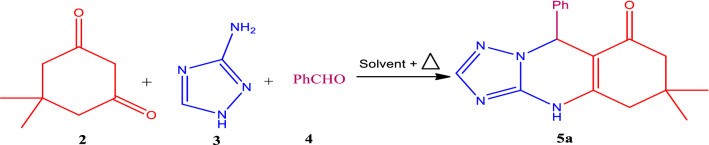
Abstract
Novel nano-ordered 1,1,3,3-tetramethylguanidine-functionalized melamine (Melamine@TMG) organocatalyst was prepared and adequately identified by various techniques including FTIR, EDX, XRD and SEM spectroscopic or microscopic methods as well as TGA and DTG analytical methods. The Melamine@TMG, as an effective multifunctional organocatalyst, was found to promote smoothly the three-component synthesis of 1,2,4-triazoloquinazolinone derivatives using cyclic dimedone, 3-amino-1,2,4-triazole and different benzaldehyde derivatives in EtOH at 40 °C. This practical method afforded the desired products in high to excellent yields (86–99%) and short reaction times (10–25 min). The main advantages of this new method are the use of heterogeneous multifunctional nanocatalyst, simple work-up procedure with no need for chromatographic purification, highly selective conversion of substrates and recyclability of the catalyst, which could be used in five consecutive runs with only a small decrease in its activity.
Subject terms: Environmental chemistry, Catalysis, Green chemistry, Materials chemistry, Medicinal chemistry, Organic chemistry, Supramolecular chemistry
Introduction
The discovery of efficient catalytic systems for synthetic chemical reactions is a milestone and key principle in green and sustainable chemistry, which has emerged as an active area of research. Organocatalysis is one of the most popular and rapidly growing research fields since its fundamental “renaissance” at the beginning of the twenty-first century1–12. Organocatalytic systems are generally homogenous in nature2,3,11–13. However, when they are changed into nano-ordered systems it has been proven to possess heterogeneous catalytic behaviour. This may lead to the designing and exploration of new carbonaceous materials which itself is a hot topic in both academia and industry14–21. In addition, a high surface to volume ratio is leading to high activity, selectivity, and often more stability. Nano-ordered organocatalysts can reduce the temperature and the amount of hazardous waste in chemical transformations, and by enhancing the selectivity of a reaction avoid undesired products as well as be simply recovered from the crude products and reused in multiple reactions. Therefore, heterogeneous organocatalysts have resulted in higher yields and cleaner synthesis22–33. In this regard, 1,3,5-triazine-2,4,6-triamine is an appropriate choice for designing new heterogeneous organocatalysts by anchoring proper organic molecules to its surface in order to produce multifunctional catalytic systems. 1,3,5-triazine-2,4,6-triamine, namely melamine or cyanuramide, is symmetric heterocyclic base and has been widely used in different industries such as plastics, resins, fertilizers, and insecticides34. Furthermore, the various catalysts consist of functionalized melamine have been reported for the synthesis of heterocyclic scaffolds having nitrogen, oxygen, or sulfur in their structures. In fact, melamine-based catalytic systems promote organic transformations smoothly, and hence are more environment friendly and cost-effective19,35–43. On the other hand, guanidine and its derivatives have been used, as homogeneous bifunctional organocatalysts, in different organic transformations in recent years4,5,44–50. Therefore, designing and exploration of new heterogeneous organocatalytic systems based on melamine and guanidine derivatives would be very desirable.
Quinazolinone derivatives have received considerable attention as a well-known family of N-containing heterocyclic scaffolds in medicinal chemistry. They possess significant biological and medicinal properties such as antimicrobial51,52, anticonvulsant53, antihistaminic54, antihypertensive55, etc. Among various reported procedures for the synthesis of 1,2,4-triazoloquinazolinone derivatives, the three-component reaction of enolizable 1,3-dicarbonyls, 3-amino-1,2,4-triazole, and aldehydes/ketones has been known as the most beneficial method in terms of obtained yield, selectivity and green chemistry principles51,56–66. Some recent examples include DABCO based ionic liquid67, TiO2 nanoparticles supported ionic liquids68, magnetic nanoparticles coated by silica with bis dicationic bridge69, sulfonic acid functionalized SBA-1570, nanoporous silica71, H4[W12SiO40] grafted on magnetic chitosan72, l-proline51, p-toluenesulfonic acid monohydrate73, sulfamic acid74, anthranilic acid75, molecular iodine76, etc77,78. However, there is a lot more room to explore new and more efficient catalytic systems for this MCR which can work under green conditions using organocatalytic systems.
In this present work, we hereby report a green approach for the synthesis of 1,2,4-triazoloquinazolinones catalyzed by the 1,1,3,3-tetramethylguanidine superbase79 anchored onto melamine (Melamine@TMG, 1) as a novel nano-ordered multifunctional and heterogeneous organocatalyst (Scheme 1). The Melamine@TMG catalyst was prepared in two definite steps: The melamine surface modification with 3-bromopropyl groups was the first step to afford Melamine@PrBr intermediate (I) 41; the second step was functionalization of the obtained Melamine@PrBr nanoparticles with the TMG base through bimolecular nucleophilic substitution (SN2). The obtained nanocatalyst was characterized by different spectroscopic, microscopic, and analytical techniques. The efficiency of the Melamine@TMG nanocatalyst was examined in the synthesis of 1,2,4-triazoloquinazolinones. After completion of reactions, the Melamine@TMG nanocatalyst was separated from the crude products by washing in EtOH during crystallization of the desired products and exhibited the best catalytic performance in the first cycle (98% isolated product) and it’s activity decreased slightly after four times of recycling (84% isolated product).
Scheme 1.
Preparation of nano-ordered multifunctional Melamine@TMG organocatalyst and its application for the three-component synthesis of 1,2,4-triazoloquinazolinones 5a–j.
Experimental
Materials and instruments
All chemicals, reagents, and solvents were supplied by Merck, Sigma-Aldrich, or local Companies and used as received, except for benzaldehyde which a fresh-distilled sample was used. X-ray diffraction (XRD) patterns were obtained using an X’Pert PRO MPD PANalytical Company. The infrared spectra of the catalyst and products were measured by a Bruker-Vector 33 Fourier transform infrared spectrometer (FTIR) using KBr discs. Scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analysis were carried out on a TESCAN-Mira III. The thermogravimetric analysis (TGA) and derivative thermogravimetric (DTG) curves were determined on a TGA/DSC Mettler Toledo apparatus. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE 500 in DMSO-d6 at ambient temperature. The CHN analysis were measured by a Thermo Scientific Eager 800. All compounds, except 5c and 5i, are known and their structures were confirmed by comparison of their melting points as well as FTIR and 1H NMR spectral data with the authentic samples (Supplementary Information).
Preparation of the Melamine@TMG catalyst (1)
Preparation of N2,N4,N6-tris(3-bromopropyl)-1,3,5-triazine-2,4,6-triamine (Melamine@PrBr, I)
Melamine (10 mmol, 1.261 g) was dispersed in EtOH (150 mL) by sonication for 10 min. Then, the obtained mixture was transferred into a round bottom flask and 1,3-dibromopropane (29.4 mmol, d = 1.98 g.cm-3, 3.0 mL) was added41. The obtained colloidal mixture was heated at 60 °C under stirring by a mechanical stirrer (400 rpm) for 24 h. Afterward, the reaction mixture was filtered off and the white solid was washed twice with hot EtOH and dried under vacuum to afford Melamine@PrBr (I) in 96% yield.
Preparation of 1,1,3,3-tetramethylguanidine-functionalized melamine nanocatalyst (Melamine@TMG, 1)
The as-prepared Melamine@PrBr (1.2 g) was dispersed in dry toluene (150 mL) in a round-bottom flask by ultrasonication for 10 min. Then, 1,1,3,3-tetramethylguanidine (TMG, 39.9 mmol, d = 0.918 g.cm-3, 5.0 mL) in triethylamine (79 mmol, d = 0.73 g.cm-3, 11 mL) and KI (1.2 mmol, 0.02 g) were added to the obtained mixture under mechanical stirring (400 rpm). The reaction mixture was then heated under N2 atmosphere for 72 h and the final product was filtered off and washed twice with EtOH and dried at 60 °C under vacuum for 2 h to afford the Melamine@TMG, as a white solid, in 91% yield.
General procedure for the synthesis of 1,2,4-triazoloquinazolinones 5a–j catalyzed by Melamine@TMG (1)
To a mixture of dimedone (2, 1 mmol, 0.140 g), 3-amino-1,2,4-triazole (3, 1 mmol, 0.084 g) and arylaldehyde (4, 1 mmol) in EtOH (3 mL), Melamine@TMG (1, 2.5 mol%, 15 mg) was added. The reaction mixture was stirred at 40 °C for times mentioned in Table 2 and the progress of the reaction was observed by TLC. After completion of the reaction, a precipitate was formed which was dissolved in additional EtOH (3 mL) by heating and filtered off to separate the catalyst 1. The filtrate was allowed to stand at room temperature to give pure products 5a–j. The separated catalyst was then washed with EtOAc and n-hexane (2 mL) for 10 min., respectively, and finally dried at 60 °C under vacuum for 0.5 h to be used for further next runs.
Table 2.
Synthesis of various derivatives of 1,2,4-triazoloquinazolinones 5a–j catalyzed by the nano-ordered Melamine@TMG organocatalyst (1).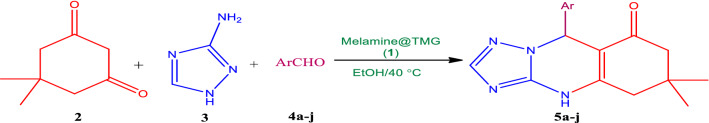
| Entry | Aldehyde 4 | Product 5 | Time (min) | Yielda (%) | Mp (°C) | ||
|---|---|---|---|---|---|---|---|
| Found | Reported | References | |||||
| 1 | PhCHO | 5a | 10 | 98 | 250–252 | 248–250 | 74,77,78 |
| 2 | 2-MeOC6H4CHO | 5b | 20 | 90 | 242–244 | 240–243 | 76,83 |
| 3 | 3,4-(MeO)2C6H3CHO | 5c | 25 | 89 | 225–227 | – | This work |
| 4 | 4-MeC6H4CHO | 5d | 20 | 86 | 262–264 | 264–269 | 74,78 |
| 5 | 4-ClC6H4CHO | 5e | 8 | 98 | 303–305 | 303–305 | 74,78 |
| 6 | 3-O2NC6H4CHO | 5f. | 5 | 97 | 267–269 | 266–269 | 74,78 |
| 7 | 4-O2NC6H4CHO | 5 g | 5 | 98 | 294–297 | 290–294 | 84 |
| 8 | 4-HOC6H4CHO | 5 h | 20 | 91 | 305–308 | > 300 | 74,78 |
| 9 | 3,4-(Cl)2C6H3CHO | 5i | 5 | 99 | 324–326 | – | This work |
| 10 | 4-FC6H4CHO | 5j | 5 | 99 | 257–259 | 258–260 | 85 |
Reaction conditions: dimedone (2, 1 mmol), 3-amino-1,2,4-triazole (3, 1 mmol), aldehyde (4a–j, 1 mmol), Melamine@TMG nanocatalyst (1, 2.5 mol%, 15 mg) in EtOH (2 mL) at 40 °C.
bIsolated yield.
Results and discussion
Characterization of the nano-ordered Melamine@TMG organocatalyst (1)
The as-prepared nano-ordered multifunctional and heterogeneous Melamine@TMG organocatalyst (1) was properly characterized by Fourier transform infrared (FTIR) and energy dispersive X-Ray (EDX) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), and thermal gravimetric analysis (TGA) and derivative thermogravimetry (DTG) techinques before investigation of its catalytic activity in the synthesis of 1,2,4-triazoloquinazolinones .
FTIR spectra of Melamine@TMG organocatalyst (1)
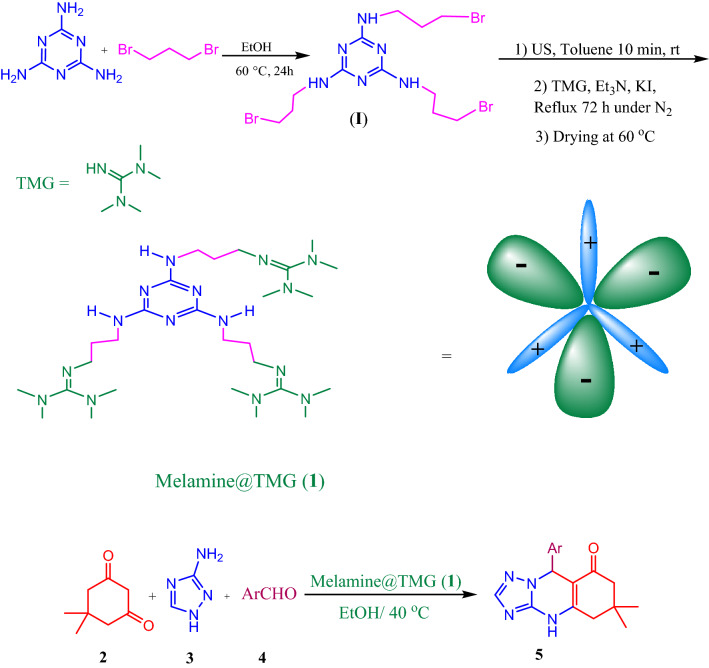
In the FTIR spectrum of melamine (Fig. 1a, A), the bands at 3483 and 3411 cm−1 are related to the asymmetric and symmetric stretching vibrations of the N–H bonds of melamine42,80,. The band at 1673 cm−1 is attributed to the bending vibrations of the NH2 and the band at 1525 cm−1 is related to the stretching vibrations of NCN of melamine42,81. For the Melamine@PrBr (Fig. 1a, B) and Melamine@TMG (Fig. 1a, C), new signals appeared at 1644, 1540, and 1014 cm−1, which are associated with the stretching and scissoring vibrations of C=N, N–H, and C–N bonds of both TMG and melamine, respectively. Appearance of these characteristic bands confirm the existence of TMG moiety in the structure of the catalyst 179. By considering this point that there are similar bands in the structure of melamine and TMG, it is not possible to determine clearly the signals of these two structures by FTIR analysis. Furthermore, the FTIR spectra of Melamine@TMG after five consecutive runs in the model reaction (Fig. 1b) demonstrated very good similarity with the fresh catalyst 1. This finding shows that the structure of Melamine@TMG organocatalyst (1) does not change during five consecutive runs.
Figure 1.
FTIR spectra of melamine (A), the Melamine@PrBr (B), and the nano-ordered Melamine@TMG (1, C, part a) and the recycled Melamine@TMG catalyst after five consecutive runs in the model reaction (part b).
XRD patterns of the Melamine@TMG organocatalyst (1)
Comparative XRD patterns of the Melamine@TMG organocatalyst (1) and its components are presented in Fig. 2. As data in Fig. 2a show, XRD pattern of the melamine has a non-amorphous and crystalline phase (2θ: 13.22, 17.78, 21.74, 22.14, 26.28, 26.51, 28.90, 29.89 and 35.91o). Furthermore, the XRD patterns of both Melamine@prBr (Fig. 2b, 2θ: 13.20, 17.77, 21.73, 22.13, 26.25, 28.89 and 29.88o) and Melamine@TMG organocatalyst (1, Fig. 2c, 2θ: 17.74, 21.70, 22.12, 26.24, 28.88, and 29.86o) show almost the same peaks compared to the melamine. Although the non-amorphous structure of the melamine has not been completely changed, but the intensity of different peaks has been altered80.
Figure 2.
XRD patterns of the melamine (A), the Melamine@PrBr (B), and the nano-ordered Melamine@TMG (1, C).
Energy dispersive X-ray spectroscopy of the nano-ordered Melamine@TMG (1)
The chemical composition of the Melamine@PrBr (I) and Melamine@TMG organocatalyst (1) was determined by energy-dispersive X-ray (EDX) spectroscopy (Fig. 3). The EDX spectra of the Melamine@PrBr and nano-ordered Melamine@TMG showed the expected elements including Br, N and C. The obtained results confirm successful anchoring of 1,3-dibromopropane, as the linker, and TMG, as an organic base, to the melamine during depicted steps for preparation of the Melamine@TMG catalyst (1) in Scheme 1.
Figure 3.
The EDX spectra of the Melamine@PrBr (a) and nano-ordered Melamine@TMG (1, b).
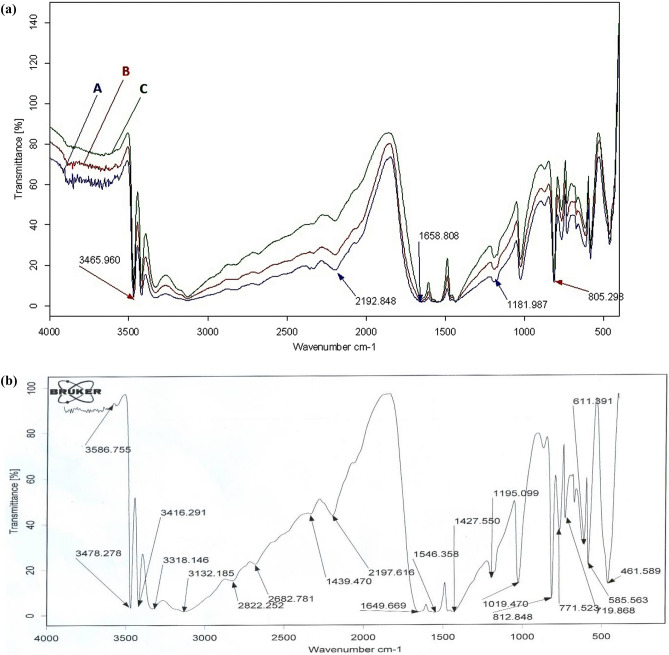
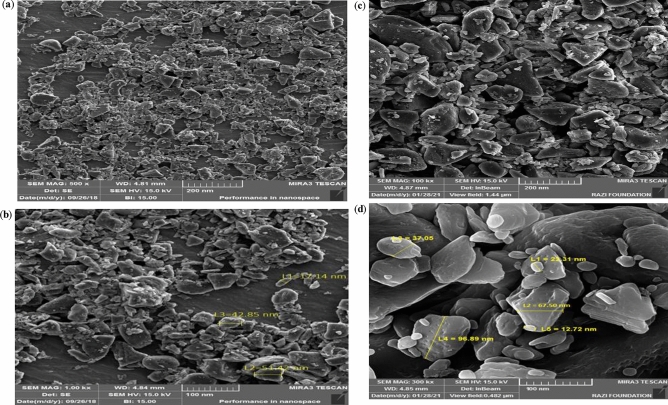
Scanning electron microscopy images of the Melamine@TMG organocatalyst (1)
The scanning electron microscopy (SEM) images demonstrated that the nano-ordered Melamine@TMG (Fig. 4a,b) has a layered structure and the size of nanoparticles are mainly between 17 and 52 nm. On the other hand, the morphology of Melamine@TMG catalyst after five cycles in the model reaction was also preserved considerably (Fig. 4c,d).
Figure 4.
SEM images of the fresh nano-ordered Melamine@TMG (1, a,b) and the recycled Melamine@TMG catalyst after five consecutive runs in the model reaction (c,d).
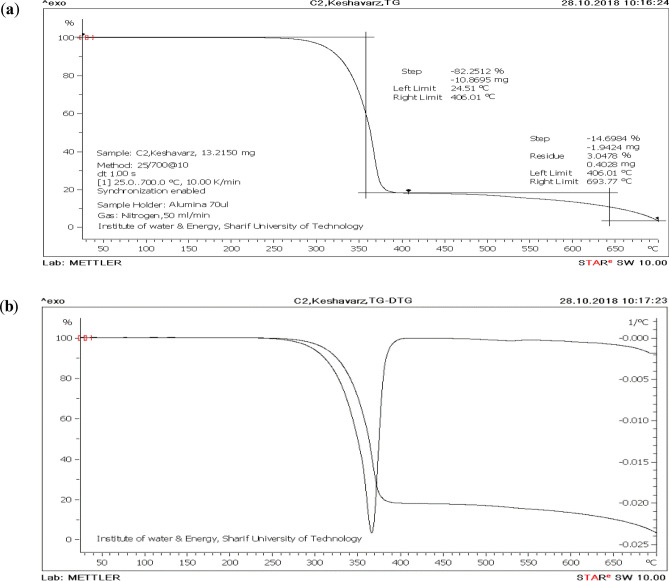
Thermal gravimetric analysis and derivative thermogravimetry of the Melamine@TMG (1)
The thermogravimetric analysis (TGA) of the Melamine@TMG organocatalyst (1) shows a two-step mass loss of the organic materials between 250–700 °C (Fig. 5a). The analysis represents that two distinct weight loss stages occur during the pyrolysis of the nanocatalyst. According to data presented in Fig. 5a, the residual mass percent of the Melamine@TMG at 700 °C is about 3.0%. Therefore, the weight loss of 82% from 250 to 406 °C can be attributed mostly to the loss and decomposition of organic TMG, its propylene linkage, and melamine’s condensation on heating with the elimination of ammonia to form "melam", "melem", "melon"82, while the second weight loss peaks of 14.69% in the range of 406–694 °C can be assigned to complete decomposition of the melamine residue. These findings are also confirmed by the values reported in the derivative thermogravimetry (DTG) analysis (Fig. 5b). The most weight loss occurs at about 370 °C, which is related to the first stage of thermal decomposition. Although this catalyst decomposes mostly at 370 °C, it can be noted that the Melamine@TMG catalyst (1) demonstrated thermal stability up to 250 °C. Hence, it can easily be used in the range of room temperature to 250 °C without any significant change in its structure and catalytic activity.
Figure 5.
TGA (a) and TG-DTG curves of the Melamine@TMG organocatalyst (1, b).
Exploration of the catalytic activity of nano-ordered Melamine@TMG organocatalyst (1)
The catalytic activity of the as-prepared nano-ordered Melamine@TMG (1) was investigated for the three-component synthesis of 1,2,4-triazoloquinazolinones (Scheme 1). Initially, the three-component reaction of benzaldehyde (4a), 3-amino-1,2,4-triazole (3), and dimedone (2) was carried out in various solvents and the absence/presence of different catalysts to find the optimized conditions for the model reaction. The obtained results are reported in Table 1. The results revealed that the model reaction in the absence of any catalyst or ordinary acidic and basic catalysts including CH3CO2H and NaOH proceeds to provide the desired product, 6,6-dimethyl-9-phenyl-5,6,7,9-tetrahydro-[1, 2, 4] triazolo[5,1-b]quinazolin-8(4H)-one (5a) in longer reaction times and lower yields (Entries 1–5). Indeed, the best results were obtained by using the novel Melamine@TMG nanocatalyst (1) in EtOH, as a green solvent, at 40 °C (Entries 6–9). The amount of the nanocatalyst loading was also screened (Entries 6–12). It is obvious from the obtained results that by employing 2.5 mol% (15 mg) nanocatalyst loading in EtOH at 40 °C, the reaction affords the desired 1,2,4-triazoloquinazolinone 5a in lower reaction time and excellent yield (> 98%, Entry 8).
Table 1.
| Entry | Catalyst (mol%) | Solvent | Temp. (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| 1 | – | EtOH | Reflux | 120 | 45 |
| 2 | – | H2O | Reflux | 120 | 34 |
| 3 | – | CH3CN | Reflux | 120 | 42 |
| 4 | CH3CO2H | CH3CO2H | 60 | 30 | 76 |
| 5 | NaOH (1 mmol) | EtOH | Reflux | 30 | 71 |
| 6 | Melamine—TMG (15 mol%) | EtOH | 40 | 20 | 83 |
| 7 | Melamine—TMG (5 mol%) | EtOH | 40 | 20 | 88 |
| 8 | Melamine—TMG (2.5 mol%) | EtOH | 40 | 10 | 98 |
| 9 | Melamine—TMG (2.5 mol%) | EtOH | r.t | 25 | 92 |
| 10 | Melamine—TMG (2.5 mol%) | EtOH | Reflux | 5 | 99 |
| 11 | Melamine—TMG (2.5 mol%) | H2O | 40 | 25 | 87 |
| 12 | Melamine—TMG (2.5 mol%) | H2O | Reflux | 15 | 90 |
Reaction conditions: dimedone (2, 1 mmol), 3-amino-1,2,4-triazole (3, 1 mmol), benzaldehyde (4a, 1 mmol), catalyst (if not otherwise specified) and solvent (2 mL).
In the next step, other carbocyclic aromatic aldehydes 5b–j with different substituents were used to develop the scope of the studied three-component reaction catalyzed by the multifunctional Melamine@TMG organocatalyst (1). The obtained results have been summarized in Table 2. As data in Table 2 show, all the studied aldehydes with both electron-withdrawing and electron-donating substituents were involved in the optimized conditions smoothly to produce the corresponding products in high to excellent yields. In general, the kind and position of functional groups on the aromatic ring of aldehyde exhibits an obvious impact on the required time for completion of the reaction. Indeed, aromatic aldehydes bearing electron-donating substituents (Entries 2–4 and 8) required longer reaction times compared to those ones containing electron-withdrawing groups (Entries 5-7 and 9,10).
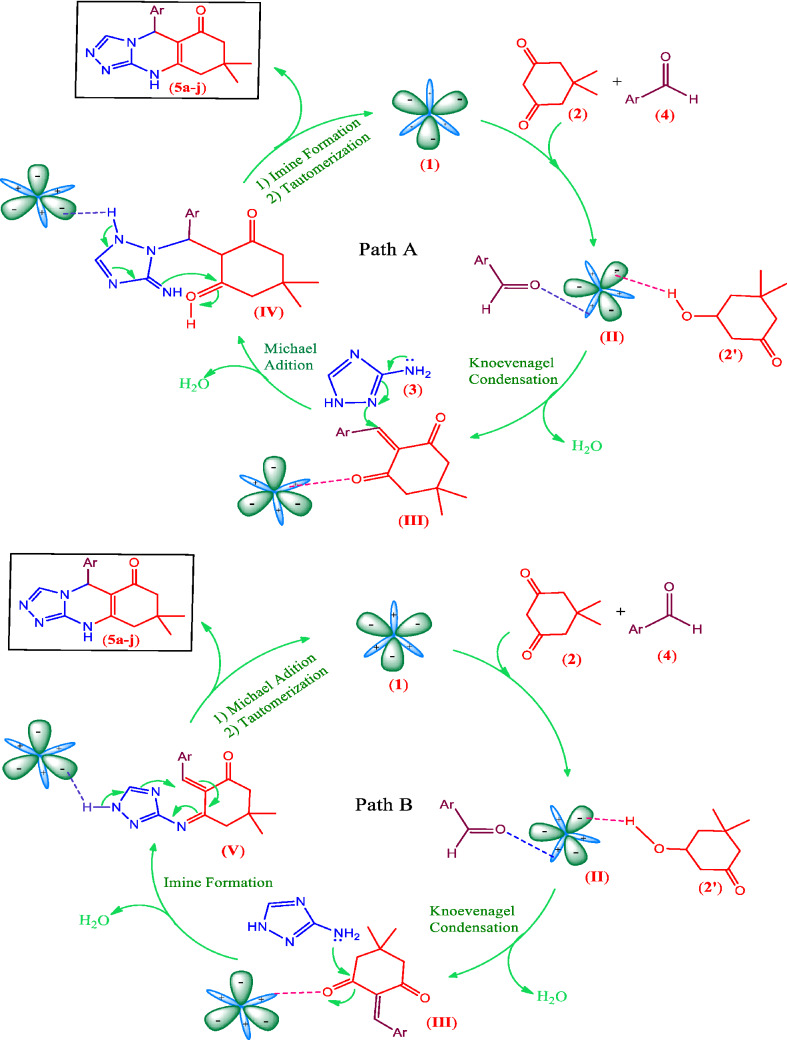
According to the functional groups existing in the structure of catalyst 1 and reactivity of different aldehydes 4a–j, a reasonable mechanism for the synthesis of 1,2,4-tria-zoloquinazolinones 5a–j catalyzed by the multifunctional Melamine@TMG organocatalyst (1) is presented in Scheme 2. In the presence of the melamine-TMG nanocatalyst, the dimedone component 2 equilibrates with its corresponding enol form 2′ and reacts with the activated aldehydes 4 to form the intermediate III through the Knoevenagel condensation. After formation of the intermediate III, there are two possible paths for the reaction: Intermediate III at first takes part in the Michael addition with 3-amino-1,2,4-triazole 3 to form the intermediate IV and subsequent cyclization by imine formation. Then, the desired products 5a–j are formed after tautomerization of the obtained cyclic imine to its corresponding enamine as the last step (Path A). Simultaneously, one of the carbonyl groups in the intermediate III can be activated by the catalyst 1 to form the corresponding imine (intermediate V). Then, hetero-annaulation occurs by the intramolecular Michael addition to afford the desired 1,2,4-triazoloquinazolinones 5a–j (Path B)11,13,33,86–90. It should be noted that the only byproducts of the reaction are water molecules with no environmental impact and can be dissolved in EtOH, as a green solvent, to promote the reaction efficiently by the nano-ordered Melamine@TMG organocatalyst (1).
Scheme 2.
Proposed mechanism for the synthesis of 1,2,4-triazoloquinazolinones 5a–j catalyzed by the nano-ordered Melamine@TMG organocatalyst (1).
As a part of our study, the recyclability of Melamine@TMG organocatalyst (1) was also examined in the synthesis of model compound 5a. The results are reported in Fig. 6. Indeed, it was observed that the catalytic activity of catalyst 1 changed a little after four consecutive runs using the recycled samples .
Figure 6.
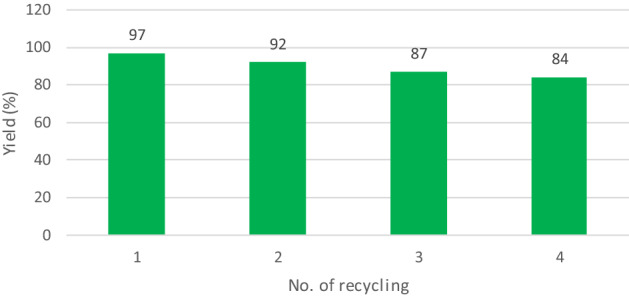
Recycling chart for the Melamine@TMG nanocatalyst 1 in the synthesis of 5a.
To show the merits of the nano-ordered Melamine@TMG organocatalyst (1) for the multicomponent synthesis of 1,2,4-triazoloquinazolinones in comparison to the previously reported catalytic systems, Table 3 compares the obtained results for the synthesis of model compound 5a. It is obvious that the excellent yield of 1,2,4-triazoloquinazolinone 5a was achieved using a low loading of the present catalyst in a green solvent or at a lower temperature compared to the other reported systems.
Table 3.
Comparison of the catalytic activity of nano-ordered Melamine@TMG organocatalyst (1) versus some introduced catalysts for the synthesis of 5a.
| Entry | Catalyst (loading/solvent) | Temperature (°C) | Time (min) | Yield (%) | References |
|---|---|---|---|---|---|
| 1 | Melamine@TMG (2.5 mol%/EtOH) | 40 | 10 | 98 | This work |
| 2 | Nano-SiO2 (15 mol%/CH3CN) | r.t | 30 | 96 | 71 |
| 3 | p-Toluenesulfonic acid (15 mol%/CH3CN) | 50 | 25 | 95 | 73 |
| 4 | [Bmim]BF4 (15 mol%/Solvent-Free) | r.t | 11 | 92 | 77 |
| 5 | [C4(H-DABCO)2] [HSO4]4 (16 mg/Solvent-Free) | 90 | 12 | 91 | 67 |
| 6 | Molecular iodine (10 mol%/CH3CN) | Reflux | 10 | 81 | 76 |
| 7 | Anthranilic acid (30 mol%/EtOH) | 80 | 10 | 95 | 75 |
Conclusion
In general, the novel nano-ordered 1,1,3,3-tetramethylguanidine-functionalized melamine (Melamine@TMG) organocatalyst was prepared and adequately characterized by various spectroscopic or microscopic methods as well as analytical techniques. The basic property of the catalyst was significantly increased by the incorporation of 1,1,3,3-tetramethylguanidine moiety while melamine itself acts as a bifunctional organocatalyst. The nano-ordered multifunctional Melamine@TMG organocatalyst was successfully used, as an efficient recyclable catalyst, for the three-component synthesis of 1,2,4-triazoloquinazolinone scaffold from 3-amino-1,2,4-triazole, dimedone, and different aryl aldehydes under green and environmentally-benign conditions. This practical method afforded the corresponding products in high to excellent yields within short reaction times. Using a heterogeneous multifunctional nanocatalyst, simple work-up procedure with no need for chromatographic purification, affording highly selective conversion, and recyclability of the catalyst with only a small decrease in its activity are other main advantages of this new practical protocol for the synthesis of 1,2,4-triazoloquinazolinones.
Supplementary Information
Acknowledgements
The authors gratefully acknowledge the partial financial support from the Islamic Azad University, Rasht Branch, as well as the Research Councils of Iran University of Science and Technology (IUST), Tehran, Iran (Grant No:160/19108) and the University of Guilan, Rasht, Iran.
Author contributions
M.K. carried out the laboratory work, performed the synthetic experiments, prepared data analyses, participated in the design of the study and drafted the manuscript; M.G.D. and M.M. conceived of the study, designed the study, coordinated the study and helped draft the manuscript. M.G.D also completely revised the manuscript. M.N. coordinated the study and helped for interpretation of data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad G. Dekamin, Email: mdekamin@iust.ac.ir
Manouchehr Mamaghani, Email: m-chem41@guilan.ac.ir.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91463-1.
References
- 1.List B. Introduction: Organocatalysis. Chem. Rev. 2007;107:5413–5415. doi: 10.1021/cr078412e. [DOI] [Google Scholar]
- 2.van der Helm MP, Klemm B, Eelkema R. Organocatalysis in aqueous media. Nat. Rev. Chem. 2019;3:491–508. doi: 10.1038/s41570-019-0116-0. [DOI] [Google Scholar]
- 3.Zhang Z, et al. Asymmetric counteranion-directed Lewis acid organocatalysis for the scalable cyanosilylation of aldehydes. Nat. Commun. 2016;7:12478. doi: 10.1038/ncomms12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wende RC, Schreiner PR. Evolution of asymmetric organocatalysis: Multi- and retrocatalysis. Green Chem. 2012;14:1821–1849. doi: 10.1039/C2GC35160A. [DOI] [Google Scholar]
- 5.Volla CMR, Atodiresei I, Rueping M. Catalytic C–C bond-forming multi-component cascade or domino reactions: Pushing the boundaries of complexity in asymmetric organocatalysis. Chem. Rev. 2014;114:2390–2431. doi: 10.1021/cr400215u. [DOI] [PubMed] [Google Scholar]
- 6.Renzi P, Bella M. Non-asymmetric organocatalysis. Chem. Commun. 2012;48:6881–6896. doi: 10.1039/C2CC31599H. [DOI] [PubMed] [Google Scholar]
- 7.De Risi C, et al. Recent advances in continuous-flow organocatalysis for process intensification. React. Chem. Eng. 2020;5:1017–1052. doi: 10.1039/D0RE00076K. [DOI] [Google Scholar]
- 8.Chakraborty Ghosal N, et al. Organocatalysis by an aprotic imidazolium zwitterion: Regioselective ring-opening of aziridines and applicable to gram scale synthesis. Green Chem. 2016;18:565–574. doi: 10.1039/C5GC01323B. [DOI] [Google Scholar]
- 9.Liu D, Chen EYX. Organocatalysis in biorefining for biomass conversion and upgrading. Green Chem. 2014;16:964–981. doi: 10.1039/C3GC41934G. [DOI] [Google Scholar]
- 10.Cokoja M, Wilhelm ME, Anthofer MH, Herrmann WA, Kühn FE. Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. Chemsuschem. 2015;8:2436–2454. doi: 10.1002/cssc.201500161. [DOI] [PubMed] [Google Scholar]
- 11.Dekamin MG, Karimi Z, Farahmand M. Tetraethylammonium 2-(N-hydroxycarbamoyl)benzoate: A powerful bifunctional metal-free catalyst for efficient and rapid cyanosilylation of carbonyl compounds under mild conditions. Catal. Sci. Technol. 2012;2:1375–1381. doi: 10.1039/C2CY20037F. [DOI] [Google Scholar]
- 12.Dekamin MG, Yazdaninia N, Mokhtari J, Naimi-Jamal MR. Tetrabutylammonium phthalimide-N-oxyl: An efficient organocatalyst for trimethylsilylation of alcohols and phenols with hexamethyldisilazane. J. Iran. Chem. Soc. 2011;8:537–544. doi: 10.1007/BF03249088. [DOI] [Google Scholar]
- 13.Dekamin MG, Sagheb-Asl S, Reza Naimi-Jamal M. An expeditious synthesis of cyanohydrin trimethylsilyl ethers using tetraethylammonium 2-(carbamoyl)benzoate as a bifunctional organocatalyst. Tetrahedron Lett. 2009;50:4063–4066. doi: 10.1016/j.tetlet.2009.04.090. [DOI] [Google Scholar]
- 14.Miller TS, et al. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017;19:15613–15638. doi: 10.1039/C7CP02711G. [DOI] [PubMed] [Google Scholar]
- 15.Benzigar MR, et al. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018;47:2680–2721. doi: 10.1039/C7CS00787F. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh A, et al. A green approach for the preparation of a surfactant embedded sulfonated carbon catalyst towards glycerol acetalization reactions. Catal. Sci. Technol. 2020;10:4827–4844. doi: 10.1039/D0CY00336K. [DOI] [Google Scholar]
- 17.Shen Y, Ge X, Chen M. Catalytic oxidation of nitric oxide (NO) with carbonaceous materials. RSC Adv. 2016;6:8469–8482. doi: 10.1039/C5RA24148K. [DOI] [Google Scholar]
- 18.Shen W, Fan W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A. 2013;1:999–1013. doi: 10.1039/C2TA00028H. [DOI] [Google Scholar]
- 19.Su Q, et al. Urea-derived graphitic carbon nitride as an efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates. Catal. Sci. Technol. 2014;4:1556–1562. doi: 10.1039/C3CY00921A. [DOI] [Google Scholar]
- 20.Dupont J. From molten salts to ionic liquids: A “Nano” journey. Acc. Chem. Res. 2011;44:1223–1231. doi: 10.1021/ar2000937. [DOI] [PubMed] [Google Scholar]
- 21.Moradi S, Zolfigol MA, Zarei M, Alonso DA, Khoshnood A. Synthesis of a biological-based glycoluril with phosphorous acid tags as a new nanostructured catalyst: Application for the synthesis of novel natural henna-based compounds. ChemistrySelect. 2018;3:3042–3047. doi: 10.1002/slct.201702544. [DOI] [Google Scholar]
- 22.Climent MJ, Corma A, Iborra S. Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv. 2012;2:16–58. doi: 10.1039/C1RA00807B. [DOI] [Google Scholar]
- 23.Chen Q, et al. Sustainable synthesis of supported metal nanocatalysts for electrochemical hydrogen evolution. Chin. J. Catal. 2020;41:1791–1811. doi: 10.1016/S1872-2067(20)63652-X. [DOI] [Google Scholar]
- 24.Gawande MB, Zboril R, Malgras V, Yamauchi Y. Integrated nanocatalysts: A unique class of heterogeneous catalysts. J. Mater. Chem. A. 2015;3:8241–8245. doi: 10.1039/C5TA00119F. [DOI] [Google Scholar]
- 25.Geetmani Singh N, et al. Synthetic, mechanistic and kinetic studies on the organo-nano catalyzed synthesis of oxygen and nitrogen containing Spiro compounds under ultrasonic condition. Green Chem. 2016;18:4216–4227. doi: 10.1039/C6GC00724D. [DOI] [Google Scholar]
- 26.Mercuri F, Baldoni M, Sgamellotti A. Towards nano-organic chemistry: Perspectives for a bottom-up approach to the synthesis of low-dimensional carbon nanostructures. Nanoscale. 2012;4:369–379. doi: 10.1039/C1NR11112D. [DOI] [PubMed] [Google Scholar]
- 27.Safaei-Ghomi J, Masoomi R, Hamadanian M, Naseh S. Magnetic nanoscaled core–shell structured Fe3O4@L-proline: An efficient, reusable and eco-friendly nanocatalyst for diastereoselective synthesis of fulleropyrrolidines. New J. Chem. 2016;40:3289–3299. doi: 10.1039/C5NJ02960K. [DOI] [Google Scholar]
- 28.Farokhian P, Mamaghani M, Mahmoodi NO, Tabatab-aeian K. A green and practical method for the synthesis of novel pyrano[2,3-c]pyrazoles and bis-pyrano[2,3-c]pyrazoles using sulfonic acid-functionalized ionic liquid. J. Iran Chem. Soc. 2018;15:11–16. doi: 10.1007/s13738-017-1203-0. [DOI] [Google Scholar]
- 29.Jahanshahi, P. & Mamaghani, M. Efficient and straightforward access to diverse and densely functionalized chromenes by 3‑amino‑1,2,4‑triazole supported on hydroxyapatite‑encapsulated‑γ‑Fe2O3 (γ‑Fe2O3@HAp@CPTMS@AT) as a new magnetic basic nanocatalyst. React. Kinet. Mech. Catal.130, 955–977. 10.1007/s11144-020-01825-5 (2020).
- 30.Mashhadinezhad, M., Shirini, F., Mamaghani, M. & Rassa, M. Green synthesis of dihydropyrimidine annulated heterocyclic systems catalyzed by nanoporous Na+-montmorillonite perchloric acid and evaluation of their biological activities. Polycycl. Aromat. Compd. 40, 1417-1433. 10.1080/10406638.2018.1553197 (2020).
- 31.Mardhiah HH, ChyuanOng H, Masjuki HH, Lim S. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sust. Energy. Rev. 2017;67:1225–1236. doi: 10.1016/j.rser.2016.09.036. [DOI] [Google Scholar]
- 32.Akbari A, Dekamin MG, Yaghoubi A, Naimi-Jamal MR. Novel magnetic propylsulfonic acid-anchored isocyanurate-based periodic mesoporous organosilica (Iron oxide@ PMO-ICS-PrSO 3 H) as a highly efficient and reusable nanoreactor for the sustainable synthesis of imidazopyrimidine derivatives. Sci. Rep. 2020;10:106461–106461. doi: 10.1038/s41598-020-67592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karami S, Dekamin MG, Valiey E, Shakib P. DABA MNPs: A new and efficient magnetic bifunctional nanocatalyst for green synthesis of biologically active pyrano [2, 3-c] pyrazole and benzylpyrazolyl coumarin derivatives. N. J. Chem. 2020;44:13952–13961. doi: 10.1039/D0NJ02666B. [DOI] [Google Scholar]
- 34.Alexander J, Benford D, Cockburn A, Cravedi J. Polycyclic aromatic hydrocarbons in food1 scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;724:1–114. [Google Scholar]
- 35.Liu Q, Mei Ai H. Ultrasound promoted synthesis of arylmethylenemalonitriles catalyzed by melamine. J. Chem. Soc. Pak. 2016;38:565–569. [Google Scholar]
- 36.Natu M, Van De Mark MR. Synthesis and characterization of an acid catalyst for acrylic-melamine resin systems based on colloidal unimolecular polymer (CUP) particles of MMA-AMPS. Prog. Org. Coat. 2015;81:35–46. doi: 10.1016/j.porgcoat.2014.12.008. [DOI] [Google Scholar]
- 37.Savateev A, Antonietti M. Heterogeneous organocatalysis for photoredox chemistry. ACS Catal. 2018;8:9790–9808. doi: 10.1021/acscatal.8b02595. [DOI] [Google Scholar]
- 38.Prasetyanto EA, Ansari MB, Min B-H, Park S-E. Melamine tri-silsesquioxane bridged periodic mesoporous organosilica as an efficient metal-free catalyst for CO2 activation. Catal. Today. 2010;158:252–257. doi: 10.1016/j.cattod.2010.03.081. [DOI] [Google Scholar]
- 39.Elavarasan S, Kala K, Muhammad I, Bhaumik A, Sasidharan M. Pd-chelated 1,3,5-triazine organosilica as an active catalyst for Suzuki and Heck reactions. Mol. Catal. 2019;476:110521. doi: 10.1016/j.mcat.2019.110521. [DOI] [Google Scholar]
- 40.Muhammad R, Mohanty P. Nitrogen enriched triazine bridged mesoporous organosilicas for CO2 capture and dye adsorption applications. J. Mol. Liq. 2017;248:127–134. doi: 10.1016/j.molliq.2017.10.036. [DOI] [Google Scholar]
- 41.Valiey E, Dekamin MG, Alirezvani Z. Melamine-modified chitosan materials: An efficient and recyclable bifunctional organocatalyst for green synthesis of densely functionalized bioactive dihydropyrano[2,3-c]pyrazole and benzylpyrazolyl coumarin derivatives. Int. J. Biol. Macromol. 2019;129:407–421. doi: 10.1016/j.ijbiomac.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 42.Alirezvani Z, Dekamin MG, Davoodi F, Valiey E. Melamine-functionalized chitosan: A new bio-based reusable bifunctional organocatalyst for the synthesis of cyanocinnamonitrile intermediates and densely functionalized nicotinonitrile derivatives. Chem. Select. 2018;3:10450–10463. [Google Scholar]
- 43.Alirezvani Z, Dekamin MG, Valiey E. Cu(II) and magnetite nanoparticles decorated melamine-functionalized chitosan: A synergistic multifunctional catalyst for sustainable cascade oxidation of benzyl alcohols/Knoevenagel condensation. Sci. Rep. 2019;9:17758. doi: 10.1038/s41598-019-53765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor JE, Bull SD, Williams JMJ. Amidines, isothioureas, and guanidines as nucleophilic catalysts. Chem. Soc. Rev. 2012;41:2109–2121. doi: 10.1039/C2CS15288F. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Gérardy R, Gauron G, Damblon C, Monbaliu J-CM. Solvent-free organocatalytic preparation of cyclic organic carbonates under scalable continuous flow conditions. React. Chem. Eng. 2019;4:17–26. doi: 10.1039/C8RE00209F. [DOI] [Google Scholar]
- 46.Ahmad SM, Braddock DC, Cansell G, Hermitage SA. Dimethylformamide, dimethylacetamide and tetramethylguanidine as nucleophilic organocatalysts for the transfer of electrophilic bromine from N-bromosuccinimide to alkenes. Tetrahedron Lett. 2007;48:915–918. doi: 10.1016/j.tetlet.2006.12.042. [DOI] [Google Scholar]
- 47.Dong S, Feng X, Liu X. Chiral guanidines and their derivatives in asymmetric synthesis. Chem. Soc. Rev. 2018;47:8525–8540. doi: 10.1039/C7CS00792B. [DOI] [PubMed] [Google Scholar]
- 48.Selig P. Guanidine organocatalysis. Synthesis. 2013;45:703–718. doi: 10.1055/s-0032-1318154. [DOI] [Google Scholar]
- 49.Lohmeijer BGG, et al. Guanidine and amidine organocatalysts for ring-opening polymerization of cyclic esters. Macromolecules. 2006;39:8574–8583. doi: 10.1021/ma0619381. [DOI] [Google Scholar]
- 50.Odagi M, Takayama K, Sato M, Yamanaka M, Nagasawa K. Development of guanidine-bisurea bifunctional organocatalysts with a chiral pyrrolidine moiety and application to α-hydroxylation of tetralone-derived β-Keto esters. Molecules. 2015;20:12590–12598. doi: 10.3390/molecules200712590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel DM, Vala RM, Sharma MG, Rajani DP, Patel HM. A Practical green visit to the functionalized [1,2,4]Triazolo[5,1-b]quinazolin-8(4H)one scaffolds using the group-assisted purification (GAP) chemistry and their pharmacological testing. ChemistrySelect. 2019;4:1031–1041. doi: 10.1002/slct.201803605. [DOI] [Google Scholar]
- 52.Patel C, et al. Synthesis and antimicrobial activity of 1,2-benzothiazine derivatives. Molecules. 2016;21:861. doi: 10.3390/molecules21070861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glasser AC, Diamond L, Combs G. Synthesis and anticonvulsant activity of substituted 2-thioquinazolin-4-ones I: Preliminary studies. J. Pharm. Sci. 1971;60:127–129. doi: 10.1002/jps.2600600128. [DOI] [PubMed] [Google Scholar]
- 54.Alagarsamy V, Solomon VR, Murugan M. Synthesis and pharmacological investigation of novel 4-benzyl-1-substituted-4H-[1,2,4]triazolo[4,3-a]quinazolin-5-ones as new class of H1-antihistaminic agents. Bioorg. Med. Chem. 2007;15:4009–4015. doi: 10.1016/j.bmc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Pathak SR, Malhotra V, Nath R, Shanker K. Synthesis and antihypertensive activity of novel quinazolin-4(3H)-one derivatives. Cent Nerv. Syst. Agents Med. Chem. 2014;14:34–38. doi: 10.2174/1871524914666140825144729. [DOI] [PubMed] [Google Scholar]
- 56.Zarganes-Tzitzikas T, Chandgude AL, Dömling A. Multicomponent reactions, union of MCRs and beyond. Chem. Rec. 2015;15:981–996. doi: 10.1002/tcr.201500201. [DOI] [PubMed] [Google Scholar]
- 57.Prasad P, Shobhashana PG, Patel MP. An efficient synthesis of 4 H-pyrano quinolinone derivatives catalysed by a versatile organocatalyst tetra-n-butylammonium fluoride and their pharmacological screening. R. Soc. Open Sci. 2017;4:170764. doi: 10.1098/rsos.170764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eckert H. Diversity oriented syntheses of conventional heterocycles by smart multi component reactions (MCRs) of the last decade. Molecules. 2012;17:1074–1102. doi: 10.3390/molecules17011074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slobbe P, Ruijter E, Orru RVA. Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm. 2012;3:1189–1218. doi: 10.1039/C2MD20089A. [DOI] [Google Scholar]
- 60.Zhi S, Ma X, Zhang W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019;17:7632–7650. doi: 10.1039/C9OB00772E. [DOI] [PubMed] [Google Scholar]
- 61.Zadmard R, Akbarzadeh A, Jalali MR. Highly functionalized calix[4]arenes via multicomponent reactions: Synthesis and recognition properties. RSC Adv. 2019;9:19596–19605. doi: 10.1039/C9RA03354H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dekamin MG, Azimoshan M, Ramezani L. Chitosan: A highly efficient renewable and recoverable bio-polymer catalyst for the expeditious synthesis of α-amino nitriles and imines under mild conditions. Green Chem. 2013;15:811–820. doi: 10.1039/C3GC36901C. [DOI] [Google Scholar]
- 63.Etivand N, Khalafy J, Dekamin MG. Fast and efficient green procedure for the synthesis of Benzo[5,6]chromene derivatives and their sulfur analogues in water by organocatalyst potassium phthalimide-<i>N</i>-oxyl. Synthesis. 2020;52:e2–e2. doi: 10.1055/s-0039-1690093. [DOI] [Google Scholar]
- 64.Dekamin MG, Eslami M, Maleki A. Potassium phthalimide-N-oxyl: A novel, efficient, and simple organocatalyst for the one-pot three-component synthesis of various 2-amino-4H-chromene derivatives in water. Tetrahedron. 2013;69:1074–1085. doi: 10.1016/j.tet.2012.11.068. [DOI] [Google Scholar]
- 65.Zolfigol MA, et al. 4-Phenyl-1,2,4-triazole-3,5-dione as a novel and reusable reagent for the aromatization of 1,4-dihydropyridines under mild conditions. Tetrahedron Lett. 2005;46:5581–5584. doi: 10.1016/j.tetlet.2005.06.031. [DOI] [Google Scholar]
- 66.Zolfigol MA, et al. Synthesis of 1,2,3-triazolylmethoxyphenyl[1,2,4]triazolo[1,2-a]indazoletrione derivatives by combining click and multicomponent reactions. Synthesis. 2016;48:1518–1524. doi: 10.1055/s-0035-1561392. [DOI] [Google Scholar]
- 67.Safari N, Shirini F, Tajik H. Preparation and characterization of a novel DABCO based tetra cationic ionic liquid as a reusable catalyst for the multi-component synthesis of 2H-indazolo[2,1-b]phthalazine-trione and [1,2,4]triazoloquinazolinone derivatives under solvent-free condition. J. Mol. Struct. 2020;1201:127143. doi: 10.1016/j.molstruc.2019.127143. [DOI] [Google Scholar]
- 68.Bakhshali-Dehkordi R, Ghasemzadeh MA, Safaei-Ghomi J. Multicomponent preparation of quinazolinone derivatives in the presence of TiO2 nanoparticles supported ionic liquids. Polycycl. Aromat. Compd. 2020 doi: 10.1080/10406638.2020.1759661. [DOI] [Google Scholar]
- 69.Karimi-Chayjani R, Daneshvar N, Nikoo Langarudi MS, Shirini F, Tajik H. Silica-coated magnetic nanoparticles containing bis dicationic bridge for the synthesis of 1,2,4-triazolo pyrimidine/quinazolinone derivatives. J. Mol. Struct. 2020;1199:126891. doi: 10.1016/j.molstruc.2019.126891. [DOI] [Google Scholar]
- 70.Mohammadi Ziarani G, Badiei A, Aslani Z, Lashgari N. Application of sulfonic acid functionalized nanoporous silica (SBA-Pr-SO3H) in the green one-pot synthesis of triazoloquinazolinones and benzimidazoquinazolinones. Arab. J. Chem. 2015;8:54–61. doi: 10.1016/j.arabjc.2011.06.020. [DOI] [Google Scholar]
- 71.Mousavi MR, Maghsoodlou MT. Nano-SiO2: A green, efficient, and reusable heterogeneous catalyst for the synthesis of quinazolinone derivatives. J. Iran. Chem. Soc. 2015;12:743–749. doi: 10.1007/s13738-014-0533-4. [DOI] [Google Scholar]
- 72.Ayati A, Daraie M, Heravi MM, Tanhaei B. H4[W12SiO40] grafted on magnetic chitosan: A green nanocatalyst for the synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone derivatives. Micro Nano Lett. 2017;12:964–969. doi: 10.1049/mnl.2017.0053. [DOI] [Google Scholar]
- 73.Mousavi MR, Maghsoodlou MT. Catalytic systems containing p-toluenesulfonic acid monohydrate catalyzed the synthesis of triazoloquinazolinone and benzimidazoquinazolinone derivatives. Monatshefte Chem. 2014;145:1967–1973. doi: 10.1007/s00706-014-1273-y. [DOI] [Google Scholar]
- 74.Heravi MM, Derikvand F, Ranjbar L. Sulfamic acid-catalyzed, three- component, one-pot synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone derivatives. Synth. Commun. 2010;40:677–685. doi: 10.1080/00397910903009489. [DOI] [Google Scholar]
- 75.Vibhute S, et al. An efficient, one-pot three components synthesis of [1,2,4] triazoloquinazolinone derivatives using anthranilic acid as green catalyst. Res. Chem. Intermed. 2017;43:4561–4574. doi: 10.1007/s11164-017-2896-5. [DOI] [Google Scholar]
- 76.Goud Puligoundla R, et al. A simple, convenient one-pot synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone derivatives by using molecular iodine. Tetrahedron Lett. 2013;54:2480–2483. doi: 10.1016/j.tetlet.2013.02.099. [DOI] [Google Scholar]
- 77.Kumari K, Raghuvanshi DS, Nand Singh K. An expeditious synthesis of tetrahydro-1,2,4- triazolo[5,1-b]quinazolin-8(4H)-ones and dihydro-1,2,4-triazolo[1,5-a]pyrimidines. Organ. Prep. Proced. Int. 2012;44:460–466. doi: 10.1080/00304948.2012.715062. [DOI] [Google Scholar]
- 78.Heravi MM, et al. A three component one-pot procedure for the synthesis of [1,2,4]triazolo/benzimidazolo-quinazolinone derivatives in the presence of H6P2W18O62 · 18H2O as a green and reusable catalyst. Mol. Divers. 2008;12:181–185. doi: 10.1007/s11030-008-9086-8. [DOI] [PubMed] [Google Scholar]
- 79.Ding Sh, et al. 1,1,3,3-Tetramethylguanidine immobilized on graphene oxide: A highly active and selective heterogeneous catalyst for Aldol reaction. Catal. Commun. 2017;92:5–9. doi: 10.1016/j.catcom.2016.12.027. [DOI] [Google Scholar]
- 80.Wang Y, et al. IR spectroscopy and theoretical vibrational calculation of the melamine molecule. J. Chem. Soc. 1997;93:3445–3451. [Google Scholar]
- 81.Yuan X, et al. Combinatorial vibration-mode assignment for the FTIR spectrum of crystalline melamine: A strategic approach toward theoretical IR vibrational calculations of triazine-based compounds. J. Phys. Chem. A. 2016;120:7427–7433. doi: 10.1021/acs.jpca.6b06015. [DOI] [PubMed] [Google Scholar]
- 82.Costa L, Camino G. Thermal behaviour of melamine. J. Therm. Anal. 1988;34:423–429. doi: 10.1007/BF01913181. [DOI] [Google Scholar]
- 83.Mourad AFE, Aly AA, Farag HH. Beshr Microwave assisted synthesis of triazoloquinazolinones and benzimidazoquinazolinones. Beilstein J. Organ. Chem. 2007;3:1–5. doi: 10.1186/1860-5397-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seyyedi N, Shirini F, Safarpoor M, Langarudi N, Jashnani S. A simple and convenient synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone and [1,2,4]triazolo[1,5-a]pyrimidine derivatives catalyzed by DABCO-based ionic liquids. J. Iran. Chem. Soc. 2017;14:1859–1867. doi: 10.1007/s13738-017-1125-x. [DOI] [Google Scholar]
- 85.Krishnamurthy G, Jagannath KV. Microwave-assisted silica-promoted solvent-free synthesis of triazoloquinazolinone and benzimidazoquinazolinones. J. Chem. Sci. 2013;125:807–811. doi: 10.1007/s12039-013-0398-6. [DOI] [Google Scholar]
- 86.Alvim HGO, da Silva Júnior EN, Neto BAD. What do we know about multicomponent reactions? Mechanisms and trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv. 2014;4:54282–54299. doi: 10.1039/C4RA10651B. [DOI] [Google Scholar]
- 87.Sharma MG, Rajani DP, Patel HM. Green approach for synthesis of bioactive Hantzsch 1,4-dihydropyridine derivatives based on thiophene moiety via multicomponent reaction. R. Soc. Open Sci. 2017;4:170006. doi: 10.1098/rsos.170006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dekamin MG, et al. Alginic acid: A highly efficient renewable and heterogeneous biopolymeric catalyst for one-pot synthesis of the Hantzsch 1,4-dihydropyridines. RSC Adv. 2014;4:56658–56664. doi: 10.1039/C4RA11801D. [DOI] [Google Scholar]
- 89.Dekamin MG, Kazemi E, Karimi Z, Mohammadalipoor M, Naimi-Jamal MR. Chitosan: An efficient biomacromolecule support for synergic catalyzing of Hantzsch esters by CuSO4. Int. J. Biol. Macromol. 2016;93:767–774. doi: 10.1016/j.ijbiomac.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 90.Yarhosseini M, Javanshir S, Dekamin MG, Farhadnia M. Tetraethylammonium 2-(carbamoyl)benzoate as a bifunctional organocatalyst for one-pot synthesis of Hantzsch 1,4-dihydropyridine and polyhydroquinoline derivatives. Monatshefte für Chemie Chem. Mon. 2016;147:1779–1787. doi: 10.1007/s00706-016-1666-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.