Abstract
Purpose of review
The COVID-pandemic has facilitated the implementation of telemedicine in both clinical practice and research. We highlight recent developments in three promising areas of telemedicine: teleconsultation, telemonitoring, and teletreatment. We illustrate this using Parkinson's disease as a model for other chronic neurological disorders.
Recent findings
Teleconsultations can reliably administer parts of the neurological examination remotely, but are typically not useful for establishing a reliable diagnosis. For follow-ups, teleconsultations can provide enhanced comfort and convenience to patients, and provide opportunities for blended and proactive care models. Barriers include technological challenges, limited clinician confidence, and a suboptimal clinician-patient relationship. Telemonitoring using wearable sensors and smartphone-based apps can support clinical decision-making, but we lack large-scale randomized controlled trials to prove effectiveness on clinical outcomes. Increasingly many trials are now incorporating telemonitoring as an exploratory outcome, but more work remains needed to demonstrate its clinical meaningfulness. Finding a balance between benefits and burdens for individual patients remains vital. Recent work emphasised the promise of various teletreatment solutions, such as remotely adjustable deep brain stimulation parameters, virtual reality enhanced exercise programs, and telephone-based cognitive behavioural therapy. Personal contact remains essential to ascertain adherence to teletreatment.
Summary
The availability of different telemedicine tools for remote consultation, monitoring, and treatment is increasing. Future research should establish whether telemedicine improves outcomes in routine clinical care, and further underpin its merits both as intervention and outcome in research settings.
Keywords: consultation, monitoring, Parkinson's disease, telemedicine, treatment
INTRODUCTION
Telemedicine is defined as the delivery of healthcare at a distance [1]. Spurred by the COVID-19 pandemic, telemedicine in its various forms has become a widely debated topic. Arguments in favour include the expanded access to multidisciplinary care, reduced travel burden, and convenience of in-home assessments [1,2]. Telemedicine also holds promise to deliver interventions remotely and to measure outcomes at home in the framework of clinical trials [3]. Counterarguments include concerns that implementation of telemedicine might interfere with the intimacy of the clinician-patient relationship, limit diagnostic accuracy, and enlarge inequalities in access to healthcare [4,5,6▪].
As the use of telemedicine increases rapidly worldwide to prevent COVID-19 transmission [7], it is crucial to critically delineate the current state of telemedicine. Here, we discuss recent developments in the various fields of telemedicine, covering a period from approximately January 2019 to February 2021. In doing so, we focus on Parkinson's disease (PD) as a model disease for other chronic neurological disorders. Specifically, we will cover three telemedicine approaches: teleconsultation, telemonitoring, and teletreatment. For each area, recent advances are highlighted and placed within a broader context. Pressing limitations and future research avenues will also be discussed.
Box 1.

no caption available
TELECONSULTATION
Teleconsultation means that the consultation between patient and clinician takes place remotely, e.g. through telephone or video conferencing (for a step-by-step guide, see [8]). In this section, we discuss the reliability and feasibility of remote neurological examinations, the experiences of patients and healthcare providers, and the opportunities for novel care models (Fig. 1).
FIGURE 1.
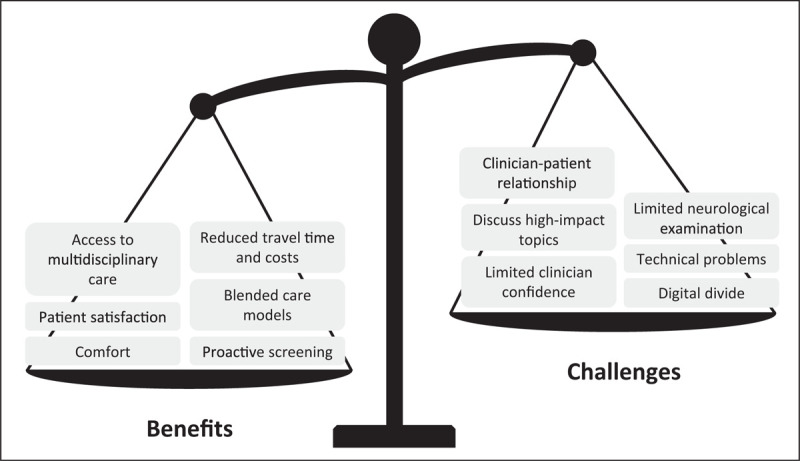
Benefits and challenges of teleconsultations compared to in-person consultations. This figure summarizes the benefits and challenges mentioned in recent studies on teleconsultations. The scale's position reflects the authors’ opinion on the overall readiness of teleconsultations for deployment in clinical practice. Source: Original.
Parts of neurological examinations can be administered during teleconsultations [9], and this provides comparable results to in-person evaluations for upper limb functioning [10] and evaluation of deep brain stimulation (DBS) candidacy [11▪]. However, remote consultations remain limited in their scope because specific assessments – such as rigidity and balance – cannot be performed remotely, and because subtle features such as bradykinesia or tremor are prone to be underdetected by video-based ratings compared to in-person ratings [12]. Indeed, a qualitative study showed that neurologists experienced reduced confidence in their decisions because of these limitations, and additional in-person examinations were often necessary to verify the remote observations [13▪]. Therefore, teleconsultations seem only suitable when the medical history or a partial neurological examination is sufficient for the neurologist to adjust the treatment plan. When a diagnosis must be newly established during a very first contact, it remains preferable to see the patient physically to allow for a thorough examination. A caveat here is that in many parts of the world, access to physical care remains restricted, e.g., due to long travel distances and limited provider capacity [14]. Under such circumstances, it is possible to perform at least a part of the neurological examination remotely, which is arguably better than no examination at all.
Overall, persons with PD were satisfied with the delivery of remote consultations [15–18]. The most commonly mentioned advantages include enhanced convenience [15,18,19], greater comfort [15,18], and reduced travel time and costs [11▪,13▪,16,19,20]. Furthermore, teleconsultations enable enhanced access to specialist care [1,19], especially for patients living in rural areas [20,21] and homebound patients with severe disability requiring palliative care [22]. Common disadvantages mentioned by both persons with RD and clinicians include technical difficulties [13▪,15,16,19], lack of hands-on examinations [13▪,19], and reduced quality of the doctor-patient contact [13▪,19]. In particular, neurologists had difficulties breaking bad news to patients through telephone or video consultations [13▪]. Taken together, teleconsultations can benefit both patients and professionals in specific situations, such as reducing travel burden for stable patients. However, teleconsultations are not suitable when clinicians must address high-impact topics, or when patients themselves prefer an in-person contact [5] or have no access to technology [6▪]. Therefore, these experiences of both patients and clinicians suggest that teleconsultations cannot replace all in-person care, but should rather be regarded as an adjunct or additional service that clinicians can use in specific situations [2,4].
Teleconsultations also offer unique possibilities to extend hospital-based care into blended care models, i.e., combining hospital- and home-based care [23]. A remarkable example was implemented in northern Italy where, during the peak of the COVID crisis in early 2020, persons with PD had limited access to in-person care by their own neurologist. These patients were offered remote access to a telenursing service via videoconferencing. Although this Parkinson nurse was a complete newcomer for the patients and could only be seen remotely, the nurse resolved over 60% of incoming requests from patients at a distance, thereby preventing unnecessary travel to the hospital [24]. When more specialized medical care was required, a teleconsultation with a specialist(s) or multidisciplinary team was scheduled during which most issues could be resolved remotely. If needed, subsequent in-person contacts or even hospital admissions were arranged.
Teleconsultations also offer opportunities to provide proactive care, i.e., aiming to identify new medical issues early on so these can be managed timely, thereby preventing avoidable disability and reducing unnecessary costs. An illustrative example is a proactive outreach program that targeted homebound and vulnerable persons with advanced PD and related disorders [25▪]. A nurse or social worker proactively called these patients to discuss topics such as home safety, physical and mental wellbeing, medical care provisions, and also lockdown restrictions or scheduling of healthcare appointments. Patients and caregivers reported that the program made them feel safe and supported [25▪]. Whether this proactive approach actually avoids medical deterioration and prevents e.g. costly admissions remains to be determined. Similarly, a case report illustrated how intense but completely remotely delivered patient contact could reduce the frequency of falls, which may have prevented fractures or other injuries [26]. The cost-effectiveness of proactive and blended care models must be evaluated in future research.
TELEMONITORING
Telemonitoring is the remote gathering of information about a patient which is used to inform healthcare providers (in a clinical setting) or researchers (in the framework of a trial). A wide and expanding spectrum of tools can be used for telemonitoring, including body-worn sensors [27,28], home sensors [29], specific apps for the smartphone [30,31], digital diaries [32], or analysis of common appliances such as computer keyboards [33] (only several selected high-quality references are given here). The promise of remote monitoring is to offer objective, continuous measures of relevant symptoms while patients are at home. This is important because hospital-based assessments can deviate considerably from daily living assessments [34]. Moreover, during in-person visits to the hospital, it remains difficult to reliably ascertain complex fluctuating events (such as response fluctuations to dopaminergic medication), rare events (such as falls [35]) or gradually developing events (such as a slowly progressive decline in physical activities, or disease progression itself) [36]. In this section, we discuss whether telemonitoring tools are ready for use in trials and clinical practice, and what persons with PD think about telemonitoring (Fig. 2).
FIGURE 2.
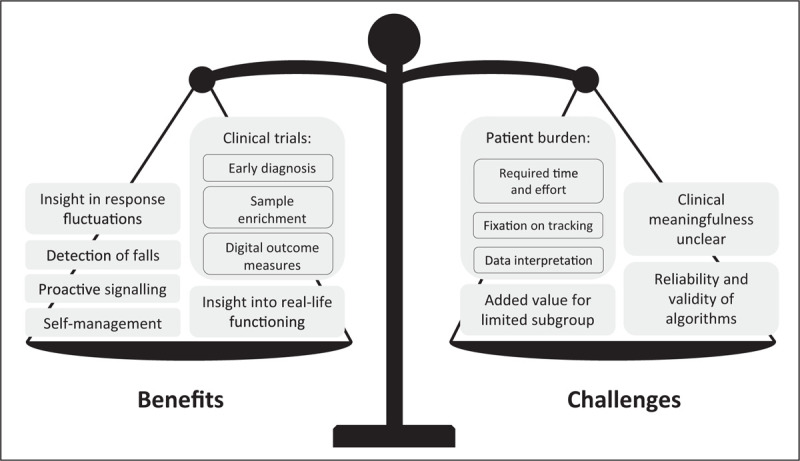
Benefits and challenges of telemonitoring compared to in-hospital measures. This figure summarizes the benefits and challenges mentioned in recent studies on telemonitoring. The scale's position reflects the authors’ opinion on the overall readiness of telemonitoring for deployment in clinical practice and trials. Source: Original.
Perhaps the most immediate application for telemonitoring is its deployment in clinical trials. Recognition is growing that the currently available clinical rating scales may be insufficiently sensitive and accurate to detect meaningful changes in patient functioning; this is particularly problematic in the setting of clinical trials where new experimental interventions are being tested. For that reason, many ongoing and planned studies are incorporating some form of telemonitoring into the overall repertoire of assessments, for now as surrogate, exploratory outcome measures. Recent examples of such studies include a phase 3 study assessing continuous subcutaneous infusion of levodopa/carbidopa [37], and a phase 2 study assessing co-administration of two compounds (CST-103 and CST-107) [38], which both use a wearable sensor to measure at home functioning as secondary outcome. A clear advantage is that telemonitoring, by virtue of the objective and longitudinal assessment in the patient's own home environment, may offer a very sensitive indication of therapeutic benefits. An important challenge is how to interpret such telemonitoring outcomes in terms of their clinical meaningfulness, even when statistically significant [39]. The increasing adoption of telemonitoring in clinical trials, alongside existing measures for patient functioning and quality of life, will help to further refine the reliability and validity of telemonitoring outcomes and support its acceptance by regulatory bodies.
In addition, telemonitoring tools could assist with subject enrolment in clinical trials by enabling early identification of people with PD or prodromal stages of PD. In a 6-year longitudinal study of prodromal individuals, specific gait characteristics such as step velocity and length were predictive of conversion to PD, even when measured as early as up to 4 years prior to the clinical diagnosis [40]. Other technologies suitable for early disease detection encompass touchscreen typing [31] or voice analysis [41]. However, voice studies often relied on high-quality data collected in controlled environments, making it difficult to apply such tools for large-scale screening based on less standardized real-life recordings. One study addressed this issue by collecting telephone-quality voice data from 1483 people with PD and 8300 healthy controls across seven countries [42▪]. Although using these real-life data reduced the classification accuracy, this study represents an important step towards analysing data as they would be captured in everyday life.
Incorporating telemonitoring into regular clinical practice faces similar challenges. Recent work indicates that it is feasible and informative to employ telemonitoring tools such as wrist-worn sensors and smartphone applications in clinical practice [43,44,45▪,46,47▪]. However, conclusive evidence of their actual impact on clinical outcomes is lacking. Telemonitoring tools often consist of a dashboard for clinicians that presents the remotely collected data. Pilot studies show positive experiences of clinicians who used such tools in clinical practice. Specifically, the information on symptom severity and medication intake displayed in these dashboards was in line with in-clinic assessments [43], enabled a clinician to make treatment decisions that were comparable to in-person evaluations in most cases [47▪], and resulted in more medication adjustments and higher medication doses [45▪]. Despite these encouraging initial findings, we lack large-scale randomized controlled trials (RCTs) assessing the effect of such dashboards on clinically relevant outcomes. A recent controlled trial showed improved scores on the Movement Disorders Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) part III and IV in the ON state when the patient's case management was supported by a telemonitoring tool [48▪]. However, since no effects were observed on the MDS-UPDRS part II and Parkinson's Disease Questionnaire (PDQ-39), more research is needed to verify whether the benefits translate into an improved patient functioning in daily life. Furthermore, for only few patients, the dashboard provided the clinician with usable information beyond that obtained during the regular clinical evaluation [46,49]. These patients had symptoms that strongly fluctuated [46] or that changed very subtly [49], or who experienced unexpected effects of multiple medications [49]. Therefore, future studies should further identify specific patient populations that may benefit most from telemonitoring tools. Finally, we note that most published work was conducted by groups that also originally developed the monitoring tools under examination. We encourage independent research groups to conduct RCTs to further test the effectiveness of such tools, which will be essential to persuade both the clinical and scientific community about the merits of telemonitoring.
Many persons with PD are motivated to monitor their symptoms, as long as there is a clear goal [50▪▪,51]. However, a mixed-methods study into the patient's perspectives on self-tracking showed that, even for the most highly motivated patients, it remains necessary to strike a balance between the perceived benefits and the inevitable burden of self-tracking [50▪▪]. Specifically, patients reported that self-tracking of e.g. their medication intake or exercise regimes helped them to better understand and manage their PD and to better inform their treating clinician. As a potential burden they mentioned difficulties understanding connections between variables, and getting too fixated on tracking. This balance between benefits and burden could explain the large differences in retention rates between studies. For example, when persons with PD were given (multiple) wearable sensors and were asked to actively provide information using a smartphone-based application, compliance was excellent for up to two weeks [44,52], but decreased steeply after three months [43]. However, when the balance between burden and benefits for patients was improved, e.g., by using only a single tool, by focussing on passive monitoring, and by providing highly personal contact (such as a readily accessible helpdesk), dropout rates could be minimized to 3% after 6 months in one study [49] or even only 1% after 1 year in another [53]. Future research should further improve the balance between benefits and burdens by tailoring the implementation of the monitoring tools to the individual patient's context, measuring only those variables that are relevant and meaningful to both patient and clinician [54▪,55].
TELETREATMENT
The development of technological devices has enabled numerous treatments to be delivered remotely. Here, we review the benefits and challenges of remotely delivered device-assisted therapies, exercise programs, and cognitive behavioural therapy (Fig. 3).
FIGURE 3.
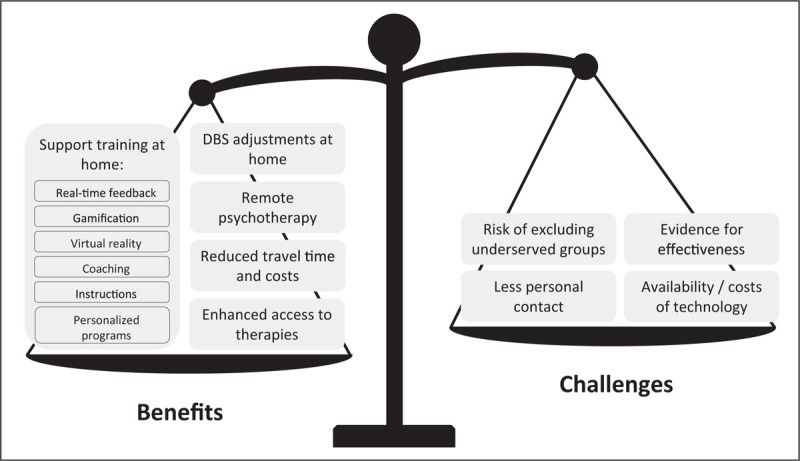
Benefits and challenges of teletreatments compared to in-person treatments. This figure summarizes the benefits and challenges mentioned in recent studies on teletreatments. The scale's position reflects the authors’ opinion on the overall readiness of teletreatments for deployment in clinical practice. Source: Original.
During the COVID-pandemic, parameters for device-assisted therapies such as DBS were successfully adjusted remotely [56▪,57]. Patients completed self-rated questionnaires about symptom severity and uploaded a video of their motor functioning at home, which were assessed by the hospital-based clinician. Then, whilst video-calling with the patient, the clinician remotely adjusted the parameters of the DBS electrodes during an online therapeutic session. Comparing their condition before and after the parameter adjustments, patients reported a decrease in symptom severity [56▪]. The patient satisfaction rates with the remote adjustment sessions were comparable to in-clinic adjustments [56▪,57]. Although patients reported some difficulty learning how to use the program, these observational results highlight the potential of teletreatment to continue care within the patient's home, even for quite markedly affected patients, and thereby prevent unnecessary travel to the hospital.
For persons with PD, it would be very helpful to be able to perform various nonpharmacological interventions at home, such as physical exercises, speech therapy, or cognitive training. Recent work has shown the feasibility and merits of home-based physical exercise programs which typically included a smartphone-based application or website that showed a personalized training program to patients, with instruction videos explaining which exercises had to be performed and what precautions should be taken [58▪,59,60]. A continued contact with a telecoach using telephone or video calls remained important so patients could ask questions, check whether they were exercising correctly, and could be motivated and supported [59,61]. A double-blind RCT exemplified how technology can further improve home-based physical exercise programs [62▪▪]. Specifically, in this study, persons with PD used a home-trainer augmented with virtual reality software and gamified elements to perform aerobic exercises at home, three times a week for six months. The results showed a stabilisation of MDS-UPDRS motor scores and an improvement in VO2 max scores, as compared to an active control group that performed only stretching exercises. Another technology-supported exercise program also appeared to be effective, but only in a more sedentary subgroup of patients [58▪]. Therefore, future research efforts should target specific patient groups, e.g., inactive patients, incorporate methods to facilitate personal contact, and continue to develop methods to enhance training programs with technology.
Remote interventions have also been tested for other allied health treatments, such as speech therapy. Specifically, delivering speech therapy remotely can enhance comfort and considerably reduce costs for persons with PD, with only a slight increase in costs for the healthcare system [63]. Technology offers new methods to possibly augment speech therapy, as is illustrated by an innovative RCT study protocol [64]. This study aims to deliver personalized, home-based, online speech therapy to 215 persons with PD. Treatment will be guided online by a speech therapist and, importantly, is supported by a visual feedback application on a smartphone or tablet that shows the patient in real-time whether their pitch is too high or low.
For various chronic neurological diseases, an online rehabilitation program was designed to strengthen both cognitive and physical skills [65]. The program combines virtual reality with a motion sensor so that patients can see their exercises on a screen and interact with them through bodily movements. The prescribed exercises target memory, dual tasking, executive functions, and movement of both upper and lower limbs. Patients received automated feedback on their performance in between exercises, whereas healthcare professionals personalized the content of each training session. Overall, adherence rates were high and patients reported a positive effect on their daily routine and functioning [65].
Finally, two studies delivered teletreatments focused on mental health. One study provided patients with various neurological disorders with a 6-week course that integrated elements from cognitive therapies. Completing the course at home and unsupervised was feasible [66]. An RCT added telephone-based cognitive behavioural therapy to treatment as usual, which led to a stronger reduction in depressive symptoms for persons with PD [67▪].
Although these studies offer some careful initial evidence that it is feasible and effective to deliver treatments and support training programs remotely, future research should investigate methods to enlarge the effectiveness and boost the patient experience of these treatments through technology.
CONCLUSION
A growing body of studies published in the last 2 years has helped to further establish the feasibility and effectiveness of a wide range of different telemedicine tools. Some of the telemedicine tools discussed here are now ready for clinical use in daily practice (e.g. videoconferencing, tools to support exercises), bearing the specific strengths and weaknesses of each approach in mind.
Other tools to remotely monitor and treat patients hold great promise, but require further development and independent evaluations to support their use in clinical practice and research. Diversity should be a specific focus of attention in these new studies, making sure that telemedicine approaches can be made widely available to patients with very different clinical and sociodemographic backgrounds.
Taken together, the time has come to seriously consider telemedicine as one of many useful tools available in our medical and research armamentarium, alongside with established services such as in-person visits to the hospital. Importantly, rather than regarding telemedicine as a panacea for challenges in research and clinical care, we encourage to consider the use of telemedicine as a supportive tool that can be applied under specific instances, for specific indications and for specific populations of eligible patients.
Acknowledgements
The Radboudumc Center of Expertise for Parkinson & Movement Disorders was supported by a center of excellence grant of the Parkinson's Foundation.
Financial support and sponsorship
This work was supported by Horizon 2020 (Grant 825785), ZonMw (Grant 91215076), and the Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships (Grant TKI-LSH-T2016-LSHM15022).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Dorsey ER, Okun MS, Bloem BR. Care, convenience, comfort, confidentiality, and contagion: The 5 C's that will shape the future of telemedicine. J Parkinsons Dis 2020; 10:893–897. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey ER, Bloem BR, Okun MS. A new day: the role of telemedicine in reshaping care for persons with movement disorders. Mov Disord 2020; 35:1897–1902. [DOI] [PubMed] [Google Scholar]
- 3.Artusi CA, Mishra M, Latimer P, et al. Integration of technology-based outcome measures in clinical trials of Parkinson and other neurodegenerative diseases. Parkinsonism Relat Disord 2018; 46:S53–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulroy E, Menozzi E, Lees AJ, et al. Reply to: ‘a new day: the role of telemedicine in reshaping care for persons with movement disorders’. Mov Disord 2020; 35:1903–1904. [DOI] [PubMed] [Google Scholar]
- 5.Mulroy E, Menozzi E, Lees AJ, et al. Telemedicine in movement disorders: Leçons du COVID-19. Mov Disord 2020; 35:1893–1896. [DOI] [PubMed] [Google Scholar]
- 6▪.Nouri SS, Khoong EC, Lyles CR, Karliner LS. Addressing equity in telemedicine for chronic disease management during the Covid-19 Pandemic. NEJM Catal 2020; 1–13. [Google Scholar]; Provides concrete recommendations on how to cross technological barriers to ensure equal access to telemedicine, based on insights gained from clinical practice.
- 7.Hassan A, Mari Z, Gatto EM, et al. Global survey on telemedicine utilization for movement disorders during the COVID-19 pandemic. Mov Disord 2020; 35:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MDS Telemedicine Study Group. Telemedicine in Your Movement Disorders Practice. April 2020. Available from: https://www.movementdisorders.org/MDS/About/Committees--Other-Groups/Telemedicine-in-Your-Movement-Disorders-Practice-A-Step-by-Step-Guide.htm. [Accessed 7 March 2021] [Google Scholar]
- 9.Schneider RB, Myers TL, Tarolli CG, et al. Remote Administration of the MDS-UPDRS in the Time of COVID-19 and Beyond. J Parkinsons Dis 2020; 10:1379–1382. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera-Martos I, Ortiz-Rubio A, Torres-Sánchez I, et al. Agreement between face-to-face and tele-assessment of upper limb functioning in patients with Parkinson Disease. PM&R 2019; 11:590–596. [DOI] [PubMed] [Google Scholar]
- 11▪.Witek N, Heath SL, Ouyang B, et al. Remote telemedicine evaluation of deep brain stimulation candidacy. Neurol Clin Pract 2020; 10:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent example of how teleconsultations can be effectively deployed for screening of DBS candidates to reduce travel time and costs.
- 12.Erb MK, Karlin DR, Ho BK, et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson's disease. NPJ Digit Med 2020; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Courtney E, Blackburn D, Reuber M. Neurologists’ perceptions of utilising tele-neurology to practice remotely during the COVID-19 pandemic. Patient Educ Couns 2021; 104:452–459. [DOI] [PubMed] [Google Scholar]; One of the first studies to provide in-depth insights into the positive and negative experiences of neurologists using teleconsultations during the COVID-pandemic.
- 14.Janca A, Aarli JA, Prilipko L, et al. WHO/WFN Survey of neurological services: a worldwide perspective. J Neurol Sci 2006; 247:29–34. [DOI] [PubMed] [Google Scholar]
- 15.Seritan AL, Heiry M, Iosif A-M, et al. Telepsychiatry for patients with movement disorders: a feasibility and patient satisfaction study. J Clin Mov Disord 2019; 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalash A, Fathy M, Dawood NL, Hamid E. Adopting virtual visits for Parkinson's disease patients during the COVID-19 pandemic in a developing country. Front Neurol 2020; 11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekimoto S, Oyama G, Hatano T, et al. A randomized crossover pilot study of telemedicine delivered via ipads in parkinson's disease. Parkinsons Dis 2019; 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarolli CG, Zimmerman GA, Goldenthal S, et al. Video research visits for atypical parkinsonian syndromes among Fox Trial Finder participants. Neurol Clin Pract 2020; 10:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spear KL, Auinger P, Simone R, et al. Patient views on telemedicine for Parkinson disease. J Parkinsons Dis 2019; 9:401–404. [DOI] [PubMed] [Google Scholar]
- 20.Singh RL, Bush EJ, Hidecker MJC, et al. Considering healthcare needs in a rural Parkinson Disease community. Prog Community Heal Partnerships Res Educ Action 2020; 14:1–11. [DOI] [PubMed] [Google Scholar]
- 21.Peacock D, Baumeister P, Monaghan A, et al. Perception of healthcare access and utility of telehealth among Parkinson's disease patients. Can J Neurol Sci/J Can des Sci Neurol 2020; 47:700–704. [DOI] [PubMed] [Google Scholar]
- 22.Katz M. Telehealth increases access to palliative care for people with Parkinson's disease and related disorders. Ann Palliat Med 2020; 9:S75–S79. [DOI] [PubMed] [Google Scholar]
- 23.Bloem BR, Henderson EJ, Dorsey ER, et al. Integrated and patient-centred management of Parkinson's disease: a network model for reshaping chronic neurological care. Lancet Neurol 2020; 19:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cilia R, Mancini F, Bloem BR, Eleopra R. Telemedicine for parkinsonism: a two-step model based on the COVID-19 experience in Milan, Italy. Parkinsonism Relat Disord 2020; 75:130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪.Sennott B, Woo K, Hess S, et al. Novel outreach program and practical strategies for patients with Parkinsonism in the COVID-19 pandemic. J Parkinsons Dis 2020; 10:1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]; Spurred by the new reality of the COVID-19 pandemic, this practical study beautifully shows how teleconsultations can be used to offer care proactively to vulnerable patients.
- 26.Mancini F, van Halteren AD, Carta T, et al. Personalized care management for persons with Parkinson's disease: a telenursing solution. Clin Park Relat Disord 2020; 3:100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Din S, Kirk C, Yarnall AJ, et al. Body-worn sensors for remote monitoring of Parkinson's disease motor symptoms: vision, state of the art, and challenges ahead. J Parkinsons Dis 2021; Pre-press:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ancona S, Faraci FD, Khatab E, et al. Wearables in the home-based assessment of abnormal movements in Parkinson's disease: a systematic review of the literature. J Neurol 2021. [DOI] [PubMed] [Google Scholar]
- 29.Tian Y, Lee G-H, He H, et al. RF-based fall monitoring using convolutional neural networks. Proc ACM Interact Mobile Wearable Ubiquitous Technol 2018; 2:1–24. [Google Scholar]
- 30.Lo C, Arora S, Baig F, et al. Predicting motor, cognitive & functional impairment in Parkinson's. Ann Clin Transl Neurol 2019; 6:1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iakovakis D, Chaudhuri KR, Klingelhoefer L, et al. Screening of Parkinsonian subtle fine-motor impairment from touchscreen typing via deep learning. Sci Rep 2020; 10:12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monje MHG, Fuller RLM, Cubo E, et al. Toward e-scales: digital administration of the international parkinson and movement disorder society rating scales. Mov Disord Clin Pract 2021; 8:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matarazzo M, Arroyo-Gallego T, Montero P, et al. Remote monitoring of treatment response in Parkinson's disease: the habit of typing on a computer. Mov Disord 2019; 34:1488–1495. [DOI] [PubMed] [Google Scholar]
- 34.Warmerdam E, Hausdorff JM, Atrsaei A, et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol 2020; 19:462–470. [DOI] [PubMed] [Google Scholar]
- 35.Silva de Lima AL, Smits T, Darweesh SKL, et al. Home-based monitoring of falls using wearable sensors in Parkinson's disease. Mov Disord 2020; 35:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet 2021; 0:1–20. [DOI] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04380142, Study Comparing Continuous Subcutaneous Infusion Of ABBV-951 With Oral Carbidopa/Levodopa Tablets For Treatment Of Motor Fluctuations In Adult Participants With Advanced Parkinson's Disease; 2020 May 8. Available from: https://clinicaltrials.gov/ct2/show/NCT04380142. [Accessed 7 March 2021] [Google Scholar]
- 38. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04739423, A Study of CST-103 Co-administered With CST-107 in Subjects With Neurodegenerative Disorders (CLIN-011); 2021 Feb 4. Available from: https://clinicaltrials.gov/ct2/show/NCT04739423. [Accessed 7 March 2021] [Google Scholar]
- 39.Stephenson D, Alexander R, Aggarwal V, et al. Precompetitive consensus building to facilitate the use of digital health technologies to support parkinson disease drug development through regulatory science. Digit Biomarkers 2020; 4:28–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Din S, Elshehabi M, Galna B, et al. Gait analysis with wearables predicts conversion to Parkinson disease. Ann Neurol 2019; 86:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracy JM, Özkanca Y, Atkins DC, Hosseini Ghomi R. Investigating voice as a biomarker: deep phenotyping methods for early detection of Parkinson's disease. J Biomed Inform 2020; 104:103362. [DOI] [PubMed] [Google Scholar]
- 42▪.Arora S, Baghai-Ravary L, Tsanas A. Developing a large scale population screening tool for the assessment of Parkinson's disease using telephone-quality voice. J Acoust Soc Am 2019; 145:2871–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]; Takes an important step toward moving voice analysis out of the clinic and into the patients natural environment. Using a large dataset of telephone recordings, the authors show the potential for the development of a population screening tool for PD.
- 43.Elm JJ, Daeschler M, Bataille L, et al. Feasibility and utility of a clinician dashboard from wearable and mobile application Parkinson's disease data. npj Digit Med 2019; 2:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gatsios D, Antonini A, Gentile G, et al. Feasibility and utility of mHealth for the remote monitoring of parkinson disease: ancillary study of the PD_manager Randomized Controlled Trial. JMIR mHealth uHealth 2020; 8:e16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Isaacson SH, Boroojerdi B, Waln O, et al. Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson's disease starting transdermal rotigotine patch: a pilot study. Parkinsonism Relat Disord 2019; 64:132–137. [DOI] [PubMed] [Google Scholar]; Small but well designed randomized study that takes the next step in systematically assessing the effect of a wearable sensor and dashboard on clinical decision making and patient functioning.
- 46.Santiago A, Langston JW, Gandhy R, et al. Qualitative evaluation of the personal KinetiGraphTM Movement recording system in a Parkinson's Clinic. J Parkinsons Dis 2019; 9:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Evans L, Mohamed B, Thomas EC. Using telemedicine and wearable technology to establish a virtual clinic for people with Parkinson's disease. BMJ Open Qual 2020; 9:e001000. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides a clear template for how telemonitoring tools could be integrated into routine clinical practice. The independent research group provides a detailed description of how a virtual clinic was implemented, including practical barriers that had to be overcome.
- 48▪.Woodrow H, Horne MK, Fernando CV, Kotschet KE. A blinded, controlled trial of objective measurement in Parkinson's disease. NPJ Park Dis 2020; 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first large scale controlled trials assessing the effect on clinical outcomes of a wearable sensor and dashboard to support clinicians in the management of response fluctuations.
- 49.Powers R, Etezadi-Amoli M, Arnold EM, et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson's disease. Sci Transl Med 2021; 13:eabd7865. [DOI] [PubMed] [Google Scholar]
- 50▪▪.Riggare S, Scott Duncan T, Hvitfeldt H, Hägglund M. You have to know why you’re doing this’: a mixed methods study of the benefits and burdens of self-tracking in Parkinson's disease. BMC Med Inform Decis Mak 2019; 19:175. [DOI] [PMC free article] [PubMed] [Google Scholar]; This well designed study uses a mixed methods approach to provide in-depth and interesting insights into PD patient's perspective on self-tracking. It highlights the need to carefully consider the balance between benefits and burdens of self-tracking for each individual patient.
- 51.de Vries NM, Smilowska K, Hummelink J, et al. Exploring the Parkinson patients’ perspective on home-based video recording for movement analysis: a qualitative study. BMC Neurol 2019; 19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heijmans M, Habets JGV, Herff C, et al. Monitoring Parkinson's disease symptoms during daily life: a feasibility study. NPJ Park Dis 2019; 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bloem BR, Evers LJW, van de Zande T, et al. The Personalized Parkinson Project – data quality enhancing strategies [abstract]. Mov Disord 2020; suppl 1: Available from: https://www.mdsabstracts.org/abstract/the-personalized-parkinson-project-data-quality-enhancing-strategies/. [Accessed 7 March 2021]. [Google Scholar]
- 54▪.Wannheden C, Revenäs Å. How people with Parkinson's disease and healthcare professionals wish to partner in care using eHealth: co-design study. J Med Internet Res 2020; 22:e19195. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a participatory design, this qualitative study provides valuable insights into e-health functionalities desired by patients and healthcare providers, and barriers to their implementation.
- 55.Espay AJ, Hausdorff JM, Sánchez-Ferro Á, et al. A roadmap for implementation of patient-centered digital outcome measures in Parkinson's disease obtained using mobile health technologies. Mov Disord 2019; 34:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Xu J, Wang J, Keith S, et al. Management of Parkinson's disease patients after DBS by remote programming: preliminary application of single center during quarantine of 2019-nCoV. J Neurol 2020; 268:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides a clear and detailed explanation of the procedures employed to perform remote DBS adjustments.
- 57.Zhang C, Zhu K, Lin Z, et al. Utility of deep brain stimulation telemedicine for patients with movement disorders during the COVID-19 outbreak in China. Neuromodul Technol Neural Interface 2021; 24:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪.Ellis TD, Cavanaugh JT, DeAngelis T, et al. Comparative effectiveness of mhealth-supported exercise compared with exercise alone for people with Parkinson Disease: randomized controlled pilot study. Phys Ther 2019; 99:203–216. [DOI] [PubMed] [Google Scholar]; This 12-month RCT exemplifies various ways in which technology can be incorporated into the design of a physical exercise program for PD patients, and highlights the need to specifically address inactive patients in such trials.
- 59.Flynn A, Preston E, Dennis S, et al. Home-based exercise monitored with telehealth is feasible and acceptable compared to centre-based exercise in Parkinson's disease: a randomised pilot study. Clin Rehabil 2020; 35:728–739. [DOI] [PubMed] [Google Scholar]
- 60.Landers MR, Ellis TD. A mobile app specifically designed to facilitate exercise in Parkinson disease: single-cohort pilot study on feasibility, safety, and signal of efficacy. JMIR mHealth uHealth 2020; 8:e18985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai B, Bond K, Kim Y, et al. Exploring the uptake and implementation of tele-monitored home-exercise programmes in adults with Parkinson's disease: A mixed-methods pilot study. J Telemed Telecare 2020; 26:53–63. [DOI] [PubMed] [Google Scholar]
- 62▪▪.van der Kolk NM, de Vries NM, Kessels RPC, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson's disease: a double-blind, randomised controlled trial. Lancet Neurol 2019; 18:998–1008. [DOI] [PubMed] [Google Scholar]; Well designed large scale RCT that exemplifies how technology can be used to enhance home-based physical exercise programs using virtual reality and gamification.
- 63.Saiyed M, Hill AJ, Russell TG, et al. Cost analysis of home telerehabilitation for speech treatment in people with Parkinson's disease. J Telemed Telecare 2020; 0:1–6. [DOI] [PubMed] [Google Scholar]
- 64. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT03963388, PERsonalized SPEeCh Therapy for actIVE Conversation (PERSPECTIVE); 2019 May 24. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03963388. [Accessed 7 March 2021] [Google Scholar]
- 65.Isernia S, Pagliari C, Jonsdottir J, et al. Efficiency and patient-reported outcome measures from clinic to home: the human empowerment aging and disability program for digital-health rehabilitation. Front Neurol 2019; 10:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gandy M, Karin E, McDonald S, et al. A feasibility trial of an internet-delivered psychological intervention to manage mental health and functional outcomes in neurological disorders. J Psychosom Res 2020; 136:110173. [DOI] [PubMed] [Google Scholar]
- 67▪.Dobkin RD, Mann SL, Gara MA, et al. Telephone-based cognitive behavioral therapy for depression in Parkinson disease. Neurology 2020; 94:e1764–e1773. [DOI] [PMC free article] [PubMed] [Google Scholar]; This RCT shows that adding remotely delivered cognitive behavioural therapy to treatment as usual outperforms treatment as usual alone on various mental health outcomes, thereby highlighting the possible advantages of teletreatment.


