Abstract
Purpose of review
To provide an update on the role of Ataxin-2 gene (ATXN2) in health and neurological diseases.
Recent findings
There is a growing complexity emerging on the role of ATXN2 and its variants in association with SCA2 and several other neurological diseases. Polymorphisms and intermediate alleles in ATXN2 establish this gene as a powerful modulator of neurological diseases including lethal neurodegenerative conditions such as motor neuron disease, spinocerebellar ataxia 3 (SCA3), and peripheral nerve disease such as familial amyloidosis polyneuropathy. This role is in fact far wider than the previously described for polymorphism in the prion protein (PRNP) gene. Positive data from antisense oligo therapy in a murine model of SCA2 suggest that similar approaches may be feasible in humans SCA2 patients.
Summary
ATXN2 is one of the few genes where a single gene causes several diseases and/or modifies several and disparate neurological disorders. Hence, understanding mutagenesis, genetic variants, and biological functions will help managing SCA2, and several human diseases connected with dysfunctional pathways in the brain, innate immunity, autophagy, cellular, lipid, and RNA metabolism.
Keywords: Ataxin-2 gene, C9ORF72, cytosine adenine guanine-repeats, spinocerebellar ataxia 2, TDP43
INTRODUCTION
Spinocerebellar ataxia 2 (SCA2) is an autosomal dominant lethal disease caused by ≥32 cytosine adenine guanine (CAG) repeats in Ataxin-2 gene (ATXN2) [1–3]. In SCA2, the main affected tissues are the cerebellum, pons, olive, brainstem, frontal lobe, medulla oblongata, cranial and peripheral nerves. The pattern of neurodegeneration extends into the substantia nigra, and affects motoneurons as well [4]. The clinical manifestations of SCA2 include progressive gait ataxia, dysarthria, dysphagia, cognitive decline, slow eye movements, ophthalmoplegia, Parkinsonism, pyramidal features, and/or neuropathy.
The pathological CAG expansion is unstable in both germinal and somatic tissues, hence offspring may inherit a shorter or larger version of the pathogenic repeats. ATXN2 CAG somatic mosaicism, i.e., the presence of different CAG, cytosine uracil guanine (CUG), and/or polyQ length stretches, exists in different body tissues. Intermediate CAG expansions in ATXN2 (≥29CAG/CAA repeats) increase the risk for many other neurological diseases. The genetic alteration locates in the first exon of ATXN2 with locus at 12:111,452,214–111,599,676 (GRCh38) harboring 25 exons and encoding a very ubiquitous RNA binding protein. In the same ATXN2 locus a novel gene, called ATXN2-AS encoding a natural antisense transcript (NATs) with a CUG tract, has been described. For this review, we cover relevant articles on ATXN2 published during the last 18 months.
Box 1.

no caption available
Spinocerebellar ataxia 2 MUTAGENESIS AND FOUNDER EFFECTS
Mutagenesis in SCA2 is thought to occur in predisposed haplotypes, particularly in those alleles with loss of 5′ CAA located within the CAG sequence [5,6]. This haplotype is universal for SCA2 families and is shared with a lower range of CAG repeats (22–31CAG), suggesting a common mutagenic mechanism [7] (Fig. 1A,B).
FIGURE 1.
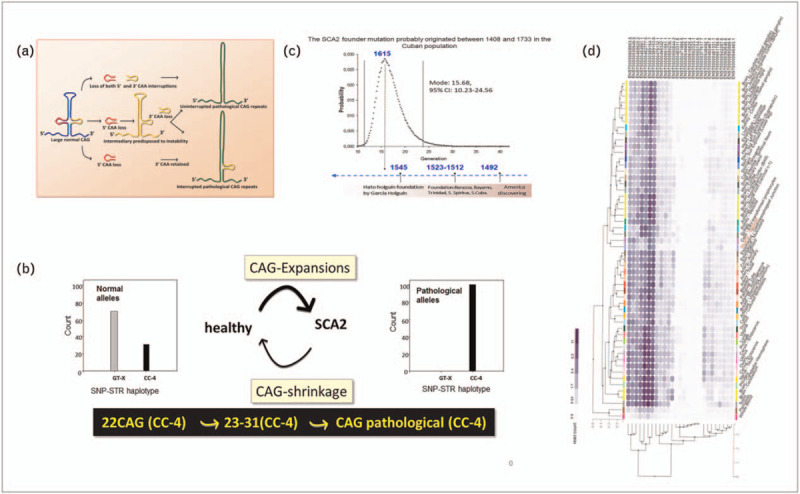
Mutagenesis, founder effect and ATXN2 isoforms. (A) Mutagenesis in SCA2 is thought to occurs under predisposed haplotypes, where 5’ CAA interruption loss occurs with more propensity [5,6]. (B) Low range CAG repeats interrupted by CAA (33–34repeats) shared the same STR-SNP haplotype, loci: D12S1333, D12S1672, rs695871 and rs695872, with uninterrupted full expansions [7]. (C) Prediction of the age for the Cuban SCA2 founder effect with DMLE [9] using six STR markers (D12S1328-D12S1332-D12S1672-D12S1333- D12S1329-88-5) in the vicinity of SCA2 mutation in 13 affected families. The curve represents the probability density of the mutation age for growth rate 0.45 within the period 1774–2007. The dashed lines correspond to the 95% credible set of values. The origin was predicted to have occurred 15 generations ago. According to this, the founder effect occurred likely 375 years ago, at year ∼1615 which is 70 years after the Holguin Villa foundation. (D) Isoform Expression of ATXN2 in different tissues (https://gtexportal.org/home/gene/ENSG00000204842.14#gene-transcript-browser-block). ATXN2, Ataxin-2 gene; CAG, cytosine adenine guanine; STR-SNP, short tandem repeat-single nucleotide polymorhphism.
The second most frequent CAG/CAA sequence pattern is (CAG)13CAA(CAG)8 and had an opposite distribution in a Chinese SCA2 cohort compared to a control population [8]. This is interesting since the allele 13CAG-CAA-8CAG shares the SCA2 risk haplotype in other populations [5] where the loss of 5′CAA was originally proposed to be the initial step for SCA2 mutagenesis. Taken together, the CAG expansions have emerged in different populations independently of each other.
SCA2 is highly prevalent in Holguin, Eastern Cuba. The original founder haplotype was introduced in this region ∼375 years ago according to predictions with Monte-Carlo simulation [9] using microsatellites markers in 13 families (Fig. 1C). This point in time corresponds to 1615, 70 years after the foundation of Holguin village. Thus, the Spanish conquista introduced either intermediate alleles and/or a founder SCA2 mutation. The genetic profiles of SCA2 Cuban families are very similar to Spanish ones (Laffita-Mesa et al. manuscript in preparation). African slaves are an unlikely source for SCA2 founder effect as they were brought into this part of Cuba several years later since sugar plantations were not so prominent. Most likely, a full penetrant mutation was not the original founder event as individuals carrying such mutations would be negatively selected. Instead, a premutation or low penetrance alleles (clinically irrelevant) within predisposing haplotype seems to have been a more reasonable event. Social conditions, cultural replicators, endogamous and closed-cycle marriages, religion, wars, syndemics resulted in a bottleneck effect increasing the number of premutation carriers among the Spanish settlers.
Ataxin-2 gene expression
Expression of ATXN2 is ubiquitous (https://gtexportal.org/home/gene/ENSG00000204842.14). At least 27 splice variants are predicted in Gtex but only few have been experimentally confirmed [10] (Fig. 1D).
Ataxin-2 gene and brain functioning, health and neurodegeneration
ATXN2 is essential for neurodevelopment in Drosophila[11]. Both ATXN2 levels and its subcellular location determine the spacing of dendritic branches for the optimal dendritic receptive fields in sensory neurons in Drosophila[12]. This may also have implications for neuronal synaptic plasticity, where FMR1 and ATX2 function together in a microRNA-dependent process mediating long-term olfactory habituation [13].
Massive Atxn2-CAG expansion in mice cause early disruption of excitability and communication in cerebellar neuronal layers [14].
In humans, an antagonistic pleiotropic role in cognition is emerging. ATXN2 and its substitute ATXN2L positively influence several cognitive domains such as verbal–numerical reasoning, reaction-time, educational attainment, and cognitive resilience [15,16]. Nevertheless, ATXN2 CAG repeat length polymorphisms, are associated with smaller volume in both the putamen and thalamus, but also with atrophy in amygdala and globus pallidus conferring cognitive decline in old age [17].
On the other hand, many lines of evidence suggest that ATXN2 genetic alterations may to lead disruption of innate immunity, autophagy, Krebs cycle, amino acid homeostasis, lipid, and RNA metabolism [18,19]. This altogether with the intracellular dynamics [20▪▪] explain the global effect in brain functioning caused by ATXN2 gain and/or loss of function.
Beyond CAG repeats, duplication in Ataxin-2 gene lowers age of onset in spinocerebellar ataxia 3 and C9-ALS
Recently, we identified a 9bp-duplication c.109_117delinsCGGAGCGGG/GCCTCGCCC in the ATXN2-S/AS region as modifier in familial neurodegeneration. Furthermore, a mosaic with a different CAG/CAA-repeat sequence pattern within the 22CAG allele associated with the 9-bp duplication was also found [21▪▪]. Other structural alterations in this region are a 17 bp deletion CCCGCCCGCTCCGCCGC, a 9 bp insertion, CCCGCCCGCT dbSNP (rs1249427887), and 18 bases insertion CCCGCCCGCTCCGCCGCG all of them located at the same loci 12–111599394-C-CCCGCCCGCTCCGCCCGCT and at frequency, 6.6e-5, 3e-3, 6.6e-5 with CADD values of 12.2. 12.6 and 22.7 (gnomAD v3.1). The 17 bp deletion encodes a polypeptide 39.85% homologous to ATXN2, when aligned with exon-1 (Fig. 2A,B).
FIGURE 2.
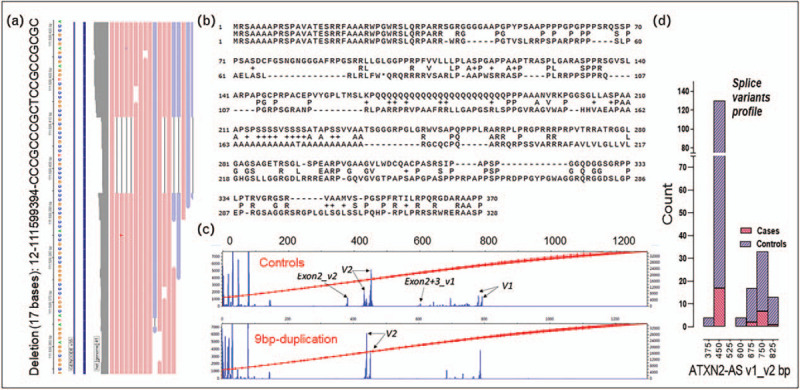
Novel ATXN2 variants and aberrant Splice variant profiles. (A) Deletion (17 bases) at 12-111599394 in ATXN2 (p.Ala35GlyfsTer39). (B) Local alignment for the first exon of wild-type ATXN2 and the predicted polypeptide resulting from the 17 bp deletion. (C) Typical fragment analysis traces for the ATXN2-AS gene expression profiling in healthy controls (top) and cases with ATXN2 duplication mutation of 9 bp duplication (bottom). Two ATXN2-AS splice variants were analyzed v1 (∼800 bp) and v2 (∼450 bp), arrows indicate the predicted length. In addition to the expected bands, other bands can be seen at 360, 480, 600–760 bp. Strand Specific-RT-PCR was performed in cDNA using primers and conditions previously published [22] with small modifications. Two rounds of PCR were done, and samples were ten-fold replicated. To obtain a pool of amplicons, 5ul of cDNA was amplified with R2/LK-F6 or LK-F7 primers and a second PCR amplification of 1/100 of the first PCR was done with LK/R3–6FAM primers. Five microliters of reaction amplicons were cleaned with 2ul ExoSAP-IT Express PCR and were mixed with GeneScan 1200 LIZ dye Size Standard (Applied Biosystems) and were ran during 4hrs with 50 cm capillary by Capillary Electrophoresis in a 3730 genetic Analyzer. Peak Scanner software was used to obtain splice variants sizes and height peaks. The data table was exported directly to be analyzed using height peaks≥150 and size ≥300. (D) Histogram with ATXN2-AS splice variant profile. Peaks showing differences of 40 bp sizes were binned together for data presentation purposes.
The 9bp-duplication [21▪▪] significantly decreased the age at onset for both spinocerebellar ataxia 3 (SCA3) and C9ORF72-amyotrophic lateral sclerosis (ALS). Droplet digital PCR experiments demonstrated its inclusion in the main transcript. In addition, in ATXN2-AS, the splice variant profile in 9bp-dup carriers differed as compared with controls. As shown in Fig. 2C,D, the controls had the predicted v1_v2 peaks at ∼800 bp and ∼440 bp [22] but mutants showed a shifted v2 profile, with no exon-2 variant and less 2+3 exon for v2 and v1 respectively suggestive for aberrant processing of the ATXN2-AS transcripts in patients with the 9bp-dup (Fig. 2C,D). Furthermore, this genetic change duplicates the phosphorylation and methylation sites on Serine and Arginine residues, Ser38-Gly-Arg40-Ser41-Gly-Arg43 located in the N-terminal intrinsically disordered region (IDRs). Indeed, this region is targeted by multiple kinases, such as PKC, GSK3, and Cdk5 [23].
Ataxin-2 gene CAG length variation in neurological diseases
The presence of at least one ATXN2 allele >22CAG decreased age at onset by 6 years in Portuguese patients with transthyretin familial amyloid polyneuropathy associated with the Val30Met variant [24]. ATXN2 intermediate alleles lowers AO in frontotemporal dementia (FTD) [25], in addition, those patients had parkinsonism and psychotic symptoms at the time of disease onset [25]. Intermediate alleles are overrepresented also in Alzheimer's disease and behavioral FTD suggesting a potential link between ATXN2 with tauopathies [26].
An autopsy performed in an individual with corticobasal syndrome recently confirmed ATXN2 genotype with 27/39CAG. The full expanded allele, which otherwise cause SCA2 at midlife, was interrupted by four CAA motifs (CAG8–CAA–CAG4–CAA–CAG4–CAA–CAG9–CAA–CAG10) and accompanied by a 27CAG intermediate allele (CAG8–CAA–CAG4–CAA–CAG4–CAA–CAG8) [27]. The patient had not cerebellar ataxia, neuroimaging abnormalities or neuropathological hallmarks for SCA2. This aggressive phenotype is in contrast with the late levodopa-responsive parkinsonism associated with interrupted expansions. It is claimed that CAA interruptions may influence variable phenotypes (Fig. 3A,B). Likewise, allelic interaction may determine a phenotype different than the expected for ATXN2 intermediate alleles in ALS and full CAG expansions in SCA2. This notion, of allelic interaction, is reinforced by appearance of late onset SCA2 resulting from homozygous 31/31CAG, and a case ALS with cognitive decline and 28/28CAG in homozygous state [28,29].
FIGURE 3.
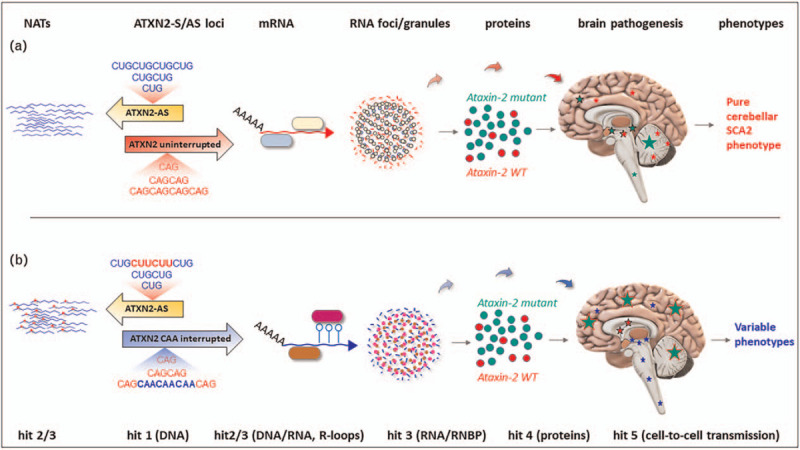
Variable phenotype may involve the CAA interruptions within the ATXN2 CAG repeats. This may entail different interactors at different pathological hits, but the most likely is the level involving RNA/RNA-binding proteins. CAG, cytosine adenine guanine.
The phenotype compatible with ALS/SCA2 in a woman with ATXN2 32CAG repeats suggests that the length of the CAG determines the timing of ALS and ataxia phenotypes in a disease continuum [30]. This continuum may also include Parkinsonism, pure cerebellar SCA2, and infantile-onset SCA2, with genetic and neuropathological overlap with TDP43 pathology.
Ataxin-2 gene as a genetic risk factor, spinocerebellar ataxia 2 mutations, variable phenotype and massive expansions in different populations and whole-genome sequencing projects
Increasingly, ATXN2 CAG repeat expansions have a prominent role in different populations either as genetic risk factor, SCA2 de novo cases or massive repetitions causing SCA2 in the childhood. Gardiner et al., 2019 analyzed 14 196 individuals with a wide age range (18–99 years) from The Netherlands, Scotland, and Ireland, and found that ∼11% carried intermediate alleles in the major nine genes causing polyQ diseases [31▪]. For ATXN2, they found both intermediate alleles and pathological expansions (30–36 units). Furthermore, Akçimen et al., 2020 used ExpansionHunter to estimate the trinucleotide repeats in whole-genome sequencing (WGS) data of unrelated healthy individuals from different geographic regions. The frequency of abnormal ATXN2 CAG repeats was 3.32% with a CAG number ranging between 27–36 units. Pathological SCA2 alleles were of low penetrance ≥34CAG repeats [32]. Likewise, Ibanez et al. identified patients with variable phenotypes, ALS (22/33CAG), levodopa-responsive early-onset Parkinson's disease (31/41CAG), progressive cerebellar ataxia (22/40CAG), and SCA2 (22/42CAG) [33].
One girl with intellectual disability had 99CAG repeats with apparently healthy parents [33]. Two little sisters with more than 180 CAG repeats presented with systemic features including global developmental delay, infantile spasms, hypotonia, seizures, dysautonomia, hearing and visual impairment, dysphagia, sleep, and mood disorders. The germinal instability started in the grandfather with 22/37CAG who transmitted an unstable ATXN2 mutation (43CAG) to one of his dizygotic sons with further massive expansions of up to ≥180CAG [8]. Another child was reported in Mexico. The maternal SCA2 mutation, 49CAG, expanded to ∼884 in her son whom at age 5yrs presented with ataxia, gaze-evoked nystagmus, and spasticity [34].
Prodromal spinocerebellar ataxia 2 and phenotype insights
Jacobi et al. described the prodromal features for European SCA1, 2, 3, and 6 cohorts and evaluated the sensibility of SARA and other clinical tools to detect changes in SCAs prodromal phase [35▪▪]. The longitudinal observation was performed between 2008–2015, with assessments at baseline, 2, 4, and 6 years. Twenty-two (59%) of SCA2 mutation carriers phenoconverted into SCA2, with a median age of onset around ∼36 years. They had an increase in the SARA from the time of inclusion, which was influenced by the age at first visit and the CAG expansion. There was a progressive cerebellar functioning deterioration determined by clinical scales and supported by volume loss in the cerebellum and pons. This was paralleled by annual decline in non-ataxia signs and sleep quality. On the other hand, Nigri et al. found isolated volume loss in the pons, and cortical thinning in specific frontal and parietal areas in preclinical SCA2 [36]. No other clinical or cognitive changes were evidenced in this one-year longitudinal study. In the prodromal phase, mutation carriers had larger variability in both gait and postural sway control [37], suggesting deterioration of the vestibulocerebellar network. Furthermore, there is a gradual worsening of speech and swallowing [38]. These preclinical abnormalities seem to be dampened with physical rehabilitation [39]. All this knowledge provides insights that will help design future disease modifying therapies (Fig. 5B,C). One challenge will be to make a fair prediction of the ‘estimated years to symptom onset’ (EYO) based on the different expanded CAG alleles and to identify gene modifiers of this stage.
FIGURE 5.
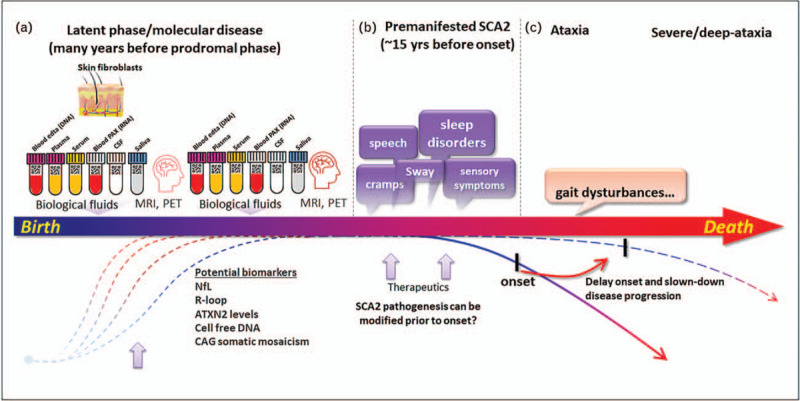
Prodromal SCA2 and biomarkers. (A) Identification of preclinical biomarkers in biological fluids and fibroblasts, paralleled with MRI and standardized clinical evaluation in asymptomatic individuals at risk [57]. Importantly, individuals are not aware of their genetic statuses. This approach, will be of great help in local or global SCA projects aimed at uncovering wet biomarkers like free circulating DNA, expanded ataxin-2 in CSF, somatic mosaicism, neurofilaments, DNA/RNA-derivatives improving the read outs for future trials. BC) SCA2 is featured by two major stages, early on it is featured by the nonmotor phase and later by a predominant and invaliding motor stage. Genetic factors or therapies acting in early stages have the potential to slowdown the neurodegeneration and postpone the onset of ataxic symptoms. Arrows in the bottom suggest potential time points for therapies targeting modifiers or disease causative factors. SCA2, spinocerebellar ataxia 2.
Antisense therapy proved to be efficient in ALS and spinocerebellar ataxia 2 preclinical models
ATXN2 and TDP43 interact through RNA molecules (Fig. 4) [40,41,42▪,43]. In spinal cord neurons of ALS patients, ataxin-2 and TDP43 are abnormally localized. Similarly, TDP-43 mislocalized in SCA2 and there is neuropathological and phenotype overlapping with Frontotemporal Lobar Degeneration (FTLD) cases manifest as pure FTLD-ALS without ataxia [44,45]. Pathological phosphorylation of TDP43 is seen in ALS, and its levels are increased in cases with ALS with C9ORF72 or ATXN2 expansions [46].
FIGURE 4.
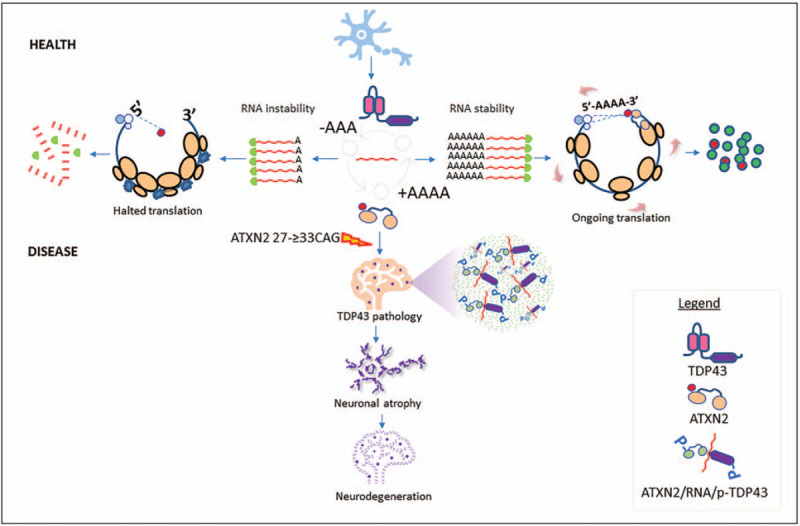
ATXN2 function and RNA metabolism in health and disease. For efficient protein synthesis, ATXN2 C-terminal PAM2 motif mediates interaction with the poly(A)-binding protein PABPC1 tethered to poly-(A) 3′tail mRNAs [40]. This complex interacts with eIF4GI at the 5′ tail, creating the required circularization mRNA linking 5′-3′ ends. Yokoshi et al. found that ATXN2 binds, stabilizes, and regulates the translation of more than 4,000 mRNA molecules [41]. For this, ataxin-2 predominantly binds to AU rich cis-regulatory elements at the 3′ tail of target mRNAs. The breadth of cellular processes targeted by this ATXN2 functions includes RNA splicing, mRNA polyadenylation, 3′tail processing and cellular metabolism. The pathological polyQ expansion perturbs this ataxin-2 physiological role, as Q31 (ALS) and Q39 (SCA2) downregulates the abovementioned processes in a dependent polyQ length manner [41]. It was not clear how ATXN2 stabilizes its target transcriptome, but Inagaki et al. showed polyadenylation enzymatic activity for ATXN2 [55]. The mechanism implies that ATXN2 binds to both cis-regulatory elements in the target mRNA and to PABP and others RNP trough the PAM2 motif. This complex may activate and stabilize their target transcriptome, for instance TDP43 and Cyclin-D1, through catalytic polyadenylation. In turn, TDP43 protein accelerates deadenylation of target mRNAs, which is a critical step in the RNA degradation. Therefore, regulating the length of the of the poly(A) tail plays a key role in the control of the mRNA stability [42▪]. This provides evidence for a common cellular process where both proteins cooperate with antagonistic functions regulating common substrates. Likewise, could suggest that an immediate pathological consequence of the abnormal interaction driven by polyQ in ATXN2, would be the loss of TDP43 function when work on stabilizing and activating their common transcriptomes. This may also affect the regulation of circadian genes as Drosophila ATX2 complex may switch distinct modes of posttranscriptional regulation through its associating factors to control circadian clocks and ATX2-related physiology [43]. It is not clear whether ATXN2 undergone any other posttranslational modification like the pathological TDP43 phosphorylation. ALS, amyotrophic lateral sclerosis.
Lowering ATXN2 expression with antisense oligo in TDP-43 ALS mice prolongs their survival which is therapeutically relevant for both ALS and SCA2 [47]. However, recent studies have shown reduced ATXN2 expression suggesting either loss of function as part of the disease process or neuroprotective role in FTLD-TDP and other TDP-43 proteinopathies [20▪▪]. The last notion is well supported as proper ataxin-2 amounts are needed it to ensure optimal dendritic arborization in Drosophila neurons. In contrast, upregulated ATXN2 levels affects the distribution of FMRP and other RNA binding proteins inducing aberrant dendritic morphology [12].
The same antisense oligo therapy reduced the levels of both wild-type and mutant ataxin-2 in SCA2 bacterial artificial chromosome mice (BAC-Q72). Moreover, ALS-related proteins Eaat2, Pcp4, TBK1 and p62/Sqstm1 were significantly dysregulated in spinal cord and cerebellum. This therapy corrected the expression levels of all but two ALS proteins and also improved motor coordination [19].
These promising preclinical results raise hope for future trials in humans. However, since ATXN2 function is pleiotropic and the long-term effect of reducing both the normal and mutated ataxin-2 alleles in humans remains unclear, an allele-specific therapy is the most desirable approach.
One future direction would be combining haplotype information with oligo antisense silencing. All SCA2 families share the universal SNPs haplotype C–C for rs695871 and rs695872 markers located in ATXN2 exon-1. Furthermore, about 45% of ALS cases with intermediate alleles have the same SCA2 haplotype [5,48]. Therefore, this information would be relevant for developing allele-specific therapies. In SCA3, another polyQ disease, Prudencio et al., 2020 demonstrated the effectiveness of this approach in silencing the pathological ATXN3 allele [49].
Of note, the novel compound naphthyridine-azaquinolone (NA) was found to specifically binds slipped-CAG DNA intermediates of Huntington's disease (HD) mutations. The therapeutic potential is highlighted as NA reduces both somatic mosaicism for the HD expanded allele and mutant HTT protein aggregates in striatum [50▪].
Genome editing technologies, such as CRISPR-Cas9, may harbor great potential for future therapies in SCA2 and other polyQ disorders. These techniques include the use of guided RNAs to either alter, excise, or insert a specific DNA sequence in a precise or predetermined manner. In one patent the use of rare cutting endonucleases and transposases for altering ATXN2 expression is claimed [51]. Another genome editing application (WO/2018/154462) comprises ex and in vivo methods for correcting abnormal ATXN2 CAG repeats [52]. Significant improvement are expected of genome editing applied for correcting the pathological ATXN2 CAG repeats and other polyQ diseases -SCA1, SCA3 and HD- [53–55]. However, finding biomarkers that may be precede the prodromal clinical changes and predictors of phenoconversion remain as other unmet needs in the field [56,57] (Fig. 5A, see Table 1).
Table 1.
Potential biomarkers, approaches and sample types
| Biomarker | Commentary | SCA2 phasesa | ATXN2 mutation specific? | Techniques | Sample type |
| ATXN2 mRNA levels | New alternative splicing variants were found lacking exon 12 and exon 24, and this could be relevant for disease progression [10]. Furthermore, genotyping of both DNA and RNA levels may shed evidence about mutant allele expression, as was recently shown in SCA3 and C9ORF72 ALS [21▪▪]. | A+++, B++, C+ | Y+++ | QPCR, DDPCR | Blood-edta, blood pax, fibroblasts. |
| ATXN2 CAG somatic mosaicism | Mosaicism of unstable CAG repeats was confirmed in SCA2 brain and lymphoblasts cell lines. The mosaicism varied within the brain and spinal cord and was smaller in the cerebellum than in other brain tissues [58]. Furthermore, CAG somatic mosaicism in DNA and mRNA are correlated in ATXN3 gene [59]. | A+++, B++, C+ | Y+++ | Fluorescent PCR, fragment analysis | Blood-edta, blood pax, fibroblasts. |
| ATXN2-AS profile | ATXN2-AS transcripts with a CUG repeat expansion are toxic in an SCA2 cell model. This was associated with aberrant splicing of amyloid beta precursor protein and N-methyl-D-aspartate receptor 1 in SCA2 brains [22]. | ? | Y+++ | Fluorescent PCR, fragment analysis | Blood-edta, blood pax, fibroblasts. |
| ATXN2 AUUUA binding function | Yokoshi et al. found that ATXN2 binds, stabilizes, and regulates the translation of more than 4,000 mRNA molecules [41]. | ? | Y | In vitro RNA binding | Blood-edta, blood pax |
| R-loop | Human ATXN2 deficiency leads to genome-destabilizing R-loops accumulation [60]. TDP-43 is also crucial in maintaining genomic stability through a co-transcriptional process that prevents aberrant R-loop accumulation [61] and controlling replication stress. | ? | Y | Qpcr, DDPCR | blood-edta, blood pax |
| Cell free DNA | Cell-free circulating DNA in plasma is significantly increased in Friedreich's ataxia, SCA2 and in SCA12 patients. It was possible to distinguish between ataxia patients and healthy controls using plasma DNA [62]. | ? | Y/N | WGS, QPCR, DDPCR, PicoGreen fluorescent assay | CSF, Plasma, serum |
| NFL | Levels of serum NfL were elevated in SCA3 individuals and correlated with disease severity. The was also high concentrations starting from early disease stage and correlated with disease severity [63]. Likewise, blood NfL levels were increased with proximity to the predicted onset of ataxia in SCA3 individuals [64]. | A+++, B++, C+ | N | Different platforms | CSF, Plasma, serum |
| Micro-RNA, and small RNAs | Bañez- et al., 2012 provide evidence involving HTT CAG repeats interfering with cell viability at the RNA level. Pathological CAG repeats ≥40 units induced neuronal cell death and increased levels of small CAG-repeated RNAs (sCAGs) of ≈21 nucleotides in a Dicer-dependent manner [65]. Furthermore, the severity of the toxic effect of HTT mRNA and sCAG generation correlated with CAG expansion length. Likewise, Creus–Muncunill et al., 2021 demonstrated that sRNA produced in the putamen of HD patients are sufficient to recapitulate HD pathophysiology in vivo[66]. | ? | Y/N | WGS, QPCR, DDPCR | Blood-pax |
| Lipid metabolism, cholesterol metabolites | ATXN2 CAG repeat variations may contribute to the missing heritability of obesity by affecting body mass index in the general population [67]. Loss of ATXN2 function by therapeutics silencing with ASO7 evidenced liver X receptors and farnesoid X receptor activation (LXR/RXR, FXR/RXR) which are nuclear receptors regulating lipid and glucose metabolism. Ligands for these receptors include fatty acids, oxysterols and bile acids as well as rexinoids, pointing to their relevance in the regulation of metabolic pathways [68]. This metabolic change agrees with the observation of ATXN2 CAG repeats as determinant of obesity-related risk parameters in Mexican adolescents whenever CAG stretch deviated from the most common 22CAG repeat [69]. Serum testosterone levels are also decreased in SCA2 male individuals and associates with disease duration [70]. Therefore, the ATXN2 loss of functions affects metabolic processes connected with underlying causes for metabolic syndrome-related diseases. | A,B,C+ | Y/N | Different platforms | Plasma, serum |
| Stau-1, Pcp2, Calb1 and RGS8 mRNA levels | Stau-1 physically interact with ATXN2 in an RNA dependent manner. Both are relocated to Stress granules and increased Stau-1 levels were found in cerebellum of SCA2 mouse model. Furthermore, Stau-1 and ATXN2 had similar effects reducing levels of Pcp2 and Calb1 [71]. | A++ | ? | QPCR, DDPCR | Blood-edta, blood pax |
ALS, amyotrophic lateral sclerosis; ATXN2, Ataxin-2 gene; CAG, cytosine adenine guanine; CUG, cytosine uracil guanine; SCA2, spinocerebellar ataxia 2.
Referred to SCA2 stages in Fig. 5A,C.
CONCLUDING REMARKS
Almost three decades ago, the goal in the field was to provide accurate SCA2 presymptomatic diagnostic, prenatal diagnosis, and symptomatic treatment [72]. At present, preimplantation genetic testing by karyomapping enabled the first birth of three healthy babies without pathogenic CAG expansions for HD, SCA2 or SCA12 [73]. Currently, drug screening, experimental disease modelling, and deep-phenotyping studies in patients with SCA2 prepare the ground for future trials. Many of the SCA2 features overlap with different diseases. Hence, if we accurately dissect all SCA2 phenotypes it may uncover features of other disorders, or human traits influenced whenever ATXN2 homeostasis and/or sequence is altered. The increasing knowledge of ATXN2 in several disparate neurological diseases resembles the major impact polymorphisms at different codons in the prion protein gene (PRNP) have over both sporadic and familial prion diseases. Research on ATXN2 is intense, productive, therapeutically driven and aimed to understand gain and loss of ATXN2 functions. Understanding the SCA2 mutagenesis and novel ATXN2 genetic alterations will help in to improve genetic counseling and to design future therapeutical trials for SCA2.
Acknowledgements
None.
Financial support and sponsorship
ParkinsonFonden and ALF support from Region Stockholm. P.S. is a Wallenberg Clinical Scholar.
Conflicts of interest
There are no conflicts of interest.
Financial Disclosure: None
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Pulst SM, Nechiporuk A, Nechiporuk T, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet 1996; 14:269–276. [DOI] [PubMed] [Google Scholar]
- 2.Imbert G, Saudou F, Yvert G, et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet 1996; 14:285–291. [DOI] [PubMed] [Google Scholar]
- 3.Sanpei K, Takano H, Igarashi S, et al. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet 1996; 14:277–284. [DOI] [PubMed] [Google Scholar]
- 4.Lastres-Becker I, Rüb U, Auburger G. Spinocerebellar ataxia 2 (SCA2). Cerebellum 2008; 7:115–124. [DOI] [PubMed] [Google Scholar]
- 5.Choudhry S, Mukerji M, Srivastava AK, et al. CAG repeat instability at SCA2 locus: anchoring CAA interruptions and linked single nucleotide polymorphisms. Hum Mol Genet 2001; 10:2437–2446. [DOI] [PubMed] [Google Scholar]
- 6.Laffita-Mesa JM, Velázquez-Pérez LC, Santos Falcón N, et al. Unexpanded and intermediate CAG polymorphisms at the SCA2 locus (ATXN2) in the Cuban population: evidence about the origin of expanded SCA2 alleles. Eur J Hum Genet 2012; 20:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos EM, Martins S, Alonso I, et al. Common origin of pure and interrupted repeat expansions in spinocerebellar ataxia type 2 (SCA2). Am J Med Genet B Neuropsychiatr Genet 2010; 153B:524–531. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Dong Y, Ma Y, et al. Genetic profile and clinical characteristics of Chinese patients with spinocerebellar ataxia type 2: A multicenter experience over 10 years. Eur J Neurol 2020; 3:955–964. [DOI] [PubMed] [Google Scholar]
- 9.Reeve JP, Rannala B. DMLE+: Bayesian linkage disequilibrium gene mapping. Bioinformatics 2002; 18:894–895. [DOI] [PubMed] [Google Scholar]
- 10.Lastres-Becker I, Nonis D, Nowock J, Auburger G. New alternative splicing variants of the ATXN2 transcript. Neurol Res Pract 2019; 1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. del Castillo U, Norkett R, Serpinskaya A, Gelfand VI. Ataxin-2 is essential for neurodevelopment in Drosophila and is a major regulator of the cytoskeleton,” bioRxiv, p. 2021.01.07.425768. 2021, doi: 10.1101/2021.01.07.425768. [Google Scholar]
- 12.Cha IJ, Lee D, Park SS, et al. Ataxin-2 dysregulation triggers a compensatory fragile X mental retardation protein decrease in Drosophila C4da meurons. Mol Cells 2020; 43:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudhakaran IP, Hillebrand J, Dervan A, et al. FMRP and Ataxin-2 function together in long-term olfactory habituation and neuronal translational control. Proc Natl Acad Sci USA 2014; 111:E99–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arsović A, Halbach MV, Canet-Pons J, et al. Mouse Ataxin-2 expansion downregulates CamKII and other calcium signaling factors, impairing granule-purkinje neuron synaptic strength. Int J Mol Sci 2020; 21:6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies G, Marioni RE, Liewald DC, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112 151). Mol Psychiatry 2016; 6:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald J, Fahey L, Holleran L, et al. Identification of 13 independent genetic loci associated with cognitive resilience in healthy aging in 330,097 individuals in the UK Biobank. bioRxiv 10.1101/2021.01.22.427640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardiner SL, Trompet S, Sabayan B, et al. Repeat variations in polyglutamine disease-associated genes and cognitive function in old age. Neurobiol Aging 2019; 84:236e17–236e28. [DOI] [PubMed] [Google Scholar]
- 18.Auburger G, Sen NE, Meierhofer D, et al. Efficient prevention of neurodegenerative diseases by depletion of starvation response factor Ataxin-2. Trends Neurosci 2017; 40:507–516. [DOI] [PubMed] [Google Scholar]
- 19.Scoles DR, Dansithong W, Pflieger LT, et al. ALS-associated genes in SCA2 mouse spinal cord transcriptomes. Hum Mol Genet 2020; 29:1658–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Watanabe R, Higashi S, Nonaka T, et al. Intracellular dynamics of Ataxin-2 in the human brains with normal and frontotemporal lobar degeneration with TDP-43 inclusions. Acta Neuropathol Commun 2020; 8:176. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article details the cellular distribution of ataxin-2 in patients with FTD and healthy controls.
- 21▪▪.Laffita-Mesa JM, Nennesmo I, Paucar M, Svenningsson P. A novel duplication in ATXN2 as modifier for spinocerebellar Ataxia 3 (SCA3) and C9ORF72-ALS. Mov Disord 2021; 36:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports a novel ATXN2 variant modulating SCA3 and MND.
- 22.Li PP, Sun X, Xia G, et al. ATXN2-AS, a gene antisense to ATXN2, is associated with spinocerebellar ataxia type 2 and amyotrophic lateral sclerosis. Ann Neurol 2016; 80:600–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asada A, Yamazaki R, Kino Y, et al. Cyclin-dependent kinase 5 phosphorylates and induces the degradation of ataxin-2. Neurosci Lett 2014; 563:112–117. [DOI] [PubMed] [Google Scholar]
- 24.Santos D, Coelho T, Alves-Ferreira M, et al. Large normal alleles of ATXN2 decrease age at onset in transthyretin familial amyloid polyneuropathy Val30Met patients. Ann Neurol 2019; 85:251–258. [DOI] [PubMed] [Google Scholar]
- 25.Rubino E, Mancini C, Boschi S, et al. ATXN2 intermediate repeat expansions influence the clinical phenotype in frontotemporal dementia. Neurobiol Aging 2019; 73:231.e7–231.e9. [DOI] [PubMed] [Google Scholar]
- 26.Rosas I, Martínez C, Clarimón J, et al. Role for ATXN1, ATXN2, and HTT intermediate repeats in frontotemporal dementia and Alzheimer's disease. Neurobiol Aging 2020; 87:139.e1–139.e7. [DOI] [PubMed] [Google Scholar]
- 27.Fournier C, Anquetil V, Camuzat A, et al. Interrupted CAG expansions in ATXN2 gene expand the genetic spectrum of frontotemporal dementias. Acta Neuropathol Commun 2018; 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tojima M, Murakami G, Hikawa R, et al. Homozygous 31 trinucleotide repeats in the SCA2 allele are pathogenic for cerebellar ataxia. Neurol Genet 2018; 4:e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borg R, Farrugia Wismayer M, Bonavia K, et al. Genetic analysis of ALS cases in the isolated island population of Malta. Eur J Hum Genet 2021; 29:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghahremani Nezhad H, Franklin JP, Alix JJP, et al. Simultaneous ALS and SCA2 associated with an intermediate-length ATXN2 CAG-repeat expansion. Amyotroph Lateral Scler Frontotemporal Degener 2020; 0:1–3. [DOI] [PubMed] [Google Scholar]
- 31▪.Gardiner SL, Boogaard MW, Trompet S, et al. Prevalence of carriers of intermediate and pathological polyglutamine disease-associated alleles among large population-based cohorts. JAMA Neurol 2019; 76:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is a larger screening for the major polyQ repeats and support the idea of premutation in different European populations.
- 32.Akçimen F, Ross JP, Liao C, et al. Expanded CAG Repeats in ATXN1, ATXN2, ATXN3, and HTT in the 1000 Genomes Project. Mov Disord 2020; 36:514–518. [DOI] [PubMed] [Google Scholar]
- 33.Ibanez K., Polke J, Hagelstrom T, et al. Whole genome sequencing for diagnosis of neurological repeat expansion disorders. bioRxiv, 2020; 2020.11.06.371716. doi: 10.1101/2020.11.06.371716. [Google Scholar]
- 34.Sánchez-Corona J, Ramirez-Garcia SA, Castañeda-Cisneros G, et al. A clinical report of the massive CAG repeat expansion in spinocerebellar ataxia type 2: Severe onset in a Mexican child and review previous cases. Genet Mol Biol 2020; 43:e20190325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪▪.Jacobi H, du Montcel ST, Romanzetti S, et al. Conversion of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 to manifest ataxia (RISCA): a longitudinal cohort study. Lancet Neurol 2020; 19:738–747. [DOI] [PubMed] [Google Scholar]; This work details the features prior to phenoconversion and early disease stages in a European SCA cohort.
- 36.Nigri A, Sarro L, Mongelli A, et al. Progression of cerebellar atrophy in spinocerebellar ataxia type 2 gene carriers: a longitudinal MRI study in preclinical and early disease stages. Front Neurol 2020; 11:616419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velázquez-Pérez L, Rodriguez-Labrada R, González-Garcés Y, et al. Prodromal spinocerebellar ataxia type 2 subjects have quantifiable gait and postural Sway Deficits. Mov Disord 2021; 36:471–480. [DOI] [PubMed] [Google Scholar]
- 38.Vogel AP, Magee M, Torres-Vega R, et al. Features of speech and swallowing dysfunction in preataxic spinocerebellar ataxia type 2. Neurology 2020; 95:e194–e205. [DOI] [PubMed] [Google Scholar]
- 39.Velázquez-Pérez L, Rodríguez-Diaz JC, Rodríguez-Labrada R, et al. Neurorehabilitation improves the motor features in prodromal sca2: a randomized, controlled trial. Mov Disord 2019; 34:1060–1068. [DOI] [PubMed] [Google Scholar]
- 40.Satterfield TF, Pallanck LJ. Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes. Hum Mol Genet 2006; 15:2523–2532. [DOI] [PubMed] [Google Scholar]
- 41.Yokoshi M, Li Q, Yamamoto M, et al. Direct binding of Ataxin-2 to distinct elements in 3’ UTRs promotes mRNA stability and protein expression. Mol Cell 2014; 55:186–198. [DOI] [PubMed] [Google Scholar]
- 42▪.Inagaki H, Hosoda N, Tsuiji H, Hoshino SI. Direct evidence that Ataxin-2 is a translational activator mediating cytoplasmic polyadenylation. J Biol Chem 2020; 295:15810–15825. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that ATXN2 directly stabilizes RNA through catalytic polyadenylation.
- 43.Lee J, Yoo E, Lee H, et al. LSM12 and ME31B/DDX6 define distinct modes of posttranscriptional regulation by ATAXIN-2 protein complex in Drosophila Circadian pacemaker neurons. Mol Cell 2017; 66:129–140.e7. [DOI] [PubMed] [Google Scholar]
- 44.Toyoshima Y, Tanaka H, Shimohata M, et al. Spinocerebellar ataxia type 2 (SCA2) is associated with TDP-43 pathology. Acta Neuropathol 2011; 122:375–378. [DOI] [PubMed] [Google Scholar]
- 45.Bäumer D, East SZ, Tseu B, et al. FTLD-ALS of TDP-43 type and SCA2 in a family with a full ataxin-2 polyglutamine expansion. Acta Neuropathol 2014; 128:597–604. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Halliday GM, Kiernan MC, Tan RH. TDP-43 levels in the brain tissue of ALS cases with and without C9ORF72 or ATXN2 gene expansions. Neurology 2019; 93:e1748–e1755. [DOI] [PubMed] [Google Scholar]
- 47.Becker LA, Huang B, Bieri G, et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 2017; 544:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Z, Zhu Y, Chen-Plotkin AS, et al. PolyQ repeat expansions in ATXN2 associated with ALS are CAA interrupted repeats. PLoS One 2011; 6:e17951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prudencio M, Garcia-Moreno H, Jansen-West KR, et al. Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3. Sci Transl Med 2020; 12:eabb7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50▪.Nakamori M, Panigrahi GB, Lanni S, et al. A slipped-CAG DNA-binding small molecule induces trinucleotide-repeat contractions in vivo. Nat Genet 2020; 52:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the use of small molecules capable to bind only expanded HTT allele and decreased the somatic mosaicism and mHTT aggregation.
- 51.Baltes N. Methods for altering gene expression for genetic disorders. WO/2020/092557. 2020. WIPO. Available at: https://patentscope.wipo.int/search/en/detail.jsf?docId=US294407028&_fid=WO2020092557 [Google Scholar]
- 52.Lundberg AS, Kulkarni S, Klein L, et al. Materials and methods for treatment of spinocerebellar ATAXIA Type 2 (SCA2) and other spinocerebellar ataxia type 2 protein (ATXN2) gene related conditions or disorders. WO/2018/154462. WIPO. Available at: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018154462&tab=PCTDOCUMENTS [Google Scholar]
- 53.Salvatori F, Pappadà M, Sicurella M, et al., 10.1101/2020.07.04.187559. [DOI] [Google Scholar]
- 54.Ouyang S, Xie Y, Xiong Z, et al. CRISPR/Cas9-targeted deletion of polyglutamine in spinocerebellar ataxia type 3-derived induced pluripotent stem cells. Stem Cells Dev 2018; 27:756–770. [DOI] [PubMed] [Google Scholar]
- 55.Ciesiolka A, Stroynowska-Czerwinska A, Joachimiak P, et al. Artificial miRNAs targeting CAG repeat expansion in ORFs cause rapid deadenylation and translation inhibition of mutant transcripts. Cell Mol Life Sci 2020; 78:1577–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 2015; 14:253–262. Erratum in: Lancet Neurol. 2015 Dec;14(12):1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012; 71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuura T, Sasaki H, Yabe I, et al. Mosaicism of unstable CAG repeats in the brain of spinocerebellar ataxia type 2. J Neurol 1999; 246:835–839. [DOI] [PubMed] [Google Scholar]
- 59.Bettencourt C, Santos C, Montiel R, et al. The (CAG)n tract of Machado–Joseph Disease gene (ATXN3): a comparison between DNA and mRNA in patients and controls. Eur J Hum Genet 2010; 18:621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ostrowski LA, Hall AC, Szafranski KJ, et al. Conserved Pbp1/Ataxin-2 regulates retrotransposon activity and connects polyglutamine expansion-driven protein aggregation to lifespan-controlling rDNA repeats. Commun Biol 2018; 1:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood M, Quinet A, Lin YL, et al. TDP-43 dysfunction results in R-loop accumulation and DNA replication defects. J Cell Sci 2020; 133:jcs244129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swarup V, Srivastava AK, Padma MV, Rajeswari MR. Quantification of circulating plasma DNA in Friedreich's ataxia and spinocerebellar ataxia types 2 and 12. DNA Cell Biol 2011; 30:389–394. [DOI] [PubMed] [Google Scholar]
- 63.Li QF, Dong Y, Yang L, et al. Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3. Mol Neurodegener 2019; 14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilke C, Haas E, Reetz K, et al. Neurofilaments in spinocerebellar ataxia type 3: blood biomarkers at the preataxic and ataxic stage in humans and mice. EMBO Mol Med 2020; 12:e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bañez-Coronel M, Porta S, Kagerbauer B, et al. A pathogenic mechanism in Huntington's disease involves small CAG-repeated RNAs with neurotoxic activity. PLoS Genet 2012; 8:e1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Creus-Muncunill J, Guisado-Corcoll A, Venturi V, et al. Huntington's disease brain-derived small RNAs recapitulate associated neuropathology in mice. Acta Neuropathol 2021; 141:565–584. [DOI] [PubMed] [Google Scholar]
- 67.Gardiner SL, de Mutsert R, Trompet S, et al. Repeat length variations in polyglutamine disease-associated genes affect body mass index. Int J Obes 2019; 43:440–449. [DOI] [PubMed] [Google Scholar]
- 68.Hiebl V, Ladurner A, Latkolik S, Dirsch VM. Natural products as modulators of the nuclear receptors and metabolic sensors LXR, FXR and RXR. Biotechnol Adv 2018; 36:1657–1698. [DOI] [PubMed] [Google Scholar]
- 69.Aguilar Aldrete ME, López-Toledo S, Caballero Avendaño A, et al. Association between nutritional risk markers and polymorphisms rs2291166 in TJP1 and VNTR (CAG)n in ATXN2 in an obese adolescent Mexican population. Endocrinol Diabetes Nutr 2021; 68:99–108. [DOI] [PubMed] [Google Scholar]
- 70.Almaguer-Mederos LE, Aguilera-Rodríguez R, Almaguer-Gotay D, et al. Testosterone levels are decreased and associated with disease duration in male spinocerebellar Ataxia type 2 patients. Cerebellum 2020; 19:597–604. [DOI] [PubMed] [Google Scholar]
- 71.Paul S, Dansithong W, Figueroa KP, et al. Staufen1 links RNA stress granules and autophagy in a model of neurodegeneration. Nat Commun 2018; 9:3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gispert S, Twells R, Orozco G, et al. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet 1993; 4:295–299. [DOI] [PubMed] [Google Scholar]
- 73.Shi D, Xu J, Niu W, et al. Live births following preimplantation genetic testing for dynamic mutation diseases by karyomapping: a report of three cases. J Assist Reprod Genet 2020; 37:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]


