Abstract
Background
Coronavirus disease 2019 (COVID-19) is widely acknowledged as a severe traumatic event, and depression, anxiety, and psychological distress are common in diagnosed patients. However, the correlations of biological indicators with emotion are rarely reported. The primary objective of this study was to explore the dysfunction of immune-inflammatory characteristics in patients with depression-anxiety symptoms.
Methods
We investigated the mental status of inpatients with COVID-19 in Wuhan and compared the differences in cytokines and lymphocytes between patients with and without depression-anxiety symptoms at admission. After two weeks of treatment, we evaluated the mental conditions and measured the cytokines and lymphocytes of the patients with depression and anxiety symptoms and explored the changes and their associations.
Results
Approximately half of the patients with COVID-19 had depression and anxiety symptoms, and the symptoms were related to the ratio of CD4+/CD8+ and the level of CD4+T lymphocytes. When compared with patients without depression-anxiety symptoms, CD4+T lymphocytes level was significantly higher in COVID-19 patients with depression-anxiety symptoms.
Conclusion
This study provided novel evidence regarding the association between depression and anxiety symptoms and immune characteristics, especially CD4+T lymphocyte levels, in COVID-19 patients. We emphasized the importance of paying attention to the dynamic immune process of patients diagnosed with COVID-19 with depression/anxiety.
Keywords: COVID-19, Anxiety, Depression, Immune-inflammatory characteristics, CD4+T lymphocytes
1. Introduction
The outbreak of a novel coronavirus disease in Wuhan, Hubei Province, China, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has attracted substantial attention globally due to its high transmission speed and serious threats to health (Sohrabi et al., 2020). On March 11, 2020, the World Health Organization (WHO) declared the outbreak a pandemic. To date, it has been reported that more than one hundred and forty-six million confirmed cases and three million deaths all over the world, and an upward trend continues (http://2019ncov.chinacdc.cn/2019-nCoV/global.html).
The symptoms of coronavirus disease 2019 (COVID-19) infection vary significantly among patients. The main manifestations include fever, chills, dry cough, and fatigue. Patients with severe cases can develop acute respiratory distress syndrome, acute respiratory failure and death (Chen et al., 2020b, Jin et al., 2020). In addition, previous studies have indicated that most patients with COVID-19 presented aberrant immune-inflammatory responses and cytokine storms, which were related to the progression of disease (Chen et al., 2020a, Zhu et al., 2020).
Apart from physical suffering, the mental health of patients with confirmed COVID-19 has gained attention. In the face of the serious infectious illness, the general public panicked, and a variety of psychiatric and mental conditions (i.e., stress, fatigue, worry, depression, anxiety, and insomnia) were reported at the early stages of the outbreak, especially among individuals with suspected symptoms (Sun et al., 2020, Wang et al., 2020). The patients were found to be under tremendous psychological stress during the period of waiting for diagnosis and treatment. Many studies have demonstrated that substantial psychological and physical stress could induce anxiety and depressive-like behaviors, which are related with the response of the immune system (Irwin and Miller 2007; Miller 2010; Cheng et al., 2015; Toben and Baune 2015; Beurel and Lowell 2018).
Earlier data showed that the patients hospitalized for an autoimmune disease or infection increased the risk of a mood disorder by 45% and 62%, respectively (Pape et al., 2019). Depression and anxiety are common in systemic inflammatory diseases patients such as chronic liver disease, rheumatoid arthritis and inflammatory bowel disease (Kurina et al., 2001, Lu et al., 2016, Mullish et al., 2014). Immune system and inflammation are considered to be involved in the pathophysiological process of mental conditions (Miller et al., 2009). On the one hand, there are many receptors on the lymphocyte including T lymphocyte surface, which enable to create “neuroimmunological synapses” with between nervous and immune cells (Pietruczuk et al., 2019). Furthermore, T lymphocytes are important in the coordination of immune and inflammatory responses through differentiation, proliferation and the release of cytokines. On the other hand, the inflammatory hypothesis of major psychiatric disorders, stressing the role of inflammation in depression (Haapakoski et al., 2015). Multiple inflammatory factors including Interleukins-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α) are over-activated in patients with major depression (Ye et al., 2018); after treatment with antidepressants, the levels of factors had reduced (Basterzi et al., 2005). Numbers of animal experiments revealed the activation of the peripheral immune system, including lymphocytes and cytokines, might contribute to anxiety and depressive-like behaviors in mice exposed to various stressors (Xu et al., 2016; Ramirez et al., 2017; Fan et al., 2019).
Considering the dual roles of mental stress and excessive immune response, the importance of mental health in patients with confirmed COVID-19 cannot be ignored. Although many studies have demonstrated the existence of a disordered immune system in patients, the association with mood characteristics is rarely reported. Therefore, the objective of this study was to investigate the immunological features including T cell subsets and inflammatory factors in patients with confirmed COVID-19 and anxiety-depression symptoms.
2. Methods
2.1. Participants and intervention
The sample was composed of sixty-one participants (57 participants included and 4 participants excluded) with confirmed COVID-19 who received care from the same therapeutic team in intensive care units of the Cancer Center of Wuhan Union Hospital, a temporary COVID-19-designated hospital.
The subjects met all the inclusion criteria: 1) age over 18 years, 2) no dyslexia, and 3) no cognitive impairment or consciousness disturbance. To ensure the quality of the questionnaires and the effectiveness of the intervention, we excluded subjects with incomplete questionnaires who had no full course of treatment. According to the previous mental health studies during COVID-19 epidemic, the patients with both Patient Health Questionnaire nine-item depression scale (PHQ-9) and Generalized Anxiety Disorder 7-item (GAD-7) scores over 5 were regarded as having symptoms of depression and anxiety (Liu et al., 2020; Ying et al., 2020) (see below).
All patients had a clinical classification diagnosis according to the New Coronavirus Pneumonia Diagnosis and Treatment Program (7th edition) published by the National Health Commission of China (http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml). The clinical classifications of COVID-19 included mild (minor clinical symptoms and no pneumonia manifestation can be found in imaging), moderate (mild infection symptoms with imaging indicating pneumonia), severe (dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen-to-fraction of inspired oxygen ratio < 300 and/or lung infiltrates > 50% within 24–48h), and critical (respiratory failure, septic shock and/or multiple organ dysfunction or failure). For statistical convenience, we combined mild and moderate cases, which were defined as non-severe; and severe and critical cases were combined as severe types.
All patients in this study not only received antiviral treatment but also had online self-help psychological guidance or a one-on-one face-to-face psychological crisis intervention including supportive psychotherapy, mindfulness exercises, relaxation training. The psychological intervention lasted for 2 weeks and was performed twice a week for 20–30 min each time. These psychotherapies were provided by a professional psychological treatment team including a chief physician, a co-chief psychotherapist, and four nurses of psychiatric wards from the First Affiliated Hospital, College of Medicine, Zhejiang University.
This study was approved by the local Medical Ethics Committee of the First Affiliated Hospital of Zhejiang University. Informed consent was obtained from all participants before the commencement of the study.
2.2. Measurement and data collection
General demographic information (age, sex, occupation, education, and marital status) and mental health status were collected through an electronic structured questionnaire. Depression and anxiety status were evaluated by PHQ-9 and GAD-7, which are quick and easy-to-administer screening tools for depression and anxiety, respectively. The item options of both of these tools range from 0 to 3, and total scores of 5, 10 and 15 represent mild, moderate, and severe depression or anxiety, respectively. The average time to complete the questionnaires ranged from 2 to 5 min. The participants were required to complete the online questionnaire through a hyperlink before and after the psychological intervention, and the researcher extracted the questionnaire responses from the database. Fasting blood samples were collected in the morning on the second day of admission and the second day after the end of the two-week psychological intervention by a nurse. All data were cross-checked for consistency before data entry and statistical analysis by two independent researchers.
2.3. Serum cytokines and lymphocytes assessment
Participants underwent a fasting blood draw in the morning. Whole blood samples were drawn from the antecubital vein and divided into two tubes. One was centrifuged at 3000 r/min for 20 min, and subsequently supernatant was collected to detect cytokine. Cytokine concentrations (INF-γ, TNF, IL-10, IL-5, IL-4 and IL-2) were determined by human Th1/Th2 kit (flow immunofluorescence method) with a FACSCalibur flow cytometer (BD, San Diego, USA). The levels of cytokine factors are expressed in pg/ml. The other tube (whole blood, 2 ml) was used to assess the level of peripheral blood T lymphocyte subsets (CD3+, CD4+, CD8+, and CD4+/CD8+ T-cell ratio) by were detected by FACS flow cytometry with BD Tritest CD4 FITC/CD8 PE/CD3 PerCP Kit (BD, San Jose, USA). The T lymphocyte subtype count was measured in cells/μl. The assay of all factors in each sample was carried out according to manufacturer instructions.
2.4. Statistical analysis
All of data were analyzed with IBM SPSS Statistics 20.0 (IBM Corporation, New York, USA). For general demographic and clinical data, descriptive statistics were performed. Quantitative variables are expressed as the mean and standard deviation when they were normally distributed; otherwise, they were expressed as the median and interquartile range. Differences in demographic and clinical characteristics between two groups were tested using t-test or Mann-Whitney U test, and the level of change between the pre- and post-psychological intervention time points in respective variables was tested by paired t-tests or paired Wilcoxon test, as appropriate. Relationships between variables were assessed with Pearson's and Spearman's correlations. All statistical tests were two-tailed, with a significance level of P < 0.05.
3. Results
3.1. Prevalence and demographic characteristics
A total of 61 patients with confirmed COVID-19 were enrolled at the Cancer Center of Wuhan Union Hospital from March 1 to March 15, 2020, of whom 57 were included in the study. Four patients who did not complete the questionnaire were excluded. According to the results of the PHQ-9 and GAD-7, 20 patients suffered from both depression and anxiety. To determine whether the mental health are related to demographic characteristics, we analyzed severity of initial infection, sex, age, education between the patients with and without depression-anxiety. There were no statistically significant differences of these data found between both groups. The detailed clinical and demographic data are shown in Table 1 .
Table 1.
Clinical demographic data of 57 inpatients with COVID-19 in Wuhan and their psychological status.
| Characteristic | Patients with depression-anxiety symptom (N = 20) | Patients without depression-anxiety symptom (N = 37) | Total (N = 57) | P | |
|---|---|---|---|---|---|
| Severity of initial infection (No., %) | severe | 7 (35.00%) | 18 (48.65%) | 25 (43.86%) | 0.406 |
| No-severe | 13 (65.00%) | 19 (51.35%) | 32 (56.14%) | ||
| Sex groups (No., %) | Male | 7 (35.00%) | 13 (35.14%) | 20 (35.09%) | 1.000 |
| Female | 13 (65.00%) | 24 (64.86%) | 37 (64.91%) | ||
| Age average (years) | 59.9511.69 | 64.8910.32 | 63.1610.97 | 0.105 | |
| Education (years) | 10.453.02 | 10.353.65 | 10.383.41 | 0.918 | |
| PHQ-9 score | 10.405.37 | 3.223.01 | 5.745.25 | 0.000 | |
| GAD-7 score | 10.754.90 | 1.891.85 | 5.00 5.34 | 0.000 | |
P values indicated the comparison between patients with and without depression-anxiety status.
Categorical variables were analyzed by chi-square test, while continuous variables were analyzed by the t-tests.
3.2. The immunologic characteristics of the participants with and without depression-anxiety symptoms
Table 2 presents the immunologic characteristics for the two groups. CD4+ T lymphocytes, IL-6 and TNF-α were found to exhibit highly significant differences between the two groups. Participants with depression-anxiety symptoms showed a higher level of CD4+ T lymphocytes than subjects without depression-anxiety symptoms. Compared to participants without depression-anxiety symptoms, the levels of IL-6 and TNF-α were lower in the participants with depression-anxiety symptoms. However, other factors (CD4+/CD8+, CD3+, CD8+, IL-2, IL-4, IL-10, IFN-γ) were not significantly different between the two groups.
Table 2.
The immunologic characteristics in the participants with and without depression-anxiety status.
| Characteristic | Patients with depression-anxiety symptom (N = 20) | Patients without depression -anxiety symptom (N = 37) | P |
|---|---|---|---|
| CD4+/CD8+ | 2.48 (1.24) | 2.13 (1.01) | 0.255 |
| CD3+ | 76.98 (72.40–79.87) | 75.50 (65.24–83.17) | 0.367 |
| CD4+ | 51.92 (8.27) | 42.90 (12.21) | 0.005 |
| CD8+ | 23.53 (8.14) | 23.77 (9.16) | 0.922 |
| IL-2 | 2.48 (2.27–2.69) | 2.50 (2.29–2.73) | 0.913 |
| IL-4 | 2.14 (0.77) | 2.14 (0.81) | 0.997 |
| IL-6 | 4.63 (2.87–16.46) | 11.16 (3.92–28.63) | 0.048 |
| IL-10 | 3.25 (1.33) | 3.02 (0.68) | 0.481 |
| IFN-γ | 2.23 (1.25) | 1.97 (0.69) | 0.387 |
| TNF-α | 2.27 (0.95) | 2.94 (1.32) | 0.050 |
Abbreviations: CD, cluster of differentiation; IL, Interleukin; IFN-γ, Interferon-γ; TNF-α, Tumor Necrosis Factor-α.
Normally distributed data (CD4+/CD8+, CD4+, CD8+, IL-4, IL-10, IFN-γ, TNF-α) were expressed as mean and standard deviation; while data (CD3+, IL-2, IL-6) were expressed as median and interquartile range.
P values indicated the comparison between patients with and without depression-anxiety status.
Differences between two groups were tested using t-test for normally distributed data and the non-normal distributed data were analyzed by Mann-Whitney U test.
The results of cytokines (IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α) are expressed in pg/ml; the results of T cells (CD3+, CD4+, CD8+) are expressed in cells/l.
3.3. The changes in scores of PHQ-9 and GAD-7 and cytokines and lymphocytes after two weeks of treatment
Following two weeks of treatment including pharmacological interventions for COVID-19 and psychological interventions for mental health, most patient conditions were improved. Fifty patients recovered and were discharged, seven patients (two patients with depression-anxiety symptoms and five patients without depression-anxiety symptoms) remaining in treatment. And after two weeks of treatment, CD4+ T lymphocytes, IL-6 and TNF-α still differed between the two groups (P < 0.05) (See Table 3 ).
Table 3.
The psychological status and immunologic characteristics in the participants with and without depression-anxiety status after 14 days of treatment.
| Characteristic | Patients with depression-anxiety symptom (N = 20) | Patients without depression -anxiety symptom (N = 37) | P |
|---|---|---|---|
| PHQ-9 | 6.85 (5.26) | 3.43 (4.06) | 0.009 |
| GAD-7 | 5.30 (4.86) | 2.37 (3.11) | 0.008 |
| CD4+/CD8+ | 2.16 (0.95) | 1.87 (0.91) | 0.259 |
| CD3+ | 76.09 (70.21–80.85) | 71.73 (63.35–77.44) | 0.367 |
| CD4+ | 47.61 (8.59) | 41.03 (9.32) | 0.012 |
| CD8+ | 24.52 (6.76) | 24.94 (8.30) | 0.847 |
| IL-2 | 3.73 (3.44–4.42) | 3.88 (3.44–4.50) | 0.913 |
| IL-4 | 3.69 (0.95) | 3.83 (0.97) | 0.593 |
| IL-6 | 7.03 (5.43–10.89) | 6.28 (5.13–8.06) | 0.048 |
| IL-10 | 5.58 (2.14) | 5.66 (1.75) | 0.869 |
| IFN-γ | 3.27 (0.61) | 3.67 (0.73) | 0.048 |
| TNF-α | 3.94 (2.16) | 3.79 (1.53) | 0.764 |
Abbreviations: CD, cluster of differentiation; IL, Interleukin; IFN-γ, Interferon-γ; TNF-α, Tumor Necrosis Factor-α.
Normally distributed data (CD4+/CD8+, CD4+, CD8+, IL-4, IL-10, IFN-γ, TNF-α) were expressed as mean and standard deviation; while data (CD3+, IL-2, IL-6) were expressed as median and interquartile range.
P values indicated the comparison between patients with and without depression-anxiety status.
Differences between two groups were tested using t-test for normally distributed data and the non-normal distributed data were analyzed by Mann-Whitney U test.
The results of cytokines (IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α) are expressed in pg/ml; the results of T cells (CD3+, CD4+, CD8+) are expressed in cells/l.
The scores of PHQ-9 and GAD-7, cytokines and T lymphocytes were altered. A statistically significant decrease was found in the PHQ-9 (P < 0.05) and GAD-7 (P < 0.01) scores in the patients with depression and anxiety. A significant decrease was found in the level of CD4+ T lymphocytes (P < 0.05) compared with the level at admission. The levels of other factors, such as IL-2, IL-4, IL-10, TNF-α and IFN-γ, were significantly elevated (P < 0.01); the other factors (CD8+ T lymphocytes, the ratio CD4+/CD8+ and IL-6) showed no significant change before and after psychotherapy.
3.4. Correlation between cytokines and lymphocytes and scores of PHQ-9 and GAD-7
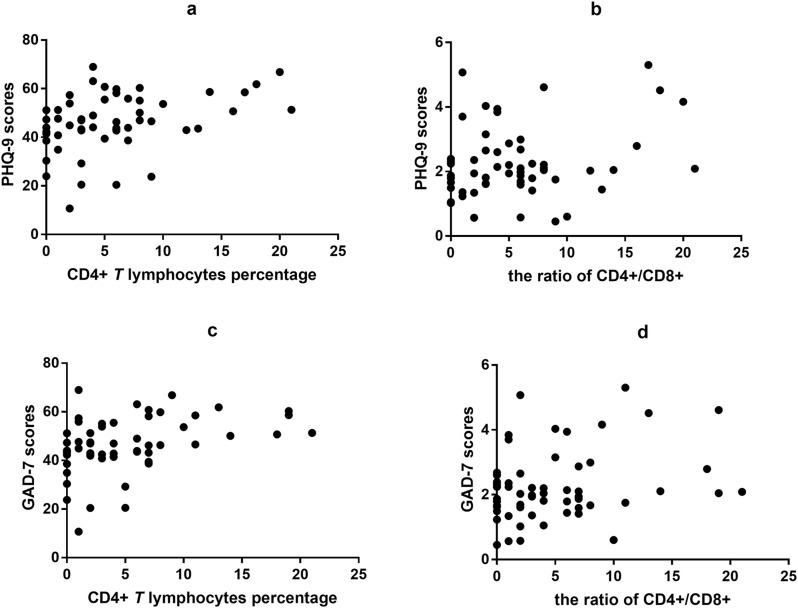
At admission, the counts of CD4+ T lymphocytes significantly correlated with the PHQ-9 (r = 0.378, P = 0.004) and GAD-7 (r = 0.403, P = 0.002) scores. The significant correlation of CD4/CD8 with PHQ-9 scores was reflected by r = 0.264 (P = 0.047), and that with GAD-7 scores was reflected by r = 0.282 (P = 0.034) (see Fig. 1 ).
Fig. 1.
Correlation between the CD4+T lymphocytes and the ratio of CD4+/CD8+ and the scores of PHQ-9 and GAD-7 at admission in patients with COVID-19.
(a) Correlation between CD4+T lymphocytes and PHQ-9 scores. There are significantly positive association between CD4+T lymphocytes and PHQ-9 scores (r = 0.378, P < 0.01).
(b) Correlation between the ratio of CD4+/CD8+ and PHQ-9 scores. There are significantly positive association between the ratio of CD4+/CD8+ and PHQ-9 scores (r = 0.264, P < 0.05).
(c) Correlation between CD4+T lymphocytes and GAD-7 scores. There are significantly positive association between CD4+T lymphocytes and GAD-7 scores (r = 0.403, P < 0.01).
(d) Correlation between the ratio of CD4+/CD8+ and GAD-7 scores. There are significantly positive association between the ratio of CD4+/CD8+ and GAD-7 scores (r = 0.282, P < 0.05).
Abbreviations: PHQ-9, Questionnaire nine-item depression scale; GAD-7, Generalized Anxiety Disorder 7-item; CD, cluster of differentiation; COVID-19, coronavirus disease 2019.
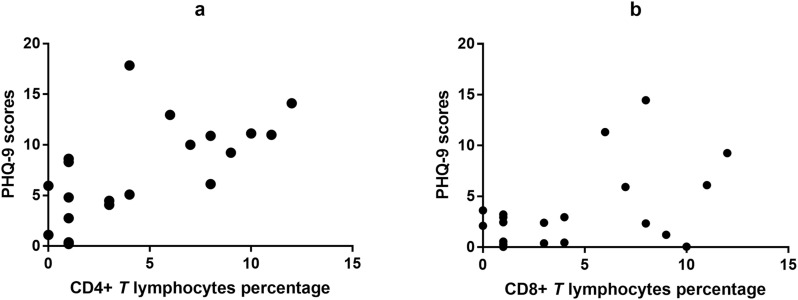
After two weeks of treatment, significant associations remained between the changes in the level of CD4+ T lymphocytes and PHQ-9 scores (r = 0.644, P = 0.002) in the patients with depression and anxiety. Furthermore, significant correlations were found between the changes in CD8+ T lymphocytes and PHQ-9 scores (r = 0.455, P = 0.044) (see Fig. 2 ). However, the changes in other cytokines and PHQ-9 scores or GAD-7 scores showed no correlation. Additionally, there was no correlation between age, severity of COVID-19, sex or PHQ-9 or GAD-7 scores.
Fig. 2.
Correlation between the changes of CD4+T lymphocytes, CD8 T lymphocytes and the changes of PHQ-9 scores before and after psychological intervention in the participants with depression-anxiety status.
(a) Correlation between the changes CD4+T lymphocytes and the changes of PHQ-9 scores. There are significantly positive association between the changes of CD4 T lymphocytes and the changes of PHQ-9 scores (r = 0.644, P < 0.01).
(b) Correlation between the changes of CD8 T lymphocytes and the changes of PHQ-9 scores. There are significantly positive association between the changes of CD8+T lymphocytes percent and the changes of PHQ-9 scores (r = 0.455, P < 0.05).
Abbreviations: PHQ-9, Questionnaire nine-item depression scale; CD, cluster of differentiation.
4. Discussion
In this study, we found that over half of the population reported different degrees of depression and anxiety, and the scores of PHQ-9 and GAD-7 were associated with the level of CD4+ T lymphocytes and the CD4+/CD8+ ratio at admission. Moreover, patients with depression-anxiety symptoms showed a higher level of CD4+ T lymphocytes than patients without depression-anxiety symptoms. After two weeks of treatment, the mean scores of PHQ-9 and GAD-7 significantly decreased as the level of CD4+ T lymphocytes decreased, and the changes in CD4+ and CD8+ T lymphocytes were significantly correlated with the changes in PHQ-9 scores. To our knowledge, although many studies have analyzed the dysregulation of inflammatory immune responses in COVID-19 patients, this is the first study to investigate the relationship with mental conditions.
Mental health is related to internal and external environmental, social and psychological factors (Meegan et al., 2017). Exposure to extreme and unavoidable situations of stress may have detrimental consequences, including emotional outbursts and compensatory immune responses in early stages, followed by endocrine, emotional and cognitive alterations (Glaser and Kiecolt-Glaser, 2005; Wu et al., 2017; Bonnefil et al., 2019). The T lymphocyte subpopulations, especially CD4+ T lymphocytes, are critical for immunological function. CD4+ T lymphocytes, which differentiate into different subtypes of T cells and secrete inflammatory factors such as cytokines, interleukins and tumor necrosis factor after antigen stimulation, have been increasingly regarded as playing an important role in the pathophysiology of mental disorders and in depression-like and anxiety-like behaviors in rodents (Beurel et al., 2013; Slyepchenko et al., 2016; Guo et al., 2017). Previous animal studies have indicated that acute physical stress caused a significant increase of peripheral CD4+ T lymphocytes levels in mice, which further led to a variety of behavioral abnormalities, including anxiety, depression, and social disorders (Dhabhar 2008; Fan et al., 2019). Studies have reported that patients with anxiety disorders showed markedly elevated CD4+ T lymphocytes and ratios of CD4+ T lymphocytes to CD8+ T lymphocytes compared to the same parameters in healthy controls (Atanackovic et al., 2004, Darko et al., 1988, Ko et al., 2013, Lynall et al., 2019). Moreover, a study reported that the levels of CD4 were significantly related to the severity of depression (r = 0.548, P < 0.05) and stress (r = 0.45, P < 0.05) among people with HIV/AIDS (Effendy et al., 2019). Consistent with these studies, in this study, statistically significant differences in CD4+ T lymphocytes were observed between the patients with and without depression-anxiety symptoms, and the level of CD4+ T lymphocytes was associated with the severity of depression and anxiety. These findings appeared to confirm the hypothesis that the immune response mechanism of patients with affective disorders in early stages was adaptive in coping with stressors.
Moreover, previous evidence has shown that the immune system dynamics was improved in patients with depression and anxiety symptoms after psychological interventions, including mindfulness and relaxation mental training, are salutogenic in regard to immune system dynamics (Andersen et al., 2004; Black and Slavich 2016; Zhao et al., 2016). Four studies enumerated lymphocytes in blood and found changes in the count of CD4+ T cell and the ratio of CD4+/CD8+ and/or % activated T cells after mindfulness meditation in patients diagnosed with HIV or breast cancer compared to the T lymphocyte cell count and/or activation in controls. Studies have revealed decreased inflammatory cytokines (i.e., IL-6 and IL-8) after stress management training or mind-body therapies (Antoni and Dhabhar 2019). Interestingly, in this study, we found a significant reduction in the level of CD4+ T lymphocytes, which was associated with changes in the PHQ-9 score; however, the levels of IL-2, IL-4, IL-10, TNF-α and IFN-γ, contrary to other results, were increased after psychotherapy. One of the main reasons we considered is the particularity of the patients diagnosed with COVID-19. Fever and respiratory symptoms are the main clinical manifestations in patients diagnosed with COVID-19, and the systemic inflammatory response is a dynamic process in the body. Previous studies have indicated that the diagnosed patients who developed severe disease exhibited a stronger inflammatory response and higher levels of inflammatory factors (Zhu et al., 2020). From this, we speculated that the difference in our results is due to the different stages of the disease, which is a dynamic process.
5. Limitations
There are certain limitations in our study. First, this study was conducted at a single hospital in China with a relatively small sample size. Second, because of the strong infectivity of novel coronavirus and shortage of health staff, all mental state assessment data were collected via self-report questionnaires, potential self-report biases cannot be ruled out. Third, the underlying diseases and comorbidities that may influence the levels of inflammatory cytokines and lymphocytes were not evaluated in our study. Finally, considering the serious harm and adverse effects of negative mood on recovery, we used a two-week psychological intervention for all patients instead of setting a control group, which was hard to confirm the effect of psychological intervention and the ascertain psychological or immune factor is causative.
6. Conclusion
In conclusion, we demonstrated that patients with confirmed COVID-19 and depression-anxiety symptoms differed from those without depression-anxiety symptoms in terms of CD4+ T lymphocytes. After two weeks of treatment, a decrease in depression scores was related with changes in CD4+ and CD8+ T lymphocytes. These findings further support that lymphocytes, particularly T lymphocytes, may play a key role in the emotional status of patients with COVID-19. It was the first assessment of mental problems and their association with T lymphocytes, providing some insight into the psychoneuroimmunology of depression and anxiety. Gaining a deeper understanding of these physiological changes can allow for better recognition of the progression of the conditions, which is of importance for the clinical mental management of COVID-19.
Contributors
All authors approved the final manuscript and substantially contributed to the article. Congchong Wu: Writing-Original draft preparation. Zhiying Zhou: Investigation. Li Ni: Data curation. Jiang Cao: Formal analysis. Meifang Tan: Visualization. Xiu Wu: Supervision. Jianbo Hu: Writing-Review and Editing. Yi Xu: Conceptualization.
Funding
This work was supported by the Analysis and test science and technology program of Zhejiang Province [grant numbers 2018C37064].
Declaration of competing interest
The author (s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Acknowledgments
All authors would sincerely thank the physician, nurses and researcher assistants at intensive care units of the Cancer Center of Wuhan Union Hospital for providing technical assistant.
References
- Andersen B.L., Farrar W.B., Golden-Kreutz D.M., Glaser R., Emery C.F., Crespin T.R., Shapiro C.L., Carson W.E. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J. Clin. Oncol. 2004;22(17):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni M.H., Dhabhar F.S. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125(9):1417–1431. doi: 10.1002/cncr.31943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D., Kröger H., Serke S., Deter H.-C. Immune parameters in patients with anxiety or depression during psychotherapy. J. Affect. Disord. 2004;81(3):201–209. doi: 10.1016/S0165-0327(03)00165-4. [DOI] [PubMed] [Google Scholar]
- Basterzi A., Aydemir C., Kisa C., Aksaray S., Tuzer V., Yazici K., Göka E. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum. Psychopharmacol. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- Beurel E., Lowell J.A. Th17 cells in depression. Brain Behav. Immun. 2018;69(undefined):28–34. doi: 10.1016/j.bbi.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Harrington L.E., Jope R.S. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol. Psychiatr. 2013;73(7):622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.S., Slavich G.M. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016;1373(1):13–24. doi: 10.1111/nyas.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefil V., Dietz K., Amatruda M., Wentling M., Aubry A.V., Dupree J.L., Temple G., Park H.J., Burghardt N.S., Casaccia P., Liu J. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. Elife. 2019;8 doi: 10.7554/eLife.40855. (undefined), undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu Haijing, Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Jope R.S., Beurel E. A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC Neurosci. 2015;16(undefined):31. doi: 10.1186/s12868-015-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darko D.F., Lucas A.H., Gillin J.C., Risch S.C., Golshan S., Hamburger R.N., Silverman M.B., Janowsky D.S. Cellular immunity and the hypothalamic-pituitary axis in major affective disorder: a preliminary study. Psychiatr. Res. 1988;25(1):1–9. doi: 10.1016/0165-1781(88)90152-7. [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology. Allergy Asthma Clin. Immunol. 2008;4(1):2–11. doi: 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effendy E., Amin M.M., de Vega L., Utami N. The association between CD-4 level, stress and depression symptoms among people living with HIV/AIDS. Open Access Maced J Med Sci. 2019;7(20):3459–3463. doi: 10.3889/oamjms.2019.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K., Li Y., Wang H., Mao X., Guo J., Wang F., Huang L., Li Y., Ma X., Gao Z., Chen Wei, Qian D., Xue W., Cao Q., Zhang L., Shen L., Zhang L., Tong C., Zhong J., Lu W., Lu L., Ren K., Zhong G., Wang Y., Tang M., Feng X., Chai R., Jin J. Stress-Induced metabolic disorder in peripheral CD4 T cells leads to anxiety-like behavior. Cell. 2019;179(4):864–879. doi: 10.1016/j.cell.2019.10.001. e19. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Guo Z., Xu P., Ge S., Zhang C., Zheng X., Xu J., Liu Z., Li B., Ge S. Ubiquitin specific peptidase 4 stabilizes interferon regulatory factor protein and promotes its function to facilitate interleukin-4 expression in T helper type 2 cells. Int. J. Mol. Med. 2017;40(4):979–986. doi: 10.3892/ijmm.2017.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49(undefined):206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R., Miller A.H. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007;21(4):374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jin X., Lian J., Hu J., Gao J., Zheng L., Zhang Y., Hao S., Jia H., Cai H., Zhang X., Yu G., Xu K., Wang X., Gu J., Zhang S., Ye C., Jin C., Lu Y., Yu X., Yu X., Huang J., Xu K., Ni Q., Yu C., Zhu B., Li Y., Liu J., Zhao H., Zhang X., Yu L., Guo Y., Su J., Tao J., Lang G., Wu X., Wu W., Qv T., Xiang D., Yi P., Shi D., Chen Y., Ren Y., Qiu Y., Li L., Sheng J., Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko F., Tsai S., Yang A.C., Zhou Y., Xu L. Association of CD8 T cells with depression and anxiety in patients with liver cirrhosis. Int. J. Psychiatr. Med. 2013;45(1):15–29. doi: 10.2190/PM.45.1.b. [DOI] [PubMed] [Google Scholar]
- Kurina L.M., Goldacre M.J., Yeates D., Gill L.E. Depression and anxiety in people with inflammatory bowel disease. J. Epidemiol. Community Health. 2001;55(10):716–720. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yang L., Zhang C., Xiang Y.-T., Liu Z., Hu S., Zhang B. Online mental health services in China during the COVID-19 outbreak. The Lancet Psychiatry. 2020;7:e17–e18. doi: 10.1016/S2215-0366(20)30077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Guo H., Lin M., Livneh H., Lai N., Tsai T. Bidirectional associations between rheumatoid arthritis and depression: a nationwide longitudinal study. Sci. Rep. 2016;6(undefined):20647. doi: 10.1038/srep20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall M.E., Turner L., Bhatti J., Cavanagh J., de Boer P., Mondelli V., Jones D., Drevets W.C., Cowen P., Harrison N.A., Pariante C.M., Pointon L., Clatworthy M.R., Bullmore E. Neuroimmunology of mood disorders and Alzheimer's disease (NIMA) consortium. Peripheral Blood Cell-Stratified Subgroups of Inflamed Depression. Biol. Psychiatry, undefined(undefined), undefined. 2019 doi: 10.1016/j.biopsych.2019.11.017. [DOI] [PubMed] [Google Scholar]
- Meegan A.P., Perry I.J., Phillips C.M. The association between dietary quality and dietary guideline Adherence with mental health outcomes in Adults: a cross-sectional analysis. Nutrients. 2017;9(3) doi: 10.3390/nu9030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H. Depression and immunity: a role for T cells? Brain Behav. Immun. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatr. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullish B.H., Kabir M.S., Thursz M.R., Dhar A. Review article: depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment. Pharmacol. Ther. 2014;40(8):880–892. doi: 10.1111/apt.12925. [DOI] [PubMed] [Google Scholar]
- Pape K., Tamouza R., Leboyer M., Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat. Rev. Neurol. 2019;15:317–328. doi: 10.1038/s41582-019-0174-4. [DOI] [PubMed] [Google Scholar]
- Pietruczuk K., Lisowska K.A., Grabowski K., Landowski J., Cubala W.J., Witkowski J.M. Peripheral blood lymphocyte subpopulations in patients with bipolar disorder type II. Sci. Rep. 2019;9:5869. doi: 10.1038/s41598-019-42482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K., Fornaguera-Trías J., Sheridan J.F. Stress-Induced microglia activation and monocyte trafficking to the brain underlie the development of anxiety and depression. Curr Top Behav Neurosci. 2017;31(undefined):155–172. doi: 10.1007/7854_2016_25. [DOI] [PubMed] [Google Scholar]
- Slyepchenko A., Maes M., Köhler C.A., Anderson G., Quevedo J., Alves G.S., Berk M., Fernandes B.S., Carvalho A.F. T helper 17 cells may drive neuroprogression in major depressive disorder: proposal of an integrative model. Neurosci. Biobehav. Rev. 2016;64(undefined):83–100. doi: 10.1016/j.neubiorev.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76(undefined):71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Yang D., Li Y., Zhou J., Wang W., Wang Q., Lin N., Cao A., Wang H., Zhang Q. Psychological impact of 2019 novel coronavirus (2019-nCoV) outbreak in health workers in China. Epidemiol. Infect. 2020;148(undefined):e96. doi: 10.1017/S0950268820001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toben C., Baune B.T. An Act of balance between adaptive and maladaptive immunity in depression: a role for T lymphocytes. J. Neuroimmune Pharmacol. 2015;10(4):595–609. doi: 10.1007/s11481-015-9620-2. [DOI] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., Ho R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Publ. Health. 2020;17(5) doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Liu C., Zhang L., Qu C., Sui X., Zhu H., Huang L., Xu Y., Han Y., Qin C. Histone deacetylase-2 is involved in stress-induced cognitive impairment via histone deacetylation and PI3K/AKT signaling pathway modification. Mol. Med. Rep. 2017;16(2):1846–1854. doi: 10.3892/mmr.2017.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Sheng H., Bao Q., Wang Y., Lu J., Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav. Immun. 2016;56(undefined):175–186. doi: 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Ye G., Yin G., Tang Z., Fu J., Chen J., Chen S., Li J., Fu T., Yu X., Xu D., Yao J., Hui L. Association between increased serum interleukin-6 levels and sustained attention deficits in patients with major depressive disorder. Psychol. Med. 2018;48:2508–2514. doi: 10.1017/S0033291718000090. [DOI] [PubMed] [Google Scholar]
- Ying Y., Ruan L., Kong F., Zhu B., Ji Y., Lou Z. Mental health status among family members of health care workers in Ningbo, China, during the coronavirus disease 2019 (COVID-19) outbreak: a cross-sectional study. BMC Psychiatr. 2020;20:379. doi: 10.1186/s12888-020-02784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Cui L., Wang W., Su Q., Li X., Wu J. Influence of psychological intervention on pain and immune functions of patients receiving lung cancer surgery. Pak J Med Sci. 2016;32(1):155–159. doi: 10.12669/pjms.321.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z., Gao G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020;95(undefined):332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]