Abstract
Objective
To compare coronavirus disease 2019 (COVID-19) acute kidney injury (AKI) to sepsis-AKI (S-AKI). The morphology and transcriptomic and proteomic characteristics of autopsy kidneys were analyzed.
Patients and Methods
Individuals 18 years of age and older who died from COVID-19 and had an autopsy performed at Mayo Clinic between April 2020 to October 2020 were included. Morphological evaluation of the kidneys of 17 individuals with COVID-19 was performed. In a subset of seven COVID-19 cases with postmortem interval of less than or equal to 20 hours, ultrastructural and molecular characteristics (targeted transcriptome and proteomics analyses of tubulointerstitium) were evaluated. Molecular characteristics were compared with archived cases of S-AKI and nonsepsis causes of AKI.
Results
The spectrum of COVID-19 renal pathology included macrophage-dominant microvascular inflammation (glomerulitis and peritubular capillaritis), vascular dysfunction (peritubular capillary congestion and endothelial injury), and tubular injury with ultrastructural evidence of mitochondrial damage. Investigation of the spatial architecture using a novel imaging mass cytometry revealed enrichment of CD3+CD4+ T cells in close proximity to antigen-presenting cells, and macrophage-enriched glomerular and interstitial infiltrates, suggesting an innate and adaptive immune tissue response. Coronavirus disease 2019 AKI and S-AKI, as compared to nonseptic AKI, had an enrichment of transcriptional pathways involved in inflammation (apoptosis, autophagy, major histocompatibility complex class I and II, and type 1 T helper cell differentiation). Proteomic pathway analysis showed that COVID-19 AKI and to a lesser extent S-AKI were enriched in necroptosis and sirtuin-signaling pathways, both involved in regulatory response to inflammation. Upregulation of the ceramide-signaling pathway and downregulation of oxidative phosphorylation in COVID-19 AKI were noted.
Conclusion
This data highlights the similarities between S-AKI and COVID-19 AKI and suggests that mitochondrial dysfunction may play a pivotal role in COVID-19 AKI. This data may allow the development of novel diagnostic and therapeutic targets.
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), and is associated with acute kidney injury (AKI) in more than one-third of hospitalized patients.1 The AKI is often moderate to severe, and is an independent risk factor for in-hospital mortality.2 , 3 Acute tubular injury is considered the pathological hallmark of COVID-19 kidney disease as shown in both native and autopsy renal studies.4, 5, 6 Microthrombi, described in heart and lungs,7, 8, 9, 10, 11 is infrequently reported in kidneys.5 , 12 Similarly, although severe COVID-19 is associated with a systemic inflammatory response and inflammation in both lungs and heart, little is known about the immune response in the kidneys.
Direct viral toxicity, hemodynamic instability, cytokine storm, and immune-mediated injury have all been proposed as likely causes of renal injury in COVID-19.5 , 13 , 14 Recent studies have drawn similarities between sepsis-associated AKI (S-AKI) and COVID-19–related kidney injury.15 , 16 To date, reports evaluating the renal pathology in COVID-19 have largely focused on light microscopy and ultrastructural studies; molecular studies have been limited.
Herein, we present a series of COVID-19 patients who underwent postmortem examination with emphasis on renal pathology findings. Our study sought to describe the pathological spectrum of COVID-19–associated renal injury, and to characterize the molecular profile of COVID-19–associated AKI as compared with S-AKI and nonsepsis-related AKI (NS-AKI) to elucidate similarities and differences in underlying pathophysiology.
Patients and Methods
Clinical Data
All cases of COVID-19 (as confirmed by antemortem SARS-CoV-2 nasopharyngeal or oropharyngeal polymerase chain reaction) undergoing research-consented postmortem examination at Mayo Clinic (Rochester, MN) between April 2 and September 9, 2020, were included in the study. Salient information for all cases was abstracted from the electronic medical record, including demographic information, disease history (if available), and the presence or absence of chronic underlying conditions.
Two autopsy-derived control cohorts with AKI were selected for comparative purposes: those who died of bronchopneumonia-associated sepsis (S-AKI, n=7) and those who died with AKI not secondary to sepsis or COVID-19 (NS-AKI, n=7).
The study was approved by and conducted according to the requirements of the Institutional Review Board at Mayo Clinic.
Morphological Analyses
A detailed light microscopic evaluation of all 17 autopsies was performed by two renal pathologists (M.P.A. and L.P.H.). Tissue sections were stained with hematoxylin and eosin, periodic acid-Schiff, trichrome, and Jones methenamine silver stains. In addition, immunohistochemical (IHC) evaluation with CD68, CD3, and CD20 to evaluate macrophages, T cells, and B cells was performed in all 17 autopsies. Detailed methods are provided in the Supplemental Appendix (available online at http://www.mayoclinicproceedings.org). Light microscopy analysis was also performed in the two control cohorts.
In a subset of seven COVID-19 cases that had postmortem interval of <20 hours and only mild autolysis, ultrastructural studies, IHC evaluation for SARS-CoV-2 antigens, and in situ hybridization for viral RNA were also performed.
A high-parameter image mass cytometry using 23 different molecular markers was performed on the COVID-19 cases. The detailed methods, including the markers, are provided in the Supplemental Appendix.
Molecular Analyses
Tissue from the well-preserved seven renal autopsy blocks from COVID-19 cases, as well as the two control groups (n=7 each) were analyzed by NanoString platform using the nCounter Human Organ Transplant Panel that profiles expression of 770 genes associated with different pathways related to immune response and tissue injury. Proteomics of the seven cases each in three groups was performed following laser capture microdissection of the tubulointerstitial compartment excluding any glomeruli and or regions of interstitial fibrosis or tubular atrophy. Apoptosis in each group was assessed by terminal deoxynucleotidyl transferase of dUTP nick end labeling assay.
Statistical Analysis
Postnormalization gene expression analysis was performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). Functional annotation gene sets were derived using NanoString-provided, literature-based annotations.17 , 18 Functional annotation gene set expression was determined using the z scores of the mean normalized counts of the constituent genes. Gene set z scores were compared between sample groups using Student t test. The top relatively upregulated and downregulated individual genes within each sample group (versus all other sample groups) were determined using fold change and Student t test. In case of quantitative proteomics data, tandem mass tag channel intensities of identified proteins were used for statistical analysis using Python 3.6.5. Channel intensities were log2 transformed and median normalized before applying independent Student t-test. P values were corrected for multiple hypotheses testing using Benjamini-Hochberg method. Differentially expressed proteins (p<.05) were analyzed using QIAGEN's Ingenuity Pathway Analysis software (IPA, QIAGEN Redwood City, CA, USA) to identify altered signaling pathways. The significance values (P value of overlap) for the canonical pathways were calculated by the right-tailed Fisher exact test. We used nonparametric rank-sum test to compare the groups because of the small number of cases per group. All statistical analyses were performed using JMP (SAS Institute, Cary, NC; version 14.1). P values less than .05 were considered to be statistically significant.
Results
Clinical Characteristics
The demographics of the 17 COVID-19 autopsy cases are provided in Table 1 . The cohort had a median age of 78 years (range, 29 to 94 years), and 14 (82.4%) were older than 70 years. There were 15 men, and 11 self-identified as White (64.7%). Three (17.6%) additional patients self-identified as Asian, none as Black. The time spent in the hospital varied widely, with 12 (70.6%) having been hospitalized for 5 or more days before death. Frequent and notable comorbidities included hypertension in 9 (52.9%), diabetes mellitus in 4 (23.5%), cardiovascular diseases (such as coronary artery disease) or cerebrovascular accident in 12 (70.6%), and chronic kidney disease in 1 (5.9%). Only 1 of 17 patients (5.9%) had no history of hypertension, diabetes mellitus, coronary artery disease, chronic kidney disease, or obesity. Seven (41.1%) were mechanically ventilated at the time of death.
Table 1.
Cohort Demographics and Relevant Clinical Characteristicsa
| Characteristics | n (%) |
|---|---|
| Median age (range): 78 (29-94), years | |
| <50 | 1 (6) |
| 50-70 | 2 (12) |
| 70-90 | 12 (70) |
| >90 | 2 (12) |
| Male | 15 (88) |
| Self-identified race | |
| Hispanic | 0 |
| Asian | 3 (18) |
| Black | 0 |
| White | 11 (64) |
| Unknown | 3 (18) |
| Comorbidities | |
| Hypertension | 9 (53) |
| Diabetes mellitus | 4 (23) |
| Obesity | 1 (6) |
| Cardiac disease (congestive heart failure/coronary artery disease) | 10 (59) |
| Cerebrovascular accident | 2 (12) |
| Cognitive decline | 7 (41) |
| Chronic obstructive pulmonary disease | 3 (18) |
| Cancer | 3 (18) |
| Chronic kidney disease | 1 (6) |
| None known | 1 (6) |
| Duration of hospitalization, d | |
| Deceased on arrival | 1 (6) |
| <1 | 4 (23) |
| 5-10 | 5 (29) |
| 10-15 | 2 (12) |
| 15-20 | 2 (12) |
| >20 | 3 (18) |
| Mechanical ventilation | 7 (41) |
| COVID-19 treatment | |
| Dexamethasone | 1 (6) |
| Remdesvir | 1 (6) |
| Remdesvir and dexamethasone | 2 (12) |
| Remdesvir and convalescent plasma | 2 (12) |
| Renal parameters | |
| AKI KDIGO stage | |
| 1 | 8 (47) |
| 2 | 2 (12) |
| 3 | 3 (18) |
| Undetermined | 4 (23) |
| Proteinuria, dipstick, mg | |
| <30 | 2 (12) |
| 30-50 | 7 (41) |
| 50-100 | 4 (23) |
| Unknown | 4 (23) |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; KDIGO, Kidney Disease Improving Global Outcomes.
All 17 patients who died with COVID-19 developed AKI. Among the patients with AKI who had a terminal serum creatinine level within 24 hours of death, eight had AKI stage 1 (47.1%), two had AKI stage 2 (11.8%), and three had AKI stage 3 (17.6%). One patient with AKI stage 3 received continuous renal replacement therapy. Urine dipstick assessment of proteinuria was unavailable in four patients, but in the remaining, all 13 patients (76.5%) had 100 mg/dL or less. Six of 17 patients had received treatment for COVID-19 including dexamethasone alone (n=1), remdesivir (n=1), a combination of dexamethasone and remdesivir (n=2), and a combination of remdesivir and convalescent plasma (n=2).
All 17 patients had a positive SARS-CoV-2 polymerase chain reaction, diagnosed at a median interval of 12 days before autopsy. At the time of autopsy, three (17.6%) were negative for SARS-CoV-2. The median postmortem interval was 18 hours and autolysis was only mild in 11 patients (64.7%). Sixteen (94.1%) died from complications of COVID-19 (all 16 had severe pulmonary disease, including acute bronchopneumonia superimposed on diffuse alveolar damage in eight [50.0%], acute and organizing pneumonia in four [25.0%], organizing pneumonia in three [18.8%], and diffuse alveolar damage alone in one [6.3%]).
Pathology Characteristics
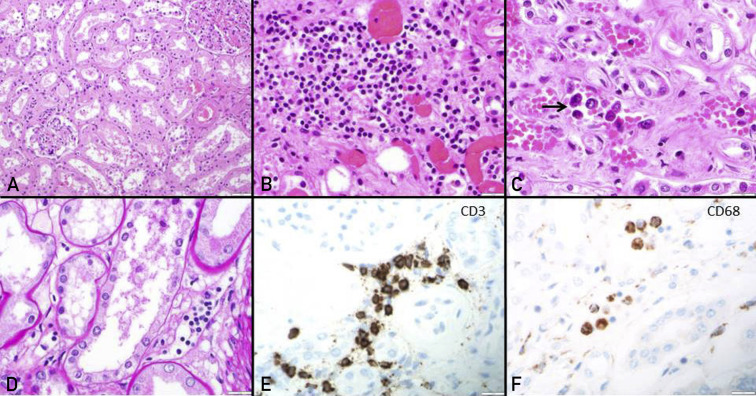
The kidney autopsy findings are characterized in Table 2 . Renal pathology was characterized by moderate-to-severe acute tubular injury in all (Figure 1 A). Most showed marked simplification of the lining epithelium and luminal ectasia. Myoglobin casts were observed in two cases and osmotic tubulopathy in one. Tubulointerstitial inflammation was mild with macrophage and CD3-positive T-cell dominance. Peritubular capillaritis, with a mixed T-cell and macrophage immunophenotype was seen in 13 (76.5%) (Figure 1B-F). Similarly, glomerulitis, with a mixed T-cell and macrophage immunophenotype was seen in the same 13 (76.5%). The four patients who did not show microvascular inflammation had been treated with a combination of remdesivir and either dexamethasone (n=2) or convalescent plasma (n=2). Patients treated with dexamethasone alone or remdesivir alone had microvascular inflammation. Neutrophilic microvascular inflammation and interstitial infiltration was not a conspicuous feature in any of the cases. Nonimmune glomerular pathology included nodular diabetic glomerulosclerosis in 1 (5.9%); whereas collapsing focal segmental glomerulosclerosis or acute thrombotic microangiopathy was not identified. Marked peritubular capillary congestion was noted in all cases (Figure 1B and C). Moderate to severe arteriosclerosis and arteriolar hyalinosis were noted in 14 (82.4%) and 11 (64.7%), respectively. Only mild glomerulosclerosis (<25%) and tubulointerstitial scarring (<25%) were noted in all 17 autopsies.
Table 2.
Summary of Salient Autopsy Findings With Emphasis on Renal Pathologya
| Autopsy characteristics | n (%) |
|---|---|
| SARS-CoV-2 polymerase chain reaction (n=17) | |
| Antemortem positivity | 17 (100) |
| Duration from diagnosis to autopsy (median; range in days) | 12 (0-67) |
| SARS-CoV-2 detected/indeterminate at autopsy | 14 (82) |
| Median postmortem interval, hours | 18 |
| Cause of death (n=17) | |
| Complications of COVID-19 | 16 (94) |
| Ischemic heart disease | 1 (6) |
| Pneumonia/diffuse alveolar damage (n=16) | |
| Acute bronchopneumonia | 8 (50) |
| Acute and organizing bronchopneumonia | 4 (25) |
| Organizing pneumonia | 3 (19) |
| Diffuse alveolar damage | 1 (6) |
| Extent of autolysis (n=17) | |
| Mild | 11 (65) |
| Moderate | 6 (35) |
| Severe | 0 (0) |
| Renal pathology (n=17) | |
| Acute tubular injury | 17 (100) |
| Glomerulitis, moderate | 13 (76) |
| Dominant immunophenotype is mixed macrophage and T cell | 13 (100) |
| Peritubular capillaritis, moderate | 13 (76) |
| Dominant immunophenotype is mixed macrophage and T cell | 13 (100) |
| Tubulointerstital inflammation | |
| Mild | 17 (100) |
| Moderate | 0 (0) |
| Severe | 0 (0) |
| Immunophenotype | |
| Neutrophil | 0 (0) |
| B cell | 3 (18) |
| Mixed macrophage and T cell | 17 (100) |
| Peritubular capillary congestion | 17 (100) |
| Nonimmune glomerular pathology | |
| Nodular diabetic glomerulosclerosis | 1 (6) |
| Acute thrombotic microangiopathy | 0 (0) |
| Vascular pathology (n=17) | |
| Arteriolar hyalinosis, moderate to severe | 11 (65) |
| Arterial sclerosis, moderate to severe | 14 (82) |
| Interstitial fibrosis and tubular atrophy (n=17) | |
| Mild | 17 (100) |
| Moderate | 0 (0) |
| Severe | 0 (0) |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 1.
Acute tubular injury and immune cell infiltration in coronavirus disease 2019 kidneys. (A) The renal cortex on light microscopy shows acute tubular injury with lumina ectasia (hematoxylin and eosin [HE] stain, original magnification ×20). (B) Mild mononuclear interstitial infiltration and peritubular capillary congestion is noted (HE stain, original magnification ×40). (C and D) Peritubular capillaritis (arrow) is a notable feature within congested capillaries (C). The microvascular inflammation is composed of CD3-positive T cells (E) and CD68-positive macrophages (F) (original magnification ×40 for E and F).
The findings of microvascular inflammation suggested immune-mediated injury in severe COVID-19–associated renal disease. We further sought to characterize the molecular and ultrastructural profile of COVID-19 kidneys. Ultrastructural, transcriptomic, and proteomic profiles of the kidneys of seven patients who had only mild autolysis and for whom glutaraldehyde fixed tissue for ultrastructural examination was available were evaluated. The findings were compared with two age-matched non–COVID-19 control cohorts with AKI: S-AKI (all with bronchopneumonia) and NS-AKI. The clinical characteristics of the three groups are shown in Table 3 . The mean age of the groups was 78, 71, and 74 years, respectively (P=.6). There were more women in the S-AKI group than in either of the other two groups. The mean terminal serum creatinine level was 3.0 mg/dL, 1.6 mg/dL, and 1.3 mg/dL, respectively (P=.32). All three groups had similar numbers of comorbidities and were comparable with respect to chronic microstructural changes in the renal parenchyma.
Table 3.
Demographic and Relevant Clinicopathology Characteristics of Three Acute Kidney Injury Cohortsa
| AKI cohort | Age, years/sex | Comorbidities | COVID-19/days from diagnosis/Rx | Serum creatinine (mg/dL) | AKI stage | Cause of AKI | ATN | Inflammation | TUNEL- positive (cells/hpf) |
Capillary congestion | Chronicity | Other organ pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 | ||||||||||||
| 1 | 71/M | HT, CAD | Positive/11/Dexamethasone | 7.19 | 3 | COVID-19 | +++ | PTC-2 G-0 Interstitial |
3 | +++ | Mild IFTA and GS | DAD, acute and organizing pneumonia |
| 2 | 86/M | HT, CAD, CHF, dementia | Positive/6/none | 6.28 | 3 | COVID-19 | +++ | PTC-2 G-2 Interstitial |
4 | +++ | Mild IFTA and GS | DAD, acute pneumonia |
| 3 | 80/M | CVA, dementia, cancer | Positive/7/none | 1.53 | 1 | COVID-19 | +++ | PTC-2 G-2 Interstitial |
3 | +++ | Mild IFTA and GS | DAD, acute pneumonia |
| 4 | 73/M | DM, Cancer | Positive/ 25/ remdesvir + convalescent plasma | 1.04 | 0 | COVID-19 | +++ | PTC-0 G-0 Interstitial-0 |
2 | +++ | Mild IFTA and GS | DAD, organizing diffuse |
| 5 | 93/M | Dementia, CHF, cancer | Positive/4/None | 2.19 | 2 | COVID-19 | +++ | PTC-2 G-2 Interstitial |
3 | +++ | Mild IFTA and Mild GS | DAD, acute pneumonia |
| 6 | 77/M | Dementia | Positive/12/None | 0.79 | 1 | COVID-19 | +++ | PTC-2 G-0 Interstitial |
3 | +++ | Mild IFTA and GS | DAD, acute pneumonia |
| 7 | 69/F | DM, CAD, HT, dementia | Positive/18/None | 2.02 | 2 | COVID-19 | +++ | PTC-0 G-0 Interstitial |
4 | +++ | Mild IFTA and GS | DAD, acute pneumonia |
| Sepsis | ||||||||||||
| 1 | 49/F | Obesity | Negative | 1.41 | 1 | E.coli sepsis with BP | +++ | PTC--2 G-2 Interstitial |
5 | + | Mild IFTA and GS | Organizing and focal acute pneumonia |
| 2 | 62/M | COPD; HT | Negative | 1.6 | 1 | E.coli sepsis with BP | +++ | PTC-2 G-1 Interstitial |
4 | + | Mild IFTA and GS | Acute bronchopneumonia with DAD |
| 3 | 62/M | Metastatic cancer; HT | Negative | 1.76 | 1 | Acute BP | +++ | PTC-2 G-2 Interstitial |
4 | + | Mild IFTA and GS | Acute BP |
| 4 | 85/F | HT/Carcinoma breast | Negative | 1.2 | 1 | Acute BP | +++ | PTC-2 G-1 Interstitial |
3 | + | Mild IFTA and GS | Acute and organizing pneumonia with DAD |
| 5 | 87/F | DM/HT/ Carcinoma lung | Negative | 1.4 | 1 | Acute BP | +++ | PTC-2 G-1 Interstitial |
3 | + | Mild IFTA and GS | Squamous cell carcinoma lung |
| 6 | 75/F | HT/ Heart failure | Negative | 2.33 | 2 | Acute BP | +++ | PTC-2 G-1 Interstitial |
6 | + | Mild IFTA and GS | Gangrene bilateral toes and acute BP |
| 7 | 78/M | HT/ Valvular heart disease | Negative | 1.35 | 1 | Acute BP | +++ | PTC-1 G-1 Interstitial |
4 | + | Mild IFTA and GS | Acute and organizing pneumonia; Pulmonary microthrombi; splenic infarct |
| Non-sepsis | ||||||||||||
| 1 | 84/M | HT, CAD, dementia | Negative | 2.55 | I | Hemodynamic | +++ | PTC-0 G-1 Interstitial-0 |
1 | _ | Mild IFTA and GS | Atherosclerotic cardiovascular disease |
| 2 | 81/F | CAD, CHF, HT | Negative | 0.55 | 0 | Hemodynamic | +++ | PTC-0 G-1 Interstitial-0 |
0 | _ | Mild IFTA and GS | Ischemic heart disease |
| 3 | 76/M | Dementia | Negative | 1.7 | I | Hemodynamic | +++ | PTC-0 G-0 Interstitial-0 |
1 | _ | Mild IFTA and GS | Atherosclerotic cardiovascular disease |
| 4 | 64/M | HT, DM, obesity | Negative | 0.85 | 0 | Hemodynamic | +++ | PTC-0 G-0 Interstitial-0 |
0 | _ | Mild IFTA and GS | Atherosclerotic cardiovascular disease |
| 5 | 66/M | CAD | Negative | 1.9 | I | Hemodynamic | +++ | PTC-0 G-0 Interstitial-0 |
2 | - | Mild IFTA and GS | Ischemic heart disease |
| 6 | 78/M | HT | Negative | 0.78 | 0 | Hemodynamic | +++ | PTC-0 G-0 Interstitial-0 |
1 | - | Mild IFTA and GS | Atherosclerotic cardiovascular disease |
| 7 | 70/M | CAD, HT, DM | Negative | 0.9 | 0 | Hemodynamic | +++ | PTC-0 G-0 Interstitial-0 |
0 | - | Mild IFTA and GS | Acute myocardial infarction |
AKI, acute kidney injury; ATN, acute tubular necrosis; BP, bronchopneumonia; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CVA, cerebrovascular accident; DAD, diffuse alveolar damage; DM, diabetes; F, female; G, glomerulitis; GS, glomerulosclerosis; hpf, high power field; HT, hypertension; IFTA, interstitial fibrosis and tubular atrophy; M, male; PTC, peritubular capillary; Rx, treatment; TUNEL, terminal deoxynucleotidyl transferase of dUTP nick end labeling.
Morphological Characteristics of the Three AKI Cohorts
Microvascular inflammation (glomerulitis and peritubular capillaritis) was more prominent in patients with COVID-19 (5 of 7 patients) and S-AKI (7 of 7 patients), as compared with NS-AKI controls (0 of 7 patients). Mononuclear inflammatory cells were the predominant inflammatory cell type in both, whereas neutrophils were noted only infrequently, and only in S-AKI. Parenchymal tubulointerstitial inflammation was patchy, mild (3 of 7 patients), or moderate (4 of 7 patients), and largely mononuclear in COVID-19 and S-AKI, as compared with minimal inflammation in the NS-AKI controls (7 of 7 patients).
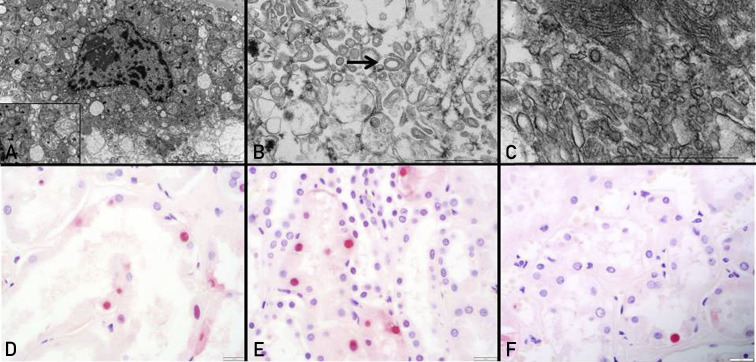
On ultrastructural evaluation of COVID-19 AKI, there was evidence of tubular injury with cytoplasmic vacuolization, loss of brush border, and mitochondrial injury. The latter was characterized by swelling, vacuolization, and distortion of cristae. Condensed mitochondria and distorted circular mitochondria were identified. Flocculent densities, and large vesicles with abundant clathrin-coated vesicles resembling viral particles were also identified (Figure 2 A-C) Tubuloreticular inclusions were not seen in the endothelial cytoplasm of COVID-19 cases. Peritubular capillary endothelial injury was noted. Multilayering of the glomerular and peritubular capillary basement membranes, features of more chronic endothelial injury, was not appreciated. Apoptosis of tubular epithelial cells was more common in COVID-19 (3.1/hpf) and S-AKI (4.1/hpf) as compared with NS-AKI controls (0.7/hpf) (P<.001) (Figure 2D-F).
Figure 2.
Evidence of tubular injury with mitochondrial insult and apoptosis in coronavirus disease 2019 kidneys. (A) Ultrastructural evaluation of proximal tubular epithelial cells show cytoplasmic vacuolization and swollen mitochondria with distorted cristae (see inset). (B) The mitochondrial injury includes ring- or donut-shaped mitochondria (arrow). (C) Clathrin-coated vesicles, easily mistaken for the virus, are also noted (arrow). Terminal deoxynucleotidyl transferase of dUTP (TUNEL) assay allows detection of TUNEL-positive cells (black arrow) in tubular epithelial cells of coronavirus disease 2019 kidneys (D), sepsis-associated acute kidney injury (E), and non-sepsis–related acute kidney injury (F).
SARS-CoV-2 IHC and in situ hybridization studies on kidney sections were negative in all seven COVID-19 cases. Definitive viral inclusions were not identified on ultrastructural studies of podocytes, endothelium, or tubules.
Molecular Characteristics of the Three AKI Cohorts
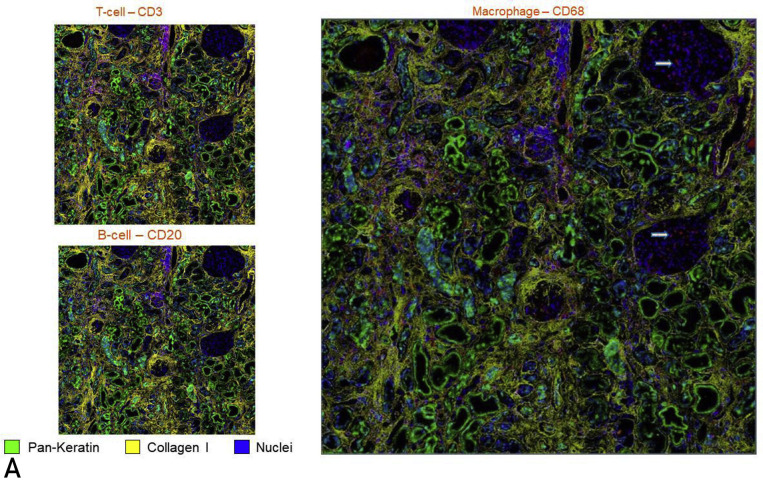
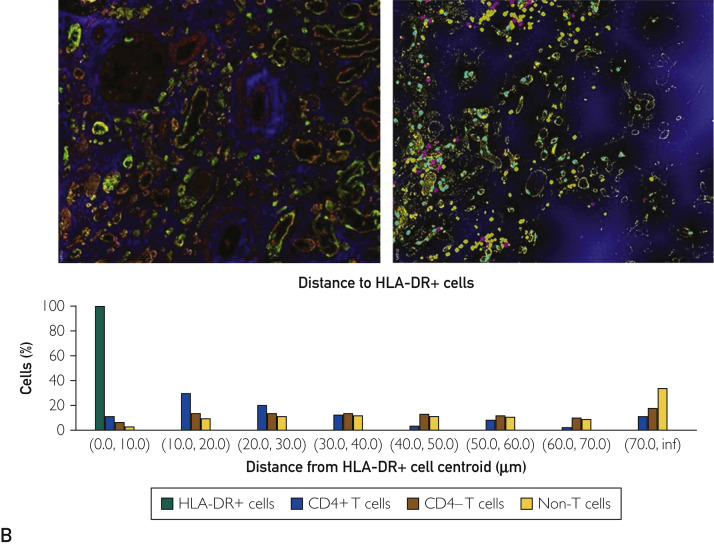
To define the phenotype of cellular infiltrates and the spatial architecture of AKI in COVID-19 kidneys, high-parameter imaging mass cytometry to detect 23 cell surface markers was performed on a Hyperion imaging system coupled to a Helios time-of-flight mass cytometer (Supplemental Table S1, available online at http://www.mayoclinicproceedings.org). Evidence of an adaptive immune response was suggested by the clustering of CD3+CD45RO+ cells in the tubulointerstitium in each case. The predominant innate immune response was underscored by an enriched population of macrophages (CD68+) both in the interstitium and glomerular capillary loops (Figure 3 A). The computational multiplex neighborhood analysis revealed that overall, the CD3+CD4+ cells were enriched in close proximity to antigen-presenting cells (HLA-DR+), compared with CD3+CD4- T cells and non–T cells (Figure 3B).
Figure 3.
Hyperion imaging mass cytometry showing simultaneous immunophenotyping of inflammatory cells and renal structural components with exploration of their spatial relation. (A) Presence of T cells (left, top) and macrophages (right) in the tubulointerstitial compartment with a conspicuous absence of B cells (left, bottom). Glomerulitis is appreciated in the right panel with margination of macrophages (arrows). All cells of interest have been tagged red. (B) Computational image neighborhood analysis was performed after labeling tubules (PanK and E-cadherin), intertubular stroma (collagen and alpha smooth muscle actin) and glomeruli (vimentin) (left). After cells were classified using a supervised approach (CD4+ T cells, CD4- T cells, and non-T cells), a distance map measuring the closest distance from the HLA-DR+ cells to each of the other cells was generated (middle, HLA-DR in yellow, distance from HLA-DR shown in increasing intensity of blue color). The percent of cells within each defined distance bin is displayed (right).
Kidney samples from COVID-19 cases and controls were analyzed by NanoString platform using the nCounter Human Organ Transplant Panel that profiles expression of 770 genes associated with different pathways related to immune response, tissue injury, and mechanisms of action for immunosuppressive drugs.17
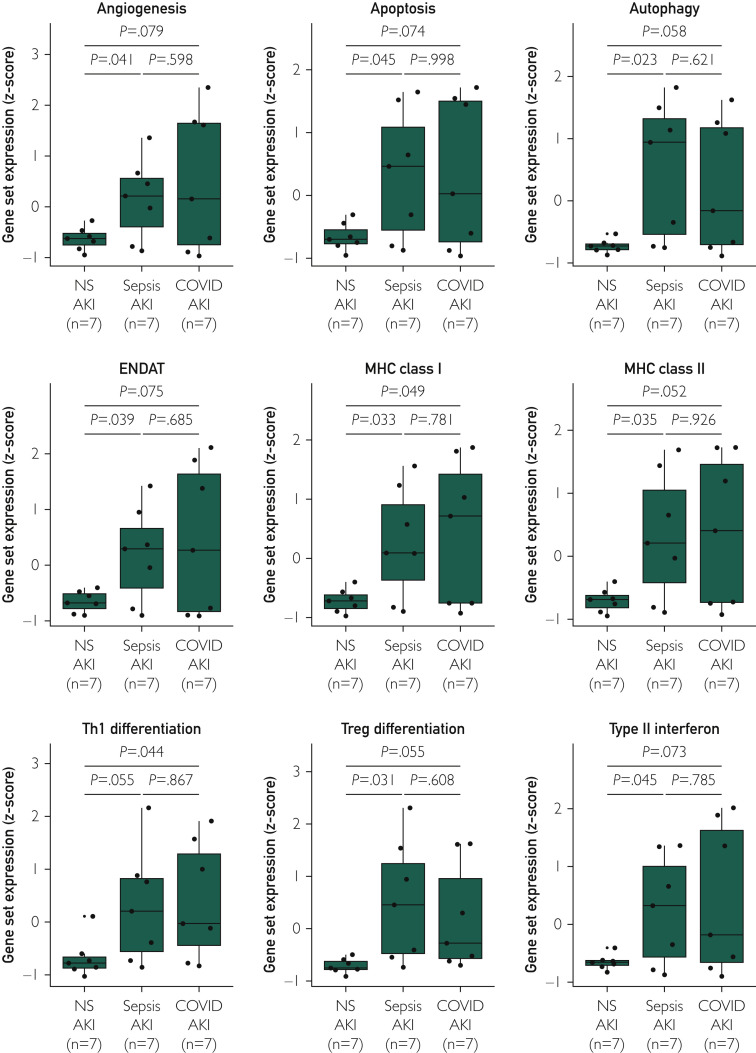
Gene expression analysis revealed that COVID-19 kidneys had significant enrichment of multiple functional annotation transcript sets, including apoptosis, autophagy, major histocompatibility complex class I and II expression, and type 1 T helper cell and regulatory T cell (Treg) differentiation. The gene expression patterns of the COVID-19 cases resembled those of S-AKI (Figure 4 ).
Figure 4.
Transcriptomic analysis. Box plots show the relative expression of select functional annotation gene sets between coronavirus disease 2019 (COVID-19) acute kidney injury (AKI), sepsis-related AKI (S-AKI) and nonsepsis-related AKI (NS-AKI). ENDAT, endothelial cell-associated transcripts; MHC, major histocompatibility complex; Th1, type 1 T helper cell; Treg, regulatory T cell.
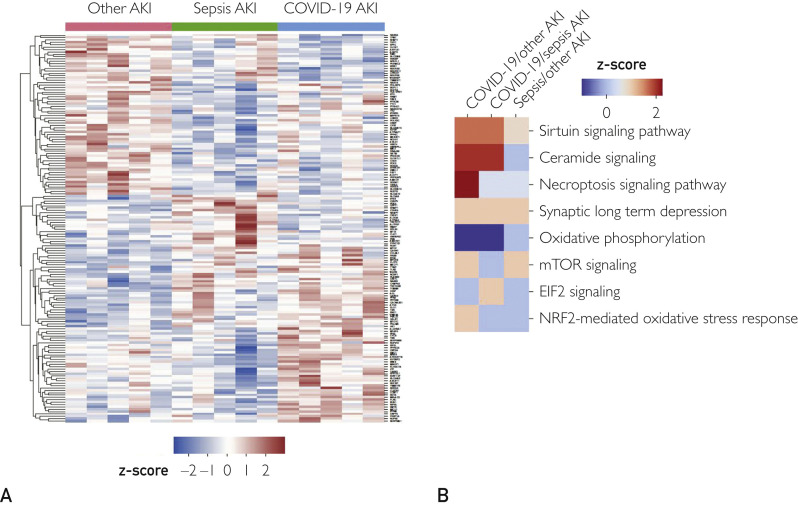
In light of the inflammation observed in COVID-19 kidneys both morphologically and at the molecular level, we wanted to identify alterations in the proteome that could suggest unique mechanisms for COVID-19 AKI. Therefore, we performed quantitative proteomics analysis from formalin-fixed paraffin-embedded kidney sections of COVID-19 and both sets of control samples. A total of 3594 proteins were identified, of which 164 were differentially expressed between the groups (P<.05): 87 between COVID-19 and NS-AKI (16 with a fold-change > 1.2), 81 between S-AKI and NS-AKI (8 with a fold-change > 1.2), and 64 between COVID-19 and S-AKI (6 with a fold-change > 1.2). A hierarchical clustering was conducted for all the proteins found in the three groups and a heat map was generated (Figure 5 A). Ingenuity Pathway Analysis demonstrated that COVID-19 AKI kidneys were enriched in proteins involved in the sirtuin and necroptosis signaling pathways, both involved in regulatory response to inflammation (Supplemental Table S2, available online at http://www.mayoclinicproceedings.org). Ceramide signaling pathway upregulation was unique in COVID-19 kidneys when compared with NS-AKI, whereas oxidative phosphorylation was downregulated in COVID-19 AKI (Figure 5B).
Figure 5.
Proteomic analysis. (A) The heat map gives a global overview of the differential expression of protein levels for each individual gene in the three acute kidney injury (AKI) cohorts. Proteins that were significantly over- (red) or under-expressed (blue) at P<.05 are shown. (B) The Ingenuity Pathway Analysis of differential protein expression in coronavirus disease 2019 (COVID-19) AKI, sepsis-associated AKI, and other AKI controls is shown. EIF2, Eukaryotic initiation factor 2; mTOR, mammalian target of rapamycin; NRF2, nuclear factor erythroid 2–related factor 2.
Discussion
Our study examined the morphologic and molecular characteristics of kidneys obtained during autopsies of patients who died of COVID-19. The morphological and molecular profile of severe COVID-19 renal injury resembles S-AKI.19 This includes microvascular dysfunction with inflammation, that is, a macrophage-dominant cellular phenotype and a prominent T cell response in the tubulointerstitium in close proximity to antigen-presenting cells noted on spatial architectural analysis. The decreased oxidative phosphorylation and upregulation of ceramide-signaling pathways with ultrastructural evidence of mitochondrial injury noted in COVID-19 AKI indicates metabolic reprogramming of tubular epithelial cells that has been described in S-AKI.
COVID-19–Associated Inflammatory Response
Overall, the COVID-19 kidneys had significantly more inflammation compared with NS-AKI controls. The molecular signals of inflammation and immune activation observed in COVID-19 kidneys suggests a global antiviral response characterized by a macrophage and T-cell–rich inflammation, and type II interferon production, as widely suggested by blood-based assays.20 , 21 The CD4+-dominant T-cell response, as we observed in the COVID-19 kidneys, has been associated with worse clinical outcomes.22 Severe acute respiratory syndrome coronavirus 2–specific CD4+ T cells directed to the spike surface glycoprotein have been detected in patients with severe COVID-19, and may account for the CD4+ T cells found in the tubulointerstitial compartment of the kidneys.23 The molecular pathways, investigated both at the genomic and proteomic level, confirmed that inflammation is the predominant driver of the renal injury seen in COVID-19, similar to S-AKI. In both groups, we found increased expression of gene sets associated with counter regulatory mechanisms normally seen in immune activation, including Treg differentiation and sirtuin signaling.24 In severe COVID-19 cases, increase in circulating Treg frequency is associated with worse outcomes, presumed secondary to suppression of antiviral T-cell responses.25
COVID-19–Associated Signaling Pathways
In COVID-19 kidneys, as well as in S-AKI, we found evidence of increased apoptosis. Although apoptosis is potentially virally mediated via the extrinsic pathways,26 the upregulated ceramide pathway noted in our COVID-19 AKI cohort coupled with the reduced oxidative phosphorylation signals suggest that ceramide-induced apoptosis via mitochondrial disruption might play a role in tubular cell death.27 Mitochondria of the proximal tubular epithelial cells of the COVID-19 kidneys showed marked vacuolization and distortion of the cristae as has been described previously.28 The upregulated ceramide pathway in COVID-19 AKI underscores the role of lipid dysregulation in severe COVID-19 as suggested by others.29 Necroptosis, an immunogenic cell death pathway that can eliminate virus-infected cells and mobilize both innate and adaptive immune responses to restrict virus replication, was also more profound in COVID-19 kidneys, compared with NS-AKI. Necroptosis plays a major role in lung injury following SARS-COV2 infection.26 , 30 , 31
COVID-19 AKI Resembles Sepsis-Associated AKI
Kellum et al16 and others have suggested that COVID-19 AKI is very similar to S-AKI.32 Our observations of microvascular dysfunction, inflammation, and the metabolic reprogramming (decreased oxidative phosphorylation and increased ceramide signaling) in COVID-19 AKI are similar to the fundamental pathophysiologic mechanisms described in S-AKI.19 , 33 , 34 We summarize the key findings in our study and draw comparisons to published literature in COVID-AKI and S-AKI in Table 4 .19 , 26 , 29 , 31 , 33, 34, 35, 36, 37, 38 These pathologies show potential therapeutic targets for COVID-19.
Table 4.
Morphological and Molecular Comparison of COVID-19 AKI to Sepsis AKIa
| COVID- 19 | Sepsis | Referencesb | |
|---|---|---|---|
| Morphology | |||
| Immune response and microvascular inflammation | ++ | ++ | 19,33,34 |
| Apoptosis | + | + | 26 |
| Molecular | |||
| Gene expression analysis | |||
| Apoptosis, autophagy, MHC class I and II expression, Th1 Treg differentiation | + | ++ | |
| Proteomic pathway analysis | |||
| Sirtuin signaling pathway | ++ | + | 35,36 |
| mTOR signaling pathway | + | + | 37 |
| Necroptosis | ++ | + | 26,30,31 |
| Oxidative phosphorylation | -- | 38 | |
| Ceramide signaling pathway | ++ | 29 |
Study Limitations
The main limitation of the current study is that although we had the ability to compare molecular characteristics of COVID-19–related pathology against S-AKI autopsies, we were not able to compare with autopsies of patients who died of other viral-mediated AKI. This requires planning and prospective studies to obtain optimal preservation of autopsy tissue for omics studies. The preservation artifacts noted on ultrastructural studies of archived formalin-fixed non–COVID-19 autopsy tissue from prior patients did not allow for comparison. Additionally, statistical analysis did not adjust for multiple testing and a larger sample size could have provided a more robust gene expression analysis. The strength of our study is that we were able to perform a detailed morphological analysis and multi-omic platform approach to study severe COVID-19 kidney injury and draw parallels to sepsis-associated injury.
Conclusion
This series is dedicated to the renal histopathologic, ultrastructural, and molecular findings in patients who had SARS-CoV-2 infection, which provides evidence for an innate and prominent T cell immune response. The morphological and molecular characteristics of COVID-19 AKI resemble those of S-AKI. Mitochondrial dysfunction may play a central role in COVID-19 AKI.
Acknowledgment
Drs Badley, Pandey, and Taner contributed equally to this work. Ms Katie Reed and Tiffany Mainella provided research for support.
Footnotes
Potential Competing Interests: The authors report no potential competing interests.
Grant Support: Research funding was obtained from the Anatomic Pathology Research Funds, Department of Laboratory Medicine and Pathology, Mayo Clinic.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pei G., Zhang Z., Peng J. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoriello D., Khairallah P., Bomback A.S. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bois M.C., Boire N.A., Layman A.J. COVID-19–associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143(3):230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso C., Leone O., Rizzo S. Pathological features of COVID-19–associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roden A.C., Bois M.C., Johnson T.F. The spectrum of histopathologic findings in lungs of patients with fatal coronavirus disease 2019 (COVID-19) infection. Arch Pathol Lab Med. 2021;145(1):11–21. doi: 10.5858/arpa.2020-0491-SA. [DOI] [PubMed] [Google Scholar]
- 10.Carsana L., Sonzogni A., Nasr A. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanley B., Naresh K.N., Roufosse C. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menter T., Haslbauer J.D., Nienhold R. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stasi A., Castellano G., Ranieri E. SARS-CoV-2 and viral sepsis: immune dysfunction and implications in kidney failure. J Clin Med. 2020;9(12):4057. doi: 10.3390/jcm9124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golmai P., Larsen C.P., DeVita M.V. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellum J.A., Nadim M.K., Forni L.G. Sepsis-associated acute kidney injury: is COVID-19 different? Kidney Int. 2020;98(6):1370–1372. doi: 10.1016/j.kint.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.nCounter Human Organ Transplant Panel https://www.nanostring.com/products/ncounter-assays-panels/immunology/human-organ-transplant/
- 18.Adam B., Afzali B., Dominy K.M. Multiplexed color-coded probe-based gene expression assessment for clinical molecular diagnostics in formalin-fixed paraffin-embedded human renal allograft tissue. Clin Transplant. 2016;30(3):295–305. doi: 10.1111/ctr.12689. [DOI] [PubMed] [Google Scholar]
- 19.Gomez H., Kellum J.A. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22(6):546–553. doi: 10.1097/MCC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Candia P., Prattichizzo F., Garavelli S., Matarese G. T cells: warriors of SARS-CoV-2 infection. Trends Immunol. 2021;42(1):18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattler A., Angermair S., Stockmann H. SARS-CoV-2–specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest. 2020;130(12):6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew D., Giles J.R., Baxter A.E. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508):eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiskopf D., Schmitz K.S., Raadsen M.P. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5(48):eabd2071. doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendes K.L., Lelis D.F., Santos S.H.S. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev. 2017;38:98–105. doi: 10.1016/j.cytogfr.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Galvan-Pena S., Leon J., Chowdhary K. Profound Treg perturbations correlate with COVID-19 severity. bioRxiv. 2020 doi: 10.1073/pnas.2111315118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S., Zhang Y., Guan Z. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther. 2020;5(1):235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda N. Ceramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phoshate. Int J Mol Sci. 2015;16(3):5076–5124. doi: 10.3390/ijms16035076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadimitriou J.C., Drachenberg C.B., Kleiner D., Choudhri N., Haririan A., Cebotaru V. Tubular epithelial and peritubular capillary endothelial injury in COVID-19 AKI. Kidney Int Rep. 2021;6(2):518–525. doi: 10.1016/j.ekir.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caterino M., Gelzo M., Sol S. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci Rep. 2021;11(1):2941. doi: 10.1038/s41598-021-82426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nailwal H., Chan F.K. Necroptosis in anti-viral inflammation. Cell Death Differ. 2019;26(1):4–13. doi: 10.1038/s41418-018-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galluzzi L., Kepp O., Chan F.K., Kroemer G. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol. 2017;12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olwal C.O., Nganyewo N.N., Tapela K. Parallels in Sepsis and COVID-19 Conditions: Implications for Managing Severe COVID-19. Front Immunol. 2021;12:602848. doi: 10.3389/fimmu.2021.602848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerolle N., Nochy D., Guerot E. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36(3):471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 34.Zarjou A., Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22(6):999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 35.Miller R., Wentzel A.R., Richards G.A. COVID-19: NAD(+) deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Med Hypotheses. 2020;144:110044. doi: 10.1016/j.mehy.2020.110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L., Chen Z., Fu W., Cai S., Zeng Z. Emerging evidence concerning the role of sirtuins in sepsis. Crit Care Res Pract. 2018;2018:5489571. doi: 10.1155/2018/5489571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Mm W., Long Y., Wang H., Han Mm W., Zhang J., Cui N. Role of the mTOR Signalling Pathway in Human Sepsis-Induced Myocardial Dysfunction. Can J Cardiol. 2019;35(7):875–883. doi: 10.1016/j.cjca.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Shenoy S. Coronavirus (COVID-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm Res. 2020;69(11):1077–1085. doi: 10.1007/s00011-020-01389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.