Abstract
Conventional melanoma therapies suffer from the toxicity and side effects of repeated treatments due to the aggressive and recurrent nature of melanoma cells. Less-invasive topical chemotherapies by utilizing polymeric microneedles have emerged as an alternative, but the sustained, long-lasting release of drug cargos remains challenged. In addition, the size of the microneedles is relatively bulky for the small, curvilinear, and exceptionally-sensitive cornea for the treatment of ocular melanoma. Here, we report a design of bioresorbable, miniaturized porous-silicon (p-Si) needles with covalently-linked drug cargos at doses comparable to those of conventional polymeric microneedles. Uniquely, the p-Si needles are built on a water-soluble film as a temporary flexible holder that can be intimately interfaced with the irregular surface of living tissues, followed by complete dissolution with saline solution within one minute. Consequently, the p-Si needles remain embedded inside tissues and then undergo gradual degradation allowing for sustained release of the drug cargos. Its utility in unobtrusive topical delivery of chemotherapy with minimal side effects is demonstrated in a murine melanoma model.
Keywords: Melanoma Treatment, Topical Drug Delivery, Bioresorbable Silicon Nanomaterials, Sustained Drug Release
Graphical Abstract
Bioresorbable, miniaturized porous silicon needles on a water-soluble patch for the management of melanoma skin cancer.
Melanoma – the most serious form of skin cancer – is typically caused by ultraviolet radiation from natural sunshine or tanning beds and developed at the stratum corneum of epidermis (15–25 μm from the skin surface).1 Given the aggressive and recurrent nature of melanoma cells, repeated treatments are often necessary, thereby increasing the risk of toxicity and side effects.2 An effective treatment involves using the topical administration of chemotherapeutics into tumor tissues using polymeric microneedles as a less-invasive and painless route.3,4 Recently, further miniaturized nanoscale needles made of porous-silicon (p-Si) emerge as an attractive candidate for intratissue injection that can offer favorable safety profile and controlled biodegradability.5–7 Compared to conventional polymeric microneedles, the miniaturized p-Si needles benefit from their amenability to existing nanofabrication processing, and therefore provide the following key advantages: (1) precise control of the size, geometry, tapering, and tip morphology at the nanoscale,8 (2) rational tuning of porosity on the surface (in turn, drug loading capacity),6,9 (3) pre-programmable dissolution rate of the p-Si needles through surface oxidations (encapsulation),10,11 (4) uniform delivery owing to the high density of the p-Si needles per projected surface area,5 and (5) long-lasting release of covalently-linked drug cargos by gradual degradation of the p-Si needles in tissue fluids over time.9,12 These attributes are important for controlled, sustained, and minimally-invasive topical delivery of therapeutics.
However, challenges still remain in that the vertically-ordered arrays of the p-Si needles are built on a rigid Si wafer that can accommodate the conditions required for conventional nanofabrication processing such as thermal annealing, corrosive chemical etching, and photolithographic patterning.13 The use of the rigid Si wafer results in a mechanical mismatch when interfaced with the soft, curvilinear, and dynamic surface of living tissues. This discordance leads to debasing of the interfacial contact quality, which is particularly problematic in their application to the small, curvilinear, and exceptionally-sensitive cornea where melanoma is occasionally formed (i.e., ocular melanomas).14 Our recent work demonstrated that these challenges can be alleviated to some extent by building the miniaturized p-Si needles on a thin, flexible backing film made of silicone elastomer such as polydimethylsiloxane (PDMS) for their use in intracellular drug delivery.15 A disadvantage of this construct is that the flexible PDMS backing film may cause irritation or discomfort to the wearer especially under vigorous deformations of tissues by body movements. Complete elimination of the backing film after the insertion of the p-Si needles into tissues may resolve the physical strains, thereby enabling unobtrusive topical delivery of therapeutics over a prolonged period of time.
Here, we introduce a bioresorbable, miniaturized p-Si needles loaded with covalently-linked drug cargos, which is built on a thin, flexible, and water-soluble film. The water-soluble film is temporarily used during the insertion of the p-Si needles into tissues, and then can be immediately dissolved within one minute by the application of saline solution. Consequently, the p-Si needles remain embedded inside tissues and, due to the nanoscale size, become unobtrusive (nearly unnoticeable) to the wearers without affecting their natural motions. The p-Si needles subsequently undergo gradual hydrolysis in tissue fluids to degrade into biocompatible byproducts, leading to sustained, long-lasting release of preloaded drug cargos over days at a controlled rate. Comprehensive experimental and computational studies provide an insight into the structural design and construction of the p-Si needles on a water-soluble backing over centimeter-scale areas and elucidate the fundamental attributes. Demonstrations of the p-Si needles for the topical delivery of chemotherapy in a murine melanoma model illustrate the utility of this concept.
RESULTS AND DISSCUSION
Design principle and fabrication process.
Figure 1A schematically illustrates the basic procedure to integrate the bioresorbable, miniaturized p-Si needles with a thin, flexible, and water-soluble medical film such as polyvinyl alcohol (PVA; medical-grade; mechanical modulus (E) = 3.4 GPa; molecular weight of ~31,000; Sigma Aldrich, USA). The first step is to fabricate an array of vertically-ordered p-Si needles on a polished monocrystalline Si wafer through sequential steps of photolithographic patterning, dry and wet etchings, and metal-assisted chemical etching (MACE) (Figure 1A, left).15 This step also formed uniform undercuts and nanopores at the bottom and on the surface of the p-Si needles, respectively (inset image). Details of the fabrication procedures are shown in the Materials and Methods section. Figure S1 shows representative scanning electron microscopy (SEM) images of the as-fabricated p-Si needles, exhibiting the minimum tip diameter (d) of 150 nm, the base diameter (D) of 2–4 μm, and the length (L) of 10–70 μm. In the next step, the entire structure was spin-cast with a 10 weight % PVA (200–300 μm-thick), allowing an air gap to form at the interface due to surface tension (Figure 1A, middle). The length of the p-Si needles was determined by this air gap that was adjusted by controlling the spin-casting speed within the range from 300 rpm to 500 rpm, leading to the consequent air gap size of ~20 μm and ~50 μm, respectively (Figure S2). The spin-casting of the PVA film was repeated until its total thickness reached about 200–300 μm to provide sufficient mechanical strength for handling. Subsequently, a thermal annealing was followed to complete the solidification of the PVA film using a convection oven maintained at 70 °C for 30 min. Finally, the fully cured PVA film was peeled from the Si wafer at constant peeling rate of 50 mm/min using an automated peeling apparatus (Mark-10, Willrich Precision Instrument) (Figure 1A, right). During this peeling process, mechanical stress can be concentrated predominantly at the bottom undercuts of the p-Si needles to generate cracks allowing for their physical liberation from the Si wafer.15
Figure 1. Design principle and fabrication process.
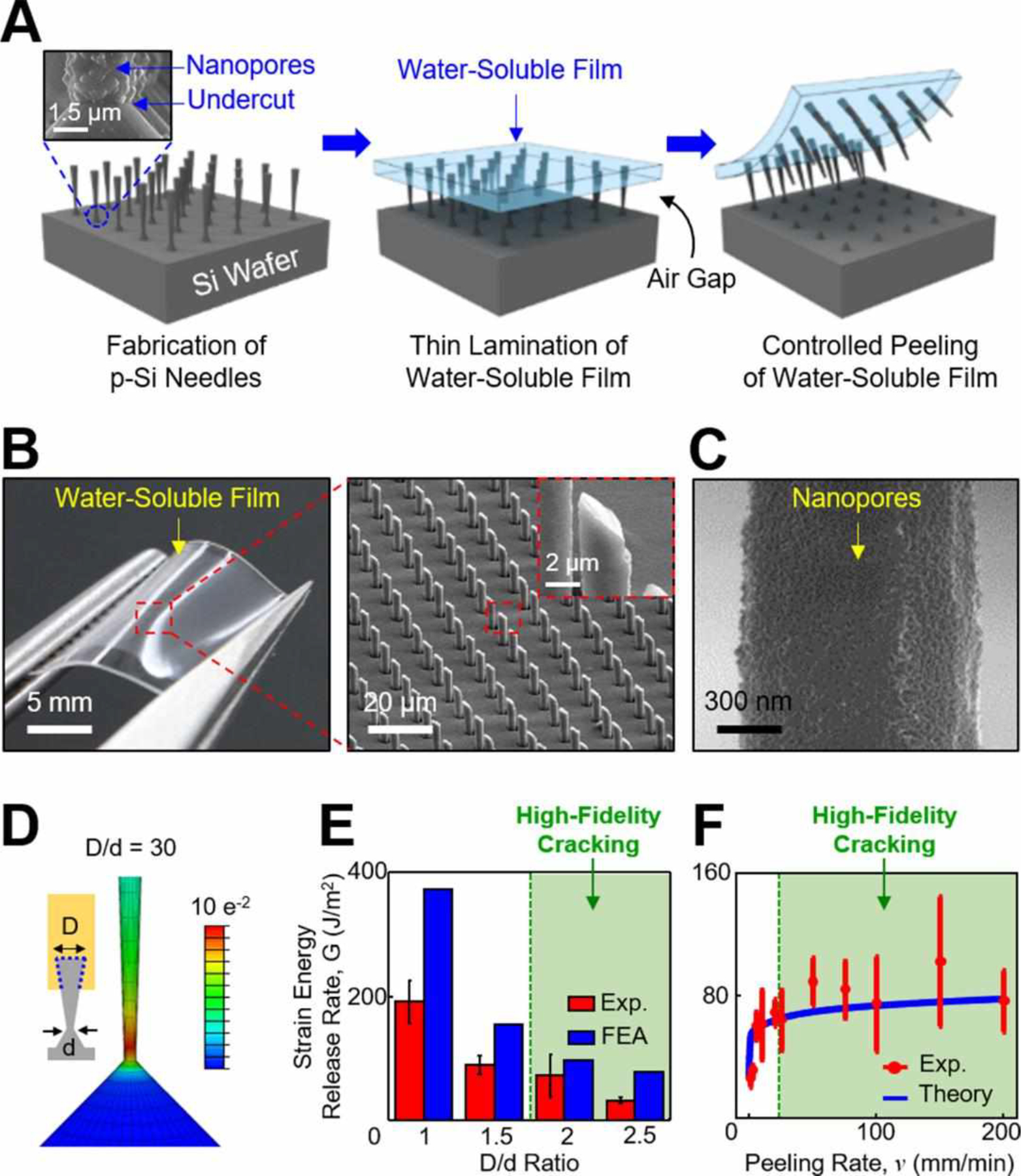
(A) Schematic illustrations for the construction of the p-Si needles on a water-soluble backing. The inset image highlights the bottom undercut and nanopores at the bottom and on the surface of the p-Si needles, respectively. (B) Optical images of the p-Si needles integrated with a PVA film. The inset image highlights the sharpened angular tip of the p-Si needles. (C) SEM image of the nanopores formed on the surface of the p-Si needles. (D) Representative FEA results showing the distribution of principal strains along the p-Si needle during constant peeling. (E) Experimental and FEA results for the effect of D/d ratio on strain energy release rate (G). (F) Experimental and theoretical results for the effect of peeling rate (v) on G.
Figure 1B shows representative photograph (left) and enlarged microscope image (right) of the physically-transferred p-Si needles to a thin layer (200–300 μm-thick) of a PVA film. The intrinsically thin and flexible property of the PVA film can facilitate intimate contact to the soft, irregular surface of tissues, while the sharpened angular tip of the p-Si needles facilitates easier penetration.15,16 The overall height, vertical arrangement, and tip morphology of the transferred p-Si needles were consistent across the entire specimen area (3×3 cm2). Figure S3 shows the fractured planes on both the donor Si wafer and the receiver PVA film, suggesting that the cracking occurred uniformly at the bottom undercut of the p-Si needles. Figure 1C shows the nanopores formed on the surface of the p-Si needles in which the porosity (in turn, drug loading capacity) can be adjusted during the MACE process. The SEM images in Figure S4 provide representative examples of the p-Si needles configured into different surface porosities (0%, ~30%, ~45%, and ~60%) that were controlled by the MACE time of 0, 30, 60, and 90 s, respectively. Figure S5 presents a series of optical images at various stages during the dissolution of the PVA film - colored with yellow food dye (McCormick & Company, USA) for visualization - when immersed in 50 ml of phosphate-buffered saline (PBS; pH 7.4; Sigma-Aldrich, USA) at 37.5 °C. The complete dissolution of the PVA film occurred typically within 10–15 min in this condition (Movie S1, Supporting Information), or it can be swabbed away using a saline-moistened cotton swab in less than 1 min.
The controlled cracking at the bottom undercut of the p-Si needles over centimeter-scale areas is crucial to successful implementation of this approach. Figure S6 shows an experimental setup and the corresponding experimental results for peeling load–peeling distance curve for a specimen (3×3 cm2, d = 1 μm, D = 4 μm, and L = 50 μm) under constant peeling of the PVA film at 50 mm/min. Movie S2 (Supporting Information) demonstrates the real-time measurement process. The results indicate that the peeling load increased rapidly to a maximum and initiate cracking, and then reached a plateau for steady-state crack propagation. Figure 1D shows finite element analysis (FEA) results, revealing that the principal strain (ε) remained localized near the bottom undercut of the p-Si needles during the constant peeling. The tendency for the localization of ε was more evident as the D/d ratio was increased, wherein the peak principal strain (εpeak) was larger than the fracture limit (~1%) of the p-Si needles when D/d > 1.5 (Figure S7). The experimental and FEA results of strain energy release rate (G) for the cracking are summarized in Figure 1E. The green-filled area denotes where the cracking typically occurred with high-fidelity. Figure 1F experimentally and theoretically reveals the dependence of G on peeling rate (v) of the PVA film. The results showed a clear power-law relationship of them (i.e., the G increased rapidly at low v and then gradually reached steady-state) due to the viscoelastic property of the PVA film.17 For instance, a rapid peeling (v > 20 mm/min) of the PVA film provided sufficiently large adhesive strength to peel the p-Si needles away from the Si wafer. On the other hand, a slow peeling (v < 20 mm/min) of the PVA film was unable to hold the p-Si needles, resulting in compressed marks left on the surface (Figure S8). For the theoretical analysis, the energy release rate was obtained by assuming that the PVA film was monolithically bonded to the p-Si needles without embedding inside, causing the discrepancy with the experimental results especially at high peeling rate (v > 100 mm/min).
Controlled dissolution of the p-Si needles in biological fluids.
Figure 2A show the gradual dissolution of a unit array (1×1 cm2) of the p-Si needles with fixed initial base diameter (D0) of 3 μm when immersed in 50 ml of PBS (pH 7.4) at 37.5 °C for 90 days, while refreshing the solution every 10 days to maintain the pH value. The magnified SEM images of the p-Si needles at predetermined time interval are shown in Figure S9. The dissolution of the p-Si needles occurred via hydrolysis of Si to silicic acid and hydrogen (i.e., Si + 4H2O ↔ Si(OH)4 + 2H2), which involves nucleophilic attack at the surface to weaken the interior bonds of Si atoms.18–20 The dissolution kinetics rely on the pH, temperature, and ionic strength of the solution as well as the pre-defined surface porosity of Si.10 Figure 2B shows measurement results of the gradual diameter reduction (D/D0) of the p-Si needles with varied surface porosities, indicating that the dissolution rate was increased from ~10 nm/day to ~20 nm/day as the surface porosity was increased from 0% to 60%. Figure 2C and D compare the dissolution of the p-Si needles in higher pH environment (PBS; pH 10.0) at 37.5 °C. The results indicate that the dissolution rate was substantially accelerated at higher pH, while the rate was non-linearly decreased over time due to enhanced dependence on the concentration of the byproducts, such as Si(OH)4, in the solution.19 In fact, the biosafety of the monocrystalline Si nanomaterials and their dissolution products has been proved in many biomedical applications, without showing measurable cytotoxic effects.10,11,19,20
Figure 2. Degradation of the p-Si needles in biofluids.
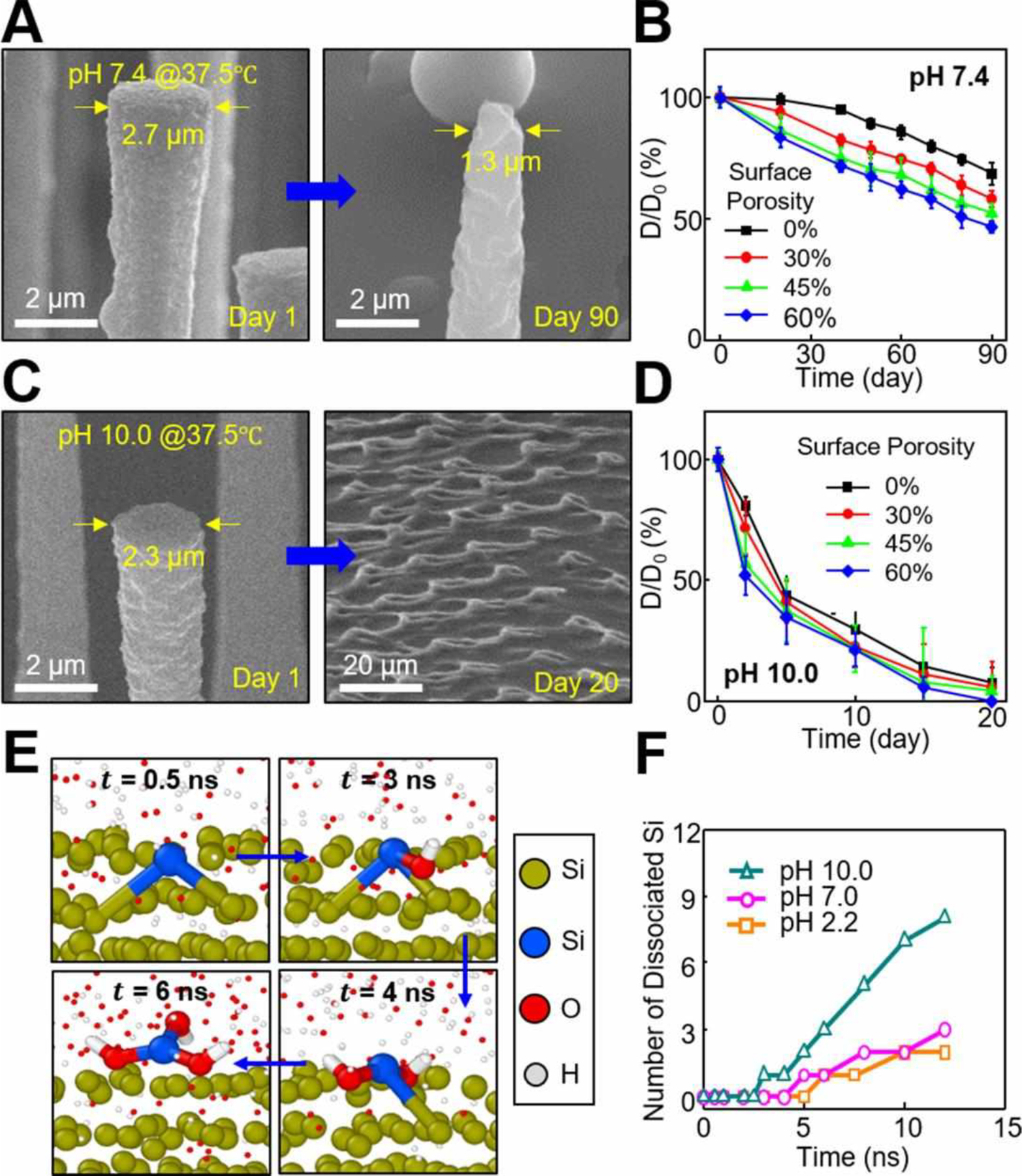
(A) SEM images of the p-Si needles at 1 day (left image) and 90 days (right image) after immersing in 50 ml of PBS (pH 7.4) at 37.5 °C. (B) Measurement results of D/D0 ratio (%) obtained from the p-Si needles with varied surface porosities of 0% (black), 30% (red), 45% (green), and 60% (blue). (C) SEM images of the p-Si needles at 1 day (left image) and 20 days (right image) after immersing in 50 ml of PBS (pH 10.0) at 37.5 °C. (D) Measurement results of D/D0 ratio (%) obtained from the p-Si needles with varied surface porosities. (E) Snapshot images of MD simulation at different time frames. (F) Results of the number of dissociated Si atoms in solutions at acidic (pH 2.2) and basic (pH 10.0) conditions formed by the addition of H+ and OH− groups, respectively, compared to a neutral condition (pH 7.0).
Figure 2E shows a series of snapshot images obtained from molecular dynamics (MD) simulation at different time frames, revealing the dissociation process of a Si atom (highlighted in blue) in water (H2O) due to the effect of nucleophile attack by OH- groups (enlarged). At the beginning (t = 0.5 ns), no bonding occurred between the Si atom and OH- groups. At t = 3 ns, an OH- group started attacking the Si atom to create a bond. At t = 4 ns, another OH- group attacked the Si atom and formed another bond to break an interior Si-Si bond. At t = 6 ns, one more OH- group attacked the Si atom and finally triggered its complete dissociation. The dissociation occurred when the maximum distance of a Si atom from its nearest neighbors remained longer than 3.0 Å.19 Figure 2F shows the variation of number of dissociated Si atoms in solutions at acidic (pH 2.2) and basic (pH 10.0) conditions formed by addition of H+ and OH− groups, respectively, compared to a neutral condition (pH 7.0). The results show that the number was increased over the simulation time from 0 ns to 12 ns, while the dissolution was accelerated at higher pH due to the increased concentration of OH− groups. It is also anticipated that the enhanced surface porosity would also affect the reaction (dissolution) rate due to the increased contact area between the Si and liquid solution. These findings are consistent with the above-described experimental observations.
Biocompatibility and controlled drug release.
To evaluate in vivo tissue compatibility, a set of real-time bioluminescence images were captured at 5 h post-injection of the p-Si needles (1×1 cm2, d = 150 nm, D = 1 μm, and L = 50 μm) to the epidermis (on top of the skin; top row) and subcutaneous muscle (under the skin; bottom row) on the backside of mice (Figure 3A). To compare the biocompatibility grade, the experiments were conducted using the p-Si needles built on a medical-grade PVA film (left column; Sigma Aldrich, USA), ones on an industrial-grade PVA film (middle column; Fibre Glast, USA), and a positive control treatment (right column) of phorbol 12-myristate 13-acetate (PMA; 1 mM, 20 μl; Sigma-Aldrich, USA) that promotes intense local inflammation.21 Both the epidermis and the subcutaneous muscle showed no evidence of inflammation following the administration of luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) that detects acute inflammation at the implemented sites,22 whereas acute inflammation appeared in the control mice. Here, the industrial-grade PVA was dissolved within 1 min after the insertion of the p-Si needles into the tissues by applying saline immediately, thereby causing no inflammation. Figure 3B shows in vitro cell viability of human dermal fibroblast (HDF) cells seeded in a 24-well plate (Fisher Scientific, USA) that contains the p-Si needles (1×1 cm2, d = 150 nm, D = 2 μm, and L = 20 μm) and medical-grade PVA film (red bar), as measured using a colorimetric MTT assay kit (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma-Aldrich, USA). For these tests, the length of the p-Si needles (20 μm-long) was tailored for the HDF cells of which the average diameter is about 20–30 μm. The cell viability remained over 99.3% during the entire period (3 days) of the assay without substantial difference compared to that of the control bare medical-grade PVA film without the p-Si needles (blue bars). Whereas, acute toxicity appeared in the industrial-grade PVA film (green bars) due to the residual ethanol and butanol. Similar results were observed in other control specimens formed by replacing the PVA film with a sheet of water-soluble poly(lactic-co-glycolic acid) (PLGA; 50/50 lactide:glycolide; molecular weight = 30,000–60,000; E = 2 GPa; Sigma Aldrich, USA), providing the cell viability of > 97.4% (Figure S10).
Figure 3. Biocompatibility and controlled drug release.
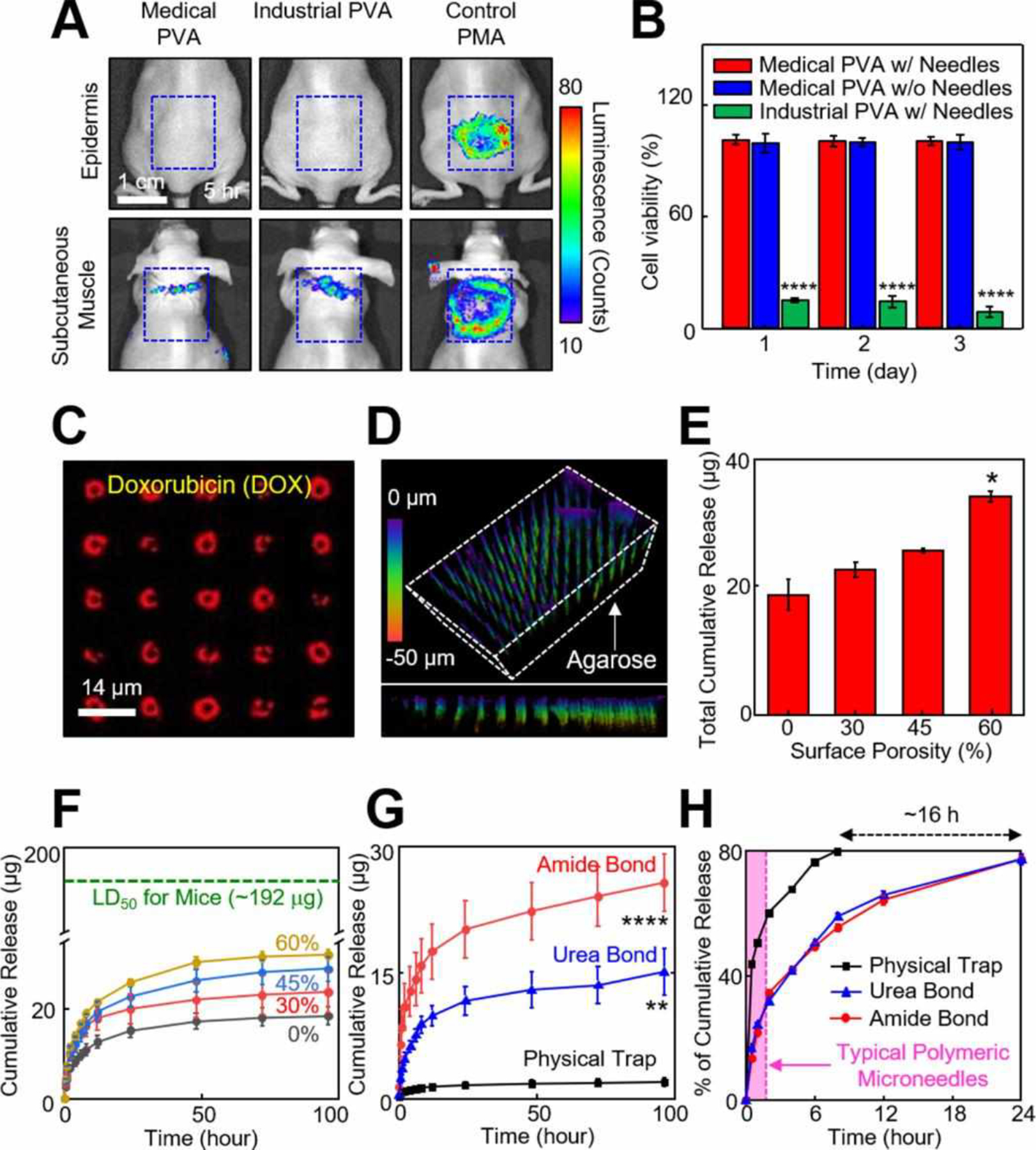
(A) Real-time bioluminescence images on the epidermis (on top of the skin) and subcutaneous muscle (under the skin) of mice at 5 h following the implementation of the p-Si needles built on a medical-grade PVA (left column), industrial-grade PVA (middle column), and control PMA treatments (right column). (B) Results of MTT assay in the cytotoxicity tests of HDF cells incubated with a medical-grade PVA with (red bars) and without (blue bars) the p-Si needles, as well as an industrial-grade PVA (green bars). Error bars represent the standard deviation (SD) of three replicates. ****p<0.0001 compared to the medical-grade PVA with the p-Si needles using one-way analysis of variance (ANOVA). (C) Confocal microscopy image of the p-Si needles loaded with DOX. (D) Confocal microscopy image of the p-Si needles embedded inside a 2.8% (w/v) agarose gel (color index by penetration length). (E) Cumulative release of DOX obtained from the p-Si needles with varied surface porosities of 0%, 30%, 45% and 60% after 20 days of the immersion in PBS (pH 7.4) at 37.5 °C. *p<0.05 compared to 0% surface porosity using ANOVA. (F) Release profiles obtained from the p-Si needles with covalently-linked DOX, compared to median lethal dose of DOX in mice (LD50, green dotted line). (G) Release profiles obtained from the p-Si needles with the surface porosity of ~45% using amide (red line), urea (blue line), and control physical (black line) bonds of DOX. **p<0.01 and ****p<0.0001 compared to the control physical bond using ANOVA. (H) Cumulative release (%) of the covalently-linked (amide and urea bonds) DOX, compared to the control physically trapped DOX.
Covalent conjugation of drug cargos to the surface of the p-Si needles is critical to reliable drug loading and sustained releasing behavior.9,12 Figure 3C shows representative fluorescence microscopy images (top view) of the p-Si needles where the surface was covalently linked with chemotherapy drug doxorubicin (DOX) via 3-Triethoxysilylpropyl succinic anhydride (TESPSA, Gelest Inc., USA) as a cross-linker. The confocal lens (40×) was focused at the bottom of the p-Si needles, resulting in ring-shaped fluorescence of the DOX. The peak fluorescence intensity of the DOX was observed on the surface of the p-Si needles due to high surface area of the nanopores. Figure 3D shows a three dimensional (3D) confocal image (tilted view) of the p-Si needles upon insertion into a soft agarose gel (2.8% w/v) that provides a comparable mechanical modulus (E = ~100 kPa) to the human tissues (E = 80–150 kPa). The experiments were performed by gently pressing the PVA film with the p-Si needles (1×1 cm2, d = 150 nm, D = 1 μm, and L = 50 μm) into the agarose gel, followed by the application of saline to completely dissolve the PVA film. The side view of the image (bottom row) highlights that the p-Si needles were embedded inside the agarose gel through the full length of 50 μm. The larger-field views of the specimen are shown in Figure S11.
Figure 3E shows the total cumulative amount of DOX released from the p-Si needles with varied surface porosities of 0–60% in PBS (pH 7.4) at 37.5 °C. The corresponding release profiles as a function of time (up to 100 h) are shown in Figure 3F, exhibiting that rapid release of DOX occurred within 24 h and then gradually reached a plateau at the predefined doses. The range of the released doses (18–35 μg) were comparable to those used in similar studies using polymeric microneedles.23,24 Importantly, the released doses remained higher than the half maximal inhibitory concentration (IC50) value (~0.3 μg/ml) for B16F10 murine melanoma cells and substantially lower than the lethal dose (LD50) value (~192 μg; green dash line) for mice. Figure 3G shows the cumulative release of covalently-linked DOX using different cross-linkers of amide (red line) and urea (blue line) in PBS (pH 7.4), as compared to that of physically-trapped DOX (black line). For these tests, the surface porosity of the p-Si needles was fixed at ~45%. The amide and urea bonds are well-known to provide strong covalent linkages of the amine group (-NH2) of DOX to the succinic anhydride of 3-(triethoxysilyl) propylsuccinic anhydride (TESPSA) and the isocyanate (-N=C=O) of 3-isocyanatepropyl triethoxysilane (ICPTS), respectively.25 The control physical bond relies on relatively weak Van der Walls force, resulting in rapid and burst release of drug molecules.26 The schematic diagrams of these bonding mechanisms are shown in Figure S12. The highest drug loading capacity occurred in the amide bond (~25 μg) followed by the urea bond (~15 μg), both of which were substantially higher than that of the physical trapping (~2 μg). These observations support that the covalently-linked DOX provides strong binding affinity for the surface of the p-Si needles to form a highly stable complex at physiological pH.37 The strong covalent boding is critical to the sustained release of DOX by allowing the release to occur predominantly by the gradual dissolution of the p-Si needles. Figure 3H presents the corresponding release profiles (%) as a function of time (up to 24 h), highlighting the longer-lasting release of the covalently-linked DOX than counterparts. For instance, more than 80% of the covalently-linked DOX was released for ~24 h (red and blue lines), which was substantially longer than control specimens with the physically-bonded DOX (~8 h; black line) and conventional polymeric microneedles (typically, 15 min-2 h; pink-filled area),27 respectively.
Unobtrusive topical application of p-Si needles.
To illuminate the utility of the p-Si needles in the envisioned scenarios of transepidermal, transmuscular, and transocular injections, a unit array of the p-Si needles (1×1 cm2, d = 150 nm, D = 1 μm, and L = 50 μm) with covalently-linked (amide) fluorescent dyes (DyLight 800, Pierce Thermo Scientific) was introduced to the epidermis, subcutaneous muscle, and cornea of athymic nude mice in vivo. Details of the experimental procedures are shown in the Materials and Methods section. Figure 4A shows representative optical images, pointing out the injection sites where the p-Si needles were embedded after the PVA film was completely dissolved with saline solution. The size of the p-Si needles was much smaller than that of conventionally-used polymeric microneedles (typically, d > 5 μm, D > 300 μm, and L > 600 μm),4,16,28,29 and remained nearly unnoticeable on the tissue surface by visual observations (Figure S13). This aspect may help reduce the risk of irritation or discomfort during/after the injection of the p-Si needles. The mice exhibited normal behaviors without showing any evidence of discomfort against natural movements for the entire period of observation (> 3 months). Figure 4B shows the corresponding IVIS images, indicating that the fluorescent dyes were uniformly localized over the irregular surface of the epidermis, subcutaneous muscle, and cornea of the mice, and maintained until the florescent dyes were completely absorbed into the body. Figure S14 shows optical images of a control unit array (1×1 cm2) of the p-Si needles integrated with a flexible, yet non-water-soluble PDMS film (200 μm-thick), which was attached on the back of a nude mouse. Of note, no wrinkles were observed on the skin over the PDMS film while other areas of the skin were easily wrinkled according to body movements. These observations imply that the skin underneath the PDMS film experienced occasional interruptions or discomforts due to the physical constraints, highlighting the rationale of eliminating the backing film after complete delivery of the p-Si needles.
Figure 4. Unobtrusive topical delivery of the p-Si needles.
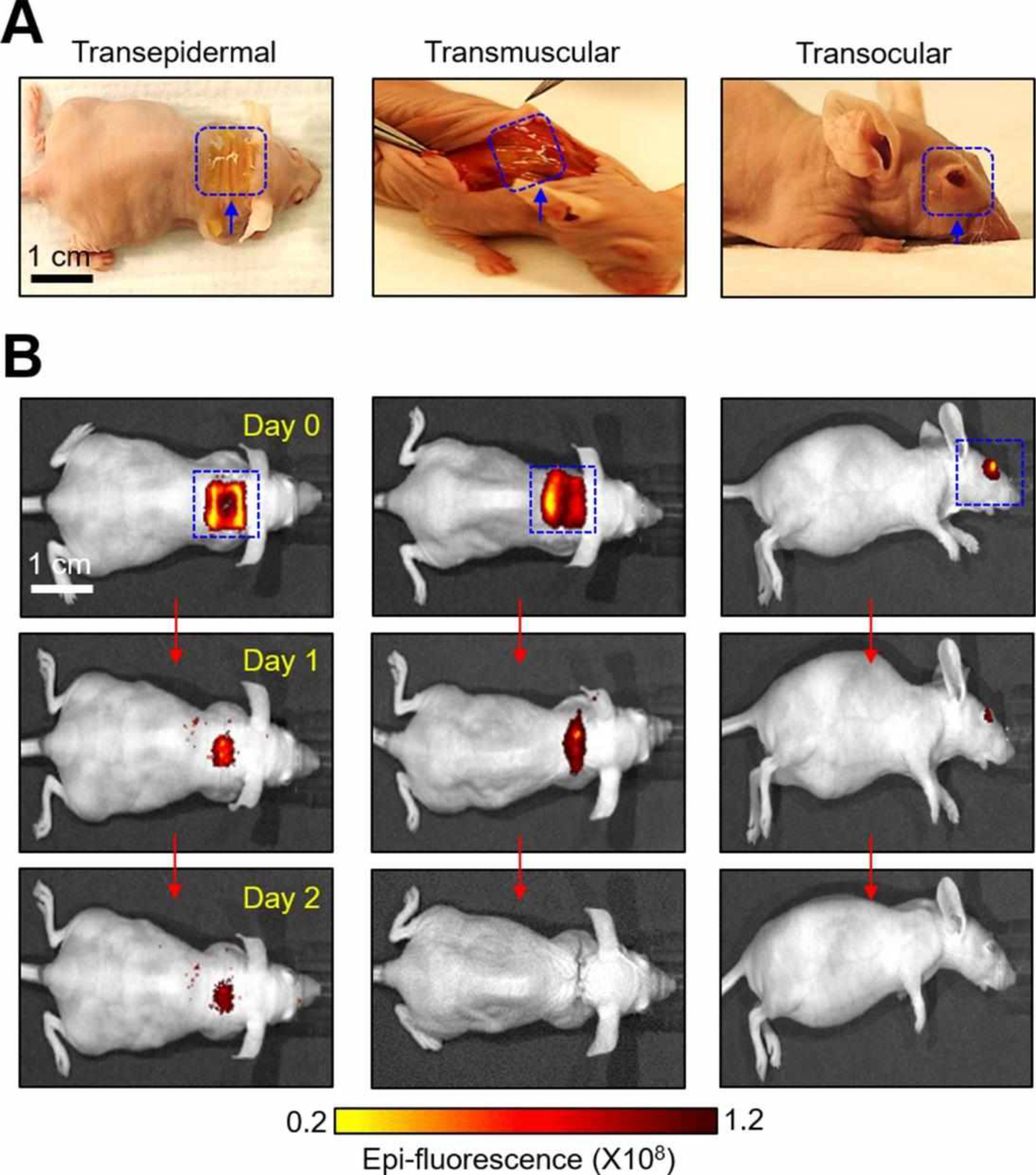
(A) Optical images of the p-Si needles with DyLight 800 fluorescent dyes on a PVA film, applied to the epidermis (left image), subcutaneous muscle (middle image), and cornea (right image) of mice. (B) IVIS images of the mice receiving the p-Si needles for 2 days following the implementations.
Demonstration on post-surgical melanoma treatments in mice in vivo.
Tumor relapse after surgical resection that often occurs by the outgrowth of residual microtumors remains a significant challenge in the treatment.30 Systemic chemotherapy and radiotherapy are often employed to prevent the recurrence of residual tumors, but these methods lead to toxic side effects and do not provide a long-lasting protection unless frequently repeated.31 Sustained topical delivery of therapeutic drug cargos with precisely controlled doses for a prolonged time, after surgical resection, may reduce the risk of tumor relapse with minimal side effects and improved convenience of patients and healthcare providers.
The utility of the p-Si needles in the inhibition of post-surgical residual melanoma was tested using C57BL/6 mice and syngeneic B16F10 melanoma cells. The mice were subcutaneously inoculated with 1×106 B16F10 melanoma cells to mimic a situation where melanoma resection is incomplete and residual cells are present. Following 2 h of the subcutaneous inoculation, an array of the p-Si needles (1×1 cm2, d = 150 nm, D = 1 μm, and L = 50 μm) covalently-linked (amide) with 50 μl of DOX (~20 μg) or without DOX (control) were applied to the tumor inoculation site (Figure 5A). The basic procedure for the injection of the p-Si needles (hereinafter referred to as “nanoinjection”) is demonstrated in Movie S3 (Supporting Information). A representative photograph in Figure 5B highlights the nanoinjection site on the shaved skin of a mouse. The mouse receiving the p-Si needles moved freely without any sign of discomfort (Movie S4, Supporting Information). Two other control groups of mice were intratumorally administered using a medical 28G insulin syringe (Fisher Scientific, USA) with a single dose of PBS (50 μl) and DOX (50 μl), representing no-treatment control and a conventional bolus injection, respectively. Figure 5C shows that, in the mice treated with the nanoinjection of DOX, tumor growth was suppressed over 10 days post-inoculation. In contrast, significant growth of the tumors occurred in 4 out of 5 mice treated with the control nanoinjection (without DOX), all 5 mice with the syringe injection of PBS, and 4 out of 5 mice with the syringe injection of DOX during the same period, followed by rapid increase to reach the endpoint tumor size of 2,000 mm3 (Figure S15).32 Figure 5D shows the comparisons of the size of the tumors at 10 days post-inoculation. All of these treatments were well tolerated by the mice with negligible weight loss during the surviving period (Figure 5E); however, local skin lesions were observed on the mice treated with the syringe injection of DOX (Figure 5F & Figure S16), which is a typical side effect of the drug.33 The superior anti-tumor efficacy of the nanoinjected DOX compared to the bolus-injected DOX with the same dose is attributable to the prolonged maintenance of effective local concentration based on the sustained drug release (Figures 3F–H). These findings support that the sustained release of DOX at the skin with residual melanoma cells by nanoinjection can provide a prolonged suppression of tumor growth without adverse effects as compared to a bolus injection. This tendency was also observed in previous reports using polymeric microneedles.27,34
Figure 5. Evaluations in a murine melanoma model in vivo.
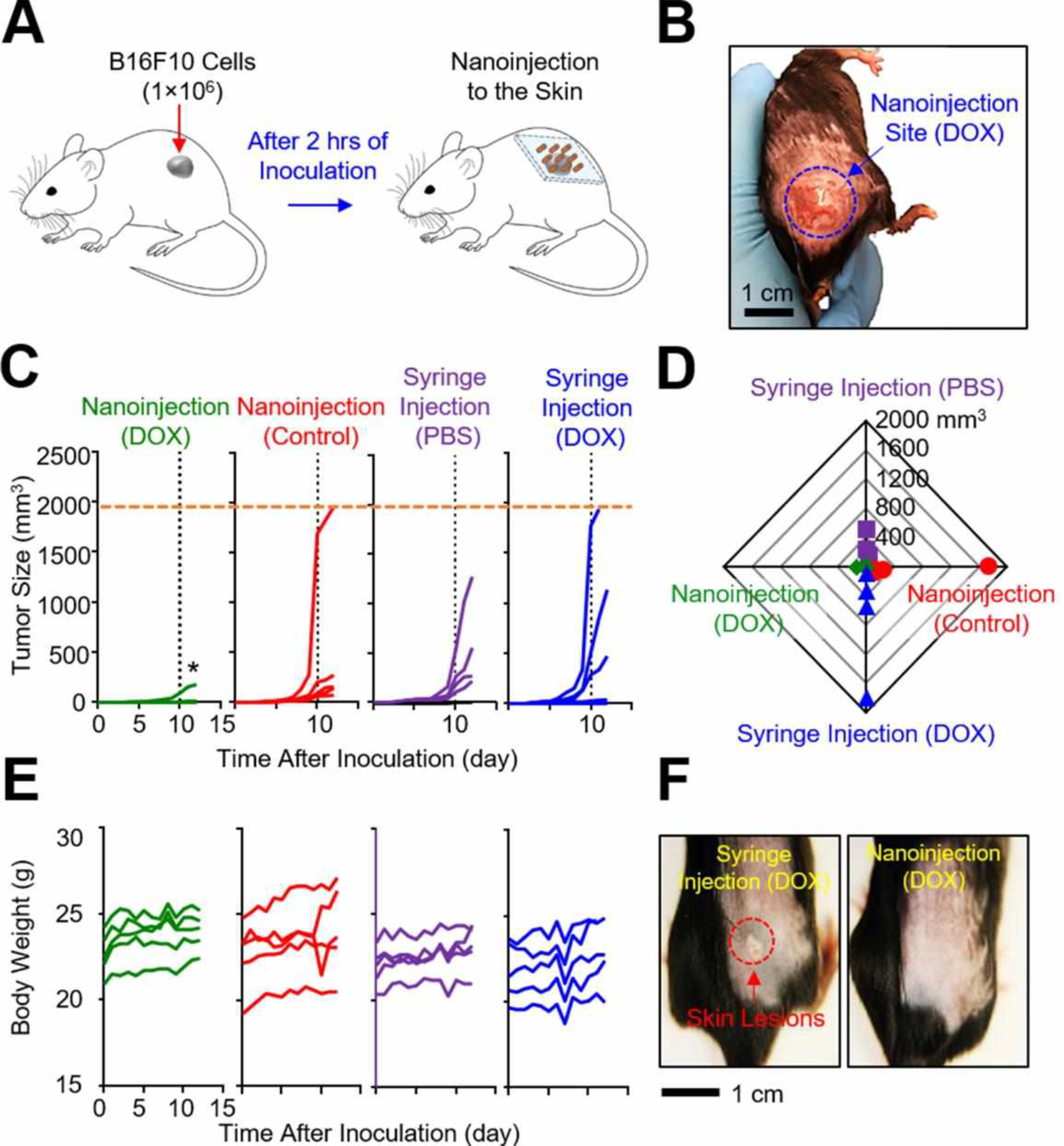
(A) Schematic illustration of experimental protocols for in vivo studies in a murine melanoma model. (B) Representative image of the shaved skin of a mouse post-nanoinjection of DOX. (C) Measurement results of the tumor size for 12 days post-inoculation (n = 5 per group). *p<0.05 compared to the control syringe injection (DOX) using ANOVA. (D) Radial shape graph of the tumor size at day 10 (n = 5 per group). (E) Measurement results of the body weight for 12 days post-inoculation (n = 5 per group). (F) Representative images highlighting the treated sites for 10 days post-injection. The red dotted circle denotes skin lesions.
CONCLUSION
Controlled cracking at the bottom undercut of vertically-ordered p-Si needles enables their physical liberation from the rigid Si wafer and subsequent integration with a thin water-soluble backing film over centimeter-scale areas. The water-soluble film serves as a temporary flexible holder that can be seamlessly interfaced with the soft, irregular surface of living tissues during the insertion of the p-Si needles, and then immadiately dissolved with saline solution. The nanoscale sharpened angular tip of the p-Si needles facilitate their penetration into the epidermis, subcutaneous muscle, and cornea of mice in vivo in a minimally invasive manner. The gradual degration of the p-Si needles in tissue fluids enables the sustained, long-lasting release of preloaded drug cargos. In vivo evaluations in a murine melanoma model support the potential utility of this concept in preventing the recurrence of residual melanoma post-surgery. The reported set of materials, structural designs, and assembly method establishes a technical foundation, which can be adjusted and tailored for minimally invasive and painless injection of drug cargos through the cornea for the managment of ocular melanomas.35
MATERIALS AND METHODS
Fabrication of the p-Si needles on a Si wafer.
The fabrication began with a bulk Si wafer (p-type; 525 μm-thick; 0–100 Ω∙cm) by immersing it in a solution of buffered oxide etch (J. T. Baker Inc., USA) for 1 min to eliminate the native oxide layer. Following a standard photolithographic patterning, a deep reactive-ion etching (DRIE) was carried out under a radiofrequency (RF) plasma power of 450 W and a platen power of 11 W using sulfur hexafluoride (SF6) gas with the flow rate of 85 sccm to create vertically-ordered Si micropillars at a prescribed aspect ratio. The deposition of (CxFy)n polymer was followed to form a partial passivation layer using octafluorocyclobutane (C4F8) gas with the flow rate of 130 sccm under the RF plasma power of 800 W. Additional isotropic dry etching under the plasma power of 450 W and platen power of 30 W by SF6 gas with the flow rate of 85 sccm was conducted to create undercuts at the bottom of the Si micropillars. The entire specimen was treated with an oxygen (O2) plasma (20 sccm, 150 W, 50 mtorr, 15 min), followed by cleaning with standard piranha solution (75% of sulfuric acid (H2SO4) and 25% of hydrogen peroxides (H2O2)) to eliminate the remaining passivation layer on the surface of the Si micropillars. Finally, the entire specimen was immersed in a solution of potassium hydroxide (KOH; 15 weight %; Fisher Scientific, USA) at 25 °C to reduce the overall size of the Si micropillars down to the nanoscale. The overall size of the miniaturized Si needles was determined by controlling the molarity of etching solution, temperature, and etching time.15 The next step involved the MACE by immersing the specimen in a mixed solution of 20 mM silver nitrate (AgNO3; Sigma-Aldrich, USA) and 49% hydrofluoric acid (HF; J. T. Baker Inc., USA) to form nanopores on the surface.36 During the MACE, the overall surface porosity was determined by controlling the etching time. The specimen was then immersed in a solution of Ag etchant (TFS, KI-I2 complex liquid, Transene Inc., USA) for 1 min to remove the remaining Ag residues on the surface. The surface porosity of the resulting p-Si needles was observed using a high-resolution SEM (S-4800, Hitachi, Japan), followed by analyzing the surface contrast using a post-image processing program (Stream Desktop 2.1, Olympus, Japan) in order to quantify the surface porosity.
Integration of the p-Si needles with a water-soluble film.
The process began by spin-coating a solution of 10 weight % PVA (Mowiol 4–88; Sigma Aldrich, USA) or 5.5 weight % PLGA (lactide:glycolide (50:50); Sigma Aldrich, USA) on the as-fabricated p-Si needles on a Si wafer, followed by curing at 70 °C for 30 min. At this stage, the thickness of the water-soluble (PVA or PLGA) film was determined by adjusting the spin-casting speed (in rpm). The spin-coating was repeated until the total thickness of the water-soluble film reached about 200 μm for enhancing mechanical strength. Finally, the film was mechanically peeled using an automated peeling apparatus (Mark-10, Willrich Precision Instrument) at constant rate of 50 mm/min, causing cracking at the bottom undercut of the p-Si needles.
Calculation of theoretical strain energy release rate (G).
By measuring the peeling force at low velocity near zero, the theoretical energy release rate was calculated according to the peeling rate. In steady-state crack propagation, the strain energy release rate (G) has the following relation by37
| (1) |
where F is measured peel force and w is width of the PVA film. By calculating the critical energy release rate (G0) at the reference velocity (v0) near zero, the theoretical energy release rate can be determined as power law equation,38
| (2) |
where n is experimentally measured constant. In the peeling tests, the range of the peeling rate of 2.4–200 mm/min, critical energy release rate (G0) of 26.15 J/m2, reference velocity (v0) of 2.4 mm/min, and experimental constant (n) of 0.14 were determined.
Finite element analysis (FEA).
The FEA was conducted using the ABAQUS/standard package to reveal the underlying mechanics of the p-Si needles under peeling process. The deformation of the p-Si needles and PVA film was modeled by linear elastic behavior with the E of 112.4 GPa and 3.4 GPa, respectively.39 The materials were modeled by 8 nodes solid elements (C3D8R). The displacement at constant peeling rate was applied on the top of the PVA film while a tie constraint was defined at the interface between the p-Si needles and PVA film.
Loading of DOX on the surface of the p-Si needles.
The process began by washing the as-fabricated p-Si needles on a Si wafer with distilled (DI) water, followed by thorough drying with nitrogen (N2) gas. The cleaned p-Si needles were immersed in a solution of 3-Triethoxysilylpropyl succinic anhydride (TESPSA; Gelest Inc., USA) for 2 h to functionalize the surface with amide.40 Alternatively, the p-Si needles were immersed in a solution of 3-Triethoxysilylpropyl isocyanate (ICPTS; Sigma-Aldrich, USA) for 2 h to functionalize the surface with urea.41 The p-Si needles were then rinsed with ethanol and then baked at 120 °C for 1 h, followed by immersing in a solution of DOX (0.5 mg/ml; Sigma-Aldrich, USA) for 24 h at room temperature. To prepare the control specimens with physically-trapped DOX, the as-fabricated p-Si needles were immersed in a solution of DOX without undergoing any treatment on the surface. The prepared specimens were dried by N2 gas and stored under an inert atmosphere.
Measurements of cumulative DOX release.
The p-Si needles loaded with DOX were immersed in 12-well plate with 4 ml of PBS (pH 7.4 or pH 10.0; Sigma-Aldrich, USA) at 37.5 °C. At the predetermined time intervals, the specimens were sampled and the PBS solution was refreshed. A 100 μl of the sampled solution was transferred to a 96-well plate, and the fluorescent intensity was measured with a plate reader (Synergy Neo Plate Reader, BioTek Instruments). The concentration of the released DOX was determined according to the fluorescent intensity measured with the excitation and emission at 480 nm and 600 nm, respectively.42
Molecular dynamics (MD) simulations.
The MD simulations were conducted to model atomic scale chemical interactions of Si with H2O and the associated dissolution dynamics with the Reaxff potential43 using a large-scale atomic/molecular massively parallel simulator (LAMMPS) package. In these simulations, Si, O, and H were used for the atomic elements.44 The initial systems included a Si layer with the thickness of 1.2 nm, which was solvated by a box of H2O with 3090 water molecules. Periodic boundary conditions were used in all directions. The temperature of the system was maintained at 300 K using the Nose-Hoover thermostat, and the time step was selected to be 0.1 fs, in order to ensure the capture of chemical reaction events between the Si and H2O species.
Cell viability tests.
For the MTT assay, approximately 5×104 human dermal fibroblast (HDF) cells were seeded on a specimen in a 24-well plate and then incubated for 24 h, 48 h, and 72 h. At each measurement point, 200 μl of MTT solution ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); Sigma-Aldrich, USA) was added to the wells and incubated for 4 h. The cell medium was removed, and 400 μl of dimethyl sulfoxide (C2H6OH; Sigma-Aldrich, USA) was added to dissolve precipitated formazan. A 100 μl of the solution was transferred to a 96-well plate and measured using a microplate reader (SpectraMax Plus 384 reader, Molecular Devices, USA) at 570 nm.
Acute inflammation tests in vivo.
All of these animal studies were approved by the Purdue Animal Care and Use Committee (PACUC; #1612001512) for the care and use of laboratory animals. Female athymic nude mice (5–6 weeks old; NCr-Fox1nu, Charles River Laboratories, USA) were used in the acute inflammation tests in vivo. As a positive control, 40 μl of PMA (100 μM; Sigma-Aldrich, USA) was rubbed on the epidermis and subcutaneous muscle to induce acute inflammation.34 The p-Si needles on a PVA film were gently applied to target sites with firm compression. The backing film was then completely dissolved by applying a saline-moistened cotton swab. Following 5 h of the implementation, 100 μl of luminol sodium salt (200 mg/kg; Sigma-Aldrich, USA) was administered by the intraperitoneal nanoinjection. Then, the mice were anesthetized with compressed air containing 2.5% isoflurane with a Classic T3 isoflurane vaporizer (Smith Medical, Dublin, OH). Bioluminescence images were acquired using an IVIS Lumina II imaging system (Caliper Life Sciences, USA) for 3 min of the exposure time at the F/stop and binning of 1 and 4, respectively.
IVIS imaging in vivo.
All of these animal studies were approved by the Purdue Animal Care and Use Committee (PACUC; #1612001512). The p-Si needles with covalently-linked (amide) DyLight 800 dyes were placed either on the epidermis, subcutaneous muscle, or cornea of mice (5–6 weeks old; NCr-Fox1nu, Charles River Laboratories, USA) with firm compression. For the transmuscular nanoinjection, 250 mg/kg of avertin (Sigma-Aldrich, USA) was administrated by an intraperitoneal injection to anesthetize mice, followed by careful incision of the skin with surgical scissors. The incisional site was then sutured using a surgical needle and thread. The mice were anesthetized with inhaled isoflurane anesthesia with a Classic T3 isoflurane vaporizer (Smith Medical, Dublin, OH) and exposed to 2.5% isoflurane delivered in O2 (2 litters/min) within a 1-litter induction chamber. The fluorescence of the injection site was measured using an IVIS Lumina II imaging system (Caliper Life Sciences, USA) at the day 0, 1, and 2 with the exposure time of 1 s each using a 150 W quartz halogen lamp, and then filtered using an indocyanine green (ICG) excitation and emission filters with the wavelengths of 710–760 nm and 810–875 nm, respectively. The data analysis was performed using the Living Image software (version 4.4, PerkinElmer Inc).
Demonstration in a murine melanoma model.
All of these animal procedures were approved by the Purdue Animal Care and Use Committee (PACUC; #1503001212). Male C57BL/6 mice (8–10 weeks old; Envigo, USA) were acclimatized for at least 1 week prior to the procedures. The p-Si needles were sterilized by an ultraviolet (UV) illumination (254 nm) for 30 min. 106 B16F10 melanoma cells (ATCC; Manassas, VA, USA) were subcutaneously inoculated in the bottom flank of the right hind leg of each mouse. After approximately 2 h, the inoculation site was treated with the nanoinjection of the covalently-linked (amide) DOX (~20 μg) and the control nanoinjection (without DOX). For comparison, two other control groups of mice were intratumorally administered using a medical 28G insulin syringe (Fisher, USA) with a single dose of PBS (50 μl) and DOX (20 μg in 50 μl), respectively. The length (L) and width (W) of each tumor were monitored every day using a digital caliper (Thorlabs Digital Calipers, Thorlabs Inc, USA), and the volume (V) was calculated according to an ellipsoid formula: V = (L×W2)/2. The body weight of the mice was monitored every other day.
Data availability.
The data that support the plots in this paper and other finding of this study are available from the corresponding authors upon reasonable request.
Supplementary Material
ACKNOWLEDGMENT
C.H.L. acknowledges funding support from the Asian Office of Aerospace Research & Development (AOARD: FA2386-16-1-4105; Program manager: Dr. Tony Kim) and the Air Force Office of Scientific Research (AFOSR: FA2386-18-1-40171; Program manager: Dr. Tony Kim). D.R.K. acknowledges funding support from the International Research and Development Program (NRF-2018K1A3A1A32055469) and the Basic Science Research Program (NRF-2018R1C1B6007938) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT of Korea. Y.Y. acknowledges funding support from the National Institute of Health (NIH: R01 CA199663). B.X. acknowledges funding support on chemomechanics of reactive MD simulations from National Science Foundation (NSF-CMMI-1728149).
Footnotes
Supporting Information.
SEM images of the p-Si needles fabricated on a donor Si wafer with varied sizes (Figure S1); SEM images of the PVA film coated on the p-Si needles at varied spin-casting speeds (Figure S2); SEM images of a donor Si wafer and receiver PVA film (Figure S3); SEM images of the p-Si needles with varied surface porosities (Figure S4); Time-dependent optical images of a yellow-colored PVA film (Figure S5); Optical image and the correspodning measurement results of the peeling of the p-Si needles (Figure S6); FEA results displaying the distribution of the principal strains for the p-Si needle during peeling process (Figure S7); Representative SEM image displaying the compressed marks leftover on the surface of a receiver PVA film peeled out of optimal conditions (Figure S8); SEM images of the p-Si needles immersed in 50 ml of PBS (pH 7.4) at 37.5 °C for 90 days (Figure S9); Photograph and SEM image of the p-Si needles built on a water-soluble PLGA film and the corresponding results of MTT assay for the cytotoxicity test of HDF cells (Figure S10); Microscope images of the p-Si needles penetrated into a 2.8% (w/v) agarose gel (Figure S11); Schematic diagrams of the amide, urea, and physical bonds of DOX to the surface of the p-Si needles (Figure S12); Enlarged optical images of the epidermis, subcutaneous muscle, and cornea of mice receiving the p-Si needles (Figure S13); Optical images of a nude mouse worn with the control p-Si needles built on a PDMS film on the backsdie (Figure S14); Measurement results of the tumor size (Figure S15); Enlarged optical images of the treated sites of the mice at 10 days post-injection (Figure S16); Visual observation (16× speed) of a yellow-colored PVA immersed in a solution of PBS (pH 7.4) at 37.5 °C (Movie S1); Real-time demonstration of the automated peeling of the p-Si needles from the donor Si wafer (Movie S2); Demonstration (26× speed) of the nanoinjection of DOX to the shaved skin of a mouse after 2 h of subcutaneous inoculation with 1×106 B16F10 melanoma cells (Movie S3); Visual observation (2× speed) of a mouse receiving an array (1×1 cm2) of the p-Si needles with covalently-linked (amide) DOX on the back side (Movie S4).
REFERENCES
- 1.Simoes MCF; Sousa JJS; Pais A, Skin Cancer and New Treatment Perspectives: A review. Cancer Lett 2015, 357, 8–42. [DOI] [PubMed] [Google Scholar]
- 2.Perera E; Gnaneswaran N; Jennens R; Sinclair R, Malignant Melanoma. Healthcare 2014, 2, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prausnitz MR; Langer R, Transdermal Drug Delivery. Nat. Biotechnology 2008, 26, 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W; Terry RN; Tang J; Feng MHR; Schwendeman SP; Prausnitz MR, Rapidly Separable Microneedle Patch for the Sustained Release of a Contraceptive. Nat. Biomed. Eng 2019, 3, 220–230. [DOI] [PubMed] [Google Scholar]
- 5.Gopal S; Chiappini C; Penders J; Leonardo V; Seong H; Rothery S; Korchev Y; Shevchuk A; Stevens MM, Porous Silicon Nanoneedles Modulate Endocytosis to Deliver Biological Payloads. Adv. Mater 2019, 31, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiappini C; De Rosa E; Martinez J; Liu X; Steele J; Stevens M; Tasciotti E, Biodegradable Silicon Nanoneedles Delivering Nucleic Acids Intracellularly Induce Localized in Vivo Neovascularization. Nat. Mater 2015, 14, 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiappini C; Martinez J; De Rosa E; Almeida C; Tasciotti E; Stevens M, Biodegradable Nanoneedles for Localized Delivery of Nanoparticles in Vivo: Exploring the Biointerface. ACS Nano 2015, 9, 5500–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y; Jiang YW; Ledesrna HA; Yi JS; Gao X; Weiss DE; Shi FY; Tian BZ, Texturing Silicon Nanowires for Highly Localized Optical Modulation of Cellular Dynamics. Nano Lett 2018, 18, 4487–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anglin EJ; Cheng LY; Freeman WR; Sailor MJ, Porous Silicon in Drug Delivery Devices and Materials. Adv. Drug Delivery Rev 2008, 60, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YK; Yu KJ; Song EM; Farimani AB; Vitale F; Xie ZQ; Yoon Y; Kim Y; Richardson A; Luan HW; Wu YX; Xie X; Lucas TH; Crawford K; Mei YF; Feng X; Huang YG; Litt B; Aluru NR; Yin L; Rogers JA, Dissolution of Monocrystalline Silicon Nanomembranes and Their Use as Encapsulation Layers and Electrical Interfaces in Water-Soluble Electronics. ACS Nano 2017, 11, 12562–12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang S; Park G; Edwards C; Corbin E; Kang S; Cheng H; Song J; Kim J; Yu S; Ng J; Lee J; Kim J; Yee C; Bhaduri B; Su Y; Omennetto F; Huang Y; Bashir R; Goddard L; Popescu G; Lee K; Rogers J, Dissolution Chemistry and Biocompatibility of Single-Crystalline Silicon Nanomembranes and Associated Materials for Transient Electronics. Acs Nano 2014, 8, 5843–5851. [DOI] [PubMed] [Google Scholar]
- 12.Santos HA; Makila E; Airaksinen AJ; Bimbo LM; Hirvonen J, Porous Silicon Nanoparticles for Nanomedicine: Preparation and Biomedical Applications. Nanomedicine 2014, 9, 535–554. [DOI] [PubMed] [Google Scholar]
- 13.Abbott J; Ye TY; Ham D; Park H, Optimizing Nanoelectrode Arrays for Scalable Intracellular Electrophysiology. Acc. Chem. Res 2018, 51, 600–608. [DOI] [PubMed] [Google Scholar]
- 14.Jovanovic P; Mihajlovic M; Djordjevic-Jocic J; Vlajkovic S; Cekic S; Stefanovic V, Ocular Melanoma: An Overview of the Current Status. Int. J. Clin. Exp. Pathol 2013, 6, 1230–1244. [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H; Jang H; Kim B; Kim MK; Wie DS; Lee HS; Kim DR; Lee CH, Flexible Elastomer Patch with Vertical Silicon Nanoneedles for Intracellular and Intratissue Nanoinjection of Biomolecules. Sci. Adv 2018, 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J; Tahk D; Yu J; Min DH; Jeon NL, Design Rules for a Tunable Merged-Tip Microneedle. Microsyst. & Nanoeng 2018, 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meitl M; Zhu Z; Kumar V; Lee K; Feng X; Huang Y; Adesida I; Nuzzo R; Rogers J, Transfer Printing by Kinetic Control of Adhesion to an Elastomeric Stamp. Nat. Mater 2006, 5, 33–38. [Google Scholar]
- 18.Wang L; Gao Y; Dai FQ; Kong DY; Wang HC; Sun PC; Shi Z; Sheng X; Xu BX; Yin L, Geometrical and Chemical-Dependent Hydrolysis Mechanisms of Silicon Nanomembranes for Biodegradable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 18013–18023. [DOI] [PubMed] [Google Scholar]
- 19.Yin L; Farimani A; Min K; Vishal N; Lam J; Lee Y; Aluru N; Rogers J, Mechanisms for Hydrolysis of Silicon Nanomembranes as Used in Bioresorbable Electronics. Adv. Mater 2015, 27, 1–8. [DOI] [PubMed] [Google Scholar]
- 20.Chang JK; Emon MAB; Li CS; Yang QS; Chang HP; Yang ZJ; Wu CI; Saif MT; Rogers JA, Cytotoxicity and in Vitro Degradation Kinetics of Foundry-Compatible Semiconductor Nanomembranes and Electronic Microcomponents. ACS Nano 2018, 12, 9721–9732. [DOI] [PubMed] [Google Scholar]
- 21.Muller AJ; Sharma MD; Chandler PR; DuHadaway JB; Everhart ME; Johnson BA; Kahler DJ; Pihkala J; Soler AP; Munn DH; Prendergast GC; Mellor AL, Chronic Inflammation that Facilitates Tumor Progression Creates Local Immune Suppression by Inducing Indoleamine 2,3 Dioxygenase. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 17073–17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross S; Gammon ST; Moss BL; Rauch D; Harding J; Heinecke JW; Ratner L; Piwnica-Worms D, Bioluminescence Imaging of Myeloperoxidase Activity in Vivo. Nat. Med 2009, 15, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pei P; Yang F; Liu JX; Hu HR; Du XY; Hanagata N; Zhao SC; Zhu YF, Composite-Dissolving Microneedle Patches for Chemotherapy and Photothermal Therapy in Superficial Tumor Treatment. Biomater. Sci 2018, 6, 1414–1423. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed KS; Shan X; Mao J; Qiu L; Chen J, Derma roller (R) Microneedles-Mediated Transdermal Delivery of Doxorubicin and Celecoxib Co-Loaded Liposomes for Enhancing the Anticancer Effect. Mater. Sci. Eng., C 2019, 99, 1448–1458. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZH; Gangarapu S; Escorihuela J; Fei GX; Zuilhof H; Xia HS, Dynamic Covalent Urea Bonds and Their Potential for Development of Self-Healing Polymer Materials. J. Mater. Chem. A 2019, 7, 15933–15943. [Google Scholar]
- 26.Wu EC; Park J-H; Park J; Segal E; Cunin F; Sailor MJ, Oxidation-Triggered Release of Fluorescent Molecules or Drugs from Mesoporous Si Microplarticles. ACS Nano 2008, 2, 2401–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatnagar S; Bankar NG; Kulkarni MV; Venuganti VVK, Dissolvable Microneedle Patch Containing Doxorubicin and Docetaxel is Effective in 4T1 Xenografted Breast Cancer Mouse Model. Int. J. Pharm 2019, 556, 263–275. [DOI] [PubMed] [Google Scholar]
- 28.Chen MC; Lin ZW; Ling MH, Near-Infrared Light-Activatable Microneedle System for Treating Superficial Tumors by Combination of Chemotherapy and Photothermal Therapy. ACS Nano 2016, 10, 93–101. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan SP; Koutsonanos DG; Martin MD; Lee JW; Zarnitsyn V; Choi SO; Murthy N; Compans RW; Skountzou I; Prausnitz MR, Dissolving Polymer Microneedle Patches for Influenza Vaccination. Nat. Med 2010, 16, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q; Wang C; Zhang X; Chen G; Hu Q; Li H; Wang J; Wen D; Zhang Y; Lu Y; Yang G; Jiang C; Wang J; Dotti G; Gu Z, In Situ Sprayed Bioresponsive Immunotherapeutic Gel for Post-Surgical Cancer Treatment. Nat. Nanotechnol 2019, 14, 89–98. [DOI] [PubMed] [Google Scholar]
- 31.Albain KS; Swann RS; Rusch VW; Turrisi AT III; Shepherd FA; Smith C; Chen Y; Livingston RB; Feins RH; Gandara DR; Fry WA; Darling G; Johnson DH; Green MR; Miller RC; Ley J; Sause WT; Cox JD, Radiotherapy Plus Chemotherapy with or without Surgical Resection for Stage III Non-Small-Cell Lung Cancer: A Phase III Randomised Controlled Trial. Lancet 2009, 374, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J; Lee SS-Y; Seo H; Pang L; Jun Y; Zhang R-Y; Zhang Z-Y; Kim P; Lee W; Kron SJ; Yeo Y, Quinic Acid-Conjugated Nanoparticles Enhance Drug Delivery to Solid Tumors via Interactions with Endothelial Selectins. Small 2018, 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotem M; Hubert A, Lyass O, Goldenhersh MA; Lngber A; Peretz T; Gabizon A, Skin Toxic Effects of Polyethylene Glycol-Coated Liposomal Doxorubicin. Arch Dermatol 2000, 136, 1475–1480. [DOI] [PubMed] [Google Scholar]
- 34.Kubicka-Wolkowska J; Kedzierska M; Lisik-Habib M; Potemski P, Skin Toxicity in a Patient with Ovarian Cancer Treated with Pegylated Liposomal Doxorubicin: A Case Report and Review of the Literature. Oncol. Lett 2016, 12, 5332–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sassalos TM; Paulus YM, Prefilled Syringes for Intravitreal Drug Delivery. Clin. Ophthalmol 2019, 13, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han H; Huang Z; Lee W, Metal-Assisted Chemical Etching of Silicon and Nanotechnology Applications. Nano Today 2014, 9, 271–304. [Google Scholar]
- 37.Feng X; Meitl MA; Bowen AM; Huang Y; Nuzzo RG; Rogers JA, Competing Fracture in Kinetically Controlled Transfer Printing. Langmuir 2007, 23, 12555–12560. [DOI] [PubMed] [Google Scholar]
- 38.Chen H; Feng X; Huang Y; Huang YG; Rogers JA, Experiments and Viscoelastic Analysis of Peel Test with Patterned Strips for Applications to Transfer Printing. J. Mech. Phys. Solids 2013, 61, 1737–1752. [Google Scholar]
- 39.Ryu S; Xiao J; Il Park W; Son K; Huang Y; Paik U; Rogers J, Lateral Buckling Mechanics in Silicon Nanowires on Elastomeric Substrates. Nano Lett 2009, 9, 3214–3219. [DOI] [PubMed] [Google Scholar]
- 40.Gang A; Gabernet G; Renner LD; Baraban L; Cuniberti G, A Simple Two-Step Silane-Based (bio-) Receptor Molecule Immobilization without Additional Binding Site Passivation. RSC Adv 2015, 5, 35631–35634. [Google Scholar]
- 41.Escorihuela J; Jose Banuls M; Garcia Castello J; Toccafondo V; Garcia-Ruperez J; Puchades R; Maquieira A, Chemical Silicon Surface Modification and Bioreceptor Attachment to Develop Competitive Integrated Photonic Biosensors. Anal. Bioanal. Chem 2012, 404, 2831–2840. [DOI] [PubMed] [Google Scholar]
- 42.Shah S; Chandra A; Kaur A; Sabnis N; Lacko A; Gryczynski Z; Fudala R; Gryczynski I, Fluorescence Properties of Doxorubicin in PBS Buffer and PVA Films. J. Photochem. Photobiol. B 2017, 170, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y; Kim BOO; Gao Y; Wie DS; Lee CH; Xu BX, Chemomechanics of Transfer Printing of Thin Films in a Liquid Environment. Int. J. Solids Struct 2019, 180, 30–44. [Google Scholar]
- 44.Fogarty JC; Aktulga HM; Grama AY; van Duin ACT; Pandit SA, A Reactive Molecular Dynamics Simulation of the Silica-Water Interface. J. Chem. Phy 2010, 132, 1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots in this paper and other finding of this study are available from the corresponding authors upon reasonable request.


