Abstract
Background:
Various prediction tools have been developed to predict biochemical recurrence (BCR) after radical prostatectomy (RP); however, few of the previous prediction tools used serum prostate-specific antigen (PSA) nadir after RP and maximum tumor diameter (MTD) at the same time. In this study, a nomogram incorporating MTD and PSA nadir was developed to predict BCR-free survival (BCRFS).
Methods:
A total of 337 patients who underwent RP between January 2010 and March 2017 were retrospectively enrolled in this study. The maximum diameter of the index lesion was measured on magnetic resonance imaging (MRI). Cox regression analysis was performed to evaluate independent predictors of BCR. A nomogram was subsequently developed for the prediction of BCRFS at 3 and 5 years after RP. Time-dependent receiver operating characteristic (ROC) curve and decision curve analyses were performed to identify the advantage of the new nomogram in comparison with the cancer of the prostate risk assessment post-surgical (CAPRA-S) score.
Results:
A novel nomogram was developed to predict BCR by including PSA nadir, MTD, Gleason score, surgical margin (SM), and seminal vesicle invasion (SVI), considering these variables were significantly associated with BCR in both univariate and multivariate analyses (P < 0.05). In addition, a basic model including Gleason score, SM, and SVI was developed and used as a control to assess the incremental predictive power of the new model. The concordance index of our model was slightly higher than CAPRA-S model (0.76 vs. 0.70, P = 0.02) and it was significantly higher than that of the basic model (0.76 vs. 0.66, P = 0.001). Time-dependent ROC curve and decision curve analyses also demonstrated the advantages of the new nomogram.
Conclusions:
PSA nadir after RP and MTD based on MRI before surgery are independent predictors of BCR. By incorporating PSA nadir and MTD into the conventional predictive model, our newly developed nomogram significantly improved the accuracy in predicting BCRFS after RP.
Keywords: Nomogram, PSA nadir, Tumor diameter, Magnetic resonance imaging, Biochemical recurrence, Radical prostatectomy
Introduction
Prostate cancer is among the most frequent cancers and the second leading cause of mortality in men. It is estimated that there might be around 191,930 new cases of prostate cancer and 33,330 deaths in the United States in 2020.[1] Approximately, 20% to 30% of the patients experience biochemical recurrence (BCR) after radical prostatectomy (RP) during follow-up.[2–4] Various prediction tools for BCR have been developed to guide the clinical decision-making for subsequent treatment. Most of these tools are developed based on clinical and pathological parameters such as pre-operative serum prostate-specific antigen (PSA), Gleason score, tumor stage, surgical margin (SM), extracapsular extension (ECE), seminal vesicle invasion (SVI), and lymph node invasion (LNI).[5–8] The cancer of the prostate risk assessment post-surgical (CAPRA-S) score is one of the most commonly used tools with good discriminative accuracy and calibration.[7] However, only few of these tools include tumor diameter and post-operative PSA nadir, simultaneously, although the prognostic value of these two characteristics in predicting BCR has been verified.[9,10]
Measurement of PSA is the cornerstone in post-operative follow-up. Serum PSA is expected to be undetectable within 6 weeks after RP and a detectable PSA in patients after RP is thought to be associated with residual cancer.[11] A persistent (detectable) PSA after RP has been proved to be a poor prognostic indicator of oncologic outcomes.[12]
Magnetic resonance imaging (MRI) has been widely used for prostate cancer diagnosis, and the prognostic potential of MRI is constantly being explored with the advancement of radiographic technologies.[13,14] Maximum tumor diameter (MTD) has been demonstrated to be an independent predictor of BCR in patients after RP.[15] However, in most studies, MTD measurement was carried out on the pathological specimens and only few of them measured MTD on MRI,[16] while the latter is considered to be more accurate and comparable. To our knowledge, no study addressing the relationship between MTD measured on MRI and BCR was conducted.
In this study, we aim to assess the prognostic power of MTD from MRI in predicting BCR-free survival (BCRFS) after RP and develop a new nomogram that incorporates MTD, PSA nadir, and other common perioperative variables.
Methods
Ethics approval
This study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee with a waiver of informed consent and compliant with the principles in the Declaration of Helsinki (S2019326).
Patients
Data of 542 patients who underwent laparoscopic RP for prostate cancer between January 2010 and March 2017 were retrospectively analyzed. The exclusion criteria were as follows: (1) patients with neoadjuvant therapy before surgery; (2) patients who had undergone transurethral resection of the prostate; (3) patients with unidentifiable lesions on MRI; (4) patients whose pathological results were not prostatic adenocarcinoma; and (5) incomplete follow-up data. Follow-ups were performed every 3 months for the first 2 years, semi-annually for the third and fourth year, and annually thereafter.
The suspicious tumor lesions were identified according to comprehensive understanding of T2-weighted images, diffusion weighted images, and apparent diffusion coefficient maps of MRI. MTD was defined as the largest tumor diameter of index lesion on axial T2-weighted images. For multifocal cases, only the largest tumor nodule was measured for analysis. PSA nadir was defined as the lowest level of serum PSA in the first two follow-ups after RP without adjuvant androgen deprivation therapy or radiotherapy. BCR was defined as post-operative PSA value >0.20 ng/mL in two consecutive measurements, and the recurrence date was assigned to the day when PSA value >0.20 ng/mL was measured for the first time. BCRFS was calculated from date of RP to date of documented BCR or date of last follow-up for those patients who did not experience BCR. Other clinical and pathological data, such as age at RP, body mass index (BMI), pre-operative PSA, Gleason score, SM, ECE, SVI, and LNI, were also collected for each patient.
Statistical analysis
Means, standard deviation, median, and interquartile ranges (IQR) were reported for continuous variables. Numbers and proportions were reported for categorical variables. The Mann-Whitney U test and Chi-square test were applied for between-group comparison. BCRFS was estimated using the Kaplan-Meier curves and log-rank test. MTD was categorized into ≤2.9 and >2.9 cm. The cutoff value of MTD that best discriminated low- and high-risk for BCR was estimated by maximally selected test with the “maxstat” package of R software.[17] PSA nadir was categorized into undetectable and detectable PSA. An undetectable PSA was defined as a PSA nadir <0.01 ng/mL. Univariable and multivariable Cox proportional hazards regression models were used to identify significant predictors of BCR. A nomogram predicting BCRFS at 3 and 5 years after RP was developed based on the multivariable model. For the validation of the nomogram, a bootstrap technique (1000 bootstrap resamples) was used for internal validation to assess the discrimination and calibration. The concordance index (c-index) was used to assess the discrimination. The calibration curve was made to assess the calibration which graphically revealed the relationship between predicted probability of BCR and actual observed events. Additionally, we compared our newly developed nomogram to the CAPRA-S score with one-shot non-parametric approach, and comparison of the two models was performed using the “compareC” package of R software.[18] Time-dependent ROC curves were illustrated using the “survivalROC” package.[19] Decision curve analyses at 3 and 5 years were performed to ascertain the clinical value of the new nomogram. Statistical analyses were performed with the R software (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism (version 7.00, GraphPad Software, San Diego, CA, USA). All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Patients’ characteristics
Overall, 337 patients were included in this study and the demographic and clinical characteristics of these patients are shown in Table 1. The median follow-up time was 42 months (IQR, 19–64 months) and 100 (29.7%) patients developed BCR during follow-up. The median age of all patients was 71 years (IQR, 65–75 years) with median BMI of 24.6 kg/m2 (IQR, 22.8–26.6 kg/m2). The median value of pre-operative PSA was 10.8 ng/mL (IQR, 7.3–19.1 ng/mL) and was divided into three groups: <10 ng/mL group, 10 to 20 ng/mL group, and >20 ng/mL group. The majority of the patients had PSA nadir <0.01 ng/mL (n = 242, 71.8%), while 95 (28.2%) patients had PSA nadir ≥0.01 ng/mL. Median PSA nadir was 0 (IQR, 0–0.01) ng/mL. The median MTD was 3.09 cm (IQR, 2.24–3.91 cm) with 45.1% of MTD ≤2.9 cm and 54.9% of MTD >2.9 cm. Comparison of clinical parameters between patients who experienced BCR or not is shown in Table 2.
Table 1.
Characteristics of prostate cancer patients treated by RP (N = 337).
| Characteristics | n | % |
| Pre-operative PSA (ng/mL) | ||
| <10 | 152 | 45.1 |
| 10–20 | 106 | 31.5 |
| >20 | 79 | 23.4 |
| PSA nadir (ng/mL) | ||
| <0.01 | 242 | 71.8 |
| ≥0.01 | 95 | 28.2 |
| Gleason score | ||
| <3 + 4 | 148 | 43.9 |
| ≥4 + 3 | 189 | 56.1 |
| Pathological tumor stage | ||
| ≤T2a | 14 | 4.2 |
| T2b | 33 | 9.8 |
| ≥T2c | 290 | 86.0 |
| SM | ||
| Negative | 222 | 65.9 |
| Positive | 115 | 34.1 |
| ECE | ||
| No | 225 | 66.8 |
| Yes | 112 | 33.2 |
| SVI | ||
| No | 295 | 87.5 |
| Yes | 42 | 12.5 |
| MTD (cm) | ||
| ≤2.9 | 152 | 45.1 |
| >2.9 | 185 | 54.9 |
| Follow-up (months), median (IQR) | 42 (19–64) | – |
BCR: Biochemical recurrence; BMI: Body mass index; ECE: Extracapsular extension; IQR: Interquartile range; MTD: Maximum tumor diameter; PSA: Prostate-specific antigen; RP: Radical prostatectomy; SD: Standard deviation; SM: surgical margin; SVI: Seminal vesicle invasion.
Table 2.
Comparison of clinical parameters between prostate cancer patients with or without BCR.
| Parameters | Non-BCR (n = 237) | BCR (n = 100) | Statistics | P |
| Age (years), median (IQR) | 71 (65, 75) | 70 (64, 75) | −0.912∗ | 0.362 |
| BMI (kg/m2), median (IQR) | 24.4 (22.5, 26.4) | 25.3 (23.5, 27.3) | −2.403∗ | 0.016 |
| Pre-operative PSA (ng/mL), median (IQR) | 10.1 (6.7, 16.1) | 14.1 (8.2, 28.9) | −3.885∗ | <0.001 |
| PSA nadir (ng/mL), median (IQR) | 0 (0, 0.003) | 0.010 (0, 0.040) | −5.024∗ | <0.001 |
| Gleason score, n | 12.846† | <0.001 | ||
| ≤3 + 4 | 119 | 29 | ||
| ≥4 + 3 | 118 | 71 | ||
| SM, n | 8.920† | 0.003 | ||
| Negative | 168 | 54 | ||
| Positive | 69 | 46 | ||
| ECE, n | 6.111† | 0.013 | ||
| No | 168 | 57 | ||
| Yes | 69 | 43 | ||
| SVI, n | 7.404† | 0.007 | ||
| No | 215 | 80 | ||
| Yes | 22 | 20 | ||
| MTD (cm), median (IQR) | 3.09 (2.30, 3.98) | 3.02 (2.02, 3.91) | −1.125∗ | 0.260 |
∗Z values. †χ2 values. BCR: Biochemical recurrence; BMI: Body mass index; ECE: Extracapsular extension; IQR: Interquartile range; MTD: Maximum tumor diameter; PSA: Prostate-specific antigen; SD: Standard deviation; SM: Surgical margin; SVI: Seminal vesicle invasion.
Development and evaluation of the novel nomogram
To identify significant predictors of BCR, we evaluated age, BMI, pre-operative PSA, Gleason score, SM, ECE, SVI, PSA nadir, and MTD in a univariable Cox proportional hazards regression model and the results are shown in Table 3. Except for age and BMI, all predictors were statistically significantly associated with BCR after RP (P < 0.01).
Table 3.
Univariable and multivariable Cox regression analyses of BCRFS.
| Univariable | Multivariable | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 0.981 (0.954–1.008) | 0.168 | – | – |
| BMI | 1.023 (0.963–1.087) | 0.459 | – | – |
| Pre-operative PSA (ng/mL) | ||||
| <10 | 1.000 (reference) | 1.000 (reference) | ||
| 10–20 | 1.090 (0.654–1.817) | 0.740 | 1.071 (0.638–1.795) | 0.921 |
| >20 | 2.773 (1.759–4.374) | <0.001 | 1.669 (0.995–2.799) | 0.075 |
| PSA nadir (ng/mL) | ||||
| <0.01 | 1.000 (reference) | 1.000 (reference) | ||
| ≥0.01 | 3.959 (2.663–5.887) | <0.001 | 4.531 (2.993–6.861) | <0.001 |
| Gleason score | ||||
| ≤3 + 4 | 1.000 (reference) | 1.000 (reference) | ||
| ≥4 + 3 | 2.310 (1.496–3.568) | <0.001 | 2.090 (1.277–3.420) | 0.003 |
| SM | ||||
| Negative | 1.000 (reference) | 1.000 (reference) | ||
| Positive | 1.966 (1.326–2.916) | <0.001 | 1.675 (1.076–2.610) | 0.007 |
| ECE | ||||
| No | 1.000 (reference) | 1.000 (reference) | ||
| Yes | 1.720 (1.155–2.560) | 0.008 | 0.791 (0.469–1.336) | 0.376 |
| SVI | ||||
| No | 1.000 (reference) | 1.000 (reference) | ||
| Yes | 2.704 (1.649–4.436) | <0.001 | 1.723 (0.897–3.307) | 0.022 |
| MTD (cm) | ||||
| ≤2.9 | 1.000 (reference) | 1.000 (reference) | ||
| >2.9 | 2.196 (1.425–3.385) | <0.001 | 1.587 (1.006–2.503) | 0.034 |
BCRFS: BCR-free survival; BMI: Body mass index; CI: Confidence interval; ECE: Extracapsular extension; HR: Hazard ratio; MTD: Maximum tumor diameter; PSA: Prostate-specific antigen; SM: Surgical margin; SVI: Seminal vesicle invasion.
As shown in Figure 1, Kaplan-Meier curves were stratified by PSA nadir (<0.01 vs. ≥0.01 ng/mL) [Figure 1B], MTD (≤2.9 vs. >2.9 cm) [Figure 1C], and the combination of PSA nadir and MTD (0 risk factor: PSA nadir <0.01 ng/mL and MTD ≤2.9 cm; one risk factor: PSA nadir <0.01 ng/mL and MTD >2.9 cm or PSA nadir ≥0.01 ng/mL and MTD ≤2.9 cm; two risk factors: PSA nadir ≥0.01 ng/mL and MTD >2.9 cm) [Figure 1D] and showed that the patients with detectable PSA or/and MTD >2.9 cm had significantly shorter BCRFS (log-rank P < 0.001).
Figure 1.
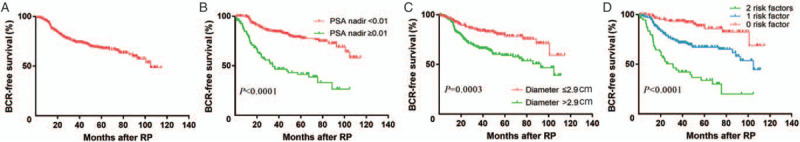
(A) Kaplan-Meier curves of BCRFS for the whole patient population, (B) patients grouped by PSA nadir (<0.01 vs. ≥0.01 ng/mL), (C) MTD (≤2.9 vs. >2.9 cm), and (D) a combination of PSA nadir and MTD. BCR: Biochemical recurrence; BCRFS: BCR-free survival; MTD: Maximum tumor diameter; PSA: Prostate-specific antigen; RP: Radical prostatectomy.
These significant predictors in univariable analyses were then assessed in a multivariable Cox regression model, and pre-operative PSA and ECE did not retain their significance and were excluded (P > 0.05) [Table 3]. Finally, PSA nadir and MTD, as well as Gleason score, SM, and SVI, were independent predictors of BCR in multivariable Cox regression analysis (P < 0.05). These variables were incorporated in a nomogram predicting BCRFS at 3 and 5 years after RP [Figure 2], which yielded a c-index of 0.76 (95% confidence interval [CI], 0.71–0.81). The calibration plots of the nomogram are shown in Figure 3 illustrating how the predicted probability of BCRFS compared with the actual outcomes.
Figure 2.
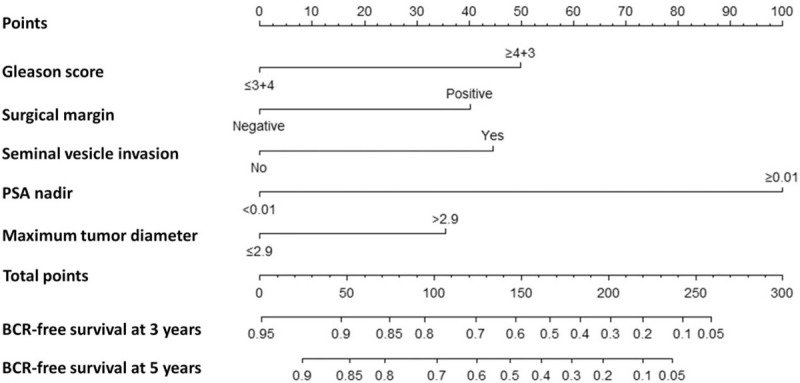
Nomogram predicting BCRFS at 3 and 5 years after RP. BCRFS: BCR-free survival; BCR: Biochemical recurrence; PSA: Prostate-specific antigen; RP: Radical prostatectomy.
Figure 3.
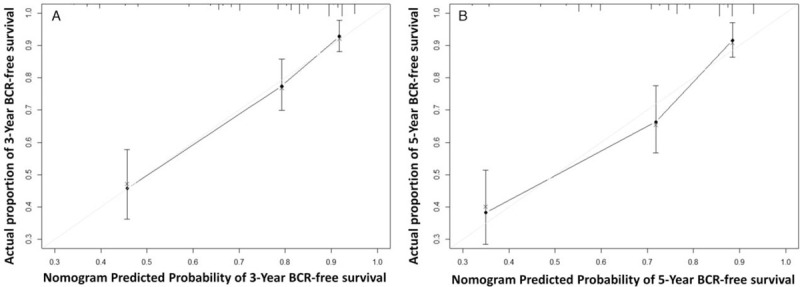
Calibration plot of the nomogram predicting BCRFS at (A) 3 years and (B) 5 years after RP. BCRFS: BCR-free survival; BCR: Biochemical recurrence; RP: Radical prostatectomy.
The c-index of the CAPRA-S score was 0.70 (95% CI, 0.64–0.75) in our study cohort, which is slightly lower than that of our nomogram (P = 0.022). To further verify the prognostic power of the combination of PSA nadir and MTD, we developed a basic model including Gleason score, SM, and SVI. It yielded a c-index of 0.66 (95% CI, 0.60–0.71), which was significantly lower than the c-index of the new nomogram (P = 0.001). The time-dependent ROC curve and decision curve analyses compared the new nomogram, the CAPRA-S score, and the basic model [Figures 4 and 5]. Our new nomogram showed an advantage in identifying patients with BCRFS in both time-dependent ROC curve and decision curve analyses.
Figure 4.
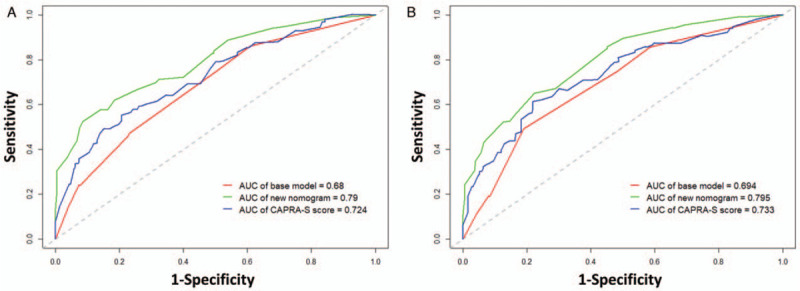
Time-dependent ROC curves comparing the base model, the new nomogram, and the CAPRA-S score in predicting BCR at (A) 3 years and (B) 5 years after RP. BCR: Biochemical recurrence; CAPRA-S: Cancer of the prostate risk assessment post-surgical; ROC: Receiver operating characteristic; RP: Radical prostatectomy.
Figure 5.
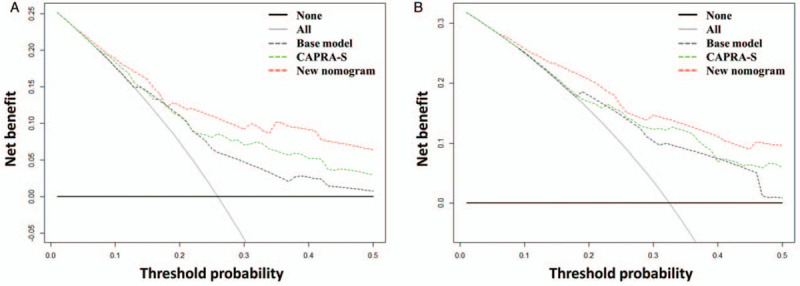
Decision curve analyses comparing the base model, the new nomogram, and the CAPRA-S score in predicting BCR at (A) 3 years and (B) 5 years after RP. BCR: Biochemical recurrence; CAPRA-S: Cancer of the prostate risk assessment post-surgical; RP: Radical prostatectomy.
Discussion
In the present study, comparing to conventional predictive models, we proposed a new nomogram by incorporating MTD and PSA nadir, which showed improved accuracy of BCR prediction for patients after RP.
After RP, PSA is expected to be undetectable within 6 weeks and it is utmost important parameter that should be monitored post-operatively.[11] Elevated PSA level after RP indicates high risk of local recurrence or metastasis.[12] If the post-operative PSA reaches 0.2 ng/mL, patient is assigned the status of BCR,[20] which was a signal of cancer activity at visual undetectable level. The relationship between PSA nadir and BCR after RP has been extensively studied. A retrospective study reported that compared to men with PSA <0.01 ng/mL after RP, the probability of BCRFS at 5 years dropped from 92.4% to 56.8% in patients with PSA ≥0.01 ng/mL.[21] In a study of 582 patients carried out by Matsumoto et al,[22] PSA persistence after RP was associated with increased BCR and overall mortality. These results are in line with the observations in the present study. In the present study, 71.8% patients had an undetectable PSA nadir and 28.2% patients had a detectable nadir during follow-up. PSA nadir after RP was found to be an independent prognostic factor (P < 0.001) in predicting BCR in univariable and multivariable analyses. Patients with PSA nadir <0.01 ng/mL had significantly longer BCRFS in our study cohort (log-rank P < 0.0001) [Figure 1B].
According to our clinical experience, tumor burden should be associated with oncological outcomes. Tumor volume and MTD as the common indicators of tumor burden have been studied by the researchers and have proved to be independent prognostic factors of BCR.[15,23] However, prostate cancer has been recognized as a multifocal disease, and the calculation of tumor volume and MTD are complicated.[24] In 2013, Billis et al[25] found that the tumor extent in a surgical specimen should be estimated with the dominant tumor and not the total tumor extent. They also reported the association of the dominant tumor with BCR prediction. Nonetheless, the calculation of tumor volume is time consuming and difficult. For the above reasons, we chose MTD as the research target and it was defined as the maximum diameter of the dominant tumor. Unlike previous studies, we measured MTD based on MRI instead of pathological specimen. MRI has better repeatability and less deformation, while on pathological specimen, deformation can vary greatly because of the shrinking of tissues after soaking in formalin. Lee et al[16] measured the diameter of the suspicious tumor lesion on diffusion weighted images of MRI and demonstrated that the diameter of tumor could increase the prediction of insignificant prostate cancer in candidates for active surveillance. In the studies of Kozal et al[26] and Müller et al,[27] MTD was an independent prognostic factor for BCR, even though they measured MTD on pathological specimens. Based on their findings, we hypothesized that the MTD on MRI could be an independent prognostic factor for prostate cancer; however, the relationship between MTD measured on MRI and BCR after RP has rarely been explored in their study as well as other previous studies. As expected, the results of the present study showed that MTD on MRI was an independently significant predictor of BCR (P = 0.0340) and the Kaplan-Meier curve depicted that men with MTD >2.9 cm had shorter BCRFS (log-rank P = 0.0003) [Figure 1C]. Interestingly, the median MTD in the present study was larger than that in the previous studies.[28] We attributed this phenomenon to shrinking of tissues after soaking in formalin which might decrease the MTD.[29] Additionally, in the present study, pathological tumor stage ≥T2c was reported in the majority of patients (n = 290, 86%) [Table 1] and it might be another reason why we have larger MTD. In the study of Eichelberger et al,[30] MTD was found to be associated with the pathological stages. With the rapid development of radiographic technologies and artificial intelligence, the identification and measurements of prostate cancer on MRI are more accurate with high repeatability for prognostic evaluation.
The CAPRA-S score is a post-operative score created by Cooperberg et al,[7] based on pre-operative PSA, Gleason score, SM, ECE, SVI, and LNI. The prognostic value of these variables was verified in our study cohort as well. All of them were significantly associated with BCR in the univariable analysis, and Gleason score, SM, and SVI were independent predictors of BCR in multivariable analysis. The c-index of our newly developed nomogram was slightly higher than that of the CAPRA-S score in our study cohort. Moreover, our nomogram predictions closely approached the actual outcome both at 3 and 5 years after RP, demonstrating good calibration, as depicted in the calibration plot. Comparing these two models, we found that our new nomogram consisted of two parts. One part was composed of the commonly used variables, namely Gleason score, SM, and SVI; and the other part was composed of PSA nadir and MTD measured on MRI. In the present study, we observed that both PSA nadir and MTD were significantly associated with BCR in univariable analysis and they were also independent prognostic factors after adjusting pre-operative PSA, Gleason score, SM, ECE, and SVI. Kaplan-Meier curve showed that the patients with these two risk factors simultaneously had the shortest BCRFS and patients with none of these two risk factors had the longest BCRFS (log-rank P < 0.0001) [Figure 1D]. However, only few of the previous prediction tools used MTD and PSA nadir at the same time. To verify the incremental predictive power of the combination of PSA nadir and MTD, we developed a basic model including Gleason score, SM, and SVI for comparison. The c-index was decreased from 0.76 to 0.66 (P < 0.001) when PSA nadir and MTD were removed from our new nomogram. The time-dependent ROC curves illustrated the advantage of our new nomogram at both 3 and 5 years after RP. The decision curve analyses also showed the advantage of our new nomogram, across the various threshold probabilities, and the new nomogram had greater net benefit than both the basic model and the CAPRA-S score in our study cohort. Our new nomogram is a promising tool to predict BCRFS and guide follow-up and decision-making of adjuvant treatment. In addition, PSA nadir and MTD improved the accuracy of our new nomogram in predicting BCR.
This study has several limitations. First, it was a retrospective study and the population was relatively smaller compared with the previous studies. Second, the present study has not yet been validated externally and the analysis of overall survival lacked because of the short-term follow-up duration.
The newly developed nomogram, which included PSA nadir, MTD measured on MRI, and several commonly used variables, shows excellent accuracy in predicting BCRFS after RP. This nomogram is a useful tool for risk stratification and follow-up planning. The combination of PSA nadir and MTD can improve the accuracy of BCR prediction.
Acknowledgements
The authors thank Mr. Li-Yuan Tao from the Research Center of Clinical Epidemiology of Peking University Third Hospital for the help in statistical analyses.
Funding
This work was supported by grants from the Beijing Natural Science Foundation (No. Z200027), National Natural Science Foundation of China (No. 61871004), National Key R&D Program of China (No. 2018YFC0115900), Innovation & Transfer Fund of Peking University Third Hospital (No. BYSYZHKC2020111), and Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (No. BMU2020PYB002). Funds were used for the collection and analysis of data.
Conflicts of interest
None.
Footnotes
How to cite this article: Xia HZ, Bi H, Yan Y, Yang B, Ma RZ, He W, Zhu XH, Zhang ZY, Zhang YT, Ma LL, Hou XF, Lu J. A novel nomogram provides improved accuracy for predicting biochemical recurrence after radical prostatectomy. Chin Med J 2021;134:1576–1583. doi: 10.1097/CM9.0000000000001607
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Rajan P, Hagman A, Sooriakumaran P, Nyberg T, Wallerstedt A, Adding C, et al. Oncologic outcomes after robot-assisted radical prostatectomy: a large European single-centre cohort with median 10-year follow-up. Eur Urol Focus 2018; 4:351–359. doi: 10.1016/j.euf.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Diaz M, Peabody JO, Kapoor V, Sammon J, Rogers CG, Stricker H, et al. Oncologic outcomes at 10 years following robotic radical prostatectomy. Eur Urol 2015; 67:1168–1176. doi: 10.1016/j.eururo.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Qu M, Lian BJ, Wang HF, Wang Y, Dong ZY, et al. Short-term therapeutic outcomes of robotic-assisted laparoscopic radical prostatectomy for oligometastatic prostate cancer: a propensity score matching study. Chin Med J 2020; 133:127–133. doi: 10.1097/CM9.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Barreras S, Sanchez-Salas R, Mejia-Monasterio C, Muttin F, Secin F, Dell’Oglio P, et al. Biochemical recurrence-free conditional probability after radical prostatectomy: a dynamic prognosis. Int J Urol 2019; 26:725–730. doi: 10.1111/iju.13982. [DOI] [PubMed] [Google Scholar]
- 6.Pompe RS, Bandini M, Preisser F, Marchioni M, Zaffuto E, Tian Z, et al. Contemporary approach to predict early biochemical recurrence after radical prostatectomy: update of the Walz nomogram. Prostate Cancer Prostatic Dis 2018; 21:386–393. doi: 10.1038/s41391-018-0033-1. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer 2011; 117:5039–5046. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Wirth GJ, Wu S, Chen J, Dahl DM, Olumi AF, et al. A close surgical margin after radical prostatectomy is an independent predictor of recurrence. J Urol 2012; 188:91–97. doi: 10.1016/j.juro.2012.02.2565. [DOI] [PubMed] [Google Scholar]
- 9.Kozminski MA, Palapattu GS, Mehra R, Montgomery JS, Weizer AZ, Skolarus TA, et al. Understanding the relationship between tumor size, gland size, and disease aggressiveness in men with prostate cancer. Urology 2014; 84:373–378. doi: 10.1016/j.urology.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Skove SL, Howard LE, Aronson WJ, Terris MK, Kane CJ, Amling CL, et al. Timing of prostate-specific antigen nadir after radical prostatectomy and risk of biochemical recurrence. Urology 2017; 108:129–134. doi: 10.1016/j.urology.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamey TA, Kabalin JN, McNeal JE, Johnstone IM, Freiha F, Redwine EA, et al. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. II. Radical prostatectomy treated patients. J Urol 1989; 141:1076–1083. doi: 10.1016/s0022-5347(17)41175-x. [DOI] [PubMed] [Google Scholar]
- 12.Preisser F, Chun FKH, Pompe RS, Heinze A, Salomon G, Graefen M, et al. Persistent prostate-specific antigen after radical prostatectomy and its impact on oncologic outcomes. Eur Urol 2019; 76:106–114. doi: 10.1016/j.eururo.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 13.Sugano D, Sidana A, Jain AL, Calio B, Gaur S, Maruf M, et al. Index tumor volume on MRI as a predictor of clinical and pathologic outcomes following radical prostatectomy. Int Urol Nephrol 2019; 51:1349–1355. doi: 10.1007/s11255-019-02168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho R, Siddiqui MM, George AK, Frye T, Kilchevsky A, Fascelli M, et al. Preoperative multiparametric magnetic resonance imaging predicts biochemical recurrence in prostate cancer after radical prostatectomy. PLoS One 2016; 11:e157313.doi: 10.1371/journal.pone.0157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose BS, Chen MH, Zhang D, Hirsch MS, Richie JP, Chang SL, et al. Maximum tumor diameter and the risk of prostate-specific antigen recurrence after radical prostatectomy. Clin Genitourin Cancer 2014; 12:e173–e179. doi: 10.1016/j.clgc.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Koo KC, Lee SH, Rha KH, Choi YD, Hong SJ, et al. Tumor lesion diameter on diffusion weighted magnetic resonance imaging could help predict insignificant prostate cancer in patients eligible for active surveillance: preliminary analysis. J Urol 2013; 190:1213–1217. doi: 10.1016/j.juro.2013.03.127. [DOI] [PubMed] [Google Scholar]
- 17.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal 2003; 43:121–137. doi: 10.1016/S0167-9473(02)00225-6. [Google Scholar]
- 18.Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med 2015; 34:685–703. doi: 10.1002/sim.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000; 56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 20.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 2007; 177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 21.Sokoll LJ, Zhang Z, Chan DW, Reese AC, Bivalacqua TJ, Partin AW, et al. Do ultrasensitive prostate specific antigen measurements have a role in predicting long-term biochemical recurrence-free survival in men after radical prostatectomy? J Urol 2016; 195:330–336. doi: 10.1016/j.juro.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Komatsuda A, Yanai Y, Niwa N, Kosaka T, Mizuno R, et al. Determining when to stop prostate specific antigen monitoring after radical prostatectomy: the role of ultrasensitive prostate specific antigen. J Urol 2017; 197:655–661. doi: 10.1016/j.juro.2016.08.098. [DOI] [PubMed] [Google Scholar]
- 23.Meyer CP, Hansen J, Boehm K, Tilki D, Abdollah F, Trinh QD, et al. Tumor volume improves the long-term prediction of biochemical recurrence-free survival after radical prostatectomy for localized prostate cancer with positive surgical margins. World J Urol 2017; 35:199–206. doi: 10.1007/s00345-016-1861-z. [DOI] [PubMed] [Google Scholar]
- 24.Salami SS, Hovelson DH, Kaplan JB, Mathieu R, Udager AM, Curci NE, et al. Transcriptomic heterogeneity in multifocal prostate cancer. JCI Insight 2018; 3:e123468.doi: 10.1172/jci.insight.123468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billis A, Meirelles LR, Freitas LL, Polidoro AS, Fernandes HA, Padilha MM, et al. Prostate total tumor extent versus index tumor extent – which is predictive of biochemical recurrence following radical prostatectomy? J Urol 2013; 189:99–104. doi: 10.1016/j.juro.2012.08.179. [DOI] [PubMed] [Google Scholar]
- 26.Kozal S, Peyronnet B, Cattarino S, Seisen T, Comperat E, Vaessen C, et al. Influence of pathological factors on oncological outcomes after robot-assisted radical prostatectomy for localized prostate cancer: results of a prospective study. Urol Oncol 2015; 33:330.e1–330.e7. doi: 10.1016/j.urolonc.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Muller G, Rieken M, Bonkat G, Gsponer JR, Vlajnic T, Wetterauer C, et al. Maximum tumor diameter adjusted to the risk profile predicts biochemical recurrence after radical prostatectomy. Virchows Arch 2014; 465:429–437. doi: 10.1007/s00428-014-1643-1. [DOI] [PubMed] [Google Scholar]
- 28.Erdem S, Verep S, Bagbudar S, Ozluk Y, Sanli O, Ozcan F. The clinical predictive factors and postoperative histopathological parameters associated with upgrading after radical prostatectomy: a contemporary analysis with grade groups. Prostate 2020; 80:225–234. doi: 10.1002/pros.23936. [DOI] [PubMed] [Google Scholar]
- 29.Turkbey B, Mani H, Aras O, Rastinehad AR, Shah V, Bernardo M, et al. Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol 2012; 188:1157–1163. doi: 10.1016/j.juro.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichelberger LE, Koch MO, Eble JN, Ulbright TM, Juliar BE, Cheng L. Maximum tumor diameter is an independent predictor of prostate-specific antigen recurrence in prostate cancer. Mod Pathol 2005; 18:886–890. doi: 10.1038/modpathol.3800405. [DOI] [PubMed] [Google Scholar]


