Abstract
Introduction
We aimed to evaluate the additional effects and safety of a Rho-associated protein kinase (ROCK) inhibitor, ripasudil hydrochloride hydrate, in Japanese patients with primary open-angle glaucoma (POAG) with an intraocular pressure (IOP) of at most 15 mmHg undergoing prostaglandin F2α (FP) receptor agonist monotherapy (FP monotherapy).
Methods
In this prospective observational study, 30 Japanese patients with POAG and IOP of at most 15 mmHg (mean age 59.4 years; 10 men) who were undergoing FP monotherapy in both eyes were administered an additional dose of ripasudil hydrochloride hydrate (GLANATEC ophthalmic solution 0.4%: ripasudil) in one eye. The following factors were investigated at 1 and 3 months after the initiation of ripasudil treatment: (1) magnitude of change in IOP between the treated and contralateral untreated eyes, (2) number of treated eyes showing 20% and 30% IOP reduction, (3) IOP difference between treated and contralateral untreated eyes, and (4) safety during the treatment period. Both (1) and (3) were analyzed using the mixed-effect model for repeated measurements.
Results
The treated eyes showed significant reduction in IOP at 1 month (− 1.92 mmHg, P < 0.001) and 3 months (− 1.81 mmHg, P < 0.001). In contrast, contralateral untreated eyes did not show IOP reduction at 1 month (0.53 mmHg, P = 0.016) and 3 months (0.38 mmHg, P = 0.15). IOP reduction of − 20% and − 30% was achieved in 9 (30%) and 3 (10%) treated eyes, respectively. There were significant differences in IOP between the treated and contralateral untreated eyes at 1 month (− 2.46 mmHg, P < 0.001) and 3 months (− 2.20 mmHg, P < 0.001). Two patients experienced local adverse events (facial edema, one patient at week 1; blepharitis, one patient at 1 month); they recovered quickly after stopping ripasudil administration.
Conclusion
In patients with POAG with an IOP of at most 15 mmHg undergoing FP monotherapy, the addition of ripasudil resulted in significant IOP lowering at 1 and 3 months. Ripasudil could be used to enhance the outcome of FP monotherapy.
Trial registration
Registered UMIN ID: UMIN000030742.
Keywords: FP receptor agonist, Intraocular pressure, Japanese, Open-angle glaucoma, Ripasudil
Key Summary Points
| Why carry out this study? |
| When baseline IOP is lower than the normal average, first-line glaucoma eye drop (FP receptor agonists) would not be enough to reach the target IOP. |
| Recently, ROCK inhibitor (ripasudil) became a candidate for glaucoma eye drops to be used in combination with FP receptor agonists. Here, we investigated its additional effect in patients with open-angle glaucoma (OAG) with an IOP of ≤ 15 mmHg undergoing FP receptor agonist monotherapy. |
| What was learned from the study? |
| There was a significant IOP reduction at 1 and 3 months with high tolerability after the addition of ripasudil. |
| Even though IOP is controlled under 15 mmHg with FP receptor agonist in patients with POAG, there may be some cases where glaucoma progression is still observed. In such cases, ripasudil might be a candidate eye drop for additional treatment. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.14535840.
Introduction
Large clinical studies conducted in the USA and Europe showed that the only evidence-based treatment for glaucoma is the lowering of intraocular pressure (IOP) [1, 2], and the target IOP must be reached during the treatment period. The Glaucoma Practice Guidelines (4th edition) recommend that the first-line treatment for primary open-angle glaucoma (POAG) is antiglaucoma eye drops, especially those containing prostaglandin-related drugs (prostaglandin F2α [FP] receptor agonists) [3]. This is because FP receptor agonists have the strongest IOP-lowering effect, have almost no systemic side effects, and can be expected to have good medical adherence [4].
A high percentage of patients with POAG (92%) in Japan have normal-tension glaucoma (NTG) with an average IOP of around 15 mmHg [5]. Long-term IOP fluctuations are thought to be associated with the progression of low-pressure glaucoma at this IOP level [6]. Intensive treatment for the lowering of IOP and efficient management of IOP will reduce these IOP fluctuations. However, in eyes with glaucoma with IOP lower than 15 mmHg, it is usually difficult to achieve IOP reduction with a single agent, such as FP receptor agonists, and concomitant use of other drugs is necessary. While β-blockers, carbonic anhydrase inhibitors, and α-2 stimulants have been candidate drugs for use along with FP receptor agonists [7–10], systemic diseases, corneal endothelial disorders, and allergic conjunctivitis, etc. may prevent concomitant use in some cases. Against this background, Rho-associated protein kinase (ROCK) inhibitors (ripasudil hydrochloride hydrate: GLANATEC ophthalmic solution 0.4%, hereinafter ripasudil) have recently become available for concomitant medication. Ripasudil was launched in Japan in 2014, and it acts directly on the trabecular meshwork–Schlemm’s canal–collective tract (main pathway) [11].
In examining the effectiveness of adding ripasudil to FP receptor agonists, the following findings were observed. The addition of ripasudil to FP receptor agonist monotherapy (FP monotherapy) in patients with POAG managed at an IOP of at least 18 mmHg resulted in significant IOP reduction at week 8, both at peak and trough in clinical trials [12]. Moreover, the addition of ripasudil to FP monotherapy in patients with POAG managed at 16.6 mmHg resulted in a significant IOP reduction at week 12 [13]. Prescriptions based on FP receptor agonists will undoubtedly continue to be one of the key therapeutic modalities for concomitant use of medical drops. However, the efficacy and safety of adding ripasudil to patients managed with IOP under 15 mmHg by FP monotherapy who require further IOP reduction have not been studied.
To enhance this type of treatment, we evaluated the efficacy and safety of ripasudil as an adjunct therapy in patients with POAG with an IOP of at most 15 mmHg undergoing FP monotherapy. This study is expected to add a new option of eye drop treatment for patients with glaucoma with relatively low IOP.
Methods
This open-label prospective study, ROCK U-15 (Ripasudil Observational study to Confirm the efficacy and safety of Rho Kinase inhibitor in Japanese patients with glaucoma with intraocular pressures of or Under 15 mmHg despite the treatment with prostaglandin analogues), included data from the clinical records of consecutive Japanese patients with POAG who were recruited between October 20, 2018, and April 30, 2020, at Yotsuya Shirato Eye Clinic (Tokyo, Japan). All procedures were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013; the protocol was approved by the Fukuda Internal Clinic IRB (ID no. 15000074). Written informed consent was obtained from all patients. The registered UMIN ID is UMIN000030742.
Inclusion Criteria
Age above 20 years.
Diagnosis of POAG according to a standardized set of criteria, as specified below*.
Patients with glaucoma who have required additional glaucoma treatment with ripasudil since December 2017 because of insufficient lowering of IOP with FP monotherapy.
IOP before the addition of ripasudil was less than or equal to 15 mmHg in both eyes during office hours on at least four consecutive IOP measurements during the 12 months before study entry, and the difference in IOP between the starting day and the date closest to the date before ripasudil was less than or equal to 2 mmHg.
Patients who have provided written consent to participate in the study.
*The diagnostic criteria for POAG were as follows: presence of glaucomatous optic neuropathy and corresponding visual field (VF) defects as determined by a Humphrey VF analyzer; observation of a normal open angle on gonioscopy; and the absence of other ocular or systemic diseases that could cause changes in the optic nerve head and/or VF. The appearance of the optic disc was assessed by direct ophthalmoscopy, and stereoscopic observation was made using a biomicroscope and appropriate lenses; furthermore, fundus photography was performed.
Exclusion Criteria
Patients with contraindications to ripasudil.
Pregnant women, maternity, and lactating patients.
Patients with best corrected visual acuity less than 0.5.
Patients with an equivalent spherical power of less than − 9.0 diopters (D) or more than 9.0 D.
Patients with equivalent spherical power difference of more than 3 D in both eyes.
Patients who cannot accurately measure IOP with a Goldmann tonometer.
Patients with insufficient mydriasis to observe the optic disc.
Patients with a history of intraocular surgery or laser treatment (cataract surgery more than 6 months was excluded).
Patients with complications of ocular trauma.
Patients with complications of retinal diseases affecting the VF.
Patients who have optic nerve disease and/or intracranial disease affecting the VF.
Any other patients who are deemed unsuitable for inclusion in the study.
Medication Protocol
After written informed consent to participate was obtained from patients, ripasudil (ripasudil hydrochloride hydrate 0.4%, Kowa Co., Ltd.) was administered twice daily to one eye while continuing the FP receptor agonist in both eyes. This approach is referred to as a one-eyed trial as indicated in the Glaucoma Practice Guidelines (4th edition) [3]. The IOP-lowering effect is usually determined after several observation points (in this case, 1 month and 3 months; total two times), and if the IOP was effectively lowered, the other eye would be administered as well.
The FP receptor agonists were administered once daily, but there was no specific time (morning or night) for application. However, the patients were required to apply FP receptor agonists at the same time during the treatment course. With an interval of at least 5 min between the FP receptor agonist and ripasudil, patients were asked to apply one drop twice daily (morning and night: 12-h interval) until 3 months. The eye that was intended to be treated with ripasudil was determined using the following criteria: the eye with a higher IOP at the time of obtaining consent was selected, and if the IOPs of the right and left eyes were the same, the right eye was selected. After the patients came to the clinic and confirmed both that FP monotherapy was applied to both eyes and ripasudil was applied to one eye in the morning, IOP was measured.
IOP Measurement
IOP was evaluated on a total of 3 days [day 1 (before adding ripasudil: baseline IOP), 1 month, and 3 months (last day)]. At each visit to the clinic (at approximately the same time on each examination day), topical anesthesia was applied, and two measurements were performed using Goldmann applanation tonometry (Haag Streit, Germany) by a well-trained examiner. If the difference between the two measurements exceeded 2 mmHg, a third measurement was performed. The average value of the two measurements with a difference of less than 2 mmHg between them was used in the analysis.
Sample Size, Power Analysis
A phase III long-term FP receptor agonist combination cohort study (K-115) reported a change of − 2.18 ± 1.87 mmHg at 8 weeks and − 2.54 ± 2.47 mmHg at 28 weeks, 2 h after the instillation of ripasudil [14]. Therefore, it was assumed that the IOP change at 2 h after ripasudil use at 12 weeks was − 2.25 ± 3.00 mmHg. Under this assumption, the number of patients that must be included to detect a significant change in IOP at two-sided significance level of 5% and power of 90% was determined as 21. In addition, we set the enrollment target for this study at 30 patients, assuming patient dropout.
Outcomes and Statistical Analysis
For the evaluation of the IOP-lowering effect, the research subjects were defined to be treated with ripasudil. However, we excluded the subjects who had seriously violated the study design or those who used glaucoma eye drops other than ripasudil or used oral medications (orally administered acetazolamide and steroid) during the observation period.
The following are the endpoints of the current study and the methods used for the analysis of the corresponding data:
-
The magnitude of IOP change in ripasudil-treated and non-ripasudil-treated eyes from baseline to 1 month and 3 months.
In each eye, IOP changes from 0 to 1 month or 0 to 3 months were calculated, and the changes were compared between treated and contralateral untreated eyes. The interocular difference is the difference between the IOP change in the treated and contralateral untreated eyes.
The IOP data were analyzed over time using the mixed-effect model for repeated measures with time, eye (treated eye or contralateral untreated eye), interaction between time and eye, and baseline IOP as fixed effects and study subjects as random effects, and the correlation structure was defined as unstructured [15].
-
Number of treated eyes showing a 20% and 30% reduction in IOP.
The number of treated eyes that achieved 20% and 30% IOP reduction from baseline IOP at 1 month and 3 months were evaluated. The percentage of IOP reduction was calculated using the following equation: -
IOP difference between ripasudil-treated and non-ripasudil-treated eyes.
Because IOPs of both eyes fluctuate, the change from baseline is not sufficient to capture the magnitude of change in IOP. Assuming that the IOP fluctuation cycle was the same in both eyes, the following measurements were performed. The IOP difference between the treated and contralateral untreated eyes was calculated at each observation point, and IOP changes from 0 to 1 month and 0 to 3 months were calculated, and its significance was tested (null hypothesis; change = 0), using a mixed-effect model for repeated measures with time and baseline difference in IOP as fixed effects and study subjects as random effects; the correlation structure was defined as unstructured [15].
-
Presence or absence of adverse events.
For safety assessment, a safety analysis population that included subjects who had received some or all of the study treatment was used. The number of patients who experienced local/systemic adverse events during the course of the treatment was calculated among the safety analysis population. This was determined from the interview and examination of the patient.
The primary endpoint of this study was (1), and the secondary endpoints were from (2) to (4). SAS ver. 9.4 (SAS Institute, NC, USA) was used for all the above statistical analyses, and a two-sided P < 0.05 was considered statistically significant.
Results
A total of 30 patients (60 eyes) with POAG with a mean age of 59.4 years (10 men and 20 women) and a mean IOP of 13.1 (SD 1.21) mmHg were included in the study. As a result of three cases of discontinuation (two cases of adverse events, one case of prolonged visit interval), 27 patients completed 3 months of follow-up (Fig. 1). The most used FP receptor agonist was latanoprost (90%), followed by tafluprost (10%). Adherence to the use of each of the eye drops was almost 100%. The baseline IOPs in treated and contralateral untreated eyes were 13.07 ± 1.21 mmHg and 13.05 ± 1.40 mmHg, respectively. Details of the patient background are shown in Table 1.
Fig. 1.
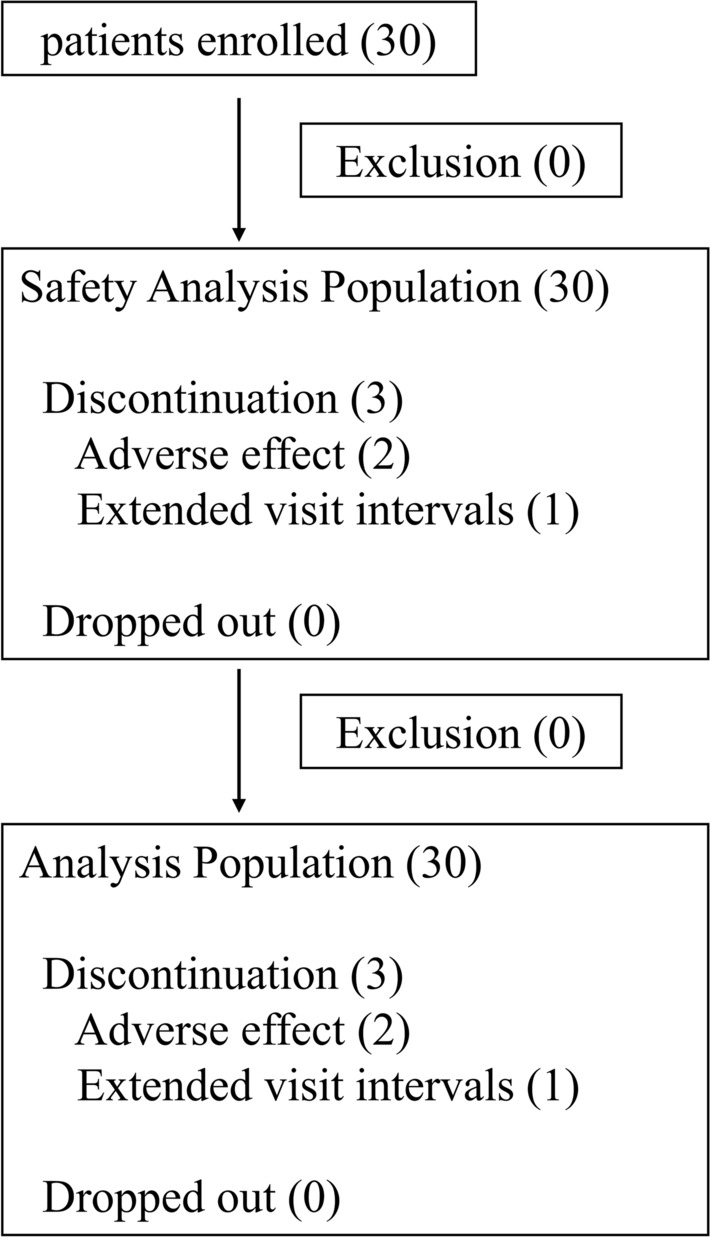
Follow-up chart of the study. Thirty patients were enrolled, two were discontinued because of the occurrence of adverse effects, and one discontinued because of the extended follow-up. There were no dropouts during the study
Table 1.
Demographic and clinical characteristics of the study patients
| Characteristics | Values |
|---|---|
| Age (years) | 59.4 ± 12.5 |
| Sex | |
| Male | 10 |
| Female | 20 |
| Baseline IOP (mmHg) | |
| Ripasudil-added side | 13.07 ± 1.21 |
| Non-added side | 13.05 ± 1.40 |
| FP receptor agonist | |
| Latanoprost | 27 (90%) |
| Travoprost | 0 |
| Tafluprost | 3 (10%) |
| Bimatoprost | 0 |
| Gonioscopy grade | |
| Shaffer grade 0 | 0 |
| Shaffer grade 1 | 0 |
| Shaffer grade 2 | 0 |
| Shaffer grade 3 | 10 (33.3%) |
| Shaffer grade 4 | 20 (66.7%) |
| Ocular history | |
| Cataract | 6 (20%) |
| Dry eye | 2 (6.7%) |
| Other | 11 (36.7%) |
| Systemic history | |
| Hypertension | 2 (6.7%) |
| Diabetes mellitus | 0 |
| Other | 1 (3.3%) |
Data are shown as the mean ± standard deviation or n (%)
IOP intraocular pressure
Magnitudes of changes in IOPs in the treated and contralateral untreated eyes were − 1.92 mmHg (95% CI − 2.41, − 1.43 mmHg; P < 0.001) and 0.53 mmHg (95% CI 0.10, 0.96 mmHg; P = 0.016) at 1 month, respectively, and − 1.81 mmHg (95% CI − 2.42, − 1.20 mmHg; P < 0.001) and 0.38 mmHg (95% CI − 0.15, 0.91 mmHg; P = 0.15) at 3 months, respectively (Fig. 2). Differences in the magnitudes of IOP changes were significant at both 1 month and 3 months (Table 2).
Fig. 2.

Intraocular pressure changes in ripasudil-treated and non-ripasudil-treated eyes at 1 month and 3 months. The IOP values at baseline and 1 month and 3 months. The vertical axis indicates IOP (mmHg). The darker and lighter bars represent ripasudil-treated and non-ripasudil-treated eyes (i.e., FP receptor agonist monotherapy), respectively. *P < 0.001
Table 2.
Intraocular pressure (IOP) difference at each observation point for the ripasudil-treated eyes and non-ripasudil-treated eyes
| n | Months | Add-on side | Non-add-on side | Difference between eyes | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted mean (95% CI) |
P value | Adjusted mean (95% CI) |
P value | Adjusted mean difference (95% CI) |
P value | |||
| Amount of IOP change (mmHg) | 30 | 0–1 | − 1.92 (− 2.41, − 1.43) | < 0.001 | 0.53 (0.10, 0.96) | 0.016 | − 2.45 (− 2.90, − 2.01) | < 0.001 |
| 0–3 | − 1.81 (− 2.42, − 1.20) | < 0.001 | 0.38 (− 0.15, 0.91) | 0.15 | − 2.19 (− 2.75, − 1.64) | < 0.001 | ||
| Percentage change of IOP (%) | 30 | 0–1 | − 14.55 (− 18.29, − 10.81) | < 0.001 | 4.19 (1.11, 7.27) | 0.009 | − 18.74 (− 21.92, − 15.56) | < 0.001 |
| 0–3 | − 13.31 (− 18.08, − 8.54) | < 0.001 | 3.17 (− 0.74, 7.08) | 0.11 | − 16.48 (− 20.53, − 12.43) | < 0.001 | ||
CI confidence interval
The numbers of treated eyes that achieved 20% IOP reduction from baseline were 11 eyes (37%) and 9 eyes (30%) at 1 month and 3 months, respectively. Similarly, those that showed a 30% IOP reduction were 1 eye (3%) and 3 eyes (10%) at 1 month and 3 months, respectively.
The magnitude of difference in IOPs between the treated and contralateral untreated eyes was − 2.46 mmHg (95% CI − 2.87, − 2.04 mmHg; P < 0.001) at 1 month and − 2.20 mmHg (95% CI − 2.71, − 1.69 mmHg; P < 0.001) at 3 months (Table 3).
Table 3.
Change from baseline to each observation point in the IOP difference between the ripasudil-treated eyes and non-ripasudil-treated eyes
| n | Months | Adjusted mean (95% CI) |
P value | |
|---|---|---|---|---|
| Difference between eyes (mmHg) | 30 | 0–1 | − 2.46 (− 2.87, − 2.04) | < 0.001 |
| 0–3 | − 2.20 (− 2.71, − 1.69) | < 0.001 |
CI confidence interval
Two patients experienced local adverse events, such as facial edema (N = 1) at week 1 and blepharitis (N = 1) at 1 month; however, both recovered quickly after discontinuing ripasudil.
Discussion
In this study, we prospectively evaluated the efficacy and safety of ripasudil in patients with POAG with IOP controlled at 13.07 mmHg using an FP monotherapy, and we found that the use of ripasudil as an adjunct drug resulted in a further reduction of IOP (− 1.8 mmHg, − 13%) from baseline to 3 months. Two patients dropped out of the study because of blepharitis and facial edema during treatment, but they improved soon after the use of ripasudil was discontinued. In response to this result, the following can be stated: in patients with POAG whose IOP was controlled to be low with FP monotherapy, if necessary, the additional administration of ripasudil was expected to provide sufficient IOP-lowering effect while ensuring safety.
A double-blind prospective study on Japanese patients with POAG whose IOP was managed with either FP receptor agonist or β-blocker and who received additional ripasudil showed significant IOP reductions both at peak time and trough time at 8 weeks. The most common side effects were conjunctival hyperemia (55.9–65.4%), followed by eye irritation and nasopharyngitis. Conjunctival hyperemia was mild in severity and resolved spontaneously after discontinuation of the drug; and other two side effects also showed improvement after discontinuation of ripasudil, all of which showed an acceptable safety level [12]. Another study, although it was not double-blinded, showed that the use of ripasudil along with FP monotherapy in patients with POAG with mean IOP managed at 16.6 mmHg led to a mean IOP reduction of 10% at 3 months [13]. Furthermore, in a 52-week long-term follow-up study, the use of ripasudil as an adjunct drug in Japanese patients with POAG undergoing FP receptor agonist therapy for IOP management showed a significant IOP reduction during the study. Conjunctival hyperemia (approximately 75%), blepharitis (approximately 20%), and allergic conjunctivitis (approximately 17%) were observed, but these symptoms were also within acceptable limits [14]. These short-term and long-term observations suggest that ripasudil may be an effective second-line agent for patients with POAG undergoing FP receptor agonist therapy.
In the current study, the mean IOP at the beginning of the study was 13.1 mmHg, but the addition of ripasudil lowered the IOP by − 14.6% at 1 month and by − 13.3% at 3 months. Although a simple comparison cannot be made because of the difference in patient backgrounds, we confirmed that ripasudil can be expected to lower the IOP further from the baseline level at the first month, even in patients whose IOP is managed at a lower level. Furthermore, approximately one-third of the patients showed an IOP reduction of − 20% after the addition of ripasudil. In patients with POAG with lower IOP, the target IOP reduction of at least 20% from the baseline level can be achieved with two drugs, an FP agonist and ripasudil. We believe that the results will offer a therapeutic solution for some patients struggling with IOP management with FP monotherapy.
IOP is known to fluctuate diurnally and seasonally and does not remain constant [16]. Because the baseline IOP in the present study was low, one might argue that the IOP variation after the addition of ripasudil might be in part related to this physiological fluctuation in IOP. Assuming that the rhythm of IOP fluctuation in both eyes was approximately the same, we investigated the IOP difference between the two eyes and found a significant difference at both 1 month and 3 months. Despite the diurnal IOP variation, the ripasudil-treated eyes showed a significant reduction in IOP.
In clinical practice, compounded drugs are often used to enhance the therapeutic effects of PG receptor agonists [10]. While this policy is not wrong and may provide an effective IOP-lowering effect, the basic recommendation is to increase the number of IOP-lowering components one at a time. Switching to a combination of PG agonist/β-blocker may maintain medication adherence but is less effective in lowering IOP than the separate use of eye drops [17, 18]. Thus, the use of additional eye drops may be preferable for some patients who can use them in combination.
ROCK is a serine-threonine protein phosphatase that has been identified as a target protein of the low molecular weight GTP-binding protein Rho. Ripasudil selectively inhibits human ROCK-1 and ROCK-2, isoforms of this Rho kinase, and is thought to promote aqueous outflow from outflow pathways [11]. Recently, we have been developing a treatment strategy to choose glaucoma eye drops according to the pathology of elevated IOP, such as steroid-induced secondary glaucoma or pseudoexfoliation glaucoma. Because ripasudil lowers IOP mainly by promoting aqueous outflow, it has been reported to lower IOP in patients with glaucoma in whom the maximum dose of eye drops was already administered [19, 20]. Ripasudil can exert its pharmacological effects on patients with pseudoexfoliation glaucoma with increased resistance to outflow of the main pathway [21] or uveitic glaucoma [22]. It was considered a good match for use with the FP agonist that acted on the secondary outflow pathway because ripasudil acted well on the main outflow pathway [23].
Although ripasudil has mostly no systemic side effects, local side effects that could lead to discontinuation of treatment have been reported. Previous reports indicate that conjunctival hyperemia, blepharitis, and allergic conjunctivitis are common [12, 14, 24], all of which recovered quickly when the use of the eye drops was discontinued. However, in our study, we had only one case of blepharitis and one case of facial edema; both occurred within a month of the addition of ripasudil. In our study, the sample size was small, and the trial period was short (3 months). Even if these ocular side effects occurred, they were spontaneously resolved after discontinuation of ripasudil. This trend was consistent with previous reports. In 103 consecutive patients treated with ripasudil, blepharitis developed in about a quarter of cases, and a history of allergy to glaucoma eye drops was reported to be a risk factor. Administering ripasudil to patients with ocular hypersensitivity should require careful monitoring [25]. Although there has been one report of systemic hypotension [13], it is essentially rare and of low severity; thus, ripasudil can be administered with confidence to patients with a systemic medical history.
While this study has the advantage of having the lowest baseline IOP of any additional eye drop studies to date, there are several limitations. First, the follow-up period was 3 months, which was somewhat short to confirm the complete IOP-lowering effect and/or safety of adding ripasudil. Regarding the former, a previous report indicated that further reductions in IOP were noted after 6 months of ripasudil use [14]. In this study, a sufficient IOP reduction was observed from the first month, but further reductions might be recorded with long-term follow-up. Because blepharitis, one of the local side effects, could occur several months after the initiation of ripasudil use, the rate of side effects could increase with the extension of the follow-up period. This is important because local side effects generally result in poor adherence to the treatment regimen, which may lead to worsening of glaucoma [26]. Second, the included subjects were relatively young. In elderly patients, adherence to the treatment regimen may worsen with an increase in the number of separately used drugs [27]; therefore, it is important to evaluate the therapeutic value of complicating the prescriptions while considering the patients’ characteristics. Third, because this study was an open-label and not a blinded trial, evaluation bias, whether conscious or unconscious, is a likely limitation. Conducting a double-blind study is the best way to resolve this matter. Lastly, in addition to the FP receptor agonist, an EP2 receptor agonist has recently become available, and because an EP2 receptor agonist is likely to be a first-line agent for glaucoma treatment because of its power of lowering IOP [28], the efficacy and safety of using ripasudil as an adjunct drug should be evaluated in the future.
Conclusion
In Japanese patients with POAG undergoing FP monotherapy of IOP below 15 mmHg, the addition of ripasudil resulted in a significant IOP reduction at 3 months. Ripasudil was generally well tolerated throughout the study, and we believe that it has great potential as an additional eye drops in line with existing medications that can be used in combination with FP receptor agonists in Japan, where there are many patients with normotensive glaucoma. Longer-term effects and safety should be explored in the future.
Acknowledgements
We thank the participants of this study.
Funding
Sponsorship for this study was offered by Kowa Co., Ltd. The Rapid Service Fee and the Open Access fee were funded by Kowa Co., Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Study conception and design: Makoto Aihara, Shiroaki Shirato, Acquisition of data: Makoto Aihara, Shiroaki Shirato, Takashi Fujishiro, Megumi Honjo, Hitomi Saito, Rei Sakata, Analysis of interpretation of data: Rei Sakata, Drafting of manuscript: Rei Sakata, Makoto Aihara, Critical revision: Makoto Aihara, Shiroaki Shirato.
Medical Writing, Editorial, and Other Assistance
We appreciate Mr. Hirokazu Yamada (Soiken Inc.) for statistical analysis and Mr. Yasunori Tomita (Soiken Inc.) and Ms. Sachi Arimura (Soiken Inc.) for the research assistance. We would like to thank Editage (http://www.editage.com) for English language editing.
Disclosures
Rei Sakata has nothing to disclose., Takashi Fujishiro has nothing to disclose., Hitomi Saito has nothing to disclose., Megumi Honjo has nothing to disclose., Shiroaki Shirato has nothing to disclose., Makoto Aihara has nothing to disclose.
Compliance with Ethics Guidelines
All procedures were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013; the protocol was approved by the Fukuda Internal Clinic IRB (ID number 15000074). Written informed consent was obtained from all patients. The registered UMIN ID is UMIN000030742.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to [them containing information that could compromise research participant privacy/consent] but are available from the corresponding author on reasonable request.
References
- 1.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 2.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–1304. doi: 10.1016/S0140-6736(14)62111-5. [DOI] [PubMed] [Google Scholar]
- 3.The Japan Glaucoma Society. Glaucoma Practice Guidelines (4th edition). J Jpn Ophthalmol Soc. 2018;122(1):5–53.
- 4.Lin L, Zhao YJ, Chew PT, et al. Comparative efficacy and tolerability of topical prostaglandin analogues for primary open-angle glaucoma and ocular hypertension. Ann Pharmacother. 2014;48(12):1585–1593. doi: 10.1177/1060028014548569. [DOI] [PubMed] [Google Scholar]
- 5.Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111(9):1641–1648. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Sakata R, Yoshitomi T, Iwase A, et al. Factors associated with progression of Japanese open-angle glaucoma with lower normal intraocular pressure. Ophthalmology. 2019;126(8):1107–1116. doi: 10.1016/j.ophtha.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Mizoue S, Nitta K, Shirakashi M, et al. Multicenter, randomized, investigator-masked study comparing brimonidine tartrate 0.1% and timolol maleate 0.5% as adjunctive therapies to prostaglandin analogues in normal-tension glaucoma. Adv Ther. 2017;34(6):1438–1448. doi: 10.1007/s12325-017-0552-5. [DOI] [PubMed] [Google Scholar]
- 8.Bournias TE, Lai J. Brimonidine tartrate 0.15%, dorzolamide hydrochloride 2%, and brinzolamide 1% compared as adjunctive therapy to prostaglandin analogs. Ophthalmology. 2009;116(9):1719–1724. doi: 10.1016/j.ophtha.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 9.Tanna AP, Rademaker AW, Stewart WC, Feldman RM. Meta-analysis of the efficacy and safety of alpha2-adrenergic agonists, beta-adrenergic antagonists, and topical carbonic anhydrase inhibitors with prostaglandin analogs. Arch Ophthalmol. 2010;128(7):825–833. doi: 10.1001/archophthalmol.2010.131. [DOI] [PubMed] [Google Scholar]
- 10.Tanna AP, Lin AB. Medical therapy for glaucoma: what to add after a prostaglandin analogs? Curr Opin Ophthalmol. 2015;26(2):116–120. doi: 10.1097/ICU.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Tanihara H. Ripasudil hydrochloride hydrate: targeting Rho kinase in the treatment of glaucoma. Expert Opin Pharmacother. 2017;18(15):1669–1673. doi: 10.1080/14656566.2017.1378344. [DOI] [PubMed] [Google Scholar]
- 12.Tanihara H, Inoue T, Yamamoto T, et al. Additive intraocular pressure-lowering effects of the Rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol. 2015;133(7):755–761. doi: 10.1001/jamaophthalmol.2015.0525. [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Ishida K, Tomita G. Effectiveness and safety of switching from prostaglandin analog monotherapy to prostaglandin/timolol fixed combination therapy or adding ripasudil. Jpn J Ophthalmol. 2018;62(4):508–516. doi: 10.1007/s10384-018-0599-0. [DOI] [PubMed] [Google Scholar]
- 14.Tanihara H, Inoue T, Yamamoto T, et al. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94(1):e26–34. doi: 10.1111/aos.12829. [DOI] [PubMed] [Google Scholar]
- 15.Diggle P. Analysis of longitudinal data: Oxford Statistical Science. Oxford: Oxford University Press; 2013.
- 16.Sultan MB, Mansberger SL, Lee PP. Understanding the importance of IOP variables in glaucoma: a systematic review. Surv Ophthalmol. 2009;54(6):643–662. doi: 10.1016/j.survophthal.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Barnebey HS, Robin AL. Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. 2017;176:61–69. doi: 10.1016/j.ajo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Cox JA, Mollan SP, Bankart J, Robinson R. Efficacy of antiglaucoma fixed combination therapy versus unfixed components in reducing intraocular pressure: a systematic review. Br J Ophthalmol. 2008;92(6):729–734. doi: 10.1136/bjo.2008.139329. [DOI] [PubMed] [Google Scholar]
- 19.Inazaki H, Kobayashi S, Anzai Y, et al. One-year efficacy of adjunctive use of ripasudil, a rho-kinase inhibitor, in patients with glaucoma inadequately controlled with maximum medical therapy. Graefes Arch Clin Exp Ophthalmol. 2017;255(10):2009–2015. doi: 10.1007/s00417-017-3727-5. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Hirooka K, Nitta E, Ukegawa K, Tsujikawa A. Additive intraocular pressure lowering effects of the rho kinase inhibitor, ripasudil in glaucoma patients not able to obtain adequate control after other maximal tolerated medical therapy. Adv Ther. 2016;33(9):1628–1634. doi: 10.1007/s12325-016-0389-3. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura R, Inoue T, Matsumura A, Tanihara H. Efficacy of ripasudil as a second-line medication in addition to a prostaglandin analog in patients with exfoliation glaucoma: a pilot study. Clin Drug Investig. 2017;37(6):535–539. doi: 10.1007/s40261-017-0509-0. [DOI] [PubMed] [Google Scholar]
- 22.Kusuhara S, Katsuyama A, Matsumiya W, Nakamura M. Efficacy and safety of ripasudil, a Rho-associated kinase inhibitor, in eyes with uveitic glaucoma. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):809–814. doi: 10.1007/s00417-018-3933-9. [DOI] [PubMed] [Google Scholar]
- 23.Komizo T, Ono T, Yagi A, Miyata K, Aihara M. Additive intraocular pressure-lowering effects of the Rho kinase inhibitor ripasudil in Japanese patients with various subtypes of glaucoma. Jpn J Ophthalmol. 2019;63(1):40–45. doi: 10.1007/s10384-018-0635-0. [DOI] [PubMed] [Google Scholar]
- 24.Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156(4):731–736. doi: 10.1016/j.ajo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Saito H, Kagami S, Mishima K, Mataki N, Fukushima A, Araie M. Long-term side effects including blepharitis leading to discontinuation of ripasudil. J Glaucoma. 2019;28(4):289–293. doi: 10.1097/IJG.0000000000001203. [DOI] [PubMed] [Google Scholar]
- 26.Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122(10):2010–2021. doi: 10.1016/j.ophtha.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen Castel O, Keinan-Boker L, Geyer O, Milman U, Karkabi K. Factors associated with adherence to glaucoma pharmacotherapy in the primary care setting. Fam Pract. 2014;31(4):453–461. doi: 10.1093/fampra/cmu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the Phase 3 AYAME Study. Am J Ophthalmol. 2020;220:53–63. doi: 10.1016/j.ajo.2020.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to [them containing information that could compromise research participant privacy/consent] but are available from the corresponding author on reasonable request.


