INTRODUCTION:
Rectal hypersensitivity is an important pathophysiological dysfunction in irritable bowel syndrome with predominant constipation (IBS-C), whose treatment remains challenging. In a randomized controlled trial, we compared the efficacy and safety of a novel sensori-behavioral treatment, sensory adaptation training (SAT) with escitalopram.
METHODS:
Patients with IBS-C (Rome III) with rectal hypersensitivity received 6 biweekly sessions of SAT or escitalopram 10 mg daily for 3 months. SAT was performed by repetitive gradual distension of 10-cm long highly compliant rectal balloon above tolerability thresholds using barostat. Treatment effects on sensory thresholds and symptoms were compared. Coprimary outcome measures were those achieving improvements in rectal hypersensitivity (≥20% increase in ≥2/3 sensory thresholds) and pain (≥30% decrease).
RESULTS:
We randomized 49 patients; 26 received SAT and 23 escitalopram. SAT significantly improved desire to defecate (Δ 13.5 ± 2.3 vs 2.2 ± 1.1 mm Hg, P = 0.0006) and maximum tolerability (Δ 14.8 ± 1.9 vs 1.6 ± 0.9 mm Hg, P < 0.0001) thresholds compared with escitalopram. There were significantly greater percentage of hypersensitivity responders with SAT than escitalopram (69% vs 17%, P < 0.001), but not pain responders (58% vs 44%, P = 0.4). Daily pain scores did not differ between groups (P = 0.8) or escitalopram (P = 0.06) but decreased with SAT (P = 0.0046) compared with baseline. SAT significantly increased rectal compliance (P < 0.019) and complete spontaneous bowel movements per week than escitalopram (P = 0.04). Five withdrew from adverse events with escitalopram and none with SAT.
DISCUSSION:
SAT was significantly more efficacious in improving hypersensitivity and bowel symptoms in IBS-C than escitalopram. SAT is a promising novel treatment for IBS with rectal hypersensitivity.
INTRODUCTION
Irritable bowel syndrome with constipation (IBS-C) is characterized by recurrent episodes of abdominal pain and constipation (1). Current treatment approaches have focused on improving symptoms (1), and although useful, a significant proportion of patients remain dissatisfied. One possibility could be a lack of mechanistic-based therapies that remedy the underlying pathophysiology.
The pathophysiology of IBS is multifactorial and includes altered gut and brain interactions, genetic factors, psychosocial dysfunction, and dysbiosis (1,2). Another important pathophysiological mechanism is visceral hypersensitivity (3). Many patients with IBS demonstrate rectal hypersensitivity (2–5) as a stable trait (6). Consequently, treatment of visceral hypersensitivity may improve IBS-C (1,7). However, there is no approved therapy for hypersensitivity, and antispasmodics (1,7), tricyclic antidepressants (7), selective serotonin reuptake inhibitors (SSRIs) (7), or gamma-aminobutyric acid inhibitors, and psychotherapies are used currently (8). Citalopram, an SSRI, decreased colonic tone and sensory thresholds in healthy volunteers (9) and improved abdominal pain in IBS (10). Whether escitalopram (an s-isomer of citalopram that works faster with fewer side-effects [11]) may reduce hypersensitivity and improve IBS is not known.
Rectal hypersensitivity is associated with enhanced cortical perception (3,4,12), hypervigilance (2,13), and activation of emotional centers that control pain (2). Whether a locally directed sensori-behavioral therapy with repeated rectal balloon distensions, in small increments, could facilitate sensory adaptation of pain responses and reduce the volley of afferent neuronal discharge in a sensitized individual has not been evaluated. A pilot observation suggested that sensory adaptation training (SAT) may improve pain and bowel symptoms in rectal hypersensitivity (14).
We hypothesized that SAT is more likely to improve rectal sensory thresholds, bowel symptoms, and abdominal pain in patients with IBS-C than escitalopram, a neuromodulator. Our aim was to investigate the feasibility, efficacy, and safety of a novel treatment using barostat-assisted rectal balloon distensions and compare with escitalopram in patients with IBS-C and rectal hypersensitivity.
METHODS
Study design and participants
Patients with IBS-C who fulfilled Rome III criteria (15) were recruited from February 2009 to December 2011 at the University of Iowa and from June 2012 to June 2015 at Augusta University because of the principal investigator moving institutions. Participants signed informed consents approved by the Institutional Review Boards: Augusta University (611345) and University of Iowa (200506800). The inclusion criteria were (i) recurrent abdominal discomfort or pain for 3 days per month over 3 months with 2 or more of the following (Rome III) (15): (a) improvement with defecation; (b) onset associated with change in stool frequency; and/or (c) onset associated with change in stool form; (ii) normal colonoscopy and no metabolic abnormalities; and (iii) on a prospective diary, patients reported (a) abdominal pain/discomfort for at least 2 days per week and (b) hard or lumpy stools >25% and loose stools ≤25% of bowel movements (BM); and (iv) on a rectal sensory test, patients had rectal hypersensitivity, defined as 2 or more thresholds of rectal sensation ≤2 SD of normal mean values (4,16) (first [≤20 cc], desire to defecate [≤80 cc], and urgency or maximum tolerable volume [≤150 cc]). Exclusion criteria were (i) taking opioids, antispasmodics, SSRI, or monoamine oxidase inhibitors (other antidepressants were allowed); (ii) mixed-IBS (Rome III); (iii) severe cardiac disease, chronic renal failure, major depression, and neurologic diseases; (iv) previous gastrointestinal surgery except cholecystectomy, appendectomy, and hysterectomy; (v) pregnancy; (vi) inflammatory bowel disease; and (vii) rectal prolapse, anal surgery, or fissure.
Randomization and masking
Subjects were randomized in 1:1 ratio to receive either SAT or escitalopram for 3 months. The randomization schedule was generated by the study biostatistician using permuted blocks of 4. The allocations were placed into sequentially numbered sealed opaque envelopes and kept with research pharmacist. Although the therapist and patient could not be blinded, the data analysts (Y.Y. and T.P.) were unaware of patient assignment.
Procedures
At study entry, patients kept a 2-week abdominal pain and bowel symptoms diary. Rectal sensory testing was performed using high-resolution anorectal manometry with a 4-cm balloon (Medtronic, Minneapolis, MN). Thresholds for first sensation, desire and urgency to defecate, and maximum tolerable volume were assessed using intermittent rectal balloon distension technique (4,16). If eligible, subjects underwent a rectal barostat (G & J Electronics, Toronto, Canada) study using a 10-cm long highly compliant balloon placed in the rectum. The balloon was inflated using the computerized intermittent rectal distension paradigm at 2 mm Hg increments followed by complete deflation with 30-second rest between distensions (17–19). Sensory thresholds for first sensation, desire to defecate, and maximum tolerable pressure or pain were assessed (17). The barostat study was repeated after 3 months. The equipment, protocols, and key personnel were similar at both sites.
Each group received similar general guidelines for management of constipation including magnesium hydroxide (Milk of Magnesia; Phillips) 1–2 tablespoons or magnesium gluconate (Magonate 500 mg; Fleming & Company, St. Louis, MO) 2–4 tablets or polyethylene glycol 17 g with instructions to titrate their use. Patients with no BM for 48 hours were instructed to use 1 glycerin suppository, after 72 hours, a tap water enema, and after 96 hours 2 bisacodyl tablets (rescue laxatives). Patients recorded their daily severity of worst pain episode, BM(s), stool consistency (Bristol Stool Form Scale 1–7), straining effort (1 = normal, 2 = moderate, and 3 = excessive), and feeling of complete evacuation during screening and last week of study. Bowel satisfaction was scored on a global bowel satisfactory score, the visual analog scale (VAS): 0 = very dissatisfied to 100 = completely satisfied. IBS-quality of life (QOL) (20) and SCL-90-R (21) questionnaires were completed.
Sensory adaptation training.
With the subject in the left lateral position, a 6-mm flexible plastic probe with a 10-cm highly compliant balloon (Mui scientific, Toronto, Canada) was placed in the rectum and connected to a barostat (G & J Electronics). Intermittent phasic distensions were performed at 2 mm Hg increments to assess baseline sensory thresholds. Subsequently, repetitive balloon inflations were performed at 1–2 mm Hg increments starting with the threshold for desire to defecate until they could no longer tolerate further distension(s). After each distension of 30–45 seconds, the patient was instructed to indicate the level of sensation on a sensory panel. The goal was to reach a threshold of 20% above the maximum tolerable threshold for that visit or 40 mm Hg, whichever occurred earlier. Successive balloon inflations were higher or lower and titrated depending on patient's tolerability. The patient was not permitted to watch the barostat monitor to prevent visual feedback that could induce hypervigilance. Thus, their responses were solely based on their perceptions. Training was terminated if patient reported persistent discomfort/pain during 2 consecutive distensions with the same pressure or activated panic button. Each training lasted 30–45 minutes, and up to 6 sessions were performed biweekly over 3 months.
Escitalopram treatment.
Patients were instructed by a research pharmacist to take escitalopram 10 mg/d. Patients returned for 3 follow-up visits at monthly intervals and received a phone call from the research coordinator every 2 weeks providing attention and maintaining parity with biweekly SAT visits.
Outcomes
The data for physiologic, symptomatic, psychologic, and QOL measures and differences between treatments were compared for before and after treatment. The primary physiologic outcome measure was rectal sensory thresholds. A rectal hypersensitivity responder was defined as an individual who showed at least 20% increase in 2 or more sensory thresholds (first, desire, and maximum tolerable pressure) after treatment when compared with baseline. Because urgency overlapped with moderate discomfort or pain or maximum tolerable pressure in 75% of subjects with IBS, the threshold for maximum tolerable pressure was used. Other measures included assessments of rectal compliance (dv/dp) (4,16). The coprimary outcome measure was 30% decrease in pain as assessed by pain logs on a Likert-like scale (0–4) when compared with baseline period. Additional secondary outcome measures included number of BMs per week, global bowel satisfaction score (VAS), number of complete spontaneous BMs (CSBMs) per week, straining effort (1–3), stool consistency (Bristol Stool Form Scale 1–7), and laxative use. The assessment of psychological symptoms focused on 9 domains (4) and QOL (IBS-QOL) on 8 domains (4,20).
Statistical analyses
The mean ± SD values for first sensation, desire to defecate, and maximum tolerable pressure using rectal barostat in healthy subjects were 15.5 ± 2.41, 24.64 ± 3.3, and 33 ± 5.64 mm Hg, respectively (17). Using repeated measures analysis with the Bonferroni correction applied for multiple pairwise comparisons of means, with 23 subjects per group, the statistical test will detect at the 0.05 significance level a mean relative change of at least 15% from baseline for all 3 sensory thresholds with 0.85 power. For comparison of mean change from baseline and after treatment between groups, the statistical test can detect at a 0.05 significance level. This is a difference in mean change of at least 20%. To allow for dropouts (∼20%), we planned a sample size of 27 subjects per group.
The 3 rectal sensory threshold measures were compared between the 2 groups and the 2 phases, baseline and after treatment. The Shapiro-Wilk test was used to assess the normality of measures. A Student t test was used to analyze the differences between the 2 treatments and for normally distributed data. Data for stool frequency, bowel satisfaction score (VAS), IBS-QOL, and SCL-90 were analyzed using the Wilcoxon rank-sum test. Significance testing for responder rates were performed using z tests with continuity correction. A false discovery rate adjusted P value of <0.05 was considered as statistically significant. Because CSBM and BM were not normally distributed, data were compared using the Mood median test. Data were analyzed as intention-to-treat, and with missing data, the last observation was carried forward.
RESULTS
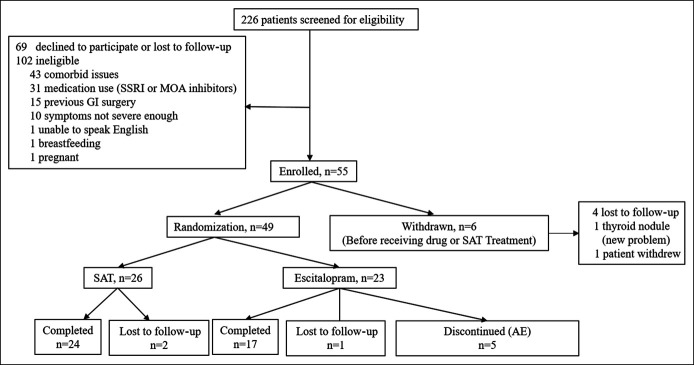
We enrolled 55 patients with IBS-C (Figure 1). Of these, 6 withdrew for personal reasons; 49 were randomized to receive either SAT (n = 26) or escitalopram (n = 23). In the SAT arm, 24/26 (92.3%) patients completed the study and 2 were lost to follow-up. In the escitalopram arm, 17/23 (73.9%) patients completed the study, 1 was lost to follow-up, and 5 withdrew because of adverse events. There were no differences between the 2 groups in baseline demographic features and sensory thresholds (Table 1). Also, at baseline, all patients with RH as identified by volumetric balloon distension were also found to be hypersensitive (2 or more thresholds of rectal sensation ≤2 SD of normal mean values (17)) with the barostat pressure distension protocol.
Figure 1.
Consort flow diagram. AE, adverse event; GI, gastrointestinal; MAO, monoamine oxidase; SAT, sensory adaptation training; SSRI, selective serotonin reuptake inhibitor.
Table 1.
Baseline characteristics and demographic data (mean ± SEM)
| SAT (n = 26) | Escitalopram (n = 23) | |
| F/M | 26/0 | 19/4 |
| Age | 45.0 ± 3.0 | 47.0 ± 3.1 |
| Duration of symptoms (yr) | 14.2 ± 5.2 | 14.4 ± 6.4 |
| Mean daily abdominal pain score (0–4) | 1.7 ± 0.2 | 1.6 ± 0.2 |
| No. of CSBMs per week, median (LQ, UQ) | 0 (0, 0.75) | 1 (0, 5.5) |
| First sensation (mm Hg) | 14.4 ± 0.9 | 13.5 ± 0.7 |
| Desire to defecate (mm Hg) | 20.7 ± 0.9 | 20.6 ± 1.0 |
| Maximum tolerable pressure (mm Hg) | 26.8 ± 1.5 | 27.3 ± 1.4 |
CSBM, complete spontaneous bowel movement; LQ, lower quartile; SAT, sensory adaptation training; UQ, upper quartile.
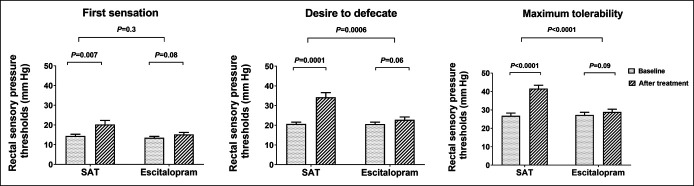
The thresholds for first sensation (P = 0.007), desire to defecate (P = 0.0001), and maximum tolerable pressure (P < 0.0001) significantly increased after SAT when compared with baseline (Figure 2). Also, thresholds for desire (P = 0.0006) and maximum tolerable pressure (P < 0.0001) were significantly higher with SAT compared with escitalopram (Figure 2). Rectal sensory thresholds were unchanged after escitalopram (Figure 2).
Figure 2.
Effects on rectal sensory pressure thresholds. Mean ± SEM. SAT, sensory adaptation training.
The mean daily abdominal pain score decreased significantly with SAT (P = 0.0046) when compared with baseline, but no difference with escitalopram (P = 0.06) and between groups (P = 0.8). The global VAS increased significantly with both SAT (P = 0.0001) and escitalopram (P = 0.002), without group differences (P = 0.6) (Table 2).
Table 2.
Effects on rectal compliance and IBS-C symptom profiles
| SAT (n = 26) | Escitalopram (n = 23) | Pa | |||||
| Baseline | After treatment | P | Baseline | After treatment | P | ||
| Rectal compliance | |||||||
| Rectal pressure | Rectal volume (mL) | ||||||
| 10 mm Hg | 92.1 ± 11.7 | 111.3 ± 12.3 | 0.003 | 96.4 ± 13.2 | 96.4 ± 14.0 | 1 | 0.019 |
| 14 mm Hg | 120.3 ± 10.4 | 145.0 ± 11.7 | 0.002 | 135.8 ± 15.9 | 130.7 ± 16.6 | 1 | 0.0003 |
| 18 mm Hg | 153.6 ± 11.0 | 184.6 ± 14.5 | 0.002 | 160.5 ± 15.8 | 161.8 ± 15.8 | 1 | 0.0014 |
| 22 mm Hg | 184.4 ± 12.6 | 225.6 ± 16.1 | 0.0008 | 195.8 ± 15.5 | 199.5 ± 16.0 | 1 | 0.0003 |
| 26 mm Hg | 223.6 ± 14.2 | 259.1 ± 18.1 | 0.002 | 216.1 ± 14.8 | 220.9 ± 17.1 | 1 | 0.006 |
| Mean daily abdominal pain score (0–4) | 1.7 ± 0.2 | 1.1 ± 0.2 | 0.0046 | 1.6 ± 0.2 | 1.3 ± 0.2 | 0.0664 | 0.8 |
| Global bowel satisfaction score (VAS) | 20.4 ± 3.9 | 50.0 ± 6.3 | 0.0001 | 22.5 ± 3.3 | 46.7 ± 5.5 | 0.002 | 0.6 |
| No. of CSBMs per week, median (LQ, UQ) | 0 (0, 0.75) | 2.5 (0, 5.75) | 0.003 | 1 (0, 5.5) | 1 (0, 6.5) | 0.306 | 0.041 |
| No. of BMs per week, median (LQ, UQ) | 6.0 (4.9,9.8) | 6.5 (5.9, 11.8) | 0.577 | 8.0 (6.2,14.1) | 7.0 (6.3,13.4) | 1.0 | 0.299 |
| Mean straining score (1–3) | 2.0 ± 0.1 | 1.8 ± 0.1 | 0.03 | 1.6 ± 0.1 | 1.8 ± 0.2 | 0.88 | 0.463 |
| Mean stool consistency—BSFS | 3.7 ± 0.3 | 3.9 ± 0.3 | 0.25 | 4.3 ± 0.3 | 3.8 ± 0.4 | 0.867 | 0.122 |
Data expressed as mean ± SEM or median.
BM, bowel movement; BSFS, Bristol Stool Form Scale; CSBM, complete spontaneous bowel movement; IBS-C, irritable bowel syndrome with predominant constipation; LQ, lower quadrant; SAT, sensory adaptation training; UQ, upper quadrant; VAS, visual analog scale.
P: SAT vs escitalopram.
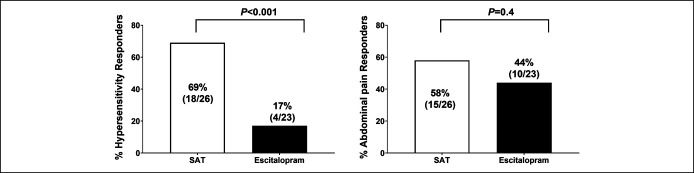
There were significantly greater (P < 0.001) percentage of rectal hypersensitivity responders (69%) with SAT compared with escitalopram (17%) (Figure 3). The percentage of abdominal pain responders was not significant between groups (P = 0.4, Figure 3), although more subjects responded with SAT (58%) than escitalopram (44%). A per-protocol analysis showed significantly greater (P = 0.002) percentage of hypersensitivity responders with SAT (18/24, 75%) compared with escitalopram (4/17, 23.5%), but no difference (P = 0.75) in the percentage of abdominal pain responders, SAT (15/24, 62.5%) vs escitalopram (9/17, 52.9%), respectively. Furthermore, among abdominal pain responders, 12/15 (80%) subjects in the SAT arm were also hypersensitivity responders compared with only 1/10 (10%) in the escitalopram arm (P < 0.001). Also, the number of subjects with ≥1 CSBM per week compared with baseline was higher with SAT than escitalopram (15/26 (57.7%) vs 6/23(26%), P = 0.05).
Figure 3.
Effects on hypersensitivity, abdominal pain, and overall responders. SAT, sensory adaptation training.
In the SAT group, the rectal compliance significantly improved at all levels of balloon distending pressures when compared with baseline and escitalopram (Table 2). There were no changes with escitalopram. The CSBM per week increased significantly with SAT compared with baseline (P = 0.003) or escitalopram (P = 0.04), but not with escitalopram (P = 0.3) (Table 2). The straining effort significantly improved (P = 0.03) with SAT when compared with baseline, but not with escitalopram (P = 0.88) or between groups (P = 0.463) (Table 2). The stool consistency and number of BMs per week were not different in both groups (Table 2). Overall, 69.2% of subjects in the SAT group and 58.9% in the escitalopram group used laxatives/enema during study without group difference.
The QOL domains improved significantly with SAT (Table 3). Likewise, most parameters improved significantly with escitalopram (Table 3), but there were no differences between groups, at baseline and after treatment. The global severity index and positive symptoms total index improved significantly with both treatments, but without any group differences (Table 3).
Table 3.
Effects on quality of life and psychological profiles
| SAT (n = 26) | Escitalopram (n = 23) | Pa | |||||
| Baseline | After treatment | P | Baseline | After treatment | P | ||
| IBS-QOL | |||||||
| Dysphoria | 41.44 ± 6.53 | 25.59 ± 6.20 | 0.0008 | 36.81 ± 5.24 | 24.48 ± 4.06 | 0.0128 | 0.722 |
| Interference with activity | 38.28 ± 6.56 | 24.77 ± 5.47 | 0.0011 | 34.52 ± 4.85 | 24.80 ± 4.89 | 0.0503 | 0.67 |
| Body image | 43.75 ± 6.15 | 27.17 ± 5.81 | 0.0003 | 36.11 ± 6.94 | 26.74 ± 6.53 | 0.0369 | 0.3427 |
| Health worry | 47.83 ± 6.97 | 32.61 ± 6.75 | 0.0038 | 41.67 ± 6.46 | 29.63 ± 4.87 | 0.0205 | 0.719 |
| Food avoidance | 51.81 ± 7.14 | 37.32 ± 7.1 | 0.0024 | 48.61 ± 7.14 | 41.20 ± 6.34 | 0.0494 | 0.265 |
| Social reaction | 39.58 ± 6.69 | 26.81 ± 5.95 | 0.01 | 24.65 ± 4.86 | 20.83 ± 5.3 | 0.261 | 0.278 |
| Sexual | 33.70 ± 8.11 | 25.00 ± 7.12 | 0.0391 | 35.42 ± 7.65 | 28.47 ± 7.62 | 0.3481 | 0.9672 |
| Relationship | 31.52 ± 6.48 | 14.86 ± 4.71 | 0.0008 | 25.46 ± 4.23 | 15.28 ± 2.72 | 0.0322 | 0.3182 |
| SCL-90R | |||||||
| GSI | 0.58 ± 0.10 | 0.42 ± 0.09 | 0.02 | 0.46 ± 0.09 | 0.28 ± 0.04 | 0.02 | 0.919 |
| PST | 27.58 ± 3.90 | 21.71 ± 3.35 | 0.02 | 25.33 ± 4.1 | 18.06 ± 2.53 | 0.0353 | 0.848 |
| Somatization | 0.97 ± 0.14 | 0.82 ± 0.13 | 0.37 | 0.78 ± 0.15 | 0.50 ± 0.08 | 0.0375 | 0.4274 |
| Obsessive-compulsive | 0.77 ± 0.15 | 0.63 ± 0.16 | 0.127 | 0.55 ± 0.10 | 0.32 ± 0.06 | 0.0062 | 0.6076 |
| Interpersonal sensitivity | 0.47 ± 0.13 | 0.26 ± 0.09 | 0.092 | 0.44 ± 0.13 | 0.27 ± 0.08 | 0.109 | 0.587 |
| Depression | 0.73 ± 0.15 | 0.47 ± 0.14 | 0.004 | 0.67 ± 0.15 | 0.41 ± 0.09 | 0.019 | 0.7593 |
| Anxiety | 0.48 ± 0.10 | 0.32 ± 0.08 | 0.124 | 0.26 ± 0.09 | 0.11 ± 0.04 | 0.083 | 0.488 |
| Hostility | 0.28 ± 0.06 | 0.18 ± 0.04 | 0.106 | 0.19 ± 0.05 | 0.18 ± 0.04 | 0.305 | 0.219 |
| Phobic anxiety | 0.29 ± 0.09 | 0.17 ± 0.08 | 0.229 | 0.17 ± 0.06 | 0.08 ± 0.03 | 0.124 | 0.867 |
| Paranoid ideation | 0.30 ± 0.11 | 0.15 ± 0.04 | 0.167 | 0.15 ± 0.05 | 0.10 ± 0.05 | 0.495 | 0.577 |
| Psychoticism | 0.27 ± 0.08 | 0.17 ± 0.05 | 0.392 | 0.17 ± 0.06 | 0.08 ± 0.02 | 0.0734 | 0.861 |
Data expressed as mean ± SEM.
CSBM, complete spontaneous bowel movement; GSI, global severity index; IBS-QOL, irritable bowel syndrome quality of life; PSDI, positive symptom distress index; PST, positive symptoms total; SAT, sensory adaptation training.
P: SAT vs escitalopram.
All patients reported transient rectal discomfort/pain during SAT. In 2 subjects, blood staining was seen on rectal balloon after treatment, but no rectal bleeding. One patient reported nausea, and another had diarrhea and vomiting for 2 days; both completed SAT study. In the escitalopram arm, 5 patients (21.7%) withdrew from adverse events within 2 weeks, and 11/23 (47.8%) experienced adverse events (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A651).
DISCUSSION
We found that patients who received SAT demonstrated significant improvement in rectal hypersensitivity as evidenced by improvements in 2 or more rectal sensory thresholds when compared with those who received escitalopram. Furthermore, escitalopram had no discernible effect on rectal hypersensitivity. Also, the daily pain score decreased in the SAT group but not in escitalopram when compared with their baseline but not between groups. The percentage of abdominal pain responders was, however, not significantly different between groups, possibly from a type II error, although more subjects responded with SAT (58%) than escitalopram (44%). Further analysis showed that 80% of the abdominal pain responders in the SAT arm were also hypersensitivity responders as compared to only 10% in the escitalopram arm. This observation suggests that the improvement in abdominal pain with SAT was associated with improvement in hypersensitivity. The rectal compliance also improved significantly with SAT but not with escitalopram. Together, these findings suggest that SAT improves rectal hypersensitivity and pain in IBS-C, possibly by inducing visceromotor changes including increased rectal compliance and nociceptor desensitization, i.e., changes similar to those observed in rodent models with 5HT4 compounds (22).
Here, we chose a higher bar for defining a hypersensitivity responder by requiring an improvement in 2 or more thresholds of hypersensitivity. To the best of our knowledge, this is the first study where changes in 2 sensory thresholds have been applied both for diagnosis of hypersensitivity and for assessing improvement. This more stringent measure allows for any intersubject and intrasubject variability and could provide greater confidence with this assessment and manometric characterization. Furthermore, the therapeutic responsiveness of this measure with SAT suggests that this could serve as a biomarker for IBS and pain.
Although visceral hypersensitivity is considered a hallmark of IBS (3,4,18), it may be more diffuse, dissimilar in different regions or localized to distal colon, and explains why rectal hypersensitivity is only demonstrable in 60% of patients with IBS (18). The mechanism(s) for rectal hypersensitivity is unclear, but previous gastroenteritis and sensitization of visceral afferents especially intraganglionic laminar nerve endings (23) and alterations in SIP syncytium (23) may lead to excessive and unregulated discharge of afferent neuronal activation during of normal luminal distension (3), causing abdominal and rectal pain, typifying IBS symptoms.
Also, perturbations involving central sensitization (24) or abnormal endogenous descending inhibitory pathways (2,3) may play a role. In patients with IBS-C who responded to noxious stimuli, there was decreased rectal sensory thresholds and an activation of the amygdala (emotional) and hippocampus (25), suggesting that the neuronal matrix controlling afferent, nociceptor input, emotional, autonomic, and descending modulatory responses to pain are disturbed in IBS-C (26). Consequently, the improvements in abdominal pain together with improvements in quality of life and psychological domains suggest that SAT may alter gut and brain interactions.
The treatment of IBS-C remains challenging in part because it is a polysymptomatic condition whose pathophysiology is multifactorial (27,28). Although current FDA-approved therapies such as linaclotide and plecanatide improve bowel symptoms and discomfort (27,28), and low-dose antidepressants/neuromodulators provide relief despite low quality of evidence (28), there is dearth of mechanistic-based treatments. Because the rectum is easily accessible for performing behavioral therapies (29), we chose this site for performing SAT. Our study showed that repeated balloon distensions, in small increments, facilitated sensory adaptation and tolerance to previously perceived noxious distensions, possibly by reducing either the volley of afferent nociceptive signals to the brain or cortical activation. Over time, SAT may downregulate the sensitized mechanoreceptors and/or afferent neurons leading to an improvement in pain and bowel symptoms. Although the balloon distensions were uncomfortable initially, over time, the subjects recognized that the luminal distensions were safe and unlikely to be harmful. Gradually, the hypervigilance subsided possibly through a combination of improved descending corticospinal inhibition, and increased tolerance of noxious stimuli, and decreased activation of hippocampus/amygdala (2). One study that performed repeated barostat studies over 1 year showed decreased hypersensitivity in 23% of subjects with IBS, but not symptoms, suggesting that physiological changes were possibly from habituation (13). However, repeat barostat studies in the escitalopram arm showed no changes in sensory thresholds, and most of the pain responders in the SAT arm were also hypersensitivity responders. Therefore it is more likely that treatment with SAT brings about sensori-neurobiologic effects through mechanisms that alter visceral sensation (27,28,30) than familiarity with experimental setup.
SAT also improved global bowel satisfaction, psychological well-being, and QOL when compared with baseline. Also, numerically, the SAT group had higher values for some psychological domains than escitalopram, but there was no difference. Escitalopram also improved these domains confirming previous observations (31), but the rectal hypersensitivity thresholds and abdominal pain were unchanged reaffirming observations of American Gastroenterology Association technical review (23). This suggests that the neuromodulatory effects of escitalopram or other SSRI (7,10) are more likely centrally mediated. Furthermore, the higher frequency of adverse events with escitalopram including 21.7% of withdrawals confirms previous studies and raises concerns for generalized use without monitoring (11,23,31).
Our study limitations include the smaller sample size and labor-intensive nature of SAT, limiting its generalizability. Furthermore, because we selected IBS patients with rectal hypersensitivity, our findings may not be generalizable. However, such patients are frequently encountered in referral centers. The SAT treatments were performed manually by titrating distending pressures, but to make this more user-friendly, further software refinements are required. The fewer visits in escitalopram arm may have resulted in lesser improvement because provider-patient interactions have been suggested to affect outcome (31). However, this seems less likely because both groups showed comparable improvements in psychological/QOL domains, and both were active interventions. The barostat is not widely used clinically, similar to other motility tools, but is commercially available, has been used in innumerable studies (2–7,9–14), and with some training can be adapted for sensori-behavioral therapy. Hence, SAT could
Study Highlights.
WHAT IS KNOWN
✓ Irritable bowel syndrome with constipation (IBS-C) is characterized by abdominal pain and constipation. Many patients remain dissatisfied with current treatments.
✓ Rectal hypersensitivity is regarded as a hallmark dysfunction of IBS-C, but it lacks effective treatment(s).
WHAT IS NEW HERE
✓ Sensory adaptation training (SAT) significantly improved rectal hypersensitivity and bowel symptoms in IBS-C, when compared with escitalopram.
✓ Most (80%) abdominal pain responders with SAT were also hypersensitivity responders unlike 10% with escitalopram. Both treatments improved quality of life and psychological profiles.
TRANSLATIONAL IMPACT
✓ SAT significantly improves symptoms in IBS-C and is better tolerated than escitalopram and could be useful in selected patients with persistent symptoms.
be a viable treatment option for selected patients with refractory IBS symptoms, but further validation is needed including comparison with sham therapy, and newer visceral analgesics (11,32), and assessment of durability of response.
In conclusion, SAT was more efficacious than a neuromodulator, escitalopram for the treatment of rectal hypersensitivity and IBS-C symptoms, and this novel sensori-behavioral therapy is both useful and safe.
CONFLICTS OF INTEREST
Guarantor of the article: Satish S.C. Rao, MD, PhD, FRCP.
Specific author contributions: S.S.C.R.: principal investigator, study concept and design, grant support, data analysis and interpretation, sensory adaptation training supervision, manuscript preparation, overall supervision, and critical revision. J.V.: study coordinator, sensory adaptation training, and data collection. Y.Y.: data analysis, tables and figures, and statistical analysis. E.C.-A.: study recruitment, coordination, sensory adaptation training, and manuscript preparation. A.E.: study recruitment, coordination, and sensory adaptation training. D.A.: statistical methods, data analysis, and manuscript preparation. All authors have approved the final version of the manuscript submitted.
Financial support: This work was supported by NIH grant R01 DK 57100-05 and grant RR00059 from the General Clinical Research Centers program, National Center for Research Resources to University of Iowa. The study was conducted at Augusta University Medical Center and the University of Iowa Hospitals and Clinics, and data analysis and manuscript writing were completed at Augusta University.
Potential competing interests: None to report.
Trial registration: Registered at ClinicalTrials.gov no NCT00584571.
Supplementary Material
ACKNOWLEDGMENT
We sincerely acknowledge the research assistance of Amanda Schmeltz and Tanisa Patcharatrakul, technical assistance of Sam Jundler with barostat programs, and the data extraction assistance of Elizabeth Dahlberg and Xuelian Xiang, and the superb secretarial support of Helen Smith. An abstract of this work was presented at DDW 2020 and published in Gastroenterology (2020;158:406).
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A651.
Contributor Information
Enrique Coss-Adame, Email: enriquecossmd@gmail.com.
Yun Yan, Email: yyan@augusta.edu.
Askin Erdogan, Email: erdoganaskin@hotmail.com.
Jessica Valestin, Email: jessica_valestin@uiowa.edu.
Deepak Nag Ayyala, Email: dayyala@augusta.edu.
REFERENCES
- 1.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407. [DOI] [PubMed] [Google Scholar]
- 2.Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simren M, Tornblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: Consistent findings from five different patient cohorts. Gut 2018;67:255–62. [DOI] [PubMed] [Google Scholar]
- 4.Rao SSC, Xiang X, Yan Y, et al. Randomised clinical trial: Linaclotide vs placebo-a study of bi-directional gut and brain axis. Aliment Pharmacol Ther 2020;51:1332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouin M, Plourde V, Boivin M, et al. Rectal distention testing in patients with irritable bowel syndrome: Sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 2002;122:1771–7. [DOI] [PubMed] [Google Scholar]
- 6.Josefsson A, Rosendahl A, Jerlstad P, et al. Visceral sensitivity remains stable over time in patients with irritable bowel syndrome, but with individual fluctuations. Neurogastroenterol Motil 2019;31:e13603. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017;66:966–74. [DOI] [PubMed] [Google Scholar]
- 8.Laird KT, Tanner-Smith EE, Russell AC, et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:937–47. [DOI] [PubMed] [Google Scholar]
- 9.Tack J, Broekaert D, Corsetti M, et al. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment Pharmacol Ther 2006;23:265–74. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auquier P, Robitail S, Llorca PM, et al. Comparison of escitalopram and citalopram efficacy: A meta-analysis. Int J Psychiatry Clin Pract 2003;7:259–68. [DOI] [PubMed] [Google Scholar]
- 12.Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005;54:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naliboff BD, Berman S, Suyenobu B, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 2006;131:352–65. [DOI] [PubMed] [Google Scholar]
- 14.Mohanty S, Schulze K, Stessman M, et al. Behavioral therapy for rectal hypersensitivity. Am J Gastroenterol 2001;96:A955. [Google Scholar]
- 15.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 16.Rao SS, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol 1999;94:773–83. [DOI] [PubMed] [Google Scholar]
- 17.Rao S, Mudipalli R, Paulson J, et al. Investigation of colonic and rectal sensory properties and compliance and its reproducibility in humans. Am J Gastroenterol 2008;103:S465. [Google Scholar]
- 18.Ludidi S, Conchillo JM, Keszthelyi D, et al. Rectal hypersensitivity as hallmark for irritable bowel syndrome: Defining the optimal cutoff. Neurogastroenterol Motil 2012;24:729–33, e345–6. [DOI] [PubMed] [Google Scholar]
- 19.Odunsi ST, Camilleri M, Bharucha AE, et al. Reproducibility and performance characteristics of colonic compliance, tone, and sensory tests in healthy humans. Dig Dis Sci 2010;55:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig Dis Sci 1998;43:400–11. [DOI] [PubMed] [Google Scholar]
- 21.Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: A step in the validation of a new self-report scale. Br J Psychiatry 1976;128:280–9. [DOI] [PubMed] [Google Scholar]
- 22.Nissen TD, Brock C, Lykkesfeldt J, et al. Pharmacological modulation of colorectal distension evoked potentials in conscious rats. Neuropharmacology 2018;140:193–200. [DOI] [PubMed] [Google Scholar]
- 23.Sanders KM, Ward SM, Koh SD. Interstitial cells: Regulators of smooth muscle function. Physiol Rev 2014;94:859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moshiree B, Zhou Q, Price DD, et al. Central sensitisation in visceral pain disorders. Gut 2006;55;905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer EA, Berman S, Derbyshire SW, et al. The effect of the 5-HT3 receptor antagonis, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther 2002;16:1357–66. [DOI] [PubMed] [Google Scholar]
- 26.Johnson A, Louwies T, Yuan T, et al. Gut and brain interactions. In: Rao SSC, et al. (eds.). Clinical and Basic Neurogastroenterology and Motility. Academic Press: Cambridge, MA, 2020, pp 17–30. [Google Scholar]
- 27.Simren M, Tack J. New treatments and therapeutic targets for IBS and other functional bowel disorders. Nat Re Gastroenterol Hepatol 2018;15:589–605. [DOI] [PubMed] [Google Scholar]
- 28.Chang L, Lembo A, Sultan S. American gastroenterological association institute technical review on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014;147:1149–72. [DOI] [PubMed] [Google Scholar]
- 29.Rao SS, Benninga MA, Bharucha AE, et al. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil 2015;27:594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenwood-van Meerveld B. Importance of 5-hydroxytryptamine receptors on intestinal afferents in the regulation of visceral sensitivity. Neurogastroenterol Motil 2007;19(Suppl 2):13–8. [DOI] [PubMed] [Google Scholar]
- 31.Kaptchuk TJ, Kelly JM, Conboy LA, et al. Components of placebo effect: Randomized controlled trial in patients with irritable bowel syndrome. BMJ 2008;336:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawoos Y, Shah A, Iqbal A, et al. Efficacy and tolerability of tianeptine versus escitalopram in patients with irritable bowel syndrome: A hospital based, randomized comparative study. Int J Contemp Med Res 2020;7:E6–E11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.