Abstract
Diabetes mellitus is a metabolic disease that predominates, nowadays. It causes hyperglycemia and consequently major health complications. Type II diabetes is the most common form and is a result of insulin resistance in the target tissues. To treat this disease, several mechanisms have been proposed. The most direct route is via inhibiting the intestinal enzymes, e.g., α-glucosidase and α-amylase, responsible for intestinal polysaccharide digestion that therefore would reduce the absorption of monosugars through the intestinal walls. In this study, we shed the light on this route by testing the inhibitory effect of Ocimum basilicum extract on the enzymes α-glucosidase and α-amylase in vitro and in silico. Experimental procedures were performed to test the effect of the O. basilicum methanol extract from aerial parts followed by the in silico docking. 500 μg/mL of the extract led to 70.2% ± 8.6 and 25.4% ± 3.3 inhibition on α-glucosidase and α-amylase activity, respectively. Similarly, the effect of caffeic acid, a major extract ingredient, was also tested, and it caused 42.7% ± 3.0 and 47.1% ± 4.0 inhibition for α-amylase and α-glucosidase, respectively. Docking experiments were performed to predict the phytochemicals responsible for this robust inhibitory activity in the O. basilicum extracts. Several compounds have shown variable levels of inhibition, e.g., caffeic acid, pyroglutamic acid, and uvasol. The results indicated that O. basilicum can be a potent antidiabetic drug.
1. Introduction
Diabetes mellitus (DM) is a metabolic disorder. It results from the resistance to insulin or the reduced secretion of insulin. A major consequence of this disorder is the distorted carbohydrate, fat, and protein metabolism and the increased levels of serum glucose. This would result in hyperglycemia and elevated plasma LDL [1], which would cause damage in blood vessels and consequently microvascular and macrovascular disorders, including atherosclerosis, retinopathy, and nephropathy. Weight loss, polyphagia, blurred vision, polyuria, and polydipsia are additional complications [2]. These complications are the major causes of mortality in patients with diabetes [3]. Two major forms of diabetes predominate. In type I diabetes, which has a low prevalence, the pancreatic β-cells are labeled for destruction by the immune system, and insulin levels are distorted [4, 5]. The more common form, type II diabetes mellitus (T2DM), mainly results from insulin resistance in the target tissues [6]. A major consequence of this insulin disorder is an imbalance in carbohydrate, fat, and protein metabolism. Fasting plasma glucose levels, the postprandial levels of plasma glucose, and hemoglobin A1C levels are elevated to ≥7 mM, ≥11 mM, and 6.5%, respectively [7]. Prolonged hyperglycemia leads to the destruction of blood vessels and destruction in the heart, eyes, kidneys, and nervous system [3].
To treat type I DM, several routes can be followed. These include insulin replacement therapy and the transplantation of pancreatic islets [8]. Type II DM can be treated with drugs that act via different modes of actions. Some drugs target hepatic enzymes involved in gluconeogenesis and glycogenolysis for inhibition. Other drugs induce an increased production and secretion of insulin in the β-pancreatic cells. Even more, some drugs act on enzymes of skeletal muscles and adipose tissues and stimulate the two organs to increase blood glucose uptake [9, 10]. Another route of action is via drugs that target the inhibition of lipolysis, where imbalanced lipolysis is known to induce the reduced secretion of insulin and consequently hyperglycemia. Furthermore, abnormal lipolysis can result in lipotoxicity, with the accumulation of toxic lipid metabolites (ceramide, diacylglycerol, and fatty acyl CoA) in the liver, muscle, adipose tissues, and pancreas [11]. Drugs that target this side effect can relieve the injury and reduce the inflammation [12]. Anti-inflammatory drugs can treat the complications of diabetes, including the inflammatory responses [13]. The route for glycemic control under a microscope in this work introduces the inhibition of intestinal enzymes responsible for intestinal polysaccharide digestion and consequently the absorption of absorbable sugar monomers through the intestinal walls [14]. Examples include the mammalian α-glucosidase and α-amylase competitive inhibitors [15]. Digestive enzymes in the small intestine hydrolyze complex polysaccharides and disaccharides into smaller fragments of monosaccharides. The inhibition of these enzymes directly prevents the escape of the resulting monosaccharides to the bloodstream and the consequent utilization by the liver, muscle, and fat tissues.
Several antidiabetic drugs are confined by their limited action and pharmacokinetic influence and have side effects, e.g., biguanides and sulfonylurea. Traditional medicine utilizes herbal-based remedies by about 80% of the world's population. Herbal-derived active compounds or chemically modified herbal phytochemicals are used to produce safer pharmaceutically active drugs [16, 17]. Indeed, many plant extracts have shown antidiabetic effects in in vitro experiments, in animal test models, and in clinical trials. [17].
Ocimum basilicum is among one of the antidiabetic herbs [18]. The genus Ocimum belongs to the family Lamiaceae [19]. A number of species of Ocimum are used to treat different types of diseases, mainly the species Ocimum basilicum, also known as sweet basil—an herbaceous, perennial plant used in traditional medicine and also an ornamental plant [20, 21]. A number of virulent metabolites exist in this species that have strong action against diseases [22, 23]. It has a wide range of pharmacological activities, much as the antimicrobial effect. This was seen against Aspergillus ochraceus [24] for extracts from the hairy root of O. basilicum against species such as P. aeruginosa strains, A. rhizogenes, P. fluorescens, X. campestris, and E. carotovora [25] and for the leaf extract against E. coli and Staphylococcus aureus [26]. The essential oil extract from O. basilicum leaves showed an insecticidal activity against larval stages of Culex tritaeniorhynchus, Aedes albopictus, and Anophelessubpictus [27, 28]. O. basilicum contains several active antioxidant compounds, e.g., polyphenoid rosmarinic acid, a derivative of cinnamic acid [29]. Extracts from Ocimum basilicum aerial parts have robust anti-inflammatory activity against macrophages and human primary chondrocytes [30]. Extracts of Ocimum basilicum aerial parts have also shown antiplatelet activity through inhibiting ADP-induced platelet aggregation [31], anticonvulsant activity [32], and antithrombotic activity [33]. O. basilicum extract significantly showed antihyperlipidemic effects and could lower both plasma triglycerides (TG) and cholesterol in rats [31]. Ocimum basilicum aerial extracts (methanol, hexane, and dichloromethane) reported recently to augment glucose transporter-4 (GLUT4) translocation to the muscle plasma membrane in vitro and thus enhance glucose uptake [18].
α-Glucosidase (α-glucosidase, EC 3.2.1.20) is a carbohydrate hydrolase that breaks down terminal nonreducing (alpha-1 ⟶ 4)-linkage to release α-glucose residues. Two families of α-glucosidase are examined so far according to the primary structure [34]. The gene coding for human lysosomal α-glucosidase is about 20 kb long, and the structure for the protein it codes for has been resolved [35]. The Trp-516 and Asp-518 residues are crucial for the enzyme's catalytic functionality [36]. It was found that the conformation of the enzyme's active site is less stable than the whole enzyme conformation [37]. Deficiency in α-glucosidase may result in several disorders, e.g., Pompe disease or glycogen storage disease type II [38].
α-Amylase (α-amylase, EC 3.2.1.1) is an enzyme that hydrolyses alpha bonds of polysaccharides, such as starch and glycogen, to produce glucose and maltose. At least, two major forms of amylase are found in humans. Salivary α-amylase breaks starch into maltose and dextrin. The pancreatic α-amylase digests sugars in the diet to small saccharides such as maltose for the uptake in the small intestine on reaching the duodenum. Pancreatic α-amylase was discovered to bind with N-linked oligosaccharides of glycoproteins, which regulates the activities of glycoproteins related to the blood glucose level [39, 40]. Three α-amylase genes, AMY1 (the salivary α-amylase gene), AMY2A, and AMY2B (pancreatic α-amylase genes), form a cluster on chromosome 1 P21, with a pairwise sequence homology of 93%–94% [41, 42].
A number of distinct protein domains make up α-amylases: The catalytic domain has an eight-stranded alpha/beta barrel containing the active site, an ∼70-amino acid calcium-binding domain protrudes in the catalytic domain between alpha helix 3 and beta strand 3, and a Greek key beta-barrel domain is the carboxyl terminal. [43–46]. Acarbose is a complex oligosaccharide that serves as an oral inhibitor of α-glucosidase and α-amylase in the treatment of T2DM [47].
The progress in drug discovery has bloomed hugely nowadays. Chemoinformatics is used to scan hundreds of plausible protein ligands. One method for screening is the ligand-based approach. With the escalating number of resolved crystal structures, modeling for the binding of ligands to the target proteins can be configured. Docking is a structure-based method that acts at the atomic resolution for the screening of robust ligand-protein interactions, including the calculation for ligand position, orientation, inhibition constants, and binding affinities. In this study, we examined the inhibitory effect of methanol O. basilicum extract on α-glucosidase and α-amylase in vitro and in silico.
2. Materials and Methods
2.1. Preparation of Plant Material
The selected plants were extracted according to Kadan et al. [18] with methanol. 10 g of grounded OB was packed in the thimble of the Soxhlet apparatus and were extracted with 150 mL of methanol (MeOH) and then refluxed for 24 h to give a dark green extract. The yield of the extract was 1.05 g (10.5%). Supernatants obtained from the extract were passed through a 0.2 μm filter and stored in aliquots at −80°C for further experimental work.
2.2. α-Amylase Inhibitory Method
The pancreatic α-amylase inhibition assay was performed according to Adisakwattana et al. [48] using acarbose as a positive control. Porcine pancreatic α-amylase (4 units/mL) was dissolved in 0.1 M sodium phosphate buffer, pH 6.9.
The plant extract, caffeic acid, or acarbose as a positive control of different dilutions was preincubated with 250 μL of the enzyme solution at 37°C for 10 min. The reaction was initiated by adding 500 μL of the substrate solution (1% starch in 0.1 M sodium phosphate buffer, pH 6.9). After 5 min of incubation, the reaction was stopped by adding 1 mL of 96 mM 3,5-dinitrosalicylic acid solution to the reaction mixture. The mixtures were heated at 100°C for 10 min in order to stop the reaction and then cooled to room temperature in a cold water bath. Subsequently, the reaction mixtures were diluted 10 times with distilled water. The absorbance was recorded at 540 nm using a spectrophotometer. The α-amylase inhibitory potential was calculated utilizing the following equation:
| (1) |
where I (%) is the α-amylase inhibitory percentage.
2.3. Intestinal α-Glucosidase Inhibitory Method
The assessment of intestinal α-glucosidase inhibitory activity was performed according to Kim et al. [49], with a slight modification. The reaction mixture consisting of plant extract, caffeic acid, or acarbose as a positive control at varying concentrations was premixed with 100 μL of 0.1 M sodium phosphate buffer, pH 6.9. 15 μL of α-glucosidase (0.1 unit/μL) was added and preincubated at 37°C for 10 min. The reaction mixture was set to 750 μL with distilled water. The reaction was initiated by adding 250 μL of 20 mM p-nitrophenyl α-D-glucopyranoside and further incubated for 10 min. The reaction was terminated by the addition of 100 μL of 0.1 M Na2CO3. The amount of released product (p-nitrophenol) was measured at 405 nm using a spectrometer.
The intestinal α-glucosidase enzyme inhibitory potential was measured utilizing the following equation:
| (2) |
where I (%) is the α-glucosidase inhibitory percentage.
2.4. Docking Experiments
Input PDB files were prepared for the natural compounds that were extracted from O. basilicum, including caffeic acid. The SMILES structures of the compounds were retrieved from the systemic IUPAC structures [50] and then converted to the PDB form using the Open Babel server [51]. These compounds were docked against the apo forms for the structures of the α-glucosidase enzyme (PDB: 5KZW) and the α-amylase enzyme (PDB: 1C8Q) with the AutoDock program, version 4.2 [52]. In each docking experiment, the receptor protein was kept rigid. Protein polar hydrogen atoms were added, and the input files were prepared using AutoDock tools [52]. Docking was performed within parallel rectangular boxes of 126 × 126 × 126 Å dimensions. The center of the grid was placed at the center of the mass of the original protein receptor in its apo form in crystal structures. A total of 20 independent docking runs were carried out for each compound against each of the enzymes starting from random positions. The PDB files were extracted and evaluated for the best-ranked fit of the enzyme-ligand interaction for each of the ligands.
3. Results
We have recently reported the phytochemical analysis of O. basilicum [18] with the objective to identify more potential antidiabetic active compounds (and test their potency in inhibiting carbohydrates digestive enzymes); the methanol O. basilicum extract from dried aerial parts was tested herein in vitro and in situ. The potential inhibitory effect of the extract on α-amylase and α-glucosidase was examined as described in the Materials and Methods section. Similarly, caffeic acid (as a major compound in the extract and one that displayed in situ inhibition) inhibitory effect was also tested.
The in vitro antidiabetic activities of O. basilicum methanol extract and for caffeic acid were investigated by the assessment of their pancreatic α-amylase and intestinal α-glucosidase inhibitory effects. Acarbose was used as a standard inhibitory drug. The results revealed that O. basilicum extract inhibited α-glucosidase in a dose-dependent manner and the IC50 value was 160 ± 10 μg/mL (Figure 1(a) and Table 1). Caffeic acid also inhibited α-glucosidase in a dose-dependent manner, and the IC50 value was 1.05 ± 0.25 mM (Figure 1(b) and Table 1).
Figure 1.
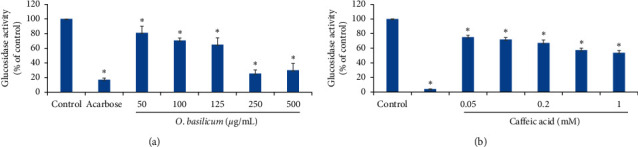
Effects of O. basilicum (a) and caffeic acid (b) on intestinal α-glucosidase. Values represent the mean ± SEM of three experiments. The t-test of statistical calculations was conducted using SPSS, version 23.0. ∗p < 0.05, which is considered significant as compared with controls.
Table 1.
The IC50 values of O. basilicum and caffeic acid for intestinal α-glucosidase and α-amylase.
| O. basilicum | Caffeic acid | |
|---|---|---|
| α-Glucosidase | 160 ± 10 μg/mL | 1.05 ± 0.25 mM |
| α-Amylase | >500 μg/mL | >1 mM |
O. basilicum inhibited also α-amylase yet at less potency compared with its inhibition to α-glucosidase. 500 μg/mL of O. basilicum extract inhibited α-amylase by only 25.4% ± 3.3 (Figure 2(a)). Caffeic acid inhibited α-amylase in a dose-dependent manner and led to 42.7% ± 3.0 inhibition at 1 mM (Figure 2(b)).
Figure 2.
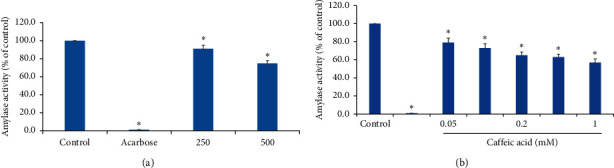
Effects of Ocimum basilicum (a) and caffeic acid (b) on pancreatic α-amylase. Values represent the mean ± SEM of three experiments. The t-test of statistical calculations was conducted using SPSS, version 23.0. ∗p < 0.05, which is considered significant as compared with controls.
Results for the docking experiments met well with the in vitro evaluation. Phytochemicals screened in the extract from OB were tested for their binding affinities, inhibition constants, and the root mean square deviation (RMSD) values for the ligand structure upon docking from the reference one. Three compounds showed potent inhibition to both α-glucosidase and α-amylase: beta-sitosterol followed by 4,7-dimethoxyindan-1-one and then caffeic acid (Tables 2 and 3). Weak nonpolar contacts accounted for most of the interactions at the binding interface, with few polar contacts seen as hydrogen bonding between the ligand and the indicated amino acid residues (Figure 3).
Table 2.
Binding free energies, inhibition constants, and the RMSD values calculated by AutoDock for ligand binding to α-glucosidase.
| OB phytochemical | AutoDock binding free energy (kcal/mol) | AutoDock inhibition constant (Ki) | RMSD for the ligand from the reference structure (Å) |
|---|---|---|---|
| 2-Hydroxymethyl-6-3,4,5-trihydroxy-2-hydroxymethyl | −3.37 | 3.36 mM | 11.463 |
| 3,3-Dihydroxyacrylic acid | −3.33 | 3.61 mM | 15.571 |
| 4,7-Dimethoxyindan-1-one | −5.79 | 56.88 μM | 8.094 |
| Alpha-hydroxyhydrocaffeic acid | −4.29 | 721.41 μM | 7.700 |
| Alpha-linolenic acid | −4.74 | 337.32 μM | 9.710 |
| Arabinitol | −1.35 | 102.52 mM | 49.393 |
| Beta-D-galactofuranose | −4.19 | 853.24 μM | 6.313 |
| Beta-D-glucopyranoside,5-methyl-2-1-methylethyl-phenyl | −5.54 | 86.74 μM | 12.272 |
| Beta-D-ribofuranose | −3.64 | 2.16 mM | 26.731 |
| Beta-sitosterol | −7.93 | 1.53 μM | 30.109 |
| Caffeic acid | −5.45 | 100.99 μM | 10.935 |
| Cyanuric acid | −4.66 | 382.74 μM | 14.773 |
| D-Xylopyranose | −3.88 | 1.42 mM | 28.285 |
| D-Xylose | −2.26 | 22.04 mM | 23.337 |
| E-but-2-ene-1-4-diol | −3.79 | 1.67 mM | 9.428 |
| E-Isoeugenol | −6.35 | 22.33 μM | 10.075 |
| Glucopyranose | −2.97 | 6.70 mM | 6.426 |
| Hydroquinone | −4.55 | 461.18 μM | 8.566 |
| Inositol | −4.48 | 520.07 μM | 38.863 |
| Linalool | −5.60 | 78.91 μM | 48.561 |
| Linoleic acid | −3.46 | 2.91 mM | 17.302 |
| Mannitol | −3.26 | 4.05 mM | 9.698 |
| Palmitic acid | −3.91 | 1.35 mM | 59.142 |
| Pentane-1,2,5-triol | −3.23 | 4.26 mM | 49.447 |
| Pyroglutamic acid | −4.81 | 298.88 μM | 16.630 |
| Talose | −2.91 | 7.34 mM | 6.730 |
| Uvasol | −5.35 | 118.93 μM | 45.174 |
| Glycerol | −1.11 | 152.58 mM | — |
| L-Valine | −2.93 | 7.10 mM | — |
| Succinate | −3.77 | 1.71 mM | — |
| Threitol | −1.58 | 69.02 mM | — |
| Urea | −2.96 | 6.82 mM | — |
Table 3.
Binding free energies, inhibition constants, and the RMSD values calculated by AutoDock for ligand binding to α-amylase.
| OB phytochemical | AutoDock binding free energy (kcal/mol) | AutoDock inhibition constant (Ki) | RMSD for the ligand from the reference structure (Å) |
|---|---|---|---|
| 2-Hydroxymethyl-6-3,4,5-trihydroxy-2-hydroxymethyl | −3.26 | 4.09 mM | 45.065 |
| 3,3-Dihydroxyacrylic acid | −3.99 | 1.20 mM | 56.124 |
| 4,7-Dimethoxyindan-1-one | −5.77 | 59.10 μM | 44.384 |
| Alpha-hydroxyhydrocaffeic acid | −4.67 | 379.57 μM | 64.753 |
| Alpha-linolenic acid | −4.03 | 1.11 mM | 50.314 |
| Arabinitol | −2.05 | 31.56 mM | 41.922 |
| Beta-D-galactofuranose | −3.52 | 2.61 mM | 48.439 |
| Beta-D-glucopyranoside,5-methyl-2-1-methylethyl-phenyl | −4.02 | 1.14 mM | 44.414 |
| Beta-D-ribofuranose | −3.35 | 3.48 mM | 44.547 |
| Beta-sitosterol | −8.38 | 719.79 nm | 48.783 |
| Caffeic acid | −5.25 | 140.92 μM | 68.360 |
| Cyanuric acid | −4.79 | 310.58 μM | 81.408 |
| D-Xylopyranose | −3.48 | 2.83 mM | 45.736 |
| D-Xylose | −2.48 | 15.29 mM | 70.312 |
| E-but-2-ene-1-4-diol | −4.30 | 708.76 μM | 39.448 |
| E-Isoeugenol | −4.64 | 397.34 μM | 48.923 |
| Glucopyranose | −3.80 | 1.65 mM | 46.170 |
| Hydroquinone | −4.13 | 933.22 μM | 44.919 |
| Inositol | −3.78 | 1.68 mM | 90.688 |
| Linalool | −4.13 | 933.83 μM | 53.531 |
| Linoleic acid | −3.75 | 1.77 mM | 79.184 |
| Mannitol | −2.41 | 17.08 mM | 44.479 |
| Palmitic acid | −4.02 | 1.13 mM | 57.625 |
| Pentane-1,2,5-triol | −2.99 | 6.45 mM | 36.675 |
| Pyroglutamic acid | −4.85 | 276.50 μM | 58.241 |
| Talose | −2.43 | 16.69 mM | 64.498 |
| Uvasol | −4.39 | 603.30 μM | 45.065 |
| Glycerol | −1.35 | 102.76 mM | — |
| L-Valine | −2.47 | 15.48 mM | — |
| Succinate | −4.00 | 1.16 mM | — |
| Threitol | −0.80 | 259.66 mM | — |
| Urea | −2.68 | 10.78 mM | — |
Figure 3.
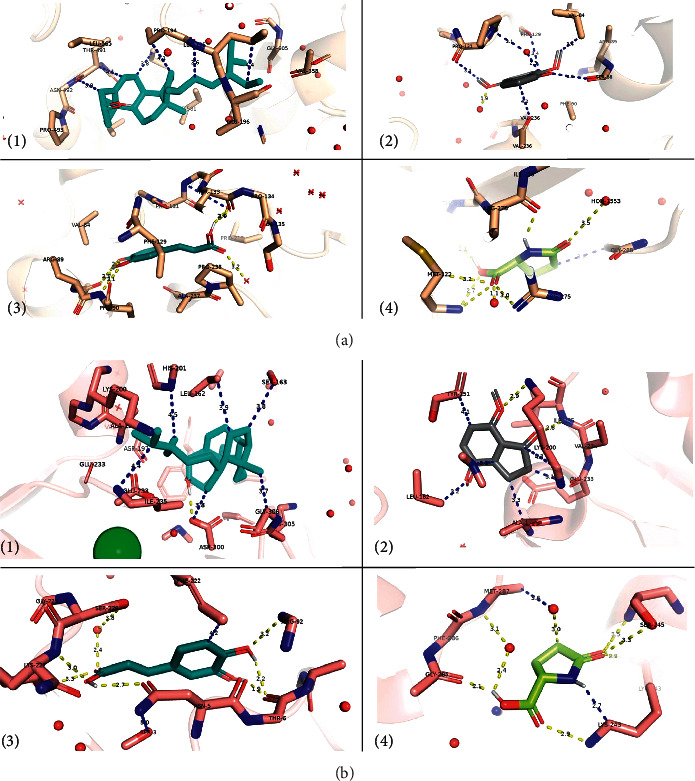
Binding interface between plausible inhibitors and (a) the α-glucosidase enzymes: (1) beta-sitosterol, (2) 4,7-dimethoxyindan-1-one, (3) caffeic acid, and (4) pyroglutamic acid; and (b) the α-amylase enzymes: (1) beta-sitosterol, (2) 4,7-dimethoxyindan-1-one, (3) caffeic acid, and (4) pyroglutamic acid. All amino acids that are within 5 Ǻ from the ligand are shown as sticks. The rest of the protein is shown in an 80% transparent cartoon model. Polar contacts are shown in yellow, whereas other possible contacts are in blue. The green ball in label (1) of subpart 3(b) refers to a chloride ion near the active site.
Other potent inhibitors also existed that showed only an inhibitory effect against α-glucosidase but not α-amylase: E-isoeugenol (Ki = 22.33 μM) followed by linalool (Ki = 78.91 μM), beta-D-glucopyranoside,5-methyl-2-1-methylethyl-phenyl (Ki = 86.74 μM), and finally uvasol (Ki = 118.93 μM) in a descending order with respect to their inhibition constants (Figure 4). This might explain why the extract had more potent effect on the enzyme α-glucosidase than the α-amylase enzyme in the in vitro experiments. Indeed, the inhibition constants for the common inhibitory phytochemicals had very similar effects.
Figure 4.
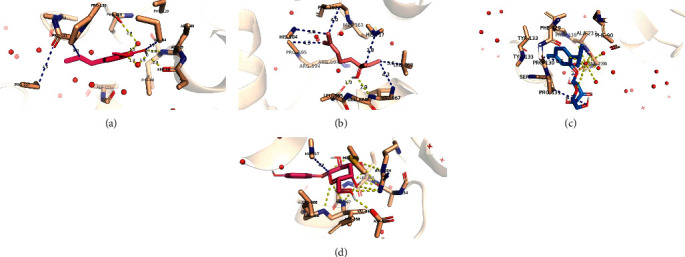
Binding interface between plausible inhibitors and the α-glucosidase enzyme: (a) E-isoeugenol, (b) linalool, (c) beta-D-glucopyranoside,5-methyl-2-1-methylethyl-phenyl, and (d) uvasol. All amino acids that are within 5 Ǻ from the ligand are shown as sticks. The rest of the protein is shown in an 80% transparent cartoon model. Polar contacts are shown in yellow, whereas other possible contacts are in blue.
Pyroglutamic acid existed in both inhibitor lists for α-glucosidase and α-amylase, but the inhibition was predicted to be weak when compared with other phytochemicals (Ki = 298.88 μM, 276.50 μM for α-glucosidase and α-amylase, respectively, see Figure 3).
4. Discussion
O. basilicum has been reported as a potential antidiabetic herb, yet the action mechanisms and the potential antidiabetic compounds in O. basilicum that inhibit intestinal digestive enzymes were not discussed and some were not identified. Here, O. basilicum methanol extract and one of the major compounds, i.e., caffeic acid inhibited α-amylase and α-glucosidase in a dose-dependent manner. Docking experiments were undertaken to understand the mechanism by which the O. basilicum extracts would inhibit the two enzymes. Phytochemicals in the O. basilicum extract were screened for their inhibitory potency, binding interface, and structural fluctuations. The number of inhibitors that were predicted to work against α-glucosidase was twofold more than those inhibiting α-amylase (8 active phytochemicals for α-glucosidase vs 4 active phytochemicals for α-amylase). Still, the inhibition level of the four common phytochemicals was comparable for the two enzymes. This would justify the higher inhibition levels for α-glucosidase when compared with α-amylase in vitro. The deviations from the reference structure (the RMSD values) were highly reasonable for all plausible inhibitors. Of the four common inhibitors, the binding interface for caffeic acid and pyroglutamic acid showed more polar contacts than beta-sitosterol and 4,7-dimethoxyindan-1-one. Thus, more polarity did not contribute to a more stable binding interface and the binding free energy as the hydrophobic contacts did. On the other hand, the four inhibitors unique to α-glucosidase (E isoeugenol, linalool, beta-D-glucopyranoside,5-methyl-2-1-methylethyl-phenyl, and uvasol) showed a good hydrophilic interface with the surrounding amino acid residues and the coordinating water molecules.
Previous works suggested that glucosidase inhibitors and amylase inhibitors are a class of compounds that help controlling diabetes by diminishing the absorption of glucose from the intestine [53]. Some research also suggested that the water/methanol extract of food materials displayed the antidiabetic activity against amylase and glucosidase [54, 55]. These results provide intense rationale for further in vivo study and drug identification.
Acknowledgments
The authors acknowledge the Palestinian-German Funding Program (PALGER), AAUP, and Al Qasimi Research Foundation for providing financial support.
Data Availability
Data are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Siba Shanak and Najlaa Basalat contributed equally to this work.
References
- 1.Shirwaikar A., Rajendran K., Kumar C. D., Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin-nicotinamide type 2 diabetic rats. Journal of Ethnopharmacology. 2004;91(1):171–175. doi: 10.1016/j.jep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Nathan D. M. Long term complications of diabetes mellitus. New England Journal of Medicine. 1993;328(23):1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 3.Zaid H., Antonescu C. N., Randhawa V. K., Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochemical Journal. 2008;413:201–215. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- 4.Kerner W., Brueckel J., German Diabetes Association Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes. 2014;122(7):384–386. doi: 10.1055/s-0034-1366278. [DOI] [PubMed] [Google Scholar]
- 5.McCrimmon R. J., Sherwin R. S. Hypoglycemia in type 1 diabetes. Diabetes. 2004;59(10):2333–2339. doi: 10.2337/db10-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaid H., Tamrakar A. K., Razzaque M. S., Efferth T. Diabetes and metabolism disorders medicinal plants: a glance at the past and a look to the future. Evidence-Based Complementary and Alternative Medicine. 2018;2018:3. doi: 10.1155/2018/5843298.5843298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman A., DeSanctis V., Yassin M., Elalaily R., Eldarsy N. E. Continuous glucose monitoring system and new era of early diagnosis of diabetes in high risk groups. Indian Journal of Endocrinology and Metabolism. 2014;18(3):274–282. doi: 10.4103/2230-8210.131130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poradzka A., Wroński J., Jasik M., Karnafel W., Fiedor P. Insulin replacement therapy in patients with type 1 diabetes by isolated pancreatic islet transplantation. Acta Poloniae Pharmaceutica. 2013;70(6):943–950. [PubMed] [Google Scholar]
- 9.Shanak S., Saad B., Zaid H. Metabolic and epigenetic action mechanisms of antidiabetic medicinal plants. Evidence-Based Complementary and Alternative Medicine. 2019;2019:18. doi: 10.1155/2019/3583067.3583067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaid H., Saad B., Mahdi A. A., Tamrakar A. K., Haddad P. S., Afifi F. U. Medicinal plants and natural active compounds for diabetes and/or obesity treatment. Evidence-Based Complementary and Alternative Medicine. 2015;2015:2. doi: 10.1155/2015/469762.469762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFronzo R. A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. the claude Bernard lecture 2009. Diabetologia. 2010;53(7):1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitada M., Zhang Z. Y., Mima A., King G. L. Molecular mechanisms of diabetic vascular complications. Journal of Diabetes Investigation. 2010;1(3):77–89. doi: 10.1111/j.2040-1124.2010.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deans K. A., Sattar N. “Anti-inflammatory” drugs and their effects on type 2 diabetes. Diabetes Technology & Therapeutics. 2006;8(1):18–27. doi: 10.1089/dia.2006.8.18. [DOI] [PubMed] [Google Scholar]
- 14.Hanhineva K., Torronen R., Bondia-Pons I., et al. Impact of dietary polyphenols on carbohydrate metabolism. International Journal of Molecular Sciences. 2010;11(4):1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Laar F. A., Lucassen P. L., Akkermans R. P., van de Lisdonk F. H., Rutten G. E., van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes—results from a cochrane systematic review and meta-analysis. Diabetes Care. 2005;28(1):154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 16.Said O., Fulder S., Khalil K., Azaizeh H., Kassis E., Saad B. Maintaining a physiological blood glucose level with glucolevel, a combination of four anti-diabetes plants used in the traditional Arab herbal medicine. Evidence-Based Complementary and Alternative Medicine. 2008;5(4):421–428. doi: 10.1093/ecam/nem047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saad B., Zaid H., Shanak S., Kadan S. Anti-Diabetes and Anti-Obesity Medicinal Plants and Phytochemicals. Berlin, Germany: Springer; 2017. [Google Scholar]
- 18.Kadan S., Saad B., Sasson Y., Zaid H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chemistry. 2016;196:1066–1074. doi: 10.1016/j.foodchem.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 19.Simon J., Morales M., Phippen W., Vieira R., Hao Z. Basil: A Source of Aroma Compounds and a Popular Culinary and Ornamental Herb. Perspectives on New Crops and New Uses Alexandria. Richmond, VA, USA: ASHS Press; 1999. [Google Scholar]
- 20.Siddiqui B. S., Bhatti H. A., Begum S., Perwaiz S. Evaluation of the antimycobacterium activity of the constituents from Ocimum basilicum against mycobacterium tuberculosis. Journal of Ethnopharmacology. 2012;144(1):220–222. doi: 10.1016/j.jep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Bantis F., Ouzounis T., Radoglou K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Scientia Horticulturae. 2016;198:277–283. [Google Scholar]
- 22.Bora K. S., Arora S., Shri R. Role of Ocimum basilicum L. in prevention of ischemia and reperfusion-induced cerebral damage, and motor dysfunctions in mice brain. Journal of Ethnopharmacology. 2011;137(3):1360–1365. doi: 10.1016/j.jep.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 23.Loughrin J. H., Kasperbauer M. J. Light reflected from colored mulches affects aroma and phenol content of sweet basil (Ocimum basilicum L.) leaves. Journal of Agricultural and Food Chemistry. 2001;49(3):1331–1335. doi: 10.1021/jf0012648. [DOI] [PubMed] [Google Scholar]
- 24.Basilico M. Z., Basilico J. C. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Letters in Applied Microbiology. 1999;29(4):238–241. doi: 10.1046/j.1365-2672.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 25.Bais H. P., Walker T. S., Schweizer H. P., Vivanco J. A. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiology and Biochemistry. 2002;40:983–995. [Google Scholar]
- 26.Khalil A. Antimicrobial activity of ethanolic extracts of Ocimum basilicum leaf from Saudi Arabia. Biotechnology. 2013;12:61–64. [Google Scholar]
- 27.Kathirvel P., Ravi S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Natural Product Research. 2012;26(12):1112–1118. doi: 10.1080/14786419.2010.545357. [DOI] [PubMed] [Google Scholar]
- 28.Hossain M. A., Kabir M. J., Salehuddin S. M., et al. Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimum basilicum occurring in Bangladesh. Pharmaceutical Biology. 2010;48(5):504–511. doi: 10.3109/13880200903190977. [DOI] [PubMed] [Google Scholar]
- 29.Phippen W. B., Simon J. E. Anthocyanins in basil (Ocimum basilicum L.) Journal of Agricultural and Food Chemistry. 1998;46(5):1734–1738. [Google Scholar]
- 30.Raina P., Deepak M., Chandrasekaran C. V., Agarwal A., Wagh N., Kaul-Ghanekar R. Comparative analysis of anti-inflammatory activity of aqueous and methanolic extracts of Ocimum basilicum (basil) in RAW264.7, SW1353 and human primary chondrocytes in respect of the management of osteoarthritis. Journal of Herbal Medicine. 2016;6(1):28–36. [Google Scholar]
- 31.Amrani S., Harnafi H., Gadi D., et al. Vasorelaxant and anti-platelet aggregation effects of aqueous Ocimum basilicum extract. Journal of Ethnopharmacology. 2020;125(1):157–162. doi: 10.1016/j.jep.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 32.Freire C. M. M., Marques M. O. M., Costa M. Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L. essential oil. Journal of Ethnopharmacology. 2006;105(1-2):161–166. doi: 10.1016/j.jep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Tohti I., Tursun M., Umar A., Turdi S., Imin H., Moore N. Aqueous extracts of Ocimum basilicum L. (sweet basil) decrease platelet aggregation induced by ADP and thrombin in vitro and rats arterio—venous shunt thrombosis in vivo. Thrombosis Research. 2006;118(6):733–739. doi: 10.1016/j.thromres.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Chiba S. Molecular mechanism in alpha-glucosidase and glucoamylase. Bioscience Biotechnology and Biochemistry. 1997;61(8):1233–1239. doi: 10.1271/bbb.61.1233. [DOI] [PubMed] [Google Scholar]
- 35.Hoefsloot L. H., Hoogeveenwesterveld M., Reuser A. J. J., Oostra B. A. Characterization of the human lysosomal alpha-glucosidase gene. Biochemical Journal. 1990;272(2):493–497. doi: 10.1042/bj2720493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermans M. M. P., Kroos M. A., Vanbeeumen J., Oostra B. A., Reuser A. J. J. Human lysosomal alpha-glucosidase—characterization of the catalytic site. Journal of Biological Chemistry. 1991;266(21):13507–13512. [PubMed] [Google Scholar]
- 37.Wu X.-Q., Xu H., Yue H., Liu K.-Q., Wang X.-Y. Inhibition kinetics and the aggregation of alpha-glucosidase by different denaturants. Protein Journal. 2009;28(9-10):448–456. doi: 10.1007/s10930-009-9213-0. [DOI] [PubMed] [Google Scholar]
- 38.Lim J. A., Li L. S., Raben N. Pompe disease: from pathophysiology to therapy and back again. Frontiers in Aging Neuroscience. 2014;6:p. 177. doi: 10.3389/fnagi.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushita H., Takenaka M., Ogawa H. Porcine pancreatic alpha-amylase shows binding activity toward N-linked oligosaccharides of glycoproteins. Journal of Biological Chemistry. 2002;277(7):4680–4686. doi: 10.1074/jbc.M105877200. [DOI] [PubMed] [Google Scholar]
- 40.Asanuma-Date K., Hirano Y., Le N., et al. Functional regulation of sugar assimilation by N-Glycan-specific interaction of pancreatic alpha-amylase with glycoproteins of duodenal brush border membrane. Journal of Biological Chemistry. 2012;287(27):23104–23118. doi: 10.1074/jbc.M111.314658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tricoli J. V., Shows T. B. Regional assignment of human amylase (AMY) to p22—p21 of chromosome-1. Somatic Cell and Molecular Genetics. 1984;10(2):205–210. doi: 10.1007/BF01534909. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter D., Dhar S., Mitchell L. M., et al. Obesity, starch digestion and amylase: association between copy number variants at human salivary (AMY1) and pancreatic (AMY2) amylase genes. Human Molecular Genetics. 2015;24(12):3472–3480. doi: 10.1093/hmg/ddv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abe A., Yoshida H., Tonozuka T., Sakano Y., Kamitori S. Complexes of Thermoactinomyces vulgaris R-47 alpha-amylase 1 and pullulan model oligossacharides provide new insight into the mechanism for recognizing substrates with alpha-(1,6) glycosidic linkages. FEBS Journal. 2005;272(23):6145–6153. doi: 10.1111/j.1742-4658.2005.05013.x. [DOI] [PubMed] [Google Scholar]
- 44.Kadziola A., Sogaard M., Svensson B., Haser R. Molecular structure of a barley alpha-amylase-inhibitor complex: implications for starch binding and catalysis. Journal of Molecular Biology. 1998;278(1):205–217. doi: 10.1006/jmbi.1998.1683. [DOI] [PubMed] [Google Scholar]
- 45.Kadziola A., Abe J., Svensson B., Haser R. Crystal and molecular-structure of barley alpha-amylase. Journal of Molecular Biology. 1994;239(1):104–121. doi: 10.1006/jmbi.1994.1354. [DOI] [PubMed] [Google Scholar]
- 46.Machius M., Wiegand G., Huber R. Crystal-structure of calcium-depleted bacillus-licheniformis alpha-amylase at 2.2-a resolution. Journal of Molecular Biology. 1995;246(4):545–559. doi: 10.1006/jmbi.1994.0106. [DOI] [PubMed] [Google Scholar]
- 47.Ademiluyi A. O., Oboh G. Aqueous extracts of roselle (Hibiscus sabdariffa linn.) varieties inhibit alpha-amylase and alpha-glucosidase activities in vitro. Journal of Medicinal Food. 2013;16(1):88–93. doi: 10.1089/jmf.2012.0004. [DOI] [PubMed] [Google Scholar]
- 48.Adisakwattana S., Ruengsamran T., Kampa P., Sompong W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complementary Medicine and Therapies. 2010;12(1):p. 110. doi: 10.1186/1472-6882-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim Y. M., Wang M. H., Rhee H. I. A novel alpha-glucosidase inhibitor from pine bark. Carbohydrate Research. 2004;339(3):715–717. doi: 10.1016/j.carres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Lowe D. M., Corbett P. T., Murray-Rust P., Glen R. C. Chemical name to structure: OPSIN, an open source solution. Journal of Chemical Information and Modeling. 2011;51(3):739–753. doi: 10.1021/ci100384d. [DOI] [PubMed] [Google Scholar]
- 51.O’Boyle N. M., Banck M., James C. A., Morley C., Vandermeersch T., Hutchison G. R. Open babel: an open chemical toolbox. Journal of Cheminformatics. 2011;3:p. 33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris G. M., Huey R., Lindstrom W., et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. Journal of Computational Chemistry. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S., Ahire M., Patil S., et al. Antidiabetic activity of gnidia glauca and dioscorea bulbifera: potent amylase and glucosidase inhibitors. Evidence-Based Complementary and Alternative Medicine. 2012;2012:10. doi: 10.1155/2012/929051.929051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malekaneh M., Zarban A. Antidiabetic activity of water extract of B-100 (a herbal formulation) in streptozotocin-induced diabetic rats. Clinical Biochemistry. 2014;44(13):p. S348. [Google Scholar]
- 55.Moradabadi L., Kouhsari S. M., Sani M. F. Hypoglycemic effects of three medicinal plants in experimental diabetes: inhibition of rat intestinal alpha-glucosidase and enhanced pancreatic insulin and cardiac glut-4 mRNAs expression. Iranian Journal of Pharmaceutical Research. 2013;12(3):387–397. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


