Supplemental Digital Content is available in the text.
Keywords: acute respiratory failure, coronavirus disease 2019, immunomodulation, infectious disease, secondary infections
Abstract
OBJECTIVES:
Since the onset of the coronavirus disease 2019 pandemic, immune modulators have been considered front-line candidates for the management of patients presenting with clinical symptoms secondary to severe acute respiratory syndrome coronavirus 2 infection. Although heavy emphasis has been placed on early clinical efficacy, we sought to evaluate the impact of pharmacologic approach to coronavirus disease 2019 within the ICU on secondary infections and clinical outcomes.
DATA SOURCES:
PubMed (inception to March 2021) database search and manual selection of bibliographies from selected articles.
STUDY SELECTION AND DATA EXTRACTION:
Articles relevant to coronavirus disease 2019, management of severe acute respiratory syndrome coronavirus 2–associated respiratory failure, and prevalence of secondary infections with pharmacotherapies were selected. The MeSH terms “COVID-19,” “secondary infection,” “SARS-CoV-2,” “tocilizumab,” and “corticosteroids” were used for article identification. Articles were narratively synthesized for this review.
DATA SYNTHESIS:
Current data surrounding the use of tocilizumab and/or corticosteroids for coronavirus disease 2019 management are limited given the short follow-up period and conflicting results between studies. Further complicating the understanding of immune modulator role is the lack of definitive understanding of clinical impact of the immune response in coronavirus disease 2019.
CONCLUSIONS:
Based on the current available literature, we suggest prolonged trials and follow-up intervals for those patients managed with immune modulating agents for the management of coronavirus disease 2019.
The report of a novel betacoronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in Wuhan, China, in December of 2019 (1). SARS-CoV-2 and its resulting disease, coronavirus disease 2019 (COVID-19), quickly spread worldwide, classified as a pandemic by the World Health Organization (WHO) in March 2020. Given the rapid spread and global impact, the medical community was forced to quickly grow and evolve to treat this novel disease. Several treatment considerations showed early signals of benefit in small clinical cohorts but have shown conflicting results in larger trials (2, 3). Many of these treatments are immunomodulatory and activate or suppress certain immunologic functions with the intent of assisting the endogenous immune response to the virus. Through increased knowledge regarding host response to the disease and further evaluation of clinical data, potential immunologic implications of both the disease and immunomodulatory therapies have been elucidated.
PATHOPHYSIOLOGY AND IMMUNOSUPPRESSION
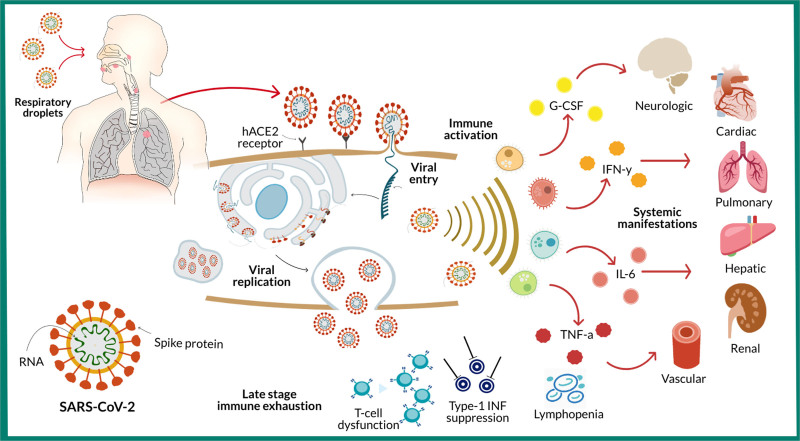
SARS-CoV-2 resembles the pathophysiology of severe acute respiratory syndrome and Middle East respiratory syndrome, the two prior coronaviruses known to cause pneumonia, respiratory failure, and death (4, 5). The way by which SARS-CoV-2 invades host cells, resulting in profound activation of the innate immune system, subsequent cytokine release, and multiple organ failure, is shown in Figure 1 (5, 6). Elevation in blood cytokine levels is a common laboratory finding in SARS-CoV-2 patients, notably interleukin (IL)–6, IL-7, and IL-10 (7).
Figure 1.
Pathophysiology of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. SARS-CoV-2 spread via respiratory droplets and enters the human cell via S-protein envelope binding with human angiotensin receptor-2 (hACE2). Once viral fusion is complete, the virus is endocytosed, and viral RNA uses host cellular components to replicate and spread. As SARS-CoV-2 viral load peaks, the innate immune system becomes activated and a cytokine storm ensues, with release of tumor necrosis factor alpha (TNF-a), interferon gamma (IFN-y), granulocyte colony-stimulating factor (G-CSF), and various other interleukins (IL). Rapid viral replication leads to epithelial and endothelial apoptosis, vascular leakage, profound inflammation resulting in multisystem organ dysfunction. As infection persists, the host enters a state of immune exhaustion characterized by T-cell dysfunction, lymphopenia, and suppressed type-1 interferon response.
An early and strong type-1 interferon (IFN) response is needed to suppress viral replication (8, 9). Type-1 IFNs constitute the largest family of IFNs in humans and include IFN-α, IFN-β, IFN-e, IFN-k, and IFN-w (10, 11). Intracellular RNA degradation, viral clearance, tissue repair, and extended adaptive immune response are key immunologic pathways facilitated by type-1 IFNs (12). Previous studies conducted with other coronavirus strains indicate these viruses exhibit a potent inhibition of early type-1 IFN response leading to hyperinflammation, and it is theorized that SARS-CoV-2 also exhibits this inhibition (13–16).
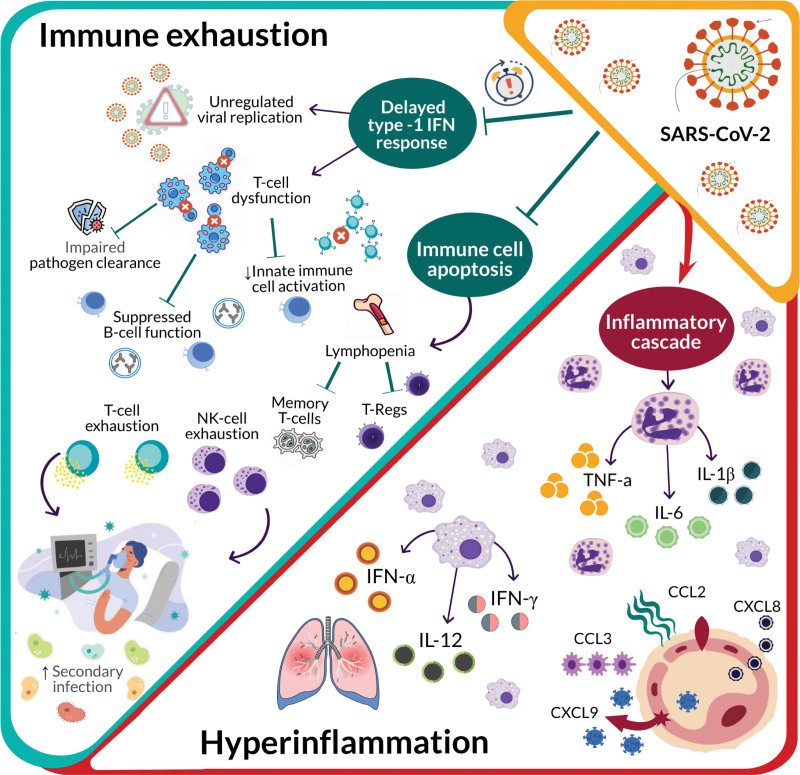
As infection persists, the host immunologic state is characterized by immune cell apoptosis, lymphocyte dysfunction, and lymphopenia (Fig. 2). T cell response to SARS-CoV-2 is weakened and predisposes the host to persistent immunologic impairment and progression to late-stage infection (17). It is clear that T-cell dysfunction is a significant contributor to immunologic status at all stages of disease, leading to persistent cytokine release, inflammatory activation, and subsequent immunosuppression.
Figure 2.
Immunologic consequences of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)–associated immunosuppression. As infection persists, the host enters a state of immune exhaustion. A delayed or suppressed type-1 interferon (IFN) response leads to T-cell dysfunction and ultimately exhaustion. Furthermore, viral-induced immune cell apoptosis results in lymphopenia with decreases in all lymphocyte subtypes. Continued activation of the inflammatory cascade leads to increasing levels of inflammatory cytokines and hyperinflammation. Hyperinflammation paired with immune exhaustion places patients at an increased risk of secondary infectious complications. CCL = C–C motif chemokine ligand, CXCL = C-X-C motif chemokine ligand, IL = interleukin, NK = natural killer, TNF-a = tumor necrosis factor alpha.
Increased susceptibility to secondary bacterial, fungal, and viral infections is a concern and has been described in respiratory viral infections (18). Various mechanisms have been implicated including endothelial cell dysfunction, vascular leakage, decreased mucus clearance, promotion of biofilm formation, and microbiome alterations (18–21). Primary viral infections can also trigger reactivation of latent viruses such as herpes simplex virus (HSV) or varicella zoster virus. Prolonged fever and cytokine release which damage neurons and tissues where these viruses remain latent has led to reactivation in various inflammatory states (22). Both are common clinical manifestations of SARS-CoV-2 infection and may present further risk. These mechanisms, in addition to the previously described immunologic dysfunction, present a significant risk for secondary infections in patients with SARS-CoV-2.
Reports of secondary bacterial infections in SARS-CoV-2 patients vary from 4% to 25%, reaching up to 50% in nonsurvivors (23, 24). Patients admitted to the ICU often require mechanical ventilation or extracorporeal membrane oxygenation (ECMO), which present additional risk factors (25–28). Common bacterial pathogens include Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus species. Data on fungal secondary infections are less abundant compared with bacterial infections, but Candida species appear to be the most common fungal pathogen encountered (29). There are also increasing reports of SARS-CoV-2–associated pulmonary aspergillosis similar to that seen with previous influenza outbreaks (30–33). However, larger studies are conflicting on the true prevalence and risk of fungal secondary infections in this population (34–36). Regarding viral secondary infections, previously reported rates of HSV reactivation range from 21% to 57% in ICU patients (37, 38). Only one study has specifically evaluated the frequency of HSV reactivation in SARS-CoV-2 patients and found a reactivation rate of 23.6% (39). Although the immunologic effects of SARS-CoV-2 impose risks for secondary infections, utilization of treatment modalities with immunosuppressive properties, such as tocilizumab and corticosteroids, may confer additional risk.
Tocilizumab is a recombinant, humanized monoclonal antibody that competitively antagonizes both soluble and membrane-bound IL-6 receptors, preventing IL-6 signal transduction and leading to significant immunosuppression (40). Current literature suggests tocilizumab may reduce the need or duration of mechanical ventilation, progression to severe disease, or death, but results are inconsistent (2, 3, 41–44). Reports of infectious complications in SARS-CoV-2 patients treated with tocilizumab are also inconsistent and limited in follow-up compared with previous tocilizumab trials (45–47).
Additionally, corticosteroids possess potent immunosuppressant properties, and infectious complications have been well-documented with use of oral corticosteroids for rheumatologic conditions (48–50). The effect is dose-dependent, with every increase of 5 mg daily of prednisone and 1,000 mg cumulative dose in the last year increasing the risk of all-cause infection by 13% and 50%, respectively. Even short-term use of prednisone has demonstrated a higher risk of sepsis within 30 days of initiation (50). Similar findings have been documented in critically ill patients receiving corticosteroids, with significant increases in pneumonia (odds ratio [OR], 2.64; CI, 1.21–5.75) and bacteremia (OR, 3.25; CI, 1.26–8.37) (51).
Considering the associations with infection previously reported with tocilizumab and corticosteroid administration, these therapies may pose similar risks following their use in patients with SARS-CoV-2. The aim of this study was to characterize the incidence of secondary infections in patients with SARS-CoV-2 infection admitted to a single-center ICU. We also provide an in-depth review of the available literature regarding secondary infections following tocilizumab and corticosteroid therapy in SARS-CoV-2 patients.
METHODS
This was a retrospective case series conducted at University of Kentucky Healthcare, which is a tertiary care referral academic medical center. This study was approved by the University of Kentucky HealthCare Institutional Review Board (approval number 47751). Due to the retrospective nature, informed consent was waived. The electronic health record for patients admitted to the medical ICU at our institution with a positive SARS-CoV-2 test from March 2020 to November 2020 was reviewed. Data including baseline demographics, clinical variables, and outcomes including secondary infections were collected in a standardized data collection form. Baseline immunosuppression was defined as presence of functional immunosuppressive disorders, autoimmune disorders, or home medications with known immunosuppressive properties prior to admission.
Secondary infections were defined as a new infection with onset greater than 48 hours from initial ICU admission, including ICUs at outside hospitals. These infections were identified via microbiologic results positive for new bacterial, viral, and fungal pathogens requiring anti-infective implementation. In addition to the aforementioned criteria, fungal secondary infections were also identified based on an elevated beta-D-glucan (BDG) that was treated with an antifungal agent. Viral secondary infections were identified based on a positive polymerized chain reaction test. Treatment variables were found via the medication administration record. Cases were adjudicated by review of the primary team notes and treatment decisions. Candida species that grew in the urinary or respiratory tract and coagulase-negative Staphylococcus blood cultures which were deemed noninfectious by the treating team were not included.
The authors also conducted a MEDLINE/PubMed database search from inception to April 2021, and bibliographies were manually selected from selected articles. The MeSH terms “COVID-19,” “secondary infection,” SARS-CoV-2,” “tocilizumab,” and “corticosteroids” were used for article identification. Articles relevant to COVID-19, management of SARS-CoV-2–associated respiratory failure, and incidence of secondary infections with pharmacotherapies were selected. Articles were narratively synthesized for this review.
RESULTS
Over a period of 6 months, we analyzed 237 patients who presented to the ICU with SARS-CoV-2 complicated by acute respiratory failure (Table 1). On average, patients in our case series were elderly, obese, and more than 60% were diagnosed with diabetes prior to admission (Table 1). One-hundred eleven patients (46.8%) were transferred from an outside hospital where they had been admitted for an average of 7 days before transfer. Baseline immunosuppression was present in 87 patients (36.7%). The need for mechanical ventilation was high at 75%, whereas ECMO was used in 12.7% of patients. Tocilizumab use was infrequent; however, corticosteroids were administered to a majority of patients. In those patients who received tocilizumab, only three (10%) did not receive concomitant corticosteroids. There were 18 patients who did not receive tocilizumab or dexamethasone during ICU admission. Seventy-six patients (32.1%) died during their admission, and the majority died while in the ICU. Overall, our patients experienced ICU and hospital lengths of stay of 16 ± 16 days and 24 ± 18 days, respectively.
TABLE 1.
Demographics and Outcomes of Patient Population
| Variables | No Secondary Infection (N = 127) | Secondary Infection (N = 110) | Overall (N = 237) |
|---|---|---|---|
| Age, yr, mean, sd | 62.1, 14.2 | 58.9, 13.3 | 61, 14 |
| Female gender, n (%) | 74 (58.3) | 67 (60.9) | 96 (40.5) |
| Body mass index, kg/m2, mean, sd | 33.9, 7.9 | 35.9, 11.2 | 34.8, 9.6 |
| Immunosuppression at baseline, n (%)a | 37 (29.1) | 50 (45.5) | 87 (36.7) |
| Diabetes, n (%) | 72 (56.7) | 73 (66.4) | 145 (61.2) |
| Source, OSH, n (%) | 52 (40.9) | 59 (53.6) | 111 (46.8) |
| OSH duration prior to transfer, days, mean, sd | 5.4, 4.4 | 8.9, 6.8 | 7.3, 6.0 |
| Source, floor, n (%) | 1 (0.8) | 0 (0) | 20 (8.4) |
| Source, emergency department, n (%) | 58 (45.7) | 38 (34.5) | 96 (40.5) |
| Source, long-term acute care facility/nursing home, n (%) | 5 (3.9) | 5 (4.5) | 10 (4.2) |
| Days since onset, mean, sd | 8.6, 5.7 | 9.7, 6.7 | 9.1, 6.2 |
| Mechanical ventilation, n (%) | 76 (59.8) | 103 (93.6) | 179 (75.5) |
| Extracorporeal membrane oxygenation, n (%) | 9 (7.0) | 21 (19.1) | 30 (12.7) |
| Reintubation, n (%) | 5 (3.9) | 18 (16.4) | 23 (9.7) |
| ICU mortality, n (%) | 26 (20.5) | 47 (42.7) | 73 (30.8) |
| Hospital mortality, n (%) | 28 (22.1) | 48 (43.6) | 76 (32.1) |
| ICU readmission, n (%) | 3 (2.4) | 5 (4.5) | 8 (3.4) |
| Hospital duration, d, mean, sd | 19.3, 16.1 | 28.7, 18.2 | 23.61, 17.73 |
| ICU duration, d, mean, sd | 11.7, 13.3 | 21.7, 17.0 | 16.31, 15.94 |
| Maximum C-reactive protein, mg/L, mean, sd | 516.9, 516.6 | 240.5, 133.8 | 228.1, 385.4 |
| Maximum d-dimer, µg/mL, mean, sd | 4.6, 5.6 | 4.7, 5.1 | 4.651, 5.4 |
| Maximum interleukin-6, pg/mL, mean, sd | 79.7, 77.6 | 49.9, 113.3 | 65, 95 |
| Minimum platelets, k/mc, mean, sd | 177.3, 68.4 | 134.1, 61.8 | 157, 68.7 |
| Minimum WBC, k/mc, mean, sd | 6.6, 4.3 | 6.8, 5.0 | 6.69, 4.64 |
| Number of infections, n (%) | |||
| One | — | 55 (50) | 55 (23.2) |
| Two | — | 31 (25.2) | 31(13.1) |
| Three | — | 14 (12.7) | 14 (5.9) |
| Four or more | — | 10 (9.1) | 10 (4.2) |
| Renal replacement therapy, n (%) | 20 (15.8) | 42 (38.2) | 62 (26.2) |
| Vasopressor, n (%) | 56 (44.1) | 95 (86.4) | 151 (63.7) |
| Steroids, n (%) | 107 (84.3) | 106 (96.4) | 213 (89.9) |
| Remdesivir, n (%) | 77 (60.6) | 71 (64.5) | 148 (62.5) |
| Tocilizumab, n (%) | 19 (15.0) | 11 (10) | 30 (12.7) |
| Hydroxychloroquine, n (%) | 10 (7.9) | 7 (6.4) | 17 (7.2) |
— = not applicable, OSH = outside hospital.
aDefined as receiving immunosuppressive therapies or presence of immunosuppressive disease state prior to admission.
Of the 237 patients included in this case series, 110 (46.4%) developed a secondary infection, and a total of 173 secondary infections were identified. In patients with a secondary infection, 55 (50%) had one secondary infection, 31 (23.2%) had two secondary infections, 14 (12.7%) had three secondary infections, and 10 (9.1%) had four or more secondary infections (Table 1). Respiratory (49%) and bloodstream (27.6%) were the most common sources of infection (Supplementary Table 1, http://links.lww.com/CCX/A717). Common respiratory pathogens included both Gram-positive and Gram-negative organisms, whereas bloodstream pathogens were mainly Gram-positive organisms. Regarding fungal secondary infections, most were identified as Candida species or an elevated BDG treated with an antifungal agent. Only one case of aspergillus was identified in a respiratory culture. The only virial secondary infection identified was HSV reactivation which occurred in 17 patients (7.2%).
Patients developing secondary infections had higher rates of baseline immunosuppression, diabetes, need for ventilator and ECMO support, and vasopressor use. These patients also experienced greater rates of reintubation, longer ICU and hospital lengths of stay, and higher mortality rates. Of the 30 patients who received tocilizumab, 11 (36.7%) experienced a secondary infection. Notably, of the three patients who received tocilizumab monotherapy, no incidences of secondary infection were seen. Additionally, 213 patients (89.9%) received corticosteroids, and 106 (49.8%) developed a secondary infection. All patients who had HSV reactivation received corticosteroids during their ICU admission. Of the 27 patients who received tocilizumab in combination with corticosteroids, 11 (40.7%) developed secondary infections. In those patients not receiving corticosteroids or tocilizumab, fours (22%) developed a secondary infection. Supplemental Table 2 (http://links.lww.com/CCX/A718) further summarizes the incidence of secondary infections by therapeutic intervention.
DISCUSSION AND REVIEW OF LITERATURE
Case Series
Our findings suggest a high prevalence of secondary infections in patients with SARS-CoV-2, especially bacterial pneumonia and bloodstream infections. In our study, 46.4% of patients developed secondary infections compared with 4–25% previously reported (23). The higher prevalence reported in our study may reflect the vast differences in patient populations. In our case series, more than 60% of patients were diagnosed with diabetes prior to admission, whereas previous reports had a much lower incidence of diabetes ranging from 16.3% to 22.6% (30). Previous studies have shown those with diabetes are three times more likely to develop bacterial infections due to defective phagocytes (52, 53). Additionally, immunomodulatory treatment with steroids was higher in our case series (30). Our case series focused only on a medical ICU population, whereas previous reports included patients outside of the ICU (30).
In a recent analysis, authors report the differences in the immune response between COVID-19 and bacterial sepsis (54). Immune cell profiles differed between mild and severe COVID-19 patients with severity being associated with unique immune profiles compared with sepsis, with persistent and profound depletion in lymphocyte subsets as well as reduction in human leukocyte antigen-DR on circulating monocytes expression. Findings supported the onset of strong and durable immune suppression together with an elevated incidence of ICU-acquired infections of 60% in those with COVID-19. Thus, using immunomodulating therapies in the treatment of SARS-CoV-2 might impact ICU-acquired infections, which supports our findings of increased risk of secondary infections in patients receiving corticosteroids or tocilizumab. Rates of HSV reactivation in our population were 7.2% which is much lower than 23.6% reported by the only published study specifically looking at this outcome (39). As previously mentioned, tocilizumab and corticosteroids exhibit immunosuppressive effects that can contribute to the development of secondary infections. We now present a review of the available literature on secondary infections associated with tocilizumab and corticosteroid use in SARS-CoV-2 patients. To our knowledge, this is the only expansive review of secondary infections with these two immunomodulatory therapies in SARS-CoV-2 patients.
Tocilizumab
A summary of studies reporting secondary infections in SARS-CoV-2 patients treated with tocilizumab is presented in Supplementary Table 3 (http://links.lww.com/CCX/A719). In small studies, tocilizumab has been associated with a 16–54% incidence of secondary infections (24, 55–62). In a large study of 3,580 patients with SARS-CoV-2, 497 patients received tocilizumab and were four times as likely to develop secondary infections compared with standard of care (63). However, two prospective, randomized, double-blind, placebo-controlled trials investigating tocilizumab compared with standard of care did not report a significant difference in secondary infections between groups (64, 65). The most recently published randomized trials also found no difference in bacterial secondary infections in patients treated with tocilizumab compared with standard of care; however, nonsevere secondary infections were not included. 43, 44, 66). It should be noted, the definition of secondary infection was not reported for a majority of these previous trials. For studies providing a definition, secondary infections were most commonly identified based on positive microbiologic cultures. Follow-up duration also varied significantly among studies ranging from 10 to 47 days, with some studies not reporting a follow-up duration.
Tocilizumab use was less frequent in our study compared with prior studies, and this could be largely due to our study period as this was conducted prior to many of the large, randomized, controlled trials showing efficacy of tocilizumab. However, our secondary infection rate of 36.7% following tocilizumab use is in line with rates previously reported.
Although much less reported in current literature, viral reactivation has also been described following administration of tocilizumab. HSV reactivation was noted in one patient from a small cohort study, whereas another study reported one patient with hepatitis B virus reactivation and four patients with HSV reactivation (55, 58). Similar results were seen in our population with only two patients experiencing HSV reactivation who received tocilizumab, further supporting a potential low risk for viral reactivation following tocilizumab therapy in SARS-CoV-2 patients. In patients with rheumatoid arthritis, a Japanese postmarketing registry estimated the rate of HSV to be 2.2 episodes per 100 patient-years, and in the United States, this was conducted using U.S. Medicare data with the incidence of HSV of 2.15 episodes per 100 patient-years with no differences compared with other biologics (67, 68).
It is unclear how long the immunosuppressive effects persist after single or subsequent doses of tocilizumab and may lead to infectious complications later during the hospital course. A 2011 meta-analysis reported a rate of serious infection of 4.9 per 100 patient-years among patients receiving tocilizumab doses of 8 mg/kg and lower for those receiving placebo or 4 mg/kg (3.5 per 100 patient-years) in a rheumatoid arthritis long-term safety analysis (69). The type of infection ranged from pneumonia, urinary tract infections, and cellulitis. Those who were older than 65 years old reached an incident rate of 8.5 episodes per 100 patient-years. Authors found the rate of serious infections remained relatively stable with continued tocilizumab treatment; thus, the potential to impact rates of serious infection is likely due to tocilizumab’s rapid onset of action leading to reductions of inflammatory markers with prompt suppression of inflammation. Furthermore, in adult patients with rheumatoid arthritis, the half-life ranges from 11 to 13 days (70). In clinical trials of rheumatoid arthritis, tocilizumab has been administered once every 4 weeks. Considering the risk of secondary infections and the apparent half-life of tocilizumab, it is logical to consider the need for long-term infection risk follow-up in patients receiving tocilizumab in the setting of COVID-19. In our case series, the overall hospital duration was 23.6 days. Considering the half-life of tocilizumab, our case series likely did not capture the true incidence of secondary infections attributed to tocilizumab, and longer duration of follow-up is needed. Additionally, lack of standardized dosing strategies for tocilizumab further complicates interpretation of secondary infection rates following administration.
Corticosteroids
Corticosteroids have emerged as a front-line treatment option for SARS-CoV-2, but despite the proposed efficacy, safety data on corticosteroids in SARS-CoV-2 remain unclear. The largest randomized trial of corticosteroids in SARS-CoV-2 to date, RECOVERY, failed to report the incidence of adverse events, including secondary infections (71). Furthermore, after the results of RECOVERY were published, additional trials were terminated early because equipoise for withholding corticosteroids was no longer present, further limiting the analysis of secondary infections. The incidence and risk of secondary infections remains unclear and is of great concern in the context of SARS-CoV-2 infection.
A summary of studies reporting secondary infections in SARS-CoV-2 patients treated with corticosteroids is presented in Table 2. Of the available studies, minimal to no increase in risks of secondary bacterial or fungal infections has been found after 28 days of follow-up, regardless of the steroid used (dexamethasone, hydrocortisone, and methylprednisolone) (72–74). Although findings were not statistically significant, most of these studies were terminated early or largely underpowered. Additionally, these studies had varying definitions and methods of detecting secondary infections ranging from positive microbiological cultures to physician discretion and use of antibiotic therapy. Corticosteroid use was common in our study population (89.9%) and reflects the published literature supporting efficacy of corticosteroids that became available during our study period. Overall, 49.8% of patients who received corticosteroids developed at least one secondary infection in our population, and this is higher than previously published rates ranging from 21.9% to 37.7% (72, 73). This could be due to the longer duration of follow-up to death or hospital discharge performed in our study than was done in previous studies.
TABLE 2.
Studies Reporting Secondary Infections in Coronavirus Disease 2019 Patients Treated With Corticosteroids
| References | Study Design/ Population | Intervention | Secondary Infections | Follow-up Duration | Secondary Infection Definition | Comments |
|---|---|---|---|---|---|---|
| Tomazini et al (72) | Multicenter, randomized, open-label trial | Dexamethasone IV vs placebo | 21.9% vs 29.1% | 28 d | Not reported | 88.1% vs 86.5% were receiving other concomitant antibiotics |
| CoDEX | 299 ICU patients | Dose: 20 mg × 5 d, then 10 mg × 5 d | BSI: 7.9% vs 9.5% | Trial halted early | ||
| 151 (51%) received dexamethasone | Duration: 10 d or until ICU discharge | |||||
| Dequin et al (73) | Multicenter, randomized, double-blind, placebo-controlled trial | Hydrocortisone continuous infusion vs placebo | 37.7% vs 41.1%; hazard ratio 0.81 (0.49–1.35) | 28 d | At discretion of provider and must have been treated with antibiotics | Could use quicker 8 d taper if respiratory status was improved by day 4 |
| CAPE COVID | 149 ICU patients | Dose: 200 mg/d × 7 d, 100 mg/d × 4 d, 50 mg/d × 3 d | Trial halted early | |||
| 76 (51%) received hydrocortisone | Duration: 14 d | |||||
| Jeronimo et al 2020 (74) | Single-center, randomized, double-blind, placebo-controlled trial | Methylprednisolone IV v placebo | BSI at day 7: 8.3% vs 8% | 28 d | Positive blood culture | Standard of care included antibiotics for community-acquired pneumonia coverage |
| MetCOVID | 393 hospitalized patients | Dose: 0.5 mg/kg bid | Sepsis at day 28: 38.1% vs 38.7% | |||
| 194 (49%) received methylprednisolone | Duration: 5 d | |||||
| Le Balc’h et al 2020 (39) | Single-center, retrospective study | — | Herpesviridae reactivation occurred in 47.4% | Not reported | Viral infections tested twice weekly using quantitative real-time polymerized chain reaction on tracheal aspirates | 44% vs 20% received steroids |
| 38 mechanically ventilated patients |
BSI = bloodstream infection.
Reactivation of latent viral infections has been described with corticosteroid use in the setting of SARS-CoV-2 infection. Although overall HSV reactivation rate was low (7.2%) in our study population, all patients experiencing HSV reactivation received corticosteroids. Limited data are available on viral reactivation following corticosteroid use in SARS-CoV-2 infection; however, the observed rate in our study is much higher than previously reported. In a retrospective study of 38 ICU patients who experienced Herpesviridae pulmonary reactivations, 44% in the reactivation group received corticosteroids compared with only 20% in the nonreactivation group (39). Although not statistically significant, the clinical significance of Herpesviridae reactivation in patients with corticosteroids should raise concern given the prevalence of this disease largely arises in immunocompromised hosts and may lead to fatal outcomes. Recently, Kuindersma et al (75) proposed tailoring immune modulation rather than targeting inflammation with corticosteroids in all COVID-19 patients. They suggest using variables such as fever, heart rate, CRP, and microbiological workup to help address whether steroids should be initiated, changed, or stopped. This suggestion stems from the concern of patients becoming more susceptible to secondary infections following the utilization of corticosteroids. Authors found that within the first wave of COVID-19 between March and May 2020, 2% of patients were diagnosed with HSV pneumonitis, and during the second wave from September to November 2020, 19% of patients were diagnosed with HSV pneumonitis. They suggest this difference in HSV pneumonitis may be due to the stark contrast of corticosteroid recommendations in early versus later phases of COVID-19.
Given the imprecisions of the data, the WHO recommends corticosteroids with low certainty in SARS-CoV-2 by extrapolating evidence from acute respiratory distress syndrome, pneumonia, and sepsis studies and continues to suggest the risk of secondary infections in these conditions is low (risk ratio, 1.01; CI, 0.9–1.13) (76).
LIMITATIONS AND FUTURE DIRECTIONS
Several limitations in our case series should be acknowledged, namely the single-center, retrospective design. Additionally, our overall sample size was relatively small. We also did not assess the association of secondary infections with clinical outcomes in our study; however, it is logical that secondary infectious complications would contribute to poor clinical outcomes. Secondary bacterial, fungal, and viral infections can be common among patients with SARS-CoV-2 due to disease pathophysiology as well as immunosuppressive therapies. Additional studies are needed to establish the impact of secondary infections on clinical prognosis and outcomes in patients with SARS-CoV-2. Several risk factors for contracting SARS-CoV-2 and developing severe disease have been identified (77, 78). Risk factors for developing secondary infections following immunosuppressive therapies for SARS-CoV-2 may certainly exist; however, identification is lacking at this time. Future studies working to identify such risk factors may help select patients who require closer monitoring for infection following treatment or patients in which certain immunosuppressive therapies should be avoided. If immunosuppressive therapies such as tocilizumab or corticosteroids are used, clinicians should vigilantly monitor for the development of secondary infections following treatment. Unfortunately, little is known regarding the chronological association between immunosuppressive therapies and secondary infections in patients with SARS-CoV-2. Future studies should aim to characterize the timeline of developing secondary infections in patients with SARS-CoV-2 who are treated with tocilizumab, corticosteroids, or other immunosuppressive therapies to aid in infection surveillance and reporting.
CONCLUSIONS
Secondary infections occur frequently in ICU patients being treated for SARS-CoV-2 infection, with tocilizumab and corticosteroid exposure potentially increasing risk. Future studies are needed with long-term follow-up of clinical outcomes to identify risk factors and mitigation strategies of secondary infections in patients with SARS-CoV-2 treated with these therapies.
ACKNOWLEDGMENTS
We thank Dr. Jamie Sturgill (University of Kentucky, College of Medicine, Department of Pulmonary, Critical Care, and Sleep Medicine) for her help with article revision.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.Solomon CG, Gandhi RT, Lynch JB, et al. Mild or moderate COVID-19. N Engl J Med 2020; 18:1757–1766 [DOI] [PubMed] [Google Scholar]
- 2.Gordon A, Mouncey P, Al-Beidh F. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384:1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 2021; 384:1503–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Zhao Z, Wang Y, et al. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. bioRxiv. Published online April 9, 2020. doi: 10.1101/2020.01.26.919985 [Google Scholar]
- 6.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020; 581:221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channappanavar R, Fehr AR, Zheng J, et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest 2019; 129:3625–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervantes-Barragán L, Kalinke U, Züst R, et al. Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J Immunol 2009; 182:1099–1106 [DOI] [PubMed] [Google Scholar]
- 10.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: A complex web of host defenses. Annu Rev Immunol 2014; 32:513–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzé G, Schreiber G, Piehler J, et al. The receptor of the type I interferon family. Interferon: The 50th Anniversary. Vol. 316. Berlin, Germany, Springer, 2007, pp 71–95 [DOI] [PubMed] [Google Scholar]
- 12.Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol 2015; 15:231–242 [DOI] [PubMed] [Google Scholar]
- 13.Rose KM, Elliott R, Martínez-Sobrido L, et al. Murine coronavirus delays expression of a subset of interferon-stimulated genes. J Virol 2010; 84:5656–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Jha BK, Wu A, et al. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe 2012; 11:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Totura AL, Baric RS. SARS coronavirus pathogenesis: Host innate immune responses and viral antagonism of interferon. Curr Opin Virol 2012; 2:264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lokugamage K, Hage A, de Vries M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. bioRxiv. Published online July 13, 2020. doi: 10.1101/2020.03.07.982264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Chen S, Yang Z, et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med 2020; 201:1435–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakaletz LO. Viral-bacterial co-infections in the respiratory tract. Curr Opin Microbiol 2017; 35:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vareille M, Kieninger E, Edwards MR, et al. The airway epithelium: Soldier in the fight against respiratory viruses. Clin Microbiol Rev 2011; 24:210–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avadhanula V, Rodriguez CA, Devincenzo JP, et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 2006; 80:1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanada S, Pirzadeh M, Carver KY, et al. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 2018; 9:2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewals BG, Machiels B, Liang X, et al. “Novel” triggers of herpesvirus reactivation and their potential health relevance. Front Microbiol 2019; 1:3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. ; COVID-19 Researchers Group. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin Microbiol Infect 2021; 27:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest 2020; 50:e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: A systematic review and meta-analysis. J Infect 2020; 81:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 2020; 6:ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luyt CE, Sahnoun T, Gautier M, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: A retrospective cohort study. Ann Intensive Care 2020; 10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razazi K, Arrestier R, Haudebourg AF, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care 2020; 24:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes S, Troise O, Donaldson H, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 2020; 26:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. ; Dutch-Belgian Mycosis study group. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir Med 2018; 6:782–792 [DOI] [PubMed] [Google Scholar]
- 31.Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi (Basel) 2020; 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Arkel ALE, Rijpstra TA, Belderbos HNA, et al. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med 2020; 202:132–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehler P, Cornely OA, Böttiger BW, et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020; 63:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fekkar A, Lampros A, Mayaux J, et al. Occurrence of invasive pulmonary fungal infections in severe COVID-19 patients admitted to the ICU. Am J Respir Crit Care Med 2020; 203:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pemán J, Ruiz-Gaitán A, García-Vidal C, et al. Revista Iberoamericana de Micología fungal co-infection in COVID-19 patients: Should we be concerned? Rev Iberoam Micol 2020; 37:41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis 2020:ciaa1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luyt CE, Combes A, Deback C, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med 2007; 175:935–942 [DOI] [PubMed] [Google Scholar]
- 38.Saugel B, Jakobus J, Huber W, et al. Herpes simplex virus in bronchoalveolar lavage fluid of medical intensive care unit patients: Association with lung injury and outcome. J Crit Care 2016; 32:138–144 [DOI] [PubMed] [Google Scholar]
- 39.Le Balc’h P, Pinceaux K, Pronier C, et al. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care 2020; 24:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardy A: Genentech Inc. Actemra (tocilizumab) Injection Package Insert. South San Francisco, CA, Genentech Inc, 2018, pp 1–40 [Google Scholar]
- 41.Lan SH, Lai CC, Huang HT, et al. Tocilizumab for severe COVID-19: A systematic review and meta-analysis. Int J Antimicrob Agents 2020; 56:106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID-19: A systematic review and meta-analysis. J Med Virol 2021; 93:1620–1630 [DOI] [PubMed] [Google Scholar]
- 43.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. ; BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 2020; 383:2333–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med 2021; 384:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calderón-Goercke M, Loricera J, Aldasoro V, et al. Tocilizumab in giant cell arteritis. Observational, open-label multicenter study of 134 patients in clinical practice. Semin Arthritis Rheum 2019; 49:126–135 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto K, Goto H, Hirao K, et al. Longterm safety of tocilizumab: Results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol 2015; 42:1368–1375 [DOI] [PubMed] [Google Scholar]
- 47.Chiu YM, Chen DY. Infection risk in patients undergoing treatment for inflammatory arthritis: Non-biologics versus biologics. Expert Rev Clin Immunol 2020; 16:207–228 [DOI] [PubMed] [Google Scholar]
- 48.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 2011; 335:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Keeley A, Mallen C, et al. Incidence of infections associated with oral glucocorticoid dose in people diagnosed with polymyalgia rheumatica or giant cell arteritis: A cohort study in England. CMAJ 2019; 191:E680–E688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017; 357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Britt RC, Devine A, Swallen KC, et al. Corticosteroid use in the intensive care unit: At what cost? Arch Surg 2006; 141:145–149 [DOI] [PubMed] [Google Scholar]
- 52.Joshi N, Caputo GM, Weitekamp MR, et al. Infections in patients with diabetes mellitus. N Engl J Med 1999; 341:1906–1912 [DOI] [PubMed] [Google Scholar]
- 53.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 54.de Roquetaillade C, Mansouri S, Brumpt C, et al. Comparison of circulating immune cells profiles and kinetics between coronavirus disease 2019 and bacterial sepsis. Crit Care Med 2021May 18. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol 2020; 92:2042–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campochiaro C, Della-Torre E, Cavalli G, et al. ; TOCI-RAF Study Group. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur J Intern Med 2020; 76:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vu CA, DeRonde KJ, Vega AD, et al. Effects of tocilizumab in COVID-19 patients: A cohort study. BMC Infect Dis 2020; 20:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol 2020; 2:e474–e484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: A multicentre observational study. Lancet Rheumatol 2020; 2:e603–e612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020; 19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossotti R, Travi G, Ughi N, et al. ; Niguarda COVID-19 Working Group. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis. J Infect 2020; 81:e11–e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2020July 11. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis TC, Adhikari S, Tatapudi V, et al. A propensity-matched cohort study of tocilizumab in patients with coronavirus disease 2019. Crit Care Explor 2020; 2:e0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia. JAMA Intern Med 2020;. 181:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: A randomized clinical trial. JAMA Intern Med 2021; 181:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon AC, Mouncey PR, Al-Beidh F. Interleukin-6 receptor antagonists in critically ill patients with Covid-19-preliminary report. MedRX. Published online 2021. doi: 10.1101/2021.01.07.21249390 [Google Scholar]
- 67.Koike T, Harigai M, Inokuma S, et al. Effectiveness and safety of tocilizumab: Postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 2014; 41:15–23 [DOI] [PubMed] [Google Scholar]
- 68.Yun H, Xie F, Delzell E, et al. Risks of Herpes Zoster in patients with rheumatoid arthritis according to biologic disease modifying therapy. Arthritis Care Res 2015; 67:731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schiff MH, Kremer JM, Jahreis A, et al. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther 2011; 13:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sebba A. Tocilizumab: The first interleukin-6-receptor inhibitor. Am J Health Syst Pharm 2008; 65:1413–1418 [DOI] [PubMed] [Google Scholar]
- 71.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 — preliminary Report. N Engl J Med 2021; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomazini BM, Maia IS, Cavalcanti AB, et al. ; COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 2020; 324:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dequin PF, Heming N, Meziani F, et al. ; CAPE COVID Trial Group and the CRICS-TriGGERSep Network. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA 2020; 324:1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): A randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 2021; 72:e373–e381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuindersma M, Diaz RR, Spronk PE. Tailored modulation of the inflammatory balance in COVID - 19 patients admitted to the ICU?— a viewpoint. Crit Care. 2021; 25:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organization. Corticosteroids or COVID-19. 2020. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1
- 77.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.