Abstract
Autoimmune phenomena and clinically apparent autoimmune diseases, including autoimmune hepatitis, are increasingly been reported not only after natural infection with the SARS-CoV-2 virus, but also after vaccination against it. We report the case of a 63-year old man without a history of autoimmunity or SARS-CoV-2 natural infection who experienced acute severe autoimmune-like hepatitis seven days after the first dose of the mRNA-1273 SARS-CoV-2 vaccine. Liver histology showed inflammatory portal infiltrate with interface hepatitis, lobular and centrilobular inflammation with centrilobular necrosis, in absence of fibrosis and steatosis. Serum immunoglobulin G was slightly elevated. Autoimmune liver serology showed an indirect immunofluorescence pattern on triple rodent tissue compatible with anti-mitochondrial antibody (AMA), but, unexpectedly, this pattern was not mirrored by positivity for primary biliary cholangitis (PBC)-specific molecular tests, indicating that this antibody is different from classical AMA. Anti-nuclear antibody (ANA) was also positive with a rim-like indirect immunofluorescence pattern on liver and HEp2 cell substrates, similar to PBC-specific ANA; however, anti-gp210 and a large panel of molecular-based assays for nuclear antigens were negative, suggesting a unique ANA in our patient. He carries the HLA DRB1*11:01 allele, which is protective against PBC. Response to prednisone treatment was satisfactory. The clinical significance of these novel specificities needs to be further evaluated in this emerging condition.
Keywords: SARS-Cov-2, mRNA vaccine, Autoimmune-like hepatitis, Atypical anti-mitochondrial antibody
1. Introduction
The ongoing pandemic caused by Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2), a highly transmissible and pathogenic virus leading to coronavirus disease 2019 (COVID-19), is having a devastating global impact, leading to an unprecedented fast development of anti-COVID-19 vaccines. Two mRNA vaccines, BNTb262 and mRNA-1273, have recently been approved in Switzerland, and a massive vaccination campaign has started at the end of 2020. These vaccines trigger the interferon pathway as part of their mechanism of action, raising concerns about potential safety issues in patients predisposed to autoimmune conditions associated with interferon activation, including autoimmune hepatitis (AIH) [1,2]. A diverse range of autoimmune diseases following COVID-19 and anti-SARS-CoV-2 vaccination are increasingly being reported [[3], [4], [5], [6]]. Recently, autoimmune-like hepatitis following mRNA Covid-19 has been reported in four women, only one of whom having pre-existing extrahepatic autoimmune conditions [[7], [8], [9], [10]]. We describe a well characterized case of autoimmune-like hepatitis in a 63-year old man arising one week after the first dose of the mRNA-1273 vaccine.
2. Case description
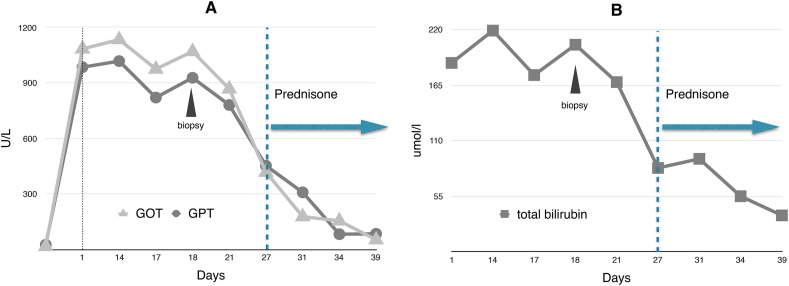
A 63-year old male Caucasian patient presented to his general practictioner with jaundice, fatigue and loss of appetite starting seven days after the first dose of the mRNA-1273 SARS-CoV-2 vaccine. His medical history included type 2 diabetes and ischemic heart disease, for which he was on metformin 500 mg/day, acetylsalicylic acid 100 mg/day and rosuvastatin 10 mg/day for over five years. He did not take herbal remedies or other drugs, and his daily amount of alcohol consumption was 10 g. He had no history of clinical apparent COVID-19. On examination, the patient was jaundiced, and had tender hepatomegaly without ascites. His body mass index was 28. An abdominal ultrasound showed normal liver parenchyma, normal portal vein flow, no ascites, normal spleen size (11 cm). Laboratory findings were: hemoglobin 14.8 g/dl, white cell count 6.3 109/l, platelets 309 109/l, INR 1.16, total immunoglobulin G (IgG) 19.96 g/l (normal range: 5.4–18.22 g/l), aspartate aminotransferase (ASAT) 1127 U/l (upper limit of normal (ULN) 34 U/l), alanine aminotransferase (ALAT) 1038 U/l (ULN 55 U/l), gammaglutamyltransferase (GGT) 536 U/l (ULN 55 U/l), alkaline phosphatase (ALP) 192 U/l (normal range: 40–150 U/l), total bilirubin 204.8 μmol/l (ULN 20.4 μmol/l) (Fig. 1 ). Polymerase chain reaction for hepatitis B, C and E viruses and anti-hepatitis A virus IgM were negative. Initial testing for autoimmune liver serology showed anti-nuclear antibody (ANA) at a titer of 1:640 with fine speckled indirect immunofluorescence pattern on HEp2 cells, positive anti-gastric parietal cells antibody at a titer of 1:320. Anti-smooth muscle antibody (ASMA), anti-neutrophil cytoplasmic antibody (ANCA), anti-soluble liver antigen (SLA) antibody, anti-liver kidney microsomal type 1 antibody (anti-LKM1), anti-liver cytosol type 1 (anti-LC1) antibody and anti-mitochondrial antibody (AMA) were all negative.
Fig. 1.
Transaminases (Panel A) and bilirubin (Panel B) serum levels.
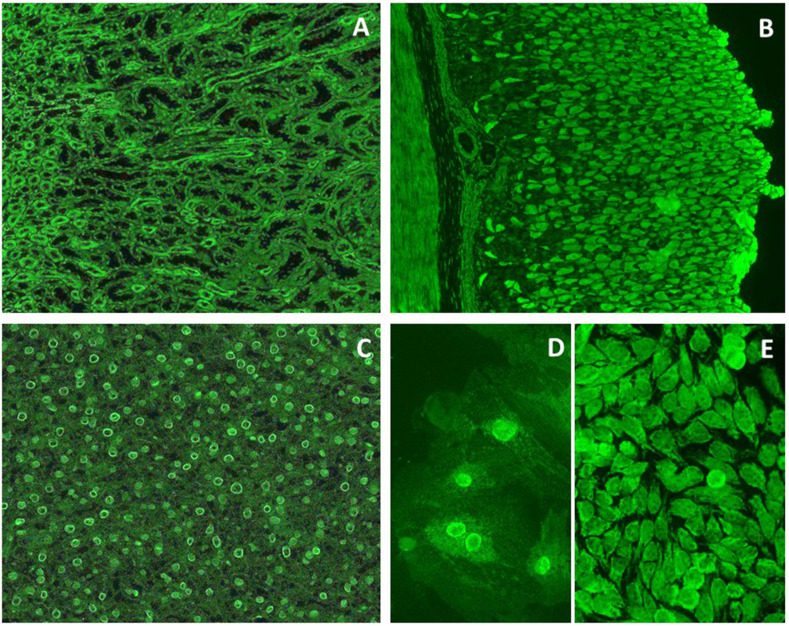
Anti-SARS-Cov 2 IgG spike 1 antibody 24 days after the first vaccine dose was positive (1392 AU/ml, positivity cut-off: 50 AU/ml). Autoimmune liver serology was repeated at referral to our center 12 days after first testing; indirect immunofluorescence on triple rodent tissue (Euroimmun) was as follows (Fig. 2 ):
-
-
Liver substrate: positive nuclear staining with a rim-like pattern, and positive faint cytoplasmic staining
-
-
Kidney substrate: bright staining of the smaller distal tubuli
-
-
Stomach substrate: positive parietal cell staining
Fig. 2.
Indirect immunofluorescence on rodent tissue, VSM47 and HEp2 cells. The staining of the mitochondria-rich distal renal tubules (Panel A) and of the gastric parietal cells (Panel B) is compatible with anti-mitochondrial antibody (AMA), a specificity however negative at molecular level (see text). Panel C shows a positive rim-like staining of the nuclei (rim-like anti-nuclear antibody (ANA)) in the liver. Panel D (VSM47) and Panel E (HEp2 cells) confirm a rim-like ANA and show a trabecular staining of the cytoplasm. A speckled pattern is also visible in the nuclei of Panels D and E.
Indirect immunofluorescence on HEp2 and VSM47 cells (Euroimmun) showed positive nuclear staining with a speckled pattern and a faint rim-like pattern, coupled with positive cytoplasmic staining with a trabecular pattern (Fig. 2). Molecular-based assays for U1-snRNP, SSA/Ro, SS-B/La, CENP-B, Scl-70, Jo-1, Sm, dsDNA, Fibrillarin, RNA Pol III, Rib-P, PM-Scl, PCNA, Mi2 were negative (CTD screen, Thermofisher Scientific). Anti-native pyruvate dehydrogenase complex E2 sudunit (PDC-E2), anti-recombinant PDC subunit E2, anti-2-oxoglutarate dehydrogenase complex E2 subunit (OGDC-E2) and anti-branched-chain 2-oxo acid dehydrogenase complex E2-subunit (BCOADC-E2) were negative (Alphadia). MIT-3 based Elisa (INOVA) was also negative. Immunoblot for anti-SLA, anti-LC1, anti-LKM1, anti-sp100 and anti-gp210 (Alphadia), anti-transglutaminase IgA (Elisa, Thermofisher Scientific), ANCA, tested by indirect immunofluorescence on fixed human neutrophils (INOVA), anti-myeloperoxidase and anti-proteinase 3 (Elisa, Thermofisher Scientific) were all negative. While anti-cardiolipin IgG and IgM antibodies were negative, IgA was positive (26.1 CU, positivity cut-off 20CU); similarly, anti-β-2 glycoprotein IgA was positive (51.3 CU, positivity cut-off 20), with negative anti-β-2 glycoprotein IgM and IgG.
Human leukocyte antigen (HLA) testing showed DRB1*01:01 11:01, DQA1*01:01 05:01, and DQB1*03:01 05:01.
Serum ceruloplasmin and alfa-1-antitrypsin levels were normal.
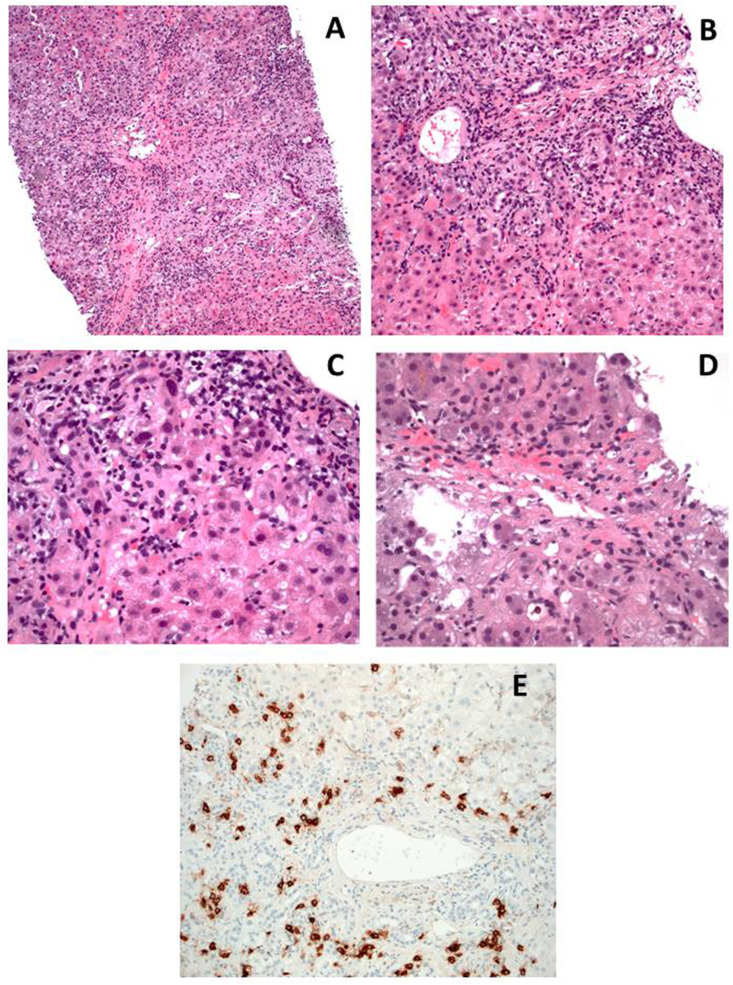
Liver histology showed inflammatory portal infiltrate with interface hepatitis, lobular and centrilobular inflammation with centrilobular necrosis, in absence of fibrosis and steatosis (Fig. 3 ).
Fig. 3.
Liver biopsy findings. Panel A and B: expanded portal tracts with intense lymphoplasmacytic infiltrate with scattered eosinophils, and interface hepatitis (HE; Panel A 10x, Panel B 20x). Panel C: higher magnification of interface hepatitis (HE 40x). Panel D: intense lobular activity associated with centrilobular necrosis (HE 40x). Panel E: CD38 staining showing plasma cellular infiltrate, also affecting the interface (20x). Courtesy of Luca Mazzucchelli and Elisabetta Merlo, Istituto Cantonale di Patologia EOC, Locarno, Switzerland.
The patient did not develop signs of encephalopathy, and after a transient worsening of INR to 1.4, he showed spontaneous improvement of serum bilirubin, INR and transaminase levels (Fig. 1). However, after the results of the liver biopsy showing severe inflammation with interface hepatitis, prednisone 40 mg/day was started, which was well tolerated and induced a good response during a follow-up of 2 weeks (Fig. 1). The dose was rapidly tapered to 20 mg/day, and the patient is strictly monitored in order to continue tapering the prednisone to the minimal dose needed to maintain disease control. The second vaccine dose was temporarily suspended.
3. Discussion
We report a well characterized case of acute severe autoimmune-like hepatitis in a 63-year old male without a history of autoimmune diseases, with symptom onset one week after the first dose of mRNA-1273 vaccine. The patient responded well to prednisone treatment over two weeks. As for other similar cases recently reported in the literature [[7], [8], [9], [10]], a causal link with the vaccine cannot be proven, but it is suggested by the temporal relationship in absence of other potential triggers or risk factors for AIH, which preferentially affects young or middle-aged women [2]. Only long-term follow up will allow to establish if this hepatitis behaves as AIH or as AIH-like drug-induced liver injury.
The autoimmune liver serology of our patient is remarkable. While indirect immunofluorescence on triple rodent tissue showed a pattern compatible with AMA, molecular testing for PDC-E2, OGDC-E2 and BCOADC-E2 was negative, indicating that this antibody is different from classical AMA, the serological hallmark of primary biliary cholangitis (PBC) [11]. AMA with specificities characteristic of PBC can be transiently expressed during acute hepatitis, but, unexpectedly, the immunofluorescent AMA-like pattern of our patient was not mirrored by positivity for PBC specific molecular tests [11]. AMA was reported as negative in the recently published cases of acute hepatitis following mRNA anti-SARS-CoV-2 vaccination: however, the methodology used was not specified. Of note, the ANA detected in our patient shows a rim-like pattern on rodent tissue and on HEp2 cells, similar to PBC-specific ANA; however, anti-gp210, the antibody responsible for rim-like ANA pattern in PBC, was negative. This, plus negativity for a large panel of molecular-based assays for nuclear antigens, suggests that our patient's ANA is also unique. ANA was positive in three of the four reported post Covid-vaccine AIH-like cases, none with a rim-like pattern (Table 1 ).
Table 1.
Reported case of acute hepatitis after mRNA vaccine.
| Age (years), sex | Type of vaccine, number of doses | Time to symptoms onset | Autoantibodies | Concomitant autoimmune diseases/drugs | IgG (g/l) |
Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 35, F | BNT162b2, 1 dose | 1 week | ANA + hom, AMA-, ASMA-, anti-LKM-, ANCA- | Post-partum state/none | 10.8 | Prednisone 20 mg | Improved | Bril et al. [8] |
| 41, F | mRNA-1273, 2 doses | 1 week after 2nd dose | ANA + pattern not reported, SMA+, anti-SLA+, anti-LC1+ | None/substitutive hormonal therapy | 20.8 | Prednisone 1 mg/kg | Improved | Londono et al. [7] |
| 80, F | BNT162b2, 2 doses |
1 week after 2nd dose | ANA + speckled; AMA-, anti-LKM-, ASMA- |
Hashimoto, glomerulonephritis/ Pravastatin |
35 | Prednisone 1 mg/kg |
Improved | Rocco et al. [9] |
| 56, F | mRNA-1273, 1 dose | 5 weeks | ANA+, SMA+ Patterns not reported |
None/ Rosuvastatin |
32.6 | Budesonide | Improved | Tan et al. [10] |
Our case has a remarkably short latency time from vaccination to symptom-onset, similarly to the case recently reported by Bril et al. [8]. This does not exclude a triggering role for the mRNA vaccine, which elicits a strong stimulus to innate immunity, due to the intrinsic immunostimulatory properties of mRNA via its recognition by intracellular innate sensors, including toll-like receptors 3 and 7 and components of the inflammasome, resulting in cellular activation leading to interferon I and other pro-inflammatory cytokine and chemokine production [1]. Of note, new onset of autoimmune diseases with a latency as short as four days after the first mRNA vaccine dose has been reported [6]. However, we cannot exclude that our patient had had asymptomatic SARS-CoV-2 infection as suggested by positive anti-phospholipid IgA antibodies, in absence of IgG and IgM, a combination that has been reported in COVID-19 patients [12].
Our patient, as well as two of the four reported cases, was on long-term statin therapy. While statins are associated with mild, asymptomatic transaminase levels elevation in 1–3% of patients, severe hepatitis with jaundice and ANA positivity has also been reported, arising after 15 weeks of therapy [13]: the long exposure time in our patient and in the case reported by Tan et al. (exposure time in the case by Rocco et al. not reported), is hardly compatible with a triggering role for statin therapy.
Susceptibility to AIH is mainly conferred by HLA alleles DRB1*03 and DRB1*04, which were not carried by our patient, who interestingly had HLA DRB1*11:01, an HLA allele which is protective against PBC [14].
In conclusion, we report a case of acute severe hepatitis mimicking AIH following mRNA anti-SARS-CoV2 vaccination, whose autoimmune serology shows novel specifities, the clinical significance of which needs to be further evaluated in this emerging condition.
Acknowledgment
We kindly acknowledge the Istituto Cantonale di Patologia EOC, Locarno, Switzerland for providing the histological pictures.
References
- 1.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat. Rev. Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mieli-Vergani G., Vergani D., Czaja A.J., Manns M.P., Krawitt E.L., Vierling J.M., Lohse A.W., Montano-Loza A.J. Autoimmune Hepat. Nat Rev Dis Prim. 2018;4:18017. doi: 10.1038/nrdp.2018.17. [DOI] [PubMed] [Google Scholar]
- 3.Novelli L., Motta F., De Santis M., Ansari A.A., Gershwin M.E., Selmi C. The JANUS of chronic inflammatory and autoimmune diseases onset during COVID-19 - a systematic review of the literature. J. Autoimmun. 2021;117:102592. doi: 10.1016/j.jaut.2020.102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novelli L., Motta F., Ceribelli A., Guidelli G.M., Luciano N., Isailovic N., Vecellio M., Caprioli M., Clementi N., Clementi M., Mancini N., Selmi C., De Santis M. A case of psoriatic arthritis triggered by SARS-CoV-2 infection. Rheumatology. 2021;60:e21–e23. doi: 10.1093/rheumatology/keaa691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021;20:102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watad A., De Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., Haddad A., Elias M., Zisman D., Naffaa M.E., Brodavka M., Cohen Y., Abu-Much A., Abu Elhija M., Bridgewood C., Langevitz P., McLorinan J., Bragazzi N.L., Marzo-Ortega H., Lidar M., Calabrese C., Calabrese L., Vital E., Shoenfeld Y., Amital H., McGonagle D. Immune-Mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Londoño M.-C., Gratacós-Ginès J., Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination. Still casualty? J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.004. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) Vaccine: causality or casualty? J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following sars-cov-2 VACCINE: MAY not be a casualty. J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 Vaccination: true causality or mere association? J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.009. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terziroli Beretta-Piccoli B., Mieli-Vergani G., Vergani D. The clinical usage and definition of autoantibodies in immune-mediated liver disease: a comprehensive overview. J. Autoimmun. 2018 doi: 10.1016/j.jaut.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Hasan Ali O., Bomze D., Risch L., Brugger S.D., Paprotny M., Weber M., Thiel S., Kern L., Albrich W.C., Kohler P., Kahlert C.R., Vernazza P., Bühler P.K., Schüpbach R.A., Gómez-Mejia A., Popa A.M., Bergthaler A., Penninger J.M., Flatz L. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin. Infect. Dis. 2020:ciaa1496. doi: 10.1093/cid/ciaa1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Famularo G., Miele L., Minisola G., Grieco A. Liver toxicity of rosuvastatin therapy. World J. Gastroenterol. 2007;13:1286–1288. doi: 10.3748/wjg.v13.i8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Invernizzi P., Ransom M., Raychaudhuri S., Kosoy R., Lleo A., Shigeta R., Franke A., Bossa F., Amos C.I., Gregersen P.K., Siminovitch K.A., Cusi D., de Bakker P.I.W., Podda M., Gershwin M.E., Seldin M.F. Italian PBC Genetics Study Group, Classical HLA-DRB1 and DPB1 alleles account for HLA associations with primary biliary cirrhosis. Gene Immun. 2012;13:461–468. doi: 10.1038/gene.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]