Abstract
Background
Several studies have reported that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can directly infect endothelial cells, and endothelial dysfunction is often found in severe cases of coronavirus disease 2019 (COVID-19). To better understand the prognostic values of endothelial dysfunction in COVID-19-associated coagulopathy, we conducted a systematic review and meta-analysis to assess biomarkers of endothelial cells in patients with COVID-19.
Methods
A literature search was conducted on online databases for observational studies evaluating biomarkers of endothelial dysfunction and composite poor outcomes in COVID-19 patients.
Results
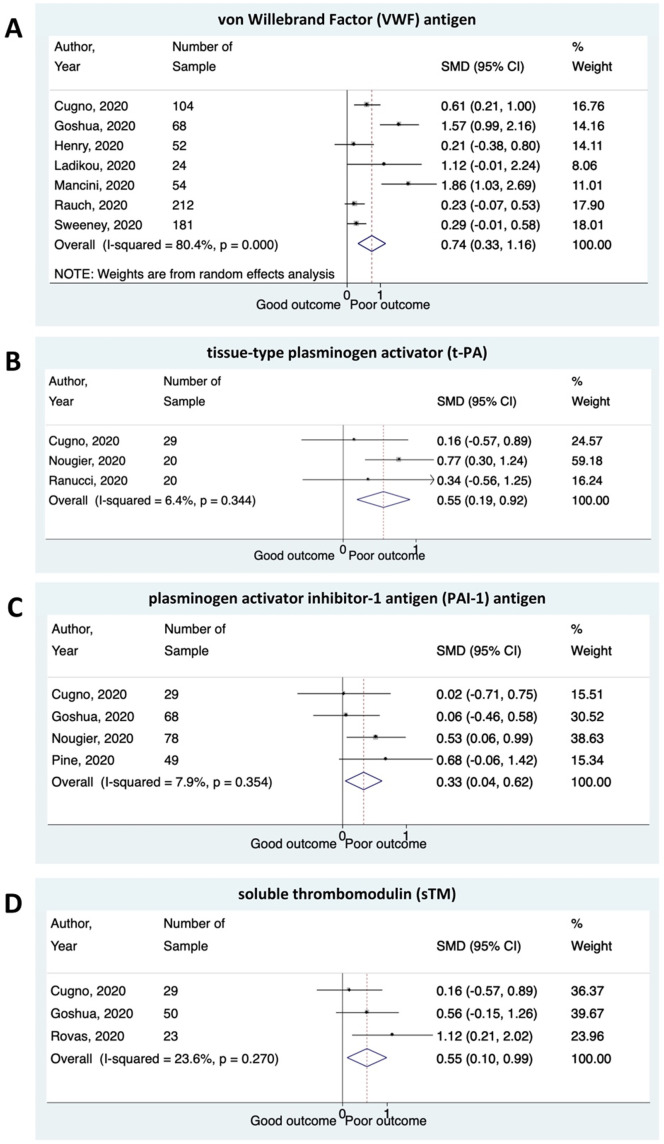
A total of 1187 patients from 17 studies were included in this analysis. The estimated pooled means for von Willebrand Factor (VWF) antigen levels in COVID-19 patients was higher compared to healthy control (306.42 [95% confidence interval (CI) 291.37–321.48], p < 0.001; I2:86%), with the highest VWF antigen levels was found in deceased COVID-19 patients (448.57 [95% CI 407.20–489.93], p < 0.001; I2:0%). Meta-analysis showed that higher plasma levels of VWF antigen, tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 antigen (PAI-1) antigen, and soluble thrombomodulin (sTM) were associated with composite poor outcome in COVID-19 patients ([standardized mean difference (SMD) 0.74 [0.33–1.16], p < 0.001; I2:80.4%], [SMD 0.55 [0.19–0.92], p = 0.003; I2:6.4%], [SMD 0.33 [0.04–0.62], p = 0.025; I2:7.9%], and [SMD 0.55 [0.10–0.99], p = 0.015; I2:23.6%], respectively).
Conclusion
The estimated pooled means show increased levels of VWF antigen in COVID-19 patients. Several biomarkers of endothelial dysfunction, including VFW antigen, t-PA, PAI-1, and sTM, are significantly associated with increased composite poor outcomes in patients with COVID-19.
PROSPERO registration number
CRD42021228821
Keywords: Endothelial dysfunction, von Willebrand Factor, Tissue-type plasminogen activator, Plasminogen activator inhibitor-1, Thrombomodulin, COVID-19
1. Introduction
Although initially recognized as a disease affecting the respiratory system, coronavirus disease 2019 (COVID-19) often manifests as cardiovascular complications such as myocarditis, myocardial injuries, arrhythmias, and venous thromboembolism events (VTE) (Evans et al., 2020). There are several possible mechanisms for these phenomena, one of which may be an unrestrained and unbalanced innate immune response, which in turn negates effective adaptive immunity, supporting the progression of COVID-19. Frequent laboratory abnormalities in patients with unfavorable progression of COVID-19, including abnormal cytokine profiles, led to the initial presumption that the immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection involved a cytokine storm (Perico et al., 2020). However, recent evidence suggests that increased inflammatory cytokines including interleukin-6 (IL-6) in patients with severe and critical COVID-19 are significantly lower compared to patients with sepsis and acute respiratory distress syndrome (ARDS) not associated with COVID-19, thus doubting the role of a cytokine storm in COVID-19-related multiple organ damage (Leisman et al., 2020).
Several studies have reported that the SARS-CoV-2 can directly infect endothelial cells, and endothelial dysfunction is often found in severe cases of COVID-19 (Nägele et al., 2020). Autopsy findings have also demonstrated endothelial dysfunction and microvascular thrombosis together with pulmonary embolism (PE) and deep-vein thrombosis in COVID-19 patients (Gavriilaki et al., 2020). These findings suggest that endothelial injury, endotheliitis, and microcirculatory dysfunction in different vascular beds contribute significantly to life-threatening complications of COVID-19, such as VTE and multiple organ involvement (Huertas et al., 2020). To better understand the prognostic values of endothelial dysfunction in COVID-19-associated coagulopathy, we conducted a systematic review and meta-analysis to assess biomarkers of endothelial cells in patients with COVID-19.
2. Methods
2.1. Search strategy and study selection
A systematic literature search of PubMed, PMC Europe, and the Cochrane Library Database from 1 January 2020 to 20 December 2020 was performed using the search strategy outlined in Supplementary Table S1. Additional records were retrieved from Google Scholar. Duplicate articles were removed after the initial search. Two authors (MJA and YA) independently screened the title and abstract of the articles. Articles that passed the screening were assessed in full text based on the eligibility criteria. Disagreements were resolved by discussion with the senior author (A). This study was conducted following the Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA) statement and registered with the International Prospective Register of Systematic Reviews (PROSPERO) database (registration number CRD42021228821).
2.2. Eligibility criteria
We included all observational studies examining biomarkers of endothelial dysfunction and outcomes from patients who tested positive for SARS-CoV-2 using the reverse transcription-polymerase chain reaction (RT-PCR) test. The following types of articles were excluded from the analysis: case reports, review articles, non-English language articles, research articles on the pediatric population, animal or in-vitro studies, unpublished studies, and studies with irrelevant or non-extractable results.
2.3. Data collection process
Data extraction was carried out by two authors (MJA and YA) independently using piloted data extraction forms consisting of the author, year of publication, study design, number and characteristics of samples, levels of several biomarkers of endothelial dysfunction, and patient outcomes. The biomarkers of endothelial dysfunction analyzed in this study were von Willebrand Factor (VWF) antigen, tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 antigen (PAI-1), and soluble thrombomodulin (sTM). The primary endpoint was composite poor outcomes consisting of ICU admission, severe illness, worsening of the respiratory status, the need for mechanical ventilation, and mortality. Moreover, if the included studies reported the data using median and quartile values, we used the formula developed by Wan et al. to estimate mean and standard deviation (Wan et al., 2014). Disease severity was defined based on the WHO R&D Blueprint on COVID-19 (World Health Organization, 2020).
2.4. Quality assessment
The quality and risk of bias assessment of included studies were conducted using the Newcastle-Ottawa score (NOS) (Wells et al., 2015) by all authors independently, and discrepancies were resolved through discussion. This scoring system consists of three domains: the selection of sample cohorts, comparability of cohorts, and assessment of outcomes (Supplementary Table S2).
2.5. Data analysis
Stata software V.14.0 (College Station) was used for meta-analysis, and figure of estimated pooled means were produced using GraphPad Prism 9. We pooled multiple means and standard errors of the same population characteristic from different studies into a single group using the fixed-effect model of the meta-analysis algorithm. Pooled effect estimates of the outcomes were reported as standardized means differences (SMD). Fixed-effects and random-effects models were used for pooled analysis with low heterogeneity (I2 statistic <50% or p-value <0.1) and high heterogeneity (I2 statistic>50% or p-value ≤0.1), respectively. Statistical significance was determined with p-value <0.05. We performed a sensitivity analysis to test the robustness of the pooled effect estimates for VWF antigen levels by excluding each study sequentially and rerunning the meta-analysis. The funnel-plot analysis was used to assess the symmetrical distribution of effect sizes, and the regression-based Egger test was performed to assess publication bias on continuous endpoints.
3. Results
3.1. Study characteristics
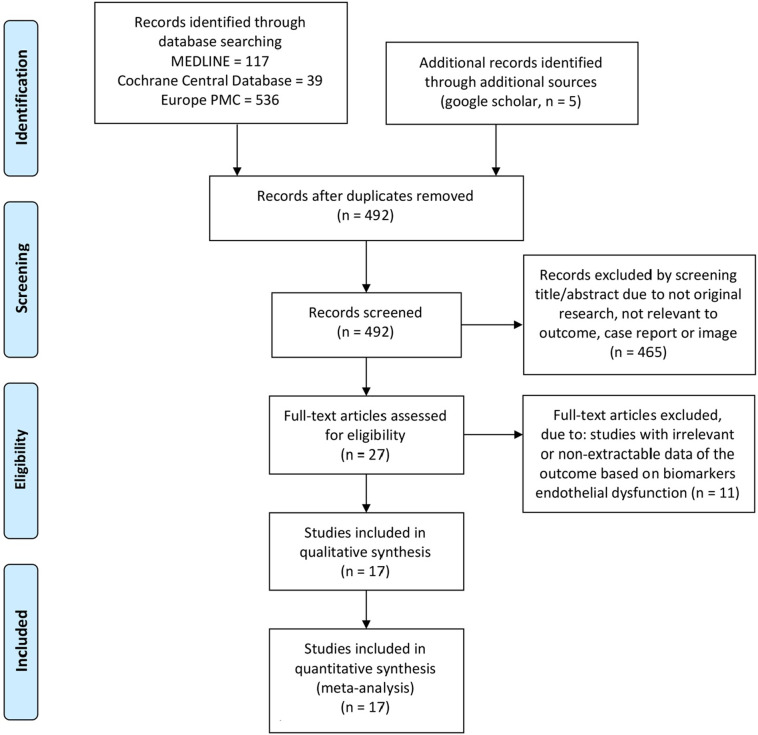
We identified 697 articles from the database search, and 492 articles remained after the duplication was removed. Screening on titles and abstracts excluded 465 articles, and the remaining 27 full-text articles were assessed according to eligibility criteria. As a result, 17 studies (Cugno et al., 2020; Fan et al., 2020; Goshua et al., 2020; Helms et al., 2020; Henry et al., 2020; Hoechter et al., 2020; Ladikou et al., 2020; Mancini et al., 2020; Morici et al., 2020; Nougier et al., 2020; Panigada et al., 2020; Pine et al., 2020; Ranucci et al., 2020; Rauch et al., 2020; Rovas et al., 2020; Sweeney et al., 2020; Taus et al., 2020) with a total of 1187 patients were subjected to qualitative analysis and meta-analysis (Fig. 1 and Table 1 ). Quality assessment with NOS showed that 13 studies (Cugno et al., 2020; Goshua et al., 2020; Henry et al., 2020; Hoechter et al., 2020; Ladikou et al., 2020; Mancini et al., 2020; Nougier et al., 2020; Pine et al., 2020; Ranucci et al., 2020; Rauch et al., 2020; Rovas et al., 2020; Sweeney et al., 2020; Taus et al., 2020) were of good quality with ≥7 NOS score and four studies (Fan et al., 2020; Helms et al., 2020; Morici et al., 2020; Panigada et al., 2020) were considered as moderate quality with six NOS score (Supplementary Table S2).
Fig. 1.
PRISMA flowchart.
Table 1.
Characteristics of the included studies.
| No | Study | Population | Study location | No. of samples | Age mean (SD) |
Male n (%) |
DM n (%) |
HT n (%) |
Hyper-lipidemia n (%) |
Comparison/end point measure | Marker examined | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cugno et al. (2020) | All hospitalized patients | Italy | 148 | 61 (13) | 87 (59) | NA | NA | NA | ICU admission | VWF antigen, sTM, PAI-1 antigen, t-PA | 8 |
| 2 | Fan et al. (2020) | ICU patients | Singapore | 12 | 51 (17) | 11 (92) | 5 (42) | 6 (50) | 4 (33) | NA | VWF antigen | 6 |
| 3 | Goshua et al. (2020) | All hospitalized patients | United States | 68 | 62 (16) | 41 (60) | 20 (29) | 38 (56) | 18 (26) | ICU admission | VWF antigen, sTM, PAI-1 antigen | 9 |
| 4 | Helms et al. (2020) | ICU patients | France | 150 | 62 (14) | 122 (81) | 30 (20) | NA | NA | NA | VWF antigen | 6 |
| 5 | Henry et al. (2020) | All hospitalized patients | United States | 52 | 52 (21) | 30 (58) | 21 (40) | 26 (50) | 15 (29) | Severe COVID-19 | VWF antigen | 9 |
| 6 | Hoechter et al. (2020) | ICU patients | Germany | 22 | 62 (14) | 19 (86) | NA | NA | NA | NA | VWF antigen | 7 |
| 7 | Ladikou et al. (2020) | ICU patients | United Kingdom | 24 | 64 (13) | 18 (75) | NA | NA | NA | ICU admission | VWF antigen | 7 |
| 8 | Mancini et al. (2020) | All hospitalized patients | Italy | 50 | 58 (13) | 32 (64) | 4 (8) | 14 (28) | NA | ICU admission | VWF antigen | 7 |
| 9 | Morici et al. (2020) | ICU patients | Italy | 6 | 62 (5) | 4 (67) | 1 (17) | 2 (33) | NA | NA | VWF antigen | 6 |
| 10 | Nougier et al. (2020) | All hospitalized patients | France | 78 | 60 (14) | 51 (65) | NA | NA | NA | ICU admission | PAI-1 antigen, t-PA | 9 |
| 11 | Panigada et al. (2020) | ICU patients | Italy | 24 | 56 (15) | NA | NA | NA | NA | NA | VWF antigen | 6 |
| 12 | Pine et al. (2020) | All hospitalized patients | United States | 49 | 63 (17) | 33 (67) | 13 (27) | 30 (61) | 12 (25) | ICU admission | PAI-1 antigen | 8 |
| 13 | Ranucci et al. (2020) | ICU patients | Italy | 20 | 64 (12) | 16 (80) | 5 (25) | 6 (30) | NA | Mortality | t-PA | 8 |
| 14 | Rauch et al. (2020) | All hospitalized patients | France | 243 | 64 (16) | 155 (64) | 56 (23) | 118 (49) | NA | Worsening of the respiratory status | VWF antigen | 7 |
| 15 | Rovas et al. (2020) | All hospitalized patients | Germany | 23 | 64 (17) | 20 (87) | 0 | 15 (65) | NA | Mechanical ventilator | sTM | 9 |
| 16 | Sweeney et al. (2020) | All hospitalized patients | United States | 181 | 66 (15) | 106 (59) | NA | NA | NA | Mortality | VWF antigen | 7 |
| 17 | Taus et al. (2020) | All hospitalized patients | Italy | 37 | 62 (13) | 18 (49) | 0 | 21 (57) | NA | NA | VWF antigen, VWF activity | 8 |
DM, diabetes mellitus; HT, hypertension; ICU, intensive care unit; NA, not available; VWF, von Willebrand Factor; PAI-1, plasminogen activator inhibitor-1 antigen; sTM, soluble thrombomodulin; t-PA, tissue-type plasminogen activator; NOS, Newcastle-Ottawa Scale; SD, standard deviation; n, number; %, percentage.
3.2. Biomarkers of endothelial dysfunction and outcome
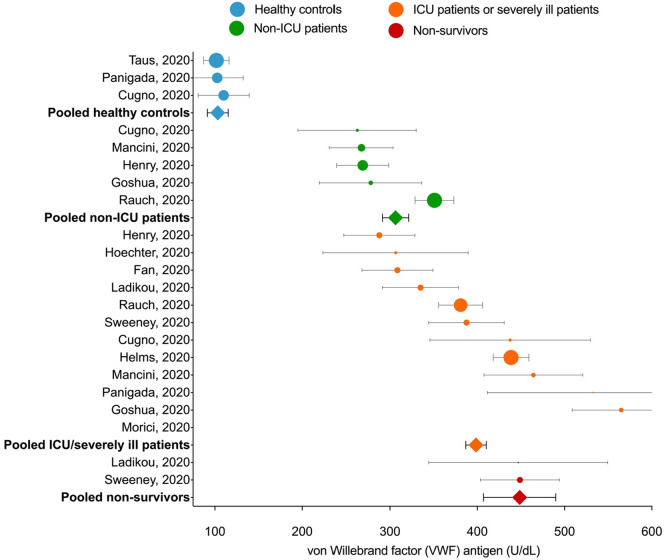
There was an increase in the VWF antigen levels in COVID-19 patients with different levels for each outcome group of COVID-19 patients. The pooled means plasma levels of VWF antigen in COVID-19 patients treated at the general wards and ICU patients or severely ill patients ([306.42 [95% confidence interval (CI) 291.37–321.48], p < 0.001; I2:86%] and [398.56 [95% CI 386.84–410.30], p < 0.001; I2:92%], respectively) were higher than healthy controls (103.24 [95% CI 91.31–115.17], p < 0.001; I2:0%). Moreover, deceased COVID-19 patients had the highest pooled means of VWF antigen levels (448.57 [95% CI 407.20–489.93], p < 0.001; I2:0%) (Fig. 2 ).
Fig. 2.
The estimated pooled mean for plasma levels of von Willebrand Factor antigen in patients with COVID-19. For individual studies, circle markers indicate study means and error bars indicate 95% confidence intervals. Markers are sized proportionately to the weight of the study in the analysis. Estimated pooled means for grouped studies are represented by the square markers.
Meta-analysis showed that higher plasma levels of VWF antigen were associated with composite poor outcome (SMD 0.74 [0.33–1.16], p < 0.001; I2:80.4%). Patients with poor outcome had significantly a higher level of t-PA and PAI-1 antigen compared to patients with good outcomes ([SMD 0.55 [0.19–0.92], p = 0.003; I2:6.4%] and [SMD 0.33 [0.04–0.62], p = 0.025; I2:7.9%], respectively). The plasma levels of sTM were found to be higher in COVID-19 patients with poor outcome ([SMD 0.55 [0.10–0.99], p = 0.015; I2:23.6%]) (Fig. 3 ).
Fig. 3.
Several biomarkers for endothelial dysfunction and the outcome of COVID-19. Patients presenting with a higher plasma levels of (A) von Willebrand Factor (VWF) antigen; (B) tissue-type plasminogen activator (t-PA); (C) plasminogen activator inhibitor-1 antigen (PAI-1) antigen; and (D) soluble thrombomodulin (sTM) have an increased risk of composite poor outcome.
We found substantial heterogeneity for VWF antigen analysis (I2:80.4%) and low heterogeneity for t-PA, PAI-1 antigen, and sTM analysis (I2:6.4%, I2:7.9%, and I2:23.6%, respectively). However, subgroup analyses to evaluate potential sources of heterogeneity of VWF levels were not performed due to the small amount of primary data included in the group analysis. The sensitivity analysis of VWF levels after excluding two studies (Goshua et al., 2020; Mancini et al., 2020) at risk of bias decreased the heterogeneity considerably while maintaining the significance of pooled effect estimate (SMD 0.34 [0.17–0.62], p < 0.001; I2:9.2%).
3.3. Publication bias
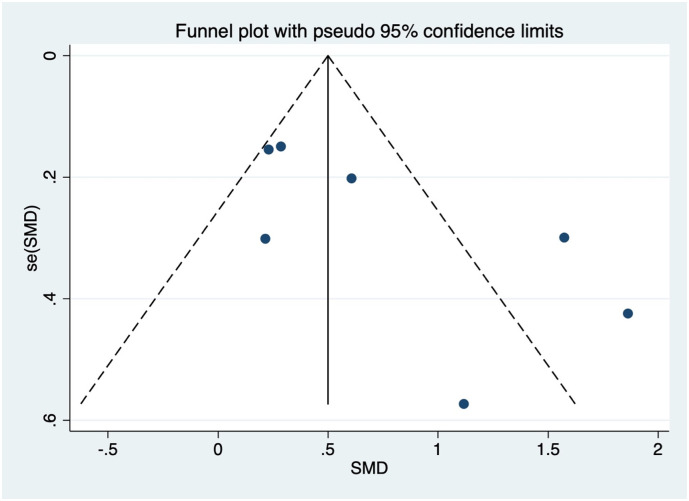
The visual assessment of the funnel plot showed an asymmetrical shape for the analysis of the vWF antigen levels, which indicated the possibility of publication bias (Fig. 4 ). This asymmetrical shape was due to the inclusion of the studies by Goshua et al. (2020) and Mancini et al. (2020). However, quantitative analysis using regression-based Egger's test for the same variable showed no significant result of small-study effects (p = 0.063). Regression-based Harbord's test for other biomarkers and composite poor outcome also showed no significant result of small-study effects.
Fig. 4.
Funnel-plot analysis for the analysis of the von Willebrand Factor (VWF) antigen. SMD, standardized mean difference.
4. Discussion
A single layer of healthy endothelial cells lines the entire vascular system and plays essential roles in maintaining laminar blood flow (Wang et al., 2018). Conditions that cause endothelial activation, such as infection and inflammation, support proatherogenic mechanisms by stimulating thrombin formation, coagulation, and fibrin deposition in blood vessel walls (Lilly, 2012). Activated endothelial cells promote coagulation and induce thrombosis by favoring the expression of antifibrinolytics (e.g., PAI-1) and procoagulants (e.g., tissue factor) over the expression of profibrinolytic mediators (e.g., t-PA) and anticoagulants (e.g., heparin-like molecules and thrombomodulin) (Lilly, 2012; Radermecker et al., 2020; Teuwen et al., 2020). Taken together, these mechanisms contribute to massive platelet binding and formation of fibrin, leading to deposition of blood clots in the microvasculature and systemic thrombosis (Perico et al., 2020). Since viral RNA of SARS-CoV-2 is rarely detected in the blood (Wölfel et al., 2020), it suggests that rather than direct viral infection of endothelial cells, additional host-dependence factors may contribute to systemic endothelial dysfunction and vasculopathy in COVID-19.
von Willebrand Factor (VWF) is a large multidomain adhesive glycoprotein produced by megakaryocytes and endothelial cells (Löf et al., 2018). Endothelial activation in COVID-19 infection and elevated VWF levels as an acute-phase protein released from endothelial cells are recognized as a response to inflammation, but high levels of VWF, in this case, indicate suspicion of endothelial disturbance (Breakey and Escher, 2020). Expression of VWF and its release from the Weibel-Palade body of endothelial cells may also be stimulated by hypoxia. Hypoxia-induced upregulation of VWF is associated with thrombus in cardiac and pulmonary vessels that promote leukocyte recruitment (Mojiri et al., 2019). Several studies have shown that COVID-19 patients have higher plasma levels of VWF antigen than healthy controls (Blasi et al., 2020; Cugno et al., 2020; Panigada et al., 2020; Taus et al., 2020). Elevated levels of the VWF antigen in COVID-19 patients are similar to those reported in studies on patients with disseminated intravascular coagulation (Habe et al., 2012), severe sepsis, and septic shock not associated with COVID-19 (Fukushima et al., 2013; Kremer Hovinga et al., 2007). In addition to the VWF antigen, VWF activity has been shown to increase in COVID-19 patients, thus further explaining the role of endothelial cell injury in COVID-19-associated coagulopathy (Helms et al., 2020; Hoechter et al., 2020; Panigada et al., 2020; Taus et al., 2020).
The procoagulant state resulting from endothelial activation can also be measured from changes in the balance between the levels of t-PA and PAI-1 (Shapiro et al., 2010). Although t-PA is recognized as a profibrinolytic mediator, t-PA is found to be higher in COVID-19 patients with poor outcomes. This paradox may be explained by a study showing that PAI-1, a major inhibitor of t-PA, facilitates the dissociation of t-PA from the surface of vascular endothelial cells (VES), which would therefore decrease cell surface-associated fibrinolytic potential (Suzuki et al., 2009). Decreased amounts of t-PA on the VEC surface and increased concentration of t-PA in the plasma due to increased PAI-1 plasma level may be the possible mechanism of hypofibrinolysis that leads to poor outcomes in COVID-19 patients.
PAI-1 is one of the acute-phase proteins that is increased during inflammatory disorders where the concentrations are affected by the changes in its production by hepatocytes (Gabay and Kushner, 1999). In patients with ARDS, increased levels of PAI-1 produced by endothelial cells and lung epithelium are associated with severe hypofibrinolytic state (Whyte et al., 2020). In addition to endothelial activation due to proinflammatory cytokines, binding of SARS-CoV-2 to the ACE2 receptor induces enhanced shredding of ACE2, thereby increasing Ang II levels which further stimulates PAI-1 expression in various cells including, endothelial cells, adipocytes, and smooth muscle cells (Gue and Gorog, 2020). In COVID-19 patients, plasma levels of PAI-1 were found to be 3.7 times higher than in healthy controls (Blasi et al., 2020). Furthermore, COVID-19 patients with severe respiratory dysfunction show significantly higher levels of PAI-1 compared to patients with burns, ARDS, and sepsis (Kang et al., 2020).
Soluble thrombomodulin (sTM) is a product of proteolytic cleavage of the intact thrombomodulin protein from the surface of endothelial cells after endothelial injury and dysfunction (Martin et al., 2013). In the hyperinflammatory state, increased sTM levels could be due to direct damage to the endothelial cells (Nawroth and Häring, 1999). Elevated plasma levels of sTM have also been reported in patients with the severe acute respiratory syndrome (SARS), indicating endothelial injury (Liu et al., 2005). The plasma levels of sTM might be too low to impact the coagulation process, but the consistent increase in sTM levels during pathologies has been widely considered a biomarker for endothelial dysfunction and vascular risk assessment (Martin et al., 2013). Furthermore, several studies have reported the role of sTM as a prognostic biomarker in COVID-19 patients (Goshua et al., 2020; Rovas et al., 2020).
4.1. Impact for clinical practice
The elevated biomarkers of endothelial cells, which indicate a manifestation of endothelial dysfunction in COVID-19, might increase the risk of vascular complications, poor outcomes, and death. Laboratory examination of VWF, t-PA, PAI-1, and sTM levels may be useful for vascular risk assessment and predicting adverse outcomes and help guide therapy in COVID-19 patients.
4.2. Limitation
One of the 17 studies included in this meta-analysis was a preprint article. Nevertheless, a thorough assessment has been made to make sure that only sound studies are included. Most of the included studies had a retrospective observational design, and the data were not sufficiently matched or adjusted for confounders. Moreover, our primary endpoints of composite poor outcomes vary widely from ICU admission, severe illness, worsening respiratory status, the need for mechanical ventilation to death. Therefore, the results must be cautiously interpreted.
5. Conclusion
There was an increase in the VWF antigen levels in COVID-19 patients, and the highest levels of VWF antigen were found in deceased COVID-19 patients. Biomarkers of endothelial dysfunction, including VWF antigen, t-PA, PAI-1 antigen, and sTM, are significantly associated with an increased composite poor outcome in patients with COVID-19.
Funding statement
None.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The corresponding author (A) can be contacted for more information.
CRediT authorship contribution statement
All the authors have participated in literature retrieval, quality assessment of the studies, and viewpoint discussion in this article. A., R.A.N., and Y.A. contributed to writing this article. Y.A. did the statistical analysis. M.J.A. and B.A.M. revised the article. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declared no conflict of interest.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mvr.2021.104224.
Appendix A. Supplementary data
Supplementary tables
References
- Blasi A., von Meijenfeldt F.A., Adelmeijer J., Calvo A., Ibañez C., Perdomo J., Reverter J.C., Lisman T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J. Thromb. Haemost. 2020;18:2646–2653. doi: 10.1111/jth.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakey N., Escher R. D-dimer and mortality in COVID-19: a self-fulfilling prophecy or a pathophysiological clue? Swiss Med. Wkly. 2020;150:w20293. doi: 10.4414/smw.2020.20293. [DOI] [PubMed] [Google Scholar]
- Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A., Lonati P., Grossi C., Borghi M.O., Novembrino C. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J. Autoimmun. 2020;102560 doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., Neil D., Hoefer I.E., Fragiadaki M., Waltenberger J., Weber C., Bochaton-Piallat M.-L., Bäck M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B.E., Ng J., Chan S.S.W., Christopher D., Tso A.C.Y., Ling L.M., Young B.E., Wong L.J.L., Sum C.L.L., Tan H.T. COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J. Thromb. Thrombolysis. 2020:1–12. doi: 10.1007/s11239-020-02318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H., Nishio K., Asai H., Watanabe T., Seki T., Matsui H., Sugimoto M., Matsumoto M., Fujimura Y., Okuchi K. Ratio of von Willebrand factor propeptide to ADAMTS13 is associated with severity of sepsis. Shock. 2013;39:409–414. doi: 10.1097/SHK.0b013e3182908ea7. [DOI] [PubMed] [Google Scholar]
- Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gavriilaki E., Anyfanti P., Gavriilaki M., Lazaridis A., Douma S., Gkaliagkousi E. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr. Hypertens. Rep. 2020;22 doi: 10.1007/s11906-020-01078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., Rinder H.M., Chun H.J., Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet. Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gue Y.X., Gorog D.A. Reduction in ACE2 may mediate the prothrombotic phenotype in COVID-19. Eur. Heart J. 2020;41:3198–3199. doi: 10.1093/eurheartj/ehaa534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habe K., Wada H., Ito-Habe N., Hatada T., Matsumoto T., Ohishi K., Maruyama K., Imai H., Mizutani H., Nobori T. Plasma ADAMTS13, von Willebrand factor (VWF) and VWF propeptide profiles in patients with DIC and related diseases. Thromb. Res. 2012;129:598–602. doi: 10.1016/j.thromres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.-M., Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Benoit S.W., de Oliveira M.H.S., Lippi G., Favaloro E.J., Benoit J.L. ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID-19: evidence of SARS-CoV-2 induced secondary thrombotic microangiopathy. Int. J. Lab. Hematol. 2020 doi: 10.1111/ijlh.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechter D.J., Becker-Pennrich A., Langrehr J., Bruegel M., Zwissler B., Schaefer S., Spannagl M., Hinske L.C., Zoller M. Higher procoagulatory potential but lower DIC score in COVID-19 ARDS patients compared to non-COVID-19 ARDS patients. Thromb. Res. 2020;196:186–192. doi: 10.1016/j.thromres.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas A., Montani D., Savale L., Pichon J., Tu L., Parent F., Guignabert C., Humbert M. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Tanaka T., Inoue H., Ono C., Hashimoto S., Kioi Y., Matsumoto H., Matsuura H., Matsubara T., Shimizu K., Ogura H., Matsuura Y., Kishimoto T. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. U. S. A. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer Hovinga J.A., Zeerleder S., Kessler P., Romani de Wit T., van Mourik J.A., Hack C.E., ten Cate H., Reitsma P.H., Wuillemin W.A., Lämmle B. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J. Thromb. Haemost. 2007;5:2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- Ladikou E.E., Sivaloganathan H., Milne K.M., Arter W.E., Ramasamy R., Saad R., Stoneham S.M., Philips B., Eziefula A.C., Chevassut T. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin. Med. 2020;20:e178–e182. doi: 10.7861/clinmed.2020-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., Hirayama A.V., Mastroiani F., Turtle C.J., Harhay M.O. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8(12):1233–1244. doi: 10.1016/s2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly L.S. Lippincott Williams & Wilkins; 2012. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty. [Google Scholar]
- Liu Z.-H., Wei R., Wu Y.-P., Lisman T., Wang Z.-X., Han J.-J., Ren D.-L., Chen Bin, Xia Z.-L., Chen Biao, Zhu Z., Zhang Y., Cui X., Hu H.-T., de Groot P.G., Xu W.-B. Elevated plasma tissue-type plasminogen activator (t-PA) and soluble thrombomodulin in patients suffering from severe acute respiratory syndrome (SARS) as a possible index for prognosis and treatment strategy. Biomed. Environ. Sci. 2005;18:260–264. [PubMed] [Google Scholar]
- Löf A., Müller J.P., Brehm M.A. A biophysical view on von Willebrand factor activation. J. Cell. Physiol. 2018;233:799–810. doi: 10.1002/jcp.25887. [DOI] [PubMed] [Google Scholar]
- Mancini I., Baronciani L., Artoni A., Colpani P., Biganzoli M., Cozzi G., Novembrino C., Boscolo Anzoletti M., De Zan V., Pagliari M.T., Gualtierotti R., Aliberti S., Panigada M., Grasselli G., Blasi F., Peyvandi F. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J. Thromb. Haemost. 2020 doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F.A., Murphy R.P., Cummins P.M. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1585–H1597. doi: 10.1152/ajpheart.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojiri A., Alavi P., Lorenzana Carrillo M.A., Nakhaei-Nejad M., Sergi C.M., Thebaud B., Aird W.C., Jahroudi N. Endothelial cells of different organs exhibit heterogeneity in von Willebrand factor expression in response to hypoxia. Atherosclerosis. 2019;282:1–10. doi: 10.1016/j.atherosclerosis.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Morici N., Bottiroli M., Fumagalli R., Marini C., Cattaneo M. Role of von Willebrand factor and ADAMTS-13 in the pathogenesis of thrombi in SARS-CoV-2 infection: time to rethink. Thromb. Haemost. 2020;120:1339–1342. doi: 10.1055/s-0040-1713400. [DOI] [PubMed] [Google Scholar]
- Nägele M.P., Haubner B., Tanner F.C., Ruschitzka F., Flammer A.J. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P.P., Häring H.-U. Thrombomodulin and coronary heart disease. Lancet. 1999;353:1722–1723. doi: 10.1016/S0140-6736(99)90039-9. [DOI] [PubMed] [Google Scholar]
- Nougier C., Benoit R., Simon M., Desmurs-Clavel H., Marcotte G., Argaud L., David J.S., Bonnet A., Negrier C., Dargaud Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020;18:2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2020;17 doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A.B., Meizlish M.L., Goshua G., Chang C.-H., Zhang H., Bishai J., Bahel P., Patel A., Gbyli R., Kwan J.M., Won C.H., Price C., Dela Cruz C.S., Halene S., van Dijk D., Hwa J., Lee A.I., Chun H.J., Pine A.B., Goshua G., Chang C.-H., Zhang H., Bishai J., Bahel P., Patel A., Gbyli R., Kwan J.M., Price C., Dela Cruz C.S., Halene S., van Dijk D., Hwa J., Lee A.I., Chun H.J. Vol. 10. 2020. Circulating Markers of Angiogenesis and Endotheliopathy in COVID-19. medRxiv Prepr. Serv. Heal. Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermecker C., Detrembleur N., Guiot J., Cavalier E., Henket M., d'Emal C., Vanwinge C., Cataldo D., Oury C., Delvenne P. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020;217 doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranucci M., Sitzia C., Baryshnikova E., Di Dedda U., Cardani R., Martelli F., Corsi Romanelli M. Covid-19-associated coagulopathy: biomarkers of thrombin generation and fibrinolysis leading the outcome. J. Clin. Med. 2020;9 doi: 10.3390/jcm9113487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A., Labreuche J., Lassalle F., Goutay J., Caplan M., Charbonnier L., Rohn A., Jeanpierre E., Dupont A., Duhamel A., Faure K., Lambert M., Kipnis E., Garrigue D., Lenting P.J., Poissy J., Susen S. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J. Thromb. Haemost. 2020;18:2942–2953. doi: 10.1111/jth.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovas A., Osiaevi I., Buscher K., Sackarnd J., Tepasse P.-R., Fobker M., Kühn J., Braune S., Göbel U., Thölking G., Gröschel A., Pavenstädt H., Vink H., Kümpers P. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2020:1–13. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro N.I., Schuetz P., Yano K., Sorasaki M., Parikh S.M., Jones A.E., Trzeciak S., Ngo L., Aird W.C. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit. Care. 2010;14 doi: 10.1186/cc9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Mogami H., Ihara H., Urano T. Unique secretory dynamics of tissue plasminogen activator and its modulation by plasminogen activator inhibitor-1 in vascular endothelial cells. Blood. 2009;113:470–478. doi: 10.1182/blood-2008-03-144279. (J. Am. Soc. Hematol.) [DOI] [PubMed] [Google Scholar]
- Sweeney J.M., Barouqa M., Krause G.J., Gonzalez-Lugo J.D., Rahman S., Gil M.R. 2020. Evidence for secondary thrombotic microangiopathy in COVID-19. medRxiv Prepr. Serv. Heal. Sci. [DOI] [Google Scholar]
- Taus F., Salvagno G., Canè S., Fava C., Mazzaferri F., Carrara E., Petrova V., Barouni R.M., Dima F., Dalbeni A., Romano S., Poli G., Benati M., De Nitto S., Mansueto G., Iezzi M., Tacconelli E., Lippi G., Bronte V., Minuz P. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020;40:2975–2989. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;1–3 doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Hao H., Leeper N.J., Zhu L. Thrombotic regulation from the endothelial cell perspectives. Arterioscler. Thromb. Vasc. Biol. 2018;38:e90–e95. doi: 10.1161/ATVBAHA.118.310367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G.A., SHEA B., O'connell D., Peterson J., Welch V., Losos M., Tugwell P., Wells G., O'Connell D., Shea B. 2015. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-analyses. [Google Scholar]
- Whyte C.S., Morrow G.B., Mitchell J.L., Chowdary P., Mutch N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. WHO R&D Blueprint-novel Coronavirus COVID-19 Therapeutic Trial Synopsis.https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The corresponding author (A) can be contacted for more information.