Abstract
Most international clinical guidelines recommend 5–10 mm clinical margins for excision of melanoma in situ (MIS). While the evidence supporting this is weak, these guidelines are generally consistent. However, as a result of the high incidence of subclinical extension of MIS, especially of the lentigo maligna (LM) subtype, wider margins will often be needed to achieve complete histologic clearance. In this review, we assessed all available contemporary evidence on clearance margins for MIS. No randomized trials were identified and the 31 non-randomized studies were largely retrospective reviews of single-surgeon or single-institution experiences using Mohs micrographic surgery (MMS) for LM or staged excision (SE) for treatment of MIS on the head/neck and/or LM specifically. The available data challenge the adequacy of current international guidelines as they consistently demonstrate the need for clinical margins > 5 mm and often > 10 mm. For LM, any MIS on the head/neck, and/or ≥ 3 cm in diameter, all may require wider clinical margins because of the higher likelihood of subclinical spread. Histologic clearance should be confirmed prior to undertaking complex reconstruction. However, it is not clear whether wider margins are necessary for all MIS subtypes. Indeed, it seems that this is unlikely to be the case. Until optimal surgical margins can be better defined in a randomized trial setting, ideally controlling for MIS subtype and including correlation with histologic excision margins, techniques such as preliminary border mapping of large, ill-defined lesions and, most importantly, sound clinical judgement will be needed when planning surgical clearance margins for the treatment of MIS.
Keywords: Melanoma, In situ, Lentigo maligna, Surgery, Excision, Margins, Treatment, Diagnosis, Pathology
Key Summary Points
| There is a high risk of subclinical spread in certain subsets of MIS (lentigo maligna, head and neck location, diameter ≥ 3 cm), requiring a wider clinical margin (10–15 mm) than for other forms of MIS to achieve histologic clearance |
| A “one size fits all” approach to surgical margins for MIS is not appropriate |
| Clinical judgement is needed to tailor surgical margins in the management of MIS |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14555106.
Introduction
One hundred years ago a 5 cm radial margin was recommended for all patients with melanoma in the hope of reducing the risk of local recurrence and improving survival. However, in the late twentieth century surgeons began selectively using narrower margins. In the past few years, the surgical management of primary melanoma has shifted from a Halstedian “more is better” philosophy to a “less is equal” approach, based on new evidence. In 2018, for example, the Swedish and Danish melanoma groups published updated results from their landmark randomized multicenter international trial of 2 cm versus 4 cm surgical excision margins for patients with invasive primary melanomas > 2 mm in thickness [1]. In their report of 936 patients with melanoma after a median follow-up of 19.6 years, the authors found no difference in overall and melanoma-specific survival between the two groups. These results definitively eliminate the need for radical surgical excision margins (> 2 cm) for melanomas ≥ 2 mm in Breslow thickness, and international guidelines will doubtless be changed to reflect this paradigm shift.
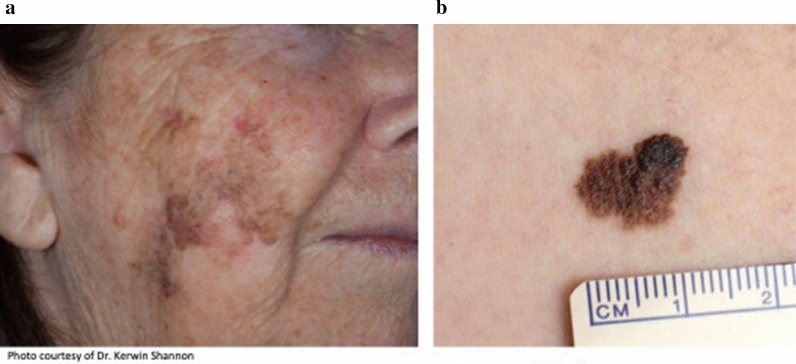
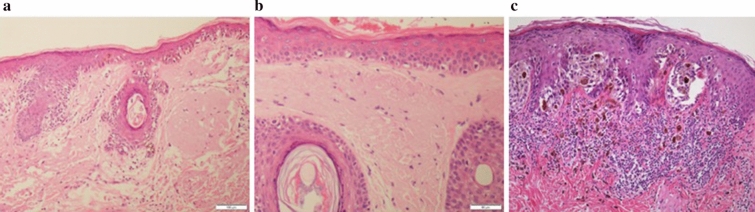
However, while significant effort has been made to study the optimal surgical excision margins for invasive primary melanoma [1–5], no randomized controlled trials (RCTs) to date have examined optimal surgical excision margins for the pre-invasive form of melanoma, known as melanoma in situ (MIS). In MIS, the malignant melanocytes are confined to the superficial layer of the skin, the epidermis, without invasion into the underlying dermis. MIS is classified into a variety of subtypes based on pathological features of both the tumor and related structures and the anatomic site of the tumor (Fig. 1). Lentigo maligna (LM) is a subtype of MIS that is characterised histologically by lentiginous and sometimes nested growth of atypical melanocytes in sun-damaged skin with associated epidermal atrophy and solar elastosis (Fig. 2a). It typically occurs on the face, scalp, and neck of elderly patients with severe chronic sun damage. In contrast to the other common subtypes of MIS (Fig. 2b), the peripheral edges of LM are often poorly defined both clinically and histologically, since the lesion often merges with other cutaneous changes of chronic sun damage [6]. This causes particular challenges for those managing patients with LM.
Fig. 1.
Clinical appearance of LM compared to non-LM melanoma in situ. a LM with indistinct borders. LM predominantly occurs on the head or neck in areas of significant sun damage. b Non-LM MIS with sharp well-defined borders; it usually occurs on non-chronically sun-exposed skin
Fig. 2.
Histologic appearance of LM compared to non-LM melanoma in situ. a Demonstrates the lentiginous and sometimes nested growth of atypical melanocytes on the background of sun damaged skin with associated epidermal atrophy and solar elastosis characteristic in of LM. The peripheral edge of LM is poorly defined both clinically and histologically, as shown in b, making complete histologic excision difficult. This is in contrast to non-LM MIS, which demonstrates sharply defined peripheral histologic margins (c)
Current international guidelines for the management of MIS are based on low-level evidence, and expert consensus that dates back to the early 1990s. They generally recommend 5 mm surgical margins for MIS (Fig. 3) with the goal of obtaining complete histologic clearance and thereby minimizing the risk of local recurrence (Table 1). However, the most recent guidelines from the USA [7, 8], Australia [9], and Canada [10] recommend clinical margins of 5–10 mm for MIS. These guidelines recognize the high incidence of subclinical extension of MIS [11], especially LM, and suggest that these lesions often require clinical clearance margins greater than 5 mm to achieve histologically clear margins.
Fig. 3.
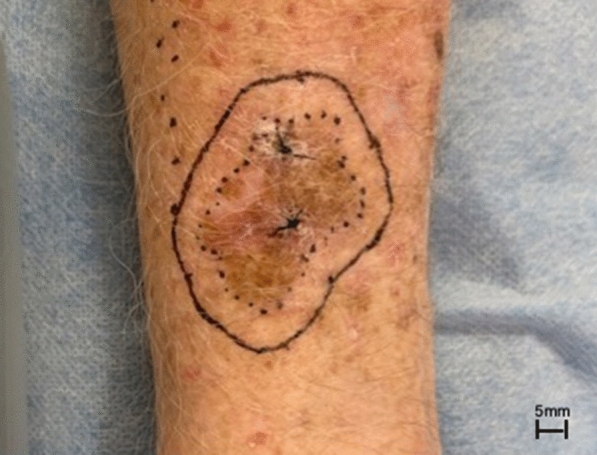
Clinical photograph of a LM on the arm showing measurement of a surgical margin at the time of wide excision, with the goal of obtaining histologic clearance. The total extent of the lesion is outlined (dotted line) and then a surgical margin is measured around the lesion (solid line)
Table 1.
Surgical excision margin recommendations for melanoma in situ in international guidelines
| Guideline group | Country | Year | Recommendation related to melanoma in situ | Level/grade of evidence |
|---|---|---|---|---|
| European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization for Research and Treatment of Cancer [49] | Europe | 2020 | Margin; 5 mm | 100% consensus, adapted from other guidelines |
| Japanese Dermatological Association Guidelines [50] | Japan | 2020 | Margin; 3–5 mm | Not stated |
| Norwegian Heath Directorate guideline [51] | Norway | 2020 | Margin; 5 mm | Level 4, grade D (case series, poor quality cohort and case–control studies, expert opinion) |
| Cancer Council Australia Melanoma Guidelines Working Group [9] | Australia and New Zealand | 2019 |
Margin; 5–10 mm Melanoma in situ of non-lentigo maligna type is likely to be completely excised with 5 mm margins whereas lentigo maligna may require wider excision. Minimum clearances from all margins should be stated/assessed. Consideration should be given to further excision if necessary; positive histological margins are unacceptable |
Level 4 (case series, poor quality cohort and case–control studies) |
| Cancer Care Ontario [10] | Canada | 2019 | Margin; 5–10 mm | Not stated |
| European Society for Medical Oncology (ESMO) [52] | Europe | 2019 | Margin; 5 mm | All margins; level II (RCT), grade B (strong or moderate evidence generally recommended) |
| Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF [53] | Germany | 2019 | A complete excision with histopathological control should be performed | Consensus 88% |
| Italian Association of Medical Oncologists [54] | Italy | 2019 | Margin; 5 mm | Low |
| Regional Cancer Centre [55] | Sweden | 2019 | Margin; about 5 mm | Not stated |
| American Academy of Dermatology [8] | USA | 2019 | Margin; 5–10 mm | Lower level (not further explained) |
| National Comprehensive Cancer Network (NCCN) [7] | USA | 2019 | Margin; 5–10 mm | Level 2B (lower level evidence, NCCN consensus) |
| European CanCer Organisation (ECCO) [56] | Europe | 2018 | Margin; 5 mm | Not stated |
| Finnish Melanoma Group [57] | Finland |
2018 2012 |
2018; NS 2012; margin; 5–10 mm |
Grade D (expert opinion) |
| Croatian Society for Medical Oncology [58] | Croatia | 2017 | NS | Not stated |
| French Dermatology Society and French National Federation of Comprehensive Cancer Centres, French National Cancer Institute [59] | France | 2017 | Margin; 5 mm | All margins; grade B (systematic review of cohort studies, individual cohort study of low quality RCT) |
| Scottish Intercollegiate Guidelines Network [13] | Scotland | 2017 |
Lentigo maligna, (a variant of melanoma in situ), should also be surgically removed, given the risk of invasion. Currently 5 mm surgical margins are recommended Consider a clinical margin of at least 5 mm when excising stage 0 melanoma. If excision for stage 0 melanoma does not achieve an adequate histological margin, discuss further management with the multidisciplinary team |
Level 3 (non-analytic studies, e.g., case series) |
| Swiss guidelines [60] | Switzerland | 2016 | Margin; 5 mm | Not stated |
| Dutch Societies of Radiology, Dermatology and Venereology, Pathology, Surgery, Internal Medicine and Radiology [61] | Netherlands | 2016 | Margin; 5 mm | Level 4, D (case series, poor quality cohort and case–control studies, expert opinion) |
| Central Oncology Institute, Warsaw [62] | Poland | 2016 | Margin; 5 mm | Taken from other guidelines (NCCN, ESMO, EORTC) |
| UK National Disciplinary guidelines for head and neck cancers [63] | UK | 2016 | Margin; 5 mm | Not stated |
| Brazilian Dermatological Society [64] | Brazil | 2015 | Margin; 5 mm | Level A (individual RCT with narrow confidence intervals) |
| National Collaborating Centre for Cancer [65] | UK | 2015 | Margin; 5 mm (stage 0, not specifically in situ) | No evidence, clinical experience |
| State expert Centre of Ministry of Health of Ukraine, National Cancer Institute of the Ministry of Health of Ukraine [12] | Ukraine | 2014 | Margins; 5 mm to ensure complete histological protrusion | Level 3 (systematic review of case–control studies, individual case–control study) |
| German Dermatologic Society and Dermatologic Cooperative Oncology Group [66] | Germany | 2013 | Margin; 5 mm | Grade D (expert consensus) |
| Melanoma cancer site team [67] | Canada | 2013 | Margin; 5–10 mm | Not stated |
| British Association of Dermatologists [68] | UK | 2010 | Margin; 5 mm to achieve complete histological excision | III [evidence obtained from well-designed nonexperimental descriptive studies, such as comparative studies, correlation studies and case studies], grade B [there is fair evidence to support the use of the procedure] (no RCT data, no references) |
| Melanoma Advisory Board [69] | South Africa | 2004 | Margin; 5 mm | Not stated |
Contemporary data on clearance margins in MIS are obtained largely from studies in which Mohs micrographic surgery (MMS) or staged excision (SE) have been used, mostly in patients with LM on the face or head/neck region. While the accuracy of MMS in the surgical treatment of MIS is both lesion- and operator-dependent, and its role remains somewhat controversial (and beyond the scope of this review), the findings from these studies are instructive, and indicate that a 5 mm clinical margin will frequently fail to achieve complete histologic clearance of MIS. In this article we critically review available reports on clearance margins for MIS and consider whether their findings are currently reflected in clinical practice guidelines around the world.
Methods
Searches of Embase and Medline to December 31, 2020 were performed to identify available clinical melanoma management guidelines using Medical Subject Heading and text word “melanoma”, limited to publication type guideline or practice guideline in Medline, and combined with the textwords guideline or practice guideline in Embase. Once identified, the section from each guideline pertaining to melanoma in situ was read in full and references cited were noted. To expand the search for international melanoma guidelines, Google Translate was used to identify words for “Melanoma guideline” in non-English languages and these words were used to perform Google searches to identify non-English language melanoma guidelines. Articles referenced by these guidelines and not previously identified, if found to relate to clearance margins or recurrence rates for MIS, were also included for review.
A literature search was also performed in PubMed, Medline, and Embase using a series of search strings as follows: “melanoma in situ” AND “surgical excision”; “lentigo maligna” AND “surgical excision; “melanoma in situ” AND “clearance margins”; lentigo maligna” AND “clearance margins”. The search related to surgical margins was limited to English language, full-length articles from January 1, 1995 to December 31, 2020 concerning human subjects exclusively. Abstracts and conference publications were excluded. Original studies consisting of case series, prospective and retrospective cohort studies, review studies, and RCTs were eligible for inclusion. If a published study was updated to include longer-term follow-up and/or additional patients, only the most recent results were included. However, if a similar dataset was used in a separate study to address different questions, both studies were included. If a study only reported defect size or number of stages of MMS or SE procedures required for histologic clearance and did not report final clearance margins, it was excluded. If a study included both in situ and invasive melanoma but did not separate them for analysis purposes, it was excluded.
Titles and abstracts were screened to check whether they met the inclusion criteria. The remaining articles were read in full and excluded if they were found not to meet inclusion/exclusion criteria. All references cited in each article were also reviewed to identify any studies not already captured by the above searches that may have met inclusion criteria. This article is based on previously conducted studies and does not contain any new studies performed by any of the authors involving human participants or animals.
Results
Clinical Guidelines
Thirty guidelines from 24 different countries were identified and all but four made a recommendation about clinical margins of excision for in situ melanoma (Table 1). The goal of surgical excision, when stated, was to achieve histologic clearance [12], reduce the risk of local recurrence [8], and reduce the risk of progression to invasive melanoma [13]. All guidelines that reported the level of evidence upon which their recommendation was made stated a low level of evidence. This included case reports or case series, expert opinion, and expert consensus.
Available Evidence on Histologic Clearance Margins in MIS
Thirty-two articles were identified that met the inclusion criteria for this review (Table 2). The majority of the articles were reviews of single-surgeon or single-institution experiences using MMS or SE for surgical treatment of MIS and/or LM. No RCTs were identified. It should be noted that a subset of studies of LM included patients with lentigo maligna melanoma (LMM); we focused our review on the subcohort of patients with LM only. As previously mentioned, if the analysis did not separate in situ from invasive lesions, the study was excluded.
Table 2.
Studies examining clearance margins in melanoma in situ
| Author | Study type | Sample size | Distinction between MIS and LM? | Anatomic site, n (%) | Clinical margin needed for histologic clearance | Follow-up | Recurrence rate |
|---|---|---|---|---|---|---|---|
| Mohs micrographic surgery | |||||||
| Robinson [15] | Prospective cohort study, single surgeon | 16 | Yes; LM only | Head: 16 (100%) | 69% clear with 6–10 mm | Range 5–9 years | 6.25% |
| Zitelli [16] | Prospective cohort study, single surgeon | 184 | No |
Head/neck: 106 (58%) Hands/feet: 6 (3%) Trunk: 34 (18%) Extremities: 38 (21%) |
83% clear with 6 mma 95% clear with 9 mma 97% clear with 12 mma |
At least 5 years | 0.5%a |
| Zalla [17] | Retrospective chart review, two surgeons | 46 | No |
Head/neck: 44 (96%) Acral: 2 (4%) |
Average 8.3 mm (range 3–19 mm) 50% clear with ≤ 6 mm 83% clear with ≤ 10 mm 96% clear with ≤ 15 mm |
Average 16 months (range 1–32) | 0% |
| Albertini [14] | Retrospective chart review, single center | 42 | No |
Head/neck: 41 (98%) Trunk: 1 (2%) |
41% clear with ≤ 6 mm 59% clear with ≤ 10 mm 88% clear with ≤ 15 mm 100% clear with ≤ 18 mm |
N/A | N/A |
| Bricca [18] | Prospective cohort study, single center | 331 | No | Head/neck: 331 (100%) |
89.1% clear with 6 mm 98.5% clear with 9 mm |
Mean 58 months (range 0–238.8) | 0.3% |
| Kunishige [23] | Retrospective database review | 1120 | No |
Head/neck: 668 (60%) Trunk: 197 (18%) Extremity: 232 (21%) Hands/feet: 20 (2%) Palm: 2 (< 1%) Genitalia: 1 (< 1%) |
86% clear with 6 mm margins 98.9% clear with 9 mm margins 99.4% clear with 12 mm margins |
Mean 4.7 years (range 0.02–23.5) | 0.3% |
| Stigall [27] | Retrospective database review | 882 | No |
Trunk: 427 (48%) Proximal extremity: 455 (52%) |
83% clear with 6 mm margins 97% clear with 9 mm margins |
Median 45 months (range 1–340) | 0.1% |
| Felton [22] | Retrospective database review, single institution | 343 | No | Head/neck: 343 (100%) |
65% clear with 5 mm margins 75% clear with 8 mm margins 92% clear with 10 mm margins 97% clear with 15 mm margin |
Mean 29 months | 0.3% |
| Valentin-Nogueras [20] | Retrospective database review, single institution | 982 | No |
Head/neck: 53.2%a Other: 46.8%a |
Mean clearance margin 7.1 mm | Mean 3.73 years (range 0–9.54) | 0.41% |
| Ellison [26] | Prospective, multicenter cohort study | 377 | No |
Head/neck: 266 (71%) Trunk: 64 (17%) Extremities: 42 (11%) Hands/feet/genitalia: 5 (1%) |
77.4% clear with 6 mm margin 92.1% clear with 9 mm margin 97.1% clear with 12 mm margin |
N/A | N/A |
| Kunishige [24] | Retrospective database review | 2335 |
Yes; LM: 1506 (64%) MIS: 829 (36%) |
Head/neck: 1362 (58%) Trunk/extremity: 973 (42%) |
79% LM/89% MIS clear with 6 mm 97% LM/MIS clear with 12 mm |
Median 4.5 years (range 0.2–23.5) |
5-year/10-year 0.27%/0.33% LM 0.00&/0.12% MIS |
| Kunishige [25] | Retrospective database review | 404 |
LM: 104 (26%) MIS: 300 (74%) |
Trunk/extremities: 404 (100%) |
< 0.8 cm in diameter 92.4% clear with 6 mm margin < 0.9 cm in diameter 94.3% clear with 6 mm margin < 1.0 cm in diameter 90.3% clear with 6 mm margin |
N/A | N/A |
| Foxton [19] | Retrospective chart review, single center | 62 | No |
Head/neck: 55 (89%) Trunk/extremities: 7 (11%) |
68% clear with 6 mm 92% clear with 9 mm 100% clear with 12 mm |
Range 3–30 months | 0% |
| Heath [21] | Retrospective database review, single institution | 529 | No |
Head/neck: 502 (95%) Trunk: 7 (1%) Extremities: 20 (4%) |
Mean surgical margin 7 mm ± 3.8 mm | Median 62.1 months | 2.5% |
| Staged excision | |||||||
| Agarwal-Antal [35] | Prospective cohort study, single center | 93 | Yes; LM only |
Head/neck: 69 (74%) Other: 24 (26%) |
42% clear with 5 mm 69% clear with 10 mm 84% clear with 15 mm 91% clear with 20 mm |
N/A | N/A |
| Huilgol [32] | Prospective cohort study, single center | 125 | Yes; LM only | Head/neck: 125 (100%) |
70% clear with 5 mm 80% of primary LM clear with 5 mm |
Mean 38 ± 25 months (range 3–100) |
5-year 0.05% |
| Jejurikar [30] | Retrospective chart review, single center | 42 | Yes; LM only | Head/neck: 42 (100%) | Mean clearance margin 10 ± 5 mm | Average 953 ± 246 days (range 473–1373) | 0% |
| Demirci [31] | Retrospective chart review, single center | 26 | Yes; LM only | Periocular 26 (100%) | Mean clearance margin 13 mm (range 5–25 mm) | Mean 49 months (range 9–112) | 2.5% |
| Hazan [34] | Retrospective chart review, single surgeon | 91 | Yes, LM only | Head/neck: 95%a | Mean clearance margin 7.1 mm (range 5–18 mm) | N/A | N/A |
| Moller [29] | Retrospective chart review, single institution | 49 | No | Head/neck: 49 (100%) | 76% clear with 5 mm margins | Median 14 months (range 1–36 | 0% |
| Shumaker [36] | Retrospective chart review, single institution | 18 | No | Periocular: 18 (100%) |
Average clearance margin 7.3 mm 33% clear with 5 mm margins |
Average 95.1 months (range 40–183) | 17% |
| Abdelmalek [33] | Retrospective chart review, single institution | 225 | Yes; LM only |
Head/neck: 67.9%a Upper extremity: 11.9%a |
Mean clearance margin 6.6 mm (range 3–35 mm) | Mean 32.3 months (range 2–96) | 1.7%a |
| Wilson [38] | Retrospective chart review, single surgeon | 58 | Yes; LM only |
Head/neck:71% Trunk: 10% Extremity: 19% |
54% (28/51) clear with 5 mm clinical margins 46% clearance rate on head and neck Of 23 not clear by 5 mm margins, average margin 10.4 ± 1.0 mm (median 9 mm, range 9–27 mm) |
Median 138 months (range 37–330) | 5.9% |
| De Vries [39] | Retrospective database review, single institution | 100 | Yes; LM only |
Head/neck: 97 (97%) Other: 3 (3%) |
88% clear with 8 mm margina 97% clear with 13 mm margina 100% clear with 18 mm margina |
Median 60 months | 4%a |
| Garcia [41] | Retrospective chart review, single institution | 29 |
Yes; LM: 8 (27.6%) MIS: 21 (72.4%) |
Head/neck: 27 (93%) Trunk/extremities: 2 (7%) |
55% clear with 5 mm margin 93% clear with 11.9 mm margin 97% clear with 12.3 mm margin |
Median 30 months (range 12–58) | 0% |
| Moyer [37] | Retrospective database review, single institution | 564 | No | Head/neck: 564 (100%) | Mean clearance margin 9.3 mm (SD 5.1) | Median 9.3 years |
1.4% 5 years 1.8% 7.5 years 2.2% 10 years |
| Beveridge [40] | Retrospective chart review, single center | 24 | Yes; LM only | Not indicated | Mean clearance margin, 9 mm | Median 18 months (range 1–63) | 0% |
| Wide local excision | |||||||
| Duffy [43] | Retrospective database review, two institutions | 36 | Yes; MIS only |
Trunk: 16 (44%) Extremity: 17 (47%) Foot: 3 (8%) |
Average excision margin 3.8 mm (range 0.2–6.5) Average margin of clearance 4.38 mm (range 1.3–7.75) |
Median 6.6 years (range 0.5–18) | 0% |
| Akhtar [44] | Retrospective database review, single institution | 192 |
Yes; LM: 75 (38%) MIS: 117 (62%) |
Head/neck: 83 (43%) Trunk: 42 (22%) Extremity: 42 (22%) |
Average primary excision margin for narrow excision group 1.51 mmb Average primary excision margin for wider excision group 4 mm |
Mean 31 months (narrow excision); mean 32.6 months (wide excision) |
1.4% (narrow excision) 1.1% (wide excision) 29% (incomplete excision) |
| Welch [42] | Retrospective chart review, two institutions | 155 | Yes; MIS only |
Trunk: 81 (52%) Extremity: 74 (48%) |
100% clear 5 mm margins | Mean 4 years (range 0.25–13) | 0% |
| Blank [70] | Retrospective chart review, single center | 86 | No |
Head/neck: 42 (46%)a Trunk/extremity: 45 (49%) Hand/foot: 1 (1%) Other: 3 (4%) |
Average margin 6.5 ± 2.4 mm | WLE | NR |
| Crouch [45] | Retrospective database review, single institution | 382 | Yes; LM only | Head/neck: 382 (100%) | 91.6% histologically clear with mean surgical margin 6.2 mm ± 3.1 mm | Median 32 months (range 12–223) | 9.9%c |
MIS melanoma in situ, LM lentigo maligna, MMS Mohs micrographic surgery, NR not recorded, SE staged excision, WLE wide local excision, SD standard deviation
aReported for entire cohort, including invasive melanomas
bExcision prior to change in 2006 UK melanoma guidelines that increased the recommended margin to 2–5 mm
cIncluded 11 cases with incomplete histologic excision after initial surgical excision
Mohs Micrographic Surgery
Mohs micrographic surgery (MMS) is a technique of surgical excision whereby a lesion is narrowly excised, with immediate en face frozen section margin assessment is undertaken. The proponents of MMS state that it is not only a tissue-conserving technique, especially important in cosmetically sensitive areas such as the face, but it allows for near-total margin assessment. However, MMS is not universally accepted as a necessary or reasonable alternative to standard wide excision for treating all MIS or invasive melanoma. This is largely due to availability, cost, and limitations in its accuracy and reliability that may result in some patients being overtreated, the very problem its proponents seek to avoid. The cells of LM are characteristically discontinuous at the periphery of the lesion [6] and hence en face sections will inevitably fail to identify margin involvement when the sections are taken from the uninvolved epidermis separating LM cells. Furthermore, MMS does not provide an opportunity to evaluate the significance of either the degree of cytological atypia or density of melanocytes which can assist in distinguishing the peripheral edge of LM from the melanocytic proliferation that normally occurs in chronically sun damaged skin. Lastly, histological interpretation of these changes is also very subjective, limiting the ability to make meaningful interpretations across studies. Standard frozen section evaluation of MIS with hematoxylin and eosin (H&E) staining can be very difficult and is quite subjective, especially in the context of extreme skin photo-damage, where it can be difficult to distinguish freezing artifact from junctional melanocytic proliferations, and therefore to interpret margin status [14]. In an effort to overcome these drawbacks, staining with melanoma-specific immunohistochemical stains such as HMB-45 or Melan-A/MART-1 was introduced, though these are not without their own set of difficulties in interpretation and likely cause “overcalling” of margins in some cases. Staining with SOX1O or MiTF for nuclear expression will possibly overcome some of these pitfalls [14]. While there remains controversy in relation to the use of MMS in the treatment of both in situ and invasive melanoma and the purpose of this article is not to enter into this controversy, the body of literature on MMS nevertheless provides highly valuable insights into the clearance margins required for MIS.
One of the earliest studies seeking to address the question of margin adequacy in LM using MMS involved a cohort of just 16 patients with LM of the head [15]. It was concluded that 5–10 mm margins would be sufficient for most LMs. However, the findings suggested stratification of surgical margins by lesion diameter, utilizing 5 mm margins for lesions < 2.0 cm in diameter and reserving 10 mm margins for lesions 2.0–3.0 cm in diameter. A surgical margin of 15 mm was suggested for lesions with a diameter > 3.0 cm.
In a much larger series seeking to measure the subclinical extent of melanoma extension, with the goal of informing clinical practice guidelines, 553 primary melanomas were treated by a single surgeon using MMS, including 184 MIS [16]. The majority of the in situ lesions (58%) were located on the head/neck. While 83% of all (in situ and invasive) lesions were cleared histologically with use of 6 mm clinical margins, clinical margins of 12 mm were required for histological clearance of 97% of melanomas. Size of clinical margin necessary to achieve histologic clearance was significantly related to both location of the lesion (p < 0.001) and diameter (p = 0.002). Melanomas on the head/neck and hands/feet required larger margins (15 mm) for 97% and 96% clearance rates, respectively. This likely reflects the growth characteristics and propensity for subclinical extension of the commonest types of MIS occurring on the head/neck and hands/feet, namely LM and acral (lentiginous) MIS, respectively.
Zalla et al. [17] reported their experience using MMS to treat 46 patients with MIS. Complete histologic clearance was achieved in only 50% of patients (n = 23) treated with ≤ 6 mm clinical margins, and ≥ 15 mm clinical margins were needed to achieve histological clearance in 96% of patients. Bricca et al. [18] undertook a prospective, single-institution study of all patients referred for MMS to treat MIS (n = 331) and invasive melanoma (n = 294). While no distinction was made between MIS and LM, only patients with head and neck melanomas were included, suggesting that LM would likely have been over-represented. On the basis of their finding that only 89.1% of MIS patients had a complete excision with 6 mm margins, the authors recommended surgical margins of 9 mm for MIS of the head/neck. Foxton et al. reported that a mean clinical margin of 6.7 mm was necessary in their cohort of 62 patients with MIS or LM, predominantly located on the head/neck (n = 55, 89%) [19]. They found that only 68% of patients achieved clearance with 6 mm margins, 92% with 9 mm margins, and 100% with 12 mm margins. Larger clinical margins required to achieve histologic clearance were related to increasing tumor diameter and recurrent tumors.
Two large retrospective single-institution studies primarily sought to determine the efficacy of MMS in the treatment of MIS and invasive melanoma, but also reported clearance margins [20, 21]. As in other reports, Valentin-Nogueras et al. found that 12 mm clinical margins were needed to achieve clearance in 97% of cases. Across the entire cohort, which included invasive melanoma and 982 patients with MIS, surgical margins were significantly related to tumor location and size (p < 000.1), with ≤ 9 mm clinical margins achieving 97% and 95% histologic clearance rates for MIS on the trunk/extremities and tumors < 1 cm, respectively [20]. Heath et al. reported their experience using a modified MMS technique to treat 529 patients with MIS, predominantly located on the head and neck [21]. Mean clinical clearance margins of 7 mm ± 3.8 mm were reported in cases of MIS.
Seeking specifically to calculate the proportion of patients requiring > 5 mm clinical margins for histological clearance, Felton et al. [22] reviewed 343 cases of MIS located on the head/neck. As per their institutional practice, all in situ lesions were designated MIS, without separate classification of LM. As in other studies of MIS on the head/neck, 5 mm clinical margins achieved complete histologic clearance in only 65% of cases. In fact, 15 mm clinical margins were required to achieve 97% histologic clearance rates. The authors did not identify any clinical factors, including lesion area, predictive of a requirement for a larger surgical margin to achieve clear histologic margins [22].
In a retrospective review of a prospectively maintained institutional database of all patients referred for MMS for treatment of melanoma, Kunishige et al. identified 1120 patients with MIS [23]. The majority of tumors were located on the head/neck and the mean tumor diameter was 2.8 cm. The authors concluded that the commonly recommended 5 mm clinical margin is inadequate, as only 86% of lesions were histologically cleared with 6 mm margins. However, the authors did not distinguish between MIS and LM, making it difficult to address whether 5 mm clinical margins may be sufficient for a certain subset of in situ melanomas. The same group specifically addressed the question of whether the margin requirements for LM differed from those for other types of MIS [24]. This was the largest cohort studied to date (1506 cases of LM and 829 cases of non-LM MIS), and the aim was to determine the minimum surgical margin needed for histologic clearance in 97% of cases. Interestingly, the authors found that the clearance rates for LM and non-LM MIS were similar, with both requiring 12 mm clinical margins for 97% histologic clearance. Subgroup analysis for location revealed a need for wider clearance clinical margins for lesions on the head/neck (both LM and non-LM MIS still required 12 mm clinical margins for 97% clearance) than for lesions on the trunk or extremity, where both LM and non-LM MIS required 9 mm clinical margins for 97% histologic clearance. Increasing diameter was found to be a predictor of subclinical spread, as larger lesions needed wider clinical margins to achieve 97% histologic clearance (diameter < 1 cm, 9 mm clinical margins; diameter 1–3 cm, 12 mm clinical margins; diameter > 3 cm, 15 mm clinical margins). In a search for lesional characteristics that might predict histologic clearance with a narrower margin, the authors analyzed a subcohort of their patients with in situ lesions < 1.0 cm in diameter located on the trunk or extremity [25]. They found that lesions < 0.9 cm in diameter approached histologic clearance rates of 95% with 6 mm clinical margins, but 9 mm clinical margins were required for 97% histologic clearance rates across all lesions > 1.0 cm. Perhaps most notable was the finding that histologic clearance rates for LM were not significantly different from those of non-LM MIS.
In the only multicenter prospective cohort study of patients with melanoma treated with MMS, including 377 non-invasive melanomas, it was found that 12 mm clinical margins were adequate for histologic clearance in 97.1% of cases [26]. Head and neck lesions required significantly wider clinical margins than lesions on the trunk or extremity (12 mm vs 10 mm, respectively, p < 0.01). While there was no discrimination between LM and non-LM MIS in the primary analysis, the two groups did not differ significantly in subgroup analysis in required clinical margins to achieve histologic clearance at any location. Larger preoperative tumor size and recurrent lesions were both positively associated with a larger clinical margin requirement [26].
Albertini et al. [14] recognized that those patients referred for MMS for management of melanomas primarily had lesions on the head/neck, were in locations of cosmetic or functional significance, or were recurrent lesions. As such, they acknowledged that they often excised lesions with asymmetric margins and were interested in minimum and maximum clinical margins required for histologic clearance of each lesion. In their review of a series of 42 patients with MIS, all but one on the head/neck, they found that the mean minimum and maximum clinical clearance margins were 5.5 mm (range 3–11 mm) and 9.4 mm (range 3–18 mm), respectively. They concluded that MIS demonstrates asymmetric lateral growth and the maximum clinical clearance margin, which exceeded 6 mm in 59% of cases, may only be necessary focally around a lesion [14].
Stigall et al. [27] sought to determine if MIS in relatively sun-protected areas, such as the trunk and proximal extremity, display subclinical extension similar to MIS in the more commonly reported sun-exposed areas such as the head/neck. All cases of MIS on the trunk and proximal extremity were retrospectively identified from a database of patients with melanoma treated with MMS. Only 23% of the 882 cases were classified as LM or non-LM subtype and no differences in clearance margins were found on subgroup analysis. Therefore, LM subtype was not considered separately for analysis purposes. Consistent with previously published data, the authors found that only 83% of MIS were cleared with 6 mm margins and 9 mm margins were necessary to achieve a 97% clearance rate, in both primary and recurrent MIS. There were no significant differences in margins when stratified by location, though lesions with a larger diameter showed a trend towards an increased margin requirement, leading to a recommendation for larger margins, at least 12 mm, for lesions > 2 cm in diameter.
Serial or Staged Excision
In an effort to eliminate the potential shortcomings of frozen section evaluation of melanoma specimens, even with the addition of immunostains, some have advocated the use of mapped serial or staged excision (SE). This technique generally entails the following process: the tumor borders are marked with the assistance of a Wood’s lamp and a 5 mm clinical margin added in a geometric [28] or contoured [29] fashion to facilitate processing and orientation. The specimen including the tumor plus margin is excised to the mid to deep subcutaneous layer, dressed or closed temporarily, and an expedited detailed histopathologic assessment of the entire formalin-fixed margin by a dermatopathologist is performed, taking care to carefully orient and diagrammatically record the margins of the specimen [28]. Additional margins are then taken from the site of any involved margins on the initial specimen. In a variant of this technique, referred to as total peripheral margin control or a “square procedure”, a thin strip of tissue (2–3 mm) beyond the 5 mm clinical margin is initially excised using a double-bladed knife and temporarily closed while the entire peripheral margin is examined histologically as permanent histologic sections. Additional targeted margins are excised until complete histologic clearance is achieved. Finally, the central tumor is excised and sent for formal pathologic review [30, 31].
Huilgol et al. [32] prospectively studied a series of 125 patients who underwent SE of LM > 1 cm in diameter on the head or neck. They found that 30% required > 5 mm excision margins for histologic clearance. Cases of recurrent LM were less likely to be histologically cleared with 5 mm clinical margins than primary LM (44% vs. 80%, respectively) [32].
In a review of 91 cases of LM and 26 cases of LMM treated with SE by a single surgeon at the Memorial Sloan Kettering Cancer Center, where 95% of tumors were located on the head or neck, lesions > 2 cm in diameter required a significantly wider clinical clearance margin than those < 1 cm in diameter (13.1 vs. 8.6 mm, p < 0.0001). Men and patients > 65 years old required a larger total clinical margin, but this was not statistically significant. A slightly smaller mean clinical margin of 6.6 mm to achieve histologically clear margins was reported in another retrospective review of 225 cases of LM [33]. While the face was the most common site of disease (67.6%), 11.9% of lesions were located on the upper extremity. It is interesting to note that 11.6% (n = 26) of LMs required less than a 5 mm clinical margin for histologic clearance, 62.2% (n = 140) required 5 mm clinical margins, and 26.2% (n = 59) required > 5 mm clinical margins [34]. Lesional characteristics, such as diameter and anatomic location, that could predict the need for wider clinical margins were not investigated.
In a study reported by Agarwal-Antal et al., 93 patients with LM were treated with SE, 75% of which were located on the head/neck, only 42% (n = 39) were histologically cleared with 5 mm clinical margins [35]. Similarly, in a case series of 18 patients with MIS located on the periocular skin, Shumaker et al. reported that only 1/3 of lesions were histologically cleared with 5 mm clinical margins [36]. In a large single-institution study of SE reported by Moyer et al., 564 cases of MIS on the head/neck were treated, with a mean clinical margin of 9.3 mm [standard deviation (SD), 5.1] [37]. Clinical margins of ≤ 5 mm achieved histologic clearance only in 41.1% of cases, and clinical margins of ≤ 10 mm achieved histologic clearance in only 72.4% of cases. A larger lesion size was identified as a predictor of clinical margin required for histologic clearance [37]. A higher histologic clearance rate (76%) was achieved with 5 mm clinical margins in a smaller single-institution retrospective review of 49 patients treated with SE for MIS of the head/neck [29]. Lesions with positive histologic margins after a single stage of excision had a larger median size than those that were cleared with 5 mm clinical margins (6.9 cm2 vs. 4.0 cm2, p = 0.03) [29].
Wilson et al. [38] published a study examining long-term recurrence after SE of LM in 58 patients (and 10 patients with LMM) with at least 5 years follow-up. While the primary outcome was local recurrence, reported to be 5.6%, all occurring in patients with LM, the authors also investigated the minimum surgical margin for histologic clearance, with their findings echoing those of earlier studies. Only 54% of LMs were histologically cleared with 5 mm clinical margins, with a histologic clearance rate of 46% in LMs on the head/neck. Of those tumors requiring more than 5 mm clinical margins, the average clearance margin was 10.4 ± 1.0 mm (median 9 mm, range 6–27 mm) [38]. In a study investigating the recurrence rate of LM after SE, de Vries reported clearance margins in a cohort of 100 patients with LM, 5 of which were diagnosed with LMM on final excision [39]. All but three of the lesions were located on the head/neck, with a mean lesion diameter of 20.1 mm (range 4–50). The initial clinical margin of 3 mm achieved histologic clearance in less than half the patients (49%). A surgical margin of 13 mm was required for histologic clearance in 97% of lesions.
Jejurikar et al. [30] from the University of Michigan reported their experience with 51 consecutive facial lesions, 42 of which were LM, treated by total peripheral margin control. The authors found that the initial 5 mm clinical margins were adequate for histologic clearance in only 35% of cases and LM required an average clinical margin of 10 ± 5 mm to obtain clear histologic margins [30]. Similarly, another group from the University of Michigan found that in 26 periocular LMs treated with total peripheral margin control, histologically tumor-free margins were obtained with a mean clinical clearance margin of 13 mm (range 5–25 mm) [31]. Larger lesion diameter was directly correlated with the clinical margin width needed for histologic clearance. In their own variant of this technique, a group from Alberta, Canada reported on their experience using staged margin-controlled excision (SMEX) for the treatment of LM in 24 patients, predominantly located on the face and anterior leg [40]. They found that a mean of 9 mm clinical margins was required for histologic clearance, in line with prior data.
Reporting on another variant of staged excision, a single-institution chart review of 29 cases of MIS (27.6% LM) predominantly located on the head/neck (93.1%) found a final mean surgical margin of 13.1 mm (SD 5.9) [41]. Forty-five percent of lesions required > 5 mm clinical margins, and 12.3 mm clinical margins were needed to achieve a 97% histologic clearance rate. Larger lesions showed a trend toward requiring larger surgical clearance margins to achieve histologically clear margins.
Wide Local Excision
There have been no prospective studies with a primary focus of determining clearance margins for MIS where wide local excision (WLE) was the form of surgical treatment. There have, however, been a number of retrospective studies. Contrary to the previously presented data that almost universally support a clinical margin greater than 5 mm to achieve histologically clear margins, three retrospective studies of non-LM MIS support the current guidelines that recommend 5 mm margins only [42–44]. In a review of 155 cases of MIS on non-chronically sun-exposed skin, conventional WLE with 5 mm margins achieved histologic clearance in 100% of cases. With a mean follow-up of 4 years, the authors reported no recurrences. It should be noted, however, that in this series, the mean clinical diameter of lesions was only 8.7 mm and 45% of cases had a negative margin in the initial biopsy specimen [42].
Similarly, Duffy et al. [43], in a database review of 36 confirmed cases of non-LM MIS, found that the average histologic excision margin was 3.8 mm (range 0.2–6.5). Median lesion size was 0.7 cm (range 0.2–1.8) and the majority of cases (56%) had negative initial biopsy margins. When summing the clear biopsy margin and excisional margin, the average histologic clearance margin was 4.38 mm (range 1.3–7.75). Notably, they reported no recurrences after a median follow-up of 6.6 years [43].
A retrospective series from the UK included 192 cases of MIS treated by WLE, of which 38% were LM [44]. Though the planned surgical margin at primary excision was not specified, the incomplete excision rate for LM was significantly higher than that of non-LM MIS (29.3% vs. 5.9%, respectively; p < 0.01). Of those with negative histologic margins at initial excision (n = 163), the average primary excision margin was noted to be 1.51 mm. As a result of a change in the UK melanoma management guidelines in 2006, with a new recommendation for excision margins of 2–5 mm for MIS, 97 patients underwent additional wide excision, with an average final excision margin of 4 mm. In both the narrow (< 2 mm) and wider excision groups, the recurrence rates were very low (1.4% and 1.1%, respectively) [44].
A recent retrospective review of the prospectively maintained database of Melanoma Institute Australia identified 379 patients with 382 LMs on the head/neck treated with WLE [45]. The mean surgical excision margin was 6.2 (± 3.1) mm with a mean histologic margin of 3.8 (± 2.6) mm. There were 32 incomplete excisions (8.4%) and 46 WLE specimens that had a close histologic margin (< 2.0 mm). A recurrence rate of 9.9% (n = 38) was observed after a median follow-up of 32 months. However, many of the recurrences occurred in cases where a negative histologic margin was not achieved and no further treatment was pursued (n = 11 of 18, 61.1%) or in cases with close histological margins (< 2.0 mm) without subsequent treatment (n = 12 of 36, 33.3%). In fact, the recurrence rate was 27.2% for lesions with a histologic margin < 3.0 mm compared to 2.6% with a histologic margin ≥ 3.0 mm, which correlated to a mean surgical margin of 6.5 (± 2.8) mm.
Conclusions
Despite varying study designs and differing surgical techniques, the available data consistently demonstrate that LM, lesions on the head/neck, and/or larger diameter lesions (> 3 cm) all require wider clinical surgical margins than are recommended in most current melanoma management guidelines to achieve complete histological disease clearance because of the higher likelihood of subclinical spread [46]. However, the question of whether this is necessary for all patients with MIS remains unanswered [42–44]. Many of the published studies described above did not separate non-LM MIS from LM, or only studied LM, making it difficult to dissect out true differences. An additional difficulty is that defining LM and determining its margins histologically requires subjective interpretation by pathologists and consequently there is likely to be inconsistent reporting of LM between pathologists [22]. Finally, it is possible that because of the difficulty in interpreting and differentiating atypical melanocytes and MIS in severely sun-damaged skin on frozen section examination, even with special immunohistochemical stains, the technique of MMS may be overtreating some of these lesions. On the basis of currently available data, as described in this review, it appears that a “one size fits all” approach to surgical margins for all forms of MIS is not appropriate. Instead, the clinical and pathological features of each case must be considered when planning surgical excision margins.
Overall, the results of the various studies challenge the adequacy of current management guidelines, with the available evidence strongly suggesting that 5 mm surgical margins as recommended in many existing guidelines will often be inadequate by failing to achieve complete histologic clearance, particularly for LM, MIS located in the head/neck region, and large (> 3 cm) lesions. The evidence also reinforces the concept that MIS on non-chronically sun-exposed skin and LM represent different biologic entities that require different clinical management strategies. Lastly, it is difficult to recommend a single surgical margin that will suffice for all lesions as data has shown that a surgical margin is a poor predictor of a histologic margin, and it is histologic clearance that is the goal of surgical excision, regardless of technique [45, 47].
On the basis of this review of available literature, suggested clinical and pathological features to be considered in margin selection for MIS are shown in Table 3. Histologic clearance of small (< 1 cm) well-circumscribed non-LM MIS on the trunk or proximal extremities will usually be achieved with 5 mm surgical margins. In the case of ill-defined lesions between 1 and 3 cm in diameter on chronically sun-exposed areas like the head/neck and distal extremities, acral MIS, or lesions of the LM subtype, 10 mm clinical margins should be obtained. In lesions with a high risk of subclinical extension, such as those > 3 cm in diameter, especially on chronically sun-exposed skin and MIS on the head/neck, 12–15 mm clinical margins should be considered to achieve reliable histologic clearance. While a comparison of the surgical options for MIS including the differences in histologic margin assessment is beyond the scope of this review, reported local recurrence rates are extremely low (Table 2), emphasizing the importance of histologically negative margins. In cases where larger clinical margins cannot be reasonably obtained because of cosmetic or functional restrictions or if there is clinical concern that the risk of subclinical spread is high, histologic clearance should be confirmed prior to complex reconstruction or tissue rearrangement. In these cases, preoperative “border mapping” can be considered using a variety of techniques such as mapping punch biopsies, total peripheral margin control/staged excision, or possibly reflectance confocal microscopy [48]. MMS may also be considered, although the difficulties assessing ill-defined MIS margins by frozen section examination must be acknowledged. In the absence of evidence from a randomized clinical trial examining optimal clinical margins in the treatment of all MIS, including LM, the treating clinician must exercise clinical judgement on a case-by-case basis.
Table 3.
Clinical considerations in margin selection for melanoma in situ
| 5 mm clinical margins may be sufficient if all of the following criteria are met |
| Well-circumscribed lesions |
| Small lesions, < 1 cm in diameter |
| Non-chronically sun-exposed skin (trunk, proximal extremities) |
| Absence of histological evidence of chronic sun damage/solar elastosis on biopsy (i.e., not LM) |
| 10 mm clinical margins should be considered for |
| Areas if sufficient skin laxity where larger margins can be achieved without untoward consequence |
| Ill-defined lesions |
| Lesions > 1 cm in diameter |
| Chronically sun-exposed skin, such as the head and neck and distal extremities |
| Diagnosis of LM on dermatopathology review of biopsy |
| Acral lesions |
| > 10 mm clinical margins should be considered in |
| Very large lesions > 3 cm in diameter, especially those on chronically sun-exposed skin |
| Head and neck location |
MIS melanoma in situ, LM lentigo maligna
Acknowledgements
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Richard A Scolyer and John F Thompson are supported by an Australian National Health and Medical Research Council (NHMRC) Program Grant (APP1093017), and RAS is the recipient of a NHMRC Practitioner Fellowship (APP1141295). The Rapid Service Fee was funded from the NHMRC Program Grant listed above.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Erica B Friedman, Gabrielle J Williams and John F Thompson made substantial contributions to the conception of the work. Erica B Friedman and Gabrielle J Williams participated in the literature search and review of literature for inclusion. All authors drafted the article and revised it critically for important intellectual content. All authors read and approved the final version of the work submitted for publication.
Disclosures
Erica B Friedman and Gabrielle J Williams have nothing to disclose. Richard A Scolyer has received fees for professional services from Qbiotics, Novartis, MSD, NeraCare, AMGEN Inc., BMS, Myriad Genetics, GSK. John F Thompson has received honoraria for advisory board participation from BMS Australia, MSD Australia, GSK and Provectus Inc, and travel support from GSK and Provectus Inc.
Compliance with Ethical Guidelines
This article is based on previously conducted studies and does not contain any new studies performed by any of the authors including human participants or animals.
References
- 1.Utjes D, Malmstedt J, Teras J, et al. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: long-term follow-up of a multicentre, randomised trial. Lancet. 2019;394(10197):471–477. doi: 10.1016/S0140-6736(19)31132-8. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg. 1991;126(4):438–441. doi: 10.1001/archsurg.1991.01410280036004. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Urist MM, Karakousis CP, et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial. Ann Surg. 1993;218(3):262–267. doi: 10.1097/00000658-199309000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn-Cedermark G, Rutqvist LE, Andersson R, et al. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8–2.0 mm. Cancer. 2000;89(7):1495–1501. doi: 10.1002/1097-0142(20001001)89:7<1495::AID-CNCR12>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Hayes AJ, Maynard L, Coombes G, et al. Wide versus narrow excision margins for high-risk, primary cutaneous melanomas: long-term follow-up of survival in a randomised trial. Lancet Oncol. 2016;17(2):184–192. doi: 10.1016/S1470-2045(15)00482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiau CJ, Thompson JF, Scolyer RA. Controversies and evolving concepts in the diagnosis, classification and management of lentigo maligna. Expert Rev Dermatol. 2013;8(2):195–214. doi: 10.1586/edm.13.17. [DOI] [Google Scholar]
- 7.Coit DG, Thompson JA, Albertini MR, et al. Cutaneous melanoma, version 22019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(4):367–402. doi: 10.6004/jnccn.2019.0018. [DOI] [PubMed] [Google Scholar]
- 8.Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208–250. doi: 10.1016/j.jaad.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 9.Sladden M, Omgo, N, Howle J, Coventry B, Cancer Council Australia Melanoma Guidelines. What are the recommended safety margins for radical excision of primary melanoma? (In situ). https://wiki.cancer.org.au/australia/Guidelines:Melanoma. 2020. Accessed 4 Mar 2020.
- 10.Wright FC, Souter LH, Kellett S, et al. Primary excision margins, sentinel lymph node biopsy, and completion lymph node dissection in cutaneous melanoma: a clinical practice guideline. Curr Oncol. 2019;26(4):e541–e550. doi: 10.3747/co.26.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne JE, Hutchinson PE. A follow-up study to investigate the efficacy of initial treatment of lentigo maligna with surgical excision. Br J Plast Surg. 2002;55(8):611–615. doi: 10.1054/bjps.2002.3967. [DOI] [PubMed] [Google Scholar]
- 12.Alexandrovich GA, Igorovich KS, Ivanovichm LA, et al. Melanoma. Adapted, evidence based clinical setting. http://old.moz.gov.ua/docfiles/dn_20151127_0784dod_akn.pdf. 2015. Accessed 20 Oct 2020.
- 13.SIGN. Cutaneous melanoma: a national clinical guideline. http://www.sign.ac.uk. 2017. Accessed 8 July 2020.
- 14.Albertini JG, Elston DM, Libow LF, Smith SB, Farley MF. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28(8):656–665. doi: 10.1046/j.1524-4725.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JK. Margin control for lentigo maligna. J Am Acad Dermatol. 1994;31(1):79–85. doi: 10.1016/s0190-9622(94)70140-7. [DOI] [PubMed] [Google Scholar]
- 16.Zitelli JA, Brown CD, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37(3 Pt 1):422–429. doi: 10.1016/s0190-9622(97)70144-0. [DOI] [PubMed] [Google Scholar]
- 17.Zalla MJ, Lim KK, Dicaudo DJ, Gagnot MM. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg. 2000;26(8):771–784. doi: 10.1046/j.1524-4725.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 18.Bricca GM, Brodland DG, Ren D, Zitelli JA. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52(1):92–100. doi: 10.1016/j.jaad.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Foxton GC, Elliott TG, Litterick KA. Treating melanoma in situ and lentigo maligna with Mohs micrographic surgery in Australia. Australas J Dermatol. 2019;60(1):33–37. doi: 10.1111/ajd.12845. [DOI] [PubMed] [Google Scholar]
- 20.Valentin-Nogueras SM, Brodland DG, Zitelli JA, Gonzalez-Sepulveda L, Nazario CM. Mohs micrographic surgery using MART-1 immunostain in the treatment of invasive melanoma and melanoma in situ. Dermatol Surg. 2016;42(6):733–744. doi: 10.1097/DSS.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heath M, Woody M, Leitenberger J, Latour E, Bar A. Invasive melanoma and melanoma in situ treated with modified Mohs micrographic surgery with en face permanent sectioning: a 10-year retrospective review. Dermatol Surg. 2019 doi: 10.1097/DSS.0000000000002246. [DOI] [PubMed] [Google Scholar]
- 22.Felton S, Taylor RS, Srivastava D. Excision margins for melanoma in situ on the head and neck. Dermatol Surg. 2016;42(3):327–334. doi: 10.1097/DSS.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 23.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66(3):438–444. doi: 10.1016/j.jaad.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. J Am Acad Dermatol. 2019;81(1):204–212. doi: 10.1016/j.jaad.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 25.Kunishige JH, Doan L, Brodland DG, Zitelli JA. 9-mm Surgical margin required for both LM and MIS as diagnosed in real-world community practice. J Am Acad Dermatol. 2019;81(4):e117–e118. doi: 10.1016/j.jaad.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 26.Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81(3):767–774. doi: 10.1016/j.jaad.2019.05.057. [DOI] [PubMed] [Google Scholar]
- 27.Stigall LE, Brodland DG, Zitelli JA. The use of Mohs micrographic surgery (MMS) for melanoma in situ (MIS) of the trunk and proximal extremities. J Am Acad Dermatol. 2016;75(5):1015–1021. doi: 10.1016/j.jaad.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Hill DC, Gramp AA. Surgical treatment of lentigo maligna and lentigo maligna melanoma. Australas J Dermatol. 1999;40(1):25–30. doi: 10.1046/j.1440-0960.1999.00311.x. [DOI] [PubMed] [Google Scholar]
- 29.Moller MG, Pappas-Politis E, Zager JS, et al. Surgical management of melanoma-in-situ using a staged marginal and central excision technique. Ann Surg Oncol. 2009;16(6):1526–1536. doi: 10.1245/s10434-008-0239-x. [DOI] [PubMed] [Google Scholar]
- 30.Jejurikar SS, Borschel GH, Johnson TM, Lowe L, Brown DL. Immediate, optimal reconstruction of facial lentigo maligna and melanoma following total peripheral margin control. Plast Reconstr Surg. 2007;120(5):1249–1255. doi: 10.1097/01.prs.0000279324.35616.72. [DOI] [PubMed] [Google Scholar]
- 31.Demirci H, Johnson TM, Frueh BR, Musch DC, Fullen DR, Nelson CC. Management of periocular cutaneous melanoma with a staged excision technique and permanent sections the square procedure. Ophthalmology. 2008;115(12):2295–2300. doi: 10.1016/j.ophtha.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Huilgol SC, Selva D, Chen C, et al. Surgical margins for lentigo maligna and lentigo maligna melanoma: the technique of mapped serial excision. Arch Dermatol. 2004;140(9):1087–1092. doi: 10.1001/archderm.140.9.1087. [DOI] [PubMed] [Google Scholar]
- 33.Abdelmalek M, Loosemore MP, Hurt MA, Hruza G. Geometric staged excision for the treatment of lentigo maligna and lentigo maligna melanoma: a long-term experience with literature review. Arch Dermatol. 2012;148(5):599–604. doi: 10.1001/archdermatol.2011.2155. [DOI] [PubMed] [Google Scholar]
- 34.Hazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58(1):142–148. doi: 10.1016/j.jaad.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47(5):743–748. doi: 10.1067/mjd.2002.124085. [DOI] [PubMed] [Google Scholar]
- 36.Shumaker PR, Kelley B, Swann MH, Greenway HT., Jr Modified Mohs micrographic surgery for periocular melanoma and melanoma in situ: long-term experience at Scripps Clinic. Dermatol Surg. 2009;35(8):1263–1270. doi: 10.1111/j.1524-4725.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 37.Moyer JS, Rudy S, Boonstra PS, et al. Efficacy of staged excision with permanent section margin control for cutaneous head and neck melanoma. JAMA Dermatol. 2017;153(3):282–288. doi: 10.1001/jamadermatol.2016.4603. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JB, Walling HW, Scupham RK, Bean AK, Ceilley RI, Goetz KE. Staged excision for lentigo maligna and lentigo maligna melanoma: analysis of surgical margins and long-term recurrence in 68 cases from a single practice. J Clin Aesthet Dermatol. 2016;9(6):25–30. [PMC free article] [PubMed] [Google Scholar]
- 39.de Vries K, Greveling K, Prens LM, et al. Recurrence rate of lentigo maligna after micrographically controlled staged surgical excision. Br J Dermatol. 2016;174(3):588–593. doi: 10.1111/bjd.14325. [DOI] [PubMed] [Google Scholar]
- 40.Beveridge J, Taher M, Zhu J, Mahmood MN, Salopek TG. Staged margin-controlled excision (SMEX) for lentigo maligna melanoma in situ. J Surg Oncol. 2018;118(1):144–149. doi: 10.1002/jso.25109. [DOI] [PubMed] [Google Scholar]
- 41.Garcia D, Eilers RE, Jiang SB. Recurrence rate of melanoma in situ when treated with serial disk staged excision: a case series. J Clin Investig Dermatol. 2017 doi: 10.13188/2373-1044.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch A, Reid T, Knox J, Wilson ML. Excision of melanoma in situ on nonchronically sun-exposed skin using 5-mm surgical margins. J Am Acad Dermatol. 2014;71(4):834–835. doi: 10.1016/j.jaad.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Duffy KL, Truong A, Bowen GM, et al. Adequacy of 5-mm surgical excision margins for non-lentiginous melanoma in situ. J Am Acad Dermatol. 2014;71(4):835–838. doi: 10.1016/j.jaad.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akhtar S, Bhat W, Magdum A, Stanley PR. Surgical excision margins for melanoma in situ. J Plast Reconstr Aesthet Surg. 2014;67(3):320–323. doi: 10.1016/j.bjps.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Crouch G, Sinha S, Lo S, et al. Clinical outcomes following surgical treatment of lentigo maligna of the head and neck. Eur J Surg Oncol. 2020 doi: 10.1016/j.ejso.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Shin TM, Etzkorn JR, Sobanko JF, et al. Clinical factors associated with subclinical spread of in situ melanoma. J Am Acad Dermatol. 2017;76(4):707–713. doi: 10.1016/j.jaad.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 47.Friedman EB, Dodds TJ, Lo S, et al. Correlation between surgical and histologic margins in melanoma wide excision specimens. Ann Surg Oncol. 2019;26(1):25–32. doi: 10.1245/s10434-018-6858-y. [DOI] [PubMed] [Google Scholar]
- 48.Waddell A, Star P, Guitera P. Advances in the use of reflectance confocal microscopy in melanoma. Melanoma Manag. 2018;5(1):04. doi: 10.2217/mmt-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma Part 2: treatment-update 2019. Eur J Cancer. 2020;126:159–177. doi: 10.1016/j.ejca.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura Y, Asai J, Igaki H, et al. Japanese Dermatological Association Guidelines: outlines of guidelines for cutaneous melanoma 2019. J Dermatol. 2020;47(2):89–103. doi: 10.1111/1346-8138.15151. [DOI] [PubMed] [Google Scholar]
- 51.Norwegian Directorate of Health. National action program with guidelines for diagnosis, treatment and follow-up of malignant melanomas. 2020. https://www.helsedirektoratet.no/retningslinjer/maligne-melanomer-handlingsprogram. Accessed 19 Sept 2019.
- 52.Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. ESMO Guidelines Committee Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 53.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (Association of Medical Societies): S3-Leitlinie zur Diagnostik, Therapie und Nachsorge des Melanoms. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Melanom/Melanom_Version_3/LL_Melanom_Langversion_3.2.pdf. 2019. Accessed 4 Mar 2020.
- 54.Italian Association of Medical Oncologists. Guidelines: melanoma. https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Melanoma-1.pdf. 2019. Accessed 8 July 2020.
- 55.Regionala Cancercentrum I Samverkan: Hudmelanom. Standardiserat vardforlopp. https://www.cancercentrum.se/samverkan/. 2017. Accessed 4 March 2020.
- 56.Wouters MW, Michielin O, Bastiaannet E, et al. ECCO essential requirements for quality cancer care: melanoma. Crit Rev Oncol Hematol. 2018;122:164–178. doi: 10.1016/j.critrevonc.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 57.[Update on current care guidelines: melanoma]. Duodecim. 2012;128(9):988–9. [PubMed]
- 58.Herceg D, Buzina DS, Ceović R, et al. Croatian Society For Medical Oncology clinical guidelines for diagnosis, treatment and follow-up of patients with melanoma. Lijec Vjesn. 2016;138(1–2):22–29. [PubMed] [Google Scholar]
- 59.Guillot B, Dalac S, Denis MG, et al. French updated recommendations in stage I to III melanoma treatment and management. J Eur Acad Dermatol Venereol. 2017;31(4):594–602. doi: 10.1111/jdv.14064. [DOI] [PubMed] [Google Scholar]
- 60.Dummer R, Siano M, Hunger RE, et al. The updated Swiss guidelines 2016 for the treatment and follow-up of cutaneous melanoma. Swiss Med Wkly. 2016;146:w14279. doi: 10.4414/smw.2016.14279. [DOI] [PubMed] [Google Scholar]
- 61.Dutch Melanoma Working Group: [Melanoma National Directive 2.1]. www.huidziekten.nl/richtlijnen/richtlijn-melanoom-2015.pdf. 2016. Accessed 20 Feb 2020.
- 62.Rutkowski P, Wysocki PJ, Nasierowska-Guttmejer A, et al. Cutaneous melanoma—guidelines for diagnostics and therapy in 2016 [Polish] Przeglad Dermatologiczny. 2016;103(1):1–18. [Google Scholar]
- 63.Ahmed O, Kelly C. Head and neck melanoma (excluding ocular melanoma): United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S133–S141. doi: 10.1017/S0022215116000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castro LGM, Messina MC, Loureiro W, et al. Guidelines of the Brazilian Dermatology Society for diagnosis, treatment and follow up of primary cutaneous melanoma-Part I. An Bras Dermatol. 2015;90(6):851–861. doi: 10.1590/abd1806-4841.20154707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Institute for Health and Care Excellence (UK): Melanoma: assessment and management. 2015. https://www.ncbi.nlm.nih.gov/books/NBK315807/. Accessed 19 Sept 2019. [PubMed]
- 66.Pflugfelder A, Kochs C, Blum A, et al. Malignant melanoma S3-guideline: “diagnosis, therapy and follow-up of melanoma”. J Dtsch Dermatol Ges. 2013;11(s6):1–116. doi: 10.1111/ddg.12113_suppl. [DOI] [PubMed] [Google Scholar]
- 67.Giacomantonio C, Morris S, Langley R, et al. Guidelines for the management of malignant melanoma. Cancer care nova Scotia. www.partnershipagainstcancer.ca/db-sage/sage20142196/. 2013. Accessed 19 Sept 2019.
- 68.Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. Br J Dermatol. 2010;163(2):238–256. doi: 10.1111/j.1365-2133.2010.09883.x. [DOI] [PubMed] [Google Scholar]
- 69.Whitaker DK, Sinclair W. Melanoma Advisory Board Guideline on the management of melanoma. J S Afr Med. 2004;94(8):699–707. [PubMed] [Google Scholar]
- 70.Blank NR, Hibler BP, Tattersall IW, et al. Melanoma and melanoma in-situ diagnosis after excision of atypical intraepidermal melanocytic proliferation: a retrospective cross-sectional analysis. J Am Acad Dermatol. 2019;80(5):1403–1409. doi: 10.1016/j.jaad.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]