Abstract
Objectives
Loss of smell or taste are early symptoms of COVID-19. Given the high asymptomatic rate of COVID-19, as well as unreliable temperature checking and contact history taking, it is important to understand the role of olfactory and gustatory dysfunction (OGD) in the diagnosis of COVID-19. The aim of this study is to determine how initial symptoms of OGD can be used to screen patients for COVID-19 laboratory testing.
Methods
We followed recommendations from the Preferred Reporting Items for Systemic Reviews and Meta-analysis (PRISMA) statement to conduct this systematic review study. We used OGD-related key words to search for literature published between January 1, 2020 and September 30, 2020 on Pubmed, Ovid Medline databases. We estimated the prevalence of OGD and compared it with that of other OGD-related symptoms. The weighted summary proportion under the fixed and random effects model was assessed using MedCalc statistical software. Whenever there was heterogeneity, a random effects model was selected. Publication bias was assessed by funnel plot asymmetry and Egger's regression test.
Results
A total of 25 articles (evidence level III:5; IV:20) were identified and reviewed. Data synthesis of 19 articles revealed that the pooled prevalence of olfactory dysfunction in COVID-19 is 53.56% (range 5.6-100%, 95% CI 40.25-66.61%). The pooled prevalence of gustatory dysfunction in COVID-19 is 43.93% (range 1.5-85.18%, 95% CI 28.72-59.74%), just behind fever (62.22%, range 18.18-95.83%, 95% CI 54.82-69.33%), cough (64.74%, range 38.89-87.5%, 95% CI 57.97-71.22%), and fatigue (56.74%, range 6.25-93.62%, 95% CI 32.53-79.35%). The prevalence of gustatory dysfunction in subgroup with objective evaluation is lower than those without (9.91% vs. 49.21%, relive risk 2.82, p<0.001).
Conclusion
Our updated systematic review attests that OGD is an important early symptom of COVID-19 infection. Screening for OGD should be further emphasized to prioritize patients for laboratory test.
Keywords: COVID-19, Olfactory and gustatory dysfunction (OGD), Systematic review, Meta-analysis, Nasal symptoms
1. Background
Coronavirus disease 2019 (COVID-19) is an airway infectious disease that was first identified in December 2019. The single-stranded RNA (ssRNA) severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2) is believed to have evolved from a viral strain found in bats [1], [2], [3]. Since the first COVID-19 case was identified, the outbreak has spread globally through international travel. To date, the pandemic shows little sign of ease; globally, billion people have been infected, and mortality is still on the rise to cause tremendous impacts on public health, economy, and society.
Diagnosis of COVID-19 relies on the detection of nucleic acid or antigen from either sputum or saliva, via nasal or oral swabbing. The reverse transcription-polymerase chain reaction (RT-PCR) is the test of choice for COVID-19 [1]. Antibody testing can be conducted in conjunction with viral testing in some cases to identify previous COVID-19 infection. However, even though laboratory and point-of-care (POC) tests are readily available on the market, many countries with high prevalence have encountered challenges related to laboratory capacity exhaustion. A stratified or targeted screening strategy can aid in the early identification of high-risk patients, and also optimize laboratory capacity utilization.
The clinical courses of COVID-19 and SARS are similar in many ways [2], [3]; patients are contagious early on in the disease course, and early clinical symptoms include fever, cough, fatigue, and dyspnea, which are also seen in seasonal flus. However, one big difference seen in COVID-19 is the high number of asymptomatic and mild cases; these patients don't usually have high fevers, as seen in SARS, which makes screening and prevention difficult. Fortunately, studies have documented symptoms of COVID-19 manifesting in a predictable order. Influenza infection often begins with cough, then fever. In contrast, SARS, Middle East Respiratory Syndrome (MERS), and COVID-19 tend to start with fever, then progress to cough and muscle pain. COVID-19 may also present with nausea and vomiting before diarrhea [4]. Understanding the symptomatology of COVID-19 can help identify important early warning signs to prevent potential community transmission.
Since Mao et al. reported the neurological manifestations of 214 COVID-19 patients [5], [6], there has been increasing image-based evidence to support the neurological involvement of COVID-19. Olfactory and gustatory dysfunction (OGD) are atypical symptoms that have drawn attention during the COVID-19 pandemic. In Mao's study, 5.1% anosmia and 5.6% ageusia were reported [5]. There is speculation that the virus may attack the CNS through retrograde olfactory pathways. Imaging studies confirm a high percentage of patients with increased olfactory cleft width and volume, indicative of olfactory bulb degeneration that is predisposed to viral upper airway infection, similar to other postviral infection olfactory loss. [7], [8], [9]. In two systematic reviews, pooled data demonstrated that the prevalence of OGD among COVID-19 patients may be as high as 41.0~52.73% and 38.2~52.73%, respectively [10,11]. As such, the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) established the COVID-19 Anosmia Reporting Tool for Clinician [12]. The initial report identified 73% prevalence of anosmia in COVID-19 patients, with 26.6% of patients experiencing anosmia as the initial symptom [13].
It would seem that OGD are symptoms that have predictive value in the early identification of potential COVID-19 patients in the community. Given the rapidly accumulated evidences and heterogeneity of existing data, it is critical to consistently update our knowledge base. We hypothesize that, next to fever, OGD are predictive initial symptoms that can be used to refer patients for COVID-19 laboratory testing. In this study, we performed qualitative and quantitative analyses on collective data.
2. Materials and methods
2.1. Study design
We followed recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [14] to conduct this systematic review.
2.2. Search strategy
On PubMed and Ovid Medline databases, we entered the search string “anosmia or hyposmia or dysosmia or olfactory dysfunction or olfaction disorder or smell dysfunction or ageusia or hypogeusia or dysgeusia or taste dysfunction or gustatory dysfunction and COVID-19 or SARS-CoV-2” in either the ``title'' or ``abstract'' fields to search for literature published between January 1, 2020 and September 30, 2020. Literature searches [DW, YHC, PCW] were conducted every month until the end of September.
2.3. Study selection
We included peer-reviewed scientific papers reporting the prevalence of OGD in patients with confirmed COVID-19 infection. Studies needed to be available in the full-text version to be included. We also used PubMed's "related link" function to extend our search manually.
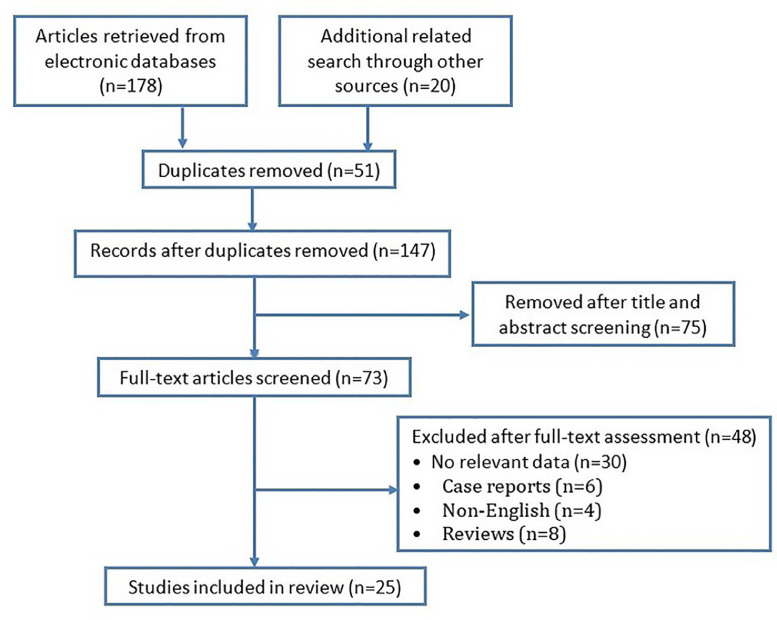
Only English publications related to human studies (i.e. no animal or laboratory studies) were included. We excluded publications in the categories ``letter to editor,'' ``opinion,'' ``editorial,'' ``case report,'' and ``systematic review.'' Search output duplicates were verified and excluded. The search flowchart is shown in Fig. 1 .
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of study selection process.
2.4. Quality evaluation
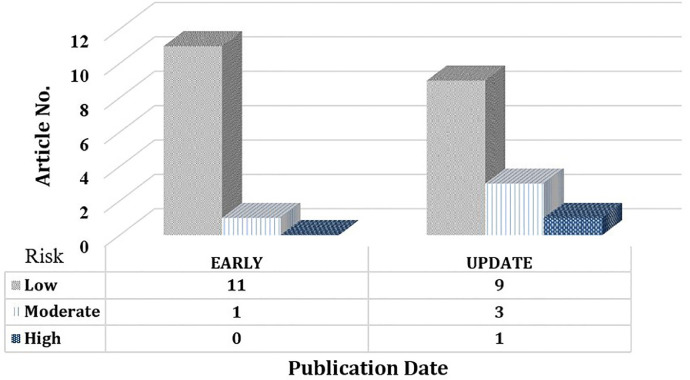
The queried publications’ quality was assessed (PCW, YHC) using the bias checklist developed by Hoy et al [15]. The checklist uses 9 items (representativeness of national population, representativeness of targeted population, use of randomization, non-response rate, data source, case definition, reliability and validity of measurement, mode of data collection, clarity of numerator and denominator) to evaluate study quality (risk of bias), and generates a total risk score ranging from low (0-3), moderate (4-6), to high (7-9). The distribution of quality scores of the EARLY (published before May 31, 2020) and UPDATE (published after June 1, 2020) groups are demonstrated and compared in Fig. 2 . We also compare the prevalence of OGD between EARLY and UPDATE groups (relative risk).
Fig. 2.
Quality (risk of bias) evaluation grouped by publication date.
2.5. Data extraction, synthesis, and meta-analysis
Three authors (DW, YHC, PCW) reviewed 25 studies and extracted data including publication specifics, patients' collective demographics (gender, age), disease characteristics (onset, duration, severity, recovery), and associated clinical information. Disagreements were solved through repetitive discussions. Studies that reported OGD both independently and with clear numerators/denominators were used for data synthesis.
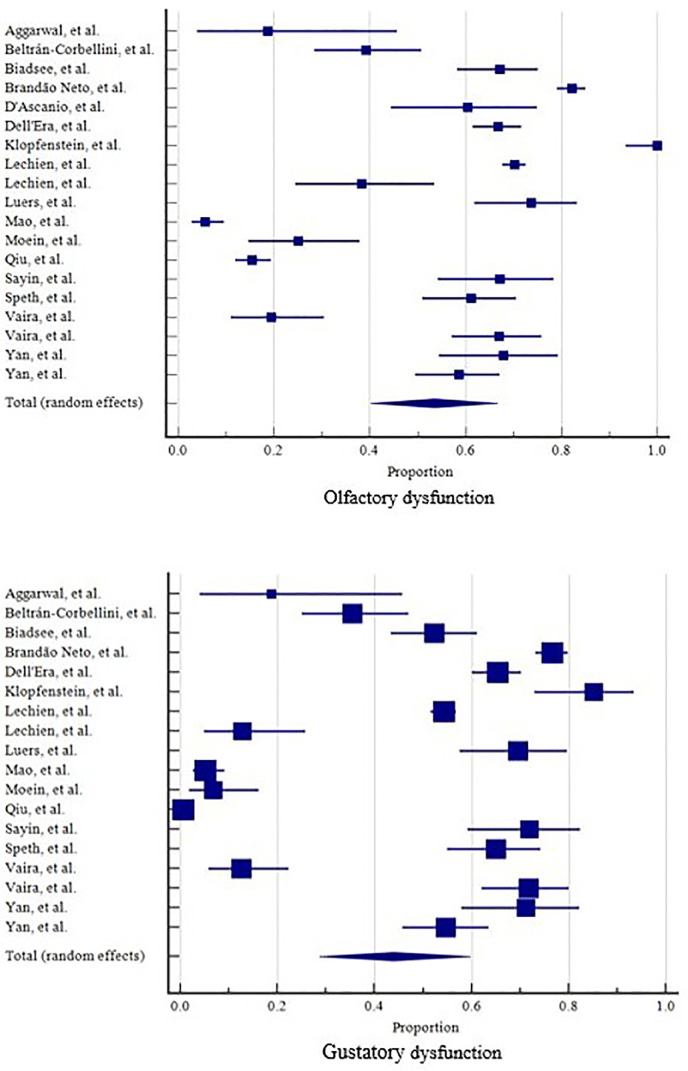
Statistical analysis was performed using MedCalc for Windows, version 19.4 (MedCalc Software, Ostend, Belgium). A forest tree plot (Fig. 3 ) was used to demonstrate the pooled-prevalence (with 95% confident interval) of OGD. We estimated the prevalence of OGD and compared it with that of other OGD-related symptoms, the weighted summary proportion under the fixed and random effects model was assessed. Publication bias was assessed by funnel plot asymmetry and Egger's regression test. Whenever there was heterogeneity, a random effects model was selected. Cochran's Q and I2 tests were used to evaluate the heterogeneity with the criteria P<0.1 or larger I2 values to show increasing heterogeneity [16]. We compare the prevalence of OGD between subgroups with and without objective smell/taste evaluation (relative risk).
Fig. 3.
Forest plots for olfactory dysfunction and gustatory dysfunction.
3. Results
3.1. Characteristics of studies
In total, we identified 198 papers using key words and an extended manual search; 25 papers were retained for full text evaluation and meta-analysis/data synthesis. The literature selection algorithm is shown in Fig. 1. All appropriate criteria, and a summary of study characteristics is detailed in Table 1 . Pooled, the studies included olfactory and gustatory information from 7,778 patients (average age 46.78, 56.9% female). The studies were conducted in Europe (16, 64%), North America (5, 20%), Asia-Other than China (3, 12%), and China (1, 4%). 12 (48%) studies were published prior to May 31, 2020 (EARLY group); while the remaining studies were published between June 1, 2020 and September 30, 2020 (UPDATE group).
Table 1.
Summary of Literature Bullet Points.
| Author/Publication date | Area | Study design/Level of evidence | No. of patients | Age, y/Female, No. | Olfactory % (95%CI) | Gustatory % (95%CI) | Other nasal symptoms, No. | Day of onset | Duration | Recovery | Objective smell test |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aggarwal, et al [50]/ 2020.05 | North America | Cross-sectional/ IV | 16 | Median:67/ 4 | 18.7(4.1-45.6) | 18.8(4.0-45.7) | - | - | - | - | No |
| Beltrán-Corbellini, et al. [30]/ 2020.04 | Europe | Case–control/ III | 79 | Mean: 61.6/ 31 | 39.2(28.4-50.8) | 35.4(25.0-47.0) | Nasal obstruction 4/31 | - | 7.1 | 12/31 | No |
| Biadsee, et al. [29]/ 2020.06 | Asia | Cross-sectional/ IV | 128 | Mean: 36.3/ 70 | 67.2(58.3-75.2) | 52.3(43.3-61.2) | Nasal obstruction: 29 Rhinorrhea: 34 |
4.6 | - | - | No |
| Brandão Neto, et al.[37] / 2020.05 | Europe | Cross-sectional/ IV | 655 | Mean: 37.7/ 424 | 82.3(79.1-85.1) | 76.6(73.2-79.8) | Nasal obstruction: 142 | - | - | 77/143 | No |
| *Boscolo-Rizzo, et al. [31] / 2020.05 | Europe | Cross-sectional/ IV | 54 | - | - | - | Nasal obstruction: 29 Rhinorrhea: 34 | - | - | - | No |
| *Chary, et al. [36]/ 2020.06 | Europe | Cross-sectional/ IV | 115 | Mean: 47/ 81 | - | - | - | 2 | - | 52 | No |
| D'Ascanio, et al. [35]/ 2020.07 | Europe | Case–control/ III | 43 | Mean: 58.1/ 14 | 60.5(44.4-75.0) | - | - | - | - | - | No |
| Dell'Era, et al. [43]/ 2020.07 | Europe | Cross-sectional/ IV | 355 | Median:50/ 163 | 66.7(61.6-71.6) | 65.4(60.1-70.3) | - | 23 | Olfactory :10 Gustatory :10 |
149 | No |
| *Haehner, et al. [23]/ 2020.06 | Europe | Cross-sectional/ IV | 22 | Mean: 38.6/ 12 | - | - | Rhinorrhea: 10 | 5.5 | - | - | No |
| *Kaye, et al. [13]/ 2020.04 | North America | Cross-sectional/ IV | 237 | Mean: 39.6/ 129 | - | - | Nasal obstruction: 60 Rhinorrhea: 42 | - | - | 64 | No |
| Klopfenstein, et al. [32]/ 2020.04 | Europe | Cross-sectional/ IV | 54 | Mean: 47/ 36 | 100(93.4-100) | 85.2(72.9-93.4) | Nasal obstruction: 16 Rhinorrhea: 31 Sneezing: 18 |
4.4 | Olfactory :8.9 |
7-13 days, 35% | No |
| Lechien, et al. [51] / 2020.06 | Europe | Cross-sectional/ IV | 1420 | Mean: 39.2/ 962 | 70.2(67.7-72.6) | 54.2(51.6-56.8) | Nasal obstruction: 963 Rhinorrhea: 854 Post nasal drip: 680 |
- | - | - | No |
| Lechien, et al. [52] / 2020.07 | Europe | Cross-sectional/ IV | 47 | Mean: 58.8/ 25 | 38.3(24.5-53.6) | 12.8(4.8-25.7) | Nasal obstruction: 30 Rhinorrhea: 33 Post nasal drip: 25 |
10.7 | - | - | Sniffin'stick tests |
| *Lee, et al. [39] / 2020.05 | Asia | Cross-sectional/ IV | 3191 | Mean: 44/ 2030 | - | - | - | - | Olfactory :7 Gustatory :6 |
recovered within 3 weeks | No |
| Luers, et al. [40]/ 2020.05 | Europe | Cross-sectional/ IV | 72 | Mean: 38 | 73.6(61.9-83.3) | 69.4(57.5-79.8) | Nasal obstruction: 39 Rhinorrhea: 38 Sneezing: 36 |
3.56 | Olfactory :3.56 Gustatory :3.38 |
- | No |
| Mao, et al. [5] / 2020.06 | China | Cross-sectional/ IV | 214 | Mean: 52.7/ 127 | 5.6(2.9-9.6) | 5.1(2.6-9.0) | - | 2 | - | - | No |
| Moein, et al. [45] / 2020.06 | North America | Case–control/ III | 60 | Mean: 46.5/ 20 | 25.0(14.7-37.8) | 6.7(1.8-16.2) | - | - | - | - | UPSIT |
| Qiu, et al. [46] / 2020.05 | Europe China |
Cross-sectional/ IV | 394 | Mean: 38.8/ 69 | 15.5(12.1-19.4) | 1.5(0.9-2.8) | - | - | - | 44/102 | No |
| Sayin, et al. [47] / 2020.06 | Asia | Case–control/ III | 64 | Mean: 37.8/ 39 | 67.2(54.3-78.4) | 71.9(59.2-82.4) | Nasal obstruction: 18 Rhinorrhea: 11 | - | - | 18 | No |
| Speth, et al. [48] / 2020.05 | Europe | Cross-sectional/ IV | 103 | Mean: 46.8/ 53 | 61.1(51.1-70.6) | 65.0(55.0-74.2) | Nasal obstruction: 51 Rhinorrhea: 36 | 12 | - | - | No |
| *Tostmann, et al. [17] / 2020.04 | Europe | Cross-sectional/ IV | 90 | Female: 71 | - | - | Rhinorrhea: 24 Sneezing: 36 |
- | - | - | No |
| Vaira, et al. [53] / 2020.04 | Europe | Cross-sectional/ IV | 72 | Mean: 49.2/ 45 | 19.4(11.1-30.5) | 12.5(5.9-22.4) | Nasal obstruction: 11 Rhinorrhea: 13 | 19.3 | - | - | No |
| Vaira, et al. [49] / 2020.08 | Europe | Cross-sectional/ IV | 106 | Mean: 49.6/ 53 | 66.9(57.2-75.8) | 71.7(62.1-80.0) | - | - | - | - | No |
| Yan, et al. [33] / 2020.06 | North America | Cross-sectional/ IV | 59 | Female: 29 | 67.8(54.4-79.3) | 71.2(57.9-82.2) | Nasal obstruction: 28 Rhinorrhea: 18 | - | - | 29/40 | No |
| Yan, et al. [34] / 2020.06 | North America | Case–control/ III | 128 | Mean: 45.1/ 67 | 58.6(49.6-67.2) | 54.7(45.7-63.5) | Nasal obstruction: 35 Rhinorrhea: 17 | 5.19 | - | - | No |
UPSIT : University of Pennsylvania Smell Identification Test *:studies show mixed OGD complaints are used for qualitative review only.
3.2. Quality of studies
In total, 20 studies were of level IV evidence, and 5 studies were of level III evidence (retrospective, case control study). The quality scores ranged from 1 to 7, with a mean score of 2.88±1.26. The risks of bias were either low (20, 80%), moderate (4, 16%), or high (1, 4%). Most of the studies lacked randomized design and had selection bias (25, 100%). In addition, most studies did not have validated criteria for testing and quantifying smell or taste (23, 92%).
Overall, the risk of bias remained notable over time (EARLY vs. UPDATE groups, 2.33±0.88 vs. 3.39±1.39, t-test, P=0.038). The UPDATE group has higher numbers of studies with moderate to high risk of research bias. The distributions of risk scores are shown in Fig 2. The prevalence of OGD in EARLY groups evaluation are 53.91% (range 15.48-100%, 95% CI 26.27-80.29%), 43.68% (range 1.78-85.18%, 95% CI 13.98-76.13%) for olfactory and gustatory dysfunctions, respectively. The prevalence of OGD in UPDATE groups evaluation are 53.21% (range 5.61-67.18%, 95% CI 37.92-68.18%), 44.71% (range 5.14-71.88%, 95% CI 29.37-60.58%) for olfactory and gustatory dysfunctions, respectively. Compare to the UPDATE group, EARLY group had higher risk having olfactory dysfunction (RR=3.59, 95% CI=3.36~3.86, P<0.001) and gustatory dysfunction (RR=3.38, 95% CI=3.12~3.65, P<0.001), respectively.
3.3. Clinical features
The pooled prevalence of COVID-19 symptoms (other than OGD) in the studies were: fever (62.22%, range 18.18-95.83%, 95% CI 54.82-69.33%), cough (64.74%, range 38.89-87.5%, 95% CI 57.97-71.22%), fatigue (56.74%, range 6.25-93.62%, 95% CI 32.53-79.35%), headache (48.28%, range 21.83-81.48%, 95% CI 35.77-60.89%), dyspnea (42.39%, range 10.16-87.23%, 95% CI 32.29-52.83%), and sore throat (39.73%, range 12.5-62.5%, 95% CI 31.41-48.36%).
3.4. Prevalence of olfactory and gustatory dysfunction
Of the 25 selected studies, 19 reported olfactory dysfunction independently, and 18 reported gustatory dysfunction independently. The pooled prevalence of olfactory dysfunction was 53.56% (range 5.6-100%, 95% CI 40.25-66.61%). The pooled prevalence of gustatory dysfunction was 43.93% (range 1.5-85.18%, 95% CI 28.72-59.74%). (Fig. 3) The prevalence of OGD in “with objective smell/taste evaluation” are 31.43% (range 25-38.29%, 95% CI 19.44-44.82%), 9.91% (range 6.67-12.77%, 95% CI 4.79-16.59%) for olfactory and gustatory dysfunctions, respectively. The prevalence of OGD in “without objective smell/taste evaluation” are 56.12% (range 5.61-100%, 95% CI 42.02-69.73%), 49.21% (range 1.78-85.18%, 95% CI 33.56-64.94%) for olfactory and gustatory dysfunctions, respectively. Relative risk for “with and without objective smell/taste evaluation” are 1.01 (95% CI 0.76-1.35, P=0.910), 2.82 (95% CI 1.56-5.10, P<0.001) for olfactory and gustatory dysfunctions, respectively.
3.5. Reliability and validity of measurement
Of the 19 studies (76%) reporting prevalence of olfactory and gustatory dysfunction independently, 17 (68%) relied on self-reporting via telephones, computers, or mobile devices; 6 (24%) used validated questionnaires; 2 (8%) used chemo-sensory strips.
3.6. Associated nasal symptoms
Associated nasal symptoms were reported in 16 studies (64%). The studies included the prevalence of nasal congestion (38.36%, 95% CI 25.16-52.49%), rhinorrhea (37.14%, 95% CI 25.38-49.71%), and sneezing (41.51%, 95% CI 32.79-50.51%).
3.7. Onset and recovery of OGD
The reported onset of OGD ranged from 2 to 23 days, with a median of 5.34 days. The mean recovery time was 7.21±12.93 days. The mean follow up period was 29.29±12.96 days, with a median of 14.5 days. At the end of the follow up period, 41.74±10.14% (range 27%~53.8%) patients had complete recovery from OGD.
3.8. Associated risk factors
Associated risk factors of OGD were reported in 11 studies (44%). They reported the prevalence of smoking (10.49%, 95% CI 6.4-15.45%), asthma (7.51%, 95% CI 4.43-11.32%), chronic obstructive pulmonary disease (3.86%, 95% CI 1.09-8.22%), and allergic rhinitis (16.29%, 95% CI 11.03-22.35%).
3.9. Heterogeneity of the studies
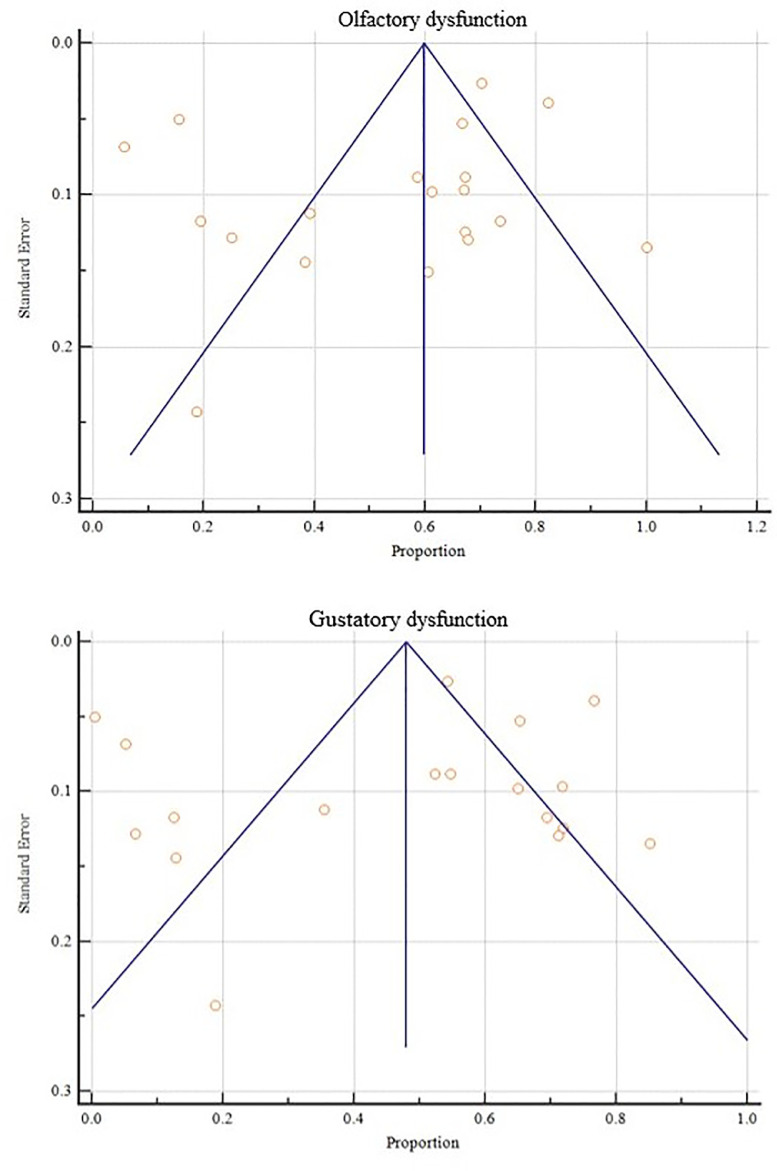
Heterogeneity tests for OGD indicated that there was high heterogeneity in both olfactory and gustatory dysfunctions, with I2 being 98.46% (98.14% ~98.73%) and 98.91% (98.69% to 99.08%), respectively. Publication bias tests (Egger's test) had intercepts of -3.6 (-10.8 ~ 3.6, P> 0.05) and -2.3 (-11.5 ~ 6.8, P> 0.05), respectively. (Fig. 4 )
Fig. 4.
Funnel plots for olfactory dysfunction and gustatory dysfunction.
4. Discussion
Since its outbreak in Wuhan, China in late 2019, the COVID-19 pandemic has been worse in severity and duration compared to SARS. Early detection of community infection is the key to containing the disease, and many fast RT-PCR test kits have been developed during the pandemic. The biochemical test is crucial in establishing diagnosis. However, test capacity has become a key issue in successful disease containment. To allocate test resources more efficiently, some have advocated for prioritizing testing based on symptomatology. For example, Larsen et al., using a Stochastic Progression Model to discern respiratory infection symptoms, suggested that early fever and late diarrhea could be used to distinguish COVID-19 from influenza, MERS, and SARS [4]. Tostmann et al. reported acceptable predictability (sensitivity 91.2%, specificity 55.6%) of symptoms for targeted COVID-19 screening [17].
Smell and taste dysfunctions can result from a variety of peripheral or systemic conditions such as nasal allergy, sinus infection, head injury, toxic exposure, neurological disorders, or certain medications. Post-viral olfactory dysfunction (PVOD) is considered one of the most common causes of anosmia. This is particularly true for COVID-19; since reported by Mao et al. [5], many studies have suggested that OGD symptoms are more prominent in COVID-19 than in other non-SARS-CoV2 coronavirus infections [18]. Several systematic reviews have reported high prevalence of olfactory (41~74.9%) and gustatory (38.2~81.3%) dysfunctions that postulate the importance of these symptoms [10,11,18,19]. In Giorli et al.’s report, the prevalence of anosmia is third only to fever (rate difference -0.316) and cough (-0.249) in non-hospitalized patients [20].
As a result, new onset OGD has been recommended as a screening tool by the American Academy of Otolaryngology - Head and Neck Surgery (AAO-HNS) [12], the British Association of Otorhinolaryngology [21], and the Centers for Disease Control and Prevention (CDC) [22]. OGD as a predictor of COVID-19 has 97% specificity, 65% sensitivity, 63% positive predictive value, and 97% negative predictive value, as reported by Haehner A et al. [23] With the rapid accumulation of studies investigating COVID-19 symptoms, particularly the unique manifestation of OGD, we felt the need to update our understanding of this matter.
Because the olfactory bulb is considered to be an immunologic barrier to viral infection, understanding the pathophysiology of COVID-19-induced anosmia is important. Emerging evidence indicates that SARS-COV-2 leads to central nervous system complications when it infiltrates the olfactory neuroepithelium [24] and blood brain barrier [25,26]. Angiotensin converting enzyme 2 (ACE2) is considered to be the primary receptor for SARS-COV-2 virus. Studies have shown that the olfactory neuroepithelium has elevated ACE2 expression, which suggests that SARS-COV-2 replication enters the central nervous system and induces anosmia via this route [27,28]. SARS-CoV-2 particles were found in the olfactory bulb of a COVID-19 patient with diffuse CD163-positive macrophage and cytotoxic T lymphocyte infiltration. CD163 is a marker of cytokine storm-induced macrophage activation [24]. More neuroepithelium biopsy specimens are required to prove this hypothesis, however, with the low prevalence of flu-like nasal symptoms (such as nasal obstruction, rhinorrhea, sneezing, etc.) shown in most studies [23,[29], [30], [31], [32], it is generally agreed that COVID-19-related OGD is sensorineural rather than conductive, though most studies were not able to verify patients' prior rhinology history.
OGD is reported more in ambulatory COVID-19 patients (mild cases), however, due to the wide range of admission criteria and the difficulty in surveying intubated patients (severe cases), the reliability of this characteristic is questionable [5,31,[33], [34], [35]. Higher OGD prevalence is observed in female and younger patients [29,30,36,37]. This sex and age difference may be attributed to sex and age-related inflammatory and hormonal responses that reflect the lifetime plasticity of OGD [36,38]. Regarding time course, whether patients can fully recover from OGD after contracting COVID-19 is a point of interest. Most studies reported acute onset and rapid recovery of OGD [39,40]. Samaranayake reported that 64.5% of olfactory and 54.0% of gustatory symptoms manifested prior to general COVID-19 symptoms [19]. Levinsen reported a recovery rate of 73.3% [41], which is encouraging. Most studies reported that OGD had significant improvement around 1-2 weeks after disease onset [30,42,43]. A 15-day recovery rate of 64% was reported by Chary et al [36], while 30-day electronic survey and quality-of-life evaluation studies reported >60% and >80% total recovery of OGD [35,44]. Since the time course might be prognostic, it is important to understand the severity grading and functional deficit of OGD, which were mentioned in only a few of the studies we reviewed [45], [46], [47], [48], [49].
Prior systematic reviews reported a wide range of OGD prevalence due to the variety of study designs, methodology, sample size, geographic location, and olfactory test validity. Owing to the nature of rapid publication, the studies were of poor quality; only 5 studies were based on controlled study designs. Aside from gender and age, limited patient demographic or clinical information were included. In addition, most of the studies failed to control for or verify other underlying causes of smell and taste dysfunction during the pandemic. The risk of bias are continuously seen in both EARLY and UPDATE publication groups. And, EARLY group had higher risks of olfactory and gustatory dysfunctions than UPDATE group. The validity of the smell test is another critical issue; few studies [5,52] used an objective chemosensory test. Our investigation shows that studies with objective smell/taste evaluation tended to have lower OGD prevalence (even though the difference for olfactory function is not statistically significant), this attests the need to obtain reliable OGD information from objective chemosensory test. In general, the extrapolation of this study is limited by the information quality we can extract from the current OGD-related COVID-19 literatures.
5. Conclusion
Our systematic review attests the importance of olfactory and gustatory dysfunctions as early symptoms of COVID-19. The predictability of OGD is as high as 53.56 and 43.93%, just behind fever, cough, and fatigue. Screening for OGD should be further emphasized so patient RT-PCR testing can be prioritized. Positive OGD can serve as a fast, sensitive, and reliable clinical alert to initiate quarantine for COVID-19 patients. Further understanding of COVID-19-related OGD's pathophysiology may provide more insight into its diagnostic and prognostic value.
Funding
None.
Declaration of Competing Interest
The Author(s) declare(s) that there is no conflict of interest.
Acknowledgement
This database study is waived from IRB approval.
References
- 1.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seladi-Schulman J. Coronavirus vs. SARS: How Do They Differ? Healthline Web sites. Available at: https://www.healthline.com/health/coronavirus-vs-sars. Accessed September 6, 2020.
- 3.Caldaria A., Conforti C., Di Meo N., Dianzani C., Jafferany M., Lotti T., et al. COVID-19 and SARS: differences and similarities. Dermatol Ther. 2020;33(4):e13395. doi: 10.1111/dth.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen J.R., Martin M.R., Martin J.D., Kuhn P., Hicks J.B. Modeling the Onset of Symptoms of COVID-19. Front Public Health. 2020;8:473. doi: 10.3389/fpubh.2020.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altundag A., Yıldırım D., Tekcan Sanli D.E., Cayonu M., Kandemirli S.G., Sanli A.N., et al. Olfactory Cleft Measurements and COVID-19-Related Anosmia. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820965920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad Radiol. 2021;28(1):28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altundag A., Temirbekov D., Haci C., Yildirim D., Cayonu M. Olfactory Cleft Width and Volume: Possible Risk Factors for Postinfectious Olfactory Dysfunction. Laryngoscope. 2021;131(1):5–9. doi: 10.1002/lary.28524. [DOI] [PubMed] [Google Scholar]
- 10.Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., Ofori-Asenso R. Smell and Taste Dysfunction in Patients with COVID-19: a Systematic Review and Meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: a Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2020;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Otolaryngology–Head and Neck Surgery. COVID-19 anosmia reporting tool for clinicians. Available at: https://www.entnet.org/content/reporting-tool-patients-anosmiarelated-covid-19. Accessed April 6, 2020. [DOI] [PubMed]
- 13.Kaye R., Chang C.W.D., Kazahaya K., Brereton J., Denneny J.C., 3rd COVID-19 Anosmia Reporting Tool: initial Findings. Otolaryngol Head Neck Surg. 2020;163(1):132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ. 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tostmann A., Bradley J., Bousema T., Yiek W.K., Holwerda M., Bleeker-Rovers C., et al. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrich B.M., Goshtasbi K., Raad R.A., Ganti A., Papagiannopoulos P., Tajudeen B.A., et al. Aggregate Prevalence of Chemosensory and Sinonasal Dysfunction in SARS-CoV-2 and Related Coronaviruses. Otolaryngol Head Neck Surg. 2020;163(1):156–161. doi: 10.1177/0194599820929278. [DOI] [PubMed] [Google Scholar]
- 19.Samaranayake L.P., Fakhruddin K.S., Panduwawala C. Sudden onset, acute loss of taste and smell in coronavirus disease 2019 (COVID-19): a systematic review. Acta Odontol Scand. 2020;78(6):467–473. doi: 10.1080/00016357.2020.1787505. [DOI] [PubMed] [Google Scholar]
- 20.Giorli A., Ferretti F., Biagini C., Salerni L., Bindi I., Dasgupta S., et al. A Literature Systematic Review with Meta-Analysis of Symptoms Prevalence in Covid-19: the Relevance of Olfactory Symptoms in Infection Not Requiring Hospitalization. Curr Treat Options Neurol. 2020;22(10):36. doi: 10.1007/s11940-020-00641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ENTUK . 2020. Loss of sense of smell as marker of COVID-19 infection.https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf Available at: Accessed July 30, 2020. [Google Scholar]
- 22.CDC's Diagnostic Test for COVID-19 Only and Supplies. Centers for disease control and prevention. centers for disease control and prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html. Accessed September 6, 2020.
- 23.Haehner A., Draf J., Dräger S., de With K., Hummel T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. ORL J Otorhinolaryngol Relat Spec. 2020;82(4):175–180. doi: 10.1159/000509143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morbini P., Benazzo M., Verga L., Pagella F.G., Mojoli F., Bruno R., et al. Ultrastructural Evidence of Direct Viral Damage to the Olfactory Complex in Patients Testing Positive for SARS-CoV-2. JAMA Otolaryngol Head Neck Surg. 2020;146(10):972–973. doi: 10.1001/jamaoto.2020.2366. [DOI] [PubMed] [Google Scholar]
- 25.Das G., Mukherjee N., Ghosh S. Neurological Insights of COVID-19 Pandemic. ACS Chem Neurosci. 2020;11(9):1206–1209. doi: 10.1021/acschemneuro.0c00201. [DOI] [PubMed] [Google Scholar]
- 26.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 27.Cascella M. Features, evaluation, and treatment of coronavirus (COVID-19). U.S. National Library of Medicine Web sites. Available at: https://www.ncbi.nlm.nih.gov/books/NBK554776/. Accessed September 6, 2020.
- 28.Butowt R., Bilinska K. SARS-CoV-2: olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem Neurosci. 2020;11(9):1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 30.Biadsee A., Biadsee A., Kassem F., Dagan O., Masarwa S., Ormianer Z. Olfactory and Oral Manifestations of COVID-19: sex-Related Symptoms-A Potential Pathway to Early Diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722–728. doi: 10.1177/0194599820934380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltrán-Corbellini Á., Chico-García J.L., Martínez-Poles J., Rodríguez-Jorge F., Natera-Villalba E., Gómez-Corral J., et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020;27(9):1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boscolo-Rizzo P., Borsetto D., Spinato G., Fabbris C., Menegaldo A., Gaudioso P., et al. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Otorhinolaryngol. 2020;277(9):2637–2640. doi: 10.1007/s00405-020-06066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.Y., Lepiller Q., Gendrin V., et al. Features of anosmia in COVID-19. Med Mal Infect. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., DeConde A.S. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020;10(7):821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Ascanio L., Pandolfini M., Cingolani C., Latini G., Gradoni P., Capalbo M., et al. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol Head Neck Surg. 2021;164(1):82–86. doi: 10.1177/0194599820943530. [DOI] [PubMed] [Google Scholar]
- 36.Chary E., Carsuzaa F., Trijolet J.P., Capitaine A.L., Roncato-Saberan M., Fouet K., et al. Prevalence and Recovery From Olfactory and Gustatory Dysfunctions in Covid-19 Infection: a Prospective Multicenter Study. Am J Rhinol Allergy. 2020;34(5):686–693. doi: 10.1177/1945892420930954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandão Neto D., Fornazieri M.A., Dib C., Di Francesco R.C., Doty R.L., Voegels R.L., et al. Chemosensory Dysfunction in COVID-19: Prevalences, Recovery Rates, and Clinical Associations on a Large Brazilian Sample. Otolaryngol Head Neck Surg. 2021;164(3):512–518. doi: 10.1177/0194599820954825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gori A., Leone F., Loffredo L., Cinicola B.L., Brindisi G., De Castro G., et al. COVID-19-Related Anosmia: the Olfactory Pathway Hypothesis and Early Intervention. Front Neurol. 2020;11:956. doi: 10.3389/fneur.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y., Min P., Lee S., Kim S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. 2020;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luers J.C., Rokohl A.C., Loreck N., Wawer Matos P.A., Augustin M., Dewald F., et al. Olfactory and Gustatory Dysfunction in Coronavirus Disease 19 (COVID-19) Clin Infect Dis. 2020;71(16):2262–2264. doi: 10.1093/cid/ciaa525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levinson R., Elbaz M., Ben-Ami R., Shasha D., Levinson T., Choshen G., et al. Time course of anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. Infect Dis (Lond) 2020;52(8):600–602. doi: 10.1080/23744235.2020.1772992. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49(1):26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dell'Era V., Farri F., Garzaro G., Gatto M., Aluffi Valletti P., Garzaro M. Smell and taste disorders during COVID-19 outbreak: Cross-sectional study on 355 patients. Head Neck. 2020;42(7):1591–1596. doi: 10.1002/hed.26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Locatello L.G., Maggiore G., Bruno C., Trotta M., Gallo O. An Integrated Care Strategy for the Follow-up of Patients With COVID-19-Associated Chemosensory Dysfunction. Otolaryngol Head Neck Surg. 2021;164(2):294–296. doi: 10.1177/0194599820950716. [DOI] [PubMed] [Google Scholar]
- 45.Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu C., Cui C., Hautefort C., Haehner A., Zhao J., Yao Q., et al. Olfactory and Gustatory Dysfunction as An Early Identifier of COVID-19 in Adults and Children: An International Multicenter Study. Otolaryngol Head Neck Surg. 2020;163(4):714–721. doi: 10.1177/0194599820934376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayin İ., Yaşar K.K., Yazici Z.M. Taste and Smell Impairment in COVID-19: An AAO-HNS Anosmia Reporting Tool-Based Comparative Study. Otolaryngol Head Neck Surg. 2020;163(3):473–479. doi: 10.1177/0194599820931820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speth M.M., Singer-Cornelius T., Oberle M., Gengler I., Brockmeier S.J., Sedaghat A.R. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol Head Neck Surg. 2020;163(1):114–120. doi: 10.1177/0194599820929185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Soma D., Giovanditto F., et al. Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J Otolaryngol Head Neck Surg. 2020;49(1):56. doi: 10.1186/s40463-020-00449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl) 2020;7(2):91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 51.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., et al. Clinical and Epidemiological Characteristics of 1,420 European Patients with mild-to-moderate Coronavirus Disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lechien J.R., Ducarme M., Place S., Chiesa-Estomba C.M., Khalife M., De Riu G., et al. Objective Olfactory Findings in Hospitalized Severe COVID-19 Patients. Pathogens. 2020;9(8):627. doi: 10.3390/pathogens9080627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaira L.A., Deiana G., Fois A.G., Pirina P., Madeddu G., De Vito A., et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 2020;42(6):1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]