Abstract
Background:
Perioperative neurocognitive disorders (PND) are a series of severe complications in the perioperative and anesthetic periods with a decline in memory, execution ability, and information processing speed as the primary clinical manifestation. This study aimed to evaluate the impact of edaravone (EDA) on PND and peripheral blood C-X-C motif chemokine ligand 13 (CXCL13) levels in elderly patients with hip replacement.
Methods:
A total of 160 elderly patients undergoing hip arthroplasty in Affiliated Dongguan People's Hospital of Southern Medical University (from March 2016 to March 2018) were randomly and double-blindly categorized into an EDA group and a control group (CON). Group EDA was administered intravenously EDA 30 min before surgery, and group CON was administered intravenously saline. The cognitive function of the two groups was evaluated 1-day before the operation and at 1 and 12 months after surgery, and the incidence of post-operative delirium was tested on days 1, 3, and 7 after surgery using the Chinese version of the confusion assessment method. Serum CXCL13 and interleukin (IL)-6 concentrations were measured before anesthesia, during surgery (30 min after skin incision), and on days 1, 3, and 7 after surgery. The continuous variables in accordance with normal distribution were tested using the Student's t test, the continuous variables without normal distribution using the Mann-Whitney U test, and categorical variables by the χ2 test or Fisher exact test.
Results:
The incidence of post-operative delirium within 7 days after surgery was significantly higher in group CON than that in group EDA (31.3% vs. 15.0%, t = −5.6, P < 0.001). The modified telephone interview for cognitive status and activities of daily life scores were significantly higher in the group EDA than those in the group CON at 1 month (39.63 ± 4.35 vs. 33.63 ± 5.81, t = −2.13, P < 0.05 and 74.3 ± 12.6 vs. 61.2 ± 13.1, t = −1.69, P < 0.05) and 12 months (40.13 ± 5.93 vs. 34.13 ± 5.36, t = −3.37, P < 0.05 and 79.6 ± 11.7 vs. 65.6 ± 16.6, t = −2.08, P < 0.05) after surgery; and the incidence of neurocognitive dysfunction was significantly lower in the group EDA than that in the group CON (P < 0.05). Serum CXCL13 and IL-6 concentrations were significantly lower in the group EDA than those in the group CON during and after surgery (P < 0.05).
Conclusion:
EDA can significantly reduce the serum concentrations of CXCL13 and IL-6 and improve the PND of patients.
Keywords: Edaravone, Perioperative neurocognitive disorder, Chemokine CXC ligand 13, Interleukin-6
Introduction
Perioperative neurocognitive disorders (PND) are a series of severe complications in the perioperative and anesthetic periods with a decline in memory, execution ability, and information processing speed as the primary clinical manifestation.[1] The exact mechanism underlying PND remains unclear. Several studies have reported that surgery itself can interfere with the stable in vivo environment, initiate the immune cascade, and secrete numerous inflammatory mediators and free oxygen, consequently leading to the inflammatory response of the central nervous system and plays a significant role in the pathogenesis of PND.[2–4] C-X-C motif chemokine ligand 13 (CXCL13), also known as the B-cell chemokine, is the main factor for recruiting B cells in the neuritic response.[5] Edaravone (EDA) is a brain-protective agent that can scavenge free radicals; inhibit lipid peroxidation; inhibit leukotriene production; and reduce damage to cells, tissues, and organs.[6–9] Animal experiments have shown that EDA can improve surgery and lipopolysaccharide-induced cognitive impairment in rats.[10] However, only few studies have reported that EDA can improve PND and reduce peripheral blood CXCL13 levels in elderly patients with hip replacement. This study used a formal PND assessment method and large-sample randomized controlled trials to clinically verify whether EDA can improve PND in elderly patients who underwent hip replacement, aiming to provide a reference for the clinical prevention and treatment of PND.
Methods
Ethical approval
This study was approved by the Medical Ethics Committee of Dongguan People's Hospital (No. 2016-76), and all patients and their families signed the informed consent form.
Case selection
Patients admitted to the Department of Orthopedics for hip replacement between March 2016 and March 2018 were enrolled with the following inclusion criteria: 65 to 80 years old, planned for hip replacement surgery, and signed the informed consent. The exclusion criteria were as follows: pre-operative Montreal cognitive assessment (MoCA) score <26 points; severe vision, hearing impairment, inability, or other reasons of inability to communicate with the study researchers; severe liver and kidney damage; neuromuscular disease; mental illness; malignant tumors; hospitalization in the past 3 months for any reason; alcoholism; drug abuse; contraindications to combined spinal and epidural anesthesia; and any other reasons inappropriate for the study. Patients who cannot wake up after surgery or are unable to undergo a cognitive test withdrew from the study. The patients were categorized into group EDA (n = 80) and group control group (CON) (n = 80) using the randomized double-blind method.
Sample size calculation
It has been shown that the incidence rate of decline in post-operative cognitive function scores in the EDA group was 11.1%, while the incidence rate of decline in post-operative cognitive function scores in the CON was 28.9%.[11] According to the test standard, α was 0.05, the test power 1-β was 0.8, the ratio between groups was 1:1, the sample size obtained was 76 people per group, a total of 152 people. The loss rate was estimated to be 5%, and the final sample size was 160 people.
Random blind grouping method
Researchers who did not participate in other parts of this study assigned patients with the random number table and patients’ enrollment order with a grouping ratio of 1:1. The grouping arrangements were sealed in opaque envelopes, which were revealed before the induction of anesthesia. Intravenous medications were arranged by a nurse in the operating room following the grouping arrangements and given to the anesthesiologist. Neither the anesthesiologist nor the patients were aware of the grouping arrangements. The grouping envelopes were kept by the researchers responsible for the random grouping and were unblinded. The flow diagram is shown in Figure 1.
Figure 1.
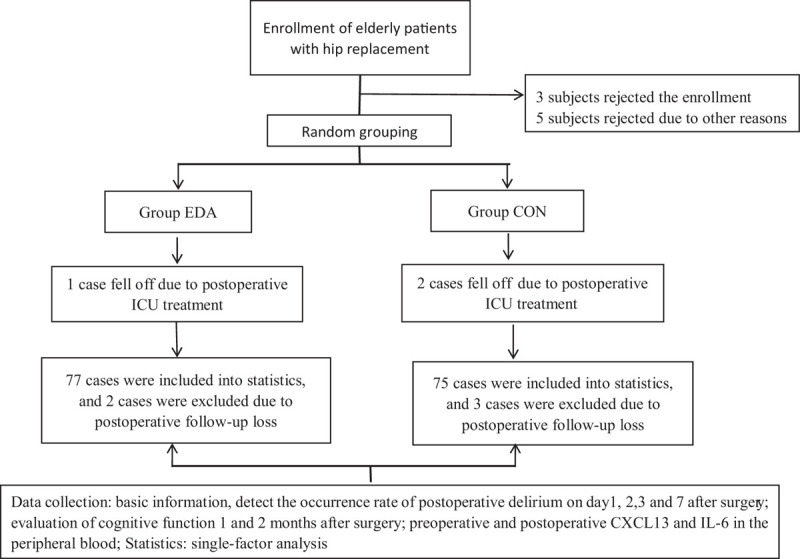
The flow diagram of the impact of EDA on PND and peripheral blood CXCL-13 levels in elderly patients with hip replacement. CON: Group control; CXCL-13: C-X-C motif chemokine ligand 13; EDA: Group edaravone; ICU: Intensive care unit; IL-6: Interleukin-6; PND: Perioperative neurocognitive disorders.
Anesthesia method
The baseline data were gender, age, educational level, American Society of Anesthesiologists classification, presence of comorbidities (diabetes, hypertension, and others), pre-operative MoCA score, anesthesia time, operation time, intra-operative bleeding, and hospital stay length.
Method of anesthesia administration
Routine diet and water restriction was performed before surgery. After entering the operating room, the peripheral vein was opened for routine monitoring of non-invasive blood pressure (NIBP), electrocardiogram (ECG), peripheral oxygen saturation (SpO2), and respiratory rate (RR). The patients in both groups were administered combined lumbar epidural anesthesia controlled between T8 and T10 in the supine position. Group EDA (Jiangsu Xiansheng Pharmaceutical Co., Ltd., Jiangsu, China) was intravenously administered 30 mg of EDA dissolved in 100 mL of physiological saline (Jiangsu Xiansheng Pharmaceutical Co., Ltd., Jiangsu, China) at an injection speed of 200 mL/h. Group CON was intravenously administered with an equal volume of normal saline. Intra-operative urine volume, bleeding volume, and blood transfusion volume were recorded; stable intra-operative hemodynamics was maintained, and all patients underwent patient-controlled intravenous analgesia (PCIA) and maintained a visual analog scale (VAS) score of ≤3 points.
Anesthesia method
Patients were required to fast for about 8 to 10 h and be on water restriction 4 h before surgery. After being pushed into the operating room, the upper limbs’ peripheral veins were opened and connected to the ECG monitor for routine monitoring of NIBP, ECG, SpO2, and RR. Each patient was subjected to combined lumbar-scleral anesthesia and placed in the lateral position with the L3 to L4 intervertebral space as the puncture point. Herein, 8 to 15 mg of 0.5% bupivacaine hydrochloride was injected into the subarachnoid space with an injection time of 30 s. The anesthesia plane was controlled at T8 to T10 when each patient was in the supine position. If the anesthesia plane was insufficient, 3 to 5 mL of 2% lidocaine was administered additionally. Within 30 min after the anesthesia plane was fixed, group EDA was intravenously infused with 30 mg of EDA in 100 mL of physiological saline at an infusion rate of 200 mL/h. Group CON was infused with an equal volume of normal saline. Intra-operative urine volume, bleeding volume, and fluid/blood transfusion volume were recorded during surgery to maintain stable hemodynamics. PCIA was performed post-operatively with the following analgesics: 10 μg/kg of fentanyl + 200 mg of flurbiprofen + 10 g of tropisetron, diluted with physiological saline to 200 mL, and the background infusion rate was 2 mL/h. Pain degree was post-operatively evaluated using the VAS scoring method (0 points for pain and 10 points for severe pain), and the VAS score should be maintained at ≤3 points.
Observation outcomes
The primary endpoints were the incidence of post-operative delirium (POD) within 7 days after surgery, the scores of the modified telephone interview for cognitive status (TICS-M), and the activities of daily life (ADL) at 1 and 12 months after surgery. The secondary endpoints were changes in the peripheral serum CXCL13 and interleukin (IL)-6 concentrations during the perioperative period.
Determination of cognitive function
On day 1 before surgery, the two groups’ cognitive function was evaluated using MoCA. At 10 AM on days 1, 2, 3, and 7 after surgery, the Chinese version of the confusion assessment scale was used to detect the occurrence rate of POD, and TICS-M was used to assess the cognitive function of the patients in the two groups at 1 and 12 months after surgery.
Blood specimen collection and testing
However, 3 mL of peripheral venous blood was collected before anesthesia, during surgery (30 min after skin removal), and on days 1, 3, and 7 after surgery, followed by centrifugation, serum sealing in eppenforf (EP) tubes, and storage at −80°C. The corresponding human CXCL-13 and IL-6 enzyme-linked immunosorbent assay kits were used for detection according to the kit instructions.
Statistical analysis
SPSS 17.0 software was used for the analysis. The continuous variables that corresponded to the normal distribution are represented as mean ± standard deviation (SD), but the continuous variables that did not conform to the normal distribution are represented as median (range or interquartile range); the count data were represented by n (%). Differences in the primary endpoints between the two groups were compared according to the intention-to-treat principle: the continuous variables that met the normal distribution were tested using the t method, and the continuous variables that did not fit the normal distribution were tested using the Mann-Whitney U test. Categorical variables were tested by the χ2 test or Fisher exact test.
Results
In this study, five patients were lost to follow-up (two in group EDA and the remaining three in group CON), and three patients had complications and were transferred to the intensive care unit for treatment (one in group EDA and the remaining two in group CON); finally, a total of 152 patients completed the study.
Comparison of baseline data
There were no significant differences in age, body mass index, educational level, pre-operative comorbidities, pre-operative MoCA score, anesthesia time, operation time, or intra-operative blood loss between the two groups (P > 0.05). The length of hospital stay was shorter in the group EDA than in the group CON (P < 0.05) [Table 1].
Table 1.
Comparison of baseline data between EDA group and control group of elderly patients with hip replacement.
| Items | EDA (n = 77) | CON (n = 75) | Statistics | P |
| Gender (M/F) | 38/39 | 32/38 | 0.19∗ | 0.695 |
| Age (years) | 73.6 ± 5.8 | 72.3 ± 5.6 | 2.90† | 0.439 |
| Ratio of ASA classification (II/III) | 51/26 | 53/22 | 0.35∗ | 0.557 |
| BMI (kg/m2) | 22.6 ± 1.3 | 22.9 ± 1.6 | 0.35 | 0.826 |
| Pre-operative MoCA score | 28.7 ± 1.8 | 28.5 ± 1.7 | 3.90 | 0.889 |
| Educational level | ||||
| <8 years | 56 (72.7) | 53 (70.7) | 1.53∗ | 0.216 |
| ≥8 years | 21 (27.3) | 22 (29.3) | 6.34∗ | 0.432 |
| Anesthesia time (min) | 108 ± 13 | 106 ± 16 | 3.8† | 0.878 |
| Surgery time (min) | 93 ± 11 | 96 ± 10 | 3.1† | 0.813 |
| Intra-operative blood loss (mL) | 108 ± 23 | 106 ± 21 | 2.9† | 0.862 |
| Hospital stay (days) | 11.3 ± 2.8 | 16.3 ± 3.3 | −2.1† | 0.023 |
| Disease history | ||||
| Hypertension | 27 (35) | 24 (33) | 1.07∗ | 0.302 |
| Heart diseases | 18 (23) | 19 (26) | 0.28∗ | 0.599 |
| DM | 20 (27) | 17 (23) | 0.15∗ | 0.703 |
Data were presented as n, n (%), mean ± SD. ∗χ2 value. †t-value. ASA: American Society of Anesthesiologists; BMI: Body mass index; CON: Group control; DM: Diabetes mellitus; EDA: Group edaravone; F: Female; M: Man; MoCA: Montreal cognitive assessment; SD: Standard deviation.
Comparison of cognitive function
The total TICS-M and ADL scores at 1 and 12 months after surgery were higher in the group EDA than in the group CON (P < 0.05) [Table 2].
Table 2.
Comparison of TICS-M and ADL scores between EDA group and control group of elderly patients with hip replacement.
| 1 month after surgery | 12 months after surgery | |||||||
| Items | EDA | CON | t | P | EDA | CON | t | P |
| TICS-M score | 39.63 ± 4.35 | 33.63 ± 5.81 | −2.13 | 0.013 | 40.13 ± 5.93 | 34.13 ± 5.36 | −3.37 | 0.009 |
| Memory | 7.73 ± 3.32 | 6.21 ± 2.67 | −1.98 | 0.023 | 7.97 ± 2.36 | 6.33 ± 2.33 | −1.91 | 0.021 |
| Orientation | 12.64 ± 1.83 | 11.62 ± 1.87 | 1.33 | 0.072 | 13.13 ± 2.16 | 12.12 ± 2.09 | 1.16 | 0.068 |
| Language and notice | 17.31 ± 2.63 | 13.96 ± 2.16 | −1.76 | 0.026 | 17.53 ± 1.73 | 14.16 ± 1.65 | −1.63 | 0.031 |
| ADL score | 74.3 ± 12.6 | 61.2 ± 13.1 | −1.69 | 0.029 | 79.6 ± 11.7 | 65.6 ± 16.6 | −2.08 | 0.019 |
Compared with group CON. ADL: Activities of daily life; CON: Group control; EDA: Group edaravone; TICS-M: Modified telephone interview for cognitive status.
Comparison of incidence of PND
At 1 month after surgery, ten patients (12.5%) in group EDA and 18 patients (22.5%) in group CON developed PND; the difference was statistically significant (t = −1.73, P < 0.05). At 12 months after surgery, four patients (5.0%) in group EDA and eight patients (10.0%) in group CON had neurocognitive disorders (NCD). The difference was statistically significant between the two groups (t = −2.16, P < 0.05).
Comparison of incidence of POD
Within 7 days after surgery, the incidence of POD in the two groups was 23.1% (37), which was 15.0% in group EDA (12 cases) and 31.3% (25 cases) in group CON. There was a statistically significant difference between the two groups (t = −5.6, P < 0.001).
Comparison of serum CXCL13 and IL-6 concentrations
The concentrations of serum CXCL13 and IL-6 at each time point in the two groups were significantly higher than those before anesthesia (P < 0.05). The concentrations of serum CXCL13 and IL-6 at each time point were significantly lower in the group EDA than in the group CON (P < 0.05) [Figures 2 and 3].
Figure 2.
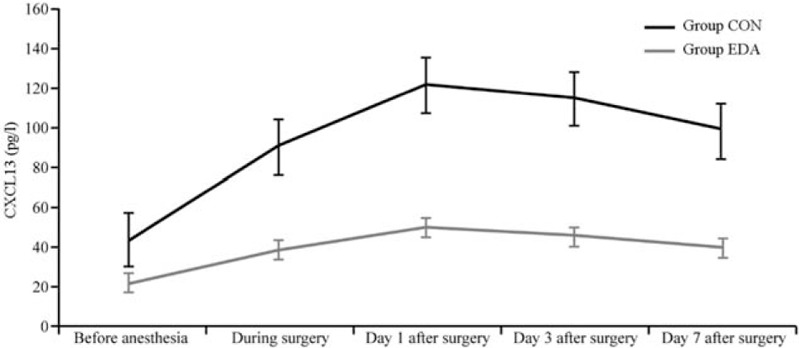
Comparison of serum CXCL13 at each time point between groups. CON: Group control; CXCL-13: C-X-C motif chemokine ligand; 13; EDA: Group edaravone.
Figure 3.
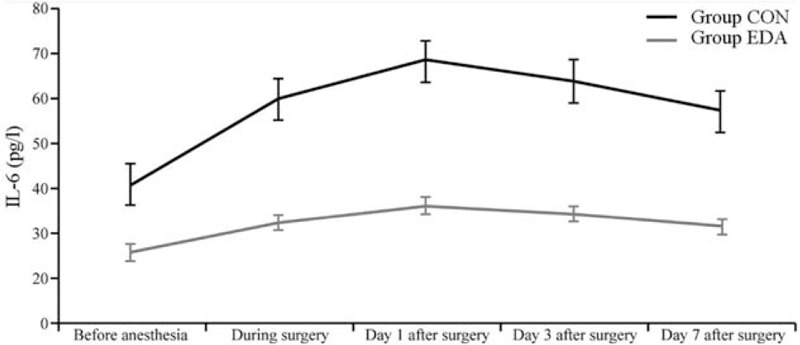
Comparison of serum IL-6 at each time point between groups (mean ± standard deviation). CON: Group control; EDA: Group edaravone; IL-6: Interleukin-6.
Discussion
The PND is a common post-operative complication in elderly patients, and the previous study has reported that the incidence of PND in the elderly after non-cardiac surgery can reach from 17% to 56%.[12] PND often manifest in various aspects, such as insanity, anxiety, social ability, and memory loss. With time, these are mainly divided into the following three types: delirium during the wake period, which usually occurs in post-anesthesia care unit; delirium after surgery, which usually occurs within 7 days after surgery; and post-operative neurocognitive dysfunction, which usually occurs within weeks to months after surgery.[13] Their pathogenesis is complicated, and anesthesia, hypoxemia, hypotension, type of surgery, anesthesia method, sleep disorders, or inflammatory reactions may all trigger PND. For the diagnosis of PND, Evered et al[1] suggested that the neurocognitive scale should be tested before surgery, on day 7 after surgery (or before discharge), on day 30 after surgery, and in the 12th month after surgery. If the neuropsychological scale cannot be used for evaluation in the next 12 months, TICS-M can be applied to reduce the lost follow-up rate. It is also recommended to add the ADL scale to assess patients’ daily activities. Furthermore, it is recommended that POD should be followed up for about 7 days or until before discharge. Therefore, the CAM scale used in this study to screen the patients for POD within 7 days after surgery, and TICS-M was also used to assess patients’ cognitive function and ADL scale on day 30 and 12 months after surgery to understand their daily activities. This study results showed that the incidence of POD was significantly lower in the group EDA than in the group CON within 7 days after surgery, and the TICS-M total score and ADL scores at 1 and 12 months, respectively, were significantly higher in the group EDA than in the group CON. Additionally, the incidence of PND at 1 and 12 months was significantly lower in the group EDA than in the group CON, suggesting that EDA can effectively improve perioperative neurocognitive function and patients’ quality of life after surgery.
The CXCL13 is an essential member of the chemokine family. It is mainly secreted by secondary lymphoid tissues, lymph nodes, or dendritic cells, and it can reflect the immune response after central nervous system infection. When CXCL13 specifically binds to the corresponding receptor C-X-C chemokine receptor 5 on the surface of B cells, it regulates the orientation of B cells. It can selectively destroy the blood-brain barrier, immune active cell-like reach the myelin sheath targeted, produce immune attacks, and release cytokines to destroy the myelin sheath. The loss of myelin sheath and B cell-mediated humoral immunity may lead to irreversible axonal damage and cause neurological dysfunction. In the cerebrospinal fluid and plasma of many patients with central nervous system immune- or inflammation-related diseases, the concentration of chemokine CXCL13 is significantly increased, suggesting that CXCL13 is an indicator of the immune inflammatory response of the central nervous system.[14] Li et al[15] reported that the concentration of CXCL13 in the serum and cerebrospinal fluid of patients with epilepsy is significantly increased, which is an essential factor involved in the development of neurocognitive dysfunction. IL-6 is secreted by activated macrophages and lymphocytes and plays an essential role in the body's immune defense and inflammatory response. It is a major acute-phase response pro-inflammatory factor and an early sensitive indicator of tissue damage. IL-6 levels in plasma can sensitively reflect the degree of tissue damage. Clinical and basic studies have shown that IL-6 expression is positively correlated with central nervous system inflammation.[16,17] This study results showed that the serum CXCL13 and IL-6 concentrations in the two groups increased significantly during and after surgery. However, the levels were significantly lower in the group EDA than in the group CON, indicating that EDA can reduce the concentrations of serum CXCL13 and IL-6, inhibits the inflammatory response of the central nervous system during the perioperative period, and effectively improve neurocognitive function during the perioperative period. The underlying mechanism may be as follows. (1) Anti-inflammatory effects: by inhibiting the nuclear facto-kappa B signaling pathway, EDA regulates the release of inflammatory cytokines that downregulate the expression of pro-inflammatory cytokines; upregulates the expression of inflammatory cytokines; reduces the inflammatory protein expression levels in the hippocampus, high mobility group protein box 1, inducible nitric oxide synthase, neuronal nitric oxide synthase, and endothelial nitric oxide synthase[18]; and reduces the immune response of CXCL13, which increases in an inflammatory environment. (2) Antioxidant effects: the permeability of EDA (owing to its small molecular weight) toward the blood-brain barrier is as high as 60%, which can inhibit the production of reactive oxygen species (ROS), reduce the production of the inflammatory transmitter leukotriene, inhibit delayed neuron death, reduce ROS oxidative damage to polyunsaturated fatty acids in the cell phospholipid membranes, confront apoptosis, inhibit peroxidation in cells (vascular endothelial cells or nerve cells), and reduce Ca2+-induced swelling of the brain's mitochondria, thereby reducing brain edema and brain tissue damage.[19] (3) Protective effects on human brain microvascular endothelial cells: EDA promotes the formation of tight junctions of vascular endothelial cells by inhibiting interleukin-1 beta induced secretion of monocyte chemotactic protein-1, thus alleviating damage to human brain microvascular endothelial cells.[20] Additionally, EDA protects neuronal cells from ischemia and hypoxia by inhibiting the activation of 5-lipoxygenase and maintaining the ultrastructure and integration of mitochondria.[21]
The limitation of this study lies in that the patients diagnosed with PND at 1 and 12 months after surgery were not further diagnosed with mild NCD or major NCD according to the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, which requires further studies.
In summary, the serum concentrations of CXCL13 and IL-6 in elderly patients with hip joint replacement increased during the perioperative period. EDA can reduce serum CXCL13 and IL-6 concentrations in such patients and improve neurocognitive function during the perioperative period.
Funding
This study was supported by the grant from the Projects of Guangdong Medical Science and Technology Research Foundation (No. C2018055).
Conflicts of interest
None.
Footnotes
How to cite this article: Xie HH, Ma HY, Zhang S, Li JW, Han Q, Chen HQ, Su BQ, Zhou JP. Impact of edaravone on serum CXC chemokine ligand-13 levels and perioperative neurocognitive disorders in elderly patients with hip replacement. Chin Med J 2021;134:1610–1615. doi: 10.1097/CM9.0000000000001492
References
- 1.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery. Can J Anaesth 2018; 65:1248–1257. doi: 10.1007/s12630-018-1216-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Zhu H, Hou Y, Gu W, Wu H, Luan Y, et al. Galantamine reversed early postoperative cognitive deficit via alleviating inflammation and enhancing synaptic transmission in mouse hippocampus. Eur J Pharmacol 2019; 846:63–72. doi: 10.1016/j.ejphar.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Hovens IB, van Leeuwen BL, Mariani MA, Kraneveld AD, Schoemaker RG. Postoperative cognitive dysfunction and neuroinflammation: cardiac surgery and abdominal surgery are not the same. Brain Behav Immun 2016; 54:178–193. doi: 10.1016/j.bbi.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microgliamediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2017; 2:e91229.doi: 10.1172/jci.insight.91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irani DN. Regulated production of CXCL13 within the central nervous system. J Clin Cell Immunol 2016; 7:460.doi: 10.4172/2155-9899.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Luo Z, Bi A, Yang W, An W, Dong X, et al. Compound edaravone alleviates lipopolysaccharide (LPS)-induced acute lung injury in mice. Eur J Pharmacol 2017; 811:1–11. doi: 10.1016/j.ejphar.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WW, Bai F, Wang J, Zheng RH, Yang LW, James EA, et al. Edaravone inhibits pressure overload-induced cardiac fibrosis and dysfunction by reducing expression of angiotensin II AT1 receptor. Drug Des Devel Ther 2017; 11:3019–3033. doi: 10.2147/DDDT.S144807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Jiang Y, Zhang G, Lin Z, Du S. Protective effect of edaravone on blood-brain barrier by affecting NRF-2/HO-1 signaling pathway. Exp Ther Med 2019; 18:2437–2442. doi: 10.3892/etm.2019.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Q, Cai Y, Li S, Liu H, Zhou X, Lu C, et al. Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood–brain barrier permeability. Theranostics 2017; 7:884–898. doi: 10.7150/thno.18219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Cao J, Liu N, Ma L, Zhou X, Zhang H, et al. Protective effects of edaravone in adult rats with surgery and lipopolysaccharide administration-induced cognitive function impairment. PLoS One 2016; 11:e0153708.doi: 10.1371/journal.pone.0153708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie H, Zhang S, Huang D, et al. Effects of edaravone on serum ADP and postoperative cognitive function in elderly patient after artificial hip replacement [in Chinese]. J Clin Anesthesiol 2015; 31:346–349. [Google Scholar]
- 12.Czyż-Szypenbejl K, Mędrzycka-Dąbrowska W, Kwiecień-Jaguś K, Lewandowska K. The occurrence of postoperative cognitive dysfunction (POCD) – systematic review. Psychiatr Pol 2019; 53:145–160. doi: 10.12740/PP/90648. [DOI] [PubMed] [Google Scholar]
- 13.Mathew JP, Welsh-Bohmer KA, Newman MF. Nomenclature for perioperative cognitive disorders: comment. Anesthesiology 2019; 131:443–444. doi: 10.1097/ALN.0000000000002831. [DOI] [PubMed] [Google Scholar]
- 14.Stuart MJ, Corrigan F, Baune BT. Knockout of CXCR5 increases the population of immature neural cells and decreases proliferation in the hippocampal dentate gyrus. J Neuroinflammation 2014; 11:31.doi: 10.1186/1742-2094-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R, Ma L, Huang H, Ou S, Yuan J, Xu T, et al. Altered expression of CXCL13 and CXCR5 in intractable temporal lobe epilepsy patients and pilocarpine-induced epileptic rats. Neurochem Res 2017; 42:526–540. doi: 10.1007/s11064-016-2102-y. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Cheng J, Yang L, Wang J, Liu H, Lv Z. Ropivacaine for intercostal nerve block improves early postoperative cognitive dysfunction in patients following thoracotomy for esophageal cancer. Med Sci Monit 2019; 25:460–465. doi: 10.12659/MSM.912328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Feng X, Valdearcos M, Lutrin D, Uchida Y, Koliwad SK, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth 2018; 120:537–545. doi: 10.1016/j.bja.2017.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Yang C, Meng X, Li Z, Lv C, Cao P. Neuroprotection of edaravone on the hippocampus of kainate-induced epilepsy rats through Nrf2/HO-1 pathway. Neurochem Int 2018; 112:159–165. doi: 10.1016/j.neuint.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Tian A, Ma H, Zhang R, Cui Y, Wan C. Edaravone improves spatial memory and modulates endoplasmic reticulum stress-mediated apoptosis after abdominal surgery in mice. Exp Ther Med 2017; 14:355–360. doi: 10.3892/etm.2017.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Xu H, Hu Y, He P, Ni Z, Xu H, et al. Edaravone protected human brain microvascular endothelial cells from methylglyoxal-induced injury by inhibiting AGEs/RAGE/oxidative stress. PLoS One 2013; 8:e76025.doi: 10.1371/journal.pone.0076025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Bei Y, Xiao Y, Tong HD, Wu XQ, Chen MT. Edaravone, a free radical scavenger, protects neuronal cells’ mitochondria from ischemia by inactivating another new critical factor of the 5-lipoxygenase pathway affecting the arachidonic acid metabolism. Brain Res 2018; 1690:96–104. doi: 10.1016/j.brainres.2018.03.006. [DOI] [PubMed] [Google Scholar]


