PURPOSE
Anaplastic thyroid cancer (ATC), an aggressive malignancy, is associated with a poor prognosis and an unmet need for effective treatment, especially for patients without BRAF mutations or NTRK or RET fusions. Lenvatinib is US Food and Drug Administration–approved for radioiodine-refractory differentiated thyroid cancer and has previously demonstrated activity in a small study of patients with ATC (n = 17). We aimed to further evaluate lenvatinib in ATC.
METHODS
This open-label, multicenter, international, phase II study enrolled patients with ATC, who had ≥ 1 measurable target lesion, to receive lenvatinib 24 mg once daily. The primary end points were objective response rate (ORR) by investigator assessment per RECIST v1.1 and safety. Responses were confirmed ≥ 4 weeks after the initial response. Additional end points included progression-free survival and overall survival (OS).
RESULTS
The study was halted for futility as the minimum ORR threshold of 15% was not met upon interim analysis. The interim analysis set included the first 20 patients. The full analysis set includes all 34 enrolled and treated patients. In the full analysis set, one patient achieved a partial response (ORR, 2.9%; 95% CI, 0.1 to 15.3). More than half of the evaluable patients experienced tumor shrinkage; three patients experienced a > 30% tumor reduction. The median progression-free survival was 2.6 months (95% CI, 1.4 to 2.8); the median overall survival was 3.2 months (95% CI, 2.8 to 8.2). The most common treatment-related adverse events (AEs) were hypertension (56%), decreased appetite (29%), fatigue (29%), and stomatitis (29%). No major treatment-related bleeding events or grade 5 treatment-related AEs occurred.
CONCLUSION
The safety profile of lenvatinib in ATC was manageable, and many AEs were attributable to the progression of ATC. The results suggest that lenvatinib monotherapy may not be an effective treatment for ATC; further investigation may be warranted.
INTRODUCTION
Anaplastic thyroid cancer (ATC) is an aggressive follicular cell–derived malignancy associated with a historically poor prognosis and a 5-year survival rate of < 10%.1 Although ATC is rare and makes up only 2% of all thyroid cancers, it accounts for up to 50% of all thyroid cancer–related deaths.2 Until recently, available treatment options had limited efficacy, and the duration of response was generally short (2-5 months).3
CONTEXT
Key Objective
To further evaluate the role of lenvatinib in the treatment of anaplastic thyroid cancer (ATC) as there is a lack of effective treatment options for patients with ATC.
Knowledge Generated
In the full analysis set, the objective response rate was low (2.9%; 95% CI, 0.1 to 15.3) with only one patient achieving a confirmed partial response. However, more than half of the evaluable patients (n = 28) experienced tumor shrinkage as evaluated by investigator assessment per RECIST v1.1; three of the 28 patients experienced a > 30% reduction in total target lesion size (sum of diameters) from baseline to postbaseline nadir.
Relevance
Based on these results, lenvatinib monotherapy may not be an effective treatment for ATC. However, further investigation of lenvatinib may be warranted. The authors would recommend future studies evaluating lenvatinib in combination with other anticancer agents.
Recent advances in the treatment of ATC have in part centered around patients with BRAF V600E–mutated tumors, who represent 20%-50% of all patients with ATC.4 An open-label, phase II, basket study evaluated the efficacy and safety of dabrafenib plus trametinib in patients with BRAF V600E–mutated tumors.5 The BRAF V600E–mutated ATC cohort (n = 16) demonstrated a confirmed overall response rate of 69% (95% CI, 41 to 89) and an estimated 12-month progression-free survival (PFS) of 79%.5 As a result, dabrafenib in combination with trametinib has been US Food and Drug Administration–approved for the treatment of BRAF V600E–mutated ATC.6 Additional tumor-agnostic US Food and Drug Administration approvals have recently occurred for NTRK fusion–driven and RET fusion–driven cancers.7-9 However, these genetic rearrangements are not as frequently seen in ATC.10
There remains an unmet need for effective treatment options for patients with metastatic ATC, especially for those without BRAF mutations or NTRK or RET fusions. A small-scale phase II study conducted in Japan evaluated lenvatinib in 17 patients with ATC, observing a 24% partial response rate.3,11 Therefore, in this multicenter, international, phase II study, we aimed to further evaluate the efficacy and safety of lenvatinib (a multikinase inhibitor of vascular endothelial growth factor receptors 1-3, fibroblast growth factor receptors 1-4, platelet-derived growth factor receptor-α, RET, and KIT12-15) in patients with ATC.
METHODS
This open-label, multicenter, international, phase II study enrolled patients with ATC to receive lenvatinib 24 mg once daily. Patients who were unable to swallow the lenvatinib capsule whole could dissolve the capsule in a small glass of liquid. Eligible patients were required to have a histologic diagnosis of ATC by central review of pathology, ≥ 1 measurable target lesion per Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1), an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 1, adequately controlled blood pressure, and adequate liver, kidney, and bone marrow function. Adequate blood coagulation with an International Normalized Ratio ≤ 1 was also required. Patients who had had prior surgery and/or radiation ≥ 2 weeks before the first dose of lenvatinib could be enrolled. Additionally, prior neoadjuvant, adjuvant, or palliative chemotherapy for ATC was allowed except for prior tyrosine kinase inhibitor therapy. Patients with brain metastases who had completed whole brain radiotherapy, stereotactic radiosurgery, or complete surgical resection were eligible if they remained asymptomatic and stable and did not receive steroid treatment within 1 month of enrollment. Patients were excluded if they had radiographic evidence of major blood vessel invasion or if they were candidates for comprehensive multimodality treatment (surgery and/or external beam radiotherapy or chemoradiotherapy).
The primary efficacy end point was confirmed objective response rate (ORR) by investigator assessment per RECIST v1.1. Secondary and exploratory end points included PFS, overall survival (OS), duration of objective response, disease control rate (DCR; complete response + partial response + stable disease [≥ 5 weeks]), and clinical benefit rate (CBR; complete response + partial response + durable stable disease of ≥ 23 weeks). Tumor assessments were conducted per RECIST v1.1 at screening and every 6 weeks ± 1 week; complete and partial responses were confirmed ≥ 4 weeks after the initial response. A post hoc multivariate analysis was conducted to identify factors that affected OS; factors evaluated included baseline ECOG PS (0 v 1), prior radiotherapy, baseline sum of tumor diameters (mm), and the percent change from baseline to postbaseline nadir in sums of tumor diameters. The hazard ratio (HR) was estimated using a Cox proportional hazard model; all P values and statistical significance were nominal. Safety was assessed by monitoring and recording of all adverse events (AEs) per Common Terminology Criteria for Adverse Events version 4.03 and routine laboratory assessments.
The Protocol (online only) specified that a sample size of 57 evaluable patients would be required to have a power of 0.932, using a binomial exact test, to demonstrate a statistical significance of .025 (1-sided alpha), with an assumed ORR of 27% compared with a historic control ORR of 10%. Additionally, the study protocol specified that a descriptive interim analysis would be performed after the first 20 patients had completed at least two tumor assessments (the baseline scan and the first on-treatment scan at 6 weeks) or discontinued treatment for any reason. If the number of responders was ≤ 3 (an ORR ≤ 15%) at the interim analysis (n = 20), then enrollment would be halted; safety and efficacy would be evaluated to determine whether the study would be terminated. Enrollment continued until the decision to terminate (based on the interim analysis) was made, and the full analysis was to be performed on the full analysis set (FAS), which included all patients enrolled and who received at least one dose of study drug.
This study was performed in full collaboration with the International Thyroid Oncology Group. The protocol was approved by the relevant institutional review boards or ethics committees and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent before study enrollment.
RESULTS
Patients
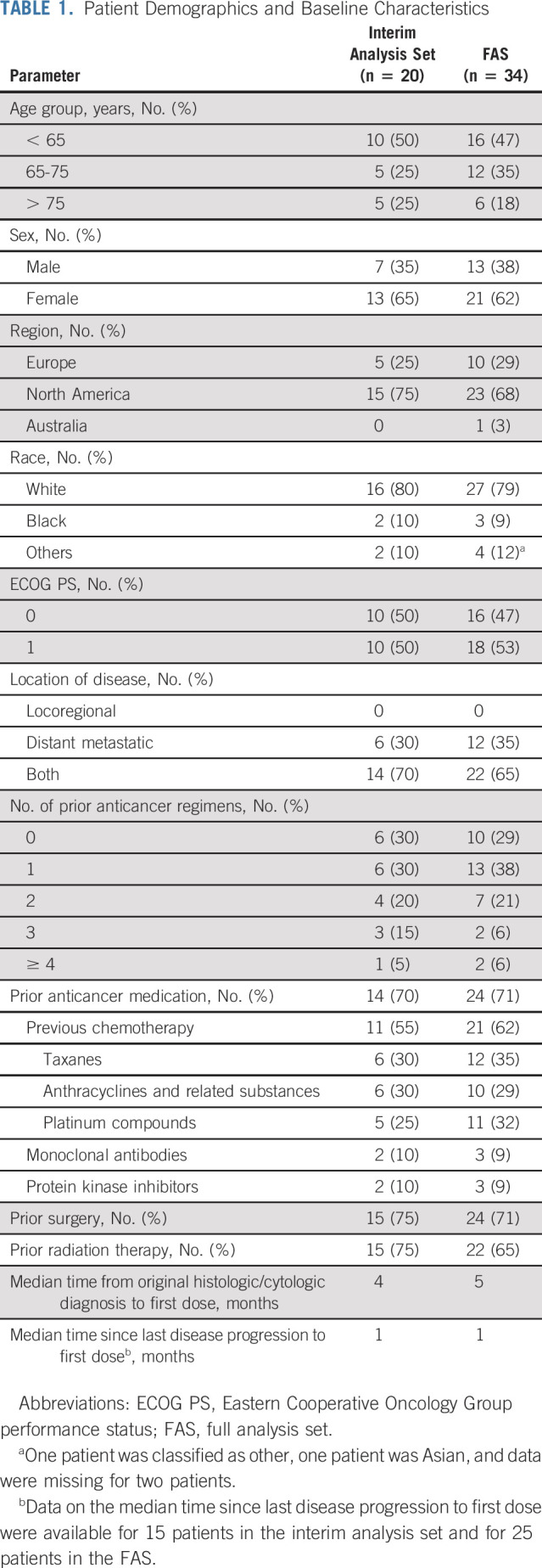
The interim analysis set included the first 20 patients, and the FAS included the 34 enrolled and treated patients (Fig 1). The total planned population of 57 patients was not reached because enrollment was halted based on the interim analysis. Central pathology review confirmed the diagnosis of ATC in 33 of 34 patients enrolled. Most patients had ≤ 1 prior anticancer therapy regimen for metastatic disease. Additionally, most patients had prior radiation therapy and/or prior surgery (Table 1).
FIG 1.
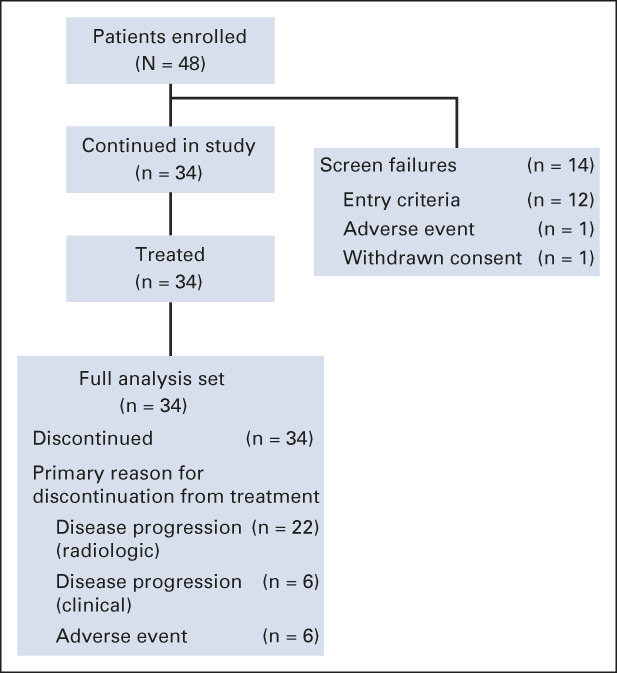
Patient disposition and primary reason for discontinuation from study treatment. The interim analysis set comprised the first 20 evaluable patients who had completed at least two tumor assessments (including the baseline scan and the first on-treatment scan at 6 weeks) or discontinued treatment because of any reason.
TABLE 1.
Patient Demographics and Baseline Characteristics
Efficacy
Upon the interim analysis, the confirmed ORR was 0% as there were no patients with a confirmed partial or complete response in the interim analysis set. There was one patient who experienced an unconfirmed partial response for an unconfirmed ORR of 5% (95% CI, 0.1 to 24.9; Table 2). Therefore, the study was halted based on the prespecified criteria for futility, as the minimum ORR threshold of 15% was not met.
TABLE 2.
Efficacy Summary
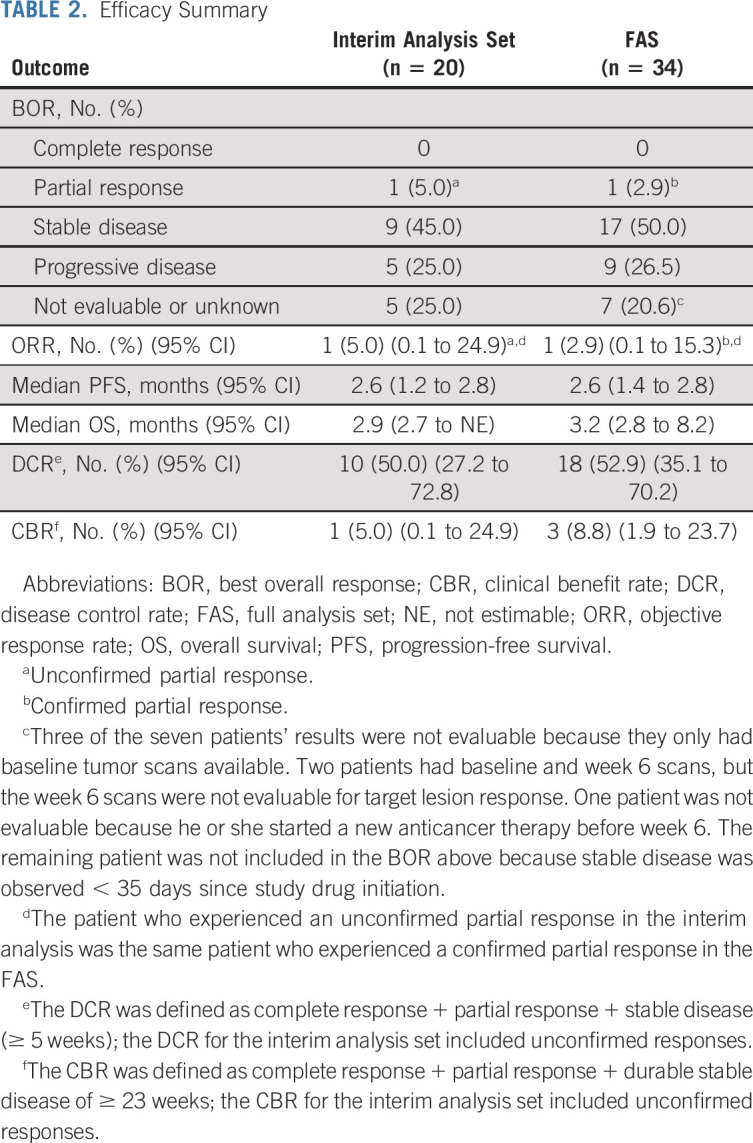
In the FAS, one patient achieved a confirmed partial response (ORR, 2.9%; 95% CI, 0.1 to 15.3; Table 2). The duration of response for this one patient was 4.1 months. There were two additional patients who also experienced a > 30% reduction in total target lesion size, but their responses could not be confirmed because of disease progression. Of note, more than half of the evaluable patients experienced tumor shrinkage (Fig 2). The median PFS was 2.6 months (95% CI, 1.4 to 2.8) (Fig 3A), and the median OS was 3.2 months (95% CI, 2.8 to 8.2) (Fig 3B). The median follow-up time for OS was 15.3 months (95% CI, 11.6 to 16.9). The DCR and CBR were 52.9% (95% CI, 35.1 to 70.2) and 8.8% (95% CI, 1.9 to 23.7), respectively (Table 2).
FIG 2.
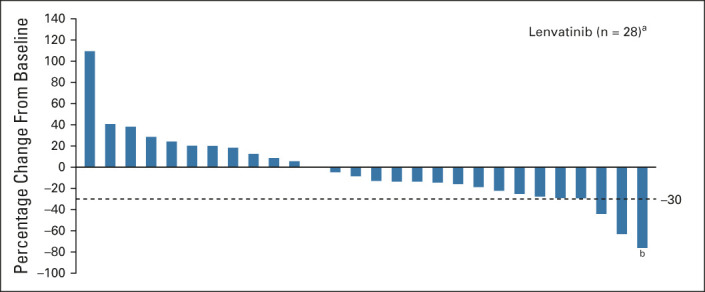
Percentage change in sum of diameters of target lesions from baseline to postbaseline nadir by investigator assessment per RECIST v1.1 (full analysis set). aEvaluable patients with both a baseline and at least one postbaseline target lesion assessment. bThis bar represents one confirmed responder.
FIG 3.
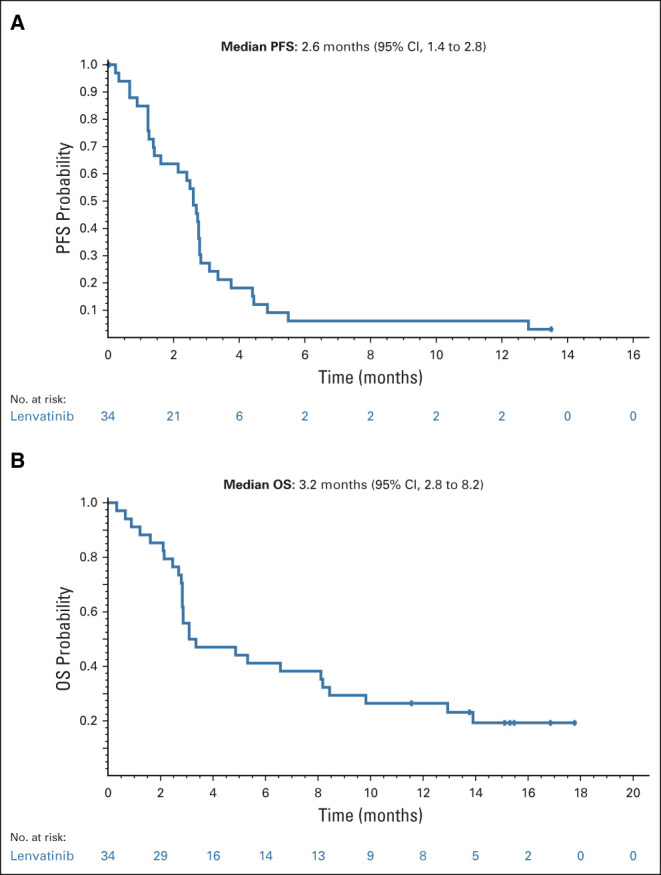
Kaplan-Meier plot of (A) PFS by investigator assessment per RECIST v1.1 (full analysis set) and (B) OS (full analysis set). OS, overall survival; PFS, progression-free survival.
A post hoc multivariate analysis of OS demonstrated that the following factors might have had a significant impact on OS: prior radiotherapy (HR, 3.65; 95% CI, 1.15 to 11.52; P = .0275), the percent change from baseline to postbaseline nadir in sums of diameters of target lesions (HR, 1.03; 95% CI, 1.01 to 1.04; P = .0009), and the baseline sums of tumor diameters (HR, 1.02; 95% CI, 1.01 to 1.04; P = .0010). Baseline ECOG PS (0 v 1) did not significantly influence OS (HR, 0.94; 95% CI, 0.38 to 2.32; P = .8924).
Study Drug Exposure
In the FAS, the median dose intensity was 21.6 mg/day per patient (range, 11.1-44.0) and the median duration of treatment was 2.3 months (range, 0.3-12.9). Dose reductions were required in 41% of patients (14 patients). The median time to first dose reduction was 4.6 weeks (range, 1.0-16.0).
Safety
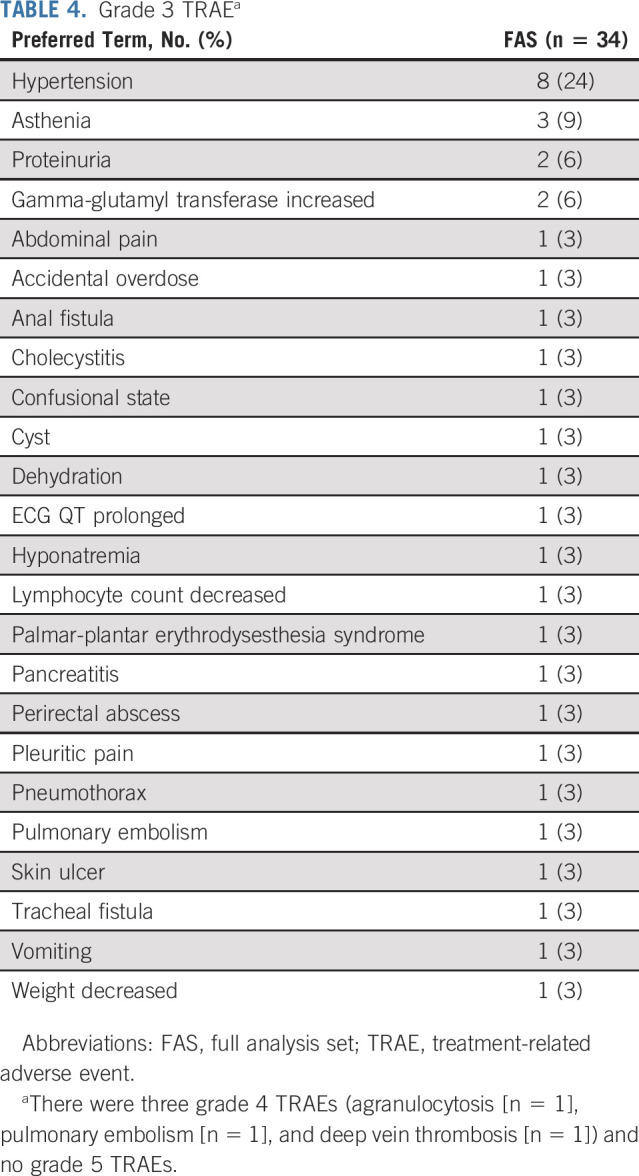
All patients in the FAS (n = 34; 100%) experienced treatment-emergent AEs (TEAEs) (Table 3). Treatment-related AEs (TRAEs) occurred in 94% of patients (n = 32). The most common TRAEs were hypertension (56%), decreased appetite (29%), fatigue (29%), and stomatitis (29%). Grade ≥ 3 TRAEs occurred in 62% of patients, and the most common TRAE was hypertension (24%) (Table 4). Of note, there were no major treatment-related bleeding events including those because of fistula formation. Two patients experienced fistula formation; one patient developed a grade 3 tracheal fistula, and the other patient developed a grade 3 anal fistula and a grade 3 perirectal abscess. At the time of data cutoff (October 30, 2018), there had been 27 deaths; 13 of these deaths occurred > 28 days after the last dose of lenvatinib. TEAEs led to 14 deaths that occurred within 28 days of the last dose, but none were considered treatment related. Grade 5 TEAEs included dysphagia (n = 1), septic shock (n = 1), hypoxia (n = 1), and malignant neoplasm progression (n = 10); one patient was reported with three grade 5 TEAEs comprising cardiopulmonary failure, renal failure, and pulmonary edema.
TABLE 3.
Safety Summary
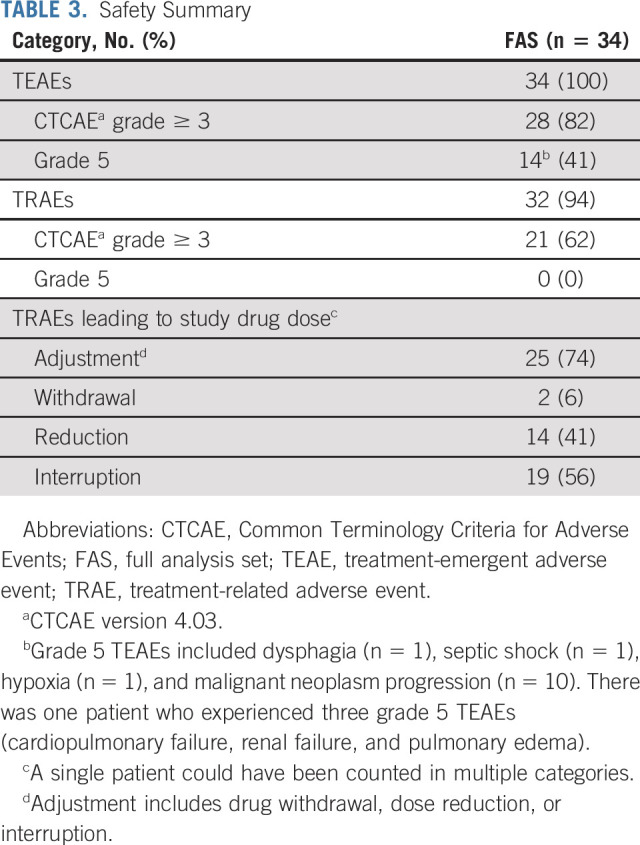
TABLE 4.
Grade 3 TRAEa
DISCUSSION
The present study was closed to enrollment following the interim analysis that demonstrated a lack of efficacy per prespecified criteria (ie, ORR < 15%) with lenvatinib treatment in patients with ATC. The response rate was approximately 3%, with half of the 34 patients achieving stable disease (≥ 5 weeks). The median PFS and OS were 2.6 and 3.2 months, respectively. Additionally, the CBR was low (8.8%; 95% CI, 1.9 to 23.7). The safety profile of lenvatinib in ATC was similar to that observed in other previous lenvatinib studies.16-18 We did observe two cases of fistula (anal and tracheal), which can be expected with potent antiangiogenic therapy, but there were no severe bleeding events likely because of strict patient selection. Of note, patients with major vessel involvement were excluded from this trial. Additionally, many of the AEs observed in this study were attributable to the progression of ATC; the grade 5 TEAEs that occurred were all likely related to underlying disease progression.
Previously, Tahara et al3 demonstrated that 24% of patients with ATC achieved a partial response with lenvatinib and the median OS was 10.6 months. However, there were several differences between our study and that of Tahara et al, which might have contributed to the poor response observed in our study compared with the activity of lenvatinib in patients with ATC that was observed in the study by Tahara et al. Unlike the previous study, the present study required a central pathology review to confirm a diagnosis of ATC. In the Tahara study, only 10 patients had a confirmed diagnosis of ATC per independent pathologic review. Furthermore, our study enrolled more patients (n = 34; v n = 17 in the study by Tahara et al3), and more patients had received prior chemotherapy (62% v 41%). In the study by Tahara et al,3,11 all patients were Japanese and tumor assessments were initially conducted more frequently (every 4 weeks up to week 16 v every 6 weeks). A post hoc analysis of the phase III Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT) suggested that people of Japanese origin may experience an increased exposure to lenvatinib because a higher rate of several AEs was reported in Japanese patients compared with non-Japanese patients.19 An increased exposure to lenvatinib because of lower body weight might have also contributed to the greater efficacy observed in the Japanese study. Additionally, our study required confirmation of response. Patients were required to experience stable disease for a longer time period because durable stable disease for the CBR was defined as ≥ 23 weeks of duration instead of 11 weeks, and stable disease for best overall response as ≥ 5 weeks of duration instead of 3 weeks.3,11
Other multikinase inhibitors of vascular endothelial growth factor receptors and other kinases, namely, sorafenib and pazopanib, have been evaluated as monotherapy in ATC.20,21 In a phase II trial assessing sorafenib, two of the 20 patients enrolled experienced a partial response; the median PFS was 1.9 months (95% CI, 1.3 to 3.6), and the median OS was 3.9 months (95% CI, 2.2 to 7.1).20 In a phase II trial assessing pazopanib, no tumor responses were observed among the 15 enrolled patients; the median time to progression was 62 days, and the median OS was 111 days.21
Combination therapies involving a multikinase inhibitor have been shown to improve survival outcomes in several tumor types, and therefore, combination therapy is a potential approach to investigate, especially given the aggressive nature of ATC. Specifically, lenvatinib in combination with programmed death (PD)-1 inhibition has demonstrated efficacy and tolerability in other tumor types.22,23 Another PD-1 inhibitor, spartalizumab, was evaluated in a phase I/II study that enrolled 42 patients with ATC.24 Spartalizumab demonstrated an ORR of 19% using RECIST v1.1, and although median duration of response was not met by the time of publication, it ranged from 16.7 weeks to 1.6 years. These results, combined with the tumor reduction seen transiently with lenvatinib in this study and in that of Tahara et al,3 suggest that investigation of lenvatinib in combination with an anti-PD-1 antibody for the treatment of ATC is warranted.
Currently (December 2020), there are two ongoing studies evaluating lenvatinib in combination with pembrolizumab for the treatment of ATC25,26: The ATLEP study is a phase II study assessing lenvatinib plus pembrolizumab in patients with ATC and poorly differentiated thyroid cancer,26 and an additional phase II study is planned to evaluate lenvatinib plus pembrolizumab in unresectable locally advanced or metastatic ATC in the United States.25
One limitation to note is that our study did not collect BRAF mutation status, and patients with this mutation should be treated with BRAF-targeted therapies such as dabrafenib and trametinib. The results from our study, along with those from two previous studies,20,21 suggest that monotherapy with the tyrosine kinase inhibitor lenvatinib may not be an effective treatment option for ATC; however, further investigation may be warranted. We hope that data from this study will provide useful information for future studies in patients with ATC, particularly the single-arm studies investigating lenvatinib in combination with other anticancer agents.
Acknowledgments
ACKNOWLEDGMENT
This study and report were conducted and prepared by the authors and funding partners, and performed in collaboration with the International Thyroid Oncology Group.
Lori J. Wirth
Consulting or Advisory Role: Merck, Loxo, Blueprint Medicines, Eisai, Lilly, Bayer, Exelixis
Research Funding: Checkmate Pharmaceuticals, Lilly, Ayala Pharmaceuticals, Eisai
Expert Testimony: Eisai
Marcia S. Brose
Honoraria: Bayer
Consulting or Advisory Role: Bayer, Eisai, Blueprint Medicines, Loxo, Exelixis
Research Funding: Bayer, Eisai, Exelixis, Blueprint Medicines, Loxo, Lilly
Eric J. Sherman
Consulting or Advisory Role: Cota Healthcare, Goldilocks, Eisai, Regeneron, UpToDate, Lilly, Blueprint Medicines
Research Funding: Plexxikon, Regeneron
Lisa Licitra
Consulting or Advisory Role: Eisai, Boehringer Ingelheim, AstraZeneca, SOBI, Novartis, Bayer, MSD, Merck Serono, Roche, Bristol Myers Squibb, Incyte, Doxapharma, GlaxoSmithKline, Nanobiotix, Debiopharm Group, Amgen, Ipsen
Research Funding: AstraZeneca, Novartis, Roche, MSD, Eisai, Merck Serono, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Exelixis, IRX Therapeutics, Medpace, Pfizer, Debiopharm Group
Travel, Accommodations, Expenses: Merck Serono, Bristol Myers Squibb, MSD, Eisai, AstraZeneca
Martin Schlumberger
Consulting or Advisory Role: Eisai, Genzyme, Bayer, Exelixis/Ipsen
Steven I. Sherman
Honoraria: Eisai
Consulting or Advisory Role: Exelixis, Loxo, Lilly
Research Funding: Exelixis
Bruce Robinson
Leadership: Cochlear, Mayne Pharma
Stock and Other Ownership Interests: Cochlear, Mayne Pharma
Consulting or Advisory Role: Loxo, Eisai
Speakers' Bureau: Eisai
Travel, Accommodations, Expenses: Eisai
Patrice Rodien
Consulting or Advisory Role: Eisai Europe
Research Funding: Pfizer
Yann Godbert
Honoraria: Eisai Europe, Bayer, Lilly
Travel, Accommodations, Expenses: Eisai Europe
Christelle De La Fouchardiere
Honoraria: Merck Serono, Roche
Consulting or Advisory Role: Lilly, Bayer, Amgen, Bristol Myers Squibb, Servier, Roche, Pierre Fabre
Research Funding: Roche
Travel, Accommodations, Expenses: Roche, Celgene, Amgen, Bristol Myers Squibb, Servier
Other Relationship: Incyte, MSD Oncology
Kate Newbold
Speakers' Bureau: Eisai Europe
Christopher Nutting
Employment: Advanced Oncotherapy
Soamnauth Misir
Employment: Eisai, Urogen Pharma
Ana Almonte
Employment: Eisai
Maria E. Cabanillas
Honoraria: Loxo/Lilly
Consulting or Advisory Role: Loxo, Ignyta
Research Funding: Kura Oncology, Eisai, Roche/Genentech, Exelixis, Merck
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part previously as congress abstracts at The Endocrine Society's Annual Meeting; Virtual Format; June 8-22, 2020 (poster no. MON-521).
SUPPORT
Supported by Eisai Inc, Woodcliff Lake, NJ, and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co Inc, Kenilworth, NJ. Medical writing support was provided by Jessica Pannu, PharmD, of Oxford PharmaGenesis Inc, Newtown, PA. This support was funded by Eisai Inc, Woodcliff Lake, NJ, and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The data will not be available for sharing at this time as the data are commercially confidential. However, Eisai will consider written requests to share the data on a case-by-case basis.
AUTHOR CONTRIBUTIONS
Conception and design: Lori J. Wirth, Marcia S. Brose, Eric J. Sherman, Lisa Licitra, Martin Schlumberger, Steven I. Sherman, Bruce Robinson, Christelle De La Fouchardiere, Christopher Nutting, Soamnauth Misir, Weifei Ye
Administrative support: Ana Almonte
Provision of study materials or patients: Eric J. Sherman, Martin Schlumberger, Keith C. Bible, Bruce Robinson, Patrice Rodien, Christelle De La Fouchardiere, Maria E. Cabanillas
Collection and assembly of data: Lori J. Wirth, Marcia S. Brose, Eric J. Sherman, Lisa Licitra, Martin Schlumberger, Steven I. Sherman, Keith C. Bible, Bruce Robinson, Patrice Rodien, Yann Godbert, Christelle De La Fouchardiere, Christopher Nutting, Soamnauth Misir, Ran Xie, Ana Almonte, Weifei Ye, Maria E. Cabanillas
Data analysis and interpretation: Lori J. Wirth, Marcia S. Brose, Eric J. Sherman, Lisa Licitra, Martin Schlumberger, Steven I. Sherman, Bruce Robinson, Yann Godbert, Christelle De La Fouchardiere, Kate Newbold, Christopher Nutting, Soamnauth Misir, Ran Xie, Weifei Ye
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Open-Label, Single-Arm, Multicenter, Phase II Trial of Lenvatinib for the Treatment of Patients With Anaplastic Thyroid Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lori J. Wirth
Consulting or Advisory Role: Merck, Loxo, Blueprint Medicines, Eisai, Lilly, Bayer, Exelixis
Research Funding: Checkmate Pharmaceuticals, Lilly, Ayala Pharmaceuticals, Eisai
Expert Testimony: Eisai
Marcia S. Brose
Honoraria: Bayer
Consulting or Advisory Role: Bayer, Eisai, Blueprint Medicines, Loxo, Exelixis
Research Funding: Bayer, Eisai, Exelixis, Blueprint Medicines, Loxo, Lilly
Eric J. Sherman
Consulting or Advisory Role: Cota Healthcare, Goldilocks, Eisai, Regeneron, UpToDate, Lilly, Blueprint Medicines
Research Funding: Plexxikon, Regeneron
Lisa Licitra
Consulting or Advisory Role: Eisai, Boehringer Ingelheim, AstraZeneca, SOBI, Novartis, Bayer, MSD, Merck Serono, Roche, Bristol Myers Squibb, Incyte, Doxapharma, GlaxoSmithKline, Nanobiotix, Debiopharm Group, Amgen, Ipsen
Research Funding: AstraZeneca, Novartis, Roche, MSD, Eisai, Merck Serono, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Exelixis, IRX Therapeutics, Medpace, Pfizer, Debiopharm Group
Travel, Accommodations, Expenses: Merck Serono, Bristol Myers Squibb, MSD, Eisai, AstraZeneca
Martin Schlumberger
Consulting or Advisory Role: Eisai, Genzyme, Bayer, Exelixis/Ipsen
Steven I. Sherman
Honoraria: Eisai
Consulting or Advisory Role: Exelixis, Loxo, Lilly
Research Funding: Exelixis
Bruce Robinson
Leadership: Cochlear, Mayne Pharma
Stock and Other Ownership Interests: Cochlear, Mayne Pharma
Consulting or Advisory Role: Loxo, Eisai
Speakers' Bureau: Eisai
Travel, Accommodations, Expenses: Eisai
Patrice Rodien
Consulting or Advisory Role: Eisai Europe
Research Funding: Pfizer
Yann Godbert
Honoraria: Eisai Europe, Bayer, Lilly
Travel, Accommodations, Expenses: Eisai Europe
Christelle De La Fouchardiere
Honoraria: Merck Serono, Roche
Consulting or Advisory Role: Lilly, Bayer, Amgen, Bristol Myers Squibb, Servier, Roche, Pierre Fabre
Research Funding: Roche
Travel, Accommodations, Expenses: Roche, Celgene, Amgen, Bristol Myers Squibb, Servier
Other Relationship: Incyte, MSD Oncology
Kate Newbold
Speakers' Bureau: Eisai Europe
Christopher Nutting
Employment: Advanced Oncotherapy
Soamnauth Misir
Employment: Eisai, Urogen Pharma
Ana Almonte
Employment: Eisai
Maria E. Cabanillas
Honoraria: Loxo/Lilly
Consulting or Advisory Role: Loxo, Ignyta
Research Funding: Kura Oncology, Eisai, Roche/Genentech, Exelixis, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society : Thyroid Cancer Survival Rates, by Type and Stage. https://www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html [Google Scholar]
- 2.Salehian B Liem SY Mojazi Amiri H, et al. : Clinical trials in management of anaplastic thyroid carcinoma; progressions and set backs: A systematic review. Int J Endocrinol Metab 17:e67759, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahara M Kiyota N Yamazaki T, et al. : Lenvatinib for anaplastic thyroid cancer. Front Oncol 7:25, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crispo F Notarangelo T Pietrafesa M, et al. : BRAF inhibitors in thyroid cancer: Clinical impact, mechanisms of resistance and future perspectives. Cancers (Basel) 11:1388, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbiah V Kreitman RJ Wainberg ZA, et al. : Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 36:7-13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration : FDA Approves Dabrafenib Plus Trametinib for Anaplastic Thyroid Cancer With BRAF V600E Mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-anaplastic-thyroid-cancer-braf-v600e-mutation [Google Scholar]
- 7.US Food and Drug Administration : FDA Approves Entrectinib for NTRK Solid Tumors and ROS-1 NSCLC. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc [Google Scholar]
- 8.US Food and Drug Administration : FDA Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions. https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0 [Google Scholar]
- 9.US Food and Drug Administration : FDA Approves Pralsetinib for Lung Cancer with RET Gene Fusions. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pralsetinib-lung-cancer-ret-gene-fusions [Google Scholar]
- 10.Cabanillas ME Zafereo M Williams MD, et al. : Recent advances and emerging therapies in anaplastic thyroid carcinoma. F1000Res 7:F1000 Faculty Rev-87, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi S Kiyota N Yamazaki T, et al. : A phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 15:717-726, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Matsui J Funahashi Y Uenaka T, et al. : Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 14:5459-5465, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Matsui J Yamamoto Y Funahashi Y, et al. : E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 122:664-671, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Okamoto K Kodama K Takase K, et al. : Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett 340:97-103, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y Matsui J Matsushima T, et al. : Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 6:18, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ Hutson TE Glen H, et al. : Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16:1473-1482, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Schlumberger M Tahara M Wirth LJ, et al. : Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621-630, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Schlumberger M Jarzab B Cabanillas ME, et al. : A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res 22:44-53, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Kiyota N Schlumberger M Muro K, et al. : Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 106:1714-1721, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savvides P Nagaiah G Lavertu P, et al. : Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 23:600-604, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bible KC Suman VJ Menefee ME, et al. : A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab 97:3179-3184, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor MH Lee CH Makker V, et al. : Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol 38:1154-1163, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makker V Taylor MH Aghajanian C, et al. : Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 38:2981-2992, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capdevila J Wirth LJ Ernst T, et al. : PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol 38:2620-2627, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov : Lenvatinib and Pembrolizumab for the Treatment of Stage IVB Locally Advanced and Unresectable or Stage IVC Metastatic Anaplastic Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT04171622 [Google Scholar]
- 26.Dierks C Miething C Thomusch O, et al. : Lenvatinib and pembrolizumab as save and effective combination treatment in 8 patients with metastasized anaplastic (ATC) or poorly differentiated thyroid carcinoma (PDTC). Ann Oncol 29(:viii646, 2018. (suppl 8; abstr 1824P) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be available for sharing at this time as the data are commercially confidential. However, Eisai will consider written requests to share the data on a case-by-case basis.


