Abstract
Background
The spectrum of neurological and psychiatric complications associated with paediatric SARS-CoV-2 infection is poorly understood. We aimed to analyse the range and prevalence of these complications in hospitalised children and adolescents.
Methods
We did a prospective national cohort study in the UK using an online network of secure rapid-response notification portals established by the CoroNerve study group. Paediatric neurologists were invited to notify any children and adolescents (age <18 years) admitted to hospital with neurological or psychiatric disorders in whom they considered SARS-CoV-2 infection to be relevant to the presentation. Patients were excluded if they did not have a neurological consultation or neurological investigations or both, or did not meet the definition for confirmed SARS-CoV-2 infection (a positive PCR of respiratory or spinal fluid samples, serology for anti-SARS-CoV-2 IgG, or both), or the Royal College of Paediatrics and Child Health criteria for paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS). Individuals were classified as having either a primary neurological disorder associated with COVID-19 (COVID-19 neurology group) or PIMS-TS with neurological features (PIMS-TS neurology group). The denominator of all hospitalised children and adolescents with COVID-19 was collated from National Health Service England data.
Findings
Between April 2, 2020, and Feb 1, 2021, 52 cases were identified; in England, there were 51 cases among 1334 children and adolescents hospitalised with COVID-19, giving an estimated prevalence of 3·8 (95% CI 2·9–5·0) cases per 100 paediatric patients. 22 (42%) patients were female and 30 (58%) were male; the median age was 9 years (range 1–17). 36 (69%) patients were Black or Asian, 16 (31%) were White. 27 (52%) of 52 patients were classified into the COVID-19 neurology group and 25 (48%) were classified into the PIMS-TS neurology group. In the COVID-19 neurology group, diagnoses included status epilepticus (n=7), encephalitis (n=5), Guillain-Barré syndrome (n=5), acute demyelinating syndrome (n=3), chorea (n=2), psychosis (n=2), isolated encephalopathy (n=2), and transient ischaemic attack (n=1). The PIMS-TS neurology group more often had multiple features, which included encephalopathy (n=22 [88%]), peripheral nervous system involvement (n=10 [40%]), behavioural change (n=9 [36%]), and hallucinations at presentation (n=6 [24%]). Recognised neuroimmune disorders were more common in the COVID-19 neurology group than in the PIMS-TS neurology group (13 [48%] of 27 patients vs 1 [<1%] of 25 patients, p=0·0003). Compared with the COVID-19 neurology group, more patients in the PIMS-TS neurology group were admitted to intensive care (20 [80%] of 25 patients vs six [22%] of 27 patients, p=0·0001) and received immunomodulatory treatment (22 [88%] patients vs 12 [44%] patients, p=0·045). 17 (33%) patients (10 [37%] in the COVID-19 neurology group and 7 [28%] in the PIMS-TS neurology group) were discharged with disability; one (2%) died (who had stroke, in the PIMS-TS neurology group).
Interpretation
This study identified key differences between those with a primary neurological disorder versus those with PIMS-TS. Compared with patients with a primary neurological disorder, more patients with PIMS-TS needed intensive care, but outcomes were similar overall. Further studies should investigate underlying mechanisms for neurological involvement in COVID-19 and the longer-term outcomes.
Funding
UK Research and Innovation, Medical Research Council, Wellcome Trust, National Institute for Health Research.
Introduction
Neurological and psychiatric complications of infection with SARS-CoV-2 have been recognised as part of COVID-19 in adults.1, 2, 3, 4, 5 In children and adolescents (younger than 18 years), single case series have identified neurological complications associated with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS; also known as multisystem inflammatory syndrome in children [MIS-C])—a rare, newly described, severe secondary inflammatory syndrome that typically occurs weeks after SARS-CoV-2 infection.6, 7, 8 Nine children with neurological complications of PIMS-TS have previously been reported from a single centre in the UK.8 To date, one international series has described the neuroimaging findings in 38 children,9 and a study of 1695 hospitalised children and young people (younger than 21 years) in the USA has shown transient neurological involvement in 365 patients and life-threatening neurological conditions in 43 patients.10 However, the prevalence of these complications is not known. We aimed to analyse the range and prevalence of neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents.
Research in context.
Evidence before this study
We searched PubMed for studies of children and adolescents with neurological or psychiatric features associated with COVID-19, published from database inception to May 5, 2021, with no language restrictions, and a focus on studies that yielded clinical descriptions and imaging findings in the cohorts described. The search yielded 183 publications, which were mostly case reports or small case series, limiting the description of the spectrum of features in the paediatric population. Two studies described larger series: one international study focused on neuroimaging findings and another described both transient and life-threatening complications from multiple centres in the USA. Two smaller studies described the manifestations of the newly described paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) from two individual centres.
Added value of this study
To our knowledge, this is the first report of a nation-wide study of neurological or psychiatric features of COVID-19 in children and adolescents. Using national hospital admission data for COVID-19 in children and adolescents allowed us to estimate the prevalence of these features for the first time. A wide range of neurological disorders and features were described, with clear differences between those who presented with and without PIMS-TS. Patients presenting without PIMS-TS had a discrete primary neurological disorder; recognised neuroimmune disorders were common in this group (present in approximately half of these patients). Other presentations included status epilepticus, movement disorders, and psychosis. Those with PIMS-TS had multiple overlapping neurological features, and encephalopathy and peripheral nervous system involvement were the most common. Only one patient with PIMS-TS had a recognised neuroimmune disorder, which suggests that perhaps different immune mechanisms underlie neurological manifestations in this newly described inflammatory syndrome. Although patients with PIMS-TS were more likely to require intensive care support, we found that early outcomes were similar in both groups; death was uncommon but one-third had a disability.
Implications of all the available evidence
By comparing children and adolescents with a primary neurological disorder associated with COVID-19 with those with PIMS-TS, we identified clear differences that could help physicians approach the management of these patients. Patients without PIMS-TS had discrete, often recognised neuroimmune, primary neurological disorders. By contrast, those with PIMS-TS had a recognisable phenotype with multiple overlapping neurological features, often with characteristic findings on brain imaging such as the splenial sign in the corpus callosum. Future studies should investigate the longer-term neurocognitive outcomes and the mechanisms underlying the cause of neurological involvement in COVID-19, particularly in those with PIMS-TS.
Methods
Study design and participants
We did a prospective national cohort study of hospitalised children and adolescents in the UK. During the first exponential phase of the COVID-19 pandemic, the CoroNerve study group was established to set up an online network of secure rapid-response notification portals via the major UK neuroscience and psychiatry bodies, including the British Paediatric Neurology Association (BPNA). From April 2, 2020, members of the BPNA were invited by the British Paediatric Neurology Surveillance Unit (BPNSU) to notify the body of any hospitalised children or adolescents they considered to have neurological or psychiatric complications associated with COVID-19. Children and adolescents aged younger than 18 years were included if they had: history of laboratory-confirmed SARS-CoV-2 infection, or suspected infection, irrespective of clinical signs and symptoms; new-onset neurological or psychiatric disorder or complication of existing neurological or psychiatric disorder occurring at the time of or following shortly after COVID-19 infection; and COVID-19 infection implicated as a possible cause of neurological or psychiatric disorder by a paediatric neurologist. Patients were still included if they had a pre-existing neurological disorder. Patients were excluded if they did not have a neurological consultation or neurological investigations, or did not meet the definition for confirmed SARS-CoV-2 infection.
This study was approved by the University of Liverpool Institute of Infection, Veterinary, and Ecological Sciences ethics committee (7725/2020) and the University of Southampton Faculty of Medicine ethics committee (56504). The electronic case report form was hosted on ALEA Clinical and managed by the Clinical Information Research Unit (Southampton, UK).
Procedures
Cases were prospectively recorded using a standardised online case report form including demographics; evidence of SARS-CoV-2 infection; clinical characteristics; comorbidities; disease course; requirement for intensive care; laboratory, imaging, and neurophysiology results; and recovery (outcomes). By requesting reporting physicians to submit their contact details at time of notification, we established confirmation of the veracity of the data in all cases and, where required, additional data to confirm the specific clinical details were obtained from the treating clinical team.
Patients were included for analysis if they had confirmed SARS-CoV-2 infection according to WHO criteria (a positive PCR of respiratory or spinal fluid samples, serology for anti-SARS-CoV-2 IgG, or both),1 or they met Royal College of Paediatrics and Child Health criteria for PIMS-TS and had neurological or psychiatric manifestations.7
Clinical neurological or psychiatric disorders were classified into: cerebrovascular event (ischaemic stroke, intracerebral or subarachnoid haemorrhage, cerebral venous sinus thrombosis, or cerebral vasculitis); altered mental status (encephalopathy, encephalitis—defined as encephalopathy with evidence of inflammation in the CNS [cerebrospinal fluid (CSF) white cell count >5 cells per μL, protein >0·45 g/dL, or MRI consistent with inflammation], seizures [clinical or electroencephalographic evidence], and psychiatric syndromes diagnosed by an attending psychiatrist [psychosis, neurocognitive dementia-like syndrome, personality change, catatonia, mania, anxiety or depression, chronic fatigue syndrome, and post-traumatic stress disorder]); or peripheral neurology (Guillain-Barré syndrome [GBS], Miller-Fisher syndrome, brachial neuritis, myasthenia gravis, peripheral neuropathy, myopathy, myositis—defined as myopathy with evidence of inflammation [eg, by MRI or biopsy of muscle with elevated plasma creatine kinase], and critical illness neuromyopathy). Further details on these classifications are available at the BPNSU website.
Patients who were diagnosed with a primary neurological or psychiatric disorder associated with COVID-19, either secondary to acute infection (eg, status epilepticus), or a recognised para-infectious or post-infectious neuroimmune syndrome (eg, GBS), were classified into the COVID-19 neurology group. Patients with neurological features in the context of PIMS-TS were classified into the PIMS-TS neurology group. The diagnosis of PIMS-TS was made in tertiary paediatric units by the multidisciplinary teams responsible for the patient's care. All cases were reviewed by a panel of paediatric neurologists, infectious diseases clinicians, and a neuroradiologist. When patients fulfilled multiple clinical case definitions, the primary diagnosis was adjudicated by the panel. Patients with GBS, acute disseminated encephalomyelitis (ADEM), other acute demyelinating syndromes, or autoimmune encephalitis,11, 12, 13, 14, 15 which are recognised para-infectious or post-infectious immune-mediated neurological disorders, were defined as having neuroimmune disorders. Combined acute-phase reactants were defined as: lactate dehydrogenase, ferritin, and D-dimers. Ethnicity was defined by National Health Service (NHS) coding criteria.16
Modified Rankin scale (mRS) score was assessed at discharge from hospital, or at the most recent clinical assessment, by a neurologist either directly or by using information from the case records.17 A good recovery was defined as an mRS score of 0–1, reflecting no substantial disability or ongoing symptoms, or back to baseline for those with a pre-existing neurological disorder; those with an mRS scale score of 2–5 were classified as having some degree of disability.17 The neurological features or imaging findings of 15 cases have been published previously (appendix pp 7–12);8, 9, 18, 19 these cases were included to reflect the full spectrum of neurological complications of paediatric COVID-19 in the UK.
Statistical analysis
The clinical data cutoff date was Feb 1, 2021. Data were transferred from the online platform to spreadsheet format (Excel 2016) using a custom Python (version 3.8.3) script. Free-text fields describing rationale and context for selections in drop-down lists were manually converted into coded data.
An estimate of the prevalence of cases in England was calculated using hospital admission data in England from the same study dates for children and adolescents.20 As admission data were only available for England, cases from Scotland, Wales, and Northern Ireland were not included in the prevalence calculation. Normality of distribution was assessed using D'Agostino-Pearson Omnibus normality tests. Data were analysed using descriptive statistics (median, range, percentages) and group comparison tests with Student's t tests or Mann-Whitney U tests for continuous variables and χ2 for categorical variables, with two-sided p values less than 0·05 considered significant. The Kruskal-Wallis test with Dunn's multiple group comparison test were used to determine any significant difference in temporality of initial COVID-19-related symptoms to onset of neurological symptoms between groups. Statistical analyses were done with Stata (version 15.1) and GraphPad Prism (version 9.0.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
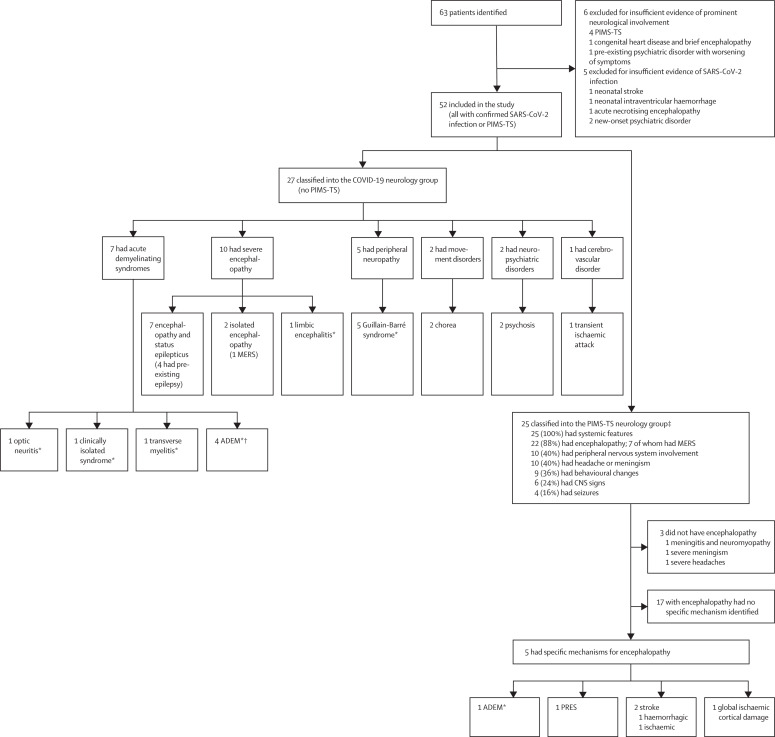
Between April 2, 2020, and Feb 1, 2021, the CoroNerve study group received notification of 63 patients who were geographically dispersed across the UK (62 in England and one in Scotland; figure 1 ). Six patients were excluded as they did not have a neurological consultation or neurological investigations (appendix p 5), and a further five patients were excluded due to insufficient evidence of SARS-CoV-2 infection (appendix pp 5–6).
Figure 1.
Study profile and classification of neurological disorders
PIMS-TS=paediatric multisystem inflammatory syndrome temporally associated with COVID-19. ADEM=acute disseminated encephalomyelitis. MERS=mild encephalopathy with reversible splenial lesion. PRES=posterior reversible encephalopathy syndrome. *Recognised para-infectious or post-infectious syndromes. †All were encephalopathic. ‡Patients could have more than one feature.
52 cases were included in the study; the median patient age was 9 years (range 1–17); 22 (42%) patients were female and 30 (58%) were male (table 1 ). 36 (69%) patients were Black (n=20 [38%]) or Asian (n=16 [31%]) and 16 (31%) were White. Ten (19%) patients had a pre-existing neurological or developmental condition and four (8%) had other pre-existing conditions (appendix pp 7–12). Respiratory symptoms were present at admission in 12 (23%) patients. Eight (15%) patients had isolated neurological or psychiatric features (PCR for respiratory secretions were positive for SARS-CoV-2). 27 (52%) patients were classified into the COVID-19 neurology group and 25 (48%) into the PIMS-TS neurology group. Patients in the PIMS-TS neurology group were more likely to be Black or Asian than those in the COVID-19 neurology group (22 [88%] of 25 patients vs 14 (52%) of 27 patients, p=0·048). During the same period, 1334 children and adolescents with COVID-19 were admitted to hospitals in England. Excluding one case from Scotland, the minimum estimated prevalence of neurological and psychiatric complications in hospitalised children and adolescents with COVID-19 in England was 51 in 1334, or 0·038 (95% CI 0·029–0·050; SE 0·99); ie, 3·8 (95% CI 2·9–5·0) cases per 100 paediatric patients admitted to hospital. The prevalence ranged from 1·8–8·1 cases per 100 patients depending on the region of referral; the highest prevalence was in London.
Table 1.
Demographics, clinical features, investigations, management, and outcomes by study group
| COVID-19 neurology group (n=27) | PIMS-TS neurology group (n=25) | p value | ||
|---|---|---|---|---|
| Age, years | 9 (1–16) | 10 (1–17) | 0·26 | |
| Sex | ||||
| Female | 10 (37%) | 12 (48%) | 0·42 | |
| Male | 17 (63%) | 13 (52%) | .. | |
| Ethnicity | ||||
| White | 13 (48%) | 3 (12%) | .. | |
| Black | 6 (22%) | 14 (56%) | .. | |
| Asian | 8 (30%) | 8 (32%) | 0·0048* | |
| Underlying comorbidity | ||||
| Neurological comorbidities | 8 (30%) | 2 (8%) | 0·048 | |
| Other comorbidities | 1 (4%) | 3 (12%) | 0·26 | |
| Clinical features | ||||
| Systemic features† | 15 (56%) | 25 (100%) | 0·0001 | |
| Respiratory involvement at presentation | 6 (22%) | 6 (24%) | 0·99 | |
| Encephalopathy | 14 (52%) | 22 (88%) | 0·0048 | |
| Seizures | 8 (30%) | 4 (16%) | 0·24 | |
| Headache or meningism | 4 (15%) | 10 (40%) | 0·041 | |
| Peripheral nervous system involvement | 7 (26%) | 10 (40%) | 0·44 | |
| Focal CNS involvement | 4 (15%) | 6 (24%) | 0·84 | |
| Behavioural change | 3 (11%) | 9 (36%) | 0·080 | |
| Hallucinations | 1 (4%) | 6 (24%) | 0·032 | |
| Recognised para-infectious or post-infectious neurological disease | 13 (48%) | 1 (4%) | 0·0003 | |
| Investigations | ||||
| SARS-CoV-2 PCR positive | 21 (78%) | 11 (44%) | 0·012 | |
| SARS-CoV-2 IgG positive | 12 (44%) | 19 (76%) | 0·021 | |
| C-reactive protein, mg/L | 1 (1–161) | 290 (80–556) | <0·0001 | |
| Elevated acute-phase reactants‡ | 4 (15%) | 25 (100%) | <0·0001 | |
| Plasma white cell count, cells per μL | 9·9 (4·0–27·3) | 20 (3·0–44·4) | <0·0001 | |
| CSF white cell count >5 cells per μL | 8 (30%) | 3 (12%) | 0·12 | |
| Abnormal neuroimaging | 11/25 (44%) | 17/23 (74%) | 0·036 | |
| Treatment | ||||
| PICU admission | 6 (22%) | 20 (80%) | <0·0001 | |
| Inotropic support | 0 | 13 (52%) | <0·0001 | |
| Immunomodulation | 12 (44%) | 22 (88%) | 0·0010 | |
| Outcome | ||||
| Disability§ | 10 (37%) | 7 (28%) | 0·48 | |
| Death | 0 | 1 (4%) | 0·29 | |
Data are n (%) or median (range), or p values. p values of less than 0·05 were considered significant. PIMS-TS=paediatric multisystem inflammatory syndrome temporally associated with COVID-19. CSF=cerebrospinal fluid. PICU=paediatric intensive care unit.
Comparison of proportion of patients with Black or Asian ethnicity in the two groups.
Systemic features were fever, shock, hypotension, or rash.
Combined acute-phase reactants were defined as lactate dehydrogenase, ferritin, and D-dimers.
Disability was defined as a modified Rankin scale score of 2–5.
Systemic features (fever, rash, hypotension, shock) were present in 40 (77%) patients; ten (19%) had cardiovascular shock (nine of whom had PIMS-TS). Systemic features were more common in the PIMS-TS neurology group than in the COVID-19 neurology group (25 [100%] of 25 patients vs 15 [56%] of 27, p=0·023). Other features included respiratory symptoms in 12 (23%) patients, seizures in 12 (23%), and status epilepticus in nine (17%).
In the COVID-19 neurology group, 14 (52%) of 27 patients had encephalopathy compared with 22 (88%) of 25 in the PIMS-TS neurology group (p=0·0048). Of these 14 patients, seven developed an encephalopathy associated with status epilepticus (three of whom did not have pre-existing epilepsy); five had encephalitis, two had an isolated encephalopathy. 13 (48%) patients in the COVID-19 neurology group presented with a recognised neuroimmune disorder (five had GBS, four had ADEM [three of whom had myelin oligodendrocyte glycoprotein (MOG) antibodies], three had other acute demyelinating syndromes, and one had autoimmune [limbic] encephalitis) compared with only one (4%) patient in the PIMS-TS neurology group (who had ADEM with MOG antibodies; p=0·0003). Two (7%) patients presented with acute psychosis and two (7%) with chorea. One patient with a previous basal ganglia stroke was diagnosed with a transient ischaemic attack (table 2 ).
Table 2.
Demographics, clinical features, investigations, management, and outcomes in the COVID-19 neurology group by condition subgroup
|
Recognised neuroimmune condition subgroup (n=13) |
Other neurological disorder subgroup (n=14) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Acute demyelinating syndromes (n=7) | GBS (n=5) | Limbic encephalitis (n=1) | Severe encephalopathy (n=9) | Psychiatric disorder (n=2) | Movement disorder (n=2) | Cerebrovascular disorder (n=1) | ||
| Age, years | 5 (1–10) | 6 (1–14) | 4 | 11 (2–16) | 12 (10–14) | 12 (9–14) | 10 | |
| Sex | ||||||||
| Female | 3 (43%) | 2 (40%) | 0 | 3 (33%) | 1 (50%) | 1 (50%) | 0 | |
| Male | 4 (57%) | 3 (60%) | 1 (100%) | 6 (67%) | 1 (50%) | 1 (50%) | 1 (100%) | |
| Ethnicity | ||||||||
| White | 2 (29%) | 2 (40%) | 1 (100%) | 5 (56%) | 0 | 2 (100%) | 1 (100%) | |
| Black | 1 (14%) | 1 (20%) | 0 | 2 (22%) | 2 (100%) | 0 | 0 | |
| Asian | 4 (57%) | 2 (40%) | 0 | 2 (22%) | 0 | 0 | 0 | |
| Underlying comorbidity | ||||||||
| Neurological comorbidities | 1 (14%) | 2 (40%) | 0 | 4 (44%) | 0 | 0 | 1 (100%) | |
| Other comorbidities | 1 (14%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Clinical features | ||||||||
| Systemic features* | 6 (86%) | 3 (60%) | 0 | 6 (67%) | 0 | 0 | 0 | |
| Respiratory involvement at presentation | 2 (29%) | 2 (40%) | 0 | 1 (11%) | 1 (50%) | 0 | 0 | |
| Encephalopathy | 4 (57%) | 0 | 1 (100%) | 9 (100%) | 0 | 0 | 0 | |
| Seizures | 0 | 0 | 1 (100%) | 7 (78%) | 0 | 0 | 0 | |
| Headache or meningism | 2 (29%) | 1 (20%) | 0 | 1 (11%) | 0 | 0 | 0 | |
| Peripheral nervous system involvement | 0 | 5 (100%) | 0 | 0 | 0 | 2 (100%) | 0 | |
| Focal CNS involvement | 3 (43%) | 0 | 0 | 0 | 0 | 0 | 1 (100%) | |
| Behavioural change | 0 | 0 | 1 (100%) | 0 | 2 (100%) | 0 | 0 | |
| Hallucinations | 0 | 0 | 0 | 0 | 1 (50%) | 0 | 0 | |
| Recognised para-infectious or post-infectious neurological disease | 7 (100%) | 5 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | |
| Investigations | ||||||||
| SARS-CoV-2 PCR positive | 5 (71%) | 4 (80%) | 1 (100%) | 9 (100%) | 1 (50%) | 0 | 1 (50%) | |
| SARS-CoV2- IgG positive | 3 (43%) | 1 (20%) | 1 (100%) | 4 (44%) | 2 (100%) | 2 (100%) | NP | |
| C-reactive protein, mg/L | 12 (0–42) | 1 (1–2) | 1 | 1 (0–158) | 9 (5–12) | 0 | NP | |
| Elevated acute-phase reactants† | 2 (29%) | 0 | 0 | 1 (11%) | 0 | 0 | NP | |
| Plasma white cell count, cells per μL | 10·0 (4·0–27·3) | 11·0 (7·0–18·0) | 7·5 | 9·4 (4·0–11·6) | 7·5 (4·0–10·5) | 6·3 (5·3–7·3) | NP | |
| CSF white cell count >5 cells per μL | 5 (71%) | 1 (20%) | 1 (100%) | 1 (11%) | 1 (50%) | NP | NP | |
| Abnormal neuroimaging | 7 (100%) | 2 (40%) | 1 (100%) | 2/8 (25%) | 0 | 0 | 0 | |
| Treatment | ||||||||
| PICU admission | 2 (29%) | 1 (20%) | 0 | 5 (56%) | 0 | 0 | 0 | |
| Inotropic support | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Immunomodulation | 6 (86%)‡ | 4 (80%) | 1 (100%) | 2 (22%) | 0 | 0 | 0 | |
| Outcome | ||||||||
| Disability§ | 4 (57%) | 2 (40%) | 0 | 1 (11%) | 1 (50%) | 1 (50%) | 0 | |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Data are n (%) or median (range). GBS=Guillain-Barré syndrome. NP=not performed. CSF=cerebrospinal fluid. PICU=paediatric intensive care unit.
Systemic features were fever, shock, hypotension, or rash.
Combined acute-phase reactants were defined as lactate dehydrogenase, ferritin, and D-dimers.
One patient did not receive immunomodulation because of underlying malignancy.
Disability was defined as a modified Rankin scale score of 2–5.
Ten (40%) of 25 patients in the PIMS-TS neurology group had features of peripheral nervous system involvement. Nine (36%) patients had behavioural changes, including six (24%) with hallucinations (at presentation) and ten (40%) with headache or meningism. Six (24%) patients had CNS signs (three had ataxia, one had hemiplegia associated with haemorrhagic stroke, one had brainstem signs associated with ischaemic stroke, and one had left hemiplegia associated with ADEM). Four (16%) patients had seizures: three had focal seizures (status epilepticus in one) and one had subtle motor seizures associated with ongoing subclinical ictal activity.
In the COVID-19 neurology group, peripheral nervous system involvement occurred independently as a separate disorder (GBS in all five cases). In the PIMS-TS neurology group, peripheral nervous system involvement was part of the multisystem presentation (appendix pp 3–4, figure 1).
Patients who presented with a recognised neuroimmune disorder in the COVID-19 neurology group (p=0·027) and patients in the PIMS-TS neurology group (p=0·035) were more likely to present later than those in the COVID-19 neurology group who did not have a recognised neuroimmune disorder.
SARS-CoV-2 was detected by PCR of nasal secretions in 32 (62%) of 52 patients; 21 (78%) in the COVID-19 neurology group and 11 (44%) in the PIMS-TS neurology group (p=0·012). SARS-CoV-2 IgG antibody was detected in 12 (44%) patients in the COVID-19 neurology group and 19 (76%) in the PIMS-TS neurology group (p=0·021; table 1).
The median C-reactive protein was higher in the PIMS-TS neurology group than in the COVID-19 neurology group (290 mg/L [range 80–556] vs 1 mg/L [0–161], p<0·0001; table 1). Combined acute-phase reactants were elevated in all 25 (100%) patients in the PIMS-neurology group compared with four (15%) in the COVID-19 neurology group (p<0·0001). Serum MOG antibodies were positive in five (63%) of eight patients with an acute demyelinating syndrome (four with ADEM [one with PIMS-TS], one with optic neuritis). 31 (60%) of 52 patients had a lumbar puncture; 11 (35%) of whom had a CSF pleocytosis, with a median white cell count of 11 per mm3 (range 6–6075). Of these 11 patients, six had encephalitis (five ADEM, one limbic), one had meningitis (PIMS-TS), one had other acute demyelinating syndrome, one had PIMS-TS and encephalopathy, one had psychosis, and one had GBS. Eight (15%) patients had an elevated CSF protein, with a median of 1·8 mg/L (range 0·43–2·63); of these, five had GBS, one had ischaemic encephalopathy associated with PIMS-TS, one had ADEM, and one had transverse myelitis. Oligoclonal bands were positive in the CSF in two (18%) of 11 patient samples analysed; one patient had ADEM and one had encephalopathy associated with PIMS-TS. Glucose analysis of the CSF was done in 26 (83%) of 31 samples and was normal (>2·5 mmol/L) in all 26. A clinically indicated viral and bacterial molecular screen of CSF was negative in all 31 CSF samples. In addition, SARS-CoV-2 RNA PCR was negative in six cases when specifically requested (three patients with ADEM and three with PIMS-TS with encephalopathy). For one child with ADEM, the admission respiratory secretion PCR was positive for SARS-CoV-2 and adenovirus, although only the COVID-19 PCR remained positive on three subsequent serial swabs (appendix pp 7–12).
Electroencephalography was done in 33 (63%) of 52 patients and the result was abnormal in 20 (61%); 18 had non-specific focal or generalised background slowing (13 in the PIMS-TS neurology group) and two had epileptic discharges (bilateral periodic lateralising epileptiform discharges in a PIMS-TS case with ischaemic encephalopathy and left posterior quadrant discharges in a COVID-19 neurology case with status epilepticus). 17 patients had clinical features of peripheral nervous system involvement: seven in the COVID-19 neurology group and ten in the PIMS-TS neurology group. Nerve conduction studies or electromyography, or both, were done in 15 (29%) of 52 patients; 12 (80%) patients had abnormal results. Five patients with PIMS-TS had diffuse myopathic or neuropathic changes, or both; four of the five patients with GBS had evidence of a primary demyelinating polyneuropathy; two with PIMS-TS had focal changes (one right peroneal and tibial neuropathy and one proximal myopathy and bilateral tibial neuropathies); and one with ADEM had evidence of a unilateral right-sided facial nerve injury secondary to brainstem involvement (appendix pp 7–12).
Cerebral imaging or spinal imaging, or both, were done in 48 (92%) of 52 patients: 46 had MRI and 11 had CT, of whom 28 (58%) had abnormal scans. In the COVID-19 neurology group, 11 (44%) of 25 patients had abnormal neuroimaging. Of these, four MRI brain scans had diffuse T2 or fluid-attenuated inversion recovery (FLAIR) signal abnormalities of the cerebral white matter or deep grey matter consistent with ADEM, one had abnormal T2 signal involving the hippocampi and cortical diffusion restriction due to limbic encephalitis, one had abnormal T2 signal in the periventricular and infratentorial regions consistent with demyelination in a child with an acute demyelinating syndrome (clinically isolated syndrome), and one had signal change in the intraorbital segment of the right optic nerve consistent with optic neuritis. Two patients had thickening and enhancement of the cauda equina nerve roots supportive of GBS, one had signal changes in the splenium of the corpus collosum consistent with mild encephalopathy with reversible splenial lesion (MERS), and one, a child with a pre-existing diagnosis of adrenal neuroblastoma, had extensive intramedullary whole spinal cord abnormal T2 signal change supportive of the clinical diagnosis of myelitis.
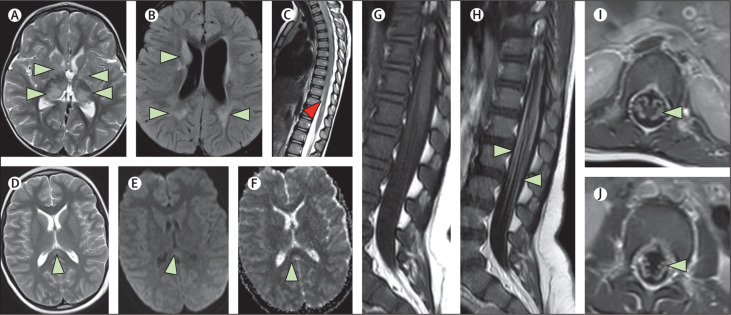
In the PIMS-TS neurology group, 17 (74%) of 23 patients had abnormal neuroimaging. Notably, seven (28%) patients had signal changes in the splenium of the corpus callosum consistent with MERS, two (8%) had findings consistent with an acute stroke (one ischaemic involving the anterior and middle right cerebral artery, one intraparenchmal haemorrhage in the right frontal lobe) and one (1%) had bilateral hyperintensities within the claustra due to ADEM. The imaging findings for three cases, described as clinical vignettes in the appendix (pp 1–2), are shown in figure 2 .
Figure 2.
MRI scans showing a range of neurological complications
(A, B, C) MRI brain and spine scans from a White girl aged 2 years with ADEM (case number 4, appendix p 7; the full patient history is given in appendix p 1). There are multiple hyperintense foci on the axial T2-weighted (A) and T2 FLAIR (B) images involving both cerebral hemispheres, including the basal ganglia, thalami, and subcortical and periventricular white matter (green arrows). Sagittal T2-weighted image of the spine (C) shows a focus of hyperintensity within the cord close to the conus (red arrow). (D, E, F) MRI brain scans from an Asian boy aged 11 years who presented with PIMS-TS, encephalopathy, and MERS (case number 48, appendix p 11; the full patient history is given in appendix p 1). Axial T2-weighted image (D) shows a focus of hyperintensity involving the splenium of the corpus callosum along the midline (green arrow). The B1000 (E) and the ADC maps (F) from the diffusion-weighted imaging shows subtle diffusion restriction involving the lesion. (G, H, I, J) MRI spine scans from an Asian boy aged 16 months who presented with Guillain-Barré syndrome (case number 8, appendix p 7; the full patient history is given in appendix p 2). Sagittal T1-weighted images before (G) and after contrast (H) show enhancement of the lumbosacral nerve roots (green arrows). The axial T1-weighted post-contrast images (I, J) show bilateral enhancement of the nerve roots. ADEM=acute disseminated encephalomyelitis. FLAIR=fluid-attenuated inversion recovery. PIMS-TS=paediatric multisystem inflammatory syndrome temporally associated with COVID-19. MERS=mild encephalopathy with reversible splenial lesion. ADC=apparent diffusion coefficient.
26 (50%) of 52 patients (20 [80%] in the PIMS-TS neurology group and six [22%] in the COVID-19 neurology group) required admission to the paediatric intensive care unit for a median of 1 days (range 1–100; p<0·0001). 34 (65%) patients (22 in the PIMS-TS neurology group and 12 in the COVID-19 neurology group; p=0·0010) received immunomodulatory medications; 27 had intravenous immunoglobulin, 25 had intravenous methylprednisolone for 3–5 days, three had high-dose oral corticosteroids, two had had anakinra, two had tocilizumab, one had infliximab, and one had five single-volume total plasma exchange. 21 (40%) patients received more than one type of treatment. 13 (22%) patients, all in the PIMS-TS neurology group (p<0·0001), required inotropic support. In addition, 16 patients treated with intravenous methylprednisolone were given an oral prednisolone steroid taper over 4–6 weeks.
In the short-term follow-up (assessed at discharge from hospital, or at the most recent clinical assessment [range 1–6 months after discharge], by a neurologist) of this cohort so far, 34 (65%) patients had an apparent good recovery (mRS score 0–1), 17 (33%) had some degree of disability (mRS score 2–5), and one (2%) died (with ischaemic stroke in the PIMS-TS neurology group; mRS score 6).
Discussion
This first nation-wide cohort study of hospitalised children and adolescents with neurological and psychiatric manifestations of SARS-CoV-2 infection in the UK, during the first 9 months of the COVID-19 pandemic, has identified a wide range of disorders and features. Although COVID-19 requiring hospital treatment is very rare in children and young people overall, we found that among hospitalised children and adolescents neurological or psychiatric manifestations are common (3·8 cases per 100 hospitalised patients). Moreover, these neurological or psychiatric manifestations disproportionately affected children from minoritised ethnic groups: 36 (69%) of the 52 patients identified were Black or Asian compared with 13% of the UK population; a similar percentage of Black or Asian patients has been reported in all children and young people admitted to hospital with COVID-19 in the UK (64%).21 Most patients presented after their acute COVID-19 illness had resolved; only 12 (23%) had respiratory symptoms on admission. However, eight (15%) patients presenting with neurological or psychiatric symptoms only did have SARS-CoV-2 detected by PCR, highlighting the importance of screening for the virus in all children and adolescents with acute neurological disorders.
All 27 patients in the COVID-19 neurology group had discrete neurological or psychiatric disorders; 20 affecting the CNS, five affecting the peripheral nervous system, and two with psychosis. In almost half of these, this was a recognised para-infectious or post-infectious immune-mediated (neuroimmune) disorder—eg, ADEM, other acute demyelinating syndromes, or GBS—with MOG antibodies in more than half of those presenting with an acute demyelinating syndrome. One case with ADEM was atypical: at presentation there were over 6000 white cells in the CSF; the initial brain imaging was normal with changes consistent with ADEM only noted during recovery on day 44. These recognised neuroimmune disorders are reported in association with a variety of preceding infections,11, 12, 13, 14, 15 but whether SARS-CoV-2 infection causes an increase in the incidence of neuroimmune disorders is not clear.
By comparison, only one child in the PIMS-TS group had a recognised neuroimmune disorder (ADEM), suggesting that different immune mechanisms are the cause of neurological manifestations in this newly described inflammatory syndrome. Immune-mediated mechanisms would also be supported by the finding that patients with recognised neuroimmune disorders and neurological manifestations associated with PIMS-TS presented later than those with other neurological or psychiatric manifestations.
Patients presenting with PIMS-TS had the most uniform features, with encephalopathy being present in 22 (88%) of 25 cases. Two-thirds of these patients had abnormal brain imaging, with the most common finding in more than 40% being a reversible splenial lesion in the corpus callosum consistent with MERS. MERS has been reported previously in PIMS-TS,8, 18 other viral infections,22 and Kawasaki disease.23
The lesions in MERS are postulated to represent intramyelinic oedema in the corpus callosum as a result of cytokine-mediated glutamate release caused by inflammation.24 Whether the same mechanism is responsible for these imaging findings in PIMS-TS remains to be seen. Ten (40%) patients with PIMS-TS additionally had peripheral nerve involvement; eight had clinical or neurophysiological features consistent with critical illness neuromyopathy in this group, which was more likely to require intensive care support. However, two patients had focal peripheral nervous system features suggesting another mechanism, perhaps similar to haemophagocytic lymphohistiocytosis, a genetic or acquired disorder characterised by a cytokine storm, which can similarly have acute CNS and peripheral nervous system involvement.18 Focal peripheral nerve imaging findings have also been reported in children with COVID-19 previously.9 Other features in this group included behavioural change in nine (36%) patients, including six with hallucinations at presentation. Comparing those with and without PIMS-TS has therefore identified differences that will help with future neurology consults. Although our sample size was small, the differences identified here might infer differing immunopathogenesis.
Our findings are consistent with those from two other paediatric series published in 2021. Both the international neuroradiological cohort9 and multicentre US cohort10 also described recognised neuroimmune disorders and PIMS-TS with life-threatening neurological features including MERS findings on brain imaging. However, those series, which were not studies done across a whole nation, reported more strokes and deaths than we found in this cohort, perhaps suggesting they were not representative of the larger patient group.
The neurological and psychiatric manifestations of COVID-19 have been reported in several studies in adults (age 18 years or older).3, 4, 5 Similarities between our study and the adult CoroNerve cohort25 include the range of disorders identified; although stroke prevalence was much higher in adults, present in almost half of the cases. Adults were also more likely to have multiple neurological diagnoses (13% of adult cases) than children and adolescents, perhaps suggesting there are multiple mechanisms linked to underlying risk factors and comorbidities. Differences also included a higher prevalence of neuroimmune disorders in the paediatric cohort. The case fatality rate was also higher in adults with almost one-quarter dying of their neurological disorder. Over the same time period of the adult CoroNerve study, 30 197 adults were admitted to hospital with COVID-19. Using this data, we estimated a prevalence of neurological and psychiatric manifestations of COVID-19 in adults of 0·9 cases per 100 hospitalised patients. Therefore, overall, neurological and psychiatric manifestations appear to be four times more common in hospitalised children and adolescents than in hospitalised adults with COVID-19 in the UK; this difference is likely to be due in part to respiratory and cardiovascular comorbidities in adults.
In the absence of detailed immunological studies investigating cell-mediated and adaptive immune responses to SARS-CoV-2 in children, the underlying pathogenesis of neurological disease of COVID-19 is unclear. There is little evidence showing direct SARS-CoV-2 neurotropism in adults,26, 27 and some evidence emerging of viral invasion of endothelial cells rather than neurons.27, 28 In this cohort, none of the patients had features suggesting viral encephalitis caused by direct invasion of brain parenchyma, although SARS-CoV-2 was only tested for in the CSF of six patients. Postulated mechanisms for neurological manifestations of SARS-CoV-2 include cytokine-driven neuroinflammation29 or secondary CNS injury from systemic hyperinflammation.26 Both mechanisms would fit the features found in the PIMS-TS neurology group in this study. Patients in this group had significantly higher peripheral inflammatory markers and were more likely to require intensive care and immunomodulatory treatment than patients in the COVID-19 neurology group. Alternatively, adaptive immune-mediated disease, which we postulate underlies the recognised para-infectious or post-infectious immune-mediated disorders within the COVID-19 neurology group, might also mediate the PIMS-TS neuropathogenesis, suggested by the majority being positive for SARS-CoV-2 IgG antibodies at presentation.
The short-term follow-up of this cohort showed almost two-thirds (65%) of patients had an apparent good recovery, one-third (33%) had some degree of disability, and only one (2%) died.
A strength of this study is that the reporting of cases is facilitated by widespread publicity of the BPNSU studies via the BPNA weekly newsletters, by all patients being managed in the national health-care system, and by close networks within the paediatric neurology community, and therefore the sample could be considered nationally representative.
This study has several limitations. The reporting system relies on paediatric neurologists reporting cases that they have been consulted about; however, cases might not have been reported due to the unprecedented workload in the pandemic. Cases might also have been missed as testing for IgG antibodies was less available at the beginning of the pandemic; the first case was reported in June, 2020. Less severe cases or cases with transient symptoms were not reported as children and adolescents are admitted to hospital under general paediatricians in the UK; severe cases might have died before a referral to a neurologist was made. As investigations were not standardised, some complications might be underestimated.
The only cases included with psychiatric features are patients who were referred to neurologists, therefore this group is likely to be under-reported. The reporting of the outcomes of children and adolescents in this study is functional, with the mRS being a crude tool. The scale being made by chart extraction in some cases is also a limitation. Although short-term outcomes were apparently good in two-thirds of this cohort, we were likely to have underestimated substantial evolving cognitive and behavioural problems.
This study has identified differences between patients presenting with a primary neurological or psychiatric disorder versus those with features associated with PIMS-TS. Recognised neuroimmune conditions were common in patients with a primary disorder; whereas those with PIMS-TS had more heterogeneous but overlapping features, with encephalopathy, neuromyopathy, behavioural change, and hallucinations being common. More patients with PIMS-TS needed intensive care, but outcomes were similar overall. The estimated prevalence of neurological or psychiatric manifestations of COVID-19 was four times more common in hospitalised children and adolescents than in hospitalised adults in the UK. Further studies are required to define the underlying neuroimmune mechanisms for these manifestations, especially in the novel PIMS-TS neurology group, and the cognitive, psychiatric, and neurological outcomes, to better determine the rehabilitation needs of these patients.
This online publication has been corrected. The corrected version first appeared at thelancet.com/child-adolescent on July 28, 2021, and further corrections were made on October 12, 2021
Data sharing
Any reasonable requests to share data will be considered by the CoroNerve studies group steering committee subject to institutional agreements and ethics approvals. Data requests should be sent to the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We are indebted to the BPNA who run the BPNSU; in particular Prof Richard Chin (University of Edinburgh, Edinburgh, UK) and Aidan Laverty (Great Ormond Street Hospital, London, UK). We are grateful to the members of the BPNA, particularly those in the UK Childhood Neurology Inflammatory Disorders specialist interest group, and their junior medical staff who reported cases and completed case report forms. We thank all NHS staff including paediatric speciality and rehabilitation teams who managed these patients who often had multisystem involvement and complex needs; particularly those in paediatric intensive care units. We would also like to thank Dr Aravinthan Varatharaj (University of Southampton, Southampton, UK) for support with setting up the case report forms, Athavan Jeyanantham (University of Southampton) for managing and coding the ALEA Clinical paediatric case report form data, and Rosie Solomon (University of Leeds, Leeds, UK) for proofreading the manuscript.
BDM is supported for COVID-19 neuroscience research by UK Research and Innovation (UKRI) and the Medical Research Council (MRC; MR/V03605X/1); and for additional neurological inflammation due to viral infection research by grants from the MRC and UKRI (MR/V007181/1), MRC (MR/T028750/1), and the Wellcome Trust (ISSF201902/3). STJR is also supported by the Wellcome Trust for paediatric neuroinfectious research (203919/Z/16/Z). IG is supported by the National Institute for Health Research (NIHR). TS is supported by the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections (IS-HPU-1112-10117 and NIHR200907), NIHR Global Health Research Group on Brain Infections (17/63/110), the UK MRC Global Effort on COVID-19 Programme (MR/V033441/1), and the EU's Horizon 2020 research and innovation programme (ZikaPLAN; 734584). MJG is supported for neuroscience and infection research internationally by MRC Newton Fund (MR/S019960/1), MRC Developmental Pathway Funding Scheme (MR/R015406/1), and NIHR (153195 17/60/67, 126156 17/63/11, and 200907).
Contributors
STJR, TS, MJG, BDM, and RK drafted the first version of the manuscript. The manuscript was revised by all authors. STJR, OA-M, YH, SA, MJG, ALRR, TS, and RK analysed the data. STJR, RK, YH, OA-M, and SA adjudicated case assignment. STJR, OA-M, MS, CF, HM, EW, DR, NE, JH, RK, SA, ML, and YH contributed data. STJR, RK, BDM, MJG, and TS accessed and verified the data. TS and LAB led the CoroNerve studies group steering committee. IG, BDM, SP, RHT, and RK led the CoroNerve studies management group. Additional members of the CoroNerve study group and their contributions are shown in the appendix (pp 14–16). All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
CoroNerve study group:
Gerome Breen, Hannah Castell, Ceryce Collie, Lilly George, Monika Hartmann, Marc Henrion, Maria Kinali, Christina Petropoulos, Sithara Ramdas, Victoria Vlachou, Brigitte Vollmer, Bethany Facer, Cordelia Dunai, and Laura Benjamin
Supplementary Material
References
- 1.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushwaha S, Seth V, Bapat P, et al. Neurological associations of COVID-19—do we know enough: a tertiary care hospital based study. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.588879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royal College of Paediatrics and Child Health Guidance—paediatric multisystem inflammatory syndrome temporally associated with COVID-19. 2020. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance
- 8.Sa M, Mirza L, Carter M, et al. Systemic inflammation is associated with neurologic involvement in pediatric inflammatory multisystem syndrome associated with SARS-CoV-2. Neurol Neuroimmunol Neuroinflamm. 2021;8:e999. doi: 10.1212/NXI.0000000000000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindan CE, Mankad K, Ram D, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5:167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaRovere KL, Riggs BJ, Poussaint TY, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 12.Cellucci T, Van Mater H, Graus F, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm. 2020;7:e663. doi: 10.1212/NXI.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacohen Y, Mankad K, Chong WK, et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology. 2017;89:269–278. doi: 10.1212/WNL.0000000000004117. [DOI] [PubMed] [Google Scholar]
- 14.Wells E, Hacohen Y, Waldman A, et al. Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol. 2018;14:433–445. doi: 10.1038/s41582-018-0024-9. [DOI] [PubMed] [Google Scholar]
- 15.Höftberger R, Lassmann H. In: Handbook of Clinical Neurology. Kovacs GG, Alafuzoff I, editors. Elsevier; Amsterdam: 2018. Inflammatory demyelinating diseases of the central nervous system; pp. 263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NHS England NHS data model and dictionary. 2021. https://www.datadictionary.nhs.uk/
- 17.Bigi S, Fischer U, Wehrli E, et al. Acute ischemic stroke in children versus young adults. Ann Neurol. 2011;70:245–254. doi: 10.1002/ana.22427. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Mannan O, Eyre M, Löbel U, et al. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol. 2020;77:1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zombori L, Bacon M, Wood H, et al. Severe cortical damage associated with COVID-19 case report. Seizure. 2021;84:66–68. doi: 10.1016/j.seizure.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NHS England COVID-19 hospital activity. 2021. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-hospital-activity/
- 21.Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63:1854–1858. doi: 10.1212/01.wnl.0000144274.12174.cb. [DOI] [PubMed] [Google Scholar]
- 23.Kontzialis M, Soares BP, Huisman TAGM. Lesions in the splenium of the corpus callosum on MRI in children: a review. J Neuroimaging. 2017;27:549–561. doi: 10.1111/jon.12455. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen C, Niculescu I, Patel S, Krishnan A. COVID-19 and involvement of the corpus callosum: potential effect of the cytokine storm? AJNR Am J Neuroradiol. 2020;41:1625–1628. doi: 10.3174/ajnr.A6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell AR, Hardwick M, Jeyanantham A, et al. Spectrum, risk factors, and outcomes of neurological and psychiatric complications of COVID-19: a UK-wide cross-sectional surveillance study. SSRN. 2021 doi: 10.2139/ssrn.3767901. published online Jan 21. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27.e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon T. Neurological infection with SARS-CoV-2—the story so far. Nat Rev Neurol. 2021;17:65–66. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any reasonable requests to share data will be considered by the CoroNerve studies group steering committee subject to institutional agreements and ethics approvals. Data requests should be sent to the corresponding author.