Abstract
The electroplating industry generates wastewater containing a variety of heavy metals which potentially contaminate water ecosystems. The available and well-known electroplating wastewater treatments are considered as an expensive and less effective method, therefore phytoremediation was used as an alternative friendly solution. This study aims to evaluate the uptake and elimination rate of heavy metals by vetiver (Chrysopogon zizanoides L.) on metal-polluted water. Vetiver was planted in artificial electroplating wastewater containing different levels (low, medium, high) of chromium (Cr) and nickel (Ni). Water, roots, and shoots were collected periodically to determine Cr and Ni contents using Atomic Absorption Spectrometry (AAS). Metal accumulation and elimination rate, Bioconcentration Factor (BCF), Biological Absorption Coefficient (BAC), and Translocation Factor (TF) were calculated to evaluate plant’s effectiveness in metal remediation processes. The results showed that vetiver (C. zizanoides L.) was able to remove 61.10% Cr and 95.65% Ni on metal-contaminated water. The highest uptake rates for Cr and Ni are 127.21 mg/kg/day and 15.60 mg/kg/day respectively, while the elimination rates for Cr and Ni tend to slow 1.09 mg/kg/day and 12.24 mg/kg/day respectively. Vetiver BCF, BAC, and TF values on Cr and Ni contaminated water were greater than 1, which indicates that vetiver work through phytoextraction and phytostabilization to treat metals. The findings showed that vetiver has promise as a phytoremediation agent thus providing implication for electroplating wastewater treatment.
Subject terms: Pollution remediation, Environmental social sciences
Introduction
Wastewater from electroplating industrial activities became major concern due to high variety and concentration of heavy metals1,2. This attracted great attention in terms of environmental impact and removal technique3. Once heavy metal contaminate the aquatic ecosystem, the metal’s toxicity, carcinogenicity, mutagenicity may adverse the aquatic life4. Metal is persisted to the environment4,5, and potentially to be biomagnified through the food chain6–9.
The discharge of metal ions such as chromium (Cr) and nickel (Ni) into surface water may impact on living organism10. Chromium has several oxidation states (− 2 to + 6), Cr (VI) and Cr (III) is the most common form in the environment, Cr (III) is less toxic than Cr (VI)11–14. While Ni is one of the plant micronutrients, it helps the formation of enzymes such as urease15. However, high Cr and Ni levels in the environment may induce plant toxicity such as chlorosis, necrosis, damage on root cells, wilting, nutritional deficiency, disruption of enzymatic activity and induce reactive oxygen species (ROS)16–18. Recent electroplating wastewater treatment is considered as an expensive and less effective method, it could produce secondary pollutants19. Therefore it is imperative to evaluate phytoremediation as an easy, inexpensive, and environmentally friendly solution in order to remove heavy metals20,21.
Phytoremediation is a green technology to remediate environmental pollutants by employing plants22–25. Plantsable to accumulate pollutants including heavy metals through phytoextraction, phytostabilization, rhizodegradation, phytotransformation, phytodegradation, and phytovolatilization processes26–29. The ability of plants to accumulate and eliminate high content of heavy metals were needed in phytoremediation processes16,30. In order to respond to heavy metal stress in the environment, plants produce chelators and organic acids to bind with toxic metal ions31–34. The complex between metal and chelator were sequestrated by cell, so the metal ions were inactivated through compartmentalization in cellular parts of plant15,35.
Vetiver (C. zizanoides L.) has exhibited the potential for polluted water treatment and phytoremediation7. As a perennial grass, it was reported that vetiver was high tolerance and effective to reduce heavy metals in wastewater7,22,35,36. Vetiver has a wide range of environmental factor tolerances. Although vetiver is terrestrial plant, it grows rapidly and is able to adapt and grow in water, acid environment and temperature stress22,26,37,38. Vetiver has erected and stiff shoots, it has massive, deep, and fast-growing root system39. These root systems provide an enormous surface area for vetiver to absorb large amounts of pollutant rather than the other species6,39. The morphological character of vetiver indicated that plants were suitable as potential phytoremediation agents. Therefore, this study was aimed to understand the metals uptake and elimination rates by vetiver in order to evaluate vetiver as a potential phytoremediation agent.
Results and discussion
Phytoremediation of Cr and Ni is shown that heavy metal content in plant growth media were reduced during 28 days remediation by vetiver grass (C. zizanoides L.). The results showed heavy metal reduction were 61.10% and 95.65% in Cr-A treatment and Ni-A treatment respectively (Table 1). The result was relatively greater than the previous report, where Cr and Ni reduction was about 21% and 38% respectively on Cr and Ni elimination from acid mines40. However, during 28 days of metal exposure, vetiver plants were affected by Cr toxicity. It was observed in the first 20 day that the grass loss on water content and turgor pressure, wilting, chlorosis, and root cells disruption. Meanwhile, in Ni treatment, the toxicity symptoms that arose in plants are chlorosis, wilting, and necrosis.
Table 1.
Cr and Ni levels on vetiver (C. zizanoides L.) growth media.
| Treatments | Uptake (mg/L) | Elimination (mg/L) | ||
|---|---|---|---|---|
| D0 | D28 | D0 | D28 | |
| Control | ||||
| Cr | 0.0000a | 0.0000a | 0.0000a | 0.0000a |
| Ni | 0.0000a | 0.0000a | 0.0000a | 0.0000a |
| Cr | ||||
| A | 50.7741b | 19.7494b | 0.0000a | 0.2292b |
| B | 155.2206c | 86.9938c | 0.0000a | 0.5737c |
| C | 346.9057d | 190.3750d | 0.0000a | 0.9380d |
| Ni | ||||
| A | 24.7387b | 1.0767a | 0.0000a | 0.2113b |
| B | 76.3505c | 12.6903b | 0.0000a | 0.5473c |
| C | 156.8409d | 34.0900c | 0.0000a | 0.6923c |
Information: A (Low concentration); B (Medium concentration); C (High concentration).
Identical letter indicated statistically no significant different (p > 0.05).
Despite the series of toxicity symptoms, the vetiver in this experiment was found to be well adapted, indicated with the growth of roots and new individuals in the Cr and Ni treatment. The ability of plants to absorb large amounts of metals and reduce their toxicity is a key factor for the remediation process. This proves that vetiver has a high potential as phytoremediation agent.
During elimination condition 0.60–0.80% Cr and 0.60–0.89% Ni were released back to the plant growth medium (Table 1). The mechanism is used by plants to reduce heavy metals toxicity by controlling the level of heavy metals accumulation in plant cells. The efflux system consisting of P1B-ATPases and CDF transporter families on roots plasma membrane is involved on this mechanism41–44.
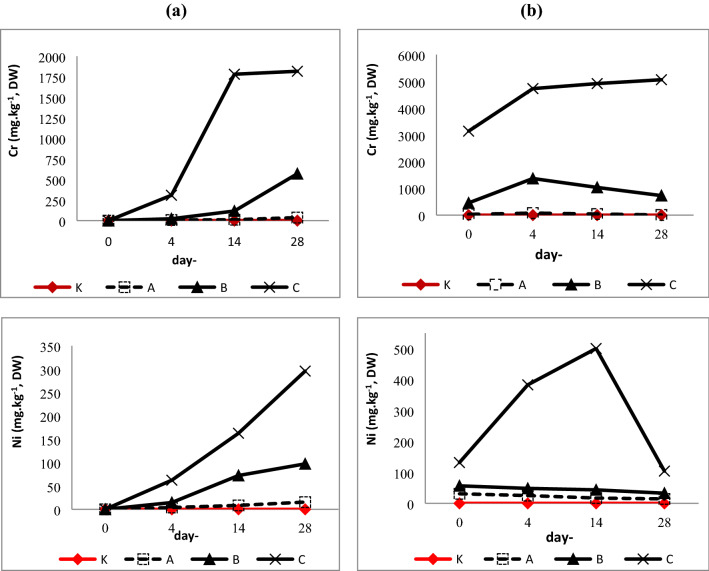
The concentration of chromium and nickel content in shoots of vetiver (C. zizanoides L.) were increased during 28 days remediation (uptake condition) (Fig. 1a). The metals uptake and translocation in vetiver (C. zizanoides L.) occurs gradually over the length of time metal exposure. The highest Cr and Ni accumulation in shoots were 1817.0894 mg/kg, DW and 295.9948 mg/kg, DW respectively. The metals uptake can be affected by metal levels on plant growth medium. High number of biomass was needed to accumulate high metal concentration, therefore NPK and fertilizer were added to support vetiver growth45. Chromium and nickel were translocated from vetiver roots to shoots through xilem46,47. The translocation process both metals tend to be slow48,49 because of Cr tend to be retained on roots vacuole and cell wall11,50–52, while more than 50% absorbed nickel were retained in roots cylinder vascular49,53.
Figure 1.
Cr and Ni content in shoots of vetiver (C. zizanoides L.): (a) uptake condition, (b) elimination condition.
Metals content in vetiver shoots during elimination condition tend to decrease after reaching the peak of metal accumulation (Fig. 1b). Nevertheless, it was found that metal accumulation in Cr-C treatment still increased until the end of the elimination condition. This indicates that the Cr concentration in Cr-C treatment is too high or not proportional to the slow development of plant biomass. Hence, a longer observation time is needed to understand the elimination of high Cr concentrations in vetiver. Vetiver transfer into non-metal contaminated medium during elimination conditions may support the plant growth. As the plant grows, new biomass will be formed and excess metal will be translocated into younger tissues, therefore the shoots metal content was reduced through the time. Study revealed that chromium and nickel were translocated into older tissue in order to protect young tissues49,54.
Rate of metals uptake in vetiver (C. zizanoides L.) strongly related with metal concentration in plant growth media. Heavy metals stress induced chelator production which is used by plants to form metals-chelator complex in order to reduce metal toxicity15. Metal accumulation in plants was accelerated with the formation of this complex, therefore the rate of metals uptake was increased as the increase of metal content in growth media (Table 2). It was reported that vetiver accumulates chromium better than nickel55, as seen on the result, the rate of chromium uptake is higher than nickel.
Table 2.
Rate of heavy metals uptake and elimination in vetiver (C. zizanoides L.).
| Samples | Rate of uptake (mg/kg/day) | Rate of elimination (mg/kg/day) | ||||
|---|---|---|---|---|---|---|
| 4 | 14 | 28 | 4 | 14 | 28 | |
| Cr-A | 1.81 | 0.79 | 1.32 | − 10.17 | − 0.41 | 1.09 |
| Cr-B | 5.61 | 8.33 | 20.38 | − 199.86 | − 33.23 | − 5.62 |
| Cr-C | 77.06 | 127.21 | 64.90 | − 728.05 | − 221.45 | − 115.87 |
| Ni-A | 0.97 | 0.60 | 0.55 | − 2.43 | − 0.12 | 0.04 |
| Ni–B | 3.64 | 5.17 | 3.48 | 12.24 | 3.84 | 2.30 |
| Ni-C | 15.60 | 11.63 | 10.57 | − 21.76 | − 14.61 | 6.85 |
Information: A (Low concentration); B (Medium concentration); C (High concentration).
The rate of metals elimination after plants were moved into non-metal contaminated water is extremely slow. Toxic metals can be eliminated by plant through sequestration metal-chelator complex into inactive form, this mechanism is aided by tonoplast antiporter such as cation diffusion facilitator (CDF), cation exchanger (CAX), and magnesium exchangers15. Although vetiver has a slow metal elimination rate, it is known to be adaptive in heavy metal stress. Plants with high ability to sequestrate metals were known as good potential phytoremediation16,22.
Vetiver (C. zizanoides L.) potential as a phytoremediation agent can be determined by some index including bioconcentration factor (BCF), biological absorption coefficient (BAC), and translocation factor (TF). Plants with BAC ≥ 1, BCF ≥ 1, TF ≥ 1 classified as metal hyperaccumulator with strong phytoextraction capacity, while plants with BAC ≥ 1, BCF ≥ 1, TF ≤ 1 classified as metal hyperaccumulator through phytostabilization mechanism56–58. Plants were classified as metal hyperaccumulators when they were able to accumulate > 1000 mg/kg, DW metals in their tissues57,59,60.
Vetiver (C. zizanoides L) able to accumulate Cr > 1000 mg/kg, DW and possess great BAC, BCF, TF values (Table 3). It is suggested that vetiver possess strong phytoextraction and phytostabilization capacity for chromium. On the other hand vetiver was not able to accumulate nickel > 1000 mg/kg, DW, it means vetiver could not be classified as a nickel hyper-accumulator. However, it has high BAC and BCF values (Table 4), so it could be classified as a good potential phytoremediation for nickel with phytostabilization capacity. Together, these results indicate that vetiver (C. zizanoides L.) tissues possess a high capacity for heavy metals accumulation, it may grow normally in a metal-polluted environment, and is thus becoming a promising metal pollution tolerant plant species.
Table 3.
Cr content in root, shoot, and plant growth media after 28 days metal exposure and 28 days moved into freshwater.
| Samples | Root (mg/kg, DW) | Shoot (mg/kg, DW) | Water (mg/kg) | BCF | BAC | TF |
|---|---|---|---|---|---|---|
| Uptake condition | ||||||
| Cr-K | 33.91a ± 1.47 | 0.00a ± 0.00 | 0.13 | |||
| Cr-A | 1776.62b ± 9.87 | 36.97b ± 0.19 | 19.75 | 89.96 | 1.87 | 0.02 |
| Cr-B | 2205.82c ± 7.33 | 570.59c ± 1.33 | 86.99 | 25.36 | 6.56 | 0.26 |
| Cr-C | 3173.70d ± 54.05 | 1817.09d ± 4.06 | 190.38 | 16.67 | 9.54 | 0.57 |
| Elimination condition | ||||||
| Cr-K | 43.61a ± 1.08 | 0.00a ± 0.00 | 0.00 | |||
| Cr-A | 668.84b ± 7.65 | 6.51b ± 0.94 | 0.23 | 2918.16 | 28.41 | 0.01 |
| Cr-B | 1734.06c ± 0.63 | 730.19c ± 3.95 | 0.57 | 3022.60 | 1268.80 | 0.42 |
| Cr-C | 2468.07d ± 24.47 | 5061.57d ± 13.82 | 0.94 | 2631.21 | 5396.13 | 2.05 |
Information: K (Control); A (Low concentration); B (Medium concentration); C (High concentration).
Identical letter indicated statistically no significant different (p > 0.05).
Table 4.
Ni content in root, shoot, and plant growth media after 28 days metal exposure and 28 days moved into freshwater.
| Samples | Root (mg/kg, DW) | Shoot (mg/kg, DW) | Water (mg/kg) | BCF | BAC | TF |
|---|---|---|---|---|---|---|
| Uptake condition | ||||||
| Ni-K | 22.30a ± 0.25 | 0.00a ± 0.00 | 0.00 | |||
| Ni-A | 1279.20b ± 5.20 | 15.49b ± 1.45 | 1.08 | 1188.08 | 14.39 | 0.01 |
| Ni–B | 1563.01c ± 10.18 | 97.35c ± 0.41 | 12.69 | 123.166 | 7.67 | 0.06 |
| Ni-C | 16,533.21d ± 9.48 | 295.99d ± 0.32 | 34.09 | 48.4954 | 8.68 | 0.18 |
| Elimination condition | ||||||
| Ni-K | 8.60a ± 0.37 | 0.00a ± 0.00 | 0.00 | |||
| Ni-A | 625.03b ± 3.57 | 14.28b ± 0.66 | 0.21 | 2958.73 | 68.05 | 0.02 |
| Ni–B | 576.00c ± 2.65 | 32.82c ± 0.66 | 0.55 | 1052.53 | 59.97 | 0.06 |
| Ni-C | 998.60d ± 8.75 | 104.29d ± 0.35 | 0.69 | 1442.43 | 150.64 | 0.10 |
Information: K (Control); A (Low concentration); B (Medium concentration); C (High concentration).
Identical letter indicated statistically no significant different (p > 0.05).
Heavy metals became an inert form inside the plant cells15, to become the part of plant biomass. Disposal strategies for metal rich senescent leaves are also needed in order to prevent secondary metals contamination issues53. Pyrolysis, gasification, incineration, and volume reduction processes such as composting and compacting are some of the methods that are usually used to manage metal rich plant biomass53.
Conclusions
Based on the results we concluded that C. zizanoides L. potentially used as a phytoremediation agent for electroplating wastewater treatment. It significantly reduced Cr and Ni levels on electroplating wastewater. Metal accumulation and elimination rate by C. zizanoides L strongly indicated the capability C. zizanoides L. to uptake and detoxify metals on its biomass. Furthermore, great BAC, BCF, and TF values suggest that C. zizanoides L. are capable of processing process phytoextraction and phytostabilization during remediation processes.
Method and materials
Preliminary
Tools experiments were soaked with HNO3 0.1 M overnight and rinsed with distilled water. Vetiver which adapted to water-growth media was selected. To obtain a solitary plant, vetiver were separated from the clump and moved to another container to be acclimatized. Electroplating wastewater was made artificially using K2Cr2O7 and NiSO4·6H2O. Chromium concentrate solution was made with diluting 5.66 g K2Cr2O7 in a 1000 mL reaction flask, while nickel standard solution was made by diluting 4.48 g NiSO4.6H2O in a 1000 mL aquabidest. Different levels concentration of chromium (Cr) and nickel (Ni) as artificial wastewater were made by dilution of concentrate solution.
Plant materials
Vetiver used in the experiment is nursery and cultivated in Green house, a collection of Laboratory for Waste and Wastewater management- Research Unit for Clean Technology—Indonesian Institute of Sciences. After the experiment all vetiver materials including samples would be ignite and the ash collected in a special container before deposit in hazardous waste deposit bureau belong to national government. The use of the plant fulfills the law according to the letter of permission no: B-390/IV/DI.01.03/5/2021 from The Secretariat of Scientific Authority for Biodiversity—Indonesian Institute of Sciences.
Heavy metal exposure
Artificial wastewater was used as plant growth media. C. zizanoides L. were moved to metal-contaminated growth media indicated that the day-0 of metal exposure. Plants were exposed to metal for 28 days indicating uptake condition, then plants were moved into non-metal contaminated growth media for 28 days indicating the elimination condition. To minimize the loss of water due to evapotranspiration, distilled water was added into plant growth media until reaches the initial volume of water growth media. Fertilizer (6.20 g NPK and 2.06 g urea diluted in 100 mL water) and compost leachates (5 g) were added on the day-0 both in uptake and elimination conditions to meet plants nutrition.
Sampling
We collected samples consisting of water (media), leaf, and root samples. Sampling site was shown on Fig. 2. Water samples were collected at the day-0 and day-28 both in uptake and elimination conditions for metal content and Chemical Oxygen Demand (COD) determination. Leaf samples were collected atday-0, 4, 14, 28 of both in uptake and elimination conditions, for metal content determination. Root samples were also taken at day-0 and day-28 both in uptake and elimination conditions for the determination of metal content. Water acidity (pH), water temperature, humidity, and air temperature were collected as additional supporting data.
Figure 2.
Sampling vetiver site.
Samples measurement
The water samples were filtered using Whatman 42 (pore size 2.5 µm) then 50 mL of water were digested using 5 mL HNO3 in 250 mL Erlenmeyer which was covered by glass funnel. Samples were heated slowly until reaching a clear color and the remaining volume is about 15–20 ml. Water-digested samples were diluted and homogenized with 50 mL distilled water before measurement. The plant samples were dried in an oven at 70 °C to a constant weight. The dried plant tissues were subsequently weighed and ground into powder. Two (2) mL of HNO3 65% were added to 0.2 g of plant samples in a 100 ml Erlenmeyer and stirred. Then 1.6 mL of H2O2 33% were carefully added and slightly stirred after the addition, followed by heating the sample on a hot plate, then a strong effervescence would be produced. At about 7–8 min the brown fumes produced were less dense and it allowed for cooling. A slightly yellow solution was obtained, filtered and washed with 5 mL of (1:1) HCl (density 1.18 g/mL) and diluted with 25 mL of distilled water. The heavy metals contents of all samples were measured using Atomic Absorption Spectrometry (Agilent Technology). Chemical Oxygen Demand (COD) was determined using Spectrophotometer UV–Vis (Agilent Technology). 1.5 mL of water sample for COD measurement were added into reagent which contain the mixture of 750 mL H2SO4, 25 mL orthophosphate, 15 g Cr2O7, 10gr Ag2SO4, then reflux for 2 h at the temperature 150 °C.
Analysis
The results were tabulated in Microsoft Excel 2016. Statistical analysis independent t-test (p < 0.05) were conducted using software IBM SPSS Statistics v.16. Bioconcentration Factor (BCF) as metal concentration ratio of plant roots to the water, Biological Absorption Coefficient (BAC) as ratio heavy metal content in plant and the water, and Translocation Factor (TF) as ratio of metal concentration in the shoot to the root were calculated using the following formula61:
The rate of metal uptake and elimination by plats were calculated as follows62:
Acknowledgements
The authors acknowledge the Research Unit for Clean Technology (LPTB)-Indonesian Institute of Sciences (LIPI), Bandung, Indonesia for the financial support.
Author contributions
All authors conceived and designed the study. Z.B.P. carried out the experiment. Z.B.P., T.S., A.P.N., and E.S.B.B. performed the statistical analysis. All authors contributed to the writing of the paper and reviewed the manuscript. All authors contributed equally.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reis MTA, Ismael MRC. Electroplating wastes. Phys. Sci. Rev. 2018 doi: 10.1515/psr-2018-0024. [DOI] [Google Scholar]
- 2.Algarra M, Jimenez MV, Rodrıguez-Castellon E, Jimenez-Lopez A, Jimenez-Jimenez J. Heavy metals removal from electroplating wastewater by aminopropyl-Si MCM-41. Chemosphere. 2005;59:779–786. doi: 10.1016/j.chemosphere.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Yao X, et al. Highly efficient removal of hexavalent chromium from electroplating wastewater using aminated wheat straw. RSC Adv. 2016;6:8797–8805. doi: 10.1039/C5RA24508G. [DOI] [Google Scholar]
- 4.Hoque E, Fritscher J. Multimetal bioremediation and biomining by a combination of new aquatic strains of Mucor hiemalis. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-46560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forstner U. Metal Pollution in the Aquatic Environment. Springer-Verlag; 1979. [Google Scholar]
- 6.Islam S, Ahmed K, Raknuzzaman M, Kundu GK. Heavy metals in the industrial sludge and their ecological risk: A case study for a developing country. J. Geochem. Explor. 2016 doi: 10.1016/j.gexplo.2016.09.006. [DOI] [Google Scholar]
- 7.Kafil M, Nasab SB, Moazed H, Bhatnagar A. Phytoremediation potential of vetiver grass irrigated with wastewater for treatment of metal contaminated soil. Int. J. Phytoremediat. 2019 doi: 10.1080/15226514.2018.1474443. [DOI] [PubMed] [Google Scholar]
- 8.Ackova DG. Heavy metals and their general toxicity on plants. Plant Sci. Today. 2018;5:14–18. doi: 10.14719/pst.2018.5.1.355. [DOI] [Google Scholar]
- 9.Algül F, Beyhan M. Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-68833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goher ME, Hassan AM, Abdel-moniem IA, Fahmy AH, El-sayed SM. Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. Egypt. J. Aquat. Res. 2014;40:225–233. doi: 10.1016/j.ejar.2014.09.001. [DOI] [Google Scholar]
- 11.Shahid M, et al. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere. 2017;178:513–533. doi: 10.1016/j.chemosphere.2017.03.074. [DOI] [PubMed] [Google Scholar]
- 12.Bandara PC, Peña-Bahamonde J, Rodrigues DF. Redox mechanisms of conversion of Cr (VI) to Cr (III) by graphene oxide-polymer composite. Sci. Rep. 2020;10:9237. doi: 10.1038/s41598-020-65534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumolo M, et al. Chromium pollution in European Water, sources, health risk, and remediation strategies: An overview. Int. J. Environ. Res. Public Health. 2020;17:5438. doi: 10.3390/ijerph17155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotfi S, Chakit M, Belghyti D. Groundwater quality and pollution index for heavy metals in Saïs Plain, Morocco. J. Health Pollut. 2020;10:200603. doi: 10.5696/2156-9614-10.26.200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, He Z, Yang X, Baligar VC. Mechanisms of nickel uptake and hyperaccumulation by plants and implications for soil remediation. Adv. Agron. 2012 doi: 10.1016/B978-0-12-394278-4.00003-9. [DOI] [Google Scholar]
- 16.Laghlimi M, Baghdad B, Hadi HE, Bouabdli A. Phytoremediation mechanisms of heavy metal contaminated soils: A review. Open J. Ecol. 2015;5:375–388. doi: 10.4236/oje.2015.58031. [DOI] [Google Scholar]
- 17.Hassan MU, et al. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities - a review. Environ. Sci. Pollut. Res. 2019;26:12673–12688. doi: 10.1007/s11356-019-04892-x. [DOI] [PubMed] [Google Scholar]
- 18.Nie J, et al. A comparative study on the uptake and toxicity of nickel added in the form of different salts to maize seedlings. Environ. Res. Public Health. 2015;12:15075–15087. doi: 10.3390/ijerph121214972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman L, Sarkar SM, Yusoff MM. Efficient removal of heavy metals from electroplating wastewater using polymer ligands. Front. Environ. Sci. Eng. 2015;10:352–361. doi: 10.1007/s11783-015-0783-0. [DOI] [Google Scholar]
- 20.Liu Z, et al. Effects of phytoremediation on industrial wastewater. Earth Environ. Sci. 2019 doi: 10.1088/1755-1315/371/3/032011. [DOI] [Google Scholar]
- 21.Suelee AL, Hasan SNMS, Kusin FM, Yusuff FM, Ibrahim ZZ. Phytoremediation potential of vetiver grass (Vetiveria zizanioides) for treatment of metal-contaminated water. Water Air Soil Pollut. 2017;228:1–15. doi: 10.1007/s11270-017-3349-x. [DOI] [Google Scholar]
- 22.Sarma H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. J. Environ. Sci. Technol. 2011;4:118–138. doi: 10.3923/jest.2011.118.138. [DOI] [Google Scholar]
- 23.Schwitzguébel J, Comino E. Is phytoremediation a sustainable and reliable approach to clean-up contaminated water and soil in Alpine areas ? Environ. Sci. Pollut. Res. 2011;18:842–856. doi: 10.1007/s11356-011-0498-0. [DOI] [PubMed] [Google Scholar]
- 24.Shmaefsky BR. Principles of phytoremediation. In: Shmaefsky BR, editor. Phytoremediation. Concepts and Strategies in Plant Sciences. Springer; 2020. [Google Scholar]
- 25.Buendía-gonzález L, Orozco-villafuerte J, Cruz-sosa F, Barrera-díaz CE, Vernon-carter EJ. Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour. Technol. 2010;101:5862–5867. doi: 10.1016/j.biortech.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Prabakaran K, et al. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019;138:28–37. doi: 10.1016/j.ecoleng.2019.07.002. [DOI] [Google Scholar]
- 27.Lee JH. An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol. Bioprocess Eng. 2013;18:431–439. doi: 10.1007/s12257-013-0193-8. [DOI] [Google Scholar]
- 28.Edao HG. Heavy metals pollution of soil; toxicity and phytoremediation techniques. Int. J. Adv. Res. Publ. 2017;1:29–41. [Google Scholar]
- 29.Fasani E, Manara A, Martini F, Furini A, Dalcorso G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2017;41:1210–1232. doi: 10.1111/pce.12963. [DOI] [PubMed] [Google Scholar]
- 30.Tangahu BV, et al. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. ofChemical Eng. 2011;2011:1–31. [Google Scholar]
- 31.Hossain MA, Piyatida P, Teixeira JA, Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012;2012:1–37. doi: 10.1155/2012/872875. [DOI] [Google Scholar]
- 32.Anjum NA, et al. Jacks of metal/metalloid chelation trade in plants-an overview. Front. Plant Sci. 2015;6:1–17. doi: 10.3389/fpls.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Wang D, Long C, Cui Z. Effect of biodegradable chelators on induced phytoextraction of uranium- and cadmium- contaminated soil by Zebrina pendula Schnizl. Sci. Rep. 2019;9:19817. doi: 10.1038/s41598-019-56262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dipu S, Kumar AA, Thanga SG. Effect of chelating agents in phytoremediation of heavy metals. Remediat. J. 2012;22:133–146. doi: 10.1002/rem.21304. [DOI] [Google Scholar]
- 35.Xia S, et al. A critical review on bioremediation technologies for Cr (VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019 doi: 10.1080/10643389.2018.1564526. [DOI] [Google Scholar]
- 36.Raman JK, Gnansounou E. A review on bioremediation potential of vetiver grass. In: Varjani SJ, Al E, editors. Waste Bioremediation. Springer Nature Singapore Pte Ltd; 2018. pp. 127–140. [Google Scholar]
- 37.Pk I, As AK, Pm N. Agrotechniques for quality planting material production in vetiver (Chrysopogon zizanioides (L.) Nash.) J. Med. Plants Stud. 2019;7:211–213. [Google Scholar]
- 38.Panja S, Sarkar D, Datta R. Removal of antibiotics and nutrients by Vetiver grass (Chrysopogon zizanioides) from secondary wastewater effluent. Int. J. Phytoremediat. 2020;22:764–773. doi: 10.1080/15226514.2019.1710813. [DOI] [PubMed] [Google Scholar]
- 39.Darajeh N, Truong P, Rezania S, Alizadeh H, Leung DWM. Effectiveness of vetiver grass versus other plants for phytoremediation of contaminated water. J. Environ. Treat. Tech. 2019;7:485–500. [Google Scholar]
- 40.Kiiskila JD, Li K, Sarkar D, Datta R. Metabolic response of vetiver grass (Chrysopogon zizanioides) to acid mine drainage. Chemosphere. 2020;240:124–961. doi: 10.1016/j.chemosphere.2019.124961. [DOI] [PubMed] [Google Scholar]
- 41.Thakur S, et al. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016;188:206. doi: 10.1007/s10661-016-5211-9. [DOI] [PubMed] [Google Scholar]
- 42.Mukta RH, Khatun MR, Huda AKMN. Calcium induces phytochelatin accumulation to cope with chromium toxicity in rice (Oryza sativa L.) J. Plant Interact. 2019;14:295–302. doi: 10.1080/17429145.2019.1629034. [DOI] [Google Scholar]
- 43.Williams LE, Mills RF. P 1B—ATPases: An ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005;10:491–502. doi: 10.1016/j.tplants.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002;53:1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- 45.Roongtanakiat N. Vetiver Phytoremediation for Heavy Metal Decontamination. PRVN Tech. Bull. ORDPB; 2009. [Google Scholar]
- 46.Page V, Feller U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy. 2015;5:447–463. doi: 10.3390/agronomy5030447. [DOI] [Google Scholar]
- 47.Centofanti T, et al. Xylem exudate composition and root-to-shoot nickel translocation in Alyssum species Xylem exudate composition and root-to-shoot nickel translocation in Alyssum species. Plant Soil. 2013;373:59–75. doi: 10.1007/s11104-013-1782-1. [DOI] [Google Scholar]
- 48.Singh HP, Mahajan P, Kaur S, Batish DR, Koh RK. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013;11:229–254. doi: 10.1007/s10311-013-0407-5. [DOI] [Google Scholar]
- 49.Chen C, Huang D, Liu J. Functions and toxicity of nickel in plants: Recent advances and future prospects. Clean. 2009;37:304–313. [Google Scholar]
- 50.Bluskov S. Distribution of Chromium and Its Effect on Morphology and Anatomy of Brassica Juncea (Indian Mustard) and Soil Microorganisms. The University of Northern British Columbia; 2004. [Google Scholar]
- 51.di Toppi LS, Fossati F, Musetti R, Mikerezi I, Favali MA. Effects of hexavalent chromium on maize, tomato, and cauliflower plants. J. Plant Nutr. 2002;25:701–717. doi: 10.1081/PLN-120002953. [DOI] [Google Scholar]
- 52.Fargasova A. Plants as models for chromium and nickel risk assessment. Ecotoxicology. 2012;21:1476–1483. doi: 10.1007/s10646-012-0901-8. [DOI] [PubMed] [Google Scholar]
- 53.Shahid M, et al. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2016;326:36–58. doi: 10.1016/j.jhazmat.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 54.Mufarrege MM, et al. Organic matter effects on the Cr (VI) removal efficiency and tolerance of Typha domingensis. Water Air Soil Pollut. 2018;229:1–12. doi: 10.1007/s11270-018-4035-3. [DOI] [Google Scholar]
- 55.V DV, Sushama PK. Heavy Metal Contamination of Dumpyard Soils and its Phytoremediation with Vetiver Grass ( Chrysopogon zizaniodes ). Nat. environment. Pollut. Technol. 2017;16:257–260. [Google Scholar]
- 56.Bader N, Alsharif E, Nassib M, Alshelmani N, Alalem A. Phytoremediation potential of Suaeda vera for some heavy metals in roadside soil in Benghazi, Libya. Asian J. Green Chem. 2019;3:82–90. [Google Scholar]
- 57.Masarovicova E, Kralova K, Kummerova M. Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiol. Plant. 2010;32:823–829. doi: 10.1007/s11738-010-0474-1. [DOI] [Google Scholar]
- 58.Anderson LL. Assessment of Thelypteris palustris, Asparagus sprengeri, and Lolium perenne for Their Potential Use in the Phytoremediation of Arsenic- Contaminated Soils. Louisiana State University; 2007. [Google Scholar]
- 59.Sinha V, Pakshirajan K, Chaturvedi R. Chromium tolerance, bioaccumulation and localization in plants: An overview. J. Environ. Manage. 2018;206:715–730. doi: 10.1016/j.jenvman.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 60.Greger M. Metal availability, uptake, transport and accumulation in plants. In: Prasad MN, editor. Heavy Metal Stress in Plants Heavy. Springer-Verlag, Berlin Heidelberg; 2004. [Google Scholar]
- 61.Sajad MA, et al. Evaluation of chromium phytoremediation potential of some plant species of Dir Lower, Khyber, Pakhtunkhwa, Pakistan. Acta Ecol. Sin. 2019 doi: 10.1016/j.chnaes.2019.12.002. [DOI] [Google Scholar]
- 62.Yap CK, Ismail A, Tan SG, Omar H. Accumulation, depuration and distribution of cadmium and zinc in the green-lipped mussel Perna viridis (Linnaeus) under laboratory conditions. Hydrobiologia. 2003;498:151–160. doi: 10.1023/A:1026221930811. [DOI] [Google Scholar]