Supplemental Digital Content is available in the text.
Keywords: anticoagulation, bivalirudin, bleeding, extracorporeal membrane oxygenation, heparin, thrombosis
Abstract
OBJECTIVES:
Extracorporeal membrane oxygenation is a potentially life-saving intervention in refractory cardiopulmonary failure, but it requires anticoagulation to prevent circuit thromboses, which exposes the patient to hemorrhagic complications. Heparin has traditionally been the anticoagulant of choice, but the direct thrombin inhibitor bivalirudin is routinely used in cases of heparin-induced thrombocytopenia and has been suggested as a superior choice. We sought to examine the timing of hemorrhagic and thrombotic complications after extracorporeal membrane oxygenation cannulation and to compare the rates of such complications between patients anticoagulated with heparin versus bivalirudin.
DESIGN:
Retrospective cohort study.
SETTING:
Johns Hopkins Hospital patients between January 2016 and July 2019.
PATIENTS:
Adult (> 18 yr) extracorporeal membrane oxygenation patients.
INTERVENTIONS:
Patients were anticoagulated either with heparin or bivalirudin.
MEASUREMENTS AND MAIN RESULTS:
We compared rates of hemorrhagic and thrombotic complications by time on heparin versus bivalirudin and characterized the average time to each complication. Of 144 extracorporeal membrane oxygenation patients (mean age 55.3 yr; 58% male), 41% were on central venoarterial extracorporeal membrane oxygenation, 40% on peripheral venoarterial extracorporeal membrane oxygenation, and 19% on venovenous extracorporeal membrane oxygenation. Thirteen patients (9%) received bivalirudin during their extracorporeal membrane oxygenation run, due to concern for (n = 8) or diagnosis of (n = 4) heparin-induced thrombocytopenia or for heparin resistance (n = 1). The rate of hemorrhagic or thrombotic complications did not differ between heparin (0.13/d) and bivalirudin (0.06/d; p = 0.633), but patients on bivalirudin received significantly fewer blood transfusions (1.0 U of RBCs/d vs 2.9/d on heparin; p < 0.001).
CONCLUSIONS:
Our results confirm the safety and efficacy of bivalirudin as an alternative anticoagulant in extracorporeal membrane oxygenation and suggest a potential benefit in less blood product transfusion, although prospective studies are needed to evaluate the true effect of bivalirudin versus the disease processes that prompted its use in our study population.
Extracorporeal membrane oxygenation (ECMO) is a rescue therapy used in the setting of refractory respiratory and/or cardiac failure. Its use continues to increase, with nearly 16,000 runs reported to the Extracorporeal Life Support Organization (ELSO) in 2019, compared with fewer than 1,900 runs in 2000 (1). Although it can be a life-saving intervention, the extracorporeal circuit causes an inflammatory response that predisposes to thromboses, necessitating the use of anticoagulation (2). Unfortunately, the patient is then vulnerable to hemorrhagic complications (3). Hemorrhage and thrombosis are the most common complications during ECMO, both of which greatly increase its associated morbidity and mortality (1, 4–8). Although studies have examined the rates of these complications, none has specifically examined the time course of when these complications occur (4, 6, 9–13).
Unfractionated heparin is the standard ECMO anticoagulant at most institutions given its inexpensive cost, long history of use, and reversibility (14). It can, however, cause heparin-induced thrombocytopenia (HIT), an immune-mediated prothrombotic state that typically occurs 5-10 days after heparin initiation (15). Furthermore, it is not a direct anticoagulant but rather works through antithrombin III, preventing heparin from having a predictable dose-response curve (16).
Bivalirudin, on the other hand, does not work through a mediator but is a direct thrombin inhibitor that has proven efficacious as a heparin-alternative in percutaneous coronary intervention and during cardiac surgery, even with cardiopulmonary bypass (17–19). Its benefits include elimination via proteolysis, which is 80% nonorgan dependent (the remaining portion renally eliminated), a short half-life, and no risk of HIT (20).
Most evidence comparing heparin to bivalirudin for anticoagulation in ECMO derives from retrospective, single-institution series or case-control studies with small sample sizes (21–26). Most authors have reported equivalent mortality and rates of thrombotic complications between ECMO patients anticoagulated on heparin versus bivalirudin (21–25); however, when differences between the two populations are found, they usually show the bivalirudin group with lower rates of bleeding (25, 26), greater time in therapeutic range (21), and less blood product transfusion (22, 27). Given that bivalirudin is rapidly proteolytically cleaved, however, there has been concern that thromboses could occur in any location with stagnant blood, most feared being a nonejecting, unvented left ventricle (LV) (24, 28).
The purpose of this study is two-fold: first, to examine the timing of individual hemorrhagic and thrombotic complications after ECMO cannulation, and second, to examine our institutional experience with anticoagulation on ECMO, where heparin is the standard anticoagulant, with bivalirudin used primarily in cases of diagnosed or suspected HIT. We hypothesized that the rate of thrombotic complications would be equivalent in patients treated with bivalirudin, but that hemorrhagic complications would be fewer.
MATERIALS AND METHODS
After Johns Hopkins Institutional Review Board approval (IRB00167056), we analyzed all adult (> 18 yr) patients placed on ECMO at our institution between January 2016 and July 2019. Our ECMO patients are routinely anticoagulated with a heparin infusion, with a goal activated partial thromboplastic time (aPTT) of 50–65 seconds unless the clinical setting (e.g., active bleeding) dictates otherwise. The heparin infusion is titrated with a nurse-managed nomogram, whereby the initial infusion dose is based on the patient’s weight. Six hours after the infusion begins, an aPTT is again drawn, and the rate of infusion is increased if subtherapeutic (< 50 s), decreased if supratherapeutic (> 65 s), or kept constant if within goal (50–65 s). Another aPTT is drawn in 6 hours until the second consecutive aPTT is within target range, at which point the aPTT is checked daily.
When HIT is suspected clinically, both antiplatelet factor 4 (PF4) and serotonin release assay (SRA) are sent. Patients with suspected HIT receive anticoagulation with a bivalirudin infusion, which is continued if PF4 and SRA are positive or transitioned back to heparin anticoagulation if negative. At our institution, bivalirudin is titrated via a prescriber-managed nomogram, also with an aPTT target range of 50–65 seconds. The initial infusion dosage is based on creatinine clearance (CrCl). If CrCl is greater than or equal to 30 mL/min, an aPTT is checked 2 hours after initiation of bivalirudin—with no change if therapeutic, an increase if subtherapeutic, or a decrease if supratherapeutic. The first aPTT check occurs at four hours if CrCl less than 30 mL/min. If the dose is changed, the aPTT is again checked 2–4 hours later. When the aPTT is in therapeutic range on two consecutive laboratory values, it is checked every 12 hours, then it is spaced to daily after another two consecutive in-range values.
For all ECMO patients, demographics, comorbidities, and ECMO characteristics are described and presented as means with sd or medians with interquartile range. These characteristics were then compared between patients anticoagulated with heparin and those anticoagulated with bivalirudin alone or a combination of bivalirudin and heparin, using Student t test, Wilcoxon rank-sum, and chi-square testing. Characteristics were also compared between patients with confirmed HIT, those with concern for HIT but ultimately tested negative (and hence received bivalirudin while laboratory confirmation was pending) and those without HIT using analysis of variance and chi-square testing. Calculated scores include the Glasgow Coma Scale prior to ECMO cannulation (29), the Acute Physiology and Chronic Health Evaluation (APACHE) II score for estimating ICU mortality on ECMO day 1 (30), and the Sequential Organ Failure Assessment (SOFA) score, also for predicting ICU mortality and also calculated on ECMO day 1 (31).
The primary outcome is a composite rate of hemorrhagic or thrombotic complications per day on heparin versus bivalirudin. Complications were “assigned” to the anticoagulant the patient was on when the event occurred. Poisson regression is used to generate incidence rate ratios (IRRs, presented with 95% CIs), which compare the rate of complications between the two groups. Complication rates were also compared between the three groups—confirmed HIT, concern for HIT, and no HIT.
Hemorrhagic complications include surgical or cannulation site bleeding requiring RBC transfusion or an intervention, gastrointestinal bleeding, pulmonary hemorrhage, disseminated intravascular coagulation (DIC; defined as an the International Society of Thrombosis and and Hemostasis Criteria for DIC score ≥ 5 in addition to documented clinical diagnosis by an attending physician) (32), intracerebral hemorrhage (ICH), subarachnoid hematoma (SAH), subdural hematoma (SDH), as well as any hemorrhage requiring transfusion or an intervention. Thrombotic complications include limb ischemia (based on absent pulses and other findings such as coolness or mottling on examination and absent flow or demonstrated thrombus on arterial duplex), ischemic stroke, intracardiac thrombus, deep venous thromboembolism, pulmonary embolus (PE), or HIT.
Secondary outcomes included the independent rates of the specific hemorrhagic and thrombotic complications. For every complication that occurred, regardless of anticoagulant, we calculated the time from ECMO cannulation to the occurrence of the complication, determining the average time to its occurrence, both in the entire ECMO cohort as well as broken down by type of ECMO (central venoarterial, peripheral venoarterial, and venovenous). An additional secondary outcome was the number of units of blood products transfused (RBC, platelets, fresh frozen plasma [FFP], and cryoprecipitate), all calculated per day on therapy (heparin vs bivalirudin). The final secondary outcome was in-hospital mortality, compared between the heparin-only and the heparin/bivalirudin groups as well as between the HIT, concern for HIT, and no HIT groups.
Finally, in the subset of patients who received both heparin and bivalirudin, we compared their time with therapeutic aPTT, calculated from the time of initiation of anticoagulant infusion to the time of the first therapeutic aPTT (50–65 s), total number of aPTTs drawn per day of therapy, the fraction of those that were in therapeutic range, and the number of dose changes per day. For all statistical analyses, a p value of less than 0.05 is considered statistically significant. Analyses were performed using Stata Version 15.1 (StataCorp, College Station, TX).
RESULTS
A total of 144 patients were placed on ECMO with an average age of 55.3 (± 15.8) years and 57.6% (n = 83) males (Table 1). The majority (n = 116; 80.6%) were placed on venoarterial ECMO, and of venoarterial ECMO patients, half (50.9%; n = 59) were cannulated centrally. The main indications for venoarterial ECMO were cardiogenic shock (53.4%) and postcardiotomy shock (46.6%). The index operations for the 54 postcardiotomy shock patients were as follows: coronary artery bypass grafting (CABG) in 32% (n = 17), CABG plus valve replacement in 13% (n = 7), valve replacement in 11% (n = 6), aortic root replacement in 22% (n = 12), aortic dissection repair in 11% (n = 6), heart transplantation in 9% (n = 5), and septal myectomy in 2% (n = 1). Median ECMO support was 4.0 days (2.5–8.9 d) for venoarterial ECMO patients and 8.6 days (3.5–16.6 d) for venovenous ECMO patients. In-hospital mortality was 68.1% (n = 79) for venoarterial ECMO patients and 39.3% (n = 11) for venovenous ECMO patients.
TABLE 1.
Demographics, Comorbidities, and Extracorporeal Membrane Oxygenation Characteristics of all Patients, Those Who Received Heparin Anticoagulation, and Those Who Received Bivalirudin or Bivalirudin and Heparin While on Extracorporeal Membrane Oxygenation
| Demographic/Characteristics | Entire Cohort, N = 144, n (%) | Heparin Only, N = 131 (91.0%), n (%) | Heparin/Bivalirudin, N = 13 (9.0%), n (%) | p (Heparin Only vs Heparin/Bivalirudin) |
|---|---|---|---|---|
| Mean age, yr (sd) | 55.3 (15.8) | 55.9 (15.4) | 49.6 (19.1) | 0.171 |
| Male gender | 83 (57.6) | 74 (56.5) | 9 (69.2) | 0.375 |
| Race | 0.519 | |||
| Caucasian | 85 (59.0) | 75 (57.3) | 10 (76.9) | |
| African American | 33 (22.9) | 30 (22.9) | 3 (23.1) | |
| Asian | 10 (6.9) | 10 (7.6) | 0 | |
| Hispanic | 6 (4.2) | 6 (4.6) | 0 | |
| Other | 10 (6.9) | 10 (7.6) | 0 | |
| Comorbidities | ||||
| Hypertension | 95 (66.0) | 86 (65.6) | 9 (69.2) | 0.823 |
| Diabetes | 41 (28.5) | 37 (28.2) | 4 (30.8) | 0.861 |
| Congestive heart failure | 37 (25.7) | 32 (24.4) | 5 (38.5) | 0.285 |
| Chronic kidney disease | 15 (10.4) | 13 (9.9) | 2 (15.4) | 0.546 |
| Atrial fibrillation | 29 (20.1) | 25 (19.1) | 4 (30.8) | 0.332 |
| Prior intracerebral hemorrhage | 4 (2.8) | 1 (7.6) | 3 (23.1) | < 0.001 |
| Prehospital medications | ||||
| Antiplatelet therapy | 67 (46.5) | 61 (46.6) | 6 (46.2) | 0.958 |
| Anticoagulation | 33 (22.9) | 26 (19.8) | 7 (53.8) | 0.005 |
| Type of ECMO | 0.279 | |||
| Venoarterial | 116 (80.6) | 107 (81.7) | 9 (69.2) | |
| Venovenous | 28 (19.4) | 24 (18.3) | 4 (30.8) | |
| Central cannulation | 60 (41.7) | 57 (43.5) | 3 (23.1) | 0.154 |
| Duration of ECMO, d (interquartile, range) | 4.8 (2.6–10.0) | 4.0 (2.4–8.6) | 12.1 (8.9–13.2) | < 0.001 |
| Pre-ECMO mechanical circulatory support | ||||
| Intra-aortic balloon pump | 33 (22.9) | 30 (22.9) | 3 (23.1) | 0.988 |
| Left ventricular assist device | 5 (3.5) | 4 (3.5) | 1 (7.7) | 0.384 |
| Pre-ECMO characteristics | ||||
| Glasgow Coma Scale score, mean (sd) | 12 (5) | 12 (5) | 10 (6) | 0.168 |
| Lactate, mmol/L, mean (sd) | 7.3 (6.0) | 6.9 (5.2) | 12.1 (12.0) | 0.025 |
| Activated partial thromboplastic time, s, mean (sd) | 44 (31) | 43 (27) | 53 (48) | 0.288 |
| Arterial blood gas, mean (sd) | ||||
| pH | 7.26 (0.14) | 7.25 (0.14) | 7.29 (0.08) | 0.392 |
| Pco2 | 47 (19) | 47 (19) | 46 (14) | 0.809 |
| Po2 | 180 (122) | 181 (123) | 165 (114) | 0.650 |
| Hco3 | 20 (7) | 20 (7) | 21 (5) | 0.685 |
| Calculated clinical scores and lab values on ECMO day 1, mean (sd) | ||||
| Acute Physiology and Chronic Health Evaluation II score, mean (sd)a | 23 (8) | 23 (9) | 23 (4) | 0.916 |
| Sequential Organ Failure Assessment score, mean sd)b | 11 (3) | 11 (3) | 13 (3) | 0.017 |
| Platelet count (/µL) | 115 (89) | 112 (89) | 140 (91) | 0.286 |
| Fibrinogen (mg/dL) | 256 (132) | 253 (119) | 290 (248) | 0.459 |
| Hemoglobin (g/dL) | 9.4 (2.1) | 9.4 (2.1) | 9.4 (1.4) | 0.989 |
| Creatinine (mg/dL) | 1.5 (1.2) | 1.5 (1.2) | 1.7 (0.8) | 0.708 |
| Lactate (mmol/L) | 5.6 (4.6) | 5.7 (4.7) | 4.1 (3.6) | 0.226 |
| Aspartate aminotransferase (U/L) | 1,053 (2,485) | 1,035 (2,540) | 1,231 (1,949) | 0.787 |
| Lactic acid dehydrogenase (U/L) | 1,726 (2,915) | 1,724 (3,036) | 1,740 (2,205) | 0.989 |
| Need for continuous renal replacement therapy on ECMO | 75 (52.1) | 64 (48.9) | 11 (84.6) | 0.015 |
| In-hospital mortality | 90 (62.5) | 81 (61.8) | 9 (69.2) | 0.722 |
| Venoarterial ECMO | 79/116 (68.1) | |||
| Venovenous ECMO | 11/28 (39.3) | |||
ECMO = extracorporeal membrane oxygenation.
aAn Acute Physiology and Chronic Health Evaluation II score of 23 (20–24) corresponds to an approximate in-hospital nonoperative mortality of 40% or postoperative mortality of 30%.
bA Sequential Organ Failure Assessment (SOFA) score of 11 (10–11) is associated with a 50% mortality rate if initial score or a 45.8% mortality if highest score. A SOFA score of 13 (12–14) is associated with a 95% mortality if initial score or a 80% mortality if highest score. Boldface values indicate statistical significant, defined as p < 0.05.
A total of 13 patients (9%) received bivalirudin at some point during their ECMO run—one (7.7%) for heparin resistance (nontherapeutic aPTT and anti-Xa level despite up-titration of the heparin infusion and administration of antithrombin for a low antithrombin III level), four (30.8%) for diagnosed HIT, and eight (61.5%) for concern for HIT while PF4/SRA was pending but, ultimately, all of which resulted negative. Average duration of bivalirudin therapy for these patients was 5.0 (± 4.2) days. Patients (n = 13) who received bivalirudin for anticoagulation during ECMO were of similar age to those (n = 131) who received heparin (49.6 ± 19.1 vs 55.9 ± 15.4 yr; p = 0.171). They also had a similar distribution of venoarterial and venovenous ECMO (p = 0.279) and central cannulation (p = 0.154) (Table 1). The groups differed as follows: more heparin/bivalirudin patients had a history of prior ICH (23.1% vs 7.6% of heparin-only patients; p < 0.001) and were on anticoagulation prior to hospitalization (53.8% vs 19.8%; p = 0.005) for reasons such as atrial fibrillation, history of PE, mechanical valves, or mechanical circulatory support. They had longer median duration on ECMO (12.1 [8.9–13.2] vs 4.0 [2.4–8.6] in heparin-only patients; p < 0.001). Heparin/bivalirudin patients had higher average lactate pre ECMO cannulation (12.1 ± 12.0 vs 6.9 ± 5.2 mmol/L; p = 0.025), but with no difference in lactate concentration on ECMO day 1 (p = 0.226). SOFA scores on ECMO day 1 were, on average, higher in the heparin/bivalirudin patients (13 ± 3 vs 11 ± 3; p = 0.017), although APACHE II scores did not significantly differ (p = 0.916) (Table 1). Interestingly, although heparin/bivalirudin patients were more likely to require continuous renal replacement therapy (CRRT) (84.6% vs 48.9%; p = 0.015), in-hospital mortality did not differ between the two groups (69.2% vs 61.8%; p = 0.722).
Supplementary Table 1 (http://links.lww.com/CCX/A708) compares the demographics, comorbidities, and ECMO characteristics of patients without HIT (all of whom were anticoagulated with heparin), those with confirmed HIT, and those suspected of but not diagnosed with HIT (received bivalirudin while test pending, then transitioned back to heparin once negative). HIT patients had significantly higher rates of atrial fibrillation, prior ICH, and were more often on anticoagulation prior to their index hospitalization. Supplementary Table 2 (http://links.lww.com/CCX/A709) compares complications and in-hospital mortality between these three groups—HIT patients had the highest need for CRRT (100%) and highest event rate of gastrointestinal bleeding (0.5/patient), but their in-hospital mortality (75%) did not significantly differ from that of patients with concern for HIT (67%) or those without HIT (64%) (p = 0.90).
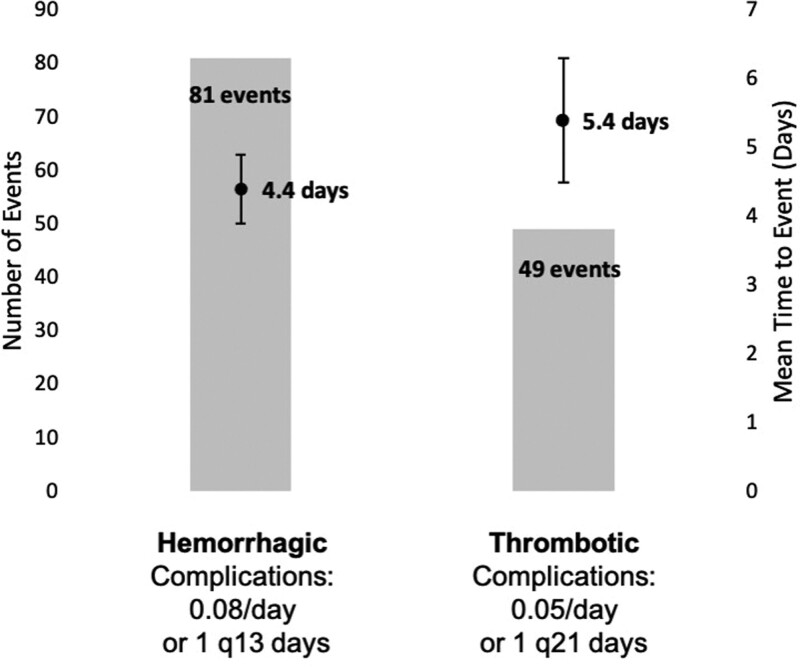
In the 144 ECMO patients, a total of 81 hemorrhagic complications occurred, with the first complication occurring on average 4.4 days from the time of cannulation. Regarding the rate of hemorrhagic complications, 0.08 hemorrhagic complications occurred per day (or one complication every 13 d) (Fig. 1). The 49 thrombotic complications occurred an average of 5.4 days after cannulation, for a rate of 0.05 per day (or one complication every 21 d). The composite rate of thrombotic and hemorrhagic complications was lower with bivalirudin, but this was not statistically significant (0.06 complications/d on bivalirudin vs 0.13/d on heparin; p = 0.130). The IRR for the occurrence of any complication on bivalirudin was 0.78 (95% CI, 0.29–2.1), meaning the incidence rate of any complication while on bivalirudin is 0.78 times the rate on heparin, although, again, not statistically significant (p = 0.633). Similarly, the IRRs for separated rates of hemorrhagic (p = 0.991) or thrombotic complications (p = 0.465) did not significantly differ (Table 2).
Figure 1.
Number of complications (hemorrhagic or thrombotic), average time to complication occurrence (in days, with error bars indicating the se of the mean), and rate of occurrence of complications per day on extracorporeal membrane oxygenation of 144 patients q = every, i.e., one hemorrhagic complication occurred for every 13 d on extracorporeal membrane oxygenation therapy..
TABLE 2.
Hemorrhagic and Thrombotic Complication Rate Per Day on Heparin and Per Day on Bivalirudin, in 144 Patients on Extracorporeal Membrane Oxygenation
| Complications | Rate Per Day on Heparin | 1 Event q x Days | Rate Per Day on Bivalirudin | 1 Event q x Days | Incidence Rate Ratio (95% CI) | p |
|---|---|---|---|---|---|---|
| All complications | 0.13 | 8 | 0.06 | 17 | 0.78 (0.29–2.1) | 0.633 |
| Hemorrhagica | 0.08 | 13 | 0.03 | 33 | 0.99 (0.24–4.0) | 0.991 |
| Thromboticb | 0.05 | 20 | 0.03 | 33 | 0.59 (0.14–2.4) | 0.465 |
| Complications after 48 hr on extracorporeal membrane oxygenation | ||||||
| Rate Per Day on Heparin | 1 Event q x Days | Rate Per Day on Bivalirudin | 1 Event q x Days | p | ||
| All complications | 0.10 | 10 | 0.08 | 13 | 0.200 | |
| Hemorrhagica | 0.06 | 16 | 0.05 | 20 | 0.083 | |
| Thromboticb | 0.04 | 27 | 0.03 | 33 | 0.140 | |
q = every, i.e., one complication event occurred for every 8 d on heparin. aHemorrhagic complications include surgical or cannulation site bleeding requiring RBC transfusion or intervention, gastrointestinal bleeding, pulmonary hemorrhage, disseminated intravascular coagulation, intracerebral hemorrhage, subarachnoid hematoma, subdural hematoma, or other hemorrhage requiring transfusion or intervention.
bThrombotic complications include limb ischemia, ischemic stroke, intracardiac thrombus, deep venous thromboembolism, pulmonary embolus, or heparin-induced thrombocytopenia.
The complication rates are also presented as one complication occurs every x days on therapy. The incidence rate ratio, calculated using Poisson regression, compares the incidence rate of the complication while on bivalirudin with the incidence rate while on heparin. Also shown is the rates of only those complications that occurred after 48 hr on extracorporeal membrane oxygenation.
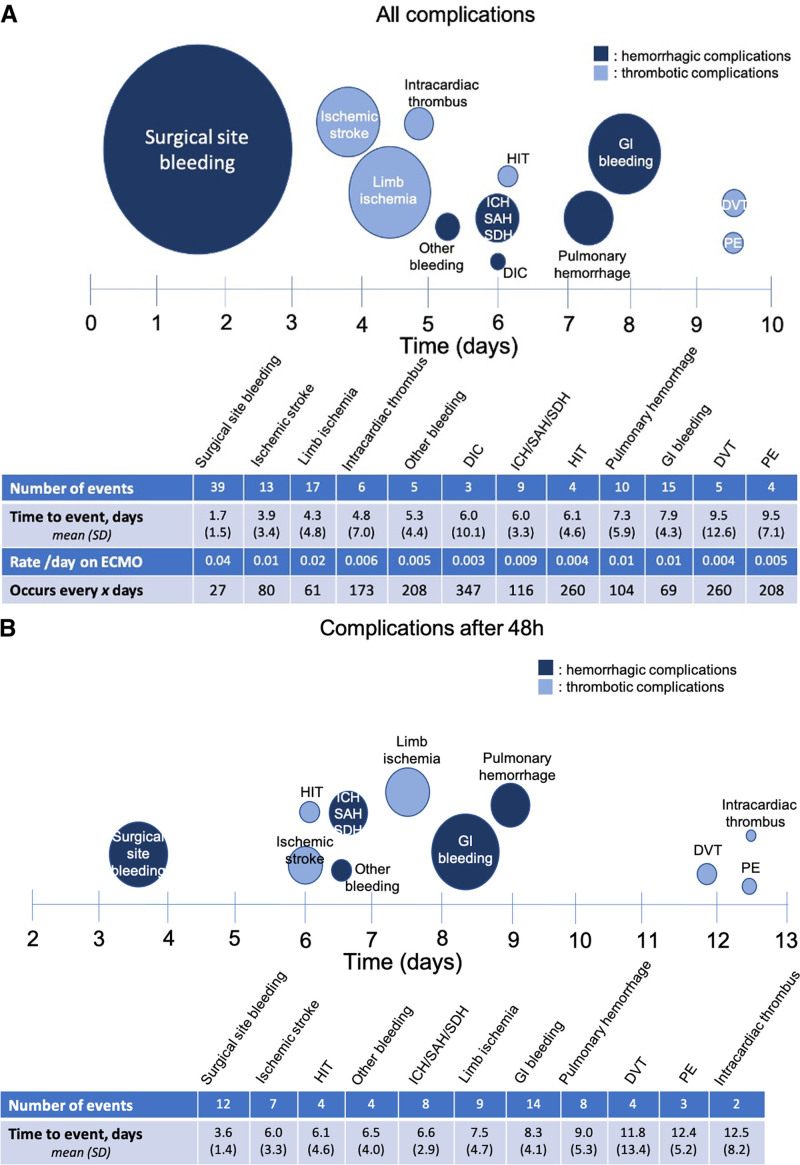
Figure 2A details the individual complications that occurred in the entire cohort of 144 patients, along with the average time post cannulation that they occurred. Surgical site bleeding was the most common complication, with 39 occurrences for a rate of 0.04 surgical site bleeding episodes requiring RBC transfusion or intervention every day on ECMO therapy. It was also the earliest complication, occurring, on average, 1.7 (± 1.5) days post cannulation. Given the large number of surgical site bleeds within 48 hours post cannulation and the general tumultuousness of the pericannulation period, we performed a subanalysis, assessing only complications that occurred 48 hours after ECMO (Fig. 2B). Two days or more after cannulation, the composite complication rate was 0.08/d on bivalirudin versus 0.10/d on heparin, which was also not statistically significant (p = 0.20) (Table 2). We performed an additional sensitivity analysis, removing limb ischemia as a thrombotic complication, given that limb ischemia was provoked by vessel instrumentation such as the ECMO cannula, an intra-aortic balloon pump (IABP), or an arterial catheter in the majority (88%; n = 15/17). Excluding limb ischemia as a complication, the composite rate of hemorrhagic and thrombotic complications still did not differ between heparin-treated and bivalirudin-treated patients (IRR, 0.60; 95% CI, 0.2–1.9; p = 0.389) nor did the rate of thrombotic complications alone (IRR, 0.54; 95% CI, 0.07–3.9; p = 0.543).
Figure 2.
A, Number of events and average time to occurrence of individual complications in all extracorporeal membrane oxygenation (ECMO) patients (n = 144), and (B) number of events and average time to occurrence of complications after 48 hr (excluding any complication that occurred before that time point). The number of events for each complication is listed in the table and is proportional to the size of the circle on the timeline. The center of the circle along the timeline indicates the average time to occurrence of that complication, with sd indicated in the table. Navy blue circles indicate hemorrhagic complications, and light blue circles indicate thrombotic complications. Other bleeding includes retroperitoneal hematomas (n = 3), rectus hematoma (n = 1), and an extremity hematoma with compartment syndrome (n = 1). DIC = disseminated intravascular coagulation, DVT = deep venous thromboembolism, GI = gastrointestinal, HIT = heparin-induced thrombocytopenia, ICH = intracerebral hemorrhage, PE = pulmonary embolus, SAH = subarachnoid hematoma, SDH = subdural hematoma.
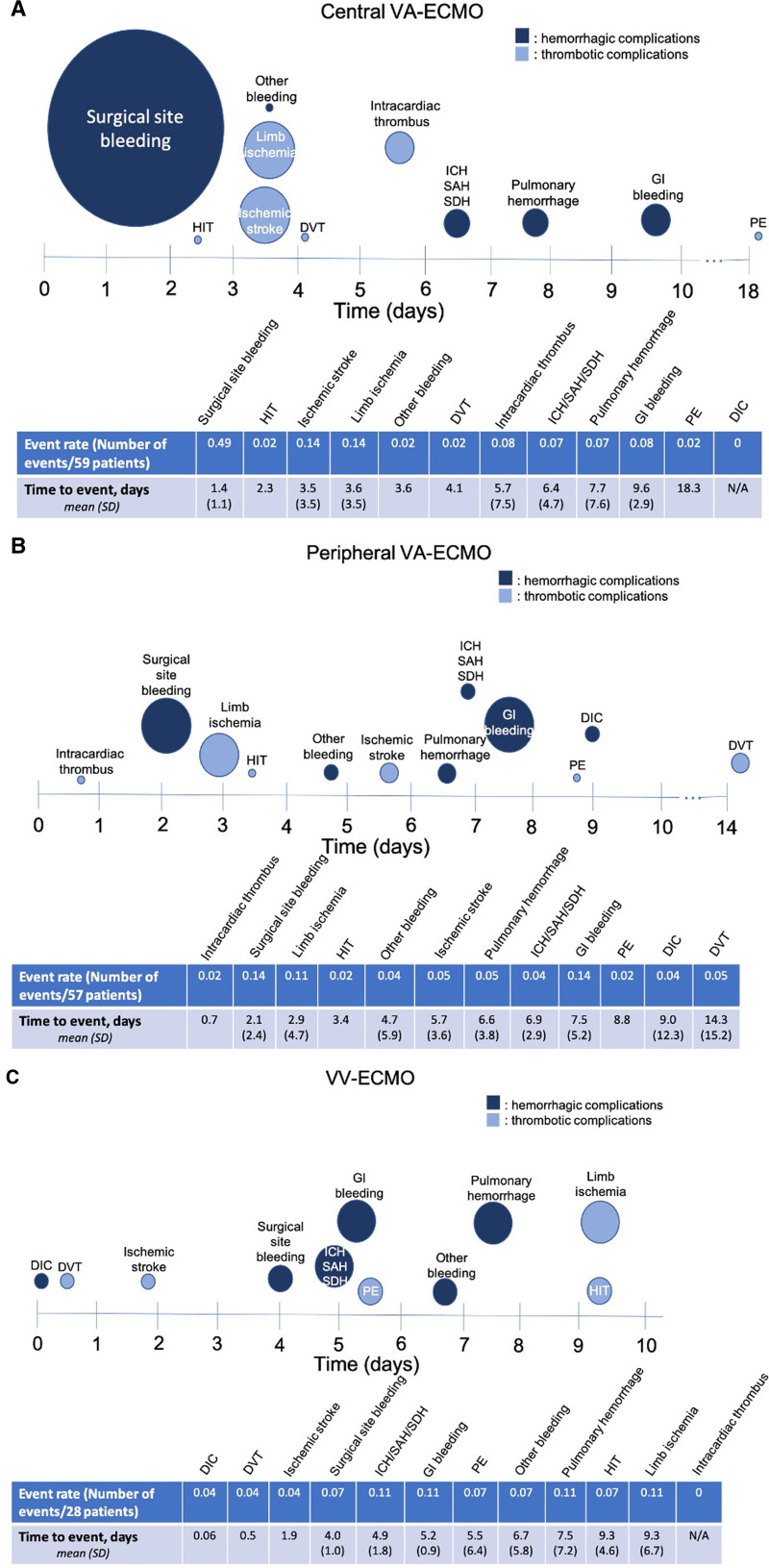
Figure 3 shows event rates of complications broken down by the type of ECMO. The 59 centrally cannulated venoarterial ECMO patients most commonly suffered surgical site bleeding, with a rate of 0.49 events per patient, on average 1.4 (± 1.1) days post cannulation (Fig. 3A). The 57 patients cannulated peripherally for venoarterial ECMO most commonly suffered surgical site bleeding and gastrointestinal bleeding, each with a rate of 0.14 events per patient, with surgical site bleeding and gastrointestinal bleeding occurring, on average, 2.1 (± 2.4) and 7.5 (± 5.2) days post cannulation, respectively (Fig. 3B). In the 28 venovenous ECMO patients, ICH/SAH/SDH, gastrointestinal bleeding, pulmonary hemorrhage, and limb ischemia occurred with equal frequency, at a rate of 0.11 event per patient (Fig. 3C).
Figure 3.
Event rate (calculated as the number of events/number of patients; the size of the circle on the timeline is proportional to the event rate) and average time to occurrence (the mean is at the center of each circle on the timeline; sd is provided in the table) of complications in (A) centrally cannulated venoarterial extracorporeal membrane oxygenation (VA-ECMO) (n = 59), (B) peripherally cannulated VA-ECMO (n = 57), and (C) venovenous extracorporeal membrane oxygenation (VV-ECMO) (n = 28) patients. Navy blue circles indicate hemorrhagic complications, and light blue circles indicate thrombotic complications. DIC = disseminated intravascular coagulation, DVT = deep venous thromboembolism, GI = gastrointestinal, HIT = heparin-induced thrombocytopenia, ICH = intracerebral hemorrhage, PE = pulmonary embolus, SAH = subarachnoid hematoma, SDH = subdural hematoma.
Per day on therapy, ECMO patients anticoagulated on heparin received significantly more blood products than those anticoagulated on bivalirudin (Table 3). Specifically, they received more RBC (2.9 vs 1.0 U/d; p < 0.001), platelets (0.7 vs 0.2 U/d; p < 0.001), FFP (1.6 vs 0.2 U/d; p < 0.001), and cryoprecipitate (0.2 vs 0.05 U/d; p = 0.004).
TABLE 3.
Blood Product Utilization Per Day on Either Heparin or Bivalirudin Anticoagulation in Extracorporeal Membrane Oxygenation Patients
| Blood Product Type | No. of Units Transfused, Per Day on Therapy | ||
|---|---|---|---|
| Heparin | Bivalirudin | p | |
| RBC | 2.9 | 1.0 | < 0.001 |
| Platelets | 0.7 | 0.2 | < 0.001 |
| Fresh frozen plasma | 1.6 | 0.2 | < 0.001 |
| Cryoprecipitate | 0.2 | 0.05 | 0.004 |
Boldface values indicate statistical significant, defined as p < 0.05.
Supplementary Table 3 (http://links.lww.com/CCX/A710) compares the aPTTs and dose changes of heparin versus bivalirudin in the 10 patients who received both. Three patients in the heparin/bivalirudin group were excluded from this analysis for receiving bivalirudin only. Time to therapeutic aPTT was only calculated for the subset of patients who began the anticoagulant infusion not yet in therapeutic range—in these patients, time to therapeutic aPTT did not differ between heparin and bivalirudin (p = 0.58). The total number of aPTTs drawn per day (p = 0.30), the percentage within therapeutic range (p = 0.88), and the number of dose changes per day (p = 0.19) also did not differ between heparin and bivalirudin.
DISCUSSION
In our cohort of 144 ECMO patients, consisting of 41% central venoarterial ECMO, 40% peripheral venoarterial ECMO, and 19% venovenous ECMO, 9% of patients received bivalirudin for anticoagulation during their ECMO run. In-hospital mortality did not significantly differ between these patients (69%) and those anticoagulated with heparin alone (62%) despite the heparin/bivalirudin patients being overall sicker than the heparin-only patients, including higher rates of prior ICH, longer median duration on ECMO, higher ECMO day one SOFA scores, and a greater need for CRRT while on ECMO. These findings were reinforced when comparing patients with confirmed HIT to those without and those suspected of but not diagnosed with HIT—with HIT patients having higher rates of comorbidities and need for CRRT but with no significant increase in in-hospital mortality. In all patients, hemorrhagic complications occurred more frequently (0.08/d) and earlier (average 4.4 d post cannulation) than thrombotic complications (rate 0.05/d, mean 5.3 d post cannulation). Surgical site bleeding was the most common and earliest complication, occurring most frequently in centrally cannulated venoarterial ECMO patients. The rates of hemorrhagic and thrombotic complications per day on ECMO did not significantly differ between heparin and bivalirudin anticoagulation, nor did the percentage of therapeutic aPTT laboratory values; however, blood product transfusion was significantly lower in patients on bivalirudin.
Overall, our 144 patients experienced 0.08 hemorrhagic complications per day (or one every 13 d on ECMO) and 0.05 thrombotic complications per day (or one every 21 d). This agrees with previous literature suggesting that bleeding events occur approximately twice as commonly as thrombotic events (6). Bleeding events occurred earlier, on average 4.4 days post cannulation, a full day earlier than thrombotic events, which occurred on average 5.3 days post cannulation. To help guide the clinician caring for a patient on ECMO, we further characterized the frequency and timing of specific complications. In all patients, surgical site bleeding was the most common complication (0.04 events/d on ECMO) and also occurred earliest, at a mean of 1.7 days post cannulation. Not surprisingly, it was most common in centrally cannulated venoarterial ECMO patients (rate of 0.49 events/patient vs 0.14 in peripherally cannulated and 0.07 in venovenous ECMO). Chung et al (6), in their analysis of nearly 12,000 adult ECMO patients from the ELSO database, found central cannulation to be a risk factor for bleeding. Limb ischemia was the second most common complication, at a rate of 0.02 events/d, on average 4 days post cannulation. Rates were more similar across configurations—0.14/patient in central venoarterial ECMO, 0.11 in peripheral venoarterial, and 0.11 in venovenous. Notably, it occurred earlier in venoarterial ECMO patients (3.6 d post cannulation for central, 2.9 d for peripheral) than in venovenous patients (9.3 d). Also notably, limb ischemia was associated with vessel instrumentation (e.g., ECMO cannula, IABP, arterial catheter) in all but two patients.
Comparing the anticoagulant groups, the heparin/bivalirudin cohort was significantly more ill than the heparin-only cohort by several markers, including higher rates of prior ICH, longer median duration on ECMO, higher average precannulation lactate and ECMO day 1 SOFA score, and a greater need for CRRT. This is not particularly surprising when one considers our institutional ECMO anticoagulation protocol—with heparin being the standard and bivalirudin being reserved for suspected or confirmed HIT. This was born out when we compared HIT patients with those without and those with suspected but not diagnosed HIT. Even with these comorbid differences, we found that the rate of any complication per day on bivalirudin (0.06 events/d), although lower in absolute terms, did not significantly differ from the rate on heparin (0.13 events/d) with a nonsignificant IRR of 0.78, nor did the specific rates of hemorrhagic or thrombotic complications alone. Our findings of equivalent mortality and rates of thrombotic complications between heparin and bivalirudin reinforce findings from previous case series and the notion that, at the very least, bivalirudin is a safe alternative to heparin for anticoagulation during ECMO (21–25).
The clinically important question—not universally agreed upon in the literature—is whether bivalirudin offers any incremental benefit above heparin. When we consider the deleterious effect on mortality that any hemorrhagic or thrombotic complication during ECMO has, the importance of preventing thromboembolism while minimizing bleeding risk becomes clear (6). Although our rates of hemorrhagic complications did not statistically significantly differ (0.03/d on bivalirudin vs 0.08/d on heparin), transfusion of all blood products—RBC, platelets, FFP, and cryoprecipitate—was significantly lower in patients on bivalirudin. This suggests that although there was no significant difference in the number of hemorrhagic complications, there was a difference in the severity of the hemorrhage.
We hypothesize that the less severe hemorrhage during bivalirudin anticoagulation—as indicated by lower blood product transfusion—is attributable to the difference in metabolism of the two anticoagulants. Bivalirudin’s rapid proteolytic cleavage has the advantages of a shorter half-life and less organ dependence. This rapid cleavage may be responsible for the finding of less severe bleeding, that is, an extravascular hemorrhage with bivalirudin clots, whereas one with heparin does not, leading to poorer hemostasis and more blood product transfusions. This mechanism for inactivation has led to concern about increased thrombus risk in intravascular areas of stagnant blood, such as with a poorly or nonejecting LV (20, 24, 28). Notably in our cohort, the six episodes of intracardiac thrombus all occurred in the heparin-only group, with no episodes in the bivalirudin group (0 thrombi in 13 patients), although not significant given our small sample size (p = 0.43).
The potential deleterious effects of blood product transfusion are well known, with greater volume of RBC transfusion independently associated with increased mortality in ECMO patients (33, 34). Hence, our finding of reduced blood product transfusion in patients anticoagulated with bivalirudin may signal a potential benefit. Importantly, however, since bivalirudin was only used in our cohort for patients with diagnosed or suspected HIT, we are unable to conclude if lower transfusion was attributable to the anticoagulant, the underlying HIT, or other underlying conditions. Ranucci et al (22) similarly reported fewer transfusions of both platelets and FFP in 13 postcardiotomy ECMO patients anticoagulated on bivalirudin, compared with eight patients who received heparin, as did Rivosecchi et al (27) (for RBCs, FFP, and platelets) in 133 bivalirudin-treated patients on venovenous ECMO (vs 162 on heparin).
Our study is limited by its single-institution, retrospective nature and particularly by the small sample size of bivalirudin patients. Only three patients received bivalirudin alone for anticoagulation during their ECMO course, whereas the remaining ten received a combination of both heparin and bivalirudin. We hence analyzed rates of complications and transfusions by duration of time on therapy, but this crossing-over between anticoagulants remains a limitation. The 10 patients who received both anticoagulants did, however, allow for a head-to-head comparison of therapeutic time between heparin and bivalirudin. We found that the time to therapeutic range, the number of aPTT labs drawn per day, the percentage that were therapeutic, and the required dose changes per day did not significantly differ between heparin and bivalirudin. This finding agrees with those reported by Berei et al (24) in their retrospective review of 72 ECMO patients but disagrees with those of Kaseer et al (21), who found more time in therapeutic range with bivalirudin versus heparin in 52 adult patients, and Pieri et al (23), who reported more dosing adjustments in the heparin-treated patients of a case-control study.
In conclusion, our study suggests equivalent rates of hemorrhagic and thrombotic complications using bivalirudin anticoagulation versus heparin in adult ECMO patients, with significantly less blood product utilization with bivalirudin, even despite bivalirudin patients being overall sicker. Although our study supports the safe use of bivalirudin as an alternative anticoagulant, it is limited by its retrospective, single-institution design with small sample sizes and points to the need for a prospective randomized study comparing the relative cost and benefits of heparin versus bivalirudin for anticoagulation in the critically ill ECMO patient population.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.Extracorporeal Life Support Organization. ECMO and ECLS > Registry > Statistics > International Summary [Internet]. Available at: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx. Accessed February 16, 2021
- 2.Millar JE, Fanning JP, McDonald CI, et al. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit Care 2016; 20:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 2009; 13:154–175 [DOI] [PubMed] [Google Scholar]
- 4.Huang M, Ong BH, Hoo AEE, et al. Prognostic factors for survival after extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J 2020; 66:141–145 [DOI] [PubMed] [Google Scholar]
- 5.Lorusso R, Barili F, Mauro MD, et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: Results from the extracorporeal life support organization registry. Crit Care Med 2016; 44:e964–e972 [DOI] [PubMed] [Google Scholar]
- 6.Chung M, Cabezas FR, Nunez JI, et al. Hemocompatibility-related adverse events and survival on venoarterial extracorporeal life support. JACC Heart Fail 2020;8:892–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho SM, Canner J, Chiarini G, et al. Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: Results from the extracorporeal life support organization registry. Crit Care Med 2020; 48:e897–e905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho SM, Canner J, Caturegli G, et al. Risk factors of ischemic and hemorrhagic strokes during venovenous extracorporeal membrane oxygenation: Analysis of data from the extracorporeal life support organization registry. Crit Care Med 2021; 49:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray BW, Haft JW, Hirsch JC, et al. Extracorporeal life support: Experience with 2,000 patients. ASAIO J 2015; 61:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzeffi M, Greenwood J, Tanaka K, et al. Bleeding, transfusion, and mortality on extracorporeal life support: ECLS working group on thrombosis and hemostasis. Ann Thorac Surg 2016; 101:682–689 [DOI] [PubMed] [Google Scholar]
- 11.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014; 97:610–616 [DOI] [PubMed] [Google Scholar]
- 12.Sklar MC, Sy E, Lequier L, et al. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. A systematic review. Ann Am Thorac Soc 2016; 13:2242–2250 [DOI] [PubMed] [Google Scholar]
- 13.Parzy G, Daviet F, Persico N, et al. Prevalence and risk factors for thrombotic complications following venovenous extracorporeal membrane oxygenation: A CT scan study. Crit Care Med 2020; 48:192–199 [DOI] [PubMed] [Google Scholar]
- 14.Sanfilippo F, Asmussen S, Maybauer DM, et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: A systematic review. J Intensive Care Med 2017; 32:312–319 [DOI] [PubMed] [Google Scholar]
- 15.Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med 2015; 373:1883–1884 [DOI] [PubMed] [Google Scholar]
- 16.Hirsh J. Heparin. N Engl J Med 1991; 324:1565–1574 [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Witzenbichler B, Guagliumi G, et al. ; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008; 358:2218–2230 [DOI] [PubMed] [Google Scholar]
- 18.Lincoff AM, Bittl JA, Harrington RA, et al. ; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863 [DOI] [PubMed] [Google Scholar]
- 19.Dyke CM, Smedira NG, Koster A, et al. A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: The EVOLUTION-ON study. J Thorac Cardiovasc Surg 2006; 131:533–539 [DOI] [PubMed] [Google Scholar]
- 20.Warkentin TE, Greinacher A, Koster A. Bivalirudin. Thromb Haemost 2008; 99:830–839 [DOI] [PubMed] [Google Scholar]
- 21.Kaseer H, Soto-Arenall M, Sanghavi D, et al. Heparin vs bivalirudin anticoagulation for extracorporeal membrane oxygenation. J Card Surg 2020; 35:779–786 [DOI] [PubMed] [Google Scholar]
- 22.Ranucci M, Ballotta A, Kandil H, et al. ; Surgical and Clinical Outcome Research Group. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care 2011; 15:R275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieri M, Agracheva N, Bonaveglio E, et al. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: A case-control study. J Cardiothorac Vasc Anesth 2013; 27:30–34 [DOI] [PubMed] [Google Scholar]
- 24.Berei TJ, Lillyblad MP, Wilson KJ, et al. Evaluation of systemic heparin versus bivalirudin in adult patients supported by extracorporeal membrane oxygenation. ASAIO J 2018; 64:623–629 [DOI] [PubMed] [Google Scholar]
- 25.Hamzah M, Jarden AM, Ezetendu C, et al. Evaluation of bivalirudin as an alternative to heparin for systemic anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med 2020; 21:827–834 [DOI] [PubMed] [Google Scholar]
- 26.Brown MA, Najam F, Pocock ES, et al. A comparison of bivalirudin and heparin for patients on extracorporeal membrane oxygenation. Thromb Res 2020; 190:76–78 [DOI] [PubMed] [Google Scholar]
- 27.Rivosecchi RM, Arakelians AR, Ryan J, et al. Comparison of anticoagulation strategies in patients requiring venovenous extracorporeal membrane oxygenation: Heparin versus bivalirudin. Crit Care Med 2021; 49:1129–1136 [DOI] [PubMed] [Google Scholar]
- 28.Ranucci M. Bivalirudin and post-cardiotomy ECMO: A word of caution. Crit Care 2012; 16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84 [DOI] [PubMed] [Google Scholar]
- 30.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med 1985; 13:818–829 [PubMed] [Google Scholar]
- 31.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 32.Taylor FB, Jr, Toh CH, Hoots WK, et al. ; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001; 86:1327–1330 [PubMed] [Google Scholar]
- 33.Smith A, Hardison D, Bridges B, et al. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion 2013; 28:54–60 [DOI] [PubMed] [Google Scholar]
- 34.Chen FT, Chen SW, Wu VC, et al. Impact of massive blood transfusion during adult extracorporeal membrane oxygenation support on long-term outcomes: A nationwide cohort study in Taiwan. BMJ Open 2020; 10:e035486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.