PURPOSE
We report the first 5-year follow-up of any first-line phase III immunotherapy trial for non–small-cell lung cancer (NSCLC). KEYNOTE-024 (ClinicalTrials.gov identifier: NCT02142738) is an open-label, randomized controlled trial of pembrolizumab compared with platinum-based chemotherapy in patients with previously untreated NSCLC with a programmed death ligand-1 (PD-L1) tumor proportion score of at least 50% and no sensitizing EGFR or ALK alterations. Previous analyses showed pembrolizumab significantly improved progression-free survival and overall survival (OS).
METHODS
Eligible patients were randomly assigned (1:1) to pembrolizumab (200 mg once every 3 weeks for up to 35 cycles) or platinum-based chemotherapy. Patients in the chemotherapy group with progressive disease could cross over to pembrolizumab. The primary end point was progression-free survival; OS was a secondary end point.
RESULTS
Three hundred five patients were randomly assigned: 154 to pembrolizumab and 151 to chemotherapy. Median (range) time from randomization to data cutoff (June 1, 2020) was 59.9 (55.1-68.4) months. Among patients initially assigned to chemotherapy, 99 received subsequent anti–PD-1 or PD-L1 therapy, representing a 66.0% effective crossover rate. Median OS was 26.3 months (95% CI, 18.3 to 40.4) for pembrolizumab and 13.4 months (9.4-18.3) for chemotherapy (hazard ratio, 0.62; 95% CI, 0.48 to 0.81). Kaplan-Meier estimates of the 5-year OS rate were 31.9% for the pembrolizumab group and 16.3% for the chemotherapy group. Thirty-nine patients received 35 cycles (ie, approximately 2 years) of pembrolizumab, 82.1% of whom were still alive at data cutoff (approximately 5 years). Toxicity did not increase with longer treatment exposure.
CONCLUSION
Pembrolizumab provides a durable, clinically meaningful long-term OS benefit versus chemotherapy as first-line therapy for metastatic NSCLC with PD-L1 tumor proportion score of at least 50%.
INTRODUCTION
Historically, patients with advanced non–small-cell lung cancer (NSCLC) have poor prognosis despite use of platinum-based chemotherapy. For patients with distant metastases, the 5-year relative survival rate is low globally, being only 6.9% in the United States between 2010 and 2016.1,2 Immunotherapy has altered the treatment approach for patients with NSCLC without oncogenic driver mutations.3 Pembrolizumab, a monoclonal antibody against programmed death 1 (PD-1), has been shown in randomized controlled studies to improve overall survival (OS) versus platinum-based chemotherapy for patients with advanced or metastatic NSCLC without sensitizing EGFR or ALK alterations when administered as monotherapy (in patients with programmed death ligand-1 [PD-L1]–positive NSCLC)4,5 and when administered in combination with chemotherapy (regardless of tumor PD-L1 expression).6-9
CONTEXT
Key Objective
Patients with metastatic non–small-cell lung cancer (NSCLC) have a poor prognosis. We examined whether first-line treatment with pembrolizumab, an anti–programmed death 1 antibody, continued to provide improved long-term outcomes over platinum-based chemotherapy in patients with programmed death ligand-1 (PD-L1) tumor proportion score (TPS) ≥ 50%, without EGFR or ALK alterations.
Knowledge Generated
Patients treated with pembrolizumab had improved 5-year overall survival rates compared with patients treated with platinum-based chemotherapy (31.9% v 16.3%).
Relevance
Pembrolizumab continues to provide long-term improved patient outcomes over chemotherapy for patients with metastatic NSCLC with PD-L1 TPS ≥ 50% in the first-line treatment setting.
KEYNOTE-024 is a randomized, open-label, phase III trial of pembrolizumab monotherapy versus platinum-based chemotherapy in patients with previously untreated NSCLC with PD-L1 tumor proportion score (TPS) of at least 50% and was, to our knowledge, the first phase III study to show superiority of immunotherapy over platinum-based chemotherapy in the first-line setting.10 The study demonstrated significantly improved progression-free survival (PFS) (hazard ratio [HR], 0.50; 95% CI, 0.37 to 0.68; P < .001) and OS (HR, 0.60; 95% CI, 0.41 to 0.89; P = .005) with pembrolizumab versus chemotherapy at the second interim analysis.10 OS continued to be improved for pembrolizumab versus chemotherapy (HR, 0.63; 95% CI, 0.47 to 0.86) in subsequent analyses.4
Five-year survival is an important landmark in cancer treatment, but until recently there have been limited data with this length of follow-up in patients with metastatic NSCLC, in part because so few patients are alive at 5 years. In KEYNOTE-001, a single-arm phase Ib study that evaluated pembrolizumab monotherapy in patients with advanced NSCLC, the 5-year OS rate was 29.6% (95% CI, 7.7 to 56.1) among previously untreated patients with PD-L1 TPS of at least 50%.11 We report 5-year efficacy and safety outcomes from KEYNOTE-024.
METHODS
Patients
As previously described,4,10 eligible patients were ≥ 18 years with previously untreated stage IV NSCLC with PD-L1 TPS of at least 50%, measurable disease by RECIST v1.1, Eastern Cooperative Oncology Group performance status of 0 or 1, and life expectancy of at least 3 months.4,10 Patients with sensitizing EGFR or ALK alterations, untreated brain metastases, or active autoimmune disease requiring systemic treatment or receiving systemic glucocorticoids or immunosuppressive therapy were excluded.
The Protocol (online only) was approved by institutional review boards or independent ethics committees at participating institutions. Patients provided written informed consent.
Study Design
Patients were randomly assigned (1:1) to receive 200 mg of intravenous pembrolizumab once every 3 weeks for 35 cycles or investigator's choice of platinum chemotherapy (carboplatin/cisplatin) plus pemetrexed or gemcitabine, or carboplatin plus paclitaxel for 4 to 6 cycles.10 Pemetrexed was permitted only for patients with nonsquamous tumors and could be administered as maintenance therapy. Random assignment was stratified by Eastern Cooperative Oncology Group performance status (0 or 1), histology (squamous or nonsquamous), and region (East Asian or non–East Asian enrollment). Treatment continued for the specified number of cycles until progressive disease (PD; per RECIST v1.1), adverse events (AEs) of unacceptable severity, or patient withdrawal. Patients with PD were permitted to continue study treatment if the investigator determined the patient to be clinically stable and deriving benefit. Patients in the chemotherapy group could cross over to receive pembrolizumab 30 days after the last chemotherapy dose if safety eligibility criteria were met per the study Protocol: patients with PD confirmed by blinded, independent central review (BICR) were eligible to cross over before the second interim analysis; after the second interim analysis, investigator-assessed PD was required.10
Patients were eligible for a second course of pembrolizumab (up to 17 cycles) if PD occurred after completion of 35 cycles of pembrolizumab or after attaining confirmed complete response (CR) with at least 6 months of treatment and an additional two cycles of pembrolizumab after CR was achieved and anticancer therapy had not been administered since their last dose of pembrolizumab and Protocol-specified eligibility criteria were met (see the Protocol).
The primary end point was PFS (time from randomization to disease progression or death from any cause, whichever occurred first, per RECIST v1.1 by BICR). Secondary end points included evaluation of OS (time from randomization to death from any cause), objective response rate (ORR) per RECIST v1.1 by BICR, safety, and tolerability. Both PFS and ORR are reported by investigator review because evaluation by BICR was discontinued after the second interim analysis (Protocol amendment 8). The exploratory end point PFS2, per RECIST v1.1 by investigator, was defined as time from randomization to subsequent disease progression after initiation of new anticancer therapy or death from any cause.
Assessments
PD-L1 expression levels and AEs were assessed as previously described.10 Imaging was performed every 9 weeks during the treatment phase (including during second-course treatment) and every 3 months during the follow-up phase, and patients were contacted every 2 months during survival follow-up.
Statistical Analysis
Efficacy was assessed in the intention-to-treat population and safety in the as-treated population (patients who were randomly assigned and received at least one dose of study treatment, analyzed by treatment received). The study Protocol provided for one planned analysis of ORR and PFS and two planned analyses of OS. Exploratory analyses included outcomes in patients who completed 35 cycles of pembrolizumab and patients who received a second course of pembrolizumab. Superiority of pembrolizumab over chemotherapy for PFS and OS was determined during the second interim analysis.10 The prespecified alpha was used at the second interim analysis. No alpha was assigned to the current analysis. The Kaplan-Meier method was used to estimate OS, PFS, and PFS2 as previously described.4,10 A stratified Cox model with Efron's method of tie handling was used to determine HRs and 95% CIs; randomization stratification factors were applied to the Cox models.
RESULTS
Patient Characteristics and Treatment
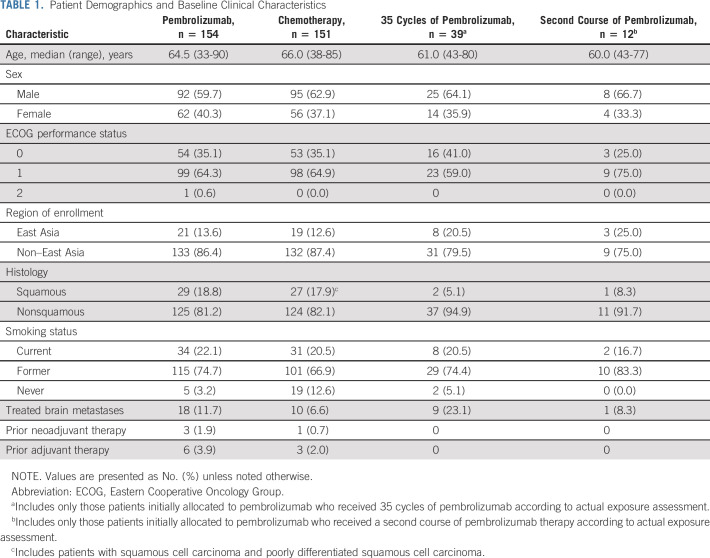
A total of 305 patients were enrolled and randomly assigned to receive pembrolizumab monotherapy (n = 154) or chemotherapy (n = 151; Fig 1). All patients except one in the chemotherapy group received study treatment. Median time from randomization to data cutoff (June 1, 2020) was 59.9 months (range, 55.1-68.4 months). Median treatment durations were 7.9 months (range, 1 day-30.2 months) for the pembrolizumab group and 3.5 months (1 day-40.4 months) for the chemotherapy group. At data cutoff, no patients were continuing to receive their initially assigned study treatment. Among patients initially assigned to chemotherapy, 99 received anti‒PD-1 or PD-L1 (PD-[L]1) therapy (83 crossed over to pembrolizumab on-study, and 16 additional patients received anti‒PD-(L)1 therapy outside the study), representing a 66.0% effective crossover rate. In the pembrolizumab group, 80 of 154 (52.9%) received additional anticancer therapy, including 12 patients who received a second course of pembrolizumab on study. Subsequent therapies for both treatment groups are summarized in Appendix Table A1 (online only). Patient demographics and baseline clinical characteristics are described in Table 1.
FIG 1.
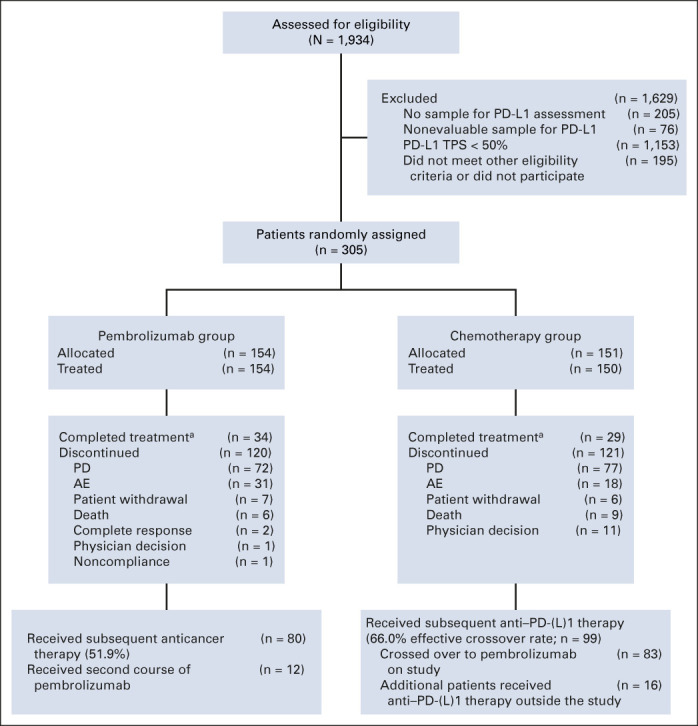
Disposition of patients. aNumber of patients who completed treatment, as reported by investigator. AE, adverse event; PD, progressive disease; PD-L1, programmed death ligand-1; TPS, tumor proportion score.
TABLE 1.
Patient Demographics and Baseline Clinical Characteristics
Efficacy Outcomes in the Intention-to-Treat Population
At data cutoff, 103 patients (66.9%) in the pembrolizumab group and 123 patients (81.5%) in the chemotherapy group had died. Median OS was 26.3 months (95% CI, 18.3 to 40.4 months) in the pembrolizumab group and 13.4 months (95% CI, 9.4 to 18.3 months) in the chemotherapy group (HR, 0.62; 95% CI, 0.48 to 0.81; Fig 2A). Kaplan-Meier estimates of OS at 5 years were 31.9% (95% CI, 24.5 to 39.5) in the pembrolizumab group and 16.3% (95% CI, 10.6 to 23.0) in the chemotherapy group.
FIG 2.
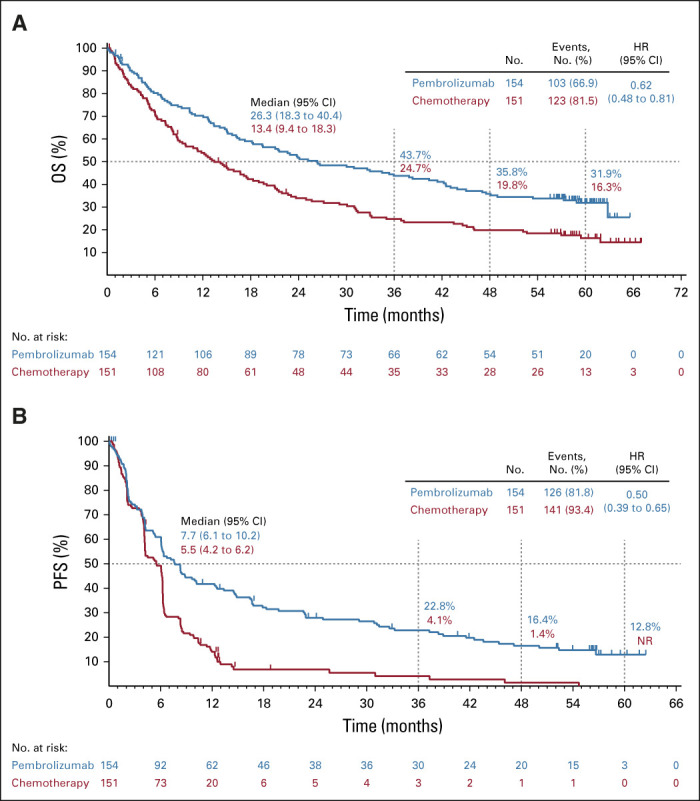
Kaplan-Meier estimates of (A) OS and (B) PFS in the pembrolizumab group and the chemotherapy group. For each treatment group, the Kaplan-Meier method was used to estimate OS and PFS, with censoring of data for patients alive or lost to follow-up at the time of last contact for OS or without disease progression or death at last disease assessment without documented disease progression prior to initiation of second-line therapy for PFS. Tick marks indicate censoring times. HR, hazard ratio; NR, not reached; OS, overall survival; PFS, progression-free survival.
In addition to patients who had CR or partial response (PR), patients who had stable disease (SD) also had OS benefit. In an exploratory analysis, median OS among patients with best response of SD was 21.1 months (95% CI, 14.1 to 34.5) for the pembrolizumab group and 14.5 months (95% CI, 8.9 to 21.1) for the chemotherapy group. In a further exploratory analysis, median OS for patients in the pembrolizumab group with treatment-related AEs leading to treatment discontinuation (n = 21) was 35.9 months (95% CI, 7.9 to NR) with a 5-year OS rate of 35.1% (95% CI, 15.7 to 55.3).
At data cutoff, 126 patients (81.8%) in the pembrolizumab group and 141 patients (93.4%) in the chemotherapy group had progressed per investigator assessment or died. Median PFS was 7.7 months (95% CI, 6.1 to 10.2 months) for patients in the pembrolizumab group versus 5.5 months (95% CI, 4.2 to 6.2 months) for patients in the chemotherapy group (HR, 0.50; 95% CI, 0.39 to 0.65; Fig 2B). Kaplan-Meier estimates of PFS at 3 and 5 years were 22.8% (95% CI, 16.3 to 29.9) and 12.8% (95% CI, 7.4 to 19.8), respectively, in the pembrolizumab group and 4.1% (95% CI, 1.3 to 9.4) and not reached (no evaluable patients) in the chemotherapy group.
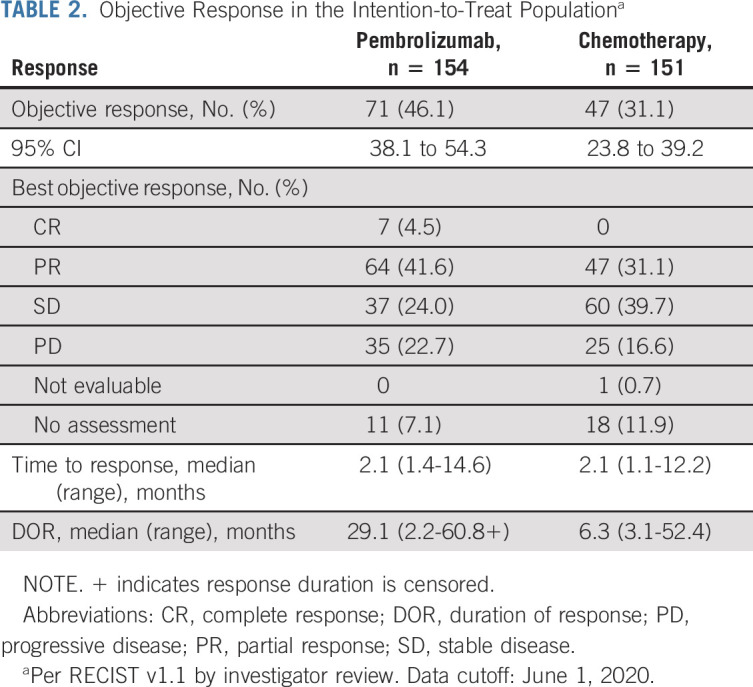
The ORR by investigator review was 46.1% (71 of 154; 95% CI, 38.1 to 54.3) among patients in the pembrolizumab group versus 31.1% (47 of 151; 95% CI, 23.8 to 39.2) in the chemotherapy group (Table 2). Seven patients (4.5%) in the pembrolizumab group versus no patients in the chemotherapy group achieved a CR (Table 2). Median duration of response was 29.1 months (range, 2.2-60.8+ months) in the pembrolizumab group and 6.3 months (range, 3.1-52.4 months) in the chemotherapy group (+ indicates the response duration is censored).
TABLE 2.
Objective Response in the Intention-to-Treat Populationa
Median PFS2 (time from randomization to subsequent disease progression after initiation of new anticancer therapy or death from any cause) was 24.1 months (95% CI, 15.0 to 31.4 months) in patients initially randomly assigned to pembrolizumab and 8.5 months (95% CI, 7.3 to 11.4 months) in patients initially randomly assigned to chemotherapy (HR, 0.51; 95% CI, 0.39 to 0.67). The PFS2 rate at 3 years was 39.5% (95% CI, 31.4 to 47.4) for pembrolizumab and 15.0% (95% CI, 9.4 to 21.8) for chemotherapy (Fig 3).
FIG 3.
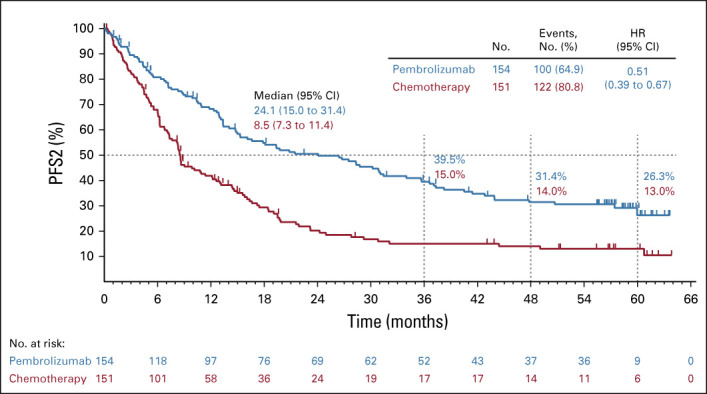
Kaplan-Meier estimates of PFS2 for each treatment group, with censoring of data at the time of last contact for patients alive without receiving second-line therapy, stopping second-line therapy without disease progression, and not initiating third-line therapy. Tick marks indicate censoring times. HR, hazard ratio; PFS, progression-free survival.
Safety
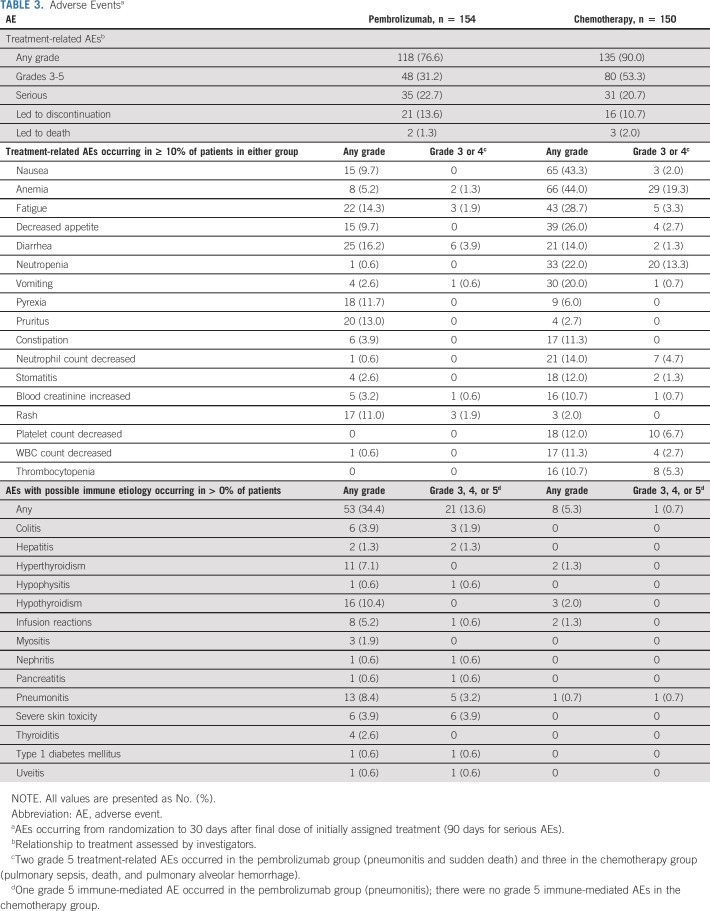
No new safety signals were identified since prior analyses. Incidence of treatment-related AEs was 76.6% (grade 3-5, 31.2%) among patients who received pembrolizumab and 90.0% (grade 3-5, 53.3%) among patients who received chemotherapy (Table 3). Serious treatment-related AEs occurred in 22.7% and 20.7% of patients in the pembrolizumab and chemotherapy groups, respectively. There were two treatment-related deaths in the pembrolizumab group and three in the chemotherapy group (pembrolizumab: sudden death of unknown cause and pneumonitis; chemotherapy: pulmonary sepsis, pulmonary alveolar hemorrhage, and death of unknown cause; all previously reported).4,10
TABLE 3.
Adverse Eventsa
Immune-mediated AEs and infusion reactions (irrespective of attribution to study treatment by the investigator) occurred in 34.4% (grade 3-5, 13.6%) of patients who received pembrolizumab and 5.3% (grade 3-5, 0.7%) of those who received chemotherapy (Table 3).
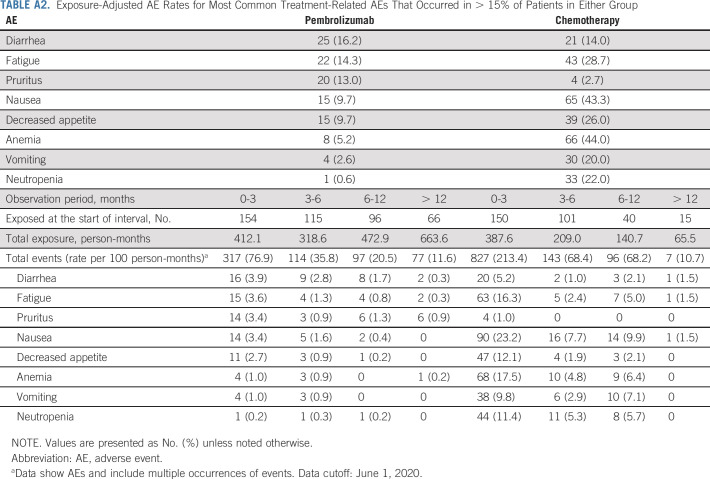
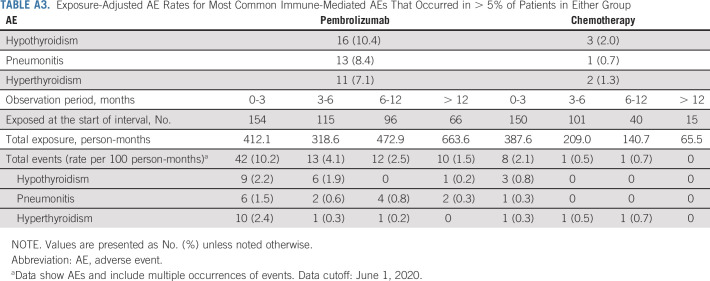
There were no new safety signals identified with long-term follow-up. Exposure-adjusted AE rates in the total study population decreased over time in both treatment groups for treatment-related AEs and immune-mediated AEs and infusion reactions (Appendix Tables A2 and A3, online only).
Outcomes in Patients Who Completed 35 Cycles of Pembrolizumab
Among the patients initially assigned to pembrolizumab, 39 of 151 (25.8%) completed 35 cycles (ie, approximately 2 years) of treatment. Median (range) time from completion of 35 cycles to data cutoff was 34.7 months (31.2-44.1). Baseline characteristics of these patients were similar to the total study population (Table 1). Among these patients, 82.1% experienced a response, per RECIST v1.1 per investigator assessment (Fig 4A). Best response was CR in four patients (10.3%), PR in 28 (71.8%), SD in six (15.4%), and PD in one (2.6%). At data cutoff, 32 of 39 patients were alive and the OS rate at 36 months from completion of 35 cycles of pembrolizumab (ie, approximately 5 years) was 81.4% (95% CI, 64.7 to 90.7). Of 39 patients, 18 (46.2%) were alive without PD or subsequent therapy for NSCLC. One patient developed a second malignancy (gastric cancer) and was treated accordingly.
FIG 4.
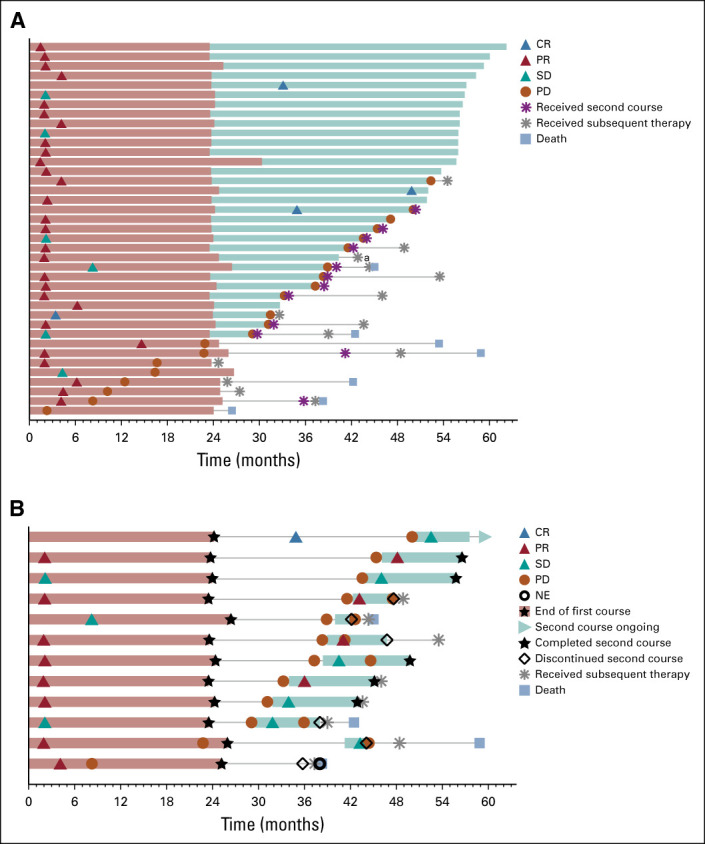
Treatment duration and time to response in (A) patients completing 35 cycles of pembrolizumab treatment and (B) patients who received a second course of pembrolizumab treatment. Light red bars indicate the first course of pembrolizumab treatment duration. Light teal bars indicate the (A) first course follow-up duration or (B) second course of treatment duration. Follow-up was defined as the time to progression or last nonprogression assessment by investigator. Response was assessed by RECIST v1.1 per investigator review. The maximum treatment duration for the second course was 17 cycles. aPatient developed a secondary malignancy. CR, complete response; NE, nonevaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Among patients who completed 35 cycles, treatment-related AEs occurred in 87.2% (grade 3-4, 15.4%; no grade 5 events), which was consistent with the overall as-treated population. Treatment-related grade 3-4 AEs were diarrhea, autoimmune hepatitis, increased gamma-glutamyltransferase, hyperglycemia, type 2 diabetes mellitus, tubulointerstitial nephritis, and rash (all n = 1). Consistent with the all patients as-treated population, incidence of immune-mediated AEs and infusion reactions was 30.8%, with grade 3-4 comprising 7.7% (one each of hepatitis, nephritis, and severe skin toxicity) and no grade 5 events.
Outcomes Among Patients Who Received a Second Course of Pembrolizumab
Twelve patients received a second course of pembrolizumab after an assessment of PD by the investigator. Median (range) time from last dose of second course to data cutoff was 15.2 months (0.4-29.6). Among these 12 patients, eight (66.7%) were alive at data cutoff. Four (33.3%) had an objective response after starting second-course pembrolizumab (all PRs) and six (50.0%) had SD as their best response per investigator assessment (Fig 4B). One patient experienced PD after receiving a second course of pembrolizumab, and one patient was not evaluable. At data cutoff, five patients (41.7%) were alive without PD and three (25.0%) had not received subsequent therapy. Five patients (41.7%) had treatment-related AEs during the second course; all were grade 1 or 2 and one event, grade 1 hypothyroidism, was an immune-mediated AE.
DISCUSSION
First-line pembrolizumab monotherapy produced clinically meaningful improvements in OS, PFS, ORR, and durable responses versus platinum-based chemotherapy among patients with metastatic NSCLC with PD-L1 TPS of at least 50% without sensitizing EGFR or ALK alterations. Notably, the 5-year OS rate in the pembrolizumab group (31.9%) was approximately double that in the chemotherapy group (16.3%), and median OS was more than 1 year longer in the pembrolizumab group (26.3 months) than in the chemotherapy group (13.4 months; HR, 0.62; 95% CI, 0.48 to 0.81). In addition to those with an objective response, pembrolizumab also improved OS among patients with a best-response assessment of SD. These data confirm the 5-year OS outcomes in the single-arm KEYNOTE-001 study among previously untreated patients with PD-L1 TPS of at least 50% and represent a clinically meaningful improvement in survival for patients whose prognosis has historically been very poor.1,11
The magnitude of treatment effect for first-line pembrolizumab is notable given that the majority of patients (66.0%) in the chemotherapy group received subsequent anti‒PD-(L)1 therapy. This high effective crossover rate may have reduced the observed treatment effect for pembrolizumab versus chemotherapy given that immunotherapy agents (including pembrolizumab) have demonstrated improved OS over chemotherapy in the second-line or later setting.12-16 In a prior analysis, we showed that when OS for patients in the chemotherapy group of KEYNOTE-024 was adjusted for crossover using a simplified 2-stage model, the HR for OS was 0.49 (95% CI, 0.34 to 0.69).4 With a doubling of the median OS and the improvement in PFS2 among patients who received pembrolizumab, these data continue to support a first-line immunotherapy approach, which permits early inhibition of PD-1 signaling, in this patient population.
The long-term efficacy of pembrolizumab was further demonstrated by analysis of outcomes in the 39 patients who completed 35 cycles of pembrolizumab: 82.1% of patients were alive at data cutoff, and 46.2% were alive without PD or subsequent therapy. These data support duration of treatment of up to 2 years with pembrolizumab.
Analysis of outcomes for patients on a second course of pembrolizumab showed such treatment was not only feasible but also associated with antitumor activity. Of the 12 patients who subsequently progressed and received a second course of pembrolizumab, two-thirds were alive at data cutoff. Notably, five of 12 were alive without PD. These data support pembrolizumab re-treatment upon PD.
Pembrolizumab was well-tolerated and was associated with a lower incidence of treatment-related AEs (any-grade and grade 3-5 toxicities) than platinum-based chemotherapy in the as-treated population and patients who completed 35 cycles. We did not identify new safety signals with long-term follow-up, and there was no evidence of increasing toxicity during long-term follow-up. Additionally, analysis of patients who discontinued pembrolizumab treatment because of treatment-related AEs revealed a median OS of 35.9 months, demonstrating favorable outcomes could occur in patients with early discontinuation because of AEs. Among patients who completed 35 cycles of pembrolizumab, treatment-related AEs were similar to those observed in the trial overall, with a similar incidence of grade 3 and 4 events and no deaths. Together with improvements in efficacy outcomes, the lower toxicity with pembrolizumab versus chemotherapy supports the improved or maintained patient-reported quality-of-life outcomes in the pembrolizumab group that have been previously reported.17
KEYNOTE-024 underscores the importance of pembrolizumab in first-line treatment of metastatic NSCLC. In an effort to extend the benefit of pembrolizumab to other patient populations, the KEYNOTE-042 study showed an OS benefit over chemotherapy in patients with advanced NSCLC and TPS > 1% without sensitizing EGFR or ALK alterations.5 Furthermore, first-line pembrolizumab plus chemotherapy also showed improved OS versus placebo plus chemotherapy for both nonsquamous (KEYNOTE-189) and squamous (KEYNOTE-407) metastatic NSCLC without EGFR or ALK alterations irrespective of tumor PD-L1 expression.8,9,18,19 Studies evaluating other anti–PD-(L)1 therapies have also shown improvements in OS among patients with advanced or metastatic NSCLC (albeit with shorter follow-up than KEYNOTE-024), either as monotherapy or in combination with chemotherapy and/or antiangiogenic or anti‒CTLA-4 agents.20-24
Epidemiologic data are beginning to show the effects of new therapies on the lung cancer disease state. Howlader et al. recently described a substantial decrease in lung cancer mortality in the United States: incidence-based mortality from 2006 to 2013 decreased annually by 3.2% for men but improved more rapidly from 2013 to 2016, with an annual 6.3% decrease.25 For women, incidence-based mortality decreased by 2.3% annually from 2006 to 2014 and then by 5.9% annually from 2014 to 2016. They concluded that this decrease in mortality was likely because of the targeted agents that became available to treat patients with EGFR and ALK mutations (only a subset of patients with NSCLC are in this category). Five-year outcomes in KEYNOTE-024 suggest that availability of immunotherapy may result in further improvement in lung cancer survival at the population level and that pembrolizumab may be transforming metastatic NSCLC into a treatable chronic disease for patients who meet eligibility criteria.
In summary, these 5-year outcomes from the phase III KEYNOTE-024 study, which, to our knowledge, is the first randomized trial evaluating pembrolizumab in this setting, show the ability of this treatment to improve long-term outcomes for patients with NSCLC. Pembrolizumab provided a durable and clinically relevant long-term OS benefit versus chemotherapy as first-line therapy for metastatic NSCLC with PD-L1 TPS of at least 50% and is a standard-of-care therapy in this setting.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Medical writing assistance was provided by Kathleen Estes, PhD, and Ali Hassan, PhD, of ICON plc (North Wales, PA). This assistance was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ.
Appendix
TABLE A1.
Subsequent Therapies
TABLE A2.
Exposure-Adjusted AE Rates for Most Common Treatment-Related AEs That Occurred in > 15% of Patients in Either Group
TABLE A3.
Exposure-Adjusted AE Rates for Most Common Immune-Mediated AEs That Occurred in > 5% of Patients in Either Group
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, Abbvie, Amgen, Mirati Therapeutics, Samsung Bioepis
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics
Delvys Rodríguez-Abreu
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, AstraZeneca Spain, Novartis
Speakers' Bureau: Roche, Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
Andrew G. Robinson
Honoraria: Merck
Consulting or Advisory Role: AstraZeneca, Merck, Amgen
Research Funding: AstraZeneca, Merck, Bristol Myers Squibb, Roche Canada
Rina Hui
Honoraria: Merck Sharp & Dohme, Novartis, Roche, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Seagen
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb, Novartis, Lilly, Pfizer, Seagen
Research Funding: AstraZeneca, Lilly, Novartis, MSD, Roche, Seagen, OncoSec
Travel, Accommodations, Expenses: Novartis
Tibor Csőszi
Consulting or Advisory Role: Novartis
Speakers' Bureau: Ipsen, Janssen-Cilag
Travel, Accommodations, Expenses: Sanofi, Pfizer
Andrea Fülöp
Research Funding: MSD, AstraZeneca, Janssen
Nir Peled
Honoraria: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, MSD, Novartis, Pfizer, Roche, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche
Research Funding: MSD Oncology, Roche/Genentech, AstraZeneca, Takeda, Merck Serono, Novartis
Travel, Accommodations, Expenses: Roche/Genentech, MSD Oncology, AstraZeneca, Pfizer
Sinead Cuffe
Travel, Accommodations, Expenses: MSD Oncology, Roche, Pfizer, Bristol Myers Squibb
Mary O'Brien
Honoraria: Merck, Merck Serono, Abbvie, Bristol Myers Squibb
Consulting or Advisory Role: Merck
Travel, Accommodations, Expenses: Bristol Myers Squibb
Suman Rao
Honoraria: Merck, AstraZeneca
Consulting or Advisory Role: Galvanize Therapeutics
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: Merck
Katsuyuki Hotta
Honoraria: AZD, MSD Oncology, Pfizer, Lilly, Taiho Pharmaceutical, Chugai Pharma, Takeda
Research Funding: MSD, Chugai Pharma, Lilly Japan, Bristol Myers Squibb, AZD
Ticiana A. Leal
Consulting or Advisory Role: BeyondSpring Pharmaceuticals, Bristol Myers Squibb, Merck, Takeda, Genentech, InvisionFirst Lung, Novocure, Jazz Pharmaceuticals, AstraZeneca, Bayer, EMD Serono, Boehringer Ingelheim, Blueprint Medicines, Daiichi Sankyo/Lilly, Debiopharm Group, Genentech, Lilly
Travel, Accommodations, Expenses: Takeda, Abbvie, Bristol Myers Squibb, Genentech, Mirati Therapeutics, AstraZeneca, Merck
Uncompensated Relationships: Mirati Therapeutics
Jonathan W. Riess
Consulting or Advisory Role: Medtronic, Boehringer Ingelheim, Novartis, Blueprint Medicines, Genentech, EcoR1 Capital, Teladoc, Daiichi Sankyo, EMD Serono
Research Funding: Merck, AstraZeneca/MedImmune, Spectrum Pharmaceuticals, Boehringer Ingelheim, Novartis, Revolution Medicines, GlaxoSmithKline
Erin Jensen
Employment: Merck
Stock and Other Ownership Interests: Merck
Bin Zhao
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
M. Catherine Pietanza
Employment: Merck
Stock and Other Ownership Interests: Merck Sharp & Dohme
Julie R. Brahmer
Honoraria: Roche/Genentech
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Merck, Amgen, Genentech, GlaxoSmithKline, AstraZeneca, Regeneron, Sanofi
Research Funding: Bristol Myers Squibb, AstraZeneca, Spectrum Pharmaceuticals, Revolution, RAPT Therapeutics, Genentech/Roche
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche/Genentech
Other Relationship: Bristol Myers Squibb, Merck, Janssen Oncology
No other potential conflicts of interest were reported.
See accompanying editorial on page 2321
PRIOR PRESENTATION
Presented as a late-breaking oral presentation at the European Society for Medical Oncology Virtual Congress 2020, September 19-21, 2020.
SUPPORT
Supported by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ.
CLINICAL TRIAL INFORMATION
M.R. and D.R.-A. contributed equally to this work.
DATA SHARING STATEMENT
Merck Sharp & Dohme Corp (MSD), a subsidiary of Merck & Co, Inc, Kenilworth, NJ, is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Martin Reck, Delvys Rodríguez-Abreu, Tibor Csőszi, Mary O'Brien, M. Catherine Pietanza, Julie R. Brahmer
Provision of study materials or patients: Rina Hui, Sinead Cuffe, Suman Rao
Collection and assembly of data: Martin Reck, Delvys Rodríguez-Abreu, Andrew G. Robinson, Rina Hui, Andrea Fülöp, Maya Gottfried, Nir Peled, Ali Tafreshi, Sinead Cuffe, Mary O'Brien, Suman Rao, Katsuyuki Hotta, Ticiana A. Leal, Jonathan W. Riess, Bin Zhao
Data analysis and interpretation: Martin Reck, Delvys Rodríguez-Abreu, Andrew G. Robinson, Rina Hui, Nir Peled, Ali Tafreshi, Mary O'Brien, Ticiana A. Leal, Jonathan W. Riess, Erin Jensen, Bin Zhao, M. Catherine Pietanza, Julie R. Brahmer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, Abbvie, Amgen, Mirati Therapeutics, Samsung Bioepis
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics
Delvys Rodríguez-Abreu
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, AstraZeneca Spain, Novartis
Speakers' Bureau: Roche, Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
Andrew G. Robinson
Honoraria: Merck
Consulting or Advisory Role: AstraZeneca, Merck, Amgen
Research Funding: AstraZeneca, Merck, Bristol Myers Squibb, Roche Canada
Rina Hui
Honoraria: Merck Sharp & Dohme, Novartis, Roche, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Seagen
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb, Novartis, Lilly, Pfizer, Seagen
Research Funding: AstraZeneca, Lilly, Novartis, MSD, Roche, Seagen, OncoSec
Travel, Accommodations, Expenses: Novartis
Tibor Csőszi
Consulting or Advisory Role: Novartis
Speakers' Bureau: Ipsen, Janssen-Cilag
Travel, Accommodations, Expenses: Sanofi, Pfizer
Andrea Fülöp
Research Funding: MSD, AstraZeneca, Janssen
Nir Peled
Honoraria: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, MSD, Novartis, Pfizer, Roche, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche
Research Funding: MSD Oncology, Roche/Genentech, AstraZeneca, Takeda, Merck Serono, Novartis
Travel, Accommodations, Expenses: Roche/Genentech, MSD Oncology, AstraZeneca, Pfizer
Sinead Cuffe
Travel, Accommodations, Expenses: MSD Oncology, Roche, Pfizer, Bristol Myers Squibb
Mary O'Brien
Honoraria: Merck, Merck Serono, Abbvie, Bristol Myers Squibb
Consulting or Advisory Role: Merck
Travel, Accommodations, Expenses: Bristol Myers Squibb
Suman Rao
Honoraria: Merck, AstraZeneca
Consulting or Advisory Role: Galvanize Therapeutics
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: Merck
Katsuyuki Hotta
Honoraria: AZD, MSD Oncology, Pfizer, Lilly, Taiho Pharmaceutical, Chugai Pharma, Takeda
Research Funding: MSD, Chugai Pharma, Lilly Japan, Bristol Myers Squibb, AZD
Ticiana A. Leal
Consulting or Advisory Role: BeyondSpring Pharmaceuticals, Bristol Myers Squibb, Merck, Takeda, Genentech, InvisionFirst Lung, Novocure, Jazz Pharmaceuticals, AstraZeneca, Bayer, EMD Serono, Boehringer Ingelheim, Blueprint Medicines, Daiichi Sankyo/Lilly, Debiopharm Group, Genentech, Lilly
Travel, Accommodations, Expenses: Takeda, Abbvie, Bristol Myers Squibb, Genentech, Mirati Therapeutics, AstraZeneca, Merck
Uncompensated Relationships: Mirati Therapeutics
Jonathan W. Riess
Consulting or Advisory Role: Medtronic, Boehringer Ingelheim, Novartis, Blueprint Medicines, Genentech, EcoR1 Capital, Teladoc, Daiichi Sankyo, EMD Serono
Research Funding: Merck, AstraZeneca/MedImmune, Spectrum Pharmaceuticals, Boehringer Ingelheim, Novartis, Revolution Medicines, GlaxoSmithKline
Erin Jensen
Employment: Merck
Stock and Other Ownership Interests: Merck
Bin Zhao
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
M. Catherine Pietanza
Employment: Merck
Stock and Other Ownership Interests: Merck Sharp & Dohme
Julie R. Brahmer
Honoraria: Roche/Genentech
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Merck, Amgen, Genentech, GlaxoSmithKline, AstraZeneca, Regeneron, Sanofi
Research Funding: Bristol Myers Squibb, AstraZeneca, Spectrum Pharmaceuticals, Revolution, RAPT Therapeutics, Genentech/Roche
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche/Genentech
Other Relationship: Bristol Myers Squibb, Merck, Janssen Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Howlader N Noone AM Krapcho M, et al. : SEER Cancer Statistics Review, 1975-2014. Bethesda, MD, National Cancer Institute, 2017 [Google Scholar]
- 2.Butler CA Darragh KM Currie GP, et al. : Variation in lung cancer survival rates between countries: Do differences in data reporting contribute? Respir Med 100:1642-1646, 2006 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-small Cell Lung Cancer. Version 2.2020. https://www.nccn.org [Google Scholar]
- 4.Reck M Rodriguez-Abreu D Robinson AG, et al. : Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37:537-546, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Mok TSK Wu YL Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-1830, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L Luft A Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-2051, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Gandhi L Rodriguez-Abreu D Gadgeel S, et al. : Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078-2092, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L Vicente D Tafreshi A, et al. : A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657-1669, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Gadgeel S Rodríguez-Abreu D Speranza G, et al. : Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J Clin Oncol 38:1505-1517, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Reck M Rodriguez-Abreu D Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Garon EB Hellmann MD Rizvi NA, et al. : Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol 37:2518-2527, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghaei H Paz-Ares L Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer J Reckamp KL Baas P, et al. : Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123-135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittmeyer A Barlesi F Waterkamp D, et al. : Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrenbacher L Spira A Ballinger M, et al. : Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 387:1837-1846, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS Garon EB Kim DW, et al. : Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1‒positive, advanced non‒small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol 38:1580-1590, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Brahmer JR Rodriguez-Abreu D Robinson AG, et al. : Health-related quality of life for pembrolizumab vs chemotherapy in advanced NSCLC with PD-L1 TPS ≥50%: Data from KEYNOTE-024. J Thorac Oncol 12:S8-S9, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Langer CJ Gadgeel SM Borghaei H, et al. : Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497-1508, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghaei H Langer CJ Gadgeel S, et al. : 24-month overall survival from KEYNOTE-021 cohort G: Pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non–small cell lung cancer. J Thorac Oncol 14:124-129, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Reck M Ciuleanu T-E Dols MC, et al. : Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 38, 2020. (suppl; abstr 9501) [Google Scholar]
- 21.Hellmann MD Paz-Ares L Bernabe Caro R, et al. : Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381:2020-2031, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Socinski MA Jotte RM Cappuzzo F, et al. : Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288-2301, 2018 [DOI] [PubMed] [Google Scholar]
- 23.West H McCleod M Hussein M, et al. : Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924-937, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Sezer A Kilickap S Gümüş M, et al. : LBA52 EMPOWER-Lung 1: Phase III first-line (1L) cemiplimab monotherapy vs platinum-doublet chemotherapy (chemo) in advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) ≥50%. Ann Oncol 31:S1182-S1183, 2020 [Google Scholar]
- 25.Howlader N Forjaz G Mooradian MJ, et al. : The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 383:640-649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme Corp (MSD), a subsidiary of Merck & Co, Inc, Kenilworth, NJ, is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.