Abstract
Sarcopenia causes functional disorders and decreases the quality of life. Thus, it has attracted substantial attention in the aging modern world. Dysbiosis of the intestinal microbiota is associated with sarcopenia; however, it remains unclear whether prebiotics change the microbiota composition and result in the subsequent recovery of muscle atrophy in elderly patients with sarcopenia. This study aimed to assess the effects of prebiotics in super-elderly patients with sarcopenia. We analyzed the effects of 1-kestose on the changes in the intestinal microbiota and body composition using a next-generation sequencer and a multi-frequency bioimpedance analysis device. The Bifidobacterium longum population was significantly increased in the intestine after 1-kestose administration. In addition, in all six patients after 12 weeks of 1-kestose administration, the skeletal muscle mass index was greater, and the body fat percentage was lower. This is the first study to show that administration of a prebiotic increased the population of B. longum in the intestinal microbiota and caused recovery of muscle atrophy in super-elderly patients with sarcopenia.
Keywords: 1-kestose, Bifidobacterium longum, intestinal microbiota, prebiotics, sarcopenia
INTRODUCTION
Sarcopenia is associated with a progressive, systemic loss of skeletal muscle mass (SMM) and strength [1]. It causes functional disorders and decreases the quality of life; hence, it has attracted substantial attention in the aging modern world. Recently, a study on germ-free mice noted that the removal of intestinal microbiota caused muscle atrophy, while the administration of microbial metabolites partly reversed skeletal muscle impairments in these mice [2]. The relationship between intestinal microbiota and muscle mass has been reported; it was noted that dysbiosis of the intestinal microbiota was associated with sarcopenia [3]. Nevertheless, it remains unclear whether prebiotics or probiotics change the intestinal microbiota composition, leading to a recovery of muscle atrophy in elderly patients with sarcopenia.
It has been reported that 1-kestose (a component of fructooligosaccharide, which is composed of one glucose and two fructose molecules) has prebiotic properties and can increase the Bifidobacterium population in the intestinal microbiota in rats [4]. Therefore, in this study, we analyzed the changes in the intestinal microbiota and body composition (such as muscle mass) as the prebiotic effects of 1-kestose in super-elderly patients with sarcopenia.
MATERIALS AND METHODS
Subjects and study protocol
The European working group on sarcopenia in older people recommends using the presence of both a low muscle mass and low muscle function (strength or performance) for the diagnosis of sarcopenia [1]. The study subjects were six elderly patients (90.8 ± 5.4 years) who were admitted to palliative care units and were diagnosed with sarcopenia due to a low muscle mass (skeletal muscle index [SMI] <5.66 kg/m2), low muscle strength (handgrip strength <20 kg), and low physical performance (usual gait speed <0.8 m/sec). Regarding underlying diseases, five patients had a history of cerebral infraction, and one had a history of cirrhosis. The Eastern Cooperative Oncology Group performance status (PS) was 3 (capable of only limited selfcare, confined to bed or chair for more than 50% of their waking hours) in all patients whose sarcopenia was closely related to their life prognosis (Table 1). All patients were stable and had experienced long hospital stays. No probiotics or antibiotics had been used during the observation period.
Table 1. Patient characteristics.
| Case | Age(yrs) | Gender | Underlyingdisease | SMI(kg/m²) | HS(kg) | Means oftransportation | PS | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 88 | F | Cirrhosis | 4.9 | 8 | Wheelchair | 3 | Death |
| 2 | 98 | F | Cerebral infraction | 2.7 | 5.5 | Wheelchair | 3 | Survival |
| 3 | 95 | F | Cerebral infraction | 2.2 | 4.5 | Wheelchair | 3 | Survival |
| 4 | 88 | M | Cerebral infraction | 4.4 | 15 | Crutch gait | 3 | Death |
| 5 | 82 | F | Cerebral infraction | 4.2 | 8.5 | Wheelchair | 3 | Survival |
| 6 | 94 | F | Cerebral infraction | 4.5 | 7.5 | Wheelchair | 3 | Survival |
SMI: skeletal muscle index; HS: handgrip strength; PS: performance status.
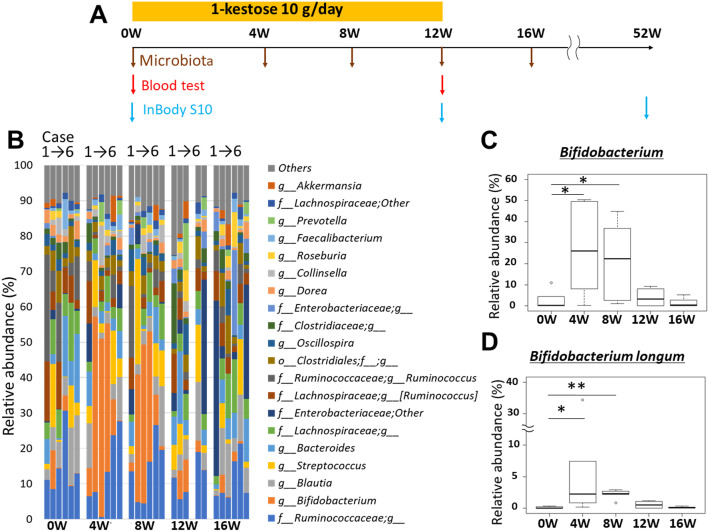
The patients were orally administered 10 g/day of 1-kestose (purify >95%, B Food Science Co., Ltd., Aichi, Japan) twice a day for 12 weeks (Fig. 1A). We assessed the body composition, blood parameters (albumin, lipids, HbA1c, etc.), and intestinal microbiota before and after 1-kestose administration. For body composition assessment, we used a multi-frequency bioimpedance analysis device (InBody S10, InBody Japan, Tokyo, Japan) and measured the SMI, SMM, trunk muscle (TM), total muscle mass (TMM), body fat mass (BFM), and body fat percentage (BFP) before and 12 and 52 weeks after 1-kestose administration. For intestinal microbiota evaluation, we used a next-generation sequencer (Illumina’s MiSeq) and conducted 16S rRNA metagenome analysis before and 4, 8, 12, and 16 weeks after 1-kestose administration (Fig. 1A). The intestinal microbiota composition was evaluated using Quantitative Insights Into Microbial Ecology (QIIME) ver. 1.9.1, and β-diversity was measured by analysis of UniFrac distances.
Fig. 1.
. Schema of the study protocol (A). Time course analysis of the intestinal microbiota. The data are shown for cases 1–6 at every time point (before and 4, 8, 12, and 16 weeks after 1-kestose administration) (B). Time course of Bifidobacterium at the genus level (C). Time course of Bifidobacterium longum at the species level (D). Bars show the SD of the data. Data were analyzed using the Friedman test, followed by the Wilcoxon signed-rank test (*p<0.05; **p<0.01).
Intestinal sample collection
Intestinal samples (approximately 100 mg) were suspended in 900 µL of guanidine thiocyanate solution (100 mM Tris–HCl [pH 9.0], 40 mM EDTA, and 4 M guanidine thiocyanate) and frozen at −80°C until further analysis [5].
DNA preparations from intestinal samples
The collected samples were sent to the laboratory of Miyarisan Pharmaceutical Co., Ltd. and stored at 4°C. DNA was extracted from collected fecal samples using a glass bead extraction method and purified, according to a previously reported method [6]. The amount of DNA was determined using a QuantiFluor ONE dsDNA System and Quantus Fluorometer (Promega, Madison, WI, USA).
PCR amplification and analysis of 16S rRNA sequences
The V3–V4 region of the 16S rRNA gene was amplified from stool DNA samples by PCR using a TaKaRa Ex Taq Hot Start PCR mixture (Takara Bio, Shiga, Japan). The primers used for PCR amplification were 341F and 805R, which contained the Illumina index and sequencing adapter overhangs [7]. PCR assays were performed using a TaKaRa PCR Thermal Cycler Dice Touch device (Takara Bio, Shiga, Japan) with the following parameters: initial denaturation at 98°C for 30 sec, followed by 35 cycles of 98°C for 10 sec and 60°C for 30 sec. The PCR products were purified and size-selected using SPRIselect (Beckman Coulter, Brea, CA, USA). DNA concentrations were quantified with a QuantiFluor ONE dsDNA System and Quantus Fluorometer (Promega, Madison, WI, USA), and equal amounts of purified PCR products were pooled for subsequent Illumina MiSeq sequencing. Sequencing was carried out with MiSeq Regent Kit V3 (600 cycles; Illumina, San Diego, CA, USA), according to the manufacturer’s instructions. Sequence processing and quality assessment were performed using the QIIME package (version 1.9.1; http://qiime.org), open-source software created to address the problem of obtaining sequencing data from raw sequences for interpretation and database deposition [8]. To obtain an overall diversity analysis for subsequent comparative and statistical evaluations, we merged the Biological Observation Matrix (BIOM) tables provided by QIIME into a unique biom table using a script included in the QIIME package. Paired-end reads were merged using the Fastq-join script in ea-utils with the parameters m =6 and p=20 and then quality-filtered using QIIME’s script split_libraries_fastq.py (r=3, p=0.75, q=20, n=0). De novo and reference-based chimera detection and removal were performed using USEARCH v6.1 with the Greengenes v13.8 database. Operational taxonomic units (OTUs) were chosen using an open reference OTU-picking pipeline against the 97% identity of the pre-clustered Greengenes v13.8 database using UCLUST. According to the manufacturer, a QIIME alpha diversity analysis script is used to perform rarefaction analysis by subsampling the OTU biom table on the basis of the minimum rarefaction depth value chosen by the user depending on the minimum number of sequences/samples obtained. For our subset, this value was 35,807. Then, using different metrics, alpha diversity was computed for each rarefied OTU table. We used two non-phylogeny-based metrics: Chao 1 and the Shannon index. After performing the rarefaction evaluation, the QIIME beta diversity analysis script was used to compute beta diversity with the rarefied OTUs table using different metrics. We used phylogeny-based metrics (unweighted and weighted UniFrac) [9]. Finally, the script was used to obtain a distance metric to compute the principal coordinate analysis (PCoA) and convert it into plots for the visualization of results. We used PERMANOVA to evaluate the statistical significance of beta diversity distances.
Statistical analysis
The relative abundance of bacterial genera and alpha-diversity indices were compared between the groups using the Friedman test following by the Wilcoxon signed-rank test in the R software. The significance of beta-diversity was evaluated by PERMNOVA in QIIME 1.9.1.
Body composition and blood parameters are expressed as the mean ± SE. A two-sided Student’s two-sample paired t-test was used for statistical analyses with IBM SPSS Statistics, version 24.0 (IBM SPSS Japan Inc., Tokyo, Japan). In this study, values of p<0.05 were considered statistically significant.
Ethics approval and consent to participate
The study was reviewed and approved by the Institutional Review Board of Niigata University.
Consent for publication
Written informed consent was obtained from the patients for publication of the report and accompanying images.
RESULTS
Intestinal microbiota
Serial analysis of intestinal microbiota showed a substantial increase in the Bifidobacterium population, and Bifidobacterium longum (at the species level) was the only intestinal organism to have increased significantly 4 (p<0.05) and 8 weeks (p<0.01) after 1-kestose administration (Fig. 1B–D) as compared with the control (0 weeks). The Supplementary Figure shows the numerical data on the serial evaluation of the intestinal microbiota in the study subjects.
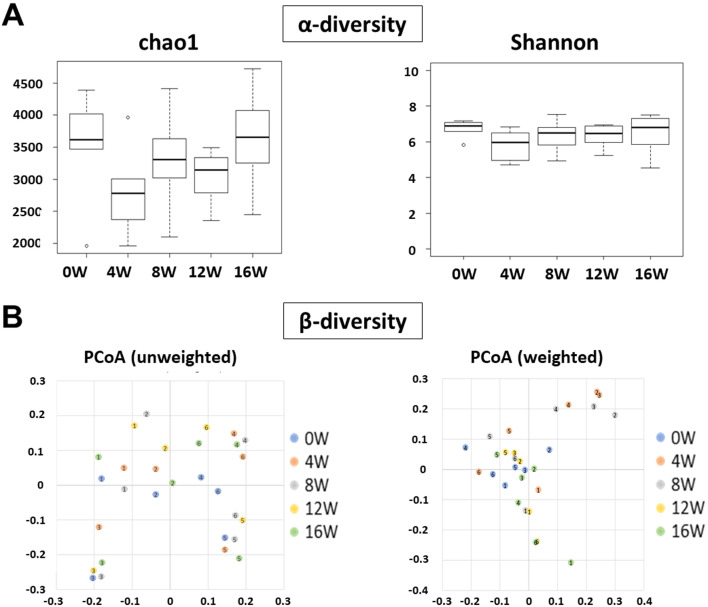
The α-diversity analysis of the species distribution is shown in Fig. 2A. Using the Chao 1 index and the Shannon index, we examined the richness and evenness of the intestinal microbiota in the study groups. There were no significant intergroup differences in the Chao1 and Shannon indices. In addition to assessing the α-diversity, we used weighted and unweighted UniFrac distances to assess the intergroup similarities in the intestinal microbial communities, i.e., the β-diversity. Using weighted distances, a significant difference was observed only between 0 and 4 weeks; however, the PCoA plots did not show a clear separation of one group from the others (Fig. 2B).
Fig. 2.
. The α-diversity and β-diversity data for this study. Bars show the SD of the data. The α-diversity was analyzed using the Friedman test, followed by the Wilcoxon signed-rank test in the R software (A). The significance of β-diversity was evaluated by PERMNOVA in QIIME 1.9.1 (B).
Body composition and blood parameters
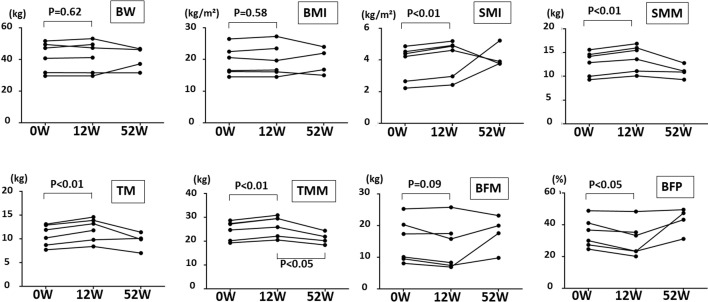
Comparison of body composition between before and 12 weeks after 1-kestose administration showed that while the body weight and body mass index did not change significantly, the average (mean ± SD) SMI, SMM, TM, and TMM values were significantly higher and the BFP was significantly lower in all patients after 12 weeks (0 weeks→12 weeks; 24.6 ± 3.6→26.4 ± 3.9, p<0.01, for TMM; 10.8 ± 2.1→12.0 ± 2.2, p<0.01, for TM; 12.8 ± 2.3→13.9 ± 2.5, p<0.01, for SMM; 3.8 ± 1.0→4.2 ± 1.1, p<0.01, SMI; and 34.8 ± 8.4→30.6 ± 9.7, p<0.05, for BFP; Fig. 3). Apart from 1-kestose administration, there were no other factors (such as antibiotic administration or a change in medication) that affected the intestinal microbiota in our subjects. There were no other factors that could increase muscle weight during the administration of 1-kestose, because the elderly people in the palliative care unit could not receive intensive rehabilitation during the 1-kestose administration period. We also evaluated the relationship between the increase in the Bifidobacterium population and muscle mass; however, we could not find a significant relationship between the two. Fifty-two weeks after 1-kestose administration, two of the six patients died (Table 1); of the remaining patients, muscle mass decreased to the baseline level in three, whereas it increased in one due to sufficient rehabilitation (Fig. 3).
Fig. 3.
. Comparison of body composition between before and 12 and 52 weeks after 1-kestose administration (*p<0.05; **p<0.01). Significant increases in the SMI (p<0.01), SMM (p<0.01), TM (p<0.01), and TMM (p<0.01) and a significant decrease in BFP (p<0.05) were detected after 12 weeks as compared with the control. BW: body weight; BMI: body mass index; SMI: skeletal muscle mass index; SMM: skeletal muscle mass; TM: trunk muscle; TMM: total muscle mass; BFM: body fat mass; BFP: body fat percentage.
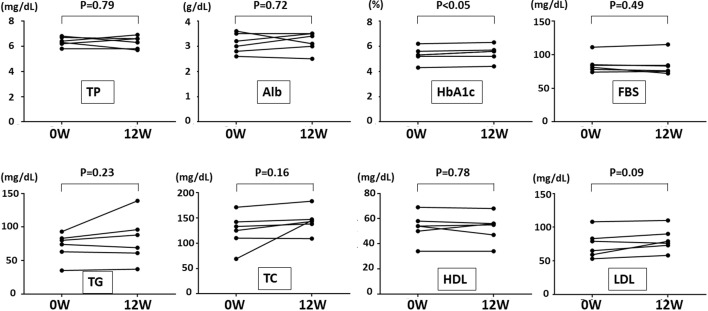
We compared the blood data between before and 12 weeks after 1-kestose administration. A slight, though significant, increase in the HbA1c level was detected after 12 weeks as compared with the control (5.4→5.5%; p<0.05). However, all changes in the HbA1c levels were also within the normal range. There were no significant differences in the other blood parameters, including the cholesterol level (Fig. 4).
Fig. 4.
. The blood data before and 12 weeks after 1-kestose administration. A significant increase in the HbA1c level (p<0.05) was detected after 12 weeks as compared with the control. TP: total protein; Alb: albumin; FBS: fasting blood sugar; TG: triglyceride; TC: total cholesterol; HDL: high-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol.
DISCUSSION
This study is the first to show evidence of the prebiotic effect of 1-kestose, which increased the population of Bifidobacterium longum in the intestinal microbiota and caused recovery from muscle atrophy in super-elderly patients with sarcopenia. Our results are consistent with those of an animal study in which Lactobacillus casei and B. longum improved physiological function and enhanced muscle strength in aged mice by regulating the intestinal microbiota [10]. Our study also supports the findings of an animal study that showed that a reduced muscle mass was associated with a distinct microbiota composition and reduced fermentative capacity in mice; the administration of probiotics and butyrate to mouse models with muscle wasting was associated with improved muscle mass [3, 11]. However, no studies have targeted the human microbiome associated with sarcopenia. While some reports have suggested interactions between the intestinal microbiota and secreted factors such as myokines in the skeletal muscle [12], it is necessary to perform detailed analyses on the underlying mechanisms by which 1-kestose improves muscle atrophy via B. longum.
Skeletal muscles collectively comprise the largest human organ. The muscle has recently attracted attention because of its functions, such as metabolism, secretion, and maintenance of morphology. Some reports have been published on the relationship between muscle atrophy and disease, emphasizing the necessity of therapeutic interventions against sarcopenia [1, 3]. It has also been reported that the combined effect of probiotics and exercise leads to an improvement in muscular strength and power [13, 14]. Carbohydrate fermentation is a core activity of the human intestinal microbiota; it produces energy and is responsible for the carbon metabolism in the colon [15]. However, there have been no previous reports regarding the relationship between 1-kestose, B. longum, and sarcopenia in humans. In this study, alteration of the intestinal microbiota (increased B. longum population) through 1-kestose administration led to the improvement of muscle atrophy in our patients with sarcopenia.
A relationship between microbiota composition and obesity has also been reported; fecal abundance of Bifidobacterium was found to be significantly lower in obese subjects than in lean subjects [16, 17]. Furthermore, the positive effects of Bifidobacterium breve strain B-3 (one of the promising anti-obesogenic strains) on body fat reduction in pre-obese adults have been reported [18]. Our study also showed that the administration of prebiotics was associated with a reduction in the BFP, thereby supporting the findings of these reports.
1-kestose has also been shown to improve lipid metabolism and insulin resistance in rats by altering the intestinal microbiota [4, 19]. In our study, no significant differences in the blood parameters were noted between before and after 1-kestose administration, with the exception of a slight increase in the HbA1c values (which were still within the normal range). These data also suggest that 1-kestose administration may have prevented the deterioration of blood parameters in the elderly patients.
In conclusion, our findings suggest an attractive supportive therapy for elderly sarcopenia patients. However, the change of gut microbial composition was subtle and transient, so further studies on a large scale are essential to conclude that 1-kestose has a beneficial effect on sarcopenia. Its combination with probiotics and/or additional support, such as rehabilitation, may further result in positive effects. We believe that there are multiple possibilities for 1-ketose to contribute to the improvement of the quality of life in the elderly.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
AUTHOR’S CONTRIBUTIONS
KT, AT, ON, YN, TY, MS, TY, SH, JY, JM, and KF diagnosed the patients and drafted the manuscript. YK, KO, AM, MA, MT, YK, TT, and ST analyzed the data. All authors critically reviewed the manuscript and approved the final draft.
FUNDING
The authors disclosed receipt of the following financial support for the research and authorship of this article: This work was supported by Miyarisan Pharmaceutical Co., Ltd. and B Food Science Co., Ltd.
CONFLICT OF INTEREST
The authors disclose the following: Kentaro Oka and Yasutoshi Kuroki are employees of Miyarisan Pharmaceutical Co., Ltd. Yoshihiro Kadota and Takumi Tochio are employees of B Food Science Co., Ltd. Shuji Terai received research funding from Miyarisan Pharmaceutical Co., Ltd. and B Food Science Co., Ltd. The remaining authors disclose no conflicts.
Supplementary Material
Acknowledgments
The authors thank Miyarisan Pharmaceutical Co., Ltd. and B Food Science Co. Ltd. for their cooperation.
REFERENCES
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People. 2010. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shawon L, Hyejin K, Isabel G, Musarrat R, Katherine M, Parag K, Laura C, Joel S, Joram P, Hongbo Z, Parasuraman P, Catherine M, Balázs G, Martin B, Johan A, Elaine H, Jeremy N, Walter W, Sven P. 2019. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med 24: 11(502) eaan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picca A, Fanelli F, Calvani R, Mulè G, Pesce V, Sisto A, Pantanelli C, Bernabei R, Landi F, Marzetti E. 2018. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators Inflamm 2018: 7026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tochio T, Kitaura Y, Nakamura S, Sugawa C, Takahashi M, Endo A, Shimomura Y. 2016. An alteration in the cecal microbiota composition by feeding of 1-kestose results in a marked increase in the cecal butyrate content in rats. PLoS One 11: e0166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Nishimoto Y, Mizutani S, Nakajima T, Hosoda F, Watanabe H, Saito Y, Shibata T, Yachida S, Yamada T. 2016. High stability of faecal microbiome composition in guanidine thiocyanate solution at room temperature and robustness during colonoscopy. Gut 65: 1574–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin JS, Touyama M, Yamada S, Yamazaki T, Benno Y. 2014. Alteration of a human intestinal microbiota under extreme life environment in the Antarctica. Biol Pharm Bull 37: 1899–1906. [DOI] [PubMed] [Google Scholar]
- 7.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. 2013. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. 2011. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinformatics Chapter 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parks DH, Beiko RG. 2012. Measuring community similarity with phylogenetic networks. Mol Biol Evol 29: 3947–3958. [DOI] [PubMed] [Google Scholar]
- 10.Ni Y, Yang X, Zheng L, Wang Z, Wu L, Jiang J, Yang T, Ma L, Fu Z. 2019. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res 63: e1900603. [DOI] [PubMed] [Google Scholar]
- 11.Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, Kinter M, Van Remmen H. 2015. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 14: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Liu B, Liang C, Li Y, Song YH. 2016. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol Metab 27: 335–347. [DOI] [PubMed] [Google Scholar]
- 13.Georges J, Lowery RP, Yaman G, Kerio C, Ormes J, McCleary SA. 2014. The effects of probiotic supplementation on lean body mass, strength, and power, and health indicators in resistance trained males: a pilot study. J Int Soc Sports Nutr 11: 38. 25132809 [Google Scholar]
- 14.Jäger R, Purpura M, Stone JD, Turner SM, Anzalone AJ, Eimerbrink MJ, Pane M, Amoruso A, Rowlands DS, Oliver JM. 2016. Probiotic Streptococcus thermophilus FP4 and bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients 8: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamprecht M, Frauwallner A. 2012. Exercise, intestinal barrier dysfunction and probiotic supplementation. Med Sport Sci 59: 47–56. [DOI] [PubMed] [Google Scholar]
- 16.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. 2010. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18: 190–195. [DOI] [PubMed] [Google Scholar]
- 17.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. 2010. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 104: 83–92. [DOI] [PubMed] [Google Scholar]
- 18.Minami J, Iwabuchi N, Tanaka M, Yamauchi K, Xiao JZ, Abe F, Sakane N. 2018. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: a randomized, double-blind, placebo-controlled trial. Biosci Microbiota Food Health 37: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe A, Kadota Y, Kamio R, Tochio T, Endo A, Shimomura Y, Kitaura Y. 2020. 1-Kestose supplementation mitigates the progressive deterioration of glucose metabolism in type 2 diabetes OLETF rats. Sci Rep 10: 15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.