Objectives:
There are no approved pharmacologic therapies for chronic sensorineural hearing loss (SNHL). The combination of CHIR99021+valproic acid (CV, FX-322) has been shown to regenerate mammalian cochlear hair cells ex vivo. The objectives were to characterize the cochlear pharmacokinetic profile of CV in guinea pigs, then measure FX-322 in human perilymph samples, and finally assess safety and audiometric effects of FX-322 in humans with chronic SNHL.
Study Designs:
Middle ear residence, cochlear distribution, and elimination profiles of FX-322 were assessed in guinea pigs. Human perilymph sampling following intratympanic FX-322 dosing was performed in an open-label study in cochlear implant subjects. Unilateral intratympanic FX-322 was assessed in a Phase 1b prospective, randomized, double-blinded, placebo-controlled clinical trial.
Setting:
Three private otolaryngology practices in the US.
Patients:
Individuals diagnosed with mild to moderately severe chronic SNHL (≤70 dB standard pure-tone average) in one or both ears that was stable for ≥6 months, medical histories consistent with noise-induced or idiopathic sudden SNHL, and no significant vestibular symptoms.
Interventions:
Intratympanic FX-322.
Main Outcome Measures:
Pharmacokinetics of FX-322 in perilymph and safety and audiometric effects.
Results:
After intratympanic delivery in guinea pigs and humans, FX-322 levels in the cochlear extended high-frequency region were observed and projected to be pharmacologically active in humans. A single dose of FX-322 in SNHL subjects was well tolerated with mild, transient treatment-related adverse events (n = 15 FX-322 vs 8 placebo). Of the six patients treated with FX-322 who had baseline word recognition in quiet scores below 90%, four showed clinically meaningful improvements (absolute word recognition improved 18–42%, exceeding the 95% confidence interval determined by previously published criteria). No significant changes in placebo-injected ears were observed. At the group level, FX-322 subjects outperformed placebo group in word recognition in quiet when averaged across all time points, with a mean improvement from baseline of 18.9% (p = 0.029). For words in noise, the treated group showed a mean 1.3 dB signal-to-noise ratio improvement (p = 0.012) relative to their baseline scores while placebo-treated subjects did not (−0.21 dB, p = 0.71).
Conclusions:
Delivery of FX-322 to the extended high-frequency region of the cochlea is well tolerated and enhances speech recognition performance in multiple subjects with stable chronic hearing loss.
Keywords: FX-322, Pharmacokinetics, Sensorineural hearing loss, Therapeutic
An estimated 1.1 billion people are at risk for disabling hearing loss worldwide (1,2) for which there is currently no pharmacologic treatment. Chronic sensorineural hearing loss (SNHL) accounts for roughly 90% of this sensory deficit (3) and is likely caused by noise, chemical, viral, and aging insults with potentially debilitating effects (4). In people with SNHL, audibility (loudness of sound) and intelligibility (clarity of words) deteriorate due to the aforementioned auditory insults. Except for “retrocochlear” hearing loss, more than 80% of chronic SNHL is due at least in part to loss of cochlear hair cells (5).
While many vertebrates such as birds and reptiles regenerate hair cells spontaneously to restore hearing after various insults, mammals do not. Mammalian progenitor cells that produce hair cells during embryonic development persist into adulthood but are quiescent (6–11). Previously published work showed that the combination of CHIR99021, a glycogen synthase kinase-3 (GSK3) inhibitor, and valproic acid (VPA) acts synergistically to activate proliferation of quiescent mammalian cochlear progenitor cells in vitro from mouse, nonhuman primate, and human (8). Additionally, a murine ex vivo study showed that application of CHIR99021+VPA (CV) following aminoglycoside ototoxicity induced supporting cells in the organ of Corti expressing the leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) to divide and regenerate hair cells (8). Others showed that hair cell transdifferentiation can improve auditory thresholds in animals and entered clinical studies, though human results have not been disclosed (12, 13, clinicaltrials.gov: NCT02132130, EudraCT 2016–004544–10).
Applying compounds that regenerated hair cells in McLean et al. (8) could provide a novel approach to improve auditory function in subjects with chronic SNHL. CV was formulated for human use as a thermoreverable poloxamer, named FX-322, that can be injected intratympanically as a liquid and transition to a gel in the middle ear to allow prolonged diffusion into the cochlea. In humans, Bird et al. (14,15) characterized the perilymph concentrations of steroid solutions, which demonstrated human cochlear PK could be assessed, but did not discuss a pharmacodynamic (PD) relationship.
Herein, we relate the preclinical and clinical cochlear pharmacokinetics (PK) of FX-322 to its clinical pharmacodynamics (PD) as a potential therapy for restoration of hearing in patients with SNHL. We evaluated spatial and temporal drug distribution in guinea pig cochlea and subsequently modeled the distribution in human cochlea. Predicted concentrations were compared to those that showed activity in ex vivo mouse and human studies (8). Drug concentrations were measured for samples of middle ear contents and perilymph to calibrate the human PK model and validate measured and modeled values. A Phase 1b clinical trial was conducted to assess safety of intratympanically administered FX-322 in adult human subjects with chronic SNHL and to study its PD effect on hearing. Collectively, these data enable the first analysis of FX-322 as a potential therapy for hearing restoration by measuring its effect on hearing improvement in relation to its cochlear distribution.
METHODS
The methods used are described here in brief. Detailed methods are provided in the Supplemental Digital Content.
Guinea Pig Pharmacokinetic Studies and Pharmacokinetic Modeling
Pharmacokinetic studies of FX-322 applied intratympanically for 3 hours were performed as terminal studies in guinea pigs to calibrate a well-established computer model predicting drug distribution over the cochlear length with time (16). Both drugs were delivered simultaneously as FX-322. Key parameters for drug entry from the middle ear and for drug elimination from perilymph were established by fitting the measured data.
The PK models established for the guinea pig were then adjusted according to the fluid and tissue compartment volumes for the human inner ear, based on human anatomy. Determinants of drug entry in the human ear (middle ear time course, entry rate into perilymph) were derived from human data analysis, interpreted by the computer model, validated in guinea pigs, and scaled to the size of the human inner ear.
Clinical Pharmacokinetic and Phase 1b Studies
These studies were conducted according to the International Conference on Harmonisation guidelines, Good Clinical Practices, and the Declaration of Helsinki. The protocols and amendments were approved by Institutional Review Boards for participating investigators and each patient provided written informed consent.
Human Perilymph Collection
The PK model was extended to humans by analysis of human middle ear contents and perilymph collected from subjects undergoing cochlear implant surgery. FX-322 (0.2 ml) was applied intratympanically and remained in place for times varying from 60 to 79 minutes. Under constant suction and irrigation, a cortical mastoidectomy with posterior tympanotomy was performed to expose the round window and stapes. All FX-322 remnants were collected for analysis, and then removed by irrigation and suction. If a pseudo membrane was present over the round window, it was carefully removed, as well as any bony overhang. Perilymph was collected with a modified micro glass capillary before cochlear implantation, as previously described (17).
Perilymph Sample Analysis
Samples were analyzed using high-pressure liquid chromatography with mass spectrometry detection (HPLC-MS) methods that were validated under matrix matched conditions.
Phase 1b Study
The study was randomized (NCT03616223), double-blind, placebo-controlled and conducted at three US sites, and as such all investigators, study staff, study subjects, and the study sponsor were blinded to treatment. Adults aged 18 to 65 years were eligible if they had an established diagnosis of stable (no documented changes of ≥10 dB at any standard frequency for >6 mo) SNHL associated with noise exposure or idiopathic sudden SNHL (SSNHL) that was confirmed by a board-certified otolaryngologist. In the case of bilateral SNHL the worse ear was typically treated, and for symmetrical SNHL the injected ear was determined at the discretion of the otolaryngologist. Briefly, subjects were excluded for conductive hearing loss; 4-frequency pure-tone average of >70 dB; vestibular symptoms; systemic autoimmune disease; or history of head or neck radiation.
Subjects were randomized to four groups in a 2:1 drug:placebo ratio to receive unilateral intratympanic injection of either: 0.05 ml placebo, 0.2 ml placebo, 0.05 ml FX-322, or 0.2 ml FX-322. The primary endpoint was safety reporting the number of participants with treatment-related adverse events as assessed by CTCAE v5.0 on Day 15. Blood samples were obtained predose and up to 24 hours post injection to measure systemic exposure to the active pharmacologic agents of FX-322. Subjects were monitored for adverse events, and underwent routine physical examination, vital signs, electrocardiogram, and clinical laboratory testing.
Exploratory audiometric assessments defined a priori per protocol, including otoscopy, tympanometry, air and bone conduction audiometry, and speech audiometry, were performed at screening and all follow-up visits for all FX-322- and placebo-injected ears (Supplemental Figure S1). Word recognition in quiet (WR) performance was assessed using the Maryland CNC word lists (18), and speech-in noise performance was assessed using the Words-in-Noise test (WIN) (19). Both speech recognition tests were administered 40 dB above the participant's speech reception threshold.
Statistical Analysis
A sample size of approximately 24 subjects was considered adequate for an initial assessment of safety and tolerability and was not based on formal statistical considerations. Prespecified statistical analyses were exclusively descriptive and included 95% confidence intervals (CIs) as appropriate. Audiometric analyses were considered exploratory and conducted without multiplicity adjustments. A mixed model for repeated measures (MMRM) was used for exploratory analyses of the relative change from baseline for audiometry at 8 kHz (in dB) and speech recognition with WR (in percentage points) and WIN (in dB signal-to-noise ratio [SNR]). Prospective patient-level and post hoc group-level analyses of speech perception changes from baseline to Day 90 were conducted per Thornton and Raffin using a binomial distribution statistic outlining test–retest variability for a 50-item list (20). Baseline and Day 90 results of the WIN test were used to fit linear mixed model cubic polynomials across the tested SNR (21). Estimates of SNR values that correspond to the 50% correct word threshold were calculated for each patient using the Spearman–Karber formula (19). These values were then analyzed via MMRM for the group differences in mean change from baseline to Day 90.
RESULTS
Guinea Pig Cochlear Sampling and PK Modeling
Guinea pig and human cochlear PK studies with FX-322 were conducted to calibrate and validate a computer model predicting drug distribution over cochlear length with time (16) to determine if there were any correlations to functional changes observed in the Phase 1b study.
Measurement of Entry Rates Into Perilymph With RW Niche Application
Drug entry into perilymph at the round window (RW) and stapes is driven by the drug concentration in the middle ear (22). Drug concentrations in samples taken from the RW niche were found to decrease with time. Exponential curves fitted to these data indicated elimination half-times of 56.4 minutes and 48.6 minutes, respectively, for CHIR99021 and VPA (Supplemental Figures S2A & S2B). Exponentially declining middle-ear concentrations were used to derive simulations of drug movement into perilymph in which calculated perilymph concentrations were compared to perilymph sample data following RW niche applications.
Perilymph samples were collected from the guinea pig LSCC or cochlear apex at either 1 or 3 hours after CV application to the RW niche. For sampling from the cochlear apex, serial samples, each 1 μl in volume, were collected as the perilymph emerged after perforating the apex. The serial samples allowed drug distribution along the length of ST from apex to base to be derived. Sample perilymph drug concentration data collected 1 hour after RW application are shown in Supplemental Figures S2C & S2D. The highest drug concentration of both FX-322 components was found in samples originating from basal regions and decreased apically. The gradient along ST was most pronounced for VPA.
Interpretation and Validation of Simulations
Experiments were simulated by configuring the delivery and sampling conditions for the experiment and varying entry permeability of the RW and stapes independently to best fit the entire data set for each drug, comprising both sampling sites (apex and LSCC) and application durations (1 and 3 hours). Kinetic parameters derived from fitting sample measurements are summarized in Supplemental Table S1. Concentration profiles along ST as a function of distance and time, calculated using the tabulated kinetic parameters, are summarized in Supplemental Figure S2. This modeling indicated that CHIR99021 and VPA achieved concentrations in the most basal, or extended high-frequency (EHF) region (Supplemental Figure S3), that were consistent with reported regenerative activity in mammalian cell and cochlear explant culture (8).
Human Cochlear Sampling and PK Modeling
Human Perilymph Sampling
Cochlear implant subjects (n = 7) were injected intratympanically with FX-322 intraoperatively, and samples of middle ear contents and 1 to 2 μl of perilymph were collected 60 to 79 minutes later (exact amounts and times shown in Supplemental Table S2). One subject's perilymph sample was contaminated with blood and therefore excluded. Measured CHIR99021 ranged from 248 to 5,850 ng/ml, while VPA concentrations ranged from 15,120 to 186,000 ng/ml, with group data analysis shown in Figure 1. While all subjects had anatomical features that have been speculated to impair drug entry, such as mucosal folds or bony overhangs, both drugs entered in all subjects (Supplemental Table S2).
FIG. 1.
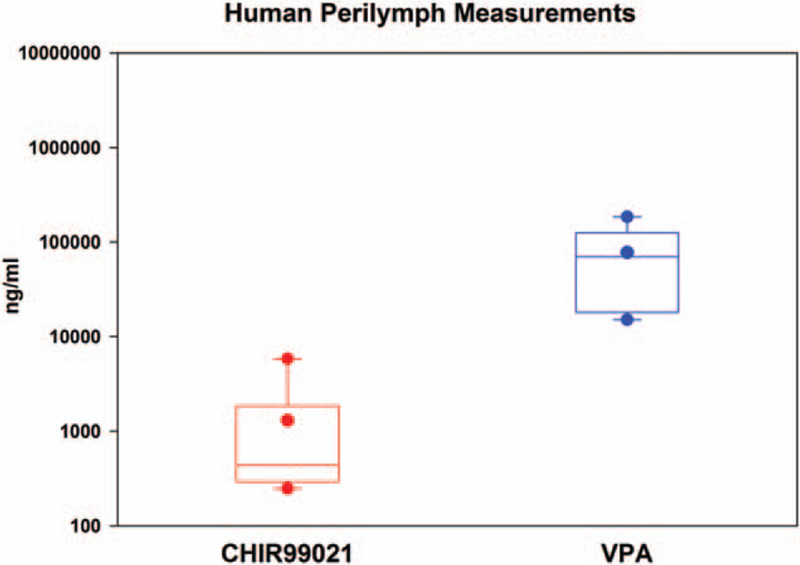
Population human perilymph measures. Box plot representation of perilymph measures of FX-322 active ingredients CHIR99021 and VPA (n = 7).
Human PK Modeling
The middle ear concentration decline rates and perilymph entry rates derived from modeling were each adjusted to fit the measured sample data from each patient (Supplemental Table S3). Calculated concentrations as a function of distance and time based on the average kinetic parameters from six subjects are shown in Figure 2. Projected drug concentrations achieved in the basal human cochlea were comparable to drug concentrations shown to be effective at proliferating Lgr5+ cells and regenerating hair cells ex vivo(8). The model predicted that the concentrations would be achieved within approximately 1 to 3 hours of drug administration and would be localized to the EHF range at the base of the cochlea.
FIG. 2.
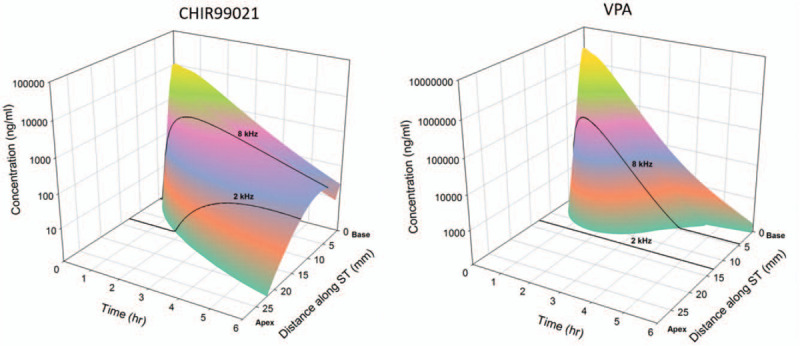
Modeled pharmacokinetics of FX-322 in the human inner ear. Calculated human cochlear distributions of CHIR99021 and valproic acid (VPA) as a function of cochlear distance and time based on elimination measurements obtained in guinea pig pharmacokinetic studies. Calculations include entry, perilymph kinetic and middle ear kinetic parameters, as summarized in Table S3.
Phase 1b Clinical Study in Stable Chronic SNHL Subjects
FX-322 Safety and Tolerability
Twenty-three subjects (3 sites contributed 13, 5, and 5 subjects) were randomized to treatment with FX-322 or placebo (Fig. 3). Demographic variables were consistent across treatment groups (Table 1). There were no notable differences in treatment-related adverse events (AEs) between the FX-322- and placebo-treated subjects (Table 1). Observed AEs were associated with the intratympanic injection procedure, including pain, discomfort, and itching (23). Most AEs were mild, transient, and resolved within minutes of dosing. One subject experienced a persistent tympanic membrane perforation that spontaneously resolved within 30 days. No drug-related systemic AEs occurred, and no clinically relevant changes were observed for clinical laboratory values, vital signs, ECG, otoscopy (except for the single perforation), or tympanometry. Plasma levels for both active agents in FX-322 were well below systemic pharmacologic levels (Supplemental Figure S4). No study participant, whether injected with FX-322 or placebo, showed a clinically meaningful decrement in hearing performance.
FIG. 3.
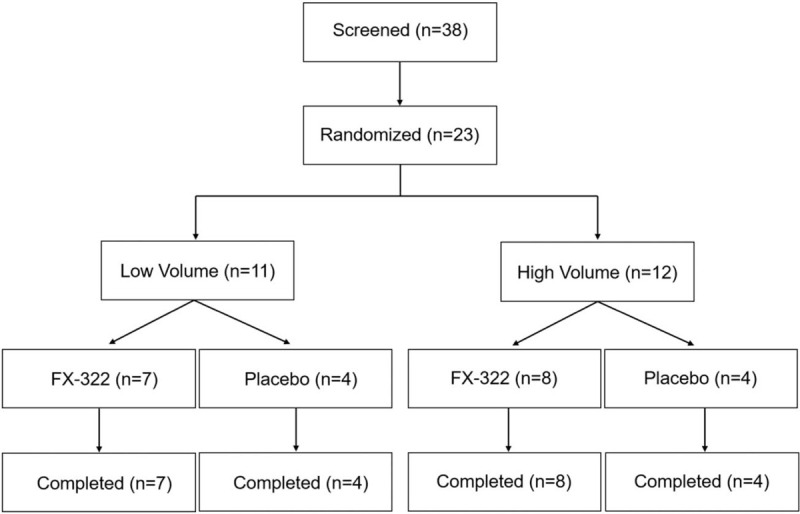
Consort diagram. Low volume = 0.05 ml; high volume = 0.2 ml.
TABLE 1.
Patient demographics and incidence of treatment-related adverse events
| Parameter | Pooled Placebo (N = 8) | FX-322 Low Volume (N = 7) | FX-322 High Volume (N = 8) | Total (N = 23) |
| Age | ||||
| Median | 58.0 | 60.0 | 52.5 | 57.0 |
| Mean | 57.0 | 59.1 | 50.5 | 55.4 |
| Std. dev. | 5.76 | 5.67 | 9.21 | 7.77 |
| Min, max | 45, 63 | 48, 64 | 33, 62 | 33, 64 |
| Sex | ||||
| Male | 4 | 5 | 5 | 14 |
| Female | 4 | 2 | 3 | 9 |
| Race | 8 | 6 | 7 | 21 |
| White | 0 | 0 | 1 | 1 |
| American Indian/Alaska Native | 0 | 1 | 0 | 1 |
| Asian | ||||
| Etiology | ||||
| NIHL | 5 | 4 | 3 | 12 |
| SSNHL | 3 | 3 | 5 | 11 |
| Median Duration | 5 | 3 | 4 | 4 |
| Mean duration | 11.88 | 5.43 | 7.63 | 8.43 |
| Duration range (yrs) | 1–43 | 1–20 | 1–21 | 1–43 |
| Adverse event | ||||
| Ear discomfort | 2 (25.0%) | 5 (71.4%) | 4 (50.0%) | 11 (47.8%) |
| Ear pain | 3 (37.5%) | 1 (14.3%) | 3 (37.5%) | 7 (30.4%) |
| Ear pruritis | 1 (12.5%) | 1 (14.3%) | 0 | 2 (8.7%) |
| Paraesthesia ear | 0 | 0 | 1 (12.5%) | 1 (4.3%) |
| Tinnitus | 0 | 0 | 1 (12.5%) | 1 (4.3%) |
| Tympanic membrane perforation | 0 | 0 | 1 (12.5%) | 1 (4.3%) |
| Dizziness | 0 | 1 (14.3%) | 0 | 1 (4.3%) |
| Headache | 1 (12.5%) | 0 | 1 (12.5%) | 2 (8.7%) |
| Parosmia | 0 | 0 | 1 (12.5%) | 1 (4.3%) |
| Oropharyngeal pain | 0 | 1 (14.3%) | 0 | 1 (4.3%) |
| Throat irritation | 0 | 0 | 1 (12.5%) | 1 (4.3%) |
NIHL, noise-induced hearing loss; SSNHL, sudden sensorineural hearing loss.
Human Hearing Assessments and Outcomes
Comparison of baseline and Day 90 pure-tone thresholds showed no statistically significant differences between groups at any frequency. However, Day 90 pure-tone assessments showed that 4/15 FX-322-treated patient ears had 10 dB improvement at the highest test frequency (8 kHz), whereas no placebo-treated ears showed this level of improvement.
Changes in individual WR performance were analyzed across sessions to determine if any clinically meaningful change had occurred based on parameters set forth by Thornton and Raffin's binomial distribution (20). Of the 23 participants, 10 displayed a deficit in WR performance (≤90%) before treatment so could be assessed for hearing improvement without a “ceiling effect” (24). Of these 10 patients, 6 were treated with FX-322 and 4 with placebo. Four of the six FX-322-treated ears showed statistically significant and clinically meaningful improvements from baseline to 90 days in the prespecified WR test, exceeding expected levels of test–retest variability for this measure (Fig. 4A). In contrast, no placebo-treated ears showed statistically significant changes. The four FX-322-treated ears that had clinically meaningful improvements had an absolute mean (SE) WR increase of 35.4 (5.5) percentage points.
FIG. 4.
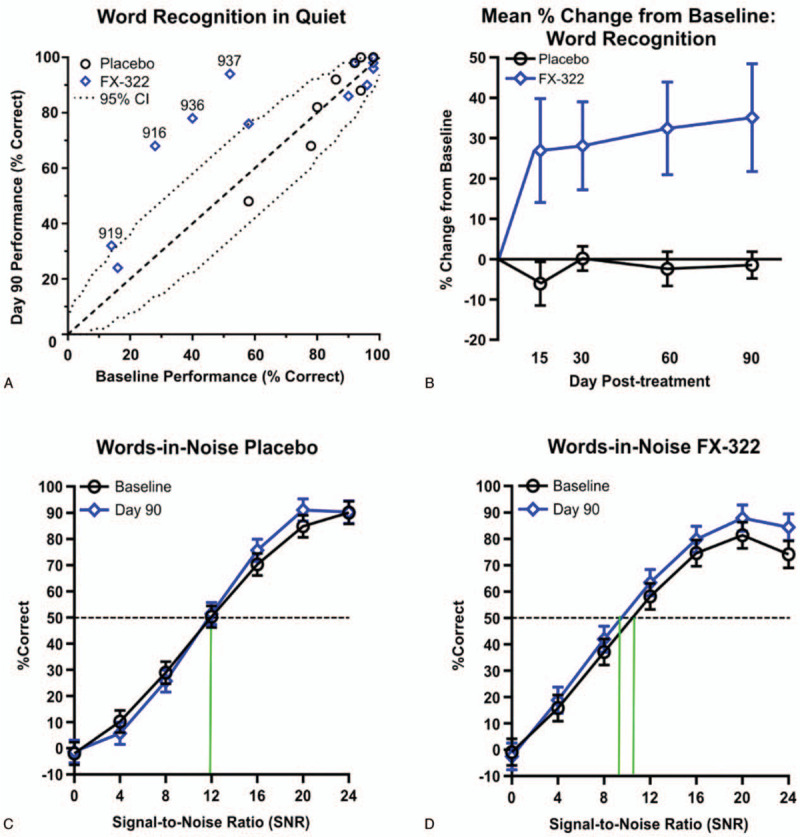
Hearing improvement in WR and WIN from a single dose of FX-322 in a Phase 1b clinical trial. A, A bivariate plot of Word Recognition in Quiet (WR) scores at Baseline and Day 90. Subjects with significant improvements by falling outside the 95% confidence interval (CI) are numbered by their patient number. Four FX-332-treated subjects and zero placebo-treated subjects fall outside of the 95% CI. B, FX-322 subjects showed increased WR scores across Days 15, 30, 60, and 90 whereas the placebo group did not improve; (2-tailed pairwise comparison of adjusted means between treatment groups averaged across all time periods, SE, p = 0.029). C, Psychometric functions for words-in-noise (WIN) data show no improvement from Baseline to Day 90 for placebo-treated subjects, (D) while FX-322-treated subjects show improvement (mean, 95% CI, p = 0.012). Data presented includes all subjects (n FX322 = 15, n placebo = 8).
FX-322-treated subjects’ speech recognition performance improved over the duration of the study while placebo-treated subjects did not. In WR assessments, FX-322-treated patient ears showed a statistical improvement in the percent change from baseline scores versus placebo on average across all time points (p = 0.029). The effects were sustained throughout the study, with the following least-square mean differences between groups: Day 15 least-square mean (SE) = 18.3% (11.0); Day 30 = 14.2% (11.4); Day 60 = 21.1% (11.4); and Day 90 = 21.9% (11.0) (Fig. 4B).
Speech recognition in a noisy background using the WIN also improved over time for FX-322-treated patient ears but not placebo-treated patient ears. Performance was quantified as the signal-to-noise ratio (SNR; 0–24 dB) consistent with 50% correct WR, with lower SNR values indicating better speech perception in background noise. Analyses showed a significant improvement in average SNR from baseline to Day 90 in FX-322-treated ears (−1.3 dB; p = 0.012) but not placebo-treated patient ears (−0.21 dB, p = 0.71 (Fig. 4C and D).
Individual responses across intelligibility tests for the four FX-322-treated patient ears that showed clinically significant improvements are listed in Table 2. Absolute improvements in WR from baseline to day 90 range from 18 to 42% in these four patients. Two of these four ears showed substantial and clinically meaningful improvements in WIN testing from baseline to Day 90, with SNR improvements that exceed the 3.1 dB threshold representing the critical difference exceeding expected test–retest variability as established by Wilson and McArdle (21). Subjects from both etiologies and dose volume cohorts responded to treatment.
TABLE 2.
Summary of intelligibility performance for most notable responders
| WR | WIN | 50% dB SNR | ||||
| Baseline | Day 90 | Baseline | Day 90 | Baseline | Day 90 | |
| Subject | ||||||
| 916 | 28% | 68% | 19% | 33% | 20.8 | 16.8 |
| 919 | 14% | 32% | 26% | 26% | 18.8 | 18.8 |
| 936 | 40% | 78% | 26% | 40% | 18.8 | 14.8 |
| 937 | 52% | 94% | 47% | 57% | 12.8 | 10.0 |
Subjects 916, 919, 936, and 937 were all treated with FX-322 and showed marked improvements from baseline to Day 90 in word recognition in quiet (WR) and words-in-noise (WIN) tests. Subjects 916 and 936 showed clinically meaningful improvement in 50% dB signal-to-noise ratio (SNR), a decrease ≥3.1 dB represents and improvement with 95% confidence.
DISCUSSION
Herein, we report intelligibility improvements in FX-322-treated subjects with chronic SNHL and relate those improvements to cochlear PK. Four FX-322-treated subjects with baseline WR scores below 90% had clinically meaningful improvements after a single injection, whereas no placebo-treated ears improved. Human cochlear PK measurements and modeling indicated that both active agents in FX-322 reached levels in the EHF region of the cochlea that regenerated hair cells ex vivo(8), and that both drugs consistently entered the human cochlea regardless of middle ear anatomical features such as pseudo membranes or prominent bony round window overhangs.
While mechanisms of inner ear damage have been extensively researched, little is known about the behavioral manifestations of therapeutically restoring hearing. In the present Phase 1b study, intratympanic application of these agents in human subjects resulted in intelligibility improvements in the absence of significant audibility improvements. These results are novel and raise questions regarding the relationship between audibility and intelligibility performance both in quiet and in noise. Loss of audibility is the direct result of damage to hair cells, particularly outer hair cells (OHCs). Healthy OHCs are known to be associated with amplification and tuning along the basilar membrane (25). Work from Liberman and Dodds (26) has shown that OHC loss elevates thresholds and flattens cochlear tuning curves, causing sound distortion. Similarly, poor OHC function and auditory nerve function in humans have been correlated with impaired speech-in-noise performance (27). The results from our trial suggest that a therapy with the potential to regenerate OHCs might help restore the sharp cochlear tuning lost by damage and thereby restore information vital to speech-in-noise intelligibility.
This work suggests a potential first correlation between human cochlear PK and observed functional hearing changes in human, a factor that is important to consider for hearing-related drug delivery and development. PK modeling showing the active agents in FX-322 concentrate primarily in the region of the cochlea where EHFs are detected is in good agreement with the audiometric observations in the Phase 1b study. FX-322 is a thermoreversible poloxamer that is injected intratympanically as a liquid and transitions to a gel, allowing prolonged cochlear diffusion. Cochlear PK measurements and modeling indicated that intratympanic injection resulted in highest drug concentration in cochlear base, which created a declining gradient along the ST toward the apical regions that encode lower frequencies. This is consistent with the observation that multiple FX-322-treated subjects had 10 dB increases in audibility at 8 kHz but not at lower frequencies where PK simulations did not show active levels of drug.
The connection of PK to intelligibility changes is supported by the growing understanding of the roles of cochlear tuning and EHF hearing. The contributions of EHFs in speech perception have been widely studied (28–37). Recent work further indicates that these frequencies are particularly critical to understanding speech-in-noise, even without changes in pure tones as measured by standard audiometry (38). Perhaps most relevant to the present study is work from Badri et al. (39) who examined speech-in-noise performance and psychophysical tuning curves in individuals with normal hearing in the standard audiometric frequencies through 8 kHz but who reported difficulty hearing in noise. Such subjects had poorer performance on speech-in-noise testing and had elevated thresholds in the ultra-high frequencies. Those subjects with elevated EHF thresholds also had broader psychophysical tuning curves at 2 kHz. This suggests that hearing loss in the EHF region removes or at least reduces information vital to speech recognition tasks and introduces distortion in the mid-frequency region deemed crucial for speech intelligibility (40).
There are several limitations in the studies we report. While baseline imbalances are not unusual in small exploratory studies, our current Phase 1b study showed an imbalance of baseline speech perception scores between treated and placebo groups and this imbalance requires careful interpretation of the data. That said, for speech perception we prospectively applied a binomial analysis (20) accounting for baseline WR performance. Additionally, the study spanned multiple levels of baseline hearing loss. Thus, improvements would only be observed in participants with moderate to moderately severe hearing loss, we think, in part because of a potential ceiling effect in mild loss. Finally, audiometry at EHFs were beyond the scope of this safety study but will be studied in trials going forward. Altogether, these results suggest that the degree and distribution of hearing loss and their impact on relative performance may be important features to consider for future studies. Lastly, since early-phase clinical safety trials inherently have small sample sizes, future randomized studies will include larger study populations to address the specific limitations observed here.
In summary, the present work provides the first evidence that a novel potential therapeutic, FX-322, is associated with clinically meaningful improvements in hearing function in subjects with chronic stable noise-induced or sudden SNHL. Data from this Phase 1b, randomized, double-blind, placebo-controlled study support the safety of intratympanic injection of FX-322 and demonstrate speech recognition improvements in the ears of subjects with stable SNHL treated with a single dose of FX-322. Multiple treated ears showed statistically significant and clinically meaningful improvements using prospectively defined and well-established WR criteria. Data derived from preclinical and clinical pharmacokinetic studies suggest that intratympanic injection of FX-322 was able to achieve therapeutically active levels of drug in the EHF region of the cochlea. Together, these data support the hypothesis that EHF function and restoration of hearing in this region contribute to intelligibility improvements that could improve communication ability in subjects with hearing loss. This work provides the first proof-of-principle that a pharmacologic treatment can improve SNHL. Further studies are warranted with larger study populations, repeat dosing, EHF testing, and longer follow-up to more fully characterize the optimal treatment regimen, effectiveness, and treatment outcomes with FX-322.
Supplementary Material
Acknowledgments
The authors thank David Friedland, William W. Chin, and Michael R Jirousek for guidance on the manuscript.
Footnotes
This study was funded by Frequency Therapeutics, Inc., Woburn, MA.
W.J.M, A.S.H., J.T.J.H., S.W., D.L. Lucchino, C. Loose, C. LeBel are employees and hold equity in Frequency Therapeutics. C.L.R., R.H.G., S.D.R., D.J. Lee, R.L., J.M.K., advise and hold equity in Frequency Therapeutics. Frequency Therapeutics has licensed IP generated by R.L. and J.M.K.
No other authors have conflicts of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1.World Health Organization. Hearing loss due to recreational exposure to loud sounds: a review. 2015. [Google Scholar]
- 2.Cunningham LL, Tucci DL. Hearing loss in adults. N Engl J Med 2017; 377:2465–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Chao T, Brant J, O’Malley B, Jr, Tsourkas A, Li D. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv Drug Deliv Rev 2017; 108:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basner M, Brink M, Bristow A, et al. ICBEN review of research on the biological effects of noise 2011-2014. Noise Health 2015; 17:57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Academic Press, David AC. Lutman ME, Haggard MP. Hearing disorders in the population: first phase findings of the MRC National Study of Hearing. Hearing Science and Hearing Disorders 1983; 35–60. [Google Scholar]
- 6.McLean WJ, McLean DT, Eatock RA, Edge AS. Distinct capacity for differentiation to inner ear cell types by progenitor cells of the cochlea and vestibular organs. Development 2016; 143:4381–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci 2012; 32:9639–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean WJ, Yin X, Lu L, et al. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep 2017; 18:1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai R, Kuo B, Wang T, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A 2012; 109:8167–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 2006; 133:2817–2826. [DOI] [PubMed] [Google Scholar]
- 11.Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol 1967; 220: suppl: 1–44. [PubMed] [Google Scholar]
- 12.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 2005; 11:271–276. [DOI] [PubMed] [Google Scholar]
- 13.Mizutari K, Fujioka M, Hosoya M, et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma [published correction appears in Neuron 2013; 77:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 2007; 28:1124–1130. [DOI] [PubMed] [Google Scholar]
- 15.Bird PA, Murray DP, Zhang M, Begg EJ. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol 2011; 32:933–936. [DOI] [PubMed] [Google Scholar]
- 16.Salt A, Hartsock J, Piu F, Hou J. Dexamethasone and dexamethasone-phosphate entry into perilymph compared for middle ear applications in guinea pigs. Audiol Neurootol 2018; 23:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt HA, Pich A, Schröder A, et al. Proteome analysis of human perilymph using an intraoperative sampling method. J Proteome Res 2017; 16:1911–1923. [DOI] [PubMed] [Google Scholar]
- 18.Causey GD, Hood LJ, Hermanson CL, Bowling LS. The Maryland CNC Test: Normative studies. Audiology 1984; 23:552–568. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RH, Harvey AB, Pillion AL. A word-recognition task in multitalker babble using a descending presentation mode from 24 db to 0 db signal to babble. J Am Acad Audiol 2003; 14:1–8. [DOI] [PubMed] [Google Scholar]
- 20.Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res 1978; 21:507–518. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RH, McArdle R. Intra- and inter-session test retest reliability words in noise test. J Am Acad Audiol 2007; 18:813–825. [DOI] [PubMed] [Google Scholar]
- 22.Piu F, Wang X, Fernandez R, et al. OTO-104: A sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol 2010; 32:171–179. [DOI] [PubMed] [Google Scholar]
- 23.Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: A randomized trial. JAMA 2011; 305:2071–2079. [DOI] [PubMed] [Google Scholar]
- 24.WMU Press, Lawson G, Peterson M. Speech Audiometry. 2011. [Google Scholar]
- 25.Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol 1978; 41:365–383. [DOI] [PubMed] [Google Scholar]
- 26.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. Hear Res 1984; 16:43–53. [DOI] [PubMed] [Google Scholar]
- 27.Hoben R, Easow G, Pevzner S, Parker MA. Outer hair cell and auditory nerve function in speech recognition in quiet and in background noise. Front Neurosci 2017; 11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayleigh L, XII. On our perception of sound direction. Phil Mag 1907; 13:214–232. [Google Scholar]
- 29.Rayleigh L, XVIII. Acoustical notes. Phil Mag 1908; 14:153–161. [Google Scholar]
- 30.Monson BB, Lotto AJ, Ternström S. Detection of high-frequency energy changes in sustained vowels produced by singers. J Acoust Soc Am 2011; 129:2263–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monson BB, Lotto AJ, Story BH. Detection of high frequency energy level changes in speech and singing. J Acoust Soc Am 2014; 135:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore BC, Stone MA, Füllgrabe C, Glasberg BR, Puria S. Spectro-temporal characteristics of speech at high frequencies and the potential for restoration of audibility to people with mild-to-moderate hearing loss. Ear Hear 2008; 29:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fullgrabe C, Baer T, Stone MA, Moore BC. Preliminary evaluation of a method for fitting hearing aids with extended bandwidth. Int J Audiol 2010; 49:741–753. [DOI] [PubMed] [Google Scholar]
- 34.Lippmann RP. Accurate consonant perception without midfrequency speech energy. IEEE Transact Speech Audio Process 1996; 4:66. [Google Scholar]
- 35.Apoux F, Bacon SP. Relative importance of temporal information in various frequency regions for consonant identification in quiet and in noise. J Acoust Soc Am 2004; 116:1671–1680. [DOI] [PubMed] [Google Scholar]
- 36.Pittman AL. Short term word learning rate in children with normal hearing and children with hearing loss in limited and extended high-frequency bandwidths. J Speech Lang Hear Res 2008; 51:785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore BC, Füllgrabe C. Evaluation of the CAMEQ2-HF method for fitting hearing aids with multichannel amplitude compression. Ear Hear 2010; 31:657–666. [DOI] [PubMed] [Google Scholar]
- 38.Motlagh Zadeh L, Silbert NH, Sternasty K, Swanepoel W, Hunter LL, Moore DR. Extended high-frequency hearing enhances speech perception in noise. Proc Natl Acad Sci U S A 2019; 116:23753–23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badri R, Siegel JH, Wright BA. Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. J Acoust Soc Am 2011; 129:852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French NR, Steinberg JC. Factors governing the intelligibility of speech sounds. J Acoust Soc Am 1947; 19:90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


