Abstract
Background:
Non-communicable chronic diseases have become the leading causes of disease burden worldwide. The trends and burden of “metabolic associated fatty liver disease” (MAFLD) are unknown. We aimed to investigate the cardiovascular and renal burdens in adults with MAFLD and non-alcoholic fatty liver disease (NAFLD).
Methods:
Nationally representative data were analyzed including data from 19,617 non-pregnant adults aged ≥20 years from the cross-sectional US National Health and Nutrition Examination Survey periods, 1999 to 2002, 2003 to 2006, 2007 to 2010, and 2011 to 2016. MAFLD was defined by the presence of hepatic steatosis plus general overweight/obesity, type 2 diabetes mellitus, or evidence of metabolic dysregulation.
Results:
The prevalence of MAFLD increased from 28.4% (95% confidence interval 26.3–30.6) in 1999 to 2002 to 35.8% (33.8–37.9) in 2011 to 2016. In 2011 to 2016, among adults with MAFLD, 49.0% (45.8–52.2) had hypertension, 57.8% (55.2–60.4) had dyslipidemia, 26.4% (23.9–28.9) had diabetes mellitus, 88.7% (87.0–80.1) had central obesity, and 18.5% (16.3–20.8) were current smokers. The 10-year cardiovascular risk ranged from 10.5% to 13.1%; 19.7% (17.6–21.9) had chronic kidney diseases (CKDs). Through the four periods, adults with MAFLD showed an increase in obesity; increase in treatment to lower blood pressure (BP), lipids, and hemoglobin A1c; and increase in goal achievements for BP and lipids but not in goal achievement for glycemic control in diabetes mellitus. Patients showed a decreasing 10-year cardiovascular risk over time but no change in the prevalence of CKDs, myocardial infarction, or stroke. Generally, although participants with NAFLD and those with MAFLD had a comparable prevalence of cardiovascular disease and CKD, the prevalence of MAFLD was significantly higher than that of NAFLD.
Conclusions:
From 1999 to 2016, cardiovascular and renal risks and diseases have become highly prevalent in adults with MAFLD. The absolute cardiorenal burden may be greater for MAFLD than for NAFLD. These data call for early identification and risk stratification of MAFLD and close collaboration between endocrinologists and hepatologists.
Keywords: Cardiovascular disease, Chronic kidney disease, Risk, Metabolic associated fatty liver disease, Non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) affects about a quarter of the world's adult population and poses major health and economic burdens to all societies.[1,2] Recently, two-position papers proposed changing the definition of NAFLD to “metabolic associated fatty liver disease” (MAFLD).[3,4] This changes an exclusive definition to an inclusive one, as present criteria require evidence of hepatic steatosis accompanied by one of three features: overweight or obesity, type 2 diabetes mellitus (T2DM), or lean or normal weight with evidence of metabolic dysregulation.[3] The prevalence of MAFLD in the US is unknown.
Among patients with NAFLD, the most common cause of death is cardiovascular disease (CVD),[5,6] while only a minority of patients develop advanced liver disease or die from liver-related causes.[7] Thus, CVD prevention may have a core role in MAFLD management. Unsurprisingly, with the new diagnosis criteria, MAFLD would be more closely associated with an increased risk of CVD, given the associations between MAFLD diagnosis and established CVD risk factors including abdominal obesity, hypertension, atherogenic dyslipidemia, and insulin resistance (IR)/dysglycemia.[8] However, the extent of these associations and their implications for MAFLD development remain unknown.
Chronic kidney disease (CKD) is another important cause of death and loss of disability-adjusted life-years,[9,10] and there is robust evidence of a strong association between NAFLD and severity with CKD stages and risks.[11] MAFLD and CKD also share similar pathophysiology risk factors, that is, IR pre-disposes patients to atherogenic dyslipidemia, etc.[12] As a new potential “driving force” for CKD development and progression, the extent of the association of MAFLD with CKD remains the subject of much scrutiny.
There are concerns that with the new definition of MAFLD, its cardiovascular and renal burden might vary with changes in the diagnostic criteria. Little is known about the cardiovascular and renal risk of MAFLD, and it is unclear as to whether identifying individuals with MAFLD would provide an opportunity to more intensively manage these common causes of morbidity and mortality. To address these questions, we analyzed nationally representative data from the US National Health and Nutrition Examination Surveys (NHANESs) from 1999 to 2016 to examine the cardiovascular and renal burdens in adults with MAFLD and NAFLD over time.
Methods
Study design and participants
The NHANES, which is conducted by the National Center for Health Statistics, comprises cross-sectional, multistage, stratified, clustered probability samples of the non-institutionalized US population. Each 2-year NHANES cycle represents American civilians that are not in an institution. The National Center for Health Statistics Research Ethics Review Board approved the NHANES (Protocol #98-12, #2005-06, and #2011-17). All participants provided informed consent before participating.
Data were obtained from nine continuous surveys over 18 years from 1999 to 2016. The response rates of the NHANESs from 1999 to 2016 ranged from 58.7% to 84.0%. Our analyses included all non-pregnant participants aged ≥20 years. Because we defined liver steatosis based on ultrasound-fatty liver index (US-FLI),[13] fasting sub-sample was used. We further excluded adults with missing data for waist circumference (n = 1127), fasting plasma glucose (FPG; n = 869), γ-glutamyl transferase (n = 249), and insulin (n = 269), and the final sample was 19,617 [Supplementary Figure 1]. Data were analyzed to examine trends in the prevalence of hypertension, dyslipidemia, smoking, diabetes, obesity, and central obesity; an average 10-year risk of CVD; and the presence of cardiovascular and renal comorbidities in the US adult populations with MAFLD or NAFLD.
Measurements
Demographic characteristics were obtained through interviewer-administered questionnaires. Demographic information regarding age, sex, race, or ethnic group, the highest level of education attained, household income, and health status was obtained via interviewer-administered questionnaires at the participants’ homes. At the NHANES Medical Examination Center (MEC), anthropometric measurements including weight, waist circumference, and height were taken by trained staff using standardized techniques. Three or four brachial arteries blood pressure (BP) (mmHg) readings were taken by physicians after 5 min of rest in a sitting position, and an average of measurements was used.
Fasting blood samples after a minimum of 8 h in a fasted state and a random urine sample were collected at the MEC. FPG (mg/dL), hemoglobin A1c (HbA1c) (%), low-density lipoprotein (LDL, mg/dL) cholesterol, and urinary creatinine (g/dL) and albumin (mg/dL) were estimated. Laboratory analyses for biochemical indicators followed standard protocols and used the following methods and kits: FPG, hexokinase enzymatic method (Roche Modular P chemistry analyzer, Roche Diagnostics, Indianapolis, IN, USA); HbA1c, glycohemoglobin analyzer (Tosoh Medics, Inc., San Francisco, CA, USA) and assays were standardized according to national reference methods; LDL cholesterol, Roche Modular P chemistry analyzer (Roche Diagnostics); urinary creatinine, enzymatic endpoint reaction that resembles high-performance liquid chromatography (Roche/Hitachi Modular P up to 2012 and Roche/Hitachi Cobas 6000 chemistry analyzer thereafter, both from Roche Diagnostics); serum γ-glutamyl transferase, enzymatic rate method (Beckman Coulter UniCel DxC 800 Synchron chemistry analyzer, Beckman Coulter, Brea, CA, USA); and urinary albumin, solid-phase fluorescent immunoassay. Regarding the needs of trend analyses, FPG within the eight consecutive NHANES cycles were adjusted into values detected by the above method and equipment according to the equations provided by the National Center for Health Statistics.
Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). Given the large sample size, the homeostasis model assessment (HOMA) was used to assess IR. HOMA-IR was calculated as FPG multiplied by fasting insulin and then divided by 22.5.
Definition of variables
MAFLD was defined by hepatic steatosis in adults (detected by US-FLI ≥30[13]) in addition to one of the following three criteria: general overweight/obesity, T2DM, or evidence of metabolic dysregulation.[3] Overweight was defined as BMI ≥25 kg/m2 in Caucasians and BMI ≥23 kg/m2 in Asians, and general obesity was defined as BMI ≥30 kg/m2 in Caucasians and BMI ≥25 kg/m2 in Asians. Central obesity was defined by waist circumference ≥102/88 cm in Caucasian men and women and ≥90/80 cm in Asian men and women, respectively. Metabolic risk abnormalities for lean/normal weight patients include central obesity, BP ≥130/85 mmHg or undergoing treatment, plasma triglycerides ≥150 mg/dL (≥1.70 mmol/L) or undergoing treatment, plasma HDL-cholesterol <40 mg/dL (<1.0 mmol/L) for men and <50 mg/dL (<1.3 mmol/L) for women or undergoing treatment, prediabetes (FPG 100–125 mg/dL [5.6–6.9 mmol/L] or HbA1c 5.7%–6.4% [39–47 mmol/mol]), and HOMA-IR score ≥2.5.
NAFLD was based on US-FLI evidence of liver steatosis (detected by US-FLI ≥30) and the exclusion of viral hepatitis (B or C), excessive alcohol consumption (an alcohol consumption ≥30 g/d for male and ≥20 g/d for female), or aspartate aminotransferase or alanine aminotransferase >500 U/L.[14]
Sex, race or ethnic group, education (<high school vs. at least high school education), and mean age were reported at the time of the survey. To categorize income (above vs. at or below poverty level), the poverty-income ratio was used as an indicator of income relative to the inflation-adjusted family need.
Cardiovascular risk factors and treatment goals
Hypertension was defined as self-reported use of anti-hypertensive drugs or measured BP ≥140/90 mmHg. The goal for control of hypertension was set at <140/90 mmHg.[15] Dyslipidemia was defined as self-reported use of lipid-lowering drugs or measured LDL cholesterol >130 mg/dL (>3.36 mmol/L) or higher. Although since 2013 the new cholesterol guidelines have abandoned specific LDL targets, we used <100 mg/dL for LDL cholesterol treatment and control status.[16] Smoking status was classified as current, former, or never. “Not currently smoking” was used as the tobacco control goal. Diabetes mellitus was defined as self-reported diabetes mellitus, HbA1c level ≥6.5% or FPG level ≥7.0 mmol/L. The goal for control for most diabetic patients was set at HbA1c 7%.[17]
Cardiovascular and renal complications of MAFLD and NAFLD
The mean 10-year risk of cardiovascular events was calculated by the modified Framingham risk score (FRS)[18] and the more contemporary American College of Cardiology and American Heart Association Atherosclerotic Cardiovascular Disease (ASCVD) risk calculator.[19] In the FRS and ASCVD risk calculator, participants aged 30 to 74 years and 40 to 79 years, respectively, were included according to the equation restrictions. Previous myocardial infarction and stroke were described based on self-reported information.
The following CKD markers were examined: mean estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 (stages III–V), and urinary albumin-to-creatinine ratio (ACR) ≥30 mg/g. Any CKD was referred to as urinary ACR ≥30 mg/g, eGFR <60 mL/min per 1.73 m2, or both. eGFR was calculated using the CKD-Epidemiology Collaboration equation.[20] The prevalence of hyperfiltration (eGFR >135 mL/min per 1.73 m2) was also estimated as an early indicator of renal damage related to hyperglycemia.[21]
Statistical analysis
The NHANES uses a complex sampling design that requires the use of sample weights to adjust for the unequal probability of selection into the survey and to adjust for the possible bias resulting from non-response, which thus provides estimates representative of the civilian, non-institutionalized US population. To account for differential probabilities of selection and non-response, we used fasting weights from individuals with US-FLI so that the sum of the sampling weights was added to the total US population. Missing data for any variable were none or <1%, and sample sizes were reduced for each specific analysis where data for the dependent variable were missing. All data analyses were performed using IBM SPSS Statistics, Version 24 (IBM Corp., Armonk, NY, USA) by the Complex Samples module. A two-tailed P value < 0.05 was considered statistically significant.
To increase the sample size in each analytic period, we combined nine cycles into four periods (1999–2002, 2003–2006, 2007–2010, and 2011–2016). Sociodemographic and clinical characteristics of individuals with MAFLD, without MAFLD, with NAFLD, and without NAFLD in each period were reported. The prevalence of hypertension, dyslipidemia, diabetes, general obesity, and central obesity and corresponding values of continuous variables were calculated, and the trends in distributional differences in these characteristics and risk factors for CVD in successive periods were tested. Tests for trends were performed by including the midpoint of each survey period as a continuous variable in a regression model.
We estimated the proportion of treatment and control of hypertension in participants with and without MAFLD. Treatment information was based on self-reports, and “control” was defined as BP <140/90 mmHg under treatment. Therefore, proportions of “treated and controlled,” “treated and uncontrolled,” and “untreated and uncontrolled” were obtained. This same approach was used for dyslipidemia and diabetes mellitus.
In each time period, we also reported the mean 10-year risk of cardiovascular events and estimated the prevalence of myocardial infarction and stroke. For CKD, we reported the mean eGFR, the prevalence of moderate-to-severe albuminuria, and reduced eGFR separately and together, and the prevalence of hyperfiltration.
For all estimates of prevalence and mean levels, we calculated the predicted changes from 1999 to 2002 to 2011 to 2016 using interaction terms of MAFLD and NAFLD status by survey periods in the regression models adjusted for age, sex, and race or ethnic group. The odds ratios (ORs) or β coefficients (95% confidence interval [CI]) were also obtained in the MAFLD and NAFLD groups and compared with the non-MAFLD and non-NAFLD groups, respectively.
Results
Over successive survey periods, the prevalence and absolute number of MAFLD cases increased significantly and were significantly greater than those of the NAFLD cases [Table 1]. In the MAFLD and non-MAFLD groups, the mean age of US adults remained stable, more women developed MAFLD over time, and higher proportions reported completing high school education in non-MAFLD but not in MAFLD. The race stays largely the same except for reduced proportions of non-Hispanic white in the non-MAFLD group. The mean BMI and waist circumference increased substantially in both groups. In the multivariable logistic model, male, older age, central obesity, diabetes, elevated systolic BP and triglyceride levels, and lower HDL cholesterol levels were significantly associated with a higher risk of MAFLD [Supplementary Table 1].
Table 1.
Characteristics of US adults aged 20 years and older by MAFLD and NAFLD status, from 1998 to 2016.
| Items | 1999–2002 | 2003–2006 | 2007–2010 | 2011–2016 | Ptrend |
| MAFLD | |||||
| n | 1367 | 1281 | 1931 | 2552 | |
| Estimated population size (millions)∗ | 11.7 (0.7) | 13.2 (0.8) | 15.5 (0.9) | 25.6 (1.2) | |
| Prevalence (%) | 28.4 (1.1) | 31.0 (1.0) | 34.4 (1.2) | 35.8 (1.0) | <0.001 |
| Mean age (years) | 50.9 (0.7) | 49.5 (0.7) | 51.0 (0.5) | 51.3 (0.4) | 0.210 |
| Male (%) | 60.1 (2.1) | 61.0 (1.4) | 59.4 (1.3) | 55.6 (1.3) | 0.022 |
| Mean BMI (kg/m2) | 33.1 (0.3) | 34.1 (0.3) | 33.4 (0.2) | 34.5 (0.2) | 0.006 |
| Mean waist circumference (cm) | 110.6 (0.8) | 113.5 (0.6) | 111.4 (0.4) | 114.1 (0.5) | 0.001 |
| Completed high-school education (%) | 75.0 (1.7) | 80.1 (1.3) | 75.6 (1.4) | 79.7 (1.4) | 0.138 |
| At or below poverty line (%) | 14.0 (1.6) | 11.2 (1.0) | 15.2 (1.4) | 15.9 (1.3) | 0.092 |
| Non-Hispanic white (%) | 72.3 (2.8) | 74.8 (2.6) | 70.0 (3.0) | 67.3 (2.5) | 0.052 |
| Non-Hispanic black (%) | 6.5 (1.0) | 7.0 (0.9) | 6.7 (0.8) | 6.5 (0.8) | 0.154 |
| Mexican American (%) | 10.8 (1.5) | 11.0 (2.2) | 13.1 (2.2) | 13.0 (1.9) | 0.456 |
| NAFLD | |||||
| n | 1286 | 1214 | 1794 | 2364 | |
| Estimated population size (millions)∗ | 10.9 (0.7) | 12.4 (0.8) | 14.5 (0.9) | 23.6 (1.2) | |
| Prevalence (%) | 26.4 (1.2) | 29.2 (1.0) | 32.2 (1.1) | 33.0 (1.0) | <0.001 |
| Mean age (years) | 51.0 (0.8) | 49.7 (0.7) | 51.2 (0.5) | 51.3 (0.4) | 0.304 |
| Male (%) | 58.3 (2.2) | 59.5 (1.4) | 58.4 (1.4) | 54.2 (1.4) | 0.040 |
| Mean BMI (kg/m2) | 33.3 (0.3) | 34.3 (0.3) | 33.5 (0.2) | 34.7 (0.2) | 0.004 |
| Mean waist circumference (cm) | 110.5 (0.8) | 113.6 (0.6) | 111.8 (0.5) | 114.4 (0.5) | <0.001 |
| Completed high-school education (%) | 74.7 (1.8) | 80.3 (1.3) | 76.1 (1.6) | 79.9 (1.4) | 0.096 |
| At or below poverty line (%) | 14.1 (1.9) | 10.8 (1.1) | 14.7 (1.5) | 15.7 (1.2) | 0.125 |
| Non-Hispanic white (%) | 71.4 (3.0) | 74.6 (2.6) | 71.0 (3.1) | 66.9 (2.5) | 0.096 |
| Non-Hispanic black (%) | 6.6 (1.0) | 6.8 (0.9) | 6.3 (0.8) | 6.3 (0.8) | 0.176 |
| Mexican American (%) | 10.8 (1.6) | 11.3 (2.2) | 12.8 (2.3) | 13.1 (1.8) | 0.581 |
| Non-MAFLD | |||||
| n | 2587 | 2508 | 3072 | 4319 | |
| Estimated population size (millions)∗ | 29.7 (1.1) | 29.4 (1.3) | 29.7 (1.4) | 45.9 (1.9) | |
| Prevalence (%) | 71.6 (1.1) | 69.0 (1.0) | 65.6 (1.2) | 64.2 (1.0) | <0.001 |
| Mean age (years) | 44.2 (0.6) | 45.1 (0.5) | 44.7 (0.5) | 45.2 (0.4) | 0.239 |
| Male (%) | 44.9 (1.1) | 44.0 (0.9) | 43.8 (1.1) | 45.5 (0.9) | 0.568 |
| Mean BMI (kg/m2) | 25.8 (0.1) | 26.2 (0.1) | 26.0 (0.1) | 26.2 (0.1) | 0.057 |
| Mean waist circumference (cm) | 89.6 (0.3) | 91.4 (0.3) | 91.0 (0.4) | 91.9 (0.3) | <0.001 |
| Completed high-school education (%) | 80.4 (1.2) | 83.8 (1.4) | 84.1 (0.9) | 86.0 (1.2) | 0.003 |
| At or below poverty line (%) | 12.8 (1.1) | 10.6 (0.9) | 13.3 (0.9) | 15.8 (1.1) | 0.011 |
| Non-Hispanic white (%) | 72.9 (2.2) | 71.2 (2.3) | 68.7 (2.1) | 65.2 (2.2) | 0.006 |
| Non-Hispanic black (%) | 11.6 (1.5) | 12.7 (1.5) | 13.1 (1.3) | 13.5 (1.4) | 0.182 |
| Mexican American (%) | 5.3 (0.7) | 6.4 (0.9) | 6.0 (0.8) | 6.0 (0.7) | 0.085 |
| Non-NAFLD | |||||
| n | 2668 | 2575 | 3209 | 4507 | |
| Estimated population size (millions)∗ | 30.5 (1.2) | 30.2 (1.3) | 30.7 (1.4) | 47.9 (1.8) | |
| Prevalence (%) | 73.6 (1.2) | 70.8 (1.0) | 67.8 (1.1) | 67.0 (1.0) | <0.001 |
| Mean age (years) | 44.3 (0.6) | 45.1 (0.5) | 44.8 (0.5) | 45.5 (0.4) | 0.152 |
| Male (%) | 45.9 (1.1) | 45.1 (0.9) | 44.8 (1.1) | 46.7 (0.8) | 0.512 |
| Mean BMI (kg/m2) | 26.0 (0.1) | 26.3 (0.1) | 26.2 (0.1) | 26.5 (0.1) | 0.019 |
| Mean waist circumference (cm) | 90.2 (0.3) | 91.9 (0.3) | 91.5 (0.3) | 92.7 (0.3) | <0.001 |
| Completed high-school education (%) | 80.4 (1.2) | 83.6 (1.4) | 83.5 (0.9) | 85.7 (1.2) | 0.005 |
| At or below poverty line (%) | 12.8 (1.1) | 10.8 (0.9) | 13.6 (0.9) | 15.9 (1.2) | 0.010 |
| Non-Hispanic white (%) | 73.2 (2.1) | 71.3 (2.4) | 68.3 (2.1) | 65.4 (2.2) | 0.004 |
| Non-Hispanic black (%) | 11.4 (1.5) | 12.6 (1.4) | 13.1 (1.3) | 13.4 (1.4) | 0.162 |
| Mexican American (%) | 5.5 (0.7) | 6.4 (0.9) | 6.4 (0.8) | 6.2 (0.8) | 0.094 |
Data are from the NHANESs between 1999 and 2016. Data are expressed as mean or percentage (standard error). P values are for the trends over time in each characteristic within each group. ∗Population size was estimated for civilian US adults aged 20 years and older who were not living in an institution, using the current population survey totals and the proportion of individuals in each MAFLD group. BMI: Body mass index; MAFLD: Metabolic associated fatty liver disease; NAFLD: Non-alcoholic fatty liver disease; NHANESs: National Health and Nutrition Examination Surveys.
The adjusted prevalence of hypertension, dyslipidemia, and diabetes remained stable in the MAFLD and NAFLD groups between the 1999 to 2002 and 2011 to 2016 surveys [Table 2]. The prevalence of general and central obesity increased greatly over time in both groups. Compared with the non-MAFLD group, the MAFLD group had significantly higher odds in all five factors, especially in diabetes (OR 5.73, 95% CI 5.10–6.45) and central obesity (OR 17.05, 95% CI 15.32–18.97).
Table 2.
Prevalence of risk diseases in US adults aged 20 years and older, by MAFLD and NAFLD status, from 1999 to 2016.
| Items | 1999–2002 | 2003–2006 | 2007–2010 | 2011–2016 | Total | Ptrend | Pinteraction | P∗ | OR (95% CI)† |
| Hypertension (%) | |||||||||
| MAFLD | 47.4 (43.9, 50.9) | 49.1 (45.9, 52.3) | 46.9 (43.8, 50.1) | 49.0 (45.8, 52.2) | 48.2 (46.5, 49.9) | 0.846 | 0.976 | 0.040 | 3.16 (2.85, 3.50) |
| NAFLD | 47.9 (44.3, 51.5) | 49.0 (45.6, 52.3) | 46.9 (43.6, 50.2) | 47.6 (44.3, 51.0) | 47.8 (46.0, 49.5) | 0.342 | 0.289 | 2.85 (2.56, 3.17) | |
| Non-MAFLD | 22.3 (20.2, 24.6) | 22.2 (20.0, 24.4) | 21.7 (19.3, 24.3) | 23.4 (21.3, 25.5) | 22.5 (21.4, 23.6) | 0.401 | |||
| Non-NAFLD | 22.8 (20.8, 25.1) | 23.0 (20.9, 25.2) | 22.6 (20.1, 25.2) | 25.1 (23.0, 27.3) | 23.6 (22.5, 24.7) | 0.942 | |||
| Dyslipidemia (%) | |||||||||
| MAFLD | 57.2 (52.9, 61.4) | 53.4 (49.8, 56.9) | 56.0 (52.7, 59.2) | 57.8 (55.2, 60.4) | 56.4 (54.8, 58.0) | 0.488 | 0.099 | 0.124 | 1.57 (1.45, 1.70) |
| NAFLD | 56.5 (52.0, 60.9) | 54.3 (50.6, 58.0) | 56.4 (53.1, 59.7) | 58.3 (55.5, 61.1) | 56.8 (55.1, 58.5) | 0.334 | 0.056 | 1.58 (1.45, 1.72) | |
| Non-MAFLD | 41.7 (38.9, 44.5) | 40.0 (37.1, 43.0) | 38.0 (35.8, 40.2) | 39.9 (38.7, 41.1) | 39.9 (38.7, 41.1) | 0.070 | |||
| Non-NAFLD | 42.3 (39.6, 45.1) | 39.9 (37.0, 42.9) | 38.4 (36.3, 40.5) | 40.4 (38.4, 42.4) | 40.3 (39.1, 41.5) | 0.062 | |||
| Diabetes (%) | |||||||||
| MAFLD | 24.2 (20.8, 28.1) | 22.5 (19.6, 25.6) | 23.9 (22.1, 25.8) | 26.4 (23.9, 28.9) | 24.6 (23.3, 26.0) | 0.408 | 0.163 | 0.347 | 5.73 (5.10, 6.45) |
| NAFLD | 24.3 (20.8, 28.1) | 22.9 (19.9, 26.3) | 24.2 (22.4, 26.2) | 26.4 (23.8, 29.1) | 24.8 (23.4, 26.3) | 0.415 | 0.104 | 5.10 (4.55, 5.73) | |
| Non-MAFLD | 3.5 (2.8, 4.3) | 5.3 (4.4, 6.3) | 5.6 (4.6, 6.8) | 5.6 (4.9, 6.4) | 5.1 (4.6, 5.5) | 0.020 | |||
| Non-NAFLD | 4.0 (3.3, 4.9) | 5.5 (4.7, 6.5) | 6.0 (5.0, 7.2) | 6.4 (5.7, 7.3) | 5.6 (5.2, 6.1) | 0.006 | |||
| General obesity (%) | |||||||||
| MAFLD | 64.6 (60.4, 68.6) | 71.2 (67.9, 74.4) | 67.1 (63.7, 70.3) | 74.0 (71.9, 76.1) | 70.2 (68.7, 71.7) | <0.001 | 0.914 | <0.001 | 15.32 (13.82, 16.99) |
| NAFLD | 65.4 (61.4, 69.2) | 72.1 (68.4, 75.6) | 67.9 (64.3, 71.3) | 75.0 (72.8, 77.2) | 71.1 (69.5, 72.6) | <0.001 | 0.923 | 13.65 (12.34, 15.10) | |
| Non-MAFLD | 14.8 (13.1, 16.6) | 17.5 (15.9, 19.3) | 17.1 (15.2, 19.2) | 20.2 (18.6, 21.8) | 17.7 (16.9, 18.6) | <0.001 | |||
| Non-NAFLD | 16.0 (14.4, 17.8) | 18.6 (17.0, 20.3) | 18.5 (16.6, 20.5) | 22.0 (20.3, 23.7) | 19.2 (18.3, 20.1) | <0.001 | |||
| Central obesity (%) | |||||||||
| MAFLD | 82.6 (79.1, 85.7) | 88.4 (86.1, 90.4) | 84.6 (82.1, 86.7) | 88.7 (87.0, 90.1) | 86.6 (85.5, 87.7) | 0.004 | 0.996 | 0.006 | 17.05 (15.32, 18.97) |
| NAFLD | 82.7 (78.8, 85.9) | 88.5 (86.1, 90.5) | 85.5 (83.0, 87.6) | 89.6 (88.0, 90.9) | 89.6 (88.0, 90.9) | <0.001 | 0.452 | 15.06 (13.55, 16.73) | |
| Non-MAFLD | 32.7 (30.8, 34.6) | 39.5 (36.8, 42.3) | 37.8 (35.2, 40.5) | 40.8 (38.1, 43.4) | 38.1 (36.8, 39.4) | <0.001 | |||
| Non-NAFLD | 34.0 (32.2, 35.9) | 40.8 (38.2, 43.3) | 38.9 (36.4, 41.5) | 42.3 (39.7, 45.0) | 39.4 (38.1, 40.7) | <0.001 | |||
Data are from the NHANESs between 1999 and 2016. Data are expressed as percentage (95% CI). All analyses were adjusted for age, sex, and race or ethnic group. Ptrend was calculated for each group by including a continuous variable for the midpoint of each survey period in logistic regression models; significant values indicate variation over time within MAFLD or NAFLD groups. Significant Pinteraction values indicate varying associations between prevalence estimates over time across groups. ∗MAFLD vs. NAFLD. †MAFLD vs. non-MAFLD or non-NAFLD. CI: Confidence interval; MAFLD: Metabolic associated fatty liver disease; NHANESs: National Health and Nutrition Examination Surveys; NAFLD: Non-alcoholic fatty liver disease; OR: Odds ratio.
Between 1999 to 2002 and 2011 to 2016, among those with hypertension [Figure 1], the proportion of adults with MAFLD using anti-hypertensive medications increased from 67.9% (95% CI 62.4–73) to 77.8% (74.5–80.7) (P = 0.006). A similar increase was also evident in the non-MAFLD group. Achievement of the BP-treatment target increased in both groups over time. The mean systolic BP over time verified these findings [Supplementary Table 2]. Among those with hypertension, the MAFLD group had lower systolic pressure, but the non-MAFLD group had a greater decreasing trend in systolic pressure (Pinteraction < 0.001).
Figure 1.
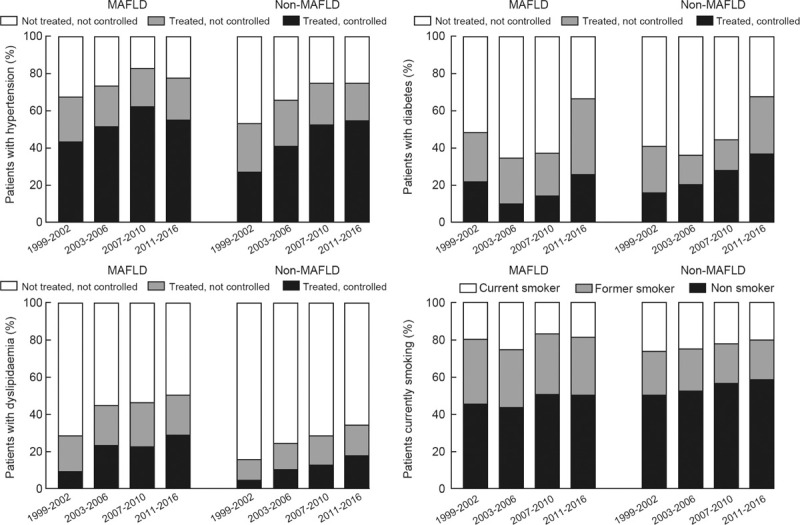
The treatment and control status of hypertension, diabetes, dyslipidemia, and smoking status in US populations with and without MAFLD. MAFLD: Metabolic associated fatty liver disease.
Among participants with dyslipidemia [Figure 1], the use of lipid-lowering medicines increased in the MAFLD group over time: 28.5% (24.4–32.9) to 50.2% (46.8–53.5). There was also a substantial increase in the proportion of LDL <100 mg/dL after treatment in the MAFLD group (9.3% [7.1–12.0] to 28.9% [25.4–32.5], P < 0.001). In the whole population and those with dyslipidemia [Supplementary Table 2], the mean LDL concentrations declined over time. However, there was still no lipid-lowering treatment in 49.8% of the population with MAFLD and dyslipidemia.
Over successive periods from 1999–2002 to 2011–2016, among eligible individuals with diabetes [Figure 1], the use of anti-diabetic medications increased in the two groups. However, in MAFLD, the proportion of reaching the control target fluctuated, and it did not significantly change between 1999 to 2002 and 2011 to 2016 (22.0–25.8% [+4.3%, 95% CI −3.1, 11.6]). Although more diabetic patients were treated, no significant decreasing trend in FPG and HbA1c was found in adults with diabetes and MAFLD [Supplementary Table 2].
The prevalence of current smokers decreased substantially between the first and last survey periods for the non-MAFLD group (26.0% [23.2–29.1] to 19.8% [17.3–22.6], P = 0.006), while there was no significant trend in the MAFLD group over time.
After adjusting for age, sex, and race or ethnic differences and excluding adults with a history of cardiovascular events, from 1999–2002 to 2011–2016, the 10-year risk of having a cardiovascular event declined for both groups, using the FRS and ASCVD risk scores within a certain age range [Table 3]. History of previous myocardial infarction and previous stroke largely remained stable over time in the MAFLD group. Compared with the non-MAFLD group, the MAFLD group has a significantly higher 10-year CVD risk and odds of myocardial infarction and stroke. Overall, a non-significant increasing trend was found in the prevalence of any CKD in both groups [Table 4]. The mean eGFR was decreasing over time in MAFLD. The MAFLD group has significantly greater odds of any CKD and ACR. We also compared the cardiorenal markers in MALFD participants with and without significant alcohol use. Participants with MAFLD and significant alcohol use were leaner and had a higher prevalence of hypertension and a greater 10-year risk of cardiovascular events as indicated by Framingham scores [Supplementary Table 3].
Table 3.
Estimates of 10-year risk of cardiovascular events, prevalence of myocardial infarction, and prevalence of strokes in US adults, by MAFLD and NAFLD status, from 1999 to 2016.
| Items | 1999–2002 | 2003–2006 | 2007–2010 | 2011–2016 | Total | Ptrend | Pinteraction | P∗ | β or OR (95% CI)† |
| 10-year risk of cardiovascular events | |||||||||
| Framingham | |||||||||
| MAFLD | 14.5 (13.5, 15.4) | 13.4 (12.6, 14.1) | 12.3 (11.5, 13.1) | 13.1 (12.3, 14.0) | 13.2 (12.8, 13,7) | 0.001 | 0.760 | 0.001 | 3.7 (3.4, 4.1) |
| NAFLD | 14.1 (13.1, 15.1) | 13.0 (12.2, 13.8) | 12.3 (11.5, 13.1) | 12.6 (11.8, 13.4) | 12.9 (12.4, 13.3) | <0.001 | 0.385 | 3.2 (2.8, 3.6) | |
| Non-MAFLD | 7.5 (7.0, 8.0) | 7.0 (6.5, 7.4) | 6.7 (6.3, 7.2) | 7.1 (6.7, 7.6) | 7.1 (6.9, 7.3) | <0.001 | |||
| Non-NAFLD | 7.9 (7.4, 8.4) | 7.3 (6.8, 7.8) | 7.0 (6.5, 7.4) | 7.7 (7.2, 8.2) | 7.5 (7.2, 7.7) | <0.001 | |||
| ASCVD | |||||||||
| MAFLD | 11.8 (10.6, 12.9) | 11.0 (10.0, 11.9) | 10.3 (9.4, 11.3) | 10.5 (9.9, 11.1) | 10.8 (10.4, 11.2) | 0.001 | 0.755 | 0.326 | 2.5 (2.2, 2.9) |
| NAFLD | 11.8 (10.5, 13.0) | 10.9 (9.8, 12.0) | 10.4 (9.4, 11.3) | 10.3 (9.7, 10.9) | 10.7 (10.3, 11.1) | <0.001 | 0.625 | 2.2 (1.8, 2.6) | |
| Non-MAFLD | 7.2 (6.7, 7.7) | 6.3 (5.8, 6.8) | 6.2 (5.7, 6.7) | 6.7 (6.2, 7.2) | 6.6 (6.4, 6.9) | <0.001 | |||
| Non-NAFLD | 7.4 (6.9, 7.9) | 6.5 (6.0, 6.9) | 6.3 (5.8, 6.8) | 7.1 (6.5, 7.6) | 6.8 (6.6, 7.1) | <0.001 | |||
| Previous myocardial infarction | |||||||||
| MAFLD | 5.4 (4.0, 7.1) | 6.3 (4.9, 8.1) | 6.1 (5.2, 7.2) | 4.9 (3.8, 6.2) | 5.5 (4.9, 6.3) | 0.297 | 0.374 | 0.360 | 1.79 (1.50, 2.13) |
| NAFLD | 5.5 (4.1, 7.4) | 6.5 (5.0, 8.4) | 6.1 (5.2, 7.1) | 5.0 (4.0, 6.2) | 5.6 (5.0, 6.3) | 0.239 | 0.316 | 1.78 (1.50, 2.11) | |
| Non-MAFLD | 2.3 (1.7, 3.1) | 2.3 (1.7, 3.1) | 2.5 (1.9, 3.3) | 2.4 (1.8, 3.2) | 2.4 (2.0, 2.7) | 0.930 | |||
| Non-NAFLD | 2.3 (1.7, 3.1) | 2.4 (1.8, 3.2) | 2.6 (2.0, 3.5) | 2.4 (1.8, 3.3) | 2.4 (2.1, 2.8) | 0.908 | |||
| Previous stroke | |||||||||
| MAFLD | 3.9 (2.6, 5.7) | 4.7 (3.6, 6.2) | 4.0 (2.9, 5.4) | 3.6 (2.9, 4.5) | 4.0 (3.4, 4.6) | 0.293 | 0.024 | 0.512 | 1.63 (1.32, 2.01) |
| NAFLD | 4.2 (2.9, 6.2) | 4.8 (3.6, 6.4) | 3.9 (2.8, 5.4) | 3.6 (2.8, 4.6) | 4.0 (3.4, 4.7) | 0.141 | 0.010 | 1.60 (1.27, 2.02) | |
| Non-MAFLD | 1.4 (1.0, 2.0) | 2.2 (1.6, 2.9) | 2.1 (1.6, 2.8) | 2.4 (1.8, 3.1) | 2.1 (1.8, 2.4) | 0.038 | |||
| Non-NAFLD | 1.3 (0.9, 1.9) | 2.2 (1.6, 3.0) | 2.2 (1.6, 3.0) | 2.5 (1.9, 3.3) | 2.1 (1.8, 2.4) | 0.022 | |||
Data are from the NHANESs between 1999 and 2016. Data are expressed as percentage (95% CI). All analyses were adjusted for age, sex, and race or ethnic group. Ten-year cardiovascular risks were calculated using Framingham and ASCVD risk scores. Ptrend was calculated for the MAFLD status group by including a continuous variable for the midpoint of each survey period in logistic regression models; significant values indicated variation over time within MAFLD groups. Significant Pinteraction values indicate varying associations between prevalence estimates over time across MAFLD groups. ∗MAFLD vs. NAFLD. †MAFLD vs. non-MAFLD or non-NAFLD. ASCVD: Atherosclerotic Cardiovascular Disease guidelines (from the American Heart Association and American College of Cardiology); CI: Confidence interval; MAFLD: Metabolic associated fatty liver disease; NHANESs: National Health and Nutrition Examination Surveys; NAFLD: Non-alcoholic fatty liver disease; OR: Odds ratio.
Table 4.
Renal markers in US adults aged 20 years and older by MAFLD and NAFLD status, from 1999 to 2016.
| Items | 1999–2002 | 2003–2006 | 2007–2010 | 2011–2016 | Total | Ptrend | Pinteraction | P∗ | β or OR (95% CI)† |
| Any CKD (%) | |||||||||
| MAFLD | 17.9 (15.4, 20.7) | 19.2 (16.2, 22.7) | 17.0 (14.9, 19.3) | 19.7 (17.6, 21.9) | 18.7 (17.5, 20.0) | 0.883 | 0.861 | 0.599 | 1.67 (1.49, 1.87) |
| NAFLD | 18.2 (15.4, 21.4) | 19.6 (16.3, 23.3) | 17.0 (14.6, 19.6) | 19.8 (17.9, 21.9) | 18.8 (17.5, 20.2) | 0.946 | 0.734 | 1.59 (1.42, 1.78) | |
| Non-MAFLD | 9.3 (8.3, 10.4) | 10.7 (9.2, 12.4) | 11.2 (10.1, 12.5) | 10.4 (9.3, 11.6) | 10.5 (9.8, 11.1) | 0.716 | |||
| Non-NAFLD | 9.5 (8.5, 10.5) | 10.9 (9.4, 12.5) | 11.5 (10.4, 12.8) | 10.8 (9.7, 12.1) | 10.7 (10.1, 11.3) | 0.572 | |||
| ACR ≥30 mg/g (%) | |||||||||
| MAFLD | 13.2 (10.9, 15.8) | 13.8 (11.5, 16.4) | 12.3 (10.4, 14.5) | 14.5 (12.7, 16.6) | 13.6 (12.5, 14.8) | 0.786 | 0.777 | 0.915 | 1.81 (1.61, 2.04) |
| NAFLD | 13.4 (11.0, 16.2) | 13.9 (11.5, 16.7) | 12.1 (10.0, 14.5) | 14.5 (12.7, 16.5) | 13.6 (12.5, 14.8) | 0.927 | 0.948 | 1.71 (1.52, 1.92) | |
| Non-MAFLD | 7.0 (6.0, 8.1) | 7.1 (5.9, 8.6) | 7.4 (6.3, 8.6) | 7.3 (6.4, 8.3) | 7.2 (6.7, 7.8) | 0.891 | |||
| Non-NAFLD | 7.0 (6.0, 8.2) | 7.3 (6.2, 8.6) | 7.7 (6.6, 8.9) | 7.6 (6.8, 8.6) | 7.4 (6.9, 8.0) | 0.835 | |||
| eGFR <60 mL/min per 1.73 m2 (%) | |||||||||
| MAFLD | 6.2 (4.9, 7.9) | 7.6 (5.7, 10.1) | 7.1 (6.0, 8.5) | 7.7 (6.6, 9.0) | 7.3 (6.6, 8.1) | 0.307 | 0.900 | 0.001 | 1.29 (1.09, 1.52) |
| NAFLD | 6.4 (5.0, 8.2) | 7.9 (5.9, 10.5) | 7.3 (6.0, 8.8) | 8.1 (6.9, 9.4) | 7.6 (6.8, 8.4) | 0.195 | 0.843 | 1.30 (1.10, 1.53) | |
| Non-MAFLD | 3.8 (3.2, 4.5) | 4.9 (4.1, 6.0) | 5.6 (4.9, 6.4) | 4.5 (3.9, 5.2) | 4.7 (4.3, 5.1) | 0.182 | |||
| Non-NAFLD | 3.8 (3.2, 4.5) | 4.9 (4.0, 5.9) | 5.5 (4.8, 6.3) | 4.5 (3.8, 5.2) | 4.6 (4.3, 5.0) | 0.208 | |||
| Mean eGFR (mL/min per 1.73 m2) | |||||||||
| MAFLD | 96.4 (94.4, 98.3) | 91.7 (89.5, 94.0) | 92.4 (91.0, 93.9) | 92.3 (91.2, 93.3) | 92.9 (92.1, 93.7) | 0.002 | 0.590 | <0.001 | 0.74 (0.11, 1.37) |
| NAFLD | 96.1 (94.0, 98.3) | 91.2 (88.9, 93.5) | 92.1 (90.6, 93.6) | 91.9 (90.8, 93.0) | 92.6 (91.7, 93.4) | 0.002 | 0.839 | 0.03 (−0.62, 0.68) | |
| Non-MAFLD | 102.3 (100.8, 103.8) | 95.4 (94.0, 96.8) | 97.6 (96.1, 99.0) | 97.4 (96.4, 98.5) | 98.1 (97.4, 98.8) | <0.001 | |||
| Non-NAFLD | 102.2 (100.7, 103.7) | 95.5 (94.1, 96.9) | 97.6 (96.1, 99.0) | 97.4 (96.4, 98.4) | 98.1 (97.4, 98.8) | <0.001 | |||
| Hyperfiltration (%) | |||||||||
| MAFLD | 3.5 (2.6, 4.8) | 0.9 (0.5, 1.8) | 0.5 (0.3, 1.0) | 1.1 (0.7, 1.9) | 1.4 (1.1, 1.8) | 0.001 | 0.414 | 0.417 | 1.75 (1.29, 2.38) |
| NAFLD | 3.6 (2.6, 5.0) | 0.9 (0.4, 1.8) | 0.5 (0.3, 1.0) | 1.2 (0.7, 2.0) | 1.4 (1.1, 1.8) | 0.003 | 0.607 | 1.67 (1.21, 2.31) | |
| Non-MAFLD | 4.3 (3.4, 5.4) | 1.5 (1.0, 2.2) | 1.7 (1.2, 2.4) | 2.0 (1.5, 2.7) | 2.3 (2.0, 2.7) | <0.001 | |||
| Non-NAFLD | 4.3 (3.4, 5.4) | 1.5 (1.0, 2.2) | 1.7 (1.2, 2.3) | 2.0 (1.5, 2.6) | 2.3 (2.0, 2.7) | <0.001 | |||
Data are from the NHANESs between 1999 and 2016. Data are expressed as percentage or mean (95% CI). Any CKD refers, urinary ACR ≥30 mg/g, eGFR <60 mL/min per 1.73 m2, or both. Hyperfiltration was defined as eGFR ≥135 mL/min per 1.73 m2. All analyses were adjusted for age, sex, and race or ethnic group. Ptrend was calculated for MAFLD or non-MAFLD group by including a continuous variable for the midpoint of each survey period in regression models. Significant Pinteraction values indicate varying associations between prevalence estimates over time across groups. ∗MAFLD vs. NAFLD. †MAFLD vs. non-MAFLD or non-NAFLD. ACR: Albumin-to-creatinine ratio; CKD: Chronic kidney disease; CI: Confidence interval; eGFR: Estimated glomerular filtration rate; MAFLD: Metabolic associated fatty liver disease; NHANESs: National Health and Nutrition Examination Surveys; NAFLD: Non-alcoholic fatty liver disease; OR: Odds ratio.
Along with the results from the MAFLD group, reciprocal results from the NAFLD group were obtained. Generally, patients with NAFLD showed similar trends to those with MAFLD in terms of cardiovascular risk factors and disease and any CKD. However, the ORs of the MAFLD group (vs. the non-MAFLD group) for general and central obesity were greater than those of the NAFLD group (vs. the non-NAFLD group). When excluding participants taking any anti-hypertensive, anti-diabetic, or lipid-lowering medication, participants with MAFLD had a significantly greater 10-year risk of cardiovascular events (as indicated by Framingham and ASCVD scores) than those with NAFLD [Supplementary Table 4]. By applying the new definition, MAFLD, we could screen more people; therefore, we further compared the characteristics of the larger population obtained using the MAFLD definition to the population obtained using the previous definition of NAFLD [Supplementary Table 5]. We can observe that the Framingham cardiovascular score of the NAFLD group is significantly lower than that of the MAFLD group.
Discussion
In the serial cross-sectional national surveys, the absolute numbers of people with MAFLD in the USA grew substantially over 18 years. By 2011 to 2016, in the USA, more than a third of adults had MAFLD, and about half of adults with MAFLD had hypertension and dyslipidemia, a quarter had diabetes, about 90% had central obesity, a fifth was smoking, about a fifth had some form of CKD, 8.5% reported a previous myocardial infarction or stroke, and the mean 10-year risk of a cardiovascular event was 13.1% (FRS) and 10.5% (a more contemporary ASCVD risk calculator). The odds of central obesity, diabetes, hypertension, and dyslipidemia in people in the MAFLD group were 17.05, 5.46, 2.90, and 1.57 times that in people in the non-MAFLD group, respectively. Our analyses also show that much work looms ahead for improvement among people with MAFLD, especially for weight management and lowering lipid and HbA1c levels, since only half of the people with dyslipidemia received treatment and a quarter of patients with diabetes mellitus were treated and controlled. Moreover, owing to a greater absolute number of patients being identified using the MAFLD definition compared to the NAFLD definition, using the MAFLD definition could lead to the early management of more patients.
Since the MAFLD definition was established, to our knowledge, this is the first study reporting the latest prevalence of MAFLD and its trends in the US population using national representative data. First, unlike a very recent article that used liver ultrasonography from NHANES III to define liver steatosis,[22] our study used the recently updated and validated US-FLI, which is an improvement over the FLI, especially in the multiethnic US population,[13] and is an important tool in epidemiological studies.[3]
Second, our data suggest that MAFLD has reached an alert level in US adults, with the potential for a major epidemic of MAFLD-related complications or outcomes, including CVD, stroke, and CKD in the USA in the near future in the absence of effective national intervention, the complications being confined not only to liver-related morbidity and mortality. Several cohort studies have shown increased CVD mortality in NAFLD patients.[23,24] Moreover, increasing attention has also been paid to NAFLD-related CKD, and a recent meta-analysis found that NAFLD was associated with an increased risk and severity of CKD.[25] Currently, the MAFLD concept includes diabetes and adiposity, which makes the casual role of MAFLD in CVD and CKD incidence and increased morbidity and mortality clearer.[16] Our study also suggests the odds of reported myocardial infarction, stroke, and any CKD in the MAFLD group, which were 75%, 58%, and 64% higher than that in the non-MAFLD group. The 10-year risk of cardiovascular events was also 3.7% (percentage points) higher in the MAFLD group. Defining MAFLD subtypes related to CVD, CKD, or liver disease may be one of the future directions, rather than being just a dichotomous state based on steatohepatitis and non-steatohepatitis MAFLD.
Third, since the MAFLD definition was released, clinicians and scientists have been discussing redefining the nosological framework for NAFLD.[26] Compared with participants with NAFLD, there was a significantly higher prevalence of hypertension and a lower prevalence of general obesity and central obesity in patients defined by MAFLD. Our findings indicate that, although NAFLD and MAFLD showed similar trends in terms of cardiovascular and renal burdens, 2 million more people had MAFLD than NAFLD in 2011 to 2016; thus, the absolute cardiovascular and renal burdens may still be greater for MAFLD patients. Moreover, participants with MAFLD may have a greater 10-year risk of cardiovascular events than those with NAFLD, especially participants receiving no medication intervention. Thus, adopting the MAFLD definition may result in more relevant patients for potential early management.
Fourth and foremost, early intervention is beneficial. In primary prevention, the most important part could be weight management, given that approximately 70% of the US population with general obesity had MAFLD in 2015 to 2016. In secondary prevention, FRS is suggested to be routinely used in MAFLD to risk-stratify patients and guide subsequent treatment of risk factors.[27] Well-established evidence confirms the significant benefit of statins in CVD.[27] However, 50% of dyslipidemia cases in MAFLD were not treated and only 29% were controlled by 2011 to 2016. Another intriguing type of medication is glucagon-like peptide-1 receptor agonist. In 2011 to 2016, more people with MAFLD and diabetes mellitus were treated but were not controlled into the target HbA1c range. As a premature consideration in NAFLD treatment,[28] GLP-1 receptor agonists could be especially appropriate in patients with both MAFLD and diabetes mellitus.[29–31] Other cutting-edge therapies such as sodium-glucose cotransporter 2 inhibitors and bariatric surgery need further exploration.[32]
The study may have important implications from the clinical and public health perspective. Our findings point to the increasing risk and burden of CVD and CKD in MAFLD patients. Given the sizable and growing number of adults with MAFLD and its potential morbidity and mortality extending beyond the liver, primary care physicians, endocrinologists, and other specialists should be aware of the long-term effect of the disease. If screening for cardiovascular and renal diseases is initiated once the MAFLD is diagnosed, we may embrace an early window of opportunity for prevention in many patients and thus help improve patient outcomes through education and early intervention.
This study had several limitations. First, considering the cross-sectional design, data could only explore secular trends but did not provide longitudinal follow-up data. Second, the application of the blood-marker equation to define liver steatosis may not be accurate enough. However, liver biopsy, the current gold standard for diagnosing hepatic steatosis, was not feasible in such a large epidemiological study. This study also had several strengths. The NHANES is a series of meticulously conducted surveys with validated and standardized methods of data collection, and the results are representative of the general population, and therefore, can be extrapolated to the entire nation. Second, comprehensive information of trends with age, sex, and race adjustment was presented including continuous, categorical, and contemporary parameters (old and new CVD risk estimator). Finally, the new US-FLI was used, which showed higher sensitivity and specificity than the FLI in the multiethnic US population.[13]
In conclusion, MAFLD and NAFLD are increasingly prevalent in US adults. Further, it is a matter of greater concern to note that a considerable number of these adults have CVD and CKD risk factors and disease, and the absolute cardiorenal burden may be greater for adults with MAFLD than those with NAFLD. These data call for early identification and risk stratification of MAFLD, close collaboration between endocrinologists and hepatologists, and application of novel medication with CVD and renal benefit. Then, we might be in a prime position to pursue a broader population of cardiometabolic health.
Funding
This study was supported by the National Natural Science Foundation of China (No. 91857117) and the Science and Technology Commission of Shanghai Municipality (Nos. 19140902400, 18410722300).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhang HJ, Wang YY, Chen C, Lu YL, Wang NJ. Cardiovascular and renal burdens of metabolic associated fatty liver disease from serial US national surveys, 1999–2016. Chin Med J 2021;134:1593–1601. doi: 10.1097/CM9.0000000000001513
Supplemental digital content is available for this article.
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, et al. Liver diseases in the Asia-Pacific region: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol 2020; 5:167–228. doi: 10.1016/S2468-1253(19)30342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020; 158:1999.e1–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 5.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015; 61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013; 10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 8.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015; 62: 1 Suppl: S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 10.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013; 382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017; 66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 12.Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol 2017; 13:297–310. doi: 10.1038/nrneph.2017.16. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015; 41:65–76. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020; 69:564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003; 42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol 2018; 6:392–403. doi: 10.1016/S2213-8587(18)30027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care 2020; 43: Suppl 1: S66–S76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 18.Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001; 285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation 2014; 129: 25 Suppl 2: S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol 2015; 10:382–389. doi: 10.2215/cjn.03080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int 2020; 40:2082–2089. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 23.Zeb I, Li D, Budoff MJ, Katz R, Lloyd-Jones D, Agatston A, et al. Nonalcoholic fatty liver disease and incident cardiac events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol 2016; 67:1965–1966. doi: 10.1016/j.jacc.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 24.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology 2018; 67:1726–1736. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001680.doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratziu V, Rinella M, Beuers U, Loomba R, Anstee QM, Harrison S, et al. The times they are a-changin’ (for NAFLD as well). J Hepatol 2020; 73:1307–1309. doi: 10.1016/j.jhep.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73:948–963. doi: 10.1016/j.jacc.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016; 387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care 2020; 43: Suppl 1: S98–S110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 31.Xian YX, Weng JP, Xu F. MAFLD vs. NAFLD: shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin Med J 2021; 134:8–19. doi: 10.1097/CM9.0000000000001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranjbar G, Mikhailidis DP, Sahebkar A. Effects of newer antidiabetic drugs on nonalcoholic fatty liver and steatohepatitis: think out of the box!. Metabolism 2019; 101:154001.doi: 10.1016/j.metabol.2019.154001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


