. Am J Psychiatry. 2019;176(1):44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3(5):472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drysdale AT, Grosenick L, Downar Jet al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ince NF, Gupte A, Wichmann Tet al. Selection of optimal programming contacts based on local field potential recordings from subthalamic nucleus in patients with Parkinson's disease. Neurosurgery. 2010;67(2):390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swann NC, de Hemptinne C, Thompson MCet al. Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing. J Neural Eng. 2018;15(4):046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Hemptinne C, Swann NC, Ostrem JLet al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci. 2015;18(5):779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alomar S, Jones J, Maldonado A, Gonzalez-Martinez J.. The stereo-electroencephalography methodology. Neurosurg Clin N Am. 2016;27(1):83-95. [DOI] [PubMed] [Google Scholar]
- 29. Bancaud J, Angelergues R, Bernouilli Cet al. Functional stereotaxic exploration (SEEG) of epilepsy. Electroencephalogr Clin Neurophysiol. 1970;28(1):85-86. https://www.ncbi.nlm.nih.gov/pubmed/4188481. [PubMed] [Google Scholar]
- 30. Chauvel P, Vignal J, Biraben A, Badier J, Scarabin J. Stereoelectroencephalography. In Pawlik G, Stefan H, eds. Multimethodological Assessment of the Epileptic Forms. New York: Springer Verlag. 1996:80-108. [Google Scholar]
- 31. Chaturvedi A, Foutz TJ, McIntyre CC.. Current steering to activate targeted neural pathways during deep brain stimulation of the subthalamic region. Brain Stimul. 2012;5(3):369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butson CR, Cooper SE, Henderson JM, McIntyre CC.. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34(2):661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mädler B, Coenen VA.. Explaining clinical effects of deep brain stimulation through simplified target-specific modeling of the volume of activated tissue. AJNR Am J Neuroradiol. 2012;33(6):1072-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aman JE, Johnson LA, Sanabria DEet al. Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson's disease patients. Neurobiol Dis. 2020;139:104819. doi:10.1016/j.nbd.2020.104819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupte AA, Shrivastava D, Spaniol MA, Abosch A.. MRI-related heating near deep brain stimulation electrodes: more data are needed. Stereotact Funct Neurosurg. 2011;89(3):131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Little S, Tripoliti E, Beudel Met al. Adaptive deep brain stimulation for Parkinson's disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry. 2016;87(12):1388-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanger TD, Liker M, Arguelles Eet al. Pediatric deep brain stimulation using awake recording and stimulation for target selection in an inpatient neuromodulation monitoring unit. Brain Sci. 2018;8(7):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang EF. Towards large-scale, human-based, mesoscopic neurotechnologies. Neuron. 2015;86(1):68-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Penfield W. The epilepsies: with a note on radical therapy. N Engl J Med. 1939;221(6):209-218. [Google Scholar]
- 40. Morrell MJ, RNS System in Epilepsy Study Group . Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304. [DOI] [PubMed] [Google Scholar]
- 41. Tandon N, Tong BA, Friedman ERet al. Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol. 2019;76(6):672-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGovern RA, Ruggieri P, Bulacio J, Najm I, Bingaman WE, Gonzalez-Martinez JA.. Risk analysis of hemorrhage in stereo-electroencephalography procedures. Epilepsia. 2019;60(3):571-580. [DOI] [PubMed] [Google Scholar]
- 43. Murray CJL, Atkinson C, Bhalla Ket al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hendriks S, Grady C, Ramos KMet al. Ethical challenges of risk, informed consent, and posttrial responsibilities in human research with neural devices: a review [published online ahead of print: October 17, 2019]. JAMA Neurol. doi:10.1001/jamaneurol.2019.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nuttin B, Wu H, Mayberg H, Hariz M.. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol. 2014;85(9):1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rabins P, Appleby BS, Brandt J.. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Arch Gen Psychiatry. 2009;66(9):931-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Widge AS, Malone DA Jr, Dougherty DD.. Closing the loop on deep brain stimulation for treatment-resistant depression. Front Neurosci. 2018;12:175. doi:10.3389/fnins.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holtzheimer PE, Husain MM, Lisanby SHet al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849. [DOI] [PubMed] [Google Scholar]