Abstract
Introduction
Bilastine is a second-generation H1 antihistamine indicated for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria. On the basis of the demonstrated efficacy and safety of the oral formulation, a new ophthalmic formulation of bilastine was recently developed. Previous preclinical studies had indicated that bilastine is mainly absorbed by the conjunctiva and shows low plasma concentration. The objective of this study was to evaluate the pharmacokinetics and safety of ophthalmic bilastine (6 mg/mL) after single and multiple dose administration at steady state in healthy adults.
Methods
This was an open-label, single-centre, phase I, bioavailability clinical trial. One drop of the bilastine ophthalmic formulation was administered once daily in each eye of the subjects for 5 days. Bilastine plasma concentrations were measured by HPLC–MS/MS. Adverse drug reactions were recorded for each subject during drug administration and follow-up visits.
Results
Twelve healthy subjects (age 18–55 years) were included in the study. After multiple dose administration, bilastine reached a mean (± SD) maximum blood concentrations of 2682.26 ± 1615.88 pg/mL at a median time of 2.50 h (range 1.25–4.00 h). The half-life of bilastine in plasma was 7.88 ± 6.72 h. Steady state AUC was 19,512.51 ± 9248.76 h·pg/mL. Adverse events were mild and transient, consisting mainly of dysgeusia.
Conclusions
Bilastine once-daily ophthalmic formulation 6 mg/mL is absorbed into the bloodstream in low amounts by the ophthalmic route. The bilastine ophthalmic formulation showed a good safety profile after multiple dose administration.
Keywords: Allergic conjunctivitis, Bilastine, H1 antihistamine, Ophthalmic formulation, Rhinoconjunctivitis
Key Summary Points
| Why carry out this study? |
| Allergic conjunctivitis is a highly prevalent disorder of the ocular conjunctive which can greatly affect quality of life. |
| Bilastine is an efficient and safe antihistamine that has been used to reduce symptoms and signs of allergic conjunctivitis. The pharmacokinetics and safety of a recently developed preservative-free, ophthalmic formulation of bilastine were investigated. |
| What was learned from the study? |
| Bilastine once-daily ophthalmic formulation 6 mg/mL is absorbed into the bloodstream in low amounts by the ophthalmic route. |
| The ophthalmic formulation of bilastine showed a good safety profile after multiple dose administration. |
| Further controlled clinical trials are warranted to evaluate the efficacy and safety of ophthalmic bilastine in the treatment of allergic conjunctivitis. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14611620.
Introduction
Allergic conjunctivitis is an highly prevalent IgE-mediated hypersensitivity disorder of the ocular conjunctive [1]. The most frequent ocular symptoms of allergic conjunctivitis are itching, tearing and conjunctival hyperemia, which can greatly affect quality of life [2, 3]. Current treatments for allergic conjunctivitis aim to control and relieve the symptoms and include systemic or topical antihistamines, mast cell stabilizers, dual-action agents, anti-inflammatories and corticosteroids [3]. Systemic antihistamines are especially recommended in the presence of other allergic comorbidities [4, 5]. Topical antihistamines and mast cell stabilizers are usually well tolerated and reduce symptoms and signs of allergic conjunctivitis, but there are limited data on their long-term efficacy [6]. Topical ocular antihistamines constitute the first-line treatment in cases of isolated ocular symptoms. Generally, topical ocular drugs for allergic conjunctivitis are preferred because they act faster and result in higher local bioavailability than systemically administered drugs [5]. Topical eye drops are considered a convenient and safe route of ocular drug administration [7], although special care must be applied in children [8].
Bilastine is a second-generation H1 antihistamine agent indicated in Europe for adults and adolescents (older than 12 years of age) at a dose of 20 mg once daily, and children (6–11 years, with a body weight of at least 20 kg) at a dose of 10 mg once daily, for symptomatic treatment of allergic rhinoconjunctivitis and urticaria, and in other regulatory agencies for children older than 2 years [9, 10]. In clinical trials, once-daily oral administration of bilastine (20 mg tablets) is effective in controlling the ocular symptoms of allergic rhinoconjunctivitis and it demonstrated an excellent safety profile and long-term tolerance. Moreover, bilastine has also demonstrated efficacy and safety in the treatment of allergic rhinoconjunctivitis and chronic idiopathic urticaria [11–14].
The pharmacokinetics and pharmacodynamics of orally administered bilastine in children [15–17] and adults [18] have been extensively analysed. After oral administration bilastine is rapidly absorbed, reaching the peak plasma concentration at 1.3 h and a mean bioavailability of 60%, mostly bound to plasma proteins [18, 19]. Bilastine is not significantly metabolized in the liver and approximately 95% is excreted unaltered in either the faeces (66.5%) or urine (28.3%), with a mean elimination half-life of 12–14.5 h [18, 20].
Pharmacokinetic and pharmacodynamic modelling complemented with non-compartmental analysis showed linear kinetics over a dose range from 2.5 to 220 mg [18]. Additionally, bilastine has been shown to be safe in patients with renal or hepatic impairment and in patients aged 65 years or older [21, 22]. A prolonged duration of action, a property that could derive from its high selectivity and affinity for the H1 receptor which results in a long residence time at the receptor, has been shown in vitro [23, 24]. However, its interaction with H1 receptors in the brain is nearly 0%, and thus it can be considered that brain penetration is negligible, unlike most other second-generation H1 antihistamines [25, 26].
On the basis of the overall superiority of topical to oral administration of treatments for allergic conjunctivitis owing to prompt onset of action and higher effectiveness [3, 27–31], and the demonstrated efficacy and safety of orally administered bilastine, a once-daily, preservative-free, ophthalmic formulation of bilastine was developed. Here, we describe the results from a phase I study carried out to evaluate the pharmacokinetics and safety of ophthalmic bilastine (6 mg/mL) after single and multiple dose administration at steady state in healthy adults.
Methods
This was an open-label, single-centre, phase I clinical trial to evaluate the relative bioavailability, tolerability and safety of an ophthalmic formulation of bilastine after multiple dose administration to healthy volunteers. The study was carried out at the Hospital Universitario de La Princesa (Madrid, Spain).
All subjects included in the study signed a written informed consent and were free to withdraw the study at any time. The protocol was approved by the Research Ethics Committee of the Hospital Universitario de La Princesa and was conducted in accordance with the ethical principles based on the Declaration of Helsinki and Good Clinical Practice. The study was registered with EudraCT number 2018-001504-11.
Study Population
Subjects were eligible for inclusion in the study if they met the following criteria: age 18–55 years, in good physical and mental health, willing to discontinue wearing contact lenses for at least 72 h prior to and during the study, calculated visual acuity of 0.7 logMAR or better in each eye as measured using an Early Treatment Diabetic Retinopathy Study (ETDRS) chart. Exclusion criteria were subjects with body mass index outside the 18.5–30.0 kg/m2 range, any ocular condition that could affect the subject’s safety or trial parameters (such as glaucoma, ocular hypertension, clinically significant blepharitis, follicular conjunctivitis, dry eye, active ocular infection, active allergic conjunctivitis), positive testing for drugs of abuse, tobacco or alcohol consumption, and pregnant or breastfeeding women. Subjects were also excluded if they had signs or symptoms of clinically active allergic conjunctivitis in either eye in the last month, a history of retinal detachment, diabetic retinopathy, active retinal disease, or eye surgery in the previous 3 months. The following medications were not allowed prior to screening and during the whole study: 7 days prior to screening (systemic or ophthalmic H1 antihistamine, H1 antihistamine/mast cell stabilizers, H1 antihistamine–vasoconstrictor drug combinations, or other ophthalmic preparations), 14 days prior to screening (inhaled, ocular, topical or systemic corticosteroids or mast cell stabilizers) and 45 days prior to screening (depo-corticosteroids).
Study Design and Procedures
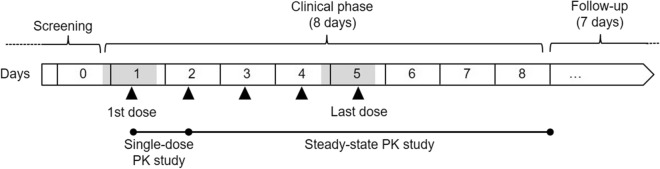
A scheme of the timeline of the study is shown in Fig. 1. Subjects received five doses of the bilastine ophthalmic formulation (one single drop of 6 mg/mL solution/eye, corresponding to 0.42 mg of bilastine) every 24 h for 5 days. The doses were administered by the investigator in the conjunctival sac of both eyes.
Fig. 1.
Design and timeline of the pharmacokinetic study. Shaded boxes indicate in-hospital study periods and black triangles time of ocular administration of bilastine ophthalmic formulation 6 mg/mL. The follow-up phase lasted 1 week after day 8
On days 1 and 5 of the procedure, the subjects remained in-hospital and 5-mL blood samples were drawn at predefined times for pharmacokinetic analysis. On days 2, 3, 4, 6, 7 and 8, and once during follow-up, the subjects attended the hospital once a day for blood sample collection. A complete ophthalmic examination was performed before the first administration of bilastine on day 0 and after the last administration on day 5. It included an assessment of visual acuity, pupil function, extraocular muscle motility, visual fields, intraocular pressure and ophthalmoscopy through a dilated pupil. A full physical examination, including an electrocardiogram (ECG), was performed before day 0, on days 1 and 5, and on the follow-up visit about 1 week after the last bilastine dose.
Bilastine was analysed in an external laboratory using a method validated according to regulatory guidelines [32]. The bilastine quantification method involved a protein precipitation extraction procedure with methanol/acetonitrile (50:50). Bilastine and its corresponding internal standard were measured by reversed-phase high-performance liquid chromatography coupled to a tandem mass spectrometry detector (HPLC–MS/MS) with a lower limit of quantification of 20 pg/mL, validated according to European Medicines Agency (EMA) guidelines.
Pharmacokinetic Analysis: Endpoints and Assessments
For the analysis of the bioavailability after the first dose, the primary endpoint was the area under the curve (AUC0–24) calculated from the plasma concentrations of bilastine. The time to Cmax (Tmax) and peak concentration (Cmax) were also determined.
For the analysis of the bioavailability under steady-state conditions, the primary endpoint was the area under the curve during a dosage interval (AUC0–τ,ss), minimum plasma concentration (Cmin,ss), maximum plasma concentration (Cmax,ss) and time until Cmax,ss was reached (Tmax,ss) at steady state calculated from the plasma concentrations of bilastine. The plasma concentration previous to the administration of each dose and concentration at the end of the dosing interval (Cτ,ss) was also calculated. The percentage fluctuation was computed as 100 × (Cmax − Cmin)/Cavg, where Cmin and Cmax were obtained between 0 and tau, and Cavg was the average concentration during a dosing interval (AUC0–τ/τ).
The pharmacokinetic data analysis was carried out according to a model-independent approach, following the recommendations of the European and American Regulatory Authorities on investigation of bioavailability and bioequivalence [33–35].
Pharmacokinetic parameters were estimated by noncompartmental analysis using validated WinNonlin Professional 7 software (Pharsight Corporation, NC, USA).
Safety and Tolerability Assessment
In addition to the ophthalmological examination, the safety and tolerability of the bilastine ophthalmic formulation were assessed by clinical evaluation of adverse events (AEs) and other parameters including vital signs, physical examination, ECG and blood and urine tests.
Throughout the study, subjects were asked about any experienced AEs. Additionally, AEs that were spontaneously notified by the volunteers were also recorded. Causality was determined using the algorithm of the Spanish pharmacovigilance system as definite, probable, possible, conditional and unrelated [36]. Only definite, probable or possible AEs were considered as adverse drug reactions (ADRs) and included in the statistical analysis. Time sequence, intensity and outcome of AEs were also recorded.
Statistical Analysis
Statistical analyses were carried out using SPSS software 15.0 (SPSS Inc., Chicago, Ill, USA). A significance level of p < 0.05 was considered statistically significant. All subjects were considered for the pharmacokinetic and safety analysis since all of them accomplished 100% of the study treatment according to protocol. Summary statistics were calculated for demographic and pharmacokinetic parameters, expressed as mean and standard deviation (SD). Owing to the exploratory nature of the study, no formal sample size calculation was performed.
No statistical tests were applied to the data on AEs. Safety and tolerability parameters were evaluated descriptively by analysing the incidence of AEs due to the bilastine ophthalmic formulation. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 21.0. Descriptive statistics (N, arithmetic mean, SD and CV) were calculated for blood pressure, heart rate, ECG intervals and laboratory values. No statistical tests were performed on these data since no clinically relevant differences between baseline and post-administration data were detected.
Results
Out of 22 subjects assessed for eligibility, 12 healthy individuals were included in the study, six men with a mean (± SD) age of 26.1 ± 3.8 years and a body mass index of 24.76 ± 4.2 kg/m2, and six women with a mean (± SD) age of 29.5 ± 8.0 years and a body mass index of 22.2 ± 2.9 kg/m2. For the total population the mean (± SD) age was 27.8 ± 6.3 years and the body mass index was 23.5 ± 3.8 kg/m2. All of them completed the clinical trial according to the protocol.
Pharmacokinetic Analysis
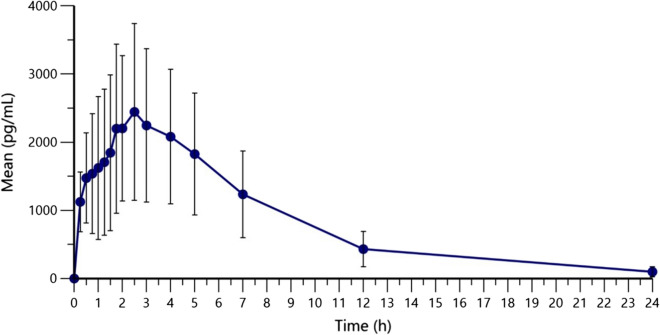
The mean plasma concentrations of bilastine up to 24 h after administration are shown in Fig. 2. After a single ophthalmic dose administration, the mean (± SD) AUC0–24 of bilastine was 19,537.81 ± 9109.72 h·pg/mL. The maximum mean plasma concentration observed (Cmax) was 2793.79 ± 1384.40 pg/mL, reached at a median Tmax of 2.13 h (range 0.25–3.00 h) and with a mean half-life (T1/2) of 4.63 ± 1.75 h (Table 1).
Fig. 2.
Bilastine plasma concentrations (pg mL−1) after single administration of one drop of 6 mg/mL per eye. Means (standard deviation) are shown
Table 1.
Summary of pharmacokinetic parameters of ophthalmic bilastine (6 mg/mL) in plasma
| Parameter N = 12 | Mean ± SD |
|---|---|
| AUC0–24 (h·pg/mL), mean ± SD* | 19,537.81 ± 9109.72 |
| Cmax (pg/mL), mean ± SD* | 2793.79 ± 1384.40 |
| AUC0–τ,ss (h·pg/mL), mean ± SD | 19,512.51 ± 9248.76 |
| Cmax,ss (pg/mL), mean ± SD | 2682.26 ± 1615.88 |
| Cmin,ss (pg/mL), mean ± SD | 54.45 ± 70.42 |
| Tmax (h), median (range)* | 2.13 (0.25–3.00) |
| Tmax,ss (h), median (range) | 2.50 (1.25–4.00) |
| T1/2,ss (h), mean ± SD | 7.88 ± 6.72 |
| T1/2,ss (h), median (range) | 5.42 (2.45–23.24 h) |
AUC0–24 area under the curve from time 0 to 24 h after drug administration, AUC0–τ,ss area under the curve from time 0 to tau in steady state, Cmax maximum concentration in plasma, Cmax,ss maximum concentration in steady state, Cmin minimum concentration in steady state, Tmax time to maximum concentration in plasma, Tmax,ss time to maximum concentration in steady state, T1/2,ss half-life in steady state
*Calculated after first dose
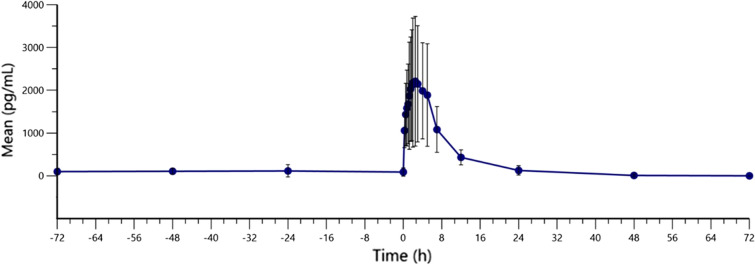
For the multiple dose study, bilastine eye drops were administered once daily for 5 days in each eye. The mean concentrations of bilastine at steady state are shown in Fig. 3. In steady-state conditions the mean (± SD) AUC0–τ,ss of bilastine was 19,512.51 ± 9248.76 h·pg/mL and maximum concentration (Cmax,ss) was 2682.26 ± 1615.88 pg/mL. Both parameters showed high interindividual variability (47.40% and 60.24%, respectively). The minimum concentration at steady state (Cmin,ss) was 54.45 ± 70.42 pg/mL with a interindividual variability of 129.32%. The median time to maximum concentration in steady state (Tmax,ss) was 2.50 h (range 1.25–4.00 h) and the mean half-life (T1/2) was 7.88 ± 6.72 h. Fluctuation was 306.16 ± 94.78% and Cτ,ss was 234.10 ± 124.60 pg/mL.
Fig. 3.
Bilastine plasma concentrations (pg mL−1) after multiple administration of eye drops. Means (standard deviation) are shown spanning from day 2 of administration to 72 h post-dose of day 5 of administration
The median terminal half-life was calculated as 4.38 h (range 2.65–9.13 h) on day 1 and 5.42 h (range 2.45–23.24 h) at steady state.
Safety and Tolerability Assessment
During the study nine subjects reported a total of 43 AEs that were considered as adverse drug reactions (ADRs) since they were definitively, probably or possibly related to the study drug (Table 2). The most frequent ADRs were dysgeusia in eight subjects, and blurred vision reported by a single subject. No serious or life-threatening AEs were reported during the study, no AEs caused premature termination of the study and all were resolved by the end of the trial. The complete ophthalmological examination performed on days 0 and 5 reported normal results and no abnormalities with clinical relevance.
Table 2.
Adverse drug reactions (ADR) reported in the study
| ADR | Number of ADR/number of subjects |
|---|---|
| Dysgeusia | 33/8 |
| Blurred vision | 4/1 |
| Abnormal sensation in eye | 2/2 |
| Photophobia | 2/1 |
| Headache | 1/1 |
| Throat irritation | 1/1 |
| Total number of ADRs/number of subjects with any ADR | 43/9 |
Discussion
In this study the pharmacokinetic parameters of a novel ophthalmic formulation of bilastine were investigated in healthy subjects. The results showed that bilastine ophthalmic formulation 6 mg/mL is absorbed via the ocular route and reaches the bloodstream. Reported adverse events during the study were mild or moderate, suggesting this route of administration is safe and well tolerated.
In this pharmacokinetic study a dose of one drop of 35 µl in each eye every 24 h was chosen because it is the dose confirmed in previous dose-finding and efficacy clinical trials. As the ophthalmic formulation has 6 mg/mL, the total dose received by the subjects was 0.42 mg every day, much lower than the therapeutic 20 mg/day oral dose. As steady‐state concentrations were achieved by the third day of once‐a‐day oral dosing [9, 18], and achievement of steady state is assessed by comparing at least three pre‐dose concentrations of the drug, in this study we administered five ophthalmic doses in 5 days. After administration of a drop containing 0.42 mg of bilastine dose by the ophthalmic route, bilastine was absorbed into the bloodstream, reaching maximum blood levels within 2.50 (1.25–4.00) h after administration at steady state, slightly later than when orally administered (approximately 1.3 h) [18, 19].
In our study very low levels of bilastine were detected in plasma (Cmax 2.79 ng/mL), which is justified because after topical ocular administration bilastine must overcome several ocular natural tissue barriers such as the cornea, the conjunctiva and other related tissues before reaching the bloodstream. This is consistent with a previous preclinical animal study, which found a Cmax of 6.10 ng/mL in plasma after a single topical ocular administration of 6 mg/mL (0.6%) of the bilastine ophthalmic formulation in each eye to male Dutch-Belted rabbits (unpublished data). This low but detectable level of bilastine in plasma suggested, in addition to the nasolacrimal route, a bulbar–stromal conjunctival route of drug absorption. In that animal study, although bilastine was distributed predominantly in the conjunctiva and cornea, consistent with administration site, the highest concentration of bilastine was observed in the conjunctiva, the intended target organ. Lower concentrations of bilastine were found in the iris/ciliary body and retina/choroid, and even lower in the aqueous humour, vitreous humour and crystalline lens. The preferential distribution of bilastine in conjunctiva may have been determined by its high molecular weight (463.6 g/mol) and high polar surface area (67.6 Å2). Further, bilastine presented an elevated conjunctiva/cornea AUC ratio (5.7), higher than for other antihistamines [37], and a high conjunctiva/plasma AUC ratio (> 800). The reduced but detectable levels of bilastine in plasma of rabbits reinforces the idea of the bulbar–stromal conjunctival route. Besides, despite the obvious differences between preclinical and clinical studies, the low levels of bilastine in plasma are comparable to those detected in the present study after a single topical administration of 6 mg/mL of the ophthalmic bilastine formulation in healthy volunteers. In contrast, the Cmax of bilastine in plasma after oral administration of a single dose of 20 mg ranges from 182.4 to 256.6 ng/mL [20]. Also, in this study the half-life after ocular administration (7.88 ± 6.72 h) was slightly lower than that observed after oral administration (9–15 h) [18, 19].
In this pharmacokinetics study the median Tmax of bilastine after single dose administration was 2.13 h, which is comparable to that found for ophthalmic formulations of olopatadine (0.5–2 h), epinastine (2 h), ketotifen (2–4 h) or azelastine (5.3 h) [38]. Ophthalmic bilastine presented a considerably longer half-life, 7.88 h, than ophthalmic olapatadine 0.77%, which had a half-life of 3.40 h [39]. The study in rabbits had shown that the long half-life of bilastine was also observed in conjunctiva 24 h after a single bilateral topical administration, similar to a study of 0.77% olapatadine in rabbits [37].
Ocular administration of antihistamines or mast cell-stabilizing agents rarely leads to systemic side effects, but in some occasions a dry mouth feeling, nausea, headache or drowsiness have been observed [8]. Given the previous safety profile of orally administered bilastine and the compared low dose administered in this study, no safety concerns were expected. No ocular toxicity was detected in animal studies performed with this new ophthalmic formulation (unpublished data). Moreover, in a previous dose-finding pharmacodynamic study with the ophthalmic formulation no safety issues were identified (ClinicalTrials.gov identifier NCT03231969). In this study bilastine ophthalmic formulation 6 mg/mL showed a good tolerability and safety profile after an ophthalmic multiple dose administration. Ophthalmic exploration (visual acuity, pupil function, extraocular muscle motility, visual fields, intraocular pressure and ophthalmoscopy) performed before and at the end of the treatment were normal, with no changes versus baseline. No serious adverse events were reported. Taste discomfort alterations were the most frequent adverse events and they were mild and transient. No previous study had found dysgeusia associated with bilastine [15, 23, 40], although there are some reports of dysgeusia caused by H1 antihistamines azelastine and emedastine [41].
It has been estimated that allergic conjunctivitis is often underdiagnosed and undertreated, as only about 10% of patients with allergic conjunctivitis seek medical treatment [3]. Allergic conjunctivitis is often treated in the context of comorbid rhinitis, but ocular symptoms without nasal involvement occur in 5–6% of patients with allergy [42, 43]. When ocular symptoms are not adequately treated, they can substantially contribute to the burden of the disease for patients with allergy. For these reasons, the recently developed preservative-free ophthalmic formulation of bilastine could become a welcome innovation in the therapeutic arsenal available for the management of allergic conjunctivitis.
As with any clinical study, there are some limitations of the study that should considered in the interpretation of the results. The subjects included in the study were healthy with an age range of 20–44 years, and a wider age range would have been desirable. However, the selection of healthy participants is characteristic for this type of study. Ongoing clinical studies testing the efficacy and safety of bilastine 6 mg/mL in the treatment of patients with allergic conjunctivitis are addressing this issue specifically.
Conclusions
This pharmacokinetic study showed that bilastine ophthalmic formulation 6 mg/mL presents low levels of plasmatic absorption and concentration. The study also showed that the bilastine ophthalmic formulation was safe and well tolerated in healthy subjects after multiple dose administration. These results suggest that the efficacy of the bilastine ophthalmic formulation for the treatment of allergic conjunctivitis may be related to tissue local permanence and direct effect on the tissue target.
Current controlled clinical trials are warranted to evaluate the efficacy and safety of ophthalmic bilastine in the treatment of allergic conjunctivitis.
Acknowledgements
The sponsor (FAES FARMA) was involved in the study design and collection, analysis and interpretation of data as well as data checking of information provided in the manuscript. Ultimate responsibility for opinions, conclusions and data interpretation lies with the authors.
Funding
This work was funded by FAES FARMA (Leioa, Spain), which sponsored the journal’s Rapid Service Fee, and partially supported by the Basque Country Government (Economic Development and Infrastructures Department) thought the HAZITEK program [grant number ZE-2018/00036, 2018].
Medical Writing Assistance
Medical writing support was provided by Francisco López de Saro, PhD (Trialance SCCL) supported by FAES FARMA according to Good Publication Practice guidelines.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for the authorship of this article and take responsibility for the integrity of the work as a whole. They have given their approval for this version to be published.
Authorship Contributions
Francisco Abad-Santos, Dolores Ochoa, Manuel Román, Gonzalo Hernández and Nieves Fernández contributed to concept and study design; Francisco Abad-Santos, Dolores Ochoa, Manuel Román, Samuel Martín-Vilchez, Gina Mejía-Abril, Paula Arranz and Lorena Elgezabal contributed to data collection; Francisco Abad-Santos, Dolores Ochoa, Manuel Román, Samuel Martín-Vilchez, Gina Mejía-Abril, Paula Arranz, Lorena Elgezabal, Gonzalo Hernández and Nieves Fernández contributed to data analysis; Francisco Abad-Santos, Dolores Ochoa, Manuel Román and Nieves Fernández drafted the manuscript.
Disclosures
Dolores Ochoa and Francisco Abad-Santos have been consultants or investigators in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Cinfa, FAES, Farmalíder, Ferrer, GlaxoSmithKline, Galenicum, Gilead, Italfarmaco, Janssen-Cilag, Kern, Normon, Novartis, Servier, Silverpharma, Teva and Zambon. Manuel Román, Carmen Belmonte, Samuel Martín-Vilchez, and Gina Mejía-Abril declare that they have nothing to disclose. Gonzalo Hernández, Paula Arranz, Lorena Elgezabal, and Nieves Fernández are employees of FAES FARMA SA (Spain)
Compliance with Ethics Guidelines
All subjects included in the study signed a written informed consent and were free to withdraw the study at any time. The protocol was approved by the Research Ethics Committee of the Hospital Universitario de La Princesa and was conducted in accordance with the ethical principles based on the Declaration of Helsinki of 1964, and its later amendments.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5. doi: 10.1186/s13223-020-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482–488. doi: 10.1097/ACI.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 3.Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol. 2020;124(2):118–134. doi: 10.1016/j.anai.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Kuna P, Jurkiewicz D, Czarnecka-Operacz MM, et al. The role and choice criteria of antihistamines in allergy management—expert opinion. Postepy Dermatol Alergol. 2016;33(6):397–410. doi: 10.5114/pdia.2016.63942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonardi A, Silva D, Perez Formigo D, et al. Management of ocular allergy. Allergy. 2019;74(9):1611–1630. doi: 10.1111/all.13786. [DOI] [PubMed] [Google Scholar]
- 6.Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A. Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst Rev. 2015(6):CD009566. [DOI] [PMC free article] [PubMed]
- 7.Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farkouh A, Frigo P, Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol. 2016;10:2433–2441. doi: 10.2147/OPTH.S118409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medicines and Healthcare products Regulatory Agency (MHRA). Summary of product characteristics Ilaxten 10 mg orodispersible tablets, bilastine 2018; https://www.medicines.org.uk/emc/product/11727/smpc#gref. Accessed 22 June 2020.
- 10.Church MK, Tiongco-Recto M, Ridolo E, Novak Z. Bilastine: a lifetime companion for the treatment of allergies. Curr Med Res Opin. 2020;36(3):445–454. doi: 10.1080/03007995.2019.1681134. [DOI] [PubMed] [Google Scholar]
- 11.Bachert C, Kuna P, Sanquer F, et al. Comparison of the efficacy and safety of bilastine 20 mg vs desloratadine 5 mg in seasonal allergic rhinitis patients. Allergy. 2009;64(1):158–165. doi: 10.1111/j.1398-9995.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuna P, Bachert C, Nowacki Z, et al. Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo for the symptomatic treatment of seasonal allergic rhinitis: a randomized, double-blind, parallel-group study. Clin Exp Allergy. 2009;39(9):1338–1347. doi: 10.1111/j.1365-2222.2009.03257.x. [DOI] [PubMed] [Google Scholar]
- 13.Horak F, Zieglmayer P, Zieglmayer R, Lemell P. The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. Inflamm Res. 2010;59(5):391–398. doi: 10.1007/s00011-009-0117-4. [DOI] [PubMed] [Google Scholar]
- 14.Bartra J, Mullol J, Montoro J, et al. Effect of bilastine upon the ocular symptoms of allergic rhinoconjunctivitis. J Investig Allergol Clin Immunol. 2011;21(Suppl 3):24–33. [PubMed] [Google Scholar]
- 15.Novák Z, Yáñez A, Kiss I, Kuna P, Tortajada-Girbés M, Valiente R. Safety and tolerability of bilastine 10 mg administered for 12 weeks in children with allergic diseases. Pediatr Allergy Immunol. 2016;27(5):493–498. doi: 10.1111/pai.12555. [DOI] [PubMed] [Google Scholar]
- 16.Vozmediano V, Lukas JC, Encinas E, et al. Model-informed pediatric development applied to bilastine: analysis of the clinical PK data and confirmation of the dose selected for the target population. Eur J Pharm Sci. 2019;1(128):180–192. doi: 10.1016/j.ejps.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez M, Vozmediano V, García-Bea A, et al. Pharmacokinetics and safety of bilastine in children aged 6 to 11 years with allergic rhinoconjunctivitis or chronic urticaria. Eur J Pediatr. 2020;179(5):801–805. doi: 10.1007/s00431-019-03559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jauregizar N, de la Fuente L, Lucero ML, Sologuren A, Leal N, Rodriguez M. Pharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) effect of bilastine. Clin Pharmacokinet. 2009;48(8):543–554. doi: 10.2165/11317180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Sadaba B, Gomez-Guiu A, Azanza JR, Ortega I, Valiente R. Oral availability of bilastine. Clin Drug Investig. 2013;33(5):375–381. doi: 10.1007/s40261-013-0076-y. [DOI] [PubMed] [Google Scholar]
- 20.Sadaba B, Azanza JR, Gomez-Guiu A, Rodil R. Critical appraisal of bilastine for the treatment of allergic rhinoconjunctivitis and urticaria. Ther Clin Risk Manag. 2013;9:197–205. doi: 10.2147/TCRM.S16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasseter KC, Sologuren A, La Noce A, Dilzer SC. Evaluation of the single-dose pharmacokinetics of bilastine in subjects with various degrees of renal insufficiency. Clin Drug Investig. 2013;33(9):665–673. doi: 10.1007/s40261-013-0110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sologuren A, Vinas R, Cordon E, et al. Open-label safety assessment of bilastine in elderly patients with allergic rhinoconjunctivitis and/or urticaria. Allergy Asthma Proc. 2018;39(4):299–304. doi: 10.2500/aap.2018.39.4136. [DOI] [PubMed] [Google Scholar]
- 23.Church MK. Safety and efficacy of bilastine: a new H(1)-antihistamine for the treatment of allergic rhinoconjunctivitis and urticaria. Expert Opin Drug Saf. 2011;10(5):779–793. doi: 10.1517/14740338.2011.604029. [DOI] [PubMed] [Google Scholar]
- 24.Bosma R, van den Bor J, Vischer HF, Labeaga L, Leurs R. The long duration of action of the second generation antihistamine bilastine coincides with its long residence time at the histamine H1 receptor. Eur J Pharmacol. 2018;5(838):107–111. doi: 10.1016/j.ejphar.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Farre M, Perez-Mana C, Papaseit E, et al. Bilastine vs. hydroxyzine: occupation of brain histamine H1-receptors evaluated by positron emission tomography in healthy volunteers. Br J Clin Pharmacol. 2014;78(5):970–980. doi: 10.1111/bcp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadopoulos NG, Zuberbier T. The safety and tolerability profile of bilastine for chronic urticaria in children. Clin Transl Allergy. 2019;9:55. doi: 10.1186/s13601-019-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abelson MB, Welch DL. An evaluation of onset and duration of action of patanol (olopatadine hydrochloride ophthalmic solution 0.1%) compared to Claritin (loratadine 10 mg) tablets in acute allergic conjunctivitis in the conjunctival allergen challenge model. Acta Ophthalmol Scand Suppl. 2000;230:60–63. doi: 10.1034/j.1600-0420.2000.078s230060.x. [DOI] [PubMed] [Google Scholar]
- 28.Crampton HJ. Comparison of ketotifen fumarate ophthalmic solution alone, desloratadine alone, and their combination for inhibition of the signs and symptoms of seasonal allergic rhinoconjunctivitis in the conjunctival allergen challenge model: a double-masked, placebo- and active-controlled trial. Clin Ther. 2003;25(7):1975–1987. doi: 10.1016/S0149-2918(03)80199-6. [DOI] [PubMed] [Google Scholar]
- 29.Marmouz F, Giralt J, Izquierdo I. Morning and evening efficacy evaluation of rupatadine (10 and 20 mg), compared with cetirizine 10 mg in perennial allergic rhinitis: a randomized, double-blind, placebo-controlled trial. J Asthma Allergy. 2011;4:27–35. doi: 10.2147/JAA.S18265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ousler GW, 3rd, Workman DA, Torkildsen GL. An open-label, investigator-masked, crossover study of the ocular drying effects of two antihistamines, topical epinastine and systemic loratadine, in adult volunteers with seasonal allergic conjunctivitis. Clin Ther. 2007;29(4):611–616. doi: 10.1016/j.clinthera.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Singh-Franco D, Ghin HL, Robles GI, Borja-Hart N, Perez A. Levocetirizine for the treatment of allergic rhinitis and chronic idiopathic urticaria in adults and children. Clin Ther. 2009;31(8):1664–1687. doi: 10.1016/j.clinthera.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 32.European Medicines Agency. Guideline on Bioanalytical Method Validation, (EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2**), 2011.
- 33.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on the investigation of bioequivalence. London, 20 January 2010. Doc. Ref.: CPMP/QWP/EWP/1401/98 Rev. 1/Corr**.
- 34.European Medicines Agency. Pharmacokinetic Studies in Man. Reference number 3CC3A. Directive 75/318/EEC, 1988.
- 35.European Medicines Agency. Note for guidance on the clinical requirements for locally applied, locally acting products containing known Constituents. CPMP/EWP/239/95 final, 1996.
- 36.Aguirre C, Garcia M. Causality assessment in reports on adverse drug reactions. Algorithm of Spanish pharmacovigilance system. Med Clin (Barc) 2016;147(10):461–464. doi: 10.1016/j.medcli.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Iyer GR, Cason MM, Womble SW, Li G, Chastain JE. Ocular pharmacokinetics comparison between 0.2% olopatadine and 0.77% olopatadine hydrochloride ophthalmic solutions administered to male New Zealand white rabbits. J Ocul Pharmacol Ther. 2015;31(4):204–210. doi: 10.1089/jop.2014.0140. [DOI] [PubMed] [Google Scholar]
- 38.Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139–1150. doi: 10.1016/j.jaci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Meier E, Narvekar A, Iyer GR, et al. Pharmacokinetics and safety of olopatadine hydrochloride 0.77% in healthy subjects with asymptomatic eyes: data from 2 independent clinical studies. Clin Ophthalmol. 2017;11:669–681. doi: 10.2147/OPTH.S126690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scaglione F. Safety profile of bilastine: 2nd generation H1-antihistamines. Eur Rev Med Pharmacol Sci. 2012;16(14):1999–2005. [PubMed] [Google Scholar]
- 41.Simons FE, Simons KJ. H1 antihistamines: current status and future directions. World Allergy Organ J. 2008;1(9):145–155. doi: 10.1097/WOX.0b013e318186fb3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibáñez MD, Garde JM. Allergy in patients under fourteen years of age in Alergologica 2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):61–68. [PubMed] [Google Scholar]
- 43.Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1994. J Allergy Clin Immunol. 2010;126(4):778–783. doi: 10.1016/j.jaci.2010.06.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.