Abstract
Faces hold a substantial value for effective social interactions and sharing. Covering faces with masks, due to COVID-19 regulations, may lead to difficulties in using social signals, in particular, in individuals with neurodevelopmental conditions. Daily-life social participation of individuals who were born preterm is of immense importance for their quality of life. Here we examined face tuning in individuals (aged 12.79 ± 1.89 years) who were born preterm and exhibited signs of periventricular leukomalacia (PVL), a dominant form of brain injury in preterm birth survivors. For assessing the face sensitivity in this population, we implemented a recently developed experimental tool, a set of Face-n-Food images bordering on the style of Giuseppe Arcimboldo. The key benefit of these images is that single components do not trigger face processing. Although a coarse face schema is thought to be hardwired in the brain, former preterms exhibit substantial shortages in the face tuning not only compared with typically developing controls but also with individuals with autistic spectrum disorders. The lack of correlations between the face sensitivity and other cognitive abilities indicates that these deficits are domain-specific. This underscores impact of preterm birth sequelae for social functioning at large. Comparison of the findings with data in individuals with other neurodevelopmental and neuropsychiatric conditions provides novel insights into the origins of deficient face processing.
Subject terms: Neuroscience, Social neuroscience
Introduction
Preterm birth is defined as any birth before 37 completed weeks of gestation, or fewer than 259 days since the first day of the woman’s last menstrual period1. It affects approximately 11% of births worldwide2,3. As a consequence of the progress in pre- and neonatal intensive care and advances in medical knowledge and technology, the survival rate of preterm infants is continuously increasing3,4. Quality of survival, therefore, has become a major concern with clear social and clinical relevance5,6. Daily-life social participation of preterm born (PB) individuals is of immense value for their quality of life. Low social competence, even more prevailing than other behavioural problems, may lead to social withdrawal7–12. Former preterms are reported to express poorer social knowledge and reasoning abilities than their term-born peers13.
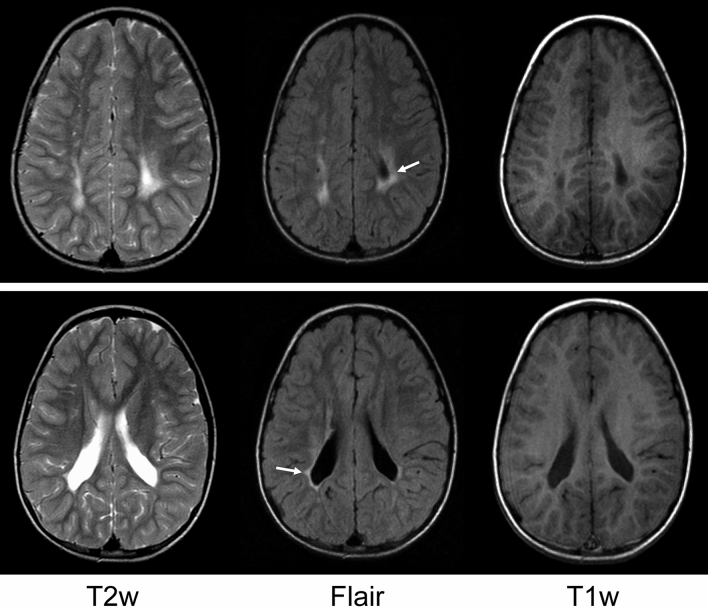
Faces and body language are two sources of non-verbal information most essential for efficient daily-life mutual interactions14–24. As argued earlier5, for a long time, both components were under-investigated in survivors of preterm birth. The visual sensitivity to body motion (represented by a set of light dots on the main joints of the invisible actor’s body) emerges early in development25–28. Already 3-day-old newborns visually prefer point-light human locomotion29. Even newly hatched chicks (Gallus gallus) are likely to be predisposed to biological motion of other species as well as to animacy in general30–33, though such predispositions are impaired in newborns at high risk of autism34,35 and in young autistic children36. The capacity for extracting information from body motion appears to be intrinsically tied with social cognitive abilities such as understanding of drives and emotions of others17. For instance, inferring affect from point-light locomotion and performance on the Reading the Mind in the Eyes Test (RMET) are strongly tangled in adult females37. Extracting social information from body motion is reported to be aberrant in survivors of preterm birth (for review, see5). However, the origins of these deficits are difficult to evaluate, since samples often include individuals with different aetiology of brain lesions, and the data are collapsed across PB individuals with and without brain injury (e.g., periventricular leukomalacia, PVL). PVL is a dominant form of brain injury in survivors of preterm birth5 (Fig. 1). Being a result of necrosis of fibres around the lateral ventricles in the peritrigonal area, PVL is characterized by periventricular gliosis in the white matter with or without tissue loss and secondary ventricular dilatation affecting connectivity of subcortical structures with cortical areas, in particular, the posterior thalamic radiation, and cortico-cortical connectivity5,38–40. Alterations are also reported in the integrity and volume of inferior fronto-occipital fasciculus, the superior longitudinal fasciculus, and frontal aslant tract41. PVL represents a bilateral pattern of lesions that constrains the compensatory capability of the brain, as it affects both hemispheres and, therefore, limits the developing brain for reorganization to preserve functions at risk5.
Figure 1.
Periventricular leukomalacia (PVL) as depicted by MRI at a later stage after completed myelination in a 6-year-old male born at 32 weeks of gestation. Axial T2-weighted (T2w), FLAIR and T1-weighted (T1w) images at the level of the centrum semiovale cutting the upper part of the ventricles (top row) and at the level of the lateral ventricles (bottom row) show the characteristic gliosis as hyperintense high signal on the T2-weighted image and hypointense low signal on the T1-weighted image. The gliosis is best seen on FLAIR images, where the periventricular hyperintensities contrast with the hypointense CSF signal. The top row illustrates gliosis, and the bottom row additional tissue loss (mild ventricular dilatation with irregularly extended borders). From Ref.5.
Pioneering work in this domain indicates that PB adolescents with signs of PVL exhibit compromised ability for visual body motion processing as compared not only with term-born peers, but also with preterms without structural brain abnormalities detectable on magnetic resonance imaging, MRI, scans, and the severity of this impairment is related to the volumetric extent of parieto-occipital PVL42,43. Shortages in body motion perception are unrelated to severity of motor disability, leg-dominated cerebral palsy (in other words, the sensitivity to body motion is not affected by the observers’ early restrictions in their own locomotion42, gestation age, and birth weight44). In former preterms aged 8–11 years with extremely low birthweight (less than 1500 g) autistic traits are reported to be positively related to the ability to reveal identity of a point-light performer (i.e., individuals with more pronounced autistic traits are more sensitive to identity of an actor defined through body motion that appears paradoxical), whereas autistic traits are negatively correlated with a capability to detect a camouflaged walking person in both typically developing (TD) and PB children45. This same cohort of children is less capable of extracting social attribution from the Heider-and-Simmel-like films with moving geometric shapes (a valuable tool for social competence examination5,17,46): they under-attribute intentionality and mental states to the shapes47. These children also turn to be less proficient in decoding non-verbal social signals from persons engaged in naturalistic dynamic social interactions in the Child and Adolescent Social Perception test (CASP48), and their difficulties are related to autistic traits49. Impairments on all these tasks suggest that PB individuals experience generalized hitches in processing of social information revealed through motion. Yet PB adolescents aged 13–16 years with PVL are substantially impaired also on the event arrangement task requiring understanding of others (that is not based on motion characteristics), whereas their PB peers with normal MRI scans perform much like term-born children. This deficit is related to the PVL extent in the right temporal brain area known to be heavily engaged in social cognition50.
Along with body language, faces hold an exceptional value for a wide range of social functioning16,21. Only a very few indications of deficits in face processing associated with prematurity were available till last decade51,52. Deficient face recognition (as assessed by the Facial Memory subtest from the Test of Memory and Learning, TOMAL53) is reported in 18% of 22 premature children aged 6–15 years with signs of (mostly) moderate parieto-occipital PVL identifiable on MRI scans54. Recent work reveals alterations in face processing as sequelae of preterm birth. PB infants examined during the first 48 h of life do not express visual preference for faces over scrambled faces as their term-born peers do55. Functional near-infrared spectroscopy (fNIRS) indicates that PB infants aged 6–10 months show smaller hemodynamic response in the right frontotemporal areas while watching their own mother’s faces as compared to unknown faces, whereas in term-born infants, mothers’ faces elicit greater hemodynamic responses than unknown faces56. Former preterms aged 6–8 months demonstrate atypical eye-gaze behaviour in regard to faces and social content at large: they look shorter at eyes and a face presented among non-face distractors than their term-born peers57. If small for gestational age, former preterms 5–15 years old perform worse on the Facial Memory subtest from the TOMAL53 showing poorer face recognition skills58. However, former preterms aged 19–20 years display intact skills in terms of accuracy (albeit slower responses) for inferring emotions from dynamic faces, with some difficulties in recognizing anger at low intensity59. Adults (26–36 years old) who were born preterm with extremely low birthweight (< 1000 g) possess lower ability for discrimination between individual faces, whether human or monkey60, indicating that deficits in face processing are long-lasting and persist into adulthood.
The present study was directed at investigation of face tuning in PB adolescents who suffer PVL. For investigation of face tuning, we applied a recently developed tool, a set of images comprising food ingredients such as fruits and vegetables61–68. The Face-n-Food images to some extent border on the style of Giuseppe Arcimboldo, a genius Italian painter known for his imaginative portraits composed of fruits, vegetables, and even roasted meat (Fig. 2). The primary advantage of these images is that single components do not trigger face processing. In other words, on the Face-n-Food task, face tuning occurs spontaneously without being explicitly cued by face elements such as eyes or mouth. For seeing a face in these images, one has to perceive an image as a Gestalt (a German word that defines a configuration of elements unified into a whole in such a way that its overall properties cannot be identified from a simple sum of its parts). The other advantage of the task is the usage of unfamiliar images that is of value for research in clinical populations69. Here we intended to clarify (i) whether former preterms exhibiting PVL express aberrant face tuning; and (ii) whether possible shortfalls in the face sensitivity are related to other visuo-perceptual abilities or rather represent a specific deficit. For assessing visuospatial and cognitive abilities, additional tasks were administered to PB individuals. All of them represent well-established tools for neuropsychological assessment54,70 (for description, see “Neuropsychological assessment” section in “Methods” section).
Figure 2.
Examples of Face-n-Food images. The least resembling face (left panel) and most resembling face (right panel) images from the Face-n-Food task (from Ref.61; the Creative Commons Attribution [CC BY] license).
Results
The Face-n-Food images were presented to all participants one by one in a predetermined order from the least to most recognizable as a face61. Similar to previous work in TD individuals and in a range of neurodevelopmental and psychiatric conditions61–68, PB individuals described a food-plate image either in terms of food (non-face response, 0) or as a face (face response, 1). Two out of 14 PB patients completely failed on the Face-n-Food task: they did not have spontaneous face impressions even from the most face-resembling images. PB individuals reported seeing a face for the first time on average only on image 7.33 ± 1.37 (mean ± SD; Mdn, 7, 95% CI, 6.56 to 8.11). TD controls gave the first face response on average on 3.56 ± 1.59 image (Mdn, 3.5; 95% CI 2.78 to 4.34), and all of them perceived images most resembling faces as a face. Thresholds for the face tuning (a median image number, on which a face response was given for the first time) were much higher in PB patients than in TD controls (Steel–Dwass test, z = 4.426, p < 0.0001; effect size, Cohen’s d = 2.744). Comparison with earlier data in autistic children of the same age and cultural background64 reveals that the face sensitivity in PB individuals is also lower than in persons with ASD (Steel–Dwass test, z = 2.375, p < 0.046; effect size, d = 0.962).
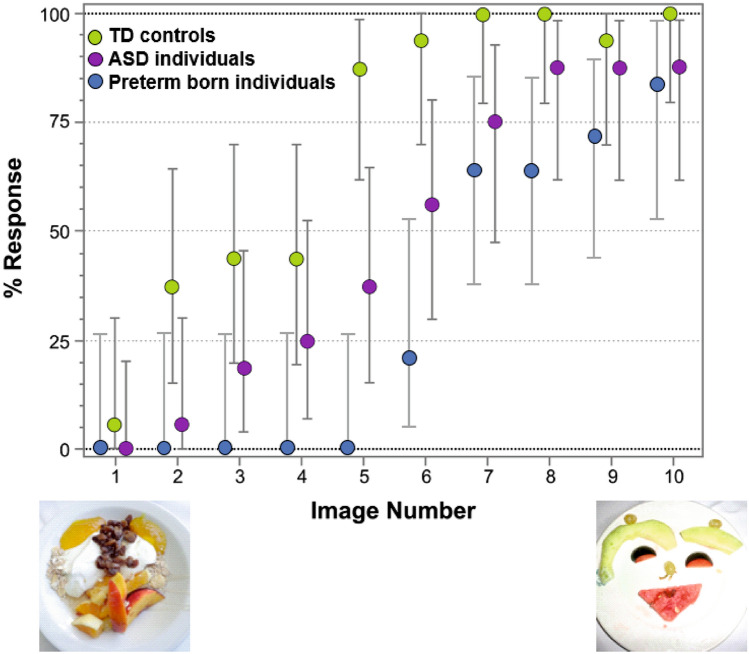
Figure 3 represents the percentage of face responses for each Face-n-Food image separately for former preterms and TD controls. Inspection of this Figure shows that former PB adolescents not only much later had a face impression, but gave overall much fewer face responses. As indicated by multiple stepwise nominal logistic regression analysis, the effect of group (TD vs. PB) is highly significant (χ2(1) = 96.01, p < 0.0001; effect size, r = 1.789). Remarkably, for the first five images that are less face resembling, all PB individuals provided no face responses at all. Starting from the image 5, TD participants reached very fast the ceiling level of performance. By contrast, even on the images strongly resembling a face (7 through 9), PB individuals attained about 70% of face responses, and only the most recognizable image 10 elicited 85.71% face responses.
Figure 3.
Percentage of face responses for each Face-n-Food image in TD controls (green), former preterms with PVL (blue), and patients with autistic spectrum disorders (ASD, violet). The image number reflects its face resemblance (1, the least recognizable as a face, through 10, the most resembling a face images from the Face-n-Food task; see Ref.61; the Creative Commons Attribution [CC BY] license). Vertical bars represent 95% confidence interval, CI. The data for individuals with ASD had been reported earlier (see Ref.64), and provided here for comparison solely.
Figure 3 enables comparison of the data in PB individuals not only with TD controls, but also with earlier data in individuals with autistic spectrum disorders, ASD64. Multiple stepwise nominal logistic regression analysis indicates that all groups of participants significantly differ from one another (χ2(2) = 78.37, p < 0.0001). Most eye-catching finding is that the performance level of PB patients on the Face-n-Food task is substantially lower not only as compared with TD controls, but also with ASD individuals (χ2(1) = 18.38, p < 0.0001; effect size, r = 0.783; with no difference in the general intelligence quotient, GIQ scores between PB and ASD persons: t(28) = 1.124, n.s.).
No correlation was found between performance of PB individuals on the Face-n-Food task (face response rate) and the GIQ (Spearman’s rho, ρ = 0.057, p = 0.85, two-tailed, n.s.). There was also a lack of correlation between face response rate and performance on the visual-perceptual tests: the Block Construction (ρ = 0.236, p = 0.416, n.s.), Street Completion (ρ = 0.213, p = 0.465, n.s.), VMIG (ρ = 0.397, p = 0.16, n.s.), Unusual Perspective (ρ = − 0.011, p = 0.969, n.s.), Unusual Lighting (ρ = − 0.134, p = 0.648, n.s.), and IF tests (ρ = 0.295, p = 0.306, n.s.). As expected, no correlation was found between the face tuning and tests assessing semantic categorization: Matching by Class (ρ = 0.077, p = 0.795, n.s) and Matching by Function (ρ = − 0.077, p = 0.794, n.s.). This indicates that impaired performance on the Face-n-Food task in PB individuals originates from specific deficits in the face sensitivity rather than from other visual-perceptual or cognitive disabilities.
Discussion
By applying a novel tool for the face sensitivity examination, a recently developed Face-n-Food task61–68, we assessed the face tuning in individuals who were born prematurely and suffer PVL, the dominant form of brain injury in prematurity5,54. PVL represents a bilateral pattern of white matter lesions equally affecting both hemispheres that leaves the brain without sufficient potential for functional reorganization. The outcome indicates that in former survivors of preterm birth with PVL, the face sensitivity is substantially lower than in term-born TD peers. In PB individuals, the face sensitivity in face-like non-face images is not related to visual-perceptual organization and other cognitive abilities assessed in the course of neuropsychological examination and, therefore, the lower performance on the Face-n-Food task appears to stem from deficits in the face tuning per se, or strictly speaking, in the sensitivity to a coarse face schema.
Face tuning in prematurity and autism
Evaluation of the outcome in PB individuals within the framework of earlier data obtained in a sample of individuals with ASD64 of similar age, cultural background (known to affect the face sensitivity66), gender (both samples are prevailed by males) and general IQ, reveals that PB individuals with PVL express a lower sensitivity to a coarse face schema in the Face-n-Food images as compared not only with TD term-born controls, but also with their peers with ASD. Although in both clinical samples, a number of patients who completely failed on the task is comparable (i.e., two out of 16 in ASD and two out of 14 in PB patients did not spontaneously recognize even the most face resembling image 10), the face recognition dynamics is different in these groups. Face recognition of the first five-six images less resembling a face is remarkably poor in PB with PVL (Fig. 3). Only starting from image 7, PB individuals reliably recognize images as a face, reaching 85.7% recognition for the most face resembling image 10. By contrast, face recognition of ASD individuals steadily improves resulting in 87.5% face responses for each of the most recognizable images 8–10. As ASD individuals are well-known to experience difficulties in several aspects of social cognition17,18,35,36,71, this discrepancy in the face sensitivity between ASD and PB patients (with substantially lower face tuning of former preterms) appears arresting. Survivors of preterm birth are reported to express autistic traits as well as difficulties in social functioning such as social withdrawal, incompetence, and communication problems72, or they can be also positively screened for ASD73–75. However, this cannot provide a satisfactory explanation for such a poor outcome in PB individuals. As establishing binding between even a couple of elements resembling eyes and mouth, i.e., building a coarse face scheme, is already sufficient for perception of non-face images as a face76, it appears stunning that PB patients are so deeply impaired on the task. Furthermore, a kind of predisposition for a coarse face schema (such as two eyes above a mouth) is believed to occur already very early in lifespan77: foetuses in the third trimester of pregnancy78, human infants79–82, and children aged 5–6 years83 are reported to demonstrate a visual preference for face-like stimuli over similar images. Furthermore, even newly hatched domestic chicks (Gallus gallus) exhibit preference to a coarse face schema84–86, while non-human primates such as the rhesus monkey are likely to share face-detection machinery with humans87–89. Yet it is reported that only 7- and 8-month-old human infants (but not infants aged 5-6 months) exhibit visual preference to Archimboldo portraits over the same images presented upside-down90 that implies a period of development of the sensitivity to faces in such images.
Impact of PVL on the face tuning
PVL lesions specifically affecting neural networks underwriting face processing might be primarily responsible for the poor outcome on the Face-n-Food task in former preterms. The periventricular area of the brain contains many interconnecting fibres, damage to which may lead to brain disintegration eliciting a number of deficits in visual perception and social cognition. Converging evidence from neuroimaging, neuropsychological and electrophysiological studies indicate that the subcortical face route, such as colliculo-pulvino-amygdalar pathway91, provides a neural substrate for the cortical social brain network87,89,92,93. Alterations of this pathway along with subcortical-cortical and cortico-cortical connectivity caused by PVL might be decisive for alterations in face processing and social cognition in PB individuals. Comparison of face tuning in PB individuals with and without PVL lesions identifiable on a MRI scan may deliver pivotal arguments for the sources of deficient face tuning. Yet one has to keep in mind that the altered brain connectivity in atypical perinatal conditions, such as Intrauterine Growth Restriction (IUGR) and prematurity itself (in former preterms without brain injury identifiable by brain imaging), have been shown to impact the brain development, affecting network connectivity in the short- and long-term and resulting in the impairment in socio-cognitive potential in childhood and adolescence94–99. In addition, fNIRS points to a negative correlation in PB individuals between oxyhemoglobin HbO2 measures during presentations of faces and grey matter volumes in the face-specific brain regions forming the social brain such as the fusiform gyrus and amygdala56,100.
Earlier data indicates that PB individuals with PVL exhibit compromised ability for visual processing of body motion as compared not only with term-born peers, but also with former preterms without structural brain abnormalities detectable on MRI scans43,101. The same holds true for the visual social cognition (namely, event arrangement) task requiring perception and understanding of others: only former preterms suffering PVL are impaired on this task, and this deficit is related to the lesion extent in the right temporal region50. By affecting structural and functional brain connectivity, PVL may lead to disintegration of neural communication inside and outside of the networks supporting social cognition. In line with this assumption, in adolescents with PVL, bursts in the spectral amplitude of the magnetoencephalographic (MEG) cortical response underlying large-scaled neural communication occur later than in TD adolescents or are even completely absent101 (see also102,103 for evidence on alterations in the topography of the evoked MEG response to body motion with and without a camouflage).
Specificity of face tuning in prematurity
Previous work revealed substantial (though rather specific for every single neurodevelopmental and neuropsychiatric condition) deficits in the face tuning in different patient populations (for comparative analysis, see67). For example, in schizophrenia, the face tuning is substantially impaired, and this deficit is related to deficits in both social cognition and visual perceptual organization67, whereas individuals with major depressive disorder do not express any shortfalls in face tuning68. Comparison of the present outcome with earlier data in patients with Down syndrome, DS65, and Williams syndrome, WS62, sheds light on the possible origins of the deficient face tuning in former preterms. By contrast with ASD patients, both WS and DS individuals are well-known for their appetitive drive for social interaction and face fascination. Yet all three samples of patients exhibit poor tuning to coarse face information in face-like non-face images: all of them did not recognize the first five images (less resembling a face) as a face. However, individuals with DS remain on rather low face recognition rate (about 0.3) even for the most recognizable image 1065 (Fig. 3) that can originate from some difficulties in abstract/symbolic reasoning, whereas PB individuals as well as those with WS steadily improve in their face recognition reaching 85.7% and 85%, respectively, for the most resembling a face image 10. This analysis suggests that face tuning deficits may be of similar origin in PB and WS individuals. One possible explanation is that difficulties in feature integration and Gestalt perception (perceiving images as a whole) known in both patient groups5,62,104–107, underlie deficient face tuning. On the same wavelength, original Arcimboldo hidden-face portraits are judged as being more ambiguous by adult TD individuals with local perceptual style108,109. The next step in clarification of the nature of aberrant face tuning in prematurity would be functional brain imaging during perception of face-like non-face images. Such examination, in particular, by means of MEG uncovering even fine-graded alterations in the time course and dynamics of bran activity, helps to identify potential deficiencies in neural communication of the underpinning neural networks. Another valuable approach would be using of multimodal integrative strategies that combine several sources of information (such as structural and functional brain connectivity) in relation to behavioural measures of performance22,24.
Face tuning in prematurity and functional brain imaging
Intact communication inside and outside of the brain networks underlying face tuning is largely unidentified and the findings available are rather controversial67. Taking together, the findings demonstrate that (a) topography and time course of the neural circuits underpinning processing of real faces and face-like images are similar; key activation includes the occipital cortices, fusiform face area (FFA), and inferior temporal brain areas110–112; and (b) corresponding brain activation is primarily right-hemispheric113,114. The right hemispheric dominance is also reported in processing of Arcimboldo-like images yielding greater functional magnetic resonance imaging (fMRI) activation, compared to Renaissance portraits and faces, in the occipito-temporal face-specific network (covering the FFA), bilateral superior and inferior parietal cortices, and the inferior frontal gyrus109. The right superior temporal sulcus (STS), a hub of the social brain, distinguishes real faces from face-like images115. Electroencephalography, EEG, suggests that already 1- to 4-day-old newborns exhibit activation in the right-lateralized network engaging lateral occipito-temporal and medial parietal areas overlapping with the face-processing circuits in adults82. However, fNIRS conducted in 7–8-month-old infants indicates that in response to upright Arcimboldo portraits compared with images of single vegetables used as a baseline, the concentration of oxy-Hb increases in the left (but not right) temporal area, whereas such effect is absent in response to inverted Arcimboldo images in the temporal areas of both hemispheres90. The findings in patients with lesions suggest that lesions to the right ocipito-temporal brain areas leave perception of Arcimboldo portraits as faces intact116,117, whereas left-hemispheric lesions severely affect face tuning118. Individuals with pre-manifest Huntington’s disease (characterized by aberrant social cognition119) show a right hemispheric decrease in the N170 component of event related potential, ERP, elicited by the face-like non-face images120.
Sex impact on the face tuning in prematurity
As predominantly male PB patients had been enrolled in the present work, one of the study’s limitations is that it left possible sex differences beyond attention. It is known that males are at a 14–20% higher risk of premature birth121–123 and its complications, being more vulnerable to white matter injury5,124,125. In the face sensitivity, female superiority has been observed by administering the Face-n-Food task to a homogeneous group of university students61. Yet in adult individuals with major depression, no gender differences occur in the face tuning68. The female brain is reported to be more responsive to face-like non-face images such as clocks or backpacks eliciting a face impression, with a greater activation in the right STS and Brodmann area 22, though sex differences are absent at earlier stages of face processing112. Therefore, the rough face schema appears to be sex-independently hardwired in the typically developing brain. Future work is required to elucidate possible sex differences in the face sensitivity of former preterms both at behavioural and brain levels. However, most likely, sex differences will be camouflaged by a more influential impact of PVL, and, therefore, clarification of this issue appears more plausible in former preterms without signs of severe brain abnormalities.
Résumé
Although a course face schema is thought to be hardwired in the brain, individuals who were born preterm with signs of brain injury (PVL) exhibit substantial deficits in the face tuning not only compared with typically developing controls but also with individuals with ASD. The lack of associations between the face sensitivity and other cognitive abilities indicates that these deficits most likely stem from alterations in face tuning per se. This underscores impact of preterm birth sequelae for social functioning at large.
Methods
Participants
Fourteen adolescents (5 females, 9 males) born preterm were enrolled in the study. They were recruited at the Unit of Child and Adolescent Neurology and Psychiatry of ASST Spedali Civili (Civil Hospital) of Brescia, Italy. PB participants were aged 12.79 ± 1.89 years (mean ± SD; age range, 10 to 16 years). Their gestational age at birth was 30.64 ± 3.13 weeks (ranging from 26 to 36 weeks); 3 of them were born extremely preterm (less than 28 weeks of gestation), 8 were born very preterm (28–32 weeks), and 3 were moderate to late preterm (32–36 weeks). Their birthweight was on average 1710 ± 756 g (median, Mdn, 1473 g, 95% confidence interval, CI, from 1313 to 2106) ranging from 940 to 3300 g. The general IQ (GIQ, Wechsler Intelligence Scale for Children, WISC, adapted to Italian population) of PB participants was on average 92.36 ± 14.17, range of 77 to 126 (6 of them had GIQ higher than 90). All of them had signs of PVL on a structural MRI scan affecting primarily parietoocipital periventricular areas, and neurologically exhibited signs of cerebral palsy in a form of diplegia or tetraplegia. Sixteen TD controls (1 female, 15 males; aged 14.13 ± 2.14; age range, 11 to 17 years; with no age differences with PB group; t(28) = 1.59, two-tailed, n.s.) had been recruited from the local community of Brescia, Italy. The data of this group was previously reported within the framework of the studies on autism and Down syndrome64,65. Participants were run individually. All of them had normal or corrected-to-normal vision. None had previous experience with such images and tasks. The study was conducted in accord with the Declaration of Helsinki and was approved by the local Ethics Committee of ASST Spedali Civili (Civil Hospital) of Brescia, Italy. Informed written consent was obtained from all participants or their care providers. Participation was voluntary, and the data were processed anonymously.
Face-n-Food task
The Face-n-Food task was administered to participants. The task is described in detail elsewhere61. In brief, for this task, all participants were presented with a set of images, one by one, in the predetermined order from the least to most resembling a face (images 1 to 10). This order was determined in the previous study with TD volunteers61. This fixed order had been used, because once seen as a face, Face-n-Food images are often processed with a strong face-dominating bias. On each trial, participants had to perform a spontaneous recognition task: they were asked to briefly describe what they saw. Their reports were recorded, and then analysed by independent experts. For further data processing, the responses were coded as either non-face (0) or face (1) report. No immediate feedback was provided. To avoid time pressure that can potentially cause stress and negative emotional and physiological reactions blocking cognitive processes, there was no time limit on the task. With each participant, the testing procedure lasted no longer than 10–20 min.
Neuropsychological assessment
Several additional tasks directed at assessment of visuospatial and cognitive abilities were routinely administered to PB individuals. All of them represent well-established tools for neuropsychological assessment54,70: (1) The Block Construction test (which is a subtest of the test battery NEPSY-II126, adapted to Italian population127) is designed to assess the visuospatial and visuomotor ability. Participants are asked to reproduce three-dimensional constructions from models or from two-dimensional drawings under time limit. (2) The Visual Motor Integration test (VMI128, adapted to Italian population129), serves for obtaining visual-motor integration quotient (VMIQ). Patients are first shown a set of progressively complex geometric shapes (in total 27), and on each item they are asked to draw it by him/herself. (3) In the Street Completion Test129,130, participants have to recognize 11 black-and-white fragmented images that represent real objects with increasing ambiguity. (4) The Unusual Perspective test and Unusual Lighting test developed from scratch130,131 contain 22 colour photographs of objects (22 images of objects photographed from unusual viewpoints and 22 images of objects presented under unusual lighting conditions) and photographs of the same objects presented in a conventional manner. Participants are asked to recognize each object. (5) The Imagery Figure (IF) test contains 20 out of the 128 items of the Birmingham Object Recognition Battery131, defined as easy in the original battery, and seemed appealing to children. Ten of them represent depictions of objects and the rest ten are imaginary fanciful images combining features of two objects or animals (e.g., a camel with a goose’s head). Participants have to indicate whether each item represents an animal or object that really exists. (6) In addition, two tests (described below) are aimed at assessment of semantic categorization129,130. Both the Matching by Class and the Matching by Function tests consist of 20 out of the 64 items of the original test (the Birmingham Object Recognition Battery131). In ten out of 20 selected items, participants are asked to match objects belonging to the same class, and in the rest to match objects that are functionally related. Each item consists of the target image and two images, one that matches the target and a distractor. For each participant on each test, z-scores were computed by using the following formula: ‘raw score’ minus ‘average score’ (for participants of this age) divided by SD (for participants of this age).
Data analysis
At outset, all data sets were routinely analysed for normality of distribution by using Shapiro–Wilk tests with subsequent usage of either parametric (for normally distributed data) or, otherwise, non-parametric statistics. For not normally distributed data sets, additionally to means and SDs, Mdns and 95% CIs are reported throughout the text.
Acknowledgements
We are grateful to participants, their families and care-providers for their engagement in this study. We appreciate kind assistance and consulting of Prof. Klaus Dietz in statistical issues.
Author contributions
Conceived and designed the study: M.A.P. and E.M.F. Performed the experiments: J.G. and F.P. Neuropsychological examination: S.M. Analyzed the data: M.A.P., A.N.S., J.G. and F.Z. Contributed reagents/materials/analysis tools: M.A.P., A.J.F. and E.M.F. Wrote the paper: M.A.P. and F.Z. All co-authors contributed to the writing and editing. Supervision and administration of the whole project: M.A.P. and E.M.F.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Reinhold Beitlich Foundation and German Research Foundation (DFG, PA847/25-1) to M.A.P. Federica Zanetti’s stay in M.A.P.’s lab was within the Program “Thesis research abroad” funded by the University of Brescia (International Student Mobility Office), Italy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quinn JA, et al. Preterm birth: Case definition and guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34(49):6047–6056. doi: 10.1016/j.vaccine.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel JP, et al. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Delnord M, Zeitlin J. Epidemiology of late preterm and early term births—An international perspective. Semin. Fetal Neonatal. Med. 2019;24(1):310. doi: 10.1016/j.siny.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics. 2010;126(2):342–351. doi: 10.1542/peds.2010-0710. [DOI] [PubMed] [Google Scholar]
- 5.Pavlova MA, Krägeloh-Mann I. Limitations on the developing preterm brain: Impact of periventricular white matter lesions on brain connectivity and cognition. Brain. 2013;136(Pt 4):998–1011. doi: 10.1093/brain/aws334. [DOI] [PubMed] [Google Scholar]
- 6.Gire C, et al. Quality of life of extremely preterm school-age children without major handicap: A cross-sectional observational study. Arch. Dis. Child. 2019;104(4):333–339. doi: 10.1136/archdischild-2018-315046. [DOI] [PubMed] [Google Scholar]
- 7.Nadeau L, Tessier R, Lefebvre F, Robaey P. Victimization: A newly recognized outcome of prematurity. Dev. Med. Child Neurol. 2004;46(8):508–513. doi: 10.1111/j.1469-8749.2004.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 8.Fan RG, Portuguez MW, Nunes ML. Cognition, behavior and social competence of preterm low birth weight children at school age. Clinics (Sao Paulo) 2013;68(7):915–921. doi: 10.6061/clinics/2013(07)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones KM, Champion PR, Woodward LJ. Social competence of preschool children born very preterm. Early Hum. Dev. 2013;89(10):795–802. doi: 10.1016/j.earlhumdev.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witt S, Weitkämper A, Neumann H, Lücke T, Zmyj N. Delayed theory of mind development in children born preterm: A longitudinal study. Early Hum. Dev. 2018;127:85–89. doi: 10.1016/j.earlhumdev.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Duncan AF, et al. Behavioral deficits at 18–22 months of age are associated with early cerebellar injury and cognitive and language performance in children born extremely preterm. J. Pediatr. 2019;204:148–156. doi: 10.1016/j.jpeds.2018.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Künz K, Juen B, Buchheim A, Kiechl-Kohlendorfer U. Unterschiede im konfliktbasierten Spielverhalten, der sozioemotionalen und kognitiven Entwicklung von Frühgeborenen und Termingeborenen im Vorschulalter (Differences in conflict-based play behavior, socio-emotional and cognitive development of preterm children compared to full-term children at preschool age) Prax. Kinderpsychol. Kinderpsychiatr. 2019;68(6):503–524. doi: 10.13109/prkk.2019.68.6.503. [DOI] [PubMed] [Google Scholar]
- 13.Lejeune F, et al. Social reasoning abilities in preterm and full-term children aged 5–7years. Early Hum. Dev. 2016;103:49–54. doi: 10.1016/j.earlhumdev.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 14.de Gelder B, et al. Standing up for the body. Recent progress in uncovering the networks involved in the perception of bodies and bodily expressions. Neurosci. Biobehav. Rev. 2010;34:513–527. doi: 10.1016/j.neubiorev.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 15.van den Stock J, de Jong SJ, Hodiamont PPG, de Gelder B. Perceiving emotions from bodily expressions and multisensory integration of emotion cues in schizophrenia. Soc Neurosci. 2011;6:537–547. doi: 10.1080/17470919.2011.568790. [DOI] [PubMed] [Google Scholar]
- 16.Kret ME, de Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012;50(7):1211–1221. doi: 10.1016/j.neuropsychologia.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Pavlova MA. Biological motion processing as a hallmark of social cognition. Cereb. Cortex. 2012;22(5):981–995. doi: 10.1093/cercor/bhr156. [DOI] [PubMed] [Google Scholar]
- 18.Pelphrey KA, Yang DY, McPartland JC. Building a social neuroscience of autism spectrum disorder. Curr. Top. Behav. Neurosci. 2014;16:215–233. doi: 10.1007/978-3-662-45758-0_253. [DOI] [PubMed] [Google Scholar]
- 19.van den Stock J, et al. Neural correlates of body and face perception following bilateral destruction of the primary visual cortices. Front. Behav. Neurosci. 2014;8:30. doi: 10.3389/fnbeh.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlova MA. Emotion science in the twenty-first century. Time, sex, and behavior in emotion science: Over and above. Front. Psychol. 2017;8:1211. doi: 10.3389/fpsyg.2017.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlova MA. Sex and gender affect the social brain: Beyond simplicity. J. Neurosci. Res. 2017;95:235–250. doi: 10.1002/jnr.23871. [DOI] [PubMed] [Google Scholar]
- 22.Sokolov AA, et al. Structural and effective brain connectivity underlying biological motion detection. Proc. Natl. Acad. Sci. U.S.A. 2018;115(51):E12034–E12042. doi: 10.1073/pnas.1812859115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tillman R, et al. Oxytocin enhances the neural efficiency of social perception. Front. Hum. Neurosci. 2019;13:71. doi: 10.3389/fnhum.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokolov AA, et al. Brain circuits signaling the absence of emotion in body language. Proc. Natl. Acad. Sci. U.S.A. 2020;117(34):20868–20873. doi: 10.1073/pnas.2007141117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox R, McDaniel C. The perception of biological motion by human infants. Science. 1982;218:486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- 26.Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. U.S.A. 2008;105(2):809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardi L, Regolin L, Simion F. Biological motion preference in humans at birth: Role of dynamic and configural properties. Dev. Sci. 2011;14(2):353–359. doi: 10.1111/j.1467-7687.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirai M, Senju A. The two-process theory of biological motion processing. Neurosci. Biobehav. Rev. 2020;111:114–124. doi: 10.1016/j.neubiorev.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Bidet-Ildei C, Kitromilides E, Orliaguet JP, Pavlova M, Gentaz E. Preference for point-light human biological motion in newborns: Contribution of translational displacement. Dev. Psychol. 2014;50(1):113–120. doi: 10.1037/a0032956. [DOI] [PubMed] [Google Scholar]
- 30.Vallortigara G, Regolin L, Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 2005;3(7):e208. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallortigara G, Regolin L. Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr. Biol. 2006;16(8):R279–R280. doi: 10.1016/j.cub.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 32.Rugani R, Rosa Salva O, Regolin L, Vallortigara G. Brain asymmetry modulates perception of biological motion in newborn chicks (Gallus gallus) Behav. Brain Res. 2015;290:1–7. doi: 10.1016/j.bbr.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Rosa-Salva O, Grassi M, Lorenzi E, Regolin L, Vallortigara G. Spontaneous preference for visual cues of animacy in naïve domestic chicks: The case of speed changes. Cognition. 2016;157:49–60. doi: 10.1016/j.cognition.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Di Giorgio E, Lunghi M, Vallortigara G, Simion F. Newborns’ sensitivity to speed changes as a building block for animacy perception. Sci. Rep. 2021;11(1):542. doi: 10.1038/s41598-020-79451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Giorgio E, et al. Difference in visual social predispositions between newborns at low-and high-risk for autism. Sci. Rep. 2016;6:26395. doi: 10.1038/srep26395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isernia S, Sokolov AN, Fallgatter AJ, Pavlova MA. Untangling the ties between social cognition and body motion: Gender Impact. Front. Psychol. 2020;11:128. doi: 10.3389/fpsyg.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoon AH, Jr, et al. Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurology. 2002;59(5):752–756. doi: 10.1212/WNL.59.5.752. [DOI] [PubMed] [Google Scholar]
- 39.Nagasunder AC, et al. Abnormal microstructure of the atrophic thalamus in preterm survivors with periventricular leukomalacia. Am. J. Neuroradiol. 2011;32(1):185–191. doi: 10.3174/ajnr.A2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batalle D, et al. Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage. 2017;149:379–392. doi: 10.1016/j.neuroimage.2017.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauer CM, Papadelis C. Alterations in the structural and functional connectivity of the visuomotor network of children with periventricular leukomalacia. Semin. Pediatr. Neurol. 2019;31:48–56. doi: 10.1016/j.spen.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlova M, Staudt M, Sokolov A, Birbaumer N, Krägeloh-Mann I. Perception and production of biological movement in patients with early periventricular brain lesions. Brain. 2003;126(Pt 3):692–701. doi: 10.1093/brain/awg062. [DOI] [PubMed] [Google Scholar]
- 43.Pavlova M, Sokolov A, Birbaumer N, Krägeloh-Mann I. Biological motion processing in adolescents with early periventricular brain damage. Neuropsychologia. 2006;44:586–593. doi: 10.1016/j.neuropsychologia.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Taylor NM, Jakobson LS, Maurer D, Lewis TL. Differential vulnerability of global motion, global form, and biological motion processing in full-term and preterm children. Neuropsychologia. 2009;47(13):2766–2778. doi: 10.1016/j.neuropsychologia.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Williamson KE, Jakobson LS, Saunders DR, Troje NF. Local and global aspects of biological motion perception in children born at very low birth weight. Child Neuropsychol. 2015;21:603–628. doi: 10.1080/09297049.2014.945407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavlova M, Guerreschi M, Lutzenberger W, Sokolov AN, Krageloh-Mann I. Cortical response to social interaction is affected by gender. Neuroimage. 2010;50:1327–1332. doi: 10.1016/j.neuroimage.2009.12.096. [DOI] [PubMed] [Google Scholar]
- 47.Williamson KE, Jakobson LS. Social attribution skills of children born preterm at very low birth weight. Dev. Psychopathol. 2014;26(4 Pt 1):889–900. doi: 10.1017/S0954579414000522. [DOI] [PubMed] [Google Scholar]
- 48.Magill-Evans J, Koning C, Cameron-Sadava A, Manyk K. The child and adolescent social perception measure. J. Nonverbal Behav. 1995;19:151–169. doi: 10.1007/BF02175502. [DOI] [Google Scholar]
- 49.Williamson KE, Jakobson LS. Social perception in children born at very low birthweight and its relationship with social/behavioral outcomes. J. Child Psychol. Psychiatry. 2014;55(9):990–998. doi: 10.1111/jcpp.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavlova M, Sokolov AN, Birbaumer N, Krägeloh-Mann I. Perception and understanding of others’ actions and brain connectivity. J. Cogn. Neurosci. 2008;20:494–504. doi: 10.1162/jocn.2008.20034. [DOI] [PubMed] [Google Scholar]
- 51.Field TM. Visual and cardiac responses to animate and inanimate faces by young term and preterm infants. Child Dev. 1979;50(1):188–194. doi: 10.2307/1129055. [DOI] [PubMed] [Google Scholar]
- 52.Rose SA, Feldman JF, Jankowski JJ. Processing speed in the 1st year of life: A longitudinal study of preterm and full-term infants. Dev. Psychol. 2002;38(6):895–902. doi: 10.1037/0012-1649.38.6.895. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds CR, Bigler ED. Test of Memory and Learning (TOMAL) Pro-Ed; 1994. [Google Scholar]
- 54.Fazzi E, et al. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev. Med. Child Neurol. 2009;51(12):974–981. doi: 10.1111/j.1469-8749.2009.03272.x. [DOI] [PubMed] [Google Scholar]
- 55.Pereira SA, et al. A comparison between preterm and full-term infants’ preference for faces. J. Pediatr (Rio J.) 2017;93:35–39. doi: 10.1016/j.jped.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Frie J, Padilla N, Ådén U, Lagercrantz H, Bartocci M. Extremely preterm-born infants demonstrate different facial recognition processes at 6–10 months of corrected age. J. Pediatr. 2016;172:96–102. doi: 10.1016/j.jpeds.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 57.Telford EJ, et al. Preterm birth is associated with atypical social orienting in infancy detected using eye tracking. J. Child Psychol. Psychiatry. 2016;57(7):861–868. doi: 10.1111/jcpp.12546. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Roche T, et al. Face recognition impairment in small for gestational age and preterm children. Res. Dev. Disabil. 2017;62:166–173. doi: 10.1016/j.ridd.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 59.Papini C, et al. Altered resting-state functional connectivity in emotion-processing brain regions in adults who were born very preterm. Psychol. Med. 2016;46(14):3025–3039. doi: 10.1017/S0033291716001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathewson KJ, et al. Visual configural processing in adults born at extremely low birth weight. Dev. Sci. 2020;23(2):e12890. doi: 10.1111/desc.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pavlova MA, Scheffler K, Sokolov AN. Face-n-Food: Gender differences in tuning to faces. PLoS ONE. 2015;10(7):e0130363. doi: 10.1371/journal.pone.0130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavlova MA, Heiz J, Sokolov AN, Barisnikov K. Social cognition in Williams syndrome: Face tuning. Front. Psychol. 2016;7:1131. doi: 10.3389/fpsyg.2016.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavlova MA, Mayer A, Hösl F, Sokolov AN. Faces on her and his mind: Female and likable. PLoS ONE. 2016;11(6):e0157636. doi: 10.1371/journal.pone.0157636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlova MA, et al. Social cognition in autism: Face tuning. Sci. Rep. 2017;7:2734. doi: 10.1038/s41598-017-02790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pavlova MA, et al. Social cognition in down syndrome: Face tuning in face-like non-face images. Front. Psychol. 2018;9:2583. doi: 10.3389/fpsyg.2018.02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavlova MA, Heiz J, Sokolov AN, Fallgatter AJ, Barisnikov K. Even subtle cultural differences affect face tuning. PLoS ONE. 2018;13(6):e0198299. doi: 10.1371/journal.pone.0198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rolf R, Sokolov AN, Rattay TW, Fallgatter AJ, Pavlova MA. Face pareidolia in schizophrenia. Schizophr. Res. 2020;218:138–145. doi: 10.1016/j.schres.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Kubon J, Sokolov AN, Popp R, Fallgatter AJ, Pavlova MA. Face tuning in major depression. Cereb. Cortex. 2021;31(5):2574–2585. doi: 10.1093/cercor/bhaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koelkebeck K, et al. Benefits of using culturally unfamiliar stimuli in ambiguous emotion identification: A cross-cultural study. Psychiatry Res. 2015;228:39–45. doi: 10.1016/j.psychres.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Fazzi E, et al. Neuro-ophthalmological disorders in cerebral palsy: Ophthalmological, oculomotor, and visual aspects. Dev. Med. Child Neurol. 2012;54(8):730–736. doi: 10.1111/j.1469-8749.2012.04324.x. [DOI] [PubMed] [Google Scholar]
- 71.Pelphrey KA, Carter EJ. Charting the typical and atypical development of the social brain. Dev. Psychopathol. 2008;20(4):1081–1102. doi: 10.1017/S0954579408000515. [DOI] [PubMed] [Google Scholar]
- 72.Johnson S, Wolke D. Behavioural outcomes and psychopathology during adolescence. Early Hum. Dev. 2013;89(4):199–207. doi: 10.1016/j.earlhumdev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Johnson S, et al. Autism spectrum disorders in extremely preterm children. J. Pediatr. 2010;156(4):525–531. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 74.Pinto-Martin JA, et al. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics. 2011;128(5):883–891. doi: 10.1542/peds.2010-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fitzallen GC, Taylor HG, Bora S. What do we know about the preterm behavioral phenotype? A narrative review. Front. Psychiatry. 2020;11:154. doi: 10.3389/fpsyt.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omer Y, Sapir R, Hatuka Y, Yovel G. What is a face? Critical features for face detection. Perception. 2019;48(5):437–446. doi: 10.1177/0301006619838734. [DOI] [PubMed] [Google Scholar]
- 77.Di Giorgio E, et al. Filial responses as predisposed and learned preferences: Early attachment in chicks and babies. Behav. Brain Res. 2017;325(Pt B):90–104. doi: 10.1016/j.bbr.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 78.Reid VM, et al. The human fetus preferentially engages with face-like visual stimuli. Curr. Biol. 2017;27(12):1825–1828. doi: 10.1016/j.cub.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 79.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1–2):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 80.Turati C, Simion F, Milani I, Umiltà C. Newborns’ preference for faces: What is crucial? Dev. Psychol. 2002;38(6):875–882. doi: 10.1037/0012-1649.38.6.875. [DOI] [PubMed] [Google Scholar]
- 81.Kato M, Mugitani R. Pareidolia in infants. PLoS ONE. 2015;10(2):e0118539. doi: 10.1371/journal.pone.0118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buiatti M, et al. Cortical route for facelike pattern processing in human newborns. Proc. Natl. Acad. Sci. U.S.A. 2019;116(10):4625–4630. doi: 10.1073/pnas.1812419116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah P, Happé F, Sowden S, Cook R, Bird G. Orienting toward face-like stimuli in early childhood. Child Dev. 2015;86(6):1693–1700. doi: 10.1111/cdev.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosa-Salva O, Regolin L, Vallortigara G. Faces are special for newly hatched chicks: Evidence for inborn domain-specific mechanisms underlying spontaneous preferences for face-like stimuli. Dev. Sci. 2010;13(4):565–577. doi: 10.1111/j.1467-7687.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- 85.Rosa Salva O, Farroni T, Regolin L, Vallortigara G, Johnson MH. The evolution of social orienting: evidence from chicks (Gallus gallus) and human newborns. PLoS ONE. 2011;6(4):e18802. doi: 10.1371/journal.pone.0018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vallortigara G. Core knowledge of object, number, and geometry: A comparative and neural approach. Cogn. Neuropsychol. 2012;29(1–2):213–236. doi: 10.1080/02643294.2012.654772. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen MN, et al. Neuronal responses to face-like and facial stimuli in the monkey superior colliculus. Front. Behav. Neurosci. 2014;8:85. doi: 10.3389/fnbeh.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taubert J, Wardle SG, Flessert M, Leopold DA, Ungerleider LG. Face pareidolia in the rhesus monkey. Curr. Biol. 2017;27(16):2505–2509. doi: 10.1016/j.cub.2017.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taubert J, Van Belle G, Vogels R, Rossion B. The impact of stimulus size and orientation on individual face coding in monkey face-selective cortex. Sci. Rep. 2018;8(1):10339. doi: 10.1038/s41598-018-28144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobayashi M, et al. Do infants recognize the Arcimboldo images as faces? Behavioral and near-infrared spectroscopic study. J. Exp. Child Psychol. 2012;111:22–36. doi: 10.1016/j.jecp.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Burra N, et al. Amygdala activation for eye contact despite complete cortical blindness. J. Neurosci. 2013;33(25):10483–10489. doi: 10.1523/JNEUROSCI.3994-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gabay S, Nestor A, Dundas E, Behrmann M. Monocular advantage for face perception implicates subcortical mechanisms in adult humans. J. Cogn. Neurosci. 2014;26(5):927–937. doi: 10.1162/jocn_a_00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diano M, Celeghin A, Bagnis A, Tamietto M. Amygdala response to emotional stimuli without awareness: Facts and interpretations. Front. Psychol. 2016;7:2029. doi: 10.3389/fpsyg.2016.02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Batalle D, et al. Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage. 2012;60(2):1352–1366. doi: 10.1016/j.neuroimage.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 95.Ball G, et al. The influence of preterm birth on the developing thalamocortical connectome. Cortex. 2013;49(6):1711–1721. doi: 10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 96.Fischi-Gómez E, et al. Structural brain connectivity in school-age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cereb. Cortex. 2015;25(9):2793–2805. doi: 10.1093/cercor/bhu073. [DOI] [PubMed] [Google Scholar]
- 97.Moiseev A, Doesburg SM, Herdman AT, Ribary U, Grunau RE. Altered network oscillations and functional connectivity dynamics in children born very preterm. Brain Topogr. 2015;28(5):726–745. doi: 10.1007/s10548-014-0416-0. [DOI] [PubMed] [Google Scholar]
- 98.Muñoz-Moreno E, et al. Structural brain network reorganization and social cognition related to adverse perinatal condition from infancy to early adolescence. Front. Neurosci. 2016;10:560. doi: 10.3389/fnins.2016.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bouyssi-Kobar M, De Asis-Cruz J, Murnick J, Chang T, Limperopoulos C. Altered functional brain network integration, segregation, and modularity in infants born very preterm at term-equivalent age. J. Pediatr. 2019;213:13–21. doi: 10.1016/j.jpeds.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Q, et al. A review of functional near-infrared spectroscopy studies of motor and cognitive function in preterm infants. Neurosci. Bull. 2020;36:321–329. doi: 10.1007/s12264-019-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pavlova M, et al. Periventricular leukomalacia specifically affects cortical MEG response to biological motion. Ann. Neurol. 2006;59:415–419. doi: 10.1002/ana.20762. [DOI] [PubMed] [Google Scholar]
- 102.Pavlova M, Bidet-Ildei C, Sokolov AN, Braun C, Krägeloh-Mann I. Neuromagnetic response to body motion and brain connectivity. J. Cogn. Neurosci. 2009;21:837–846. doi: 10.1162/jocn.2009.21050. [DOI] [PubMed] [Google Scholar]
- 103.Pavlova M, Lutzenberger W, Sokolov AN, Birbaumer N, Krägeloh-Mann I. Oscillatory MEG response to human locomotion is modulated by periventricular lesions. Neuroimage. 2007;35(3):1256–1263. doi: 10.1016/j.neuroimage.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 104.Karmiloff-Smith A, et al. Exploring the Williams syndrome face-processing debate: The importance of building developmental trajectories. J. Child Psychol. Psychiatry. 2004;45(7):1258–1274. doi: 10.1111/j.1469-7610.2004.00322.x. [DOI] [PubMed] [Google Scholar]
- 105.Annaz D, Karmiloff-Smith A, Johnson MH, Thomas MS. A cross-syndrome study of the development of holistic face recognition in children with autism, Down syndrome, and Williams syndrome. J. Exp. Child Psychol. 2009;102(4):456–486. doi: 10.1016/j.jecp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 106.Leonard HC, Annaz D, Karmiloff-Smith A, Johnson MH. Developing spatial frequency biases for face recognition in autism and Williams syndrome. J. Autism Dev. Disord. 2011;41(7):968–973. doi: 10.1007/s10803-010-1115-7. [DOI] [PubMed] [Google Scholar]
- 107.D’Souza D, et al. Face processing in Williams syndrome is already atypical in infancy. Front. Psychol. 2015;6:760. doi: 10.3389/fpsyg.2015.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boccia M, et al. Why do you like Arcimboldo’s portraits? Effect of perceptual style on aesthetic appreciation of ambiguous artworks. Atten. Percept. Psychophys. 2014;76(6):1516–1521. doi: 10.3758/s13414-014-0739-7. [DOI] [PubMed] [Google Scholar]
- 109.Boccia M, et al. Do you like Arcimboldo’s? Esthetic appreciation modulates brain activity in solving perceptual ambiguity. Behav. Brain Res. 2015;278:147–154. doi: 10.1016/j.bbr.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 110.Liu J, et al. Seeing Jesus in toast: Neural and behavioral correlates of face pareidolia. Cortex. 2014;53:60–77. doi: 10.1016/j.cortex.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, et al. Neural correlates of covert face processing: fMRI evidence from a prosopagnosic patient. Cereb. Cortex. 2014;24(8):2081–2092. doi: 10.1093/cercor/bht059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Proverbio AM, Galli J. Women are better at seeing faces where there are none: An ERP study of face pareidolia. Soc. Cogn. Affect. Neurosci. 2016;11(9):1501–1512. doi: 10.1093/scan/nsw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rossion B, Dricot L, Goebel R, Busigny T. Holistic face categorization in higher order visual areas of the normal and prosopagnosic brain: Toward a non-hierarchical view of face perception. Front. Hum. Neurosci. 2011;4:225. doi: 10.3389/fnhum.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caharel S, et al. Early holistic face-like processing of Arcimboldo paintings in the right occipito-temporal cortex: Evidence from the N170 ERP component. Int. J. Psychophysiol. 2013;90(2):157–164. doi: 10.1016/j.ijpsycho.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 115.Hadjikhani N, Kveraga K, Naik P, Ahlfors SP. Early (M170) activation of face-specific cortex by face-like objects. NeuroReport. 2009;20(4):403–407. doi: 10.1097/WNR.0b013e328325a8e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rivest J, Moscovitch M, Black S. A comparative case study of face recognition: The contribution of configural and part-based recognition systems, and their interaction. Neuropsychologia. 2009;47(13):2798–2811. doi: 10.1016/j.neuropsychologia.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Busigny T, Joubert S, Felician O, Ceccaldi M, Rossion B. Holistic perception of the individual face is specific and necessary: Evidence from an extensive case study of acquired prosopagnosia. Neuropsychologia. 2010;48(14):4057–4092. doi: 10.1016/j.neuropsychologia.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 118.Steeves JK, et al. The fusiform face area is not sufficient for face recognition: Evidence from a patient with dense prosopagnosia and no occipital face area. Neuropsychologia. 2006;44(4):594–609. doi: 10.1016/j.neuropsychologia.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 119.Bora E, Velakoulis D, Walterfang M. Social cognition in Huntington’s disease: A meta-analysis. Behav. Brain Res. 2016;297:131–140. doi: 10.1016/j.bbr.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 120.Martínez-Horta S, et al. Impaired face-like object recognition in premanifest Huntington’s disease. Cortex. 2020;123:162–172. doi: 10.1016/j.cortex.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 121.Melamed N, Yogev Y, Glezerman M. Fetal gender and pregnancy outcome. J. Matern. Fetal Neonatal Med. 2010;23(4):338–344. doi: 10.3109/14767050903300969. [DOI] [PubMed] [Google Scholar]
- 122.Khalil MM, Alzahra E. Fetal gender and pregnancy outcomes in Libya: A retrospective study. Libyan J. Med. 2013;8:20008. doi: 10.3402/ljm.v8i0.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peelen MJ, et al. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet. Gynecol. Scand. 2016;95(9):1034–1041. doi: 10.1111/aogs.12929. [DOI] [PubMed] [Google Scholar]
- 124.Gano D. White matter injury in premature newborns. Neonatal Netw. 2016;35(2):73–77. doi: 10.1891/0730-0832.35.2.73. [DOI] [PubMed] [Google Scholar]
- 125.Schneider J, Miller SP. Preterm brain injury: White matter injury. Handb. Clin. Neurol. 2019;162:155–172. doi: 10.1016/B978-0-444-64029-1.00007-2. [DOI] [PubMed] [Google Scholar]
- 126.Korkman M, Kirk U, Kemp S. NEPSY–II. A Developmental Neuropsychology Assessment. The Psychological Corporation; 2007. [Google Scholar]
- 127.Urgesi C, Campanella F, Fabbro F. NEPSY–II: Second Edition. Contributo alla taratura italiana (NEPSY–II: Second Edition. Italian Standardization) Giunti O.S. Organizzazioni Speciali; 2011. [Google Scholar]
- 128.Beery KE, Buktenica NA. VMI Developmental Test of Visual-Motor Integration. Giunti OS; 2000. [Google Scholar]
- 129.Bova SM, et al. The development of visual object recognition in school-age children. Dev. Neuropsychol. 2007;31(1):79–102. doi: 10.1207/s15326942dn3101_5. [DOI] [PubMed] [Google Scholar]
- 130.Galli J, et al. White matter changes associated with cognitive visual dysfunctions in children with cerebral palsy: A diffusion tensor imaging study. J. Neurosci. Res. 2018;96(11):1766–1774. doi: 10.1002/jnr.24307. [DOI] [PubMed] [Google Scholar]
- 131.Riddoch MJ, Humphreys GW. Birmingham Object Recognition Battery. Lawrence Erlbaum Assoc., Inc.; 1993. [Google Scholar]