Abstract
Noise overexposure leads to hair cell loss, synaptic ribbon reduction, and auditory nerve deterioration, resulting in transient or permanent hearing loss depending on the exposure severity. Oxidative stress, inflammation, calcium overload, glutamate excitotoxicity, and energy metabolism disturbance are the main contributors to noise-induced hearing loss (NIHL) up to now. Gene variations are also identified as NIHL related. Glucocorticoid is the only approved medication for NIHL treatment. New pharmaceuticals targeting oxidative stress, inflammation, or noise-induced neuropathy are emerging, highlighted by the nanoparticle-based drug delivery system. Given the complexity of the pathogenesis behind NIHL, deeper and more comprehensive studies still need to be fulfilled.
1. Introduction
Hair cells (HCs) in the inner ear cochlea function in transducing sound waves into electric signals [1–4], while supporting cells function in supporting the HCs and providing the potential pool for HC regeneration [5–9]. Damages from a variety of sources can impair HC function, including mutations in deafness genes, aging, ototoxic drugs, chronic cochlear infections, and noise exposure [10–13]. Acoustic overexposure would result in sensorineural hearing loss characterized by high-frequency hearing threshold shift mainly, termed as noise-induced hearing loss (NIHL)[14], which is due to loss or damage of sensory HCs and degeneration of the spiral ganglion neurons (SGNs). Though noise susceptibility is quite individual, it is normally acknowledged that noise above 85 dB would be considered as hearing harmful [15, 16]. Studies [17–19] have shown that mild or moderate noise would only trigger temporary hearing threshold shift (TTS), because the noise-induced hair cell damage and auditory nerve fiber degeneration were still reversible. Hearing ability is commonly measured by auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE) test [20]. The ABR threshold and DPOAE level would recover to prenoise level over time in TTS patients. However, severe noise exposure, referring as strong sound vibrations, or longtime exposure to harmful noise, or both, can lead to cochlear hair cell necrosis and apoptosis, causing permanent threshold shift (PTS)[16, 21]. Although the neonatal mammals still have very limited HC regeneration ability, the adults have lost this ability [22–26], leading to the irreversible loss of HCs after noise damage. Besides decrease of auditory sensitivity and language recognition ability, NIHL patients could suffer from headache, tinnitus, dizziness, hypertension, etc. [14, 17].
It is estimated that about 5% of the world's population suffer from disabling hearing loss [27]. Modern people are exposed to acoustic trauma with increasingly higher risk as the industrialization of society. Strikingly, evidence has shown that acoustic trauma-induced hearing impairment aggravates microglial deterioration around the hippocampus, indicating its potential causal role in the pathogenesis of dementia, which exacerbates the already overloading public health burden of NIHL [28]. Therefore, more and more attention has been focused on revealing the pathogenesis mechanisms and effective therapeutics of NIHL. We review the updates on molecular targets about NIHL, summarize the approved and potential medications for NIHL treatment, and propose the controversies and foreseeing on NIHL.
2. Cellular Pathologies
Acoustic shock with strong sonic energy caused temporary or permanent cellular pathologies. Hair cells, especially outer hair cells (OHCs), are the main targets of noise trauma. Noise overexposure causes hair cells swelling and eventual irreversible death [29]. Noise-induced sensory hair cell impairment also accompanies by collapse and loss of stereocilia and destruction of their tip connection. Interestingly, the loss of tip links connecting adjacent stereocilia would increase noise susceptibility reversely [30–32]. Acoustic overstimulation can also cause the reduction of synaptic connections between hair cells and spiral ganglion cells, afferent fiber swelling, and auditory nerve deterioration [33–37].
It is widely recognized that noise with sound intensity > 85 dB is hearing harmful, while extremely high intensity noise like blast or gunshot could do much more harm to the hearing system within very short exposing time. The latter has been given a special name termed blast-induced hearing loss to highlight its difference from chronic NIHL [38]. Besides the damage of hair cells and spiral ganglion neurons, high intensity noise can result in middle ear damage like tympanic membrane perforation, ossicular chain dislocation, oval/round window rupture, and thus external lymph fistula [39]. The stria vascularis and spiral ligament are also attacked by intense noise, resulting in lower blood flow and lower vessel diameter in the stria vascularis [40]. Hearing impairment is the foremost clinical symptom of blast overexposure injury. Besides that, blast may also cause otitis media because of the rupture of tympanic membrane and secondary infection [41]. Vertigo and balance disorder occur in patients accompanied by labyrinthine damage and hemorrhage after blast exposure, which leaves headache and dizziness for a long time after the accident. Therefore, the treatment of blast-induced hearing loss often involves operations like tympanoplasty to repair tympanic membrane and rebuild ossicular chain [42].
3. Molecular Pathogenesis
Acoustic trauma features hair cell loss, synaptic ribbon deterioration, and acoustic nerve degeneration. Previous studies [43, 44] have proved that after noise exposure, both necrosis and apoptosis occurred in the sensory epithelium. However, the causative mechanisms inducing necrosis and apoptosis are much complex and intertwined (Figure 1).
Figure 1.
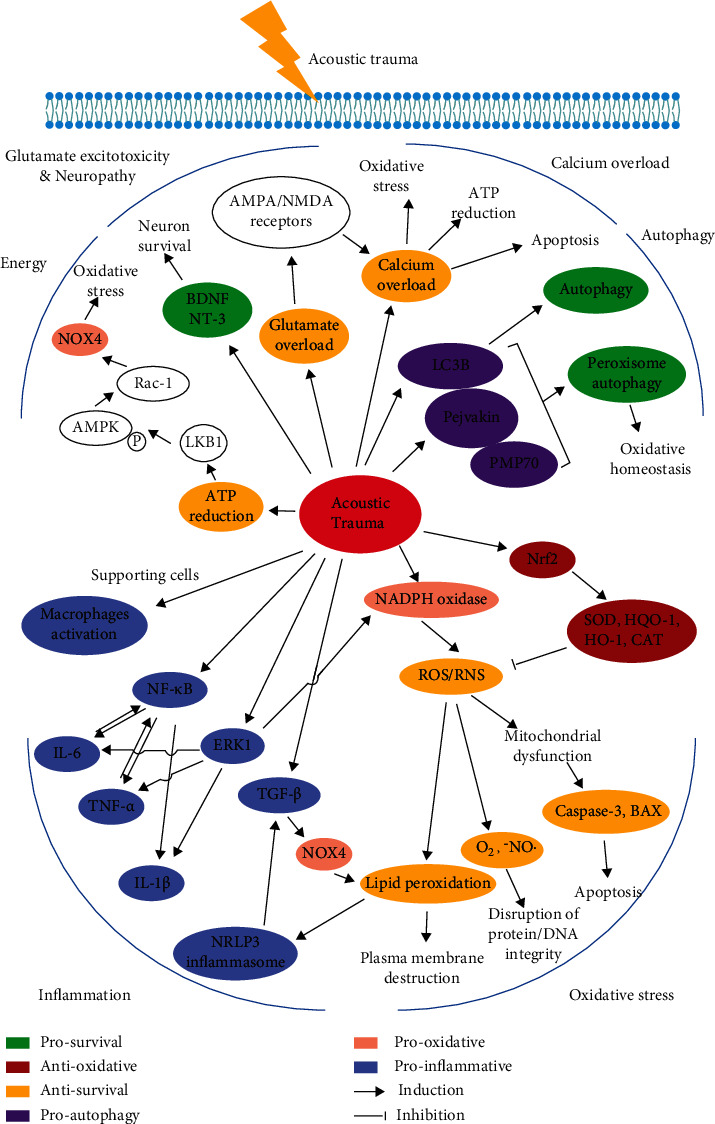
The molecular pathogenesis of NIHL.
3.1. Oxidative Stress
Though the exact mechanism behind NIHL is not fully explained yet, various studies [45–47] have suggested that oxidative stress in the cochlea contributes to the noise-induced hearing impairment. Ohlemiller et al. [45] have proved that the production of hydroxyl radical, a main kind of reactive oxygen species (ROS), increases over four times upon acoustic trauma in cochlear perilymph. ROS, mainly generated from the leaking electrons in the mitochondrial electron transport chain, is crucial for multiple physiological behaviors like cell proliferation and differentiation in relative low content [46, 48]. Under physiological circumstances, the production and scavenging of ROS are maintained at balance subtly and dynamically. Many stress stimulators like extensive sound exposure as well as ototoxic drugs like cisplatin [47] and aminoglycosides [49–56] can increase ROS production in hair cells, which is beyond the maximal cellular antioxidative ability, and threaten the integrity of DNA, protein, and other survival-crucial macromolecules [57]. Similar to ROS, reactive nitrogen species (RNS) also contribute to the noise-induced cochlear oxidative imbalance [58]. Overproduction of ROS/RNS in hair cells and spiral ganglion neurons is well studied [59, 60], whereas the role of oxidative stress in supporting cells still needs further explanation. Styrene was applied to induce specifically oxidative damage in cochlear supporting cells, and hearing ability was also apparently impaired [61]. However, how oxidative stress in supporting cells participates in NIHL progression requires deeper exploration.
The production and scavenging of ROS/RNS are mediated by various endogenous antioxidants and antioxidative enzymes [57]. The enzymes producing ROS/RNS are usually kept at low expressing level in hypermetabolic cells like acoustic hair cells, tumor cells, and cardiac myocytes, which are oxidative stress sensitive. The NADPH oxidase family (NOX) has been proved to catalyze the electron transport from NADPH to oxygen molecules to speed up ROS production [62]. The NOX4 transgenic mice which constitutively expressed human NOX4 were found to be noise susceptible while they had normal hearing threshold without acoustic stimulation, stressing the vital role of imbalance of redox homeostasis in NIHL [63].
Besides ROS/RNS overproduction, the inducible transient upregulation of antioxidative genes also features the pathogenesis of NIHL as a physical feedback mechanism. Nrf2, which is widely expressed in multiple tissues such as the heart, liver, and cochlea, can respond to oxidative stress and activate downstream antioxidative gene expression like glutathione peroxidase (GPx), NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and catalase (CAT) [64–66]. Nrf2 signaling can also activate autophagy in hair cells via p62 protein for oxidative stress amelioration [67]. More and more researches [67, 68] have proved that targeting Nrf2 signaling is a promising therapeutic solution for NIHL.
Given the fact that oxidative stress is one of the key contributors to the pathogenesis of NIHL, it is reasonable to assume that genes involved in the redox homeostasis correlate to noise vulnerability of the cochlea [69]. Superoxide dismutase (SOD) and paraoxonases (PONs) are two antioxidative enzymes found in the cochlea; the former directly catalyzes superoxide radicals to less toxic hydrogen peroxide while the latter decreases lipid peroxidation activity [70]. It is reported that the polymorphisms of SOD2, PON1, and PON2 are related to susceptibility to NIHL in workers with high occupational noise trauma risk [69, 71]. Meanwhile, overexpression of SOD1 was found to provide a protective effect on noise-related hearing loss [72]. Another newly emerging medication named nicotinamide, the NAD+ precursor, can boost NAD+ content and ameliorate NIHL in a SIRT3-dependent way [73]. SIRT3 plays an important role in maintaining the function of various antioxidative enzymes while impaired SIRT3 resulted in ROS overproduction and decreased levels of GSH [74, 75].
3.2. Autophagy
Autophagy, as a recently found endogenous self-defense mechanism, degrades damaged organelles by lysosome to maintain internal homeostasis and preserve energy providing for cell survival upon stress like nutrient deficiency, pathogen infection, protein misfolding, and oxidative stress [76–80]. Previous studies [81, 82] have shown that autophagy is upregulated in auditory hair cells after intense noise exposure. The immunostaining results show the upregulation of LC3b, the indispensable component of autophagosome, and the colocalization of LC3b and Lamp1, indicating the enhanced fusion of autophagosome and lysosome. To test the hypothesis of autophagy as the cochlear endogenous defenses against NIHL, researchers analyzed whether the disruption of autophagy could exacerbate NIHL with autophagy direct inhibitor 3-MA and mTOR signaling agonist RAP. mTOR protein is one of the main negative regulators for autophagy induction because it can phosphorylate Atg13 to cut off the beginning phase of autophagosome formation [83]. Notably, the inhibition of autophagy worsened the noise-induced oxidative imbalance and the eventual hair cell loss and impaired hearing ability [81].
Pejvakin protein is recently identified as the oxidative sensor in auditory hair cells. Researchers applied immunostaining colocalization analysis and in situ PLA technology to testify the enhanced interactions between pejvakin and LC3B, as well as pejvakin and PMP70 (the marker of peroxisome) in hair cells after acoustic trauma or simply H2O2 treatment. Furthermore, pejvakin is found to mediate peroxisome autophagy after noise exposure, which is vital to secondary peroxisome proliferation and oxidative homeostasis maintenance afterwards. Pejvakin-mutated mice show impaired peroxisome proliferation and more severe oxidative stress in auditory hair cells after noise exposure [84].
3.3. Inflammation
The cochlea was used to be considered as immune exemption organ because of the blood-perilymph barrier [85]. However back in 2008, Okano et al. [86] transplanted the EGFP-positive hematopoietic stem cells (HSCs) into wide-type C57BL/6 mice to label the bone marrow-derived cells (BMDCs) with green fluorescence and found EGFP-positive cells in cochlear spiral ganglion and spiral ligament regions of the HSC transplanted mice. Interestingly, EGFP-positive cells were colocalized with F4/80, Iba1, and CD68 immunostaining marks, indicating the presence of bone marrow-derived cochlear resident macrophage-like cells. Like microglia in the nervous system, supporting cells can also be stained with various macrophage markers surprisingly. LPS treatment can induce phagocytosis of supporting cells, which is the direct functional evidence linking cochlear supporting cells with macrophages [87]. Recently, more and more researches suggest the key role of inflammation in aging, drug, and noise-induced ototoxicity [10]. It is notable to point out that inflammation is a dual sword that requires a very complicated and delicate regulatory network involving multiple genes and transcription factors. After acoustic trauma, inflammatory cells representing CD45, CD68, and Iba1 positive infiltrate and proliferate in the cochlea, especially in the stria vascularis, with high expression of CX3CR1, the receptor of a classic chemokine [88, 89]. These inflammatory cells can synthesize and release a variety of proinflammatory cytokines like interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)[90]. IL-1β and IL-6, the markers of acute inflammation belonging to the interleukin superfamily, contribute to the mononuclear phagocyte infiltration and subsequent release of elastase and prostaglandin, bringing to excessive inflammation cascade activation resulting in microcirculation disturbance, tissue destruction, and eventual hearing impairment [88, 89, 91].
Targeting TNF-α inhibition by molecules or siRNA also attenuates the noise-induced cochlear inflammation and thus hearing threshold shift [92–94]. It is reported that noise-induced increase of TNF-α can be regulated by nuclear factor of activated T cells 4 (Nfatc4), a well-researched transcription factor served as proinflammatory cytokine inducer. Nfatc4 knockout mice show resistance to TNF-α-mediated hair cell apoptosis triggered by intense noise [95]. Notably, TNF-α is testified to be able to induce calcium influx through ERK-dependent activation of TRPV1. Desensitization of TRPV1 by capsaicin ameliorates TNF-α-induced calcium influx and hair cell apoptosis in NIHL modeling mice [96].
Tumor growth factor-β (TGF-β) has been proved to be pluri-regulatory in cochlear development and homeostasis maintenance such as otic capsule formation [97], spiral ganglion neuron survival [98, 99], and inflammation responses [100]. TGF-β initially serves as the inducer of adhesion and chemoattractive molecules for the chemotaxis and activation of leukocytes, and downregulates the interleukin production reversely as the inflammation progresses, indicating its double effects on inflammation regulation [101–103]. Reportedly, the mRNA expression level of TGF-β is upregulated in the cochleae upon noise trauma. Using P7 and P144, two peptides targeting TGF-β inhibition, significantly improves hearing compared to the control group in NIHL mouse model [104]. Further evidence indicates that TGF-β inhibitor decreased the NOX4 content, which is proved to promote NLRP3 inflammasome activation by oxidating fatty acids [105].
It is well testified that nuclear factor kappa B (NF-κB) and its target proinflammatory genes play the central roles in inflammation regulation. Previous researches [94, 106, 107] have found that NF-κB signaling cascade can be activated by noise trauma and inhibition of NF-κB can decrease the content of proinflammatory cytokines. Glucocorticoid is the most commonly used medication for the treatment of NIHL, and its pharmaceutical activity is reported to be partly mediated by NF-κB inhibition [108]. Another interesting discovery about NIHL is that mice show greater sensitivity to noise overexposure during the night [109]. The circadian sensitivity alteration of the cochlea is proved to be adrenal gland-derived endogenous glucocorticoid dependent for its key role on inflammation regulation [110]. Besides, proinflammatory meditators like cyclooxygenase-2 (COX2) and NADPH oxidase 3 (NOX3) are also found upregulated after noise exposure, verifying the indispensable role of inflammation in the pathogenesis of NIHL [94].
3.4. Glutamate Excitotoxicity and Neuropathy
The acute cochlear synaptic ribbon disruption and lasting acoustic neural degeneration also underlie the NIHL pathogenesis. Studies [111, 112] have proved that glutamate excessive accumulation serves as the contributor to acute and chronic deterioration of the auditory nervous system. Glutamate is subtly regulated and remains at a very low level in the synaptic cleft. The overloading of glutamate might dysregulate the cystine-glutamate transporter and result in cystine and thus GSH deficiency, or constantly activate the glutamate receptor in the postsynaptic membrane and damage the afferent neural fibers [113, 114]. GLAST, one of the five classic glutamate transporters, is found to be expressed in supporting cells located around the inner hair cells. GLAST plays a crucial role in glutamate uptake for its rapid recirculation in the synaptic cleft between the inner hair cells and afferent auditory neural fibers, and depletion of GLAST leads to excessive accumulation of glutamate, which might trigger calcium overload in spiral ganglion neurons. The knockout of GLAST exacerbates the hearing loss in transgenic mice, indicating the potential role of glutamate excitotoxicity in NIHL [111]. The release of glutamate from inner hair cells is also vesicular glutamate transporter type-3 (vGlut3) dependent. Despite the fact that glutamate excitotoxicity relates to NIHL pathogenesis, glutamate also has its dual effects on the noise-induced synapses loss. vGlut3-KO mice show moderate to severe hearing loss due to the impaired synaptic connection development for IHCs and acoustic nerve fibers. And Vglut3+/- mice show disrupted synaptic ribbon recovery after acoustic trauma, indicating that glutamate also participates in the repair of ribbon synapses in TTS model of NIHL [112].
Previous studies [115, 116] have suggested that calcium overload as a vital contributor to glutamate excitotoxicity. There are two main kinds of glutamate receptors in the postsynaptic region, termed as N-methyl-d-aspartate (NMDA) receptor and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) receptor [117]. Though NMDA receptors play the key role in calcium influx and consequent excitotoxicity in the central nervous system, intratympanic injection of NMDA receptor antagonist AM-101 shows no obvious protective effect on the tone ABR threshold at any frequencies, indicating that calcium influx mediated by NMDA receptors is not the most important contributor of glutamate excitotoxicity in the cochlea [115].
As for AMPA receptor, it is an ionic channel type receptor of glutamate, containing four subunits, namely, GluA1, GluA2, GluA3, and GluA4. Once activated, AMPA receptors mediate fast moving of multiple ions crossing cellular membrane [117, 118]. GluA2 has been testified to decrease Ca2+ permeability greatly [119–121]. Recent studies have revealed the existence of GluA2-lacking calcium-permeable AMPA receptors (CP-AMPARs) [116, 122]. Interestingly, IEM-1460, a CP-AMPAR inhibitor, significantly ameliorates the noise-induced decline of synapse integrity, as well as ABR threshold shift, shedding light on the therapeutic potential of NIHL by targeting CP-AMPARs [116].
The reduction of synaptic ribbons and acoustic neural degeneration is reversible in noise-induced transient hearing loss. It is reported that the recovery of cochlear synaptic ribbons after noise exposure is regulated by some endogenous neural factors named neurotrophins. Neurotrophins are endogenous molecules which are vital to the morphogenesis of nervous system development and neuron survival. Brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) are highly expressed in the inner hair cells and supporting cells to guide the auditory neural fibers to form precise synaptic connection in postnatal mice and stabilize the connection in adult mice [123]. Knockout of Ntf3 in supporting cells using Plp1-CreER mouse line impairs the high-frequency hearing while Bdnf depletion does not. Reversely, overexpression of Ntf3 or Bdnf in supporting cells and hair cells both attenuate the noise-induced synaptic ribbon reduction and eventual hearing loss [124]. Direct administration of NT-3 also attenuates NIHL progression [125]. Interestingly, as the detection technology develops into more microcosmic level, NT-3 is also found in the extracellular vesicles from human multipotent stromal cells (MSC-EVs). And administration of MSC-EVs not only raises the survival rate of spiral ganglion neurons cultured in vitro but also ameliorates the NIHL in vivo [126].
The effect of BDNF on NIHL is quite complicated and controversial. Many studies have reported that BDNF administration can enhance auditory neuron survival [127–129]. Meanwhile, tropomyosin-related kinase type B (TrkB, a selective BDNF receptor) agonist DHF [109], amitriptyline, and 7,8-dihydroxyflavone [130] increase resistance to acoustic trauma. However, genetically, depletion of Bdnf gene in adult mice shows protective effect against NIHL because of impaired glutamate release from IHCs, indicating the essential role of BDNF for maintenance of IHC function as well [131].
3.5. Calcium Overload
Calcium is one of the most important ions for the development and physiological function of the inner ear [132, 133]. Under physiological circumstances, calcium content in hair cells and spiral ganglion neurons is quite low [134, 135]. In response to the sound stimulation, calcium influx and calcium-induced calcium release (CICR) from the endoplasmic reticulum significantly increase intracellular calcium and trigger the release of neurotransmitter from the IHCs to transform mechanical signals to electrical signals [136, 137]. Moreover, the spontaneous calcium spike from the IHCs also participates in the formation and functional maturation of the afferent auditory nerve [138]. Calcium takes part in various physiological functions as one of the main second messenger molecules, while excessive accumulation of calcium under stress like ischemia-reperfusion injury [139] contributes to cell death in several pathways. Previous studies show that calcium is also involved in the acoustic ototoxicity [135, 140, 141]. Intracellular calcium concentration is significantly upregulated in auditory hair cells, especially outer hair cells shortly after acoustic overstimulation though returns to basal level within 30-45 minutes [135]. The calcium concentration is also found increased abruptly in endolymph of the cochlea after intense noise exposure [141]. Minami et al. have reported that the expression of calcineurin, a calcium/calmodulin-dependent phosphatase, is restrictedly increased in dying hair cells marked by propidium iodide after noise overstimulation [142]. Calcineurin can dephosphorylate and activate the classic proapoptotic protein BAD, and the systemic administration of calcineurin inhibitor FK506 reduced the apoptosis of hair cells evidently [142, 143].
Though strong evidence has proved that excessive calcium overload contributes to noise-induced hair cell death, the pharmacological drug development targeting calcium overload is still contentious. Voltage-gated calcium channels (VGCCs) facilitating calcium influx are proved to be responsible for the noise-induced intracellular calcium overload. There are five different types of VGCCs, namely, L-, T-, N-, P/Q-, and R-type [144]. It is reported that administration of T-type calcium channel blockers like ethosuximide and trimethadione prior to noise exposure can effectively reduce the hearing threshold shift [145]. However, other researchers suggest that L-type calcium channel blockers [146] attenuate NIHL while T-type calcium channel blockers cannot.
3.6. Energy Metabolism
Transient ATP depletion in the cochlear perilymph following acoustic trauma has been proved by studies [147, 148]. Likewise, the extracellular ATP content in the inner ear also decreased upon ischemia stress [149]. Since either the production of endogenous antioxidants or the recovery of spiral ganglion cells upon noise exposure is energy consuming, researchers proposed the hypothesis that transient ATP depletion might weaken the defense ability of the cochlea against acoustic trauma. 5′-Adenosine monophosphate- (AMP-) activated protein kinase (AMPK), the energy sensor protein responding to fluctuation of intracellular AMP/ATP ration [150], has been reported to be activated by noise-induced ATP depletion and responsible for the pathogenesis of NIHL [44]. Parallel to transient energy exhaustion, AMPK activates Rac-1, belonging to GTPase family which can phosphate its downstream targets via hydrolyzing GTP and trigger p67 protein phosphorylation [151]. p67 is one of the functional subunits of NADPH oxidase 3 (NOX3), which contributes to noise overexposure-induced ROS overproduction in hair cells and eventual cell death [152]. Reversely, excessive ROS accumulation also activates the AMPKα signaling, marked by enhanced phosphorylated AMPKα, while systematical administration of antioxidant N-acetyl-L-cysteine (NAC) decreases both phosphorylated AMPKα and oxidative stress [153]. Noise-induced phosphorylation of AMPKα is also regulated by liver kinase B1 (LKB1), mediated by the transient intracellular ATP depletion. Indirect inhibition of AMPKα or its upstream regulatory gene LKB1 can ameliorate noise-induced necrosis of acoustic hair cells [147].
However, there is hardly any effective way to boost energy generation in hair cells after noise exposure. It is reported that transient increasing serum glucose level with administration of glucose can strengthen the cochlear defense system against NIHL by increasing ATP and NADPH content in hair cells [154]. Nevertheless, the diabetic mice with constant high blood glucose show much more severe noise-induced hair cell death and spiral ganglion neuron loss, which might be due to the blood flow ration reduction induced by chronic inflammation [155].
3.7. Others
3.7.1. Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) play a key role in the extracellular matrix (ECM) homeostasis and remodeling. For example, MMP-2 is reported to be involved in synaptic remodeling after cochlear lesion [156]. Either overactivation or excessive inhibition of MMPs can lead to multiple disorders like carcinoma invasion and migration [157]. RNA sequencing data reveal that MMPs are upregulated in the acute phase after noise exposure and decreased gradually over time. A short-term administration of doxycycline, an inhibitor of MMPs, can attenuate noise-induced hearing loss while a seven-day application regimen exacerbates the hair cell death reversely [158]. MMPs are regulated by cysteinyl leukotriene and its type 1 receptor (CysLTR1), which is also increased in the cochlea after acoustic overstimulation. A four-day application of montelukast, a leukotriene receptor antagonist, can ameliorate NIHL damage [159].
3.7.2. Pannexins
Like the well-researched connexin channels, pannexin protein is also involved in the intracellular communication channel formation allowing the passage of the small molecules and ions. Panx3 is one of the main kinds of pannexin protein expressed in the cochlea [160]. A previous study [161] has proved that genetic depletion of paxn3 has no impact on the proper morphogenesis of the inner ear and hearing though the cochlear bone is slightly impaired. Strikingly, the panx3 knockout mice show decreased susceptibility to NIHL while the mechanism underlying its acoustic protective effect still requires further exploration.
4. Gene Variations Related to NIHL
Up to now, there are more than 30 gene variations reported to be relevant to NIHL in animal studies or human epidemiology investigations.
4.1. Oxidative Stress and DNA Repair-Related Gene Variations
Since oxidative stress contributes to the pathogenesis of NIHL, mutations of multiple genes involving in the antioxidative system have been proved to alter noise susceptibility. Catalase (CAT), superoxide dismutase (SOD2), and the antioxidant paraoxonase (PON) are three main proteins playing important roles in scavenging free radicals; mutation of which weakens the intracellular defensive ability against noise-induced oxidative damage [71, 162, 163]. NFL2E2 (also known as Nrf2) is a vital transcriptional factor regulating various antioxidative protein expressions such as HO-1, CAT, SOD, and GPx [65]. Polymorphisms of NFL2E2 (rs6726395 and rs77684420) have been confirmed as related to genetic vulnerability for NIHL in a Chinese population-based study [164]. Other genes, like NOX3 encoding NADPH oxidase-3 catalyzing the generation of superoxide [165], HSPA1L encoding chaperons assisting antioxidative proteins to fold correctly [166], and GST (glutathione S-transferase) family [167, 168] which participate in the metabolism of an intracellular antioxidant GSH, have been reported as NIHL-related genes.
Moreover, free radicals and lipid peroxide induced by acoustic trauma could do harm to DNA integrity and might cause mutations of certain sequences. APE1 [169] and OGG1 [170], encoding DNA repair enzymes to remove specific abnormal bases, are found to be associated with NIHL susceptibility as well.
4.2. Apoptosis-Related Gene Variations
Another case-control study [171] involving Chinese workers with or without noise exposure history identifies two SNPs of CASP3 (rs1049216 and rs6948) significantly correlated to decreased NIHL risk, due to impaired apoptosis of hair cells since caspase3 serves as the central role of apoptosis induction.
4.3. Gap Junction-Related Gene Variations
Connexin 26, which is encoded by GJB2, is involved in the gap junction establishment between hair cells, allowing intercellular communications of ions and other small molecules [172, 173]. GJB2 SNP (rs3751385) is identified as a NIHL susceptible genetic mutation in a study applied in Polish workers [174]. Meanwhile, another study including Chinese workers reports that GJB2 SNP (rs137852540) combined with SOD2 and CAT mutations increased the vulnerability to NIHL [162]. The correlation between GJB mutation and NIHL vulnerability is also confirmed in Cx26 knockdown mice [174].
4.4. Potassium Channel-Related Gene Variations
Gene polymorphisms associated with K ion circulation in the inner ear are also associated with resistance to noise-induced hearing loss. KCNQ1/KCNE1 variation affecting potassium recycling is identified related to NIHL [175, 176]. Mutations of other potassium channel-related genes like KCNQ4 [175], KCNJ10 [176], and KCNMA1 [177] are also related to NIHL vulnerability. Potassium channels located in the outer hair cells in the cochlea which are responsible for the K+ influx regulate the excitability of OHCs. Reportedly, the potassium concentration in the cochlear endolymph of the guinea pig is upregulated shortly after acoustic injury [141, 178]. However, the exact mechanism behind potassium circulation disruption and NIHL is still lack of strong evidence.
4.5. Tip-Link-Related Gene Variations
The maturation of stereocilia in the hair cells is vital for hearing onset because it is where mechanical signals of sound waves are transformed into electrical signals that could be transported through hair cells and neurons. Tip-link, made of cadherins, plays an important part in the maintenance of ordered structure of stereocilia. A study revealed that mutations of PCDH15 and CDH23, two genes encoding cadherins for the integrity of stereocilia especially tip-link, are related to susceptibility to NIHL [179, 180].
4.6. Others
The purinergic receptor 2 (P2X2 receptor) is highly expressed in the cochlear sensory epithelium, especially in supporting cells of the greater epithelial ridge. P2X2 receptor can be activated by ATP [181] which is released from hair cells through connexins, and plays an important role in the development of the cochlea and hearing function [182]. A mutation of P2X2 gene (c.178G>T) is found responsible for progressive hearing loss and NIHL susceptibility in a Chinese family [183].
Another genome-wide association study suggested that AUTS2 SNP (rs35075890) and PTPRN2 SNP (rs10081191) are associated with NIHL vulnerability [184], which are commonly thought to be related to mental disorders and metabolic diseases according to previous studies [185].
There are quite more genes, with multiple physiological functions, reported to be NIHL-associated genetic factors, while the exact roles of which in the pathogenesis of NIHL still require deeper explorations (see more details in Table 1).
Table 1.
NIHL-related gene variations.
| Class | Gene name | Variations | Function | |
|---|---|---|---|---|
| Oxidative stress | NOX3 | NADPH oxidase-3 | rs33652818 [165] | Generation of superoxide (O2·-) |
| CAT | Catalase | rs7943316 [162, 163] | Hydrogen peroxide decomposition | |
| GSTM1 | Glutathione S-transferase mu 1 | Null [167, 168] | GSH metabolism | |
| GSTP1 | Glutathione S-transferase pi 1 | Ile (105)/Ile (105) [167, 168] | GSH metabolism | |
| GSTT1 | Glutathione S-transferase theta 1 | Null [167, 168] | GSH metabolism | |
| NFL2E2 | Nuclear factor erythroid-derived 2-like 2 | rs6726395 rs77684420 [164] |
Transcriptional factor regulating various antioxidative protein expression | |
| HSPA1L | Heat shock protein family A member 1 like | rs2227956 [166] | Antioxidative protein stabilizer | |
| PON2 | Paraoxonase 2 | S311C [71] | Antioxidative protein | |
| SOD2 | Superoxide dismutase 2 | IVS3-23T/G IVS3-60T/G [71] |
Catalyzing the destruction of free radical | |
|
| ||||
| Apoptosis | CASP3 | Caspase3 | rs1049216 rs6948 [171] |
Intracellular apoptosis promoter |
|
| ||||
| DNA methylation | DNMT1 | DNA methyltransferase 1 | rs2228611 [186] | DNA methylation |
| DNMT3A | DNA methyltransferase 3 alpha | rs749131 [186] | DNA methylation | |
|
| ||||
| Gap junction | GJB1 | Gap junction protein beta1 | rs1997625 [176] | Gap junction channels |
| GJB2 | Gap junction protein beta2 | rs3751385 [162, 176] | Gap junction channels | |
| GJB4 | Gap junction protein beta4 | rs755931 [176] | Gap junction channels | |
|
| ||||
| DNA repair | APE1 | Apurinic/apyrimidinic endodeoxy-ribonuclease 1 | T1349G T656G [169] |
Encoding the endonuclease, a DNA repair enzyme |
| OGG1 | 8-Oxoguanine DNA glycosylase | Ser326Cys [170] | DNA repair enzyme | |
|
| ||||
| Ion channels | KCNMA1 | Potassium calcium-activated channel subfamily M alpha1 | rs696211 [177] | Ca2+-activated K+ channel |
| KCNE1 | Potassium voltage-gated channel subfamily E regulatory subunit 1 | rs2070358 rs1805128 [175, 176] |
K+ channel | |
| KCNJ10 | Potassium inwardly-rectifying channel subfamily J member 10 | rs1130183 [176] | K+ channel | |
| KCNQ1 | Potassium voltage-gated channel subfamily Q member 1 | rs7945327 rs11022922 rs718579 rs163171 rs2056892 [175, 176] |
K+ channel | |
| KCNQ4 | Potassium voltage-gated channel subfamily Q member 4 | rs4660468 [175] | K+ channel | |
|
| ||||
| Stereocilia structure | PCDH15 | Protocadherin related 15 | rs11004085 rs7095441 [179] |
Encoding cadherin for the integrity of stereocilia, especially tip-link |
| CDH23 | Cadherin 23 | rs3752752 [180] | Encoding cadherin for the integrity of stereocilia, especially tip-link | |
| MYH14 | Myosin heavy chain 14 | rs667907 rs588035 [179] |
Might contribute to tip-link integrity | |
|
| ||||
| Others | EYA4 | EYA transcriptional coactivator and phosphatase 4 | rs3813346 [187] | Multifunctional transcriptional factor |
| FOXO3 | Forkhead box O3 | rs2802292 rs3777781 rs212769 [188] |
Multifunctional transcriptional factor | |
| GRHL2 | Grainyhead-like 2 | rs611419 [189, 190] | Multifunctional transcriptional factor | |
| HOTAIR | HOX transcript antisense RNA | rs4759314 [191] | Regulatory lncRNA | |
| MYO1A | Myosin 1a | rs1552245 [177] | Actin-based molecular motors | |
| NOTCH1 | Notch receptor 1 | rs3124594 rs3124603 [192] |
Notch signaling transduction | |
| NCL | Nucleolin | rs7598759 [193] | Ribosome biogenesis | |
| P2RX2 | Purinergic receptor P2X2 | V60L [183] | Purinergic signaling transduction | |
| AUTS2 | Autism susceptibility candidate 2 | rs35075890 [185] | Multifunctions | |
| PTPRN2 | Protein tyrosine phosphatase receptor type N2 | rs10081191 [184] | Multifunctions | |
5. Therapeutics
It is widely acknowledged the lack of approved medications for NIHL treatment, and recent pharmaceutical explorations are heralding a new era in the field.
5.1. Approved Medications
Clinically, few medications have been identified as effective except glucocorticoid for NIHL. Glucocorticoid has been proved to exhibit various protective effects against NIHL, highlighted by its anti-inflammatory activity via inhibiting the synthesis and release of inflammatory molecules like prostaglandins and leukotrienes. Dexamethasone, as the most commonly used glucocorticoid in clinical practice, was administered postintense white noise with intratympanic or intraperitoneal injection. And two routines of administration show similar hearing recovery effects; both are significantly better than the saline group [194].
5.2. Drugs in Clinical Trials
The phase 2 trial report of ebselen has assessed its safety and efficacy on NIHL. Ebselen, the GPx1 mimic, aims at oxidative stress reduction. In this randomized, double-blind, placebo-controlled phase 2 trial, subjects receiving 400 mg ebselen show significantly protective effect on transient noise-induced hearing impairment with no side effects reported [195].
5.3. Potential Medications
5.3.1. Medications Targeting Oxidative Stress
Qter, the artificial analog of an endogenous antioxidant coenzyme Q10, was administered systematically to noise-exposed mice and significantly reduced the threshold shift after acoustic trauma and promoted outer hair cell survival [196]. Moreover, the dendrites of spiral ganglion cells and cortical neuronal morphology are significantly restored by Qter application for its role involved in free radicals scavenging and regeneration of antioxidants like reduced glutathione (GSH)[197]. And redox homeostasis is maintained by Qter treatment demonstrated by reduction of 4-HNE or DHE (the markers of lipid peroxidation) immunostaining-positive cells in the cochleae.
Parallel to Qter, a peptide derived from human telomerase called GV1001 attenuates sensory hair cell death targeting the noise-induced excessive ROS/RNS accumulation, as suggested by the byproducts of lipid peroxidation (4-HNE) and protein nitration (3-NT) [58]. Moreover, GV1001 can also ameliorate kanamycin-induced hearing loss in mice, indicating that excessive ROS/RNS and its downstream cascades like inflammation and apoptosis underlie the sharing pathological contributors for noise and aminoglycoside-induced ototoxicity. Taking into consideration previous studies regarding the therapeutic effects of GV1001, we hypothesize that GV1001 might also exert otoprotective effects against NIHL, because excessive ROS/RNS generation is thought to underlie the causative mechanisms of NIHL.
The systematical administration of methylene blue prior to noise trauma promotes the acoustic hair cell survival via ameliorating the oxidative stress-induced dysfunction of respiratory electron transport chain, featured by the reservation of complex IV activity and ATP production [198]. Moreover, methylene blue induces generation of neurotrophin-3 (NT-3) to preserve the synaptic ribbons around inner hair cells upon noise exposure [199].
5.3.2. Medications Targeting Inflammation
Avenanthramide-C (AVN-C), a natural flavonoid purified from oats, has shown anti-inflammatory and antioxidant activities in in vitro experiments. Notably, AVN-C has great water solubility and blood-labyrinth barrier permeability, suggesting pharmaceutical potential for NIHL treatment. Researchers have found that peritoneal injection of AVN-C protects hair cells from noise trauma by increasing antioxidant defense and decreasing proinflammatory cytokines like IL-1β and Tnf [200].
5.4. Medications Targeting Hearing Protective Genes
Isl1, which is highly expressed in prosensory region during otocyst development and no longer expressed in the postnatal cochlea, attenuates noise-triggered hair cell loss when artificially overexpressed specifically in postnatal hair cells. Though the exact mechanism behind the protective effect of Isl1 is not fully understood, it proposes a bold hypothesis that overexpression of acoustic progenitor developmental genes in the adult cochlea might increase the hair cell defense system against noise stress [201].
5.5. Emerging Nanoparticle Medications
Nanomedication, as a newly emerged therapy, was applied in the NIHL treatment. Nanosystems, including polyethylene glycol- (PEG-) coated poly lactic acid (PLA) nanoparticles and zeolitic imidazolate nanoparticles, were invented and applied to deliver steroid drugs to the inner ear. They show significant protective effect, even better than free steroid drugs, when administered systematically with better stability and biocompatibility [202, 203]. However, the efficacy and safety of nanoparticle-based medications still need further verification.
6. Conclusions
In this article, we systematically and comprehensively reviewed the epidemiology, mechanical and molecular pathogenesis, and therapeutic inventions about NIHL. Oxidative stress, inflammation, calcium overload, glutamate excitotoxicity, and energy metabolism are the main contributors to NIHL up to now. These pharmaceutical exploring studies not only shed light on the clinical treatment for NIHL but also make further steps on the systematical understanding of NIHL pathogenesis.
Controversies are engendered on certain disputations of NIHL: Firstly, the oxidative stress in supporting cells upon acoustic trauma has been verified, but how to promote hair cell survival through regulating supporting cells is still blank. Secondly, the excitotoxicity of excessive glutamate exacerbates the destruction of acoustic nerve while Vglut3 knockout mice show impaired neural fiber recovery. Similarly, administration of BDNF ameliorates NIHL, but depletion of Bdnf receptor gene also shows protective effect by inhibiting glutamate release. Therefore, given the complexity of glutamate and neurotrophins, what is the proper way of modulating glutamate and neurotrophins for the maximum protective effect? Thirdly, because of the blood-perilymph barrier, most medications administered systematically are hard to penetrate into the cochlea. The development of nanomedication would alter the stability and permeability of drugs, which might bring hope to the emergency of more efficacious and less toxic medication. Moreover, with the research progresses in the field of regeneration of cochlear hair cells and neural fibers [24, 204–208], NIHL might be curable one day in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82071049, 81771010, and 81570911).
Conflicts of Interest
The authors claim that there are no conflicts of interest.
References
- 1.Liu Y., Qi J., Chen X., et al. Critical role of spectrin in hearing development and deafness. Science Advances. 2019;5(4) doi: 10.1126/sciadv.aav7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Li J., Yao X., et al. Loss of CIB2 causes profound hearing loss and abolishes mechanoelectrical transduction in mice. Frontiers in Molecular Neuroscience. 2017;10:p. 401. doi: 10.3389/fnmol.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi J., Liu Y., Chu C., et al. A cytoskeleton structure revealed by super-resolution fluorescence imaging in inner ear hair cells. Cell Discovery. 2019;5(1):p. 12. doi: 10.1038/s41421-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Q., Zhang Y., da P., et al. Deletion of Limk1 and Limk2 in mice does not alter cochlear development or auditory function. Scientific Reports. 2019;9(1, article 39769):p. 3357. doi: 10.1038/s41598-019-39769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S., Zhang Y., Dong Y., et al. Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea. Cellular and Molecular Life Sciences : CMLS. 2020;77(7, article 3291):1401–1419. doi: 10.1007/s00018-019-03291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng C., Wang Y., Guo L., et al. Age-related transcriptome changes in Sox2+ supporting cells in the mouse cochlea. Stem Cell Research & Therapy. 2019;10(1, article 1437):p. 365. doi: 10.1186/s13287-019-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X., Sun S., Qi J., et al. Bmi1 regulates the proliferation of cochlear supporting cells via the canonical Wnt signaling pathway. Molecular Neurobiology. 2017;54(2, article 9686):1326–1339. doi: 10.1007/s12035-016-9686-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S., Qiang R., Dong Y., et al. Hair cell regeneration from inner ear progenitors in the mammalian cochlea. American Journal of Stem Cells. 2020;9(3):25–35. [PMC free article] [PubMed] [Google Scholar]
- 9.Cox B., Chai R., Lenoir A., et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development (Cambridge, England) 2014;141(4):816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Z., Zou S. Y., Li M., et al. The nuclear transcription factor FoxG1 affects the sensitivity of mimetic aging hair cells to inflammation by regulating autophagy pathways. Redox Biology. 2020;28, article 101364 doi: 10.1016/j.redox.2019.101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang P., Zhang S., Cheng C., et al. The roles of exosomes in visual and auditory systems. Frontiers in Bioengineering and Biotechnology. 2020;8:p. 525. doi: 10.3389/fbioe.2020.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian F., Wang X., Yin Z., et al. The slc4a2b gene is required for hair cell development in zebrafish. Aging. 2020;12(19, article 103840):18804–18821. doi: 10.18632/aging.103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv J., Fu X., Li Y., et al. Deletion of Kcnj16 in mice does not alter auditory function. Frontiers in Cell and Development Biology. 2021;9, article 630361 doi: 10.3389/fcell.2021.630361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark W., Bohne B. Effects of noise on hearing. JAMA. 1999;281(17):1658–1659. doi: 10.1001/jama.281.17.1658. [DOI] [PubMed] [Google Scholar]
- 15.Noise and hearing loss. Lancet (London, England) 1991;338(8758):21–22. [PubMed] [Google Scholar]
- 16.The Cochrane Collaboration, Kateman E., Verbeek J. H., et al. Interventions to prevent occupational noise induced hearing loss. The Cochrane Database of Systematic Reviews. 2017;7 doi: 10.1002/14651858.CD006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cochrane Collaboration, Verbeek J. H., Kateman E., Morata T. C., Dreschler W., Sorgdrager B. Interventions to prevent occupational noise induced hearing loss. The Cochrane Database of Systematic Reviews. 2009;3 doi: 10.1002/14651858.CD006396.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Lynch E., Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discovery Today. 2005;10(19):1291–1298. doi: 10.1016/S1359-6446(05)03561-0. [DOI] [PubMed] [Google Scholar]
- 19.Fetoni A., Paciello F., Rolesi R., Paludetti G., Troiani D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: oxidative stress and ROS signaling. Free Radical Biology & Medicine. 2019;135:46–59. doi: 10.1016/j.freeradbiomed.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Harding G., Bohne B., Ahmad M. DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hearing Research. 2002;174(1-2):158–171. doi: 10.1016/S0378-5955(02)00653-6. [DOI] [PubMed] [Google Scholar]
- 21.Liberman M. Noise-induced hearing loss: permanent versus temporary threshold shifts and the effects of hair cell versus neuronal degeneration. Advances in Experimental Medicine and Biology. 2016;875:1–7. doi: 10.1007/978-1-4939-2981-8_1. [DOI] [PubMed] [Google Scholar]
- 22.Wang T., Chai R., Kim G. S., et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nature Communications. 2015;6(1):p. 6613. doi: 10.1038/ncomms7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., You D., Chen Y., Chai R., Li H. Regeneration of hair cells in the mammalian vestibular system. Frontiers in Medicine. 2016;10(2):143–151. doi: 10.1007/s11684-016-0451-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S., Liu D., Dong Y., et al. Frizzled-9+ supporting cells are progenitors for the generation of hair cells in the postnatal mouse cochlea. Frontiers in Molecular Neuroscience. 2019;12:p. 184. doi: 10.3389/fnmol.2019.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., Guo L., Lu X., et al. Characterization of Lgr6+ cells as an enriched population of hair cell progenitors compared to Lgr5+ cells for hair cell generation in the neonatal mouse cochlea. Frontiers in Molecular Neuroscience. 2018;11:p. 147. doi: 10.3389/fnmol.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Lu X., Guo L., et al. Hedgehog signaling promotes the proliferation and subsequent hair cell formation of progenitor cells in the neonatal mouse cochlea. Frontiers in Molecular Neuroscience. 2017;10:p. 426. doi: 10.3389/fnmol.2017.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson B., Tucci D. L., Merson M. H., O'Donoghue G. M. Global hearing health care: new findings and perspectives. Lancet (London, England) 2017;390(10111):2503–2515. doi: 10.1016/S0140-6736(17)31073-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang H., Yang J., Huang Z., et al. Accelerated age-related decline in hippocampal neurogenesis in mice with noise-induced hearing loss is associated with hippocampal microglial degeneration. Aging. 2020;12(19, article 103898):19493–19519. doi: 10.18632/aging.103898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S., Back S. A., Park K. H., et al. Comparison of functional and morphologic characteristics of mice models of noise-induced hearing loss. Auris, Nasus, Larynx. 2013;40(1):11–17. doi: 10.1016/j.anl.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Han W., Shin J. O., Ma J. H., et al. Distinct roles of stereociliary links in the nonlinear sound processing and noise resistance of cochlear outer hair cells. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(20):11109–11117. doi: 10.1073/pnas.1920229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu C., Cheng C., Wang Y., et al. Loss of ARHGEF6 causes hair cell stereocilia deficits and hearing loss in mice. Frontiers in Molecular Neuroscience. 2018;11:p. 362. doi: 10.3389/fnmol.2018.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi J., Zhang L., Tan F., et al. Espin distribution as revealed by super-resolution microscopy of stereocilia. American Journal of Translational Research. 2020;12(1):130–141. [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen J., Lysaght A. C., Liberman M. C., Qvortrup K., Stankovic K. M. Immediate and delayed cochlear neuropathy after noise exposure in pubescent mice. PLoS One. 2015;10(5, article e0125160) doi: 10.1371/journal.pone.0125160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi L., Liu K., Wang H., et al. Noise induced reversible changes of cochlear ribbon synapses contribute to temporary hearing loss in mice. Acta Oto-Laryngologica. 2015;135(11):1093–1102. doi: 10.3109/00016489.2015.1061699. [DOI] [PubMed] [Google Scholar]
- 35.Liu W., Xu X., Fan Z., et al. Wnt signaling activates TP53-induced glycolysis and apoptosis regulator and protects against cisplatin-induced spiral ganglion neuron damage in the mouse cochlea. Antioxidants & Redox Signaling. 2019;30(11):1389–1410. doi: 10.1089/ars.2017.7288. [DOI] [PubMed] [Google Scholar]
- 36.Yan W., Liu W., Qi J., et al. A three-dimensional culture system with Matrigel promotes purified spiral ganglion neuron survival and function in vitro. Molecular Neurobiology. 2018;55(3):2070–2084. doi: 10.1007/s12035-017-0471-0. [DOI] [PubMed] [Google Scholar]
- 37.Guo R., Ma X., Liao M., et al. Development and application of cochlear implant-based electric-acoustic stimulation of spiral ganglion neurons. ACS Biomaterials Science & Engineering. 2019;5(12):6735–6741. doi: 10.1021/acsbiomaterials.9b01265. [DOI] [PubMed] [Google Scholar]
- 38.Mayorga M. The pathology of primary blast overpressure injury. Toxicology. 1997;121(1):17–28. doi: 10.1016/S0300-483X(97)03652-4. [DOI] [PubMed] [Google Scholar]
- 39.Patterson J., Hamernik R. Blast overpressure induced structural and functional changes in the auditory system. Toxicology. 1997;121(1):29–40. doi: 10.1016/S0300-483X(97)03653-6. [DOI] [PubMed] [Google Scholar]
- 40.Shin S., Lyu A. R., Jeong S. H., Kim T. H., Park M. J., Park Y. H. Acoustic trauma modulates cochlear blood flow and vasoactive factors in a rodent model of noise-induced hearing loss. International Journal of Molecular Sciences. 2019;20(21):p. 5316. doi: 10.3390/ijms20215316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Littlefield P., Brungart D. Long-term sensorineural hearing loss in patients with blast-induced tympanic membrane perforations. Ear and Hearing. 2020;41(1):165–172. doi: 10.1097/AUD.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 42.Mizutari K. Update on treatment options for blast-induced hearing loss. Current Opinion in Otolaryngology & Head and Neck Surgery. 2019;27(5):376–380. doi: 10.1097/MOO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 43.Nicotera T., Hu B., Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. Journal of the Association for Research in Otolaryngology: JARO. 2003;4(4):466–477. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng H., Chen J., Sha S. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death & Disease. 2014;5(5, article e1262) doi: 10.1038/cddis.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohlemiller K., Wright J., Dugan L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiology & Neuro-Otology. 1999;4(5):229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- 46.Cobley J. Mechanisms of mitochondrial ROS production in assisted reproduction: the known, the unknown, and the intriguing. Antioxidants (Basel, Switzerland) 2020;9(10):p. 933. doi: 10.3390/antiox9100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W., Xiong H., Pang J., et al. Nrf2 activation protects auditory hair cells from cisplatin-induced ototoxicity independent on mitochondrial ROS production. Toxicology Letters. 2020;331:1–10. doi: 10.1016/j.toxlet.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Chong S., Low I., Pervaiz S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion. 2014;19:39–48. doi: 10.1016/j.mito.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Ojano-Dirain C., Antonelli P., le Prell C. G. Mitochondria-targeted antioxidant MitoQ reduces gentamicin-induced ototoxicity. American Neurotology Society [and] European Academy of Otology and Neurotology. 2014;35(3):533–539. doi: 10.1097/MAO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 50.He Z., Guo L., Shu Y., et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy. 2017;13(11):1884–1904. doi: 10.1080/15548627.2017.1359449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao S., Cheng C., Wang M., et al. Blebbistatin inhibits neomycin-induced apoptosis in hair cell-like HEI-OC-1 cells and in cochlear hair cells. Frontiers in Cellular Neuroscience. 2020;13:p. 590. doi: 10.3389/fncel.2019.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun S., Sun M., Zhang Y., et al. In vivo overexpression of X-linked inhibitor of apoptosis protein protects against neomycin-induced hair cell loss in the apical turn of the cochlea during the ototoxic-sensitive period. Frontiers in Cellular Neuroscience. 2014;8:p. 248. doi: 10.3389/fncel.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong Z., Fu X., Li H., et al. Citicoline protects auditory hair cells against neomycin-induced damage. Frontiers in Cell and Developmental Biology. 2020;8:p. 712. doi: 10.3389/fcell.2020.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y., Li W., He Z., et al. Pre-treatment with fasudil prevents neomycin-induced hair cell damage by reducing the accumulation of reactive oxygen species. Frontiers in Molecular Neuroscience. 2019;12:p. 264. doi: 10.3389/fnmol.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu X., Liu W., Fan Z., et al. c-Myb knockdown increases the neomycin-induced damage to hair-cell-like HEI- OC1 cells in vitro. Scientific Reports. 2017;7(1, article 41094) doi: 10.1038/srep41094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan M., Fang Q., He Z., et al. Inhibition of ARC decreases the survival of HEI-OC-1 cells after neomycin damage in vitro. Oncotarget. 2016;7(41, article 11336):66647–66659. doi: 10.18632/oncotarget.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasad K., Bondy S. Increased oxidative stress, inflammation, and glutamate: potential preventive and therapeutic targets for hearing disorders. Mechanisms of Ageing and Development. 2020;185, article 111191 doi: 10.1016/j.mad.2019.111191. [DOI] [PubMed] [Google Scholar]
- 58.Lee S., Han J. J., Lee S. Y., et al. Outcomes of peptide vaccine GV1001 treatment in a murine model of acute noise-induced hearing loss. Antioxidants (Basel, Switzerland) 2020;9(2):p. 112. doi: 10.3390/antiox9020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li A., You D., Li W., et al. Novel compounds protect auditory hair cells against gentamycin-induced apoptosis by maintaining the expression level of H3K4me2. Drug Delivery. 2018;25(1):1033–1043. doi: 10.1080/10717544.2018.1461277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He Z., Sun S., Waqas M., et al. Reduced TRMU expression increases the sensitivity of hair-cell-like HEI-OC-1 cells to neomycin damage in vitro. Scientific Reports. 2016;6(1, article 29621) doi: 10.1038/srep29621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fetoni A., Rolesi R., Paciello F., et al. Styrene enhances the noise induced oxidative stress in the cochlea and affects differently mechanosensory and supporting cells. Free Radical Biology & Medicine. 2016;101:211–225. doi: 10.1016/j.freeradbiomed.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Wenzel P., Kossmann S., Münzel T., Daiber A. Redox regulation of cardiovascular inflammation - immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radical Biology & Medicine. 2017;109:48–60. doi: 10.1016/j.freeradbiomed.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 63.Morioka S., Sakaguchi H., Yamaguchi T., et al. Hearing vulnerability after noise exposure in a mouse model of reactive oxygen species overproduction. Journal of Neurochemistry. 2018;146(4):459–473. doi: 10.1111/jnc.14451. [DOI] [PubMed] [Google Scholar]
- 64.Galicia-Moreno M., Lucano-Landeros S., Monroy-Ramirez H. C., et al. Roles of Nrf2 in liver diseases: molecular, pharmacological, and epigenetic aspects. Antioxidants (Basel, Switzerland) 2020;9(10):p. 980. doi: 10.3390/antiox9100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cuadrado A., Rojo A. I., Wells G., et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Drug Discovery. 2019;18(4):295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 66.Moretti D., Tambone S., Cerretani M., et al. NRF2 activation by reversible KEAP1 binding induces the antioxidant response in primary neurons and astrocytes of a Huntington’s disease mouse model. Free Radical Biology & Medicine. 2021;162:243–254. doi: 10.1016/j.freeradbiomed.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi K., Dan K., Goto F., et al. The autophagy pathway maintained signaling crosstalk with the Keap1-Nrf2 system through p62 in auditory cells under oxidative stress. Cellular Signalling. 2015;27(2):382–393. doi: 10.1016/j.cellsig.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 68.Fetoni A., Paciello F., Rolesi R., et al. Rosmarinic acid up-regulates the noise-activated Nrf2/HO-1 pathway and protects against noise-induced injury in rat cochlea. Free Radical Biology & Medicine. 2015;85:269–281. doi: 10.1016/j.freeradbiomed.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 69.Forsberg L., de Faire U., Morgenstern R. Oxidative stress, human genetic variation, and disease. Archives of Biochemistry and Biophysics. 2001;389(1):84–93. doi: 10.1006/abbi.2001.2295. [DOI] [PubMed] [Google Scholar]
- 70.Chen Q., Reis S. E., Kammerer C. M., et al. Association between the severity of angiographic coronary artery disease and paraoxonase gene polymorphisms in the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. American Journal of Human Genetics. 2003;72(1):13–22. doi: 10.1086/345312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fortunato G., Marciano E., Zarrilli F., et al. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clinical Chemistry. 2004;50(11):2012–2018. doi: 10.1373/clinchem.2004.037788. [DOI] [PubMed] [Google Scholar]
- 72.Coling D. E., Yu K. C. Y., Somand D., et al. Effect of SOD1 overexpression on age- and noise-related hearing loss. Free Radical Biology and Medicine. 2003;34(7):873–880. doi: 10.1016/S0891-5849(02)01439-9. [DOI] [PubMed] [Google Scholar]
- 73.Brown K., Maqsood S., Huang J. Y., et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metabolism. 2014;20(6):1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J., Yang Y., Li W. SIRT3 deficiency increases mitochondrial oxidative stress and promotes migration of retinal pigment epithelial cells. Experimental Biology and Medicine (Maywood, N.J.) 2021;246(8):877–887. doi: 10.1177/1535370220976073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Someya S., Yu W., Hallows W. C., et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mizushima N., Levine B. Autophagy in human diseases. The New England Journal of Medicine. 2020;383(16):1564–1576. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 77.Zhou H., Qian X., Xu N., et al. Disruption of Atg7-dependent autophagy causes electromotility disturbances, outer hair cell loss, and deafness in mice. Cell Death & Disease. 2020;11(10):p. 913. doi: 10.1038/s41419-020-03110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding Y., Meng W., Kong W., He Z., Chai R. The role of FoxG1 in the inner ear. Frontiers in Cell and Development Biology. 2020;8, article 614954 doi: 10.3389/fcell.2020.614954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He Z., Fang Q., Li H., et al. The role of FOXG1 in the postnatal development and survival of mouse cochlear hair cells. Neuropharmacology. 2019;144:43–57. doi: 10.1016/j.neuropharm.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 80.Li H., Song Y., He Z., et al. Meclofenamic acid reduces reactive oxygen species accumulation and apoptosis, inhibits excessive autophagy, and protects hair cell-like HEI-OC1 cells from cisplatin-induced damage. Frontiers in Cellular Neuroscience. 2018;12:p. 139. doi: 10.3389/fncel.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan H., Wang X., Hill K., et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxidants & Redox Signaling. 2015;22(15):1308–1324. doi: 10.1089/ars.2014.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li P., Bing D., Wang S., et al. Sleep deprivation modifies noise-induced cochlear injury related to the stress hormone and autophagy in female mice. Frontiers in Neuroscience. 2019;13:p. 1297. doi: 10.3389/fnins.2019.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pattingre S., Espert L., Biard-Piechaczyk M., Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90(2):313–323. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 84.Defourny J., Aghaie A., Perfettini I., Avan P., Delmaghani S., Petit C. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(16):8010–8017. doi: 10.1073/pnas.1821844116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujioka M., Okano H., Ogawa K. Inflammatory and immune responses in the cochlea: potential therapeutic targets for sensorineural hearing loss. Frontiers in Pharmacology. 2014;5:p. 287. doi: 10.3389/fphar.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okano T., Nakagawa T., Kita T., et al. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. Journal of Neuroscience Research. 2008;86(8):1758–1767. doi: 10.1002/jnr.21625. [DOI] [PubMed] [Google Scholar]
- 87.Hayashi Y., Suzuki H., Nakajima W., et al. Cochlear supporting cells function as macrophage-like cells and protect audiosensory receptor hair cells from pathogens. Scientific Reports. 2020;10(1, article 63654):p. 6740. doi: 10.1038/s41598-020-63654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirose K., Discolo C. M., Keasler J. R., Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. The Journal of Comparative Neurology. 2005;489(2):180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- 89.Tornabene S., Sato K., Pham L., Billings P., Keithley E. M. Immune cell recruitment following acoustic trauma. Hearing Research. 2006;222(1-2):115–124. doi: 10.1016/j.heares.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Fujioka M., Kanzaki S., Okano H. J., Masuda M., Ogawa K., Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. Journal of Neuroscience Research. 2006;83(4):575–583. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- 91.Wang W., Zhang L. S., Zinsmaier A. K., et al. Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biology. 2019;17(6, article e3000307) doi: 10.1371/journal.pbio.3000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satoh H., Firestein G. S., Billings P. B., Harris J. P., Keithley E. M. Tumor necrosis factor-alpha, an initiator, and etanercept, an inhibitor of cochlear inflammation. The Laryngoscope. 2002;112(9):1627–1634. doi: 10.1097/00005537-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 93.Rodrigues J., Bachi A. L. L., Silva G. A. V., et al. New insights on the effect of TNF alpha blockade by gene silencing in noise-induced hearing loss. International Journal of Molecular Sciences. 2020;21(8):p. 2692. doi: 10.3390/ijms21082692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paciello F., di Pino A., Rolesi R., et al. Anti-oxidant and anti-inflammatory effects of caffeic acid: in vivo evidences in a model of noise-induced hearing loss. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 2020;143, article 111555 doi: 10.1016/j.fct.2020.111555. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y., Chen D., Zhao L., et al. Nfatc4 deficiency attenuates ototoxicity by suppressing Tnf-mediated hair cell apoptosis in the mouse cochlea. Frontiers in Immunology. 2019;10:p. 1660. doi: 10.3389/fimmu.2019.01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dhukhwa A., Bhatta P., Sheth S., et al. Targeting inflammatory processes mediated by TRPVI and TNF-α for treating noise-induced hearing loss. Frontiers in Cellular Neuroscience. 2019;13:p. 444. doi: 10.3389/fncel.2019.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frenz D., Galinovic-Schwartz V., Liu W., Flanders K. C., van de Water T. R. Transforming growth factor β1 is an epithelial-derived signal peptide that influences otic capsule formation. Developmental Biology. 1992;153(2):324–336. doi: 10.1016/0012-1606(92)90117-Y. [DOI] [PubMed] [Google Scholar]
- 98.Kim H., Kang K. Y., Baek J. G., Jo H. C., Kim H. Expression of TGFbeta family in the developing internal ear of rat embryos. Journal of Korean Medical Science. 2006;21(1):136–142. doi: 10.3346/jkms.2006.21.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okano J., Takigawa T., Seki K., Suzuki S., Shiota K., Ishibashi M. Transforming growth factor beta 2 promotes the formation of the mouse cochleovestibular ganglion in organ culture. The International Journal of Developmental Biology. 2005;49(1):23–31. doi: 10.1387/ijdb.041905jo. [DOI] [PubMed] [Google Scholar]
- 100.Stolfi C., Troncone E., Marafini I., Monteleone G. Role of TGF-beta and Smad7 in gut inflammation, fibrosis and cancer. Biomolecules. 2021;11(1):p. 17. doi: 10.3390/biom11010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wahl S. Transforming growth factor beta (TGF-beta) in inflammation: a cause and a cure. Journal of Clinical Immunology. 1992;12(2):61–74. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 102.Yoshimura A., Wakabayashi Y., Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. Journal of Biochemistry. 2010;147(6):781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nolte M., Margadant C. Controlling immunity and inflammation through integrin-dependent regulation of TGF-β. Trends in Cell Biology. 2020;30(1):49–59. doi: 10.1016/j.tcb.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Murillo-Cuesta S., RodrÃguez-de la Rosa L., Contreras J., et al. Transforming growth factor β1 inhibition protects from noise-induced hearing loss. Frontiers in Aging Neuroscience. 2015;7:p. 32. doi: 10.3389/fnagi.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moon J., Nakahira K., Chung K. P., et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nature Medicine. 2016;22(9):1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Sai N., Shi X., Zhang Y., et al. Involvement of cholesterol metabolic pathways in recovery from noise-induced hearing loss. Neural Plasticity. 2020;2020:17. doi: 10.1155/2020/6235948.6235948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang G., Zheng H., Pyykko I., Zou J. The TLR-4/NF-κB signaling pathway activation in cochlear inflammation of rats with noise-induced hearing loss. Hearing Research. 2019;379:59–68. doi: 10.1016/j.heares.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 108.Tahera Y., Meltser I., Johansson P., et al. NF-kappaB mediated glucocorticoid response in the inner ear after acoustic trauma. Journal of Neuroscience Research. 2006;83(6):1066–1076. doi: 10.1002/jnr.20795. [DOI] [PubMed] [Google Scholar]
- 109.Meltser I., Cederroth C. R., Basinou V., Savelyev S., Lundkvist G. S., Canlon B. TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Current Biology : CB. 2014;24(6):658–663. doi: 10.1016/j.cub.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cederroth C., Park J. S., Basinou V., et al. Circadian regulation of cochlear sensitivity to noise by circulating glucocorticoids. Current Biology: CB. 2019;29(15):2477–2487.e6. doi: 10.1016/j.cub.2019.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hakuba N., Koga K., Gyo K., Usami S. I., Tanaka K. Exacerbation of noise-induced hearing loss in mice lacking the glutamate transporter GLAST. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(23):8750–8753. doi: 10.1523/JNEUROSCI.20-23-08750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim K., Payne S., Yang-Hood A., et al. Vesicular glutamatergic transmission in noise-induced loss and repair of cochlear ribbon synapses. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2019;39(23):4434–4447. doi: 10.1523/JNEUROSCI.2228-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chamorro Á., Dirnagl U., Urra X., Planas A. M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. The Lancet. Neurology. 2016;15(8):869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 114.Dong X., Wang Y., Qin Z. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacologica Sinica. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bing D., Lee S. C., Campanelli D., et al. Cochlear NMDA receptors as a therapeutic target of noise-induced tinnitus. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology. 2015;35(5):1905–1923. doi: 10.1159/000374000. [DOI] [PubMed] [Google Scholar]
- 116.Hu N., Rutherford M., Green S. Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(7):3828–3838. doi: 10.1073/pnas.1914247117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hanada T. Ionotropic glutamate receptors in epilepsy: a review focusing on AMPA and NMDA receptors. Biomolecules. 2020;10(3):p. 464. doi: 10.3390/biom10030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niedzielski A., Wenthold R. Expression of AMPA, kainate, and NMDA receptor subunits in cochlear and vestibular ganglia. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995;15(3):2338–2353. doi: 10.1523/JNEUROSCI.15-03-02338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tanaka H., Grooms S. Y., Bennett M. V. L., Zukin R. S. The AMPAR subunit GluR2: still front and center-stage1. Brain Research. 2000;886(1-2):190–207. doi: 10.1016/S0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- 120.Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science (New York, N.Y.) 1991;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 121.Verdoorn T., Burnashev N., Monyer H., Seeburg P., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science (New York, N.Y.) 1991;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- 122.Sebe J., Cho S., Sheets L., Rutherford M. A., von Gersdorff H., Raible D. W. Ca2+-permeable AMPARs mediate glutamatergic transmission and excitotoxic damage at the hair cell ribbon synapse. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2017;37(25):6162–6175. doi: 10.1523/JNEUROSCI.3644-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fritzsch B., Tessarollo L., Coppola E., Reichardt L. F. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Progress in Brain Research. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- 124.Wan G., Gómez-Casati M. E., Gigliello A. R., Liberman M. C., Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. eLife. 2014;3 doi: 10.7554/eLife.03564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duan M., Agerman K., Ernfors P., Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7597–7602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Warnecke A., Harre J., Staecker H., et al. Extracellular vesicles from human multipotent stromal cells protect against hearing loss after noise trauma in vivo. Clinical and Translational Medicine. 2020;10(8, article e262) doi: 10.1002/ctm2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wise A., Richardson R., Hardman J., Clark G., O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. The Journal of Comparative Neurology. 2005;487(2):147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 128.Rejali D., Lee V. A., Abrashkin K. A., Humayun N., Swiderski D. L., Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hearing Research. 2007;228(1-2):180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pettingill L., Wise A. K., Geaney M. S., Shepherd R. K. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. PLoS One. 2011;6(4, article e18733) doi: 10.1371/journal.pone.0018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fernandez K., Watabe T., Tong M., et al. Trk agonist drugs rescue noise-induced hidden hearing loss. JCI Insight. 2021;6(3) doi: 10.1172/jci.insight.142572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zuccotti A., Kuhn S., Johnson S. L., et al. Lack of brain-derived neurotrophic factor hampers inner hair cell synapse physiology, but protects against noise-induced hearing loss. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(25):8545–8553. doi: 10.1523/JNEUROSCI.1247-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fettiplace R., Nam J. Tonotopy in calcium homeostasis and vulnerability of cochlear hair cells. Hearing Research. 2019;376:11–21. doi: 10.1016/j.heares.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sziklai I. The significance of the calcium signal in the outer hair cells and its possible role in tinnitus of cochlear origin. European Archives of Oto-Rhino-Laryngology. 2004;261(10):517–525. doi: 10.1007/s00405-004-0745-9. [DOI] [PubMed] [Google Scholar]
- 134.Ferrary E., Tran Ba Huy P., Roinel N., Bernard C., Amiel C. Calcium and the inner ear fluids. Acta Oto-Laryngologica. Supplementum. 1988;460:13–17. doi: 10.3109/00016488809125130. [DOI] [PubMed] [Google Scholar]
- 135.Zuo H., Cui B., She X., Wu M. Changes in guinea pig cochlear hair cells after sound conditioning and noise exposure. Journal of Occupational Health. 2008;50(5):373–379. doi: 10.1539/joh.L8032. [DOI] [PubMed] [Google Scholar]
- 136.Castellano-Muñoz M., Ricci A. Role of intracellular calcium stores in hair-cell ribbon synapse. Frontiers in Cellular Neuroscience. 2014;8:p. 162. doi: 10.3389/fncel.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fettiplace R. The role of calcium in hair cell transduction. Society of General Physiologists Series. 1992;47:343–356. [PubMed] [Google Scholar]
- 138.Brandt A., Striessnig J., Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(34):10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fiolet J., Baartscheer A. Cellular calcium homeostasis during ischemia; a thermodynamic approach. Cardiovascular Research. 2000;45(1):100–106. doi: 10.1016/S0008-6363(99)00294-1. [DOI] [PubMed] [Google Scholar]
- 140.Fridberger A., Flock A., Ulfendahl M., Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):7127–7132. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikeda K., Kusakari J., Takasaka T. Ionic changes in cochlear endolymph of the guinea pig induced by acoustic injury. Hearing Research. 1988;32(2-3):103–110. doi: 10.1016/0378-5955(88)90081-0. [DOI] [PubMed] [Google Scholar]
- 142.Minami S., Yamashita D., Schacht J., Miller J. M. Calcineurin activation contributes to noise-induced hearing loss. Journal of Neuroscience Research. 2004;78(3):383–392. doi: 10.1002/jnr.20267. [DOI] [PubMed] [Google Scholar]
- 143.Vicente-Torres M., Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. Journal of Neuroscience Research. 2006;83(8):1564–1572. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Neumaier F., Dibué-Adjei M., Hescheler J., Schneider T. Voltage-gated calcium channels: determinants of channel function and modulation by inorganic cations. Progress in Neurobiology. 2015;129:1–36. doi: 10.1016/j.pneurobio.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 145.Shen H., Zhang B., Shin J. H., et al. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hearing Research. 2007;226(1-2):52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heinrich U., Maurer J., Mann W. Alteration of loosely bound calcium in the guinea pig organ of Corti after treatment with diltiazem as calcium channel blocker. European Archives of Oto-Rhino-Laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology-Head and Neck Surgery. 1997;254(5):223–229. doi: 10.1007/BF00874093. [DOI] [PubMed] [Google Scholar]
- 147.Hill K., Yuan H., Wang X., Sha S. H. Noise-induced loss of hair cells and cochlear synaptopathy are mediated by the activation of AMPK. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2016;36(28):7497–7510. doi: 10.1523/JNEUROSCI.0782-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vlajkovic S., Housley G. D., Muñoz D. J. B., et al. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 2004;126(3):763–773. doi: 10.1016/j.neuroscience.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 149.Thalmann R., Miyoshi T., Thalmann I. The influence of ischemia upon the energy reserves of inner ear tissues. The Laryngoscope. 1972;82(12):2249–2272. doi: 10.1288/00005537-197212000-00013. [DOI] [PubMed] [Google Scholar]
- 150.Winder W. W., Thomson D. M. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochemistry and Biophysics. 2007;47(3):332–347. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- 151.Chen F., Zheng H. W., Hill K., Sha S. H. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(36):12421–12430. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sumimoto H., Minakami R., Miyano K. Soluble regulatory proteins for activation of NOX family NADPH oxidases. Methods in Molecular Biology (Clifton, N.J.) 2019;1982:121–137. doi: 10.1007/978-1-4939-9424-3_8. [DOI] [PubMed] [Google Scholar]
- 153.Wu F., Xiong H., Sha S. Noise-induced loss of sensory hair cells is mediated by ROS/AMPKα pathway. Redox Biology. 2020;29, article 101406 doi: 10.1016/j.redox.2019.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiong H., Lai L., Ye Y., Zheng Y. Glucose protects cochlear hair cells against oxidative stress and attenuates noise-induced hearing loss in mice. Neuroscience Bulletin. 2021;37(5):657–668. doi: 10.1007/s12264-020-00624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fujita T., Yamashita D., Katsunuma S., Hasegawa S., Tanimoto H., Nibu K. I. Increased inner ear susceptibility to noise injury in mice with streptozotocin-induced diabetes. Diabetes. 2012;61(11):2980–2986. doi: 10.2337/db11-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fredrich M., Illing R. MMP-2 is involved in synaptic remodeling after cochlear lesion. Neuroreport. 2010;21(5):324–327. doi: 10.1097/WNR.0b013e328335b518. [DOI] [PubMed] [Google Scholar]
- 157.Overall C., López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nature Reviews. Cancer. 2002;2(9):657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 158.Hu B., Cai Q., Hu Z., et al. Metalloproteinases and their associated genes contribute to the functional integrity and noise-induced damage in the cochlear sensory epithelium. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(43):14927–14941. doi: 10.1523/JNEUROSCI.1588-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park J. S., Kang S. J., Seo M. K., Jou I., Woo H. G., Park S. M. Role of cysteinyl leukotriene signaling in a mouse model of noise-induced cochlear injury. Proceedings of the National Academy of Sciences. 2014;111(27):9911–9916. doi: 10.1073/pnas.1402261111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X., Streeter M., Liu Y. P., Zhao H. B. Identification and characterization of pannexin expression in the mammalian cochlea. The Journal of Comparative Neurology. 2009;512(3):336–346. doi: 10.1002/cne.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abitbol J., Kelly J. J., Barr K., Schormans A. L., Laird D. W., Allman B. L. Differential effects of pannexins on noise-induced hearing loss. The Biochemical Journal. 2016;473(24):4665–4680. doi: 10.1042/BCJ20160668. [DOI] [PubMed] [Google Scholar]
- 162.Wang S., Yu L. G., Liu R. P., et al. Gene-gene interaction of GJB2, SOD2, and CAT on occupational noise-induced hearing loss in Chinese Han population. Biomedical and Environmental Sciences: BES. 2014;27(12):965–968. doi: 10.3967/bes2014.131. [DOI] [PubMed] [Google Scholar]
- 163.Konings A., van Laer L., Pawelczyk M., et al. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Human Molecular Genetics. 2007;16(15):1872–1883. doi: 10.1093/hmg/ddm135. [DOI] [PubMed] [Google Scholar]
- 164.Wang B. S., Xu K., Zhang H., et al. Association between NFE2L2 gene polymorphisms and noise-induced hearing loss in a Chinese population. Biomedical and Environmental Sciences. 2019;32(6):465–470. doi: 10.3967/bes2019.063. [DOI] [PubMed] [Google Scholar]
- 165.Lavinsky J., Crow A. L., Pan C., et al. Genome-wide association study identifies nox3 as a critical gene for susceptibility to noise-induced hearing loss. PLoS Genetics. 2015;11(4, article e1005094) doi: 10.1371/journal.pgen.1005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Konings A., van Laer L., Michel S., et al. Variations in HSP70 genes associated with noise-induced hearing loss in two independent populations. European Journal of Human Genetics. 2009;17(3):329–335. doi: 10.1038/ejhg.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin C. Y., Wu J. L., Shih T. S., Tsai P. J., Sun Y. M., Guo Y. L. Glutathione S-transferase M1, T1, and P1 polymorphisms as susceptibility factors for noise-induced temporary threshold shift. Hearing Research. 2009;257(1-2):8–15. doi: 10.1016/j.heares.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 168.Shen H., Huo X., Liu K., et al. Genetic variation in GSTM1 is associated with susceptibility to noise-induced hearing loss in a Chinese population. Journal of Occupational and Environmental Medicine. 2012;54(9):1157–1162. doi: 10.1097/JOM.0b013e31825902ce. [DOI] [PubMed] [Google Scholar]
- 169.Shen H., Dou J., Han L., et al. Genetic variation in APE1 gene promoter is associated with noise-induced hearing loss in a Chinese population. International Archives of Occupational and Environmental Health. 2016;89(4, article 1100):621–628. doi: 10.1007/s00420-015-1100-8. [DOI] [PubMed] [Google Scholar]
- 170.Shen H., Cao J., Hong Z., et al. A functional Ser326Cys polymorphism in hOGG1 is associated with noise-induced hearing loss in a Chinese population. PLoS One. 2014;9(3, article e89662) doi: 10.1371/journal.pone.0089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu Y., Ni J., Qi M., et al. Associations of genetic variation in CASP3 gene with noise-induced hearing loss in a Chinese population: a case–control study. Environmental Health. 2017;16(1):p. 78. doi: 10.1186/s12940-017-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Elfgang C., Eckert R., Lichtenberg-Fraté H., et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. The Journal of Cell Biology. 1995;129(3):805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhao H., Yu N., Fleming C. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.van Eyken E., van Laer L., Fransen E., et al. The contribution of GJB2 (Connexin 26) 35delG to age-related hearing impairment and noise-induced hearing loss. Otology & Neurotology. 2007;28(7):970–975. doi: 10.197/MAO.0b013e3180dca1b9. [DOI] [PubMed] [Google Scholar]
- 175.van Laer L., Carlsson P. I., Ottschytsch N., et al. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Human Mutation. 2006;27(8):786–795. doi: 10.1002/humu.20360. [DOI] [PubMed] [Google Scholar]
- 176.Pawelczyk M., van Laer L., Fransen E., et al. Analysis of gene polymorphisms associated with K ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Annals of Human Genetics. 2009;73(4):411–421. doi: 10.1111/j.1469-1809.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 177.Zhang X., Ni Y., Liu Y., et al. Screening of noise-induced hearing loss (NIHL)-associated SNPs and the assessment of its genetic susceptibility. Environmental Health. 2019;18(1):p. 30. doi: 10.1186/s12940-019-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnstone B. M., Patuzzi R., Syka J., Syková E. Stimulus-related potassium changes in the organ of Corti of guinea-pig. The Journal of Physiology. 1989;408(1):77–92. doi: 10.1113/jphysiol.1989.sp017448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Konings A., van Laer L., Wiktorek-Smagur A., et al. Candidate gene association study for noise-induced hearing loss in two independent noise-exposed populations. Annals of Human Genetics. 2009;73(2):215–224. doi: 10.1111/j.1469-1809.2008.00499.x. [DOI] [PubMed] [Google Scholar]
- 180.Kowalski T. J., Pawelczyk M., Rajkowska E., Dudarewicz A., Sliwinska-Kowalska M. Genetic variants of CDH23 associated with noise-induced hearing loss. Otology & Neurotology. 2014;35(2):358–365. doi: 10.1097/MAO.0b013e3182a00332. [DOI] [PubMed] [Google Scholar]
- 181.Brake A., Wagenbach M., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 182.Mazzarda F., Delia A., Massari R., et al. Organ-on-chip model shows that ATP release through connexin hemichannels drives spontaneous Ca2+signaling in non-sensory cells of the greater epithelial ridge in the developing cochlea. Lab on a Chip. 2020;20(16):3011–3023. doi: 10.1039/D0LC00427H. [DOI] [PubMed] [Google Scholar]
- 183.Yan D., Zhu Y., Walsh T., et al. Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proceedings of the National Academy of Sciences. 2013;110(6):2228–2233. doi: 10.1073/pnas.1222285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Niu Y., Xie C., Du Z., et al. Genome-wide association study identifies 7q11.22 and 7q36.3 associated with noise-induced hearing loss among Chinese population. Journal of Cellular and Molecular Medicine. 2021;25(1):411–420. doi: 10.1111/jcmm.16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hori K., Nagai T., Shan W., et al. Cytoskeletal regulation by AUTS2 in neuronal migration and neuritogenesis. Cell Reports. 2014;9(6):2166–2179. doi: 10.1016/j.celrep.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 186.Ding E., Liu J., Guo H., et al. DNMT1 and DNMT3A haplotypes associated with noise-induced hearing loss in Chinese workers. Scientific Reports. 2018;8(1, article 12193) doi: 10.1038/s41598-018-29648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang X., Liu Y., Zhang L., et al. Associations of genetic variations in EYA4, GRHL2 and DFNA5 with noise-induced hearing loss in Chinese population: a case-control study. Environmental Health. 2015;14(1):p. 77. doi: 10.1186/s12940-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guo H., Ding E., Bai Y., et al. Association of genetic variations in FOXO3 gene with susceptibility to noise induced hearing loss in a Chinese population. PLoS One. 2017;12(12, article e0189186) doi: 10.1371/journal.pone.0189186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li X., Huo X., Liu K., et al. Association between genetic variations in GRHL2 and noise-induced hearing loss in Chinese high intensity noise exposed workers: a case-control analysis. Industrial Health. 2013;51(6):612–621. doi: 10.2486/indhealth.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu X., Yang Q., Jiao J., et al. Genetic variation in POU4F3 and GRHL2 associated with noise-induced hearing loss in Chinese population: a case-control study. International Journal of Environmental Research and Public Health. 2016;13(6):p. 561. doi: 10.3390/ijerph13060561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang B., Ding E., Shen H., et al. Association of TagSNP in lncRNA HOTAIR with susceptibility to noise-induced hearing loss in a Chinese population. Hearing Research. 2017;347:41–46. doi: 10.1016/j.heares.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 192.Ding E., Liu J., Shen H., et al. Notch polymorphisms associated with sensitivity of noise induced hearing loss among Chinese textile factory workers. BMC Medical Genetics. 2018;19(1):p. 168. doi: 10.1186/s12881-018-0676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grondin Y., Bortoni M. E., Sepulveda R., et al. Genetic polymorphisms associated with hearing threshold shift in subjects during first encounter with occupational impulse noise. PLoS One. 2015;10(6, article e0130827) doi: 10.1371/journal.pone.0130827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Han M. A. Therapeutic effect of dexamethasone for noise-induced hearing loss: systemic versus intratympanic injection in mice. Otology & Neurotology. 2015;36(5):755–762. doi: 10.1097/MAO.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 195.Kil J., Lobarinas E., Spankovich C., et al. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (London, England) 2017;390(10098):969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
- 196.Fetoni A., de Bartolo P., Eramo S. L. M., et al. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(9):4011–4023. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lenaz G., Fato R., Formiggini G., Genova M. L. The role of coenzyme Q in mitochondrial electron transport. Mitochondrion. 2007;7:S8–S33. doi: 10.1016/j.mito.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 198.Oz M., Lorke D. E., Hasan M., Petroianu G. A. Cellular and molecular actions of methylene blue in the nervous system. Medicinal Research Reviews. 2011;31(1):93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Park J., Jou I., Park S. Attenuation of noise-induced hearing loss using methylene blue. Cell Death & Disease. 2014;5(4, article e1200) doi: 10.1038/cddis.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Umugire A., Lee S., Kim D., Choi M., Kim H. S., Cho H. H. Avenanthramide-C prevents noise- and drug-induced hearing loss while protecting auditory hair cells from oxidative stress. Cell Death Discovery. 2019;5(1):p. 115. doi: 10.1038/s41420-019-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang M., Kantardzhieva A., Scheffer D., Liberman M. C., Chen Z. Y. Hair cell overexpression of Islet1 reduces age-related and noise-induced hearing loss. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(38):15086–15094. doi: 10.1523/JNEUROSCI.1489-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Horie R., Sakamoto T., Nakagawa T., Ishihara T., Higaki M., Ito J. Stealth-nanoparticle strategy for enhancing the efficacy of steroids in mice with noise-induced hearing loss. Nanomedicine (London, England) 2010;5(9):1331–1340. doi: 10.2217/nnm.10.88. [DOI] [PubMed] [Google Scholar]
- 203.Xu X., Lin K., Wang Y., et al. A metal-organic framework based inner ear delivery system for the treatment of noise-induced hearing loss. Nanoscale. 2020;12(30):16359–16365. doi: 10.1039/D0NR04860G. [DOI] [PubMed] [Google Scholar]
- 204.Liu L., Chen Y., Qi J., et al. Wnt activation protects against neomycin-induced hair cell damage in the mouse cochlea. Cell Death & Disease. 2016;7(3, article e2136) doi: 10.1038/cddis.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang Y., Zhang S., Zhang Z., et al. Knockdown of Foxg1 in Sox9+ supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse utricle. Aging (Albany NY) 2020;12(20, article 104009):19834–19851. doi: 10.18632/aging.104009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guo R., Zhang S., Xiao M., et al. Accelerating bioelectric functional development of neural stem cells by graphene coupling: implications for neural interfacing with conductive materials. Biomaterials. 2016;106:193–204. doi: 10.1016/j.biomaterials.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 207.Guo R., Xiao M., Zhao W., et al. 2D Ti3C2TxMXene couples electrical stimulation to promote proliferation and neural differentiation of neural stem cells. Acta Biomaterialia. 2020;S1742-7061(20):30749–30752. doi: 10.1016/j.actbio.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 208.Guo R., Li J., Chen C., et al. Biomimetic 3D bacterial cellulose-graphene foam hybrid scaffold regulates neural stem cell proliferation and differentiation. Colloids and Surfaces. B, Biointerfaces. 2021;200, article 111590 doi: 10.1016/j.colsurfb.2021.111590. [DOI] [PubMed] [Google Scholar]


