Abstract
Objectives.
Primary cardiac involvement in systemic sclerosis (SSc) is prevalent and morbid, however the influence of traditional cardiovascular risk factors such as essential hypertension (HTN) are unclear. In the present study, we sought to understand the effects of HTN on left ventricular (LV) contractility in SSc patients using echocardiographic speckle-derived global longitudinal strain (GLS).
Methods.
56 SSc patients with HTN (SSc+HTN+) and 82 SSc patients without HTN (SSc+ HTN−) were compared with 40 non-SSc controls with HTN (SSc−HTN+) and 40 non-SSc controls without HTN (SSc−HTN−), matched by age and sex. All HTN patients were on stable antihypertensive therapies. Echocardiographic measures included LV ejection fraction (LVEF), left atrial volume index (LAVi), and LV diastolic function. LV contractility was assessed by GLS, averaged across the 18 LV segments.
Results.
SSc patients had diminished GLS regardless of HTN status when compared to both control groups despite normal LVEF, p<0.001. SSc+HTN+ had the highest prevalence of diastolic dysfunction with significantly higher septal E/e’, a marker of LV filling pressures (p<0.05), as well as the largest reduction in GLS compared to SSc+HTN− and both control groups.
Conclusion.
Speckle-derived strain revealed diminished LV contractility in SSc patients despite normal LVEF. SSc+HTN+ had more prominent reductions in GLS associated with evidence of LV remodeling and worsened diastolic function. Our findings demonstrate the presence of subclinical LV contractile dysfunction in SSc that is further exacerbated by concomitant HTN, thereby identifying HTN as an important modifiable cardiovascular risk factor that should be managed aggressively in this at-risk population.
Key Indexing Terms. systemic sclerosis, essential hypertension, echocardiography, global longitudinal strain
INTRODUCTION
Systemic sclerosis (SSc) is a complex heterogeneous autoimmune disease characterized by widespread fibrosis of multiple organ systems, prominent vasculopathy, and dysregulation of the immune system [1]. Cardiac involvement is highly prevalent in SSc, ranging from 10–30%, however, is largely dependent on the diagnostic technique employed [1–4]. The presence of clinically overt cardiac involvement in SSc is associated with a 2.8-fold increased risk of mortality [5], underscoring the importance of using clinical suspicion to guide the appropriate application of screening and diagnostic tools before symptoms develop [4]. With increasing utilization of noninvasive imaging techniques, subclinical cardiac involvement has been estimated as high as 70% in SSc. [6]
Although cardiopulmonary complications from pulmonary arterial hypertension (PAH) are the leading cause of mortality in SSc [7], left ventricular (LV) diastolic dysfunction is common in SSc patients and may reflect myocardial involvement secondary to microvascular coronary ischemia, myocardial inflammation, and/or myocardial fibrosis [1,8]. In the general population, diastolic dysfunction is estimated at 21% and related to common risk factors of age, essential hypertension (HTN), diabetes, and coronary artery disease [9]. SSc patients may also be affected by these traditional cardiovascular comorbidities in addition to disease-specific risk factors [10]. Diastolic function may appear before clinical symptoms of heart failure, regardless of SSc disease subtype [11], and is an important and highly prevalent early manifestation of cardiac involvement in SSc [12]. In SSc, worsening diastolic dysfunction is associated with older age, SSc disease duration, diffuse SSc subtype, and presence of cardiovascular risk factors such as essential hypertension and ischemic heart disease [10].
Despite frequent serial clinical examination and echocardiographic monitoring, scleroderma heart disease is often underdiagnosed until late in the disease course when clinically symptomatic heart failure develops [13]. Speckle-tracking derived longitudinal systolic strain is a relatively new imaging modality used in conjunction with conventional 2D echocardiography that is not user or Doppler angle dependent. Utilizing a software derived algorithm, speckle-derived strain is able to provide a noninvasive estimation of regional and global myocardial contractile function [14]. The analysis of longitudinal strain provides important additional information on regional and global contractility that is not detectable by standard measures alone [14]. LV global longitudinal strain (GLS) has been extensively studied over the past decade in a wide variety of clinical settings and is thought to be a more sensitive measure of contractile function when compared to LV ejection fraction, additionally providing prognostic information [15]. Speckle-derived strain has already shown its relevance in the detection of regional heterogeneity of right ventricular (RV) contractility in SSc [16], in prediction of mortality in SSc patients with PAH [17], and in following improvement in RV function after initiation of PAH-directed therapies in SSc [18]. Investigation in scleroderma heart disease, however, has largely focused on RV failure, despite the fact that early myocardial disease in SSc may manifest as LV diastolic dysfunction. Furthermore, the effects of traditional cardiovascular risk factors, such as HTN, on the scleroderma myocardium have not been well defined. In the present study, we sought to understand the ability of novel speckle-based strain techniques in detecting early myocardial alterations in normotensive patients with SSc, and to explore the additional effects of HTN on LV myocardial contractility in this at-risk population. Preliminary results from this study have been previously reported in abstract form [19].
MATERIALS AND METHODS
Study Population.
In this prospective single center study, patients classified with SSc according to the American College of Rheumatology/European League Against Rheumatism criteria [20] who enrolled in the Institutional Review Board-approved Johns Hopkins Scleroderma Center Research Registry (IRB00226995) were studied. All patients enrolled in the Johns Hopkins Scleroderma Center Research Registry have provided written and informed consent to have their deidentified clinical data utilized for investigation. Center standard practice includes annual echocardiograms to screen for the development of PAH, regardless of clinical symptoms and therefore SSc patients who had a clinically indicated echocardiogram performed at the Johns Hopkins Bayview Medical Center were eligible for inclusion in this cross-sectional analysis. If multiple echocardiograms were performed on the same patient, the first study performed with the highest technical quality was chosen for analysis. Of the 162 SSc patients who met these inclusion criteria, 138 (85%) patients had adequate 2D image quality to allow for complete visualization of the LV chamber and strain mapping.
A cohort of age- and sex-matched non-SSc controls, who underwent clinically indicated echocardiograms during the study period, was also evaluated. Exclusion criteria included history of hospitalization for heart failure, hemodynamically significant valvular disease (any stenosis and regurgitation greater than mild in severity), coronary artery disease (segmental wall motion abnormality and history of myocardial infarction), ischemic, dilated, or hypertrophic cardiomyopathy, primary pulmonary disease, and systemic disease associated with secondary pulmonary disease (eg, sarcoidosis, SSc, and connective tissue disease), evidence of intracardiac shunting, and evidence of congenital heart disease.
Control subjects underwent extensive chart review to establish two non-SSc control groups: non-SSc patients without hypertension (SSc−HTN−) and non-SSc patients with hypertension (SSc−HTN+). Additional exclusion criteria for the first control group (SSc−HTN−) included HTN, diabetes mellitus, atherosclerotic cardiovascular disease, atrial fibrillation, and any known history of arrhythmia, stroke, peripheral vascular disease, chronic obstructive pulmonary disease, or sleep apnea. The second control group (SSc−HTN+), however, were not excluded based on these additional features. All control patients had to have echocardiographic study quality that was technically adequate to allow for off-line strain analysis.
A cardiologist (MM), board certified in echocardiography, was blinded to disease status and analyzed each study including 2D measures and speckle-based strain. To assess for intraobserver and interobserver variability, 20 studies with adequate study quality were randomized for reanalysis 6 months after initial analysis by 2 independent cardiologists (MM, VM), again blinded to disease status.
Clinical assessment.
Demographic data, disease characteristics, smoking history, medication exposures, history of cardiovascular and pulmonary comorbidities, pulmonary function testing, and clinically obtained autoantibody tests results were obtained from the scleroderma database and clinical records closest to the time of echocardiography. Diagnosis of HTN was based on a documented history of HTN, defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, provided by the primary care physician or rheumatologist and/or treatment with any antihypertensive medication if not prescribed for the treatment of Raynaud’s phenomenon. Blood pressure at the time of echocardiogram was also obtained. SSc cutaneous subtype was defined by established criteria,11 and SSc disease duration was calculated as the time interval between the first SSc symptom (either Raynaud’s or first non-Raynaud’s symptom) and the echocardiogram date. For the non-SSc control group, individuals with HTN were identified based on a documented history of HTN, defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg based on comprehensive chart review.
Echocardiographic analysis.
Echocardiographic examinations were performed at a single clinical site using Phillips ie33 ultrasound machine (Phillips Healthcare, Andover, MA) with subjects in the left lateral decubitus position. Images were obtained with a 3.4 MHz sector transducer. Standard parasternal, apical, and subxiphoid windows were utilized in order to obtain 2D imaging of cardiac chambers, as well as color, pulsed- and continuous-wave Doppler measurements, according with American Society of Echocardiography (ASE) guidelines [21]. 2D-directed methods were used to obtain linear measurements of LV chamber size and wall thickness from the parasternal long-axis view, and then used to calculate LV mass [21]. LV ejection fraction (LVEF) was calculated according the modified Simpson’s rule using the apical four- and two-chamber views, with normal defined as ≥ 55%. Left atrial volume index (LAVi) was obtained as the average of volumetric measurement of the left atrium from the 2- and 4-chamber views and indexed to body surface area. Evaluation of LV diastolic function was based on pulsed-wave Doppler imaging of mitral valve inflow, measuring peak early diastolic velocity (E) and peak late diastolic velocity (A) to calculate the E/A ratio, and the E-wave deceleration. Using tissue Doppler imaging, the early diastolic velocity (e’) was measured at the level of the interventricular basal segment (septal e’). The septal E/e’ ratio was used to estimate LV filling pressures [22]. In accordance with the ASE Guidelines, the diagnosis of diastolic dysfunction was defined by the presence of more than two of the following abnormal cutoff values for these four recommended variables: septal e’ velocity < 7 cm/ sec, septal E/e’ ratio > 15, LAVi > 34 mL/m2, and peak tricuspid regurgitation velocity > 2.8 m/sec. LV diastolic function was defined as normal if more than two of the available variables did not meet the cutoff values for identifying abnormal function. The study was considered inconclusive if two of the parameters did not meet the cutoff values [22].
Functional assessment of the RV was performed by analyzing M-mode derived tricuspid annular systolic plane excursion (TAPSE), and, in the absence of RV outflow tract obstruction and tricuspid or pulmonic stenosis, the tricuspid regurgitant velocity was used to estimate RV systolic pressure (RVSP) using the modified Bernoulli equation and adding estimated right atrial pressure based on inferior vena cava dimension and collapsibility with sniff [21,23]. Echocardiographic analysis was performed using FUJIFILM Medical Systems Synapse Cardiovascular Software (V4.0.8, USA) for conventional analysis of 2D images.
For the assessment of peak LV longitudinal systolic strain, standard 2D cine-loops from apical four-chamber view (inferoseptal and anterolateral walls), two-chamber view (anterior and inferior walls), and three-chamber view (inferolateral and anteroseptal walls) were obtained and stored digitally for offline speckle-tracking analysis using a commercially available vendor-independent strain software (Epsilon, EchoInsight, Milwaukee, WI). Each LV wall was divided into three segments (basal, midventricular, and apical) to construct an 18-segment model. Peak systolic longitudinal strain of the LV for each segment was obtained by tracing the LV chamber endocardial borders in end-systolic still frames. Frame rates were obtained between 50–70 fps. In post-processing, automated tracking was visually verified and manually adjusted to ensure adequate border delineation. Longitudinal strain is traditionally defined as the percentage shortening of a region of interest (ROI) relative to its original length and by convention is expressed as a negative percentage [24,25]. Worsening strain refers to a less negative number (a lower absolute value) than expected for a ROI or diminished deformation along the longitudinal axis. Improved strain, on the contrary, refers to a more negative number (a higher absolute value) than expected for an ROI or enhanced deformation along the longitudinal axis. Peak global longitudinal strain (GLS) was calculated as the average value of longitudinal systolic strain for the 18 LV segments [25].
Statistical analysis.
Data are presented as mean ± standard deviation, median and interquartile range (IQR), absolute numbers or percentage, as appropriate. The independent unpaired t-test for parametric data and Wilcoxon-Mann-Whitney test for non-parametric data were used to compare continuous variables between SSc patients and control subjects, while Chi-square test and Fisher exact test were used to compare categorical data. Multivariable linear regression analysis was performed to evaluate the impact of comorbidities and therapies on GLS. The intra- and inter-observer variability was calculated by means of intraclass correlation coefficient. Statistical analysis was performed with the software SPSS for Windows (Version 22.0, IBM Corporation, Armonk, NY). Differences between groups were considered statistically significant if the p value was < 0.05.
RESULTS
Clinical characteristics.
Our SSc cohort consisted in 138 patients that were mostly females (87.7%), with mean age of 54.3 ± 12.6 years. Among them, 83 patients had limited SSc, while 55 had diffuse SSc. A majority of our SSc cohort was positive for antinuclear antibodies. The median SSc disease duration was 13.5 (IQR 6.7, 22.4) years. Control patients were intentionally frequency matched by age and sex and, by inclusion criteria, control patients either did not have any known cardiovascular and/or pulmonary disease (SSc−HTN−) or had HTN with or without other cardiovascular risk factors (e.g. diabetes, hypercholesterolemia, or atrial fibrillation) (SSc−HTN+).
We further dichotomized our SSc cohort by presence, or absence of HTN or other cardiovascular risk factors, defined as SSC+HTN− and SSc+HTN+ respectively. There were 82 SSc+HTN− patients and 56 SSc+HTN+ patients. SSc+HTN+ patients were older and more often Black in comparison to SSc+HTN− patients, as shown in Table 1. There were no significant differences in terms of disease duration, SSc subtype, and autoantibody status between SSc groups. There were also no significant differences in terms of age and gender given our frequency matched study design, or smoking status between both non-HTN groups (SSc+HTN− and SSc−HTN−) and HTN groups (SSc+HTN+ and SSC−HTN+). SSc+HTN− patients tended to have lower body mass index and body surface area in comparison to SSc−HTN− patients (Table 1).
Table 1.
Patient Characteristics of Systemic Sclerosis versus non-Systemic Sclerosis Patients with and Without Hypertension. Demographics, important cardiovascular comorbidities, medications, and SSc disease specific characteristics are presented for SSc patients versus non-SSc controls based on the presence of essential hypertension.
| SSc−HTN− (n=40) |
SSc+HTN− (n=82) |
p value1 | SSc−HTN+ (n=40) |
SSc+HTN+ (n=56) |
p value2 | p value3 | |
|---|---|---|---|---|---|---|---|
| Age, years | 53.5 ± 14.0 | 51.5 ± 13.1 | NS | 54.2 ± 16.9 | 58.5 ± 10.5 | NS | 0.001 |
| Women, n (%) | 35 (87.7) | 75 (91.5) | NS | 33 (82.5) | 46 (82.1) | NS | NS |
| Race, n (%) | |||||||
| White | 33 (82.5) | 66 (81.7) | NS | 27 (67.5) | 37 (64) | NS | 0.007 |
| Black | 4 (10) | 10 (12.2) | 11 (27.5) | 16 (32) | |||
| Other | 3 (7.5) | 6 (7.3) | 2 (5) | 1 (2) | |||
| Ever smoker, n (%) | 15 (37.5) | 30 (36.6) | NS | 14 (35) | 21 (37.5) | NS | NS |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | -- | 7 (17.5) | 9 (16.1) | NS | 0.007 |
| Atrial fibrillation | 0 (0) | 0 (0) | -- | 2 (5) | 3 (5.4) | NS | NS |
| Hypercholesterolemia | 0 (0) | 0 (0) | -- | 16 (40) | 25 (44.6) | NS | <0.0001 |
| Anti-HTN medications, n (%) | |||||||
| Beta-blocker | NA | NA | NA | 12 (30) | 14 (28) | NS | NA |
| Calcium channel blocker | NA | NA | NA | 8 (20) | 48 (85.7) | <0.0001 | NA |
| ACE-inhibitor/ARB | NA | NA | NA | 10 (25) | 44 (78.6) | <0.0001 | NA |
| Diuretics | NA | NA | NA | 10 (25) | 20 (38) | NS | NA |
| Body mass index, kg/m2 | 28.1 ± 7.2 | 25.0 ± 5.6 | <0.05 | 27.8 ± 5.7 | 27.4 ± 5.8 | NS | 0.02 |
| Body surface area, m2 | 1.82 ± 0.17 | 1.73 ± 0.21 | <0.05 | 1.98 ± 0.47 | 1.82 ± 0.24 | 0.05 | 0.01 |
| Scleroderma disease duration, median years | NA | 15.7 ± 10.7 | NA | NA | 16.5 ±11.9 | NA | NS |
| Scleroderma subtype, n (%) | |||||||
| Limited | NA | 50 (61.0) | NA | NA | 33 (58.9) | NA | NS |
| Diffuse | NA | 32 (39.0) | NA | 23 (41.1) | |||
| Autoantibody status, n | |||||||
| ANA [N=138] | NA | 82 | NA | NA | 53 | NA | NA |
| Centromere [N=137] | NA | 26 | NA | NA | 14 | NA | NS |
| Topoisomerase 1 [N=136] | NA | 26 | NA | NA | 12 | NA | NS |
| RNA polymerase III [N=115] | NA | 12 | NA | NA | 7 | NA | NS |
| Pulmonary function data, % predicted | |||||||
| FVC % predicted [N=134] | NA | 84.1 ± 16.6 | NA | NA | 80.3 ± 21.0 | NA | NS |
| DLCO % predicted [N=132] | NA | 83.6 ± 24.4 | NA | NA | 78.0 ± 26.7 | NA | NS |
List of abbreviations: HTN, systemic hypertension, SSc, systemic sclerosis; NS, not statistically significant; NA, not available/not applicable; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor type 1 blocker; ANA, anti-nuclear antibodies; RNA, ribonucleic acid; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide. Groups are defined as non-SSc controls without HTN (SSc−HTN−), SSc patients without HTN (SSc+HTN−), non-SSc controls with HTN (SSc−HTN+), and SSc patients with HTN (SSc+HTN+). Data are expressed as mean ± standard deviation, absolute number, percentage, or median, if specified. Independent unpaired t-test and Wilcoxon-Mann-Whitney test for non-parametric data were used to compare continuous variables between SSc patients and control subjects, while Chi-square test and Fisher exact test were used to compare categorical data. A cut-off p value < 0.05 was considered as statistically significant.
p value: comparison between SSc−HTN− and SSc+HTN−.
p value: comparison between SSc−HTN+ and SSc+HTN+.
p value: comparison between SSc+HTN− and SSc+HTN+.
There was no significant difference in terms of cardiovascular comorbidities between HTN groups (SSc−HTN+ and SSc+HTN+). SSc patients with HTN were more frequently treated with calcium channel blockers (as expected, since these drugs are often prescribed in SSc patients also for the treatment of Raynaud’s phenomenon). There was a higher prevalence of ACE-inhibitor/ARB treatment in the SSc patients with HTN (SSc+HTN+) compared to non-SSc controls with HTN (SSc−HTN+), while no significant differences were observed in terms of treatment with diuretics and beta-blockers.
SSc patients without HTN have decreased LV systolic function and GLS relative to age- and sex-matched control patients without HTN.
In our initial analysis, we compared SSc patients without HTN (SSc+HTN−) to age- and sex-matched healthy controls without known cardiopulmonary disease (SSc−HTN−). While LVEF was within the normal range across groups, SSc+HTN− had lower LVEF compared to SSc−HTN− by statistical significance (p<0.005), as shown in Table 2. We also found that SSc+HTN− had lower GLS when compared to SSc−HTN− (p<0.001), mostly due to diminished regional strain of the basal and midventricular LV regional segments, as shown in Figure 1. Diastolic parameters of mitral inflow such as E/e’, as a measure of LV filling pressures, and LAVi were similar between both SSc+HTN− and SSc−HTN− groups.
Table 2.
Standard and Speckle-tracking Derived Echocardiographic Characteristics for Systemic Sclerosis Patients and Non-Systemic Sclerosis Controls according to the Presence of Systemic Hypertension.
| SSc−HTN− (n=40) |
SSc+HTN− (n=82) |
P value1 | SSc−HTN+ (n=40) |
SSc+HTN+ (n=56) |
p value2 | p value3 | |
|---|---|---|---|---|---|---|---|
| Conventional Echocardiographic Measures | |||||||
| LV ejection fraction, % | 62.9 ± 5.9 | 58.3± 6.3 | < 0.001 | 59.4 ± 4.3 | 58.7 ± 6.3 | NS | NS |
| E wave, cm/sec | 79.0 ± 15.8 | 81.9 ± 18.8 | NS | 89.1 ± 23.8 | 79.7 ± 22.9 | 0.06 | NS |
| A wave, cm/sec | 70.1 ± 17.3 | 72.1 ± 19.9 | NS | 81.1 ± 30.2 | 80.9 ± 23.8 | NS | 0.02 |
| E deceleration time, msec | 218.6 ± 53.5 | 216.1 ± 57.6 | NS | 213.7 ± 69.6 | 226.6 ± 56.1 | NS | NS |
| Mitral E/A | 1.38 ± 1.39 | 1.21 ± 0.40 | NS | 1.2 ± 0.44 | 1.0 ± 0.32 | <0.05 | 0.005 |
| E′ septal, cm/sec | 8.8 ± 2.3 | 8.6 ± 2.3 | NS | 8.4 ± 2.7 | 7.1 ± 2.0 | 0.01 | <0.0001 |
| E/E′ septal | 9.4 ± 2.8 | 10.2 ± 3.3 | NS | 10.7 ± 2.8 | 12.1 ± 5.3 | 0.07 | 0.02 |
| LAVi, ml/m2 | 31.7 ± 13.6 | 33.9 ± 8.4 | NS | 31.0 ± 7.7 | 35.1 ± 10.9 | 0.045 | NS |
| RVSP, mmHg | 22.6 ± 4.4 | 29.4 ± 11.7 | <0.001 | 23.0 ± 4.7 | 34.5 ± 15.0 | <0.001 | 0.04 |
| TAPSE, cm | 2.25 ± 0.40 | 2.18 ± 0.42 | NS | 2.26 ± 0.43 | 2.14 ± 0.53 | NS | NS |
| Present | 2 (5) | 10 (12.2) | 6 (15) | 15 (26.8) | |||
| Echo-Based Strain Measures | |||||||
| Basal inferoseptal, % | −19.0 ± 2.9 | −17.0 ± 4.6 | <0.01 | −18.3 ± 4.0 | −16.1 ± 4.1 | <0.02 | NS |
| Mid inferoseptal, % | −18.7 ± 3.1 | −17.7 ± 5.1 | NS | −19.2 ± 3.6 | −17.1 ± 3.7 | <0.01 | NS |
| Apical inferoseptal, % | −22.5 ± 4.9 | −24.3 ± 5.5 | NS | −23.0 ± 5.4 | −22.6 ± 5.1 | NS | NS |
| Basal anterolateral, % | −21.4 ± 5.3 | −19.7 ± 4.9 | NS | −19.9 ± 5.5 | −18.1 ± 4.6 | NS | NS |
| Mid anterolateral, % | −19.6 ± 3.0 | −17.8 ± 4.0 | <0.01 | −18.2 ± 3.2 | −16.7 ± 3.9 | 0.06 | NS |
| Apical anterolateral, % | −21.0 ± 4.4 | −20.8 ± 4.9 | NS | −19.4 ± 4.7 | −19.6 ± 5.1 | NS | NS |
| Basal inferior, % | −19.5 ± 3.3 | −17.6 ± 5.2 | <0.05 | −18.5 ± 4.5 | −16.8 ± 4.9 | NS | NS |
| Mid inferior, % | −19.2 ± 3.1 | −18.1 ± 4.1 | NS | −17.7 ± 4.0 | −17.6 ± 4.6 | NS | NS |
| Apical inferior, % | −22.8 ± 3.8 | −23.5 ± 5.3 | NS | −23.5 ± 6.0 | −22.6 ± 7.0 | NS | NS |
| Basal anterior, % | −21.4 ± 4.6 | −19.3 ± 5.4 | <0.05 | −18.6 ± 4.9 | −18.0 ± 5.4 | NS | NS |
| Mid anterior, % | −20.0 ± 2.9 | −18.0 ± 4.2 | <0.005 | −17.3 ± 3.5 | −16.4 ± 4.5 | NS | NS |
| Apical anterior, % | −20.2 ± 3.1 | −19.7 ± 5.3 | NS | −19.2 ± 4.6 | −18.8 ± 5.9 | NS | NS |
| Basal inferolateral, % | −22.5 ± 5.5 | −19.6 ± 6.2 | <0.02 | −19.6 ± 4.8 | −17.3 ± 4.5 | <0.05 | 0.03 |
| Mid inferolateral, % | −19.7 ± 3.1 | −16.3 ± 5.1 | <0.0001 | −17.2 ± 3.7 | −17.2 ± 4.2 | NS | NS |
| Apical inferolateral, % | −19.7 ± 4.3 | −18.5 ± 5.1 | NS | −20.2 ± 5.0 | −19.2 ± 5.2 | NS | NS |
| Basal anteroseptal, % | −18.4 ± 7.2 | −17.0 ± 4.2 | NS | −17.1 ± 4.6 | −15.8 ± 4.7 | NS | NS |
| Mid anteroseptal, % | −19.9 ± 3.7 | −18.3 ± 4.2 | <0.05 | −18.0 ± 6.7 | −17.7 ± 5.2 | NS | NS |
| Apical anteroseptal, % | −21.0 ± 4.1 | −21.6 ± 7.9 | NS | −21.6 ± 4.9 | −19.2 ± 5.9 | <0.05 | NS |
| GLS, % | −20.3 ± 1.2 | −19.1 ± 2.2 | <0.001 | −19.2 ± 1.3 | −18.2 ± 2.3 | <0.01 | 0.02 |
List of abbreviations: HTN, systemic hypertension, SSc, systemic sclerosis; left ventricular; LAVi, left atrial volume indexed for body surface area; LS, longitudinal strain; GLS, global longitudinal strain; NS, not statistically significant; NA, not available/not applicable. Groups are defined as non-SSc controls without HTN (SSc−HTN−), SSc patients without HTN (SSc+HTN−), non-SSc controls with HTN (SSc−HTN+), and SSc patients with HTN (SSc+HTN+). Data are expressed as mean ± standard deviation, absolute number or percentage. Independent unpaired t-test and Wilcoxon-Mann-Whitney test for non-parametric data were used to compare continuous variables between SSc patients and control subjects, while Chi-square test and Fisher exact test were used to compare categorical data. A cut-off p value < 0.05 was considered as statistically significant.
p value: comparison between SSc−HTN− and SSc+HTN−.
p value: comparison between SSc−HTN+ and SSc+HTN+.
p value: comparison between SSc+HTN− and SSc+HTN+.
Figure 1.
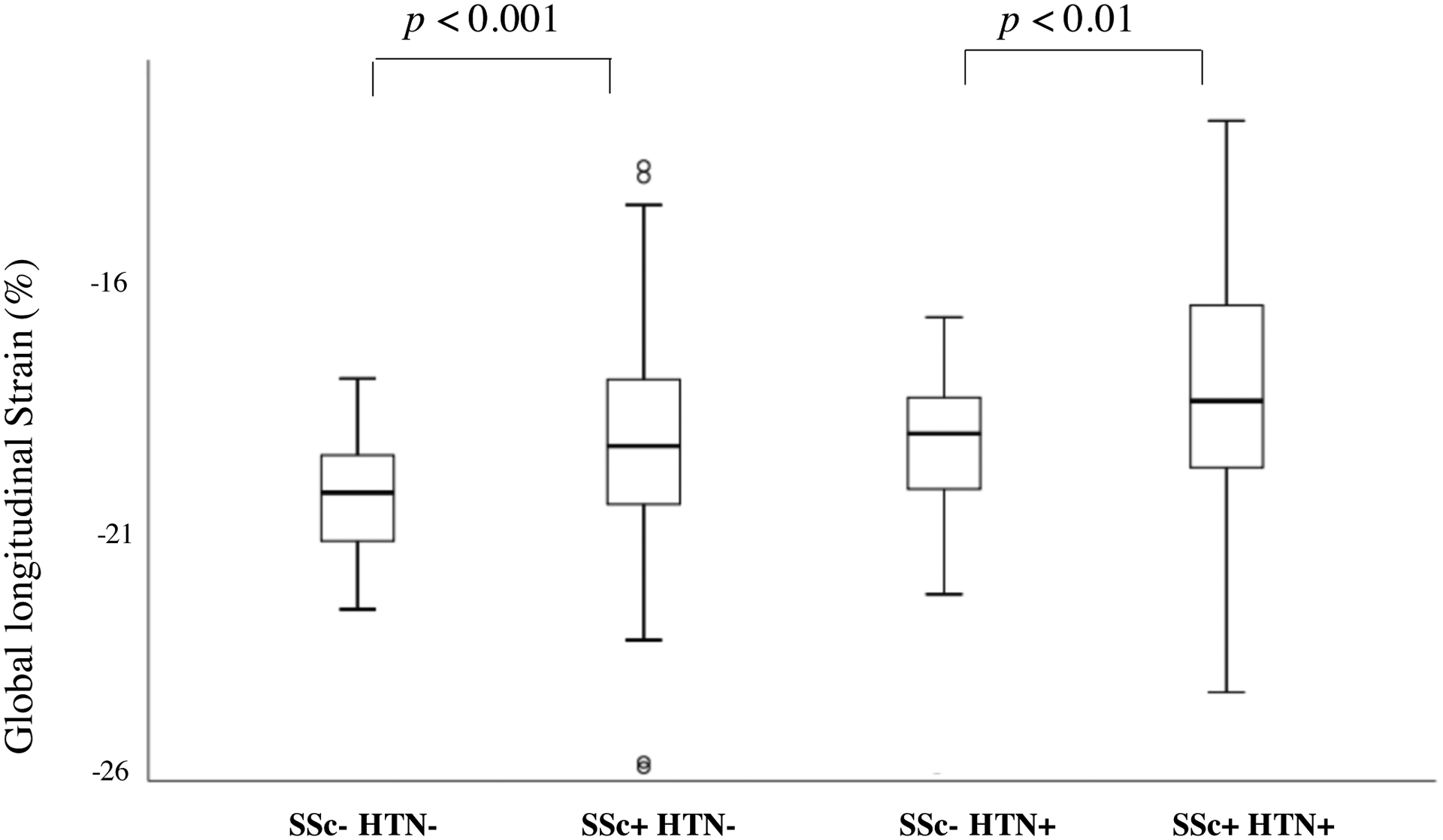
Box-Plot Graphic Presentation of Differences in Left Ventricular Global Longitudinal Strain Between Systemic Sclerosis Patients without Systemic Hypertension (SSc+HTN−) and Age and Sex-Matched Controls (SSc−HTN−), and Between Systemic Sclerosis Patients with Systemic Hypertension (SSc+HTN+) and Age and Sex-Matched Controls with Systemic Hypertension (SSc−HTN+).
SSc patients with HTN have worse parameters of diastolic function and have decreased GLS relative to age- and sex-matched control patients with HTN.
We then compared SSc patients with HTN (SSc+HTN+) to age- and sex-matched non-SSc controls with HTN (SSc−HTN+). While LVEF was similar between HTN groups, SSc+HTN+ patients had lower GLS in comparison to Control Group with HTN (p<0.01), as shown in Table 2 and in Figure 1. We also found that diastolic parameters were significantly different. In particular, SSc+HTN+ patients had a lower mitral E/A ratio (p <0.05), mainly because of a lower early diastolic E wave velocity (p=0.06) in comparison to SSc−HTN+ patients. E’ septal wave velocity was significantly reduced in SSc+HTN+ patients as well when compared to SSc−HTN+ patients (p=0.01), with a borderline significant increase in E/e’ septal ratio (p=0.07). SSc Group with HTN had significantly higher LAVi 35.1 ± 10.9 ml/m2 when compared to sex-matched control patients with HTN, p=0.045.
Multivariable linear regression analysis revealed that there was no significant impact of different antihypertensive medications on GLS, and that the presence of HTN was the only significant contributor to alterations in GLS in SSc patients, as shown in Table 3. Differences in GLS amongst groups were not affected by loading conditions as estimated by systolic blood pressure, β coefficient 0.041 (95% CI −0.012−0.023), p=0.543.
Table 3.
Multivariable linear regression analysis to explore the impact of age, comorbidities, and therapies on GLS in SSc patients.
| Variable | Beta-coefficient | 95% CI | p value |
|---|---|---|---|
| Age | −0.126 | −0.057, 0.011 | 0.184 |
| Smoking | −0.075 | −0.898, 0.362 | 0.401 |
| Dyslipidemia | −0.113 | −1.618, 0.516 | 0.309 |
| Diabetes | −0.137 | −3.057, 0.514 | 0.161 |
| Hypertension | 0.337 | 0.488, 2.664 | 0.005 |
| Calcium channel blocker | −0.010 | −0.874, 0.781 | 0.912 |
| Beta-blocker | 0.041 | −1.371, 2.137 | 0.667 |
| ACE-inhibitor | −0.130 | −2.020, 0.450 | 0.211 |
| Angiotensin receptor antagonist | 0.048 | −1.233, 2.080 | 0.614 |
| Diuretic | −0.008 | −1.110, 1.022 | 0.935 |
| Statin | 0.083 | −0.797, 1.726 | 0.468 |
Intra- and inter-observer variability and power calculation.
Intra- and inter-observer variability for GLS was excellent, with ICC of 0.95 (0.86–0.98) and 0.92 (0.70–0.98), respectively. Sample size power calculation revealed a power of 0.86.
DISCUSSION
To our knowledge, our study is the first to demonstrate the effects of systemic HTN on LV contractile function in SSc patients utilizing innovative speckle-based strain techniques. Our enriched study design evaluated four well-characterized groups including SSc patients with HTN (SSc+HTN+), SSc patients without HTN (SSc−HTN−), non-SSc patients with HTN (SSc−HTN+), and non-SSc patients without HTN (SSc−HTN−). Despite normal LVEF by conventional echo analysis, we observed the presence of regional and global abnormalities in LV myocardial contractility in SSc patients that were not detected by conventional echocardiographic techniques alone. Specifically, we found a reduction in global LV contractility, mainly due to reduction in regional basal and midventricular strain across both SSc groups. The significant impairment of GLS in SSc patients even in the absence of HTN, indicates that SSc disease is an important factor in the presence of regional and global myocardial contractility abnormalities. Although the difference between SSc and controls (both with HTN and without HTN) may seem small, this variation represents at least a 10% difference in absolute value of strain, which could be clinically important [26]. Our results are in agreement with previous studies using speckle tracking strain analysis, which have shown differences in GLS between SSc subjects and age- and comorbid-disease matched controls [27–29]. Similar to Spethman et al., we found that impairments in LV strain was most notable in the basal segments whereas the mid- and apical segments were relatively preserved [27]. Impaired GLS have previously been associated with a decrease in exercise functional capacity (measured by 6-minute walk test and peak VO2 on cardiopulmonary exercise testing) [28,30], autonomic dysfunction [31], and ventricular tachycardia or ectopic beats on 24 hour Holter monitoring [28]. Additionally, abnormal GLS has been associated with an increase in cardiovascular events (symptoms and/or signs of heart failure, coronary artery disease, atrial fibrillation, or cardiovascular death) at 20-month follow-up [29].
SSc patients with concomitant HTN had evidence of abnormal GLS, a marker of LV contractile function, as well as greater evidence of cardiac remodeling when compared to SSc patients without HTN or non-SSc patients with HTN as evidenced by greater LA dimensions and higher elevations in LV end-diastolic pressures by medial E/e’ estimation. Our findings demonstrate the significant impact of HTN, a common and prevalent cardiovascular risk factor, on the SSc myocardium and suggest that HTN has additional and unfavorable effects on the vulnerable SSc myocardium that results in more pronounced adverse remodeling and LV diastolic and systolic function when compared to other groups.
Diastolic dysfunction is an early noninvasive manifestation of cardiac involvement in SSc and suggestive of underlying myocardial fibrosis, occuring early in SSc independent of other cardiac cormobidities with an estimated prevalence of 20–60% [10]. Echocardiography is an important diagnostic tool for the identification of diastolic dysfunction in SSc, and previous studies utilizing tissue Doppler septal e’ velocities have suggested that diminished e’ velocities are associated with increasing mortality [10]. Impairment of several diastolic echocardiographic variables have all been shown to correlate with disease duration and can occur as early as Raynaud’s onset [11]. HTN is known to accelerate adverse remodeling in the general population and results in diastolic dysfunction, underling the clinical syndrome of heart failure with preserved ejection fraction (HFpEF) [13, 32]. HFpEF is highly prevalent in SSc and thought to be due to fibrotic processes inherent to the SSc disease process. HFpEF in SSc is a highly morbid condition and when associated with pulmonary hypertension, has a two-fold increase in mortality [33]. Given the increased morbidity and mortality of HFpEF in SSc, we sought to understand the impact of traditional risk factors such as HTN on the SSc myocardium as the early management of HTN in SSc patients may have significant clinical implications.
We importantly demonstrated that SSc patients with HTN have a greater degree of diastolic dysfunction and LV remodeling when compared to age- and gender-matched non-SSc patients with HTN. In addition to diastolic abnormalities, SSc+HTN+ patients had speckle-based strain abnormalities in global LV contractility not appreciable by conventional echocardiographic measures alone. While there are no guideline-based therapies for the management of HFpEF in the general population or in SSc, the presence of conventional cardiac risk factors such HTN in SSc signifies an important clinical finding that should be managed aggressively due to the increased risk of adverse cardiac remodeling in these patients. Systolic blood pressure should be managed in accordance with guidelines utilizing beta-blockade, ACE inhibitors, and ARBs [34]. Dihydropyridine calcium channel blockers may have additional vasodilatory benefits in SSc patients with active Raynaud’s phenomenon. In the general population, there continues to be considerable controversy on the use of mineralocorticoid-receptor antagonists, such as Spironolactone, and its utility in HFpEF [35]. Anti-fibrotic agents are not currently indicated for management of diastolic dysfunction and HFpEF in SSc however the role of hypertension management in the prevention of HFpEF in the SSc population with abnormal LV GLS needs to be evaluated in prospective longitudinal studies.
There were several limitations to our study. First, the ability to perform speckle-tracking strain is largely dependent on 2D image quality. Nevertheless, the strain analysis in our cohort was feasible in most of the cases and patients were excluded if image quality precluded endocardial border delineation. Additionally, there is well-described vendor-specific variability in strain measures [36,37]; to limit this, all analysis was performed using a single software by two experienced readers. In addition, while important associations can be made between the impact of HTN on the scleroderma heart as detected by innovative echocardiographic methods, our findings cannot be extrapolated as cause and effect.
In summary, speckle-tracking derived longitudinal systolic strain analysis revealed regional and global abnormalities in left ventricular myocardial contractility in SSc patients compared with age- and sex-matched controls that were not detected by standard echocardiography alone. Furthermore, SSc patients with HTN showed greater abnormalities both diastolic function, LV remodeling, and LV myocardial contractility in comparison to non-SSc subjects with HTN. These findings highlight that subclinical cardiac involvement in SSc is common and that the presence of HTN may have an additional clinically important effect on the vulnerable SSc myocardium. Our findings therefore underscoring the need for early screening and aggressive management of cardiovascular risk factors such as HTN in this at-risk population.
Sources of Support.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. VM: ERS Research Fellowship; AMH: National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (T32AR048522); FMW: Scleroderma Research Foundation and Martha McCoory Professorship; AAS: Staurulakis Family Discovery Fund, Donald B. and Dorothy L. Stabler Foundation; MM: Scleroderma Foundation, Staurulakis Family Discovery Fund, Donald B. and Dorothy L. Stabler Foundation, CHEST Foundation, Johns Hopkins University Clinician Scientist Award
Abbreviations.
- SSc
systemic sclerosis
- PAH
pulmonary arterial hypertension
- LV
left ventricle
- LAVi
left atrial volume index
- HTN
hypertension
- RV
left ventricle
- SSc+HTN−
SSc patients without hypertension
- SSc+HTN+
SSc patients with hypertension
- SSc−HTN−
non-SSc control patients without hypertension
- SSc−HTN+
non-SSc control patients with hypertension
- ASE
American Society of Echocardiography
- LVEF
left ventricular ejection fraction
- TAPSE
tricuspid annular systolic plane excursion
- RVSP
right ventricular systolic pressure
- ROI
region of interest
- GLS
global longitudinal strain
- IQR
interquartile range
Footnotes
Conflict of Interest.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Parks JL, Taylor MH, Parks LP, Silver RM. Systemic sclerosis and the heart. Rheum Dis Clin North Am 2014;40:87–102. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 3.Desai CS, Lee DC, Shah SJ. Systemic sclerosis and the heart: current diagnosis and management. Curr Opin Rheumatol 2011;23:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung G, Mercurio V, Hsu S, Mathai SC, Shah AA, Mukherjee M. Progress in Understanding, Diagnosing, and Managing Cardiac Complications of Systemic Sclerosis. Curr Rheumatol Rep. 2019;21:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, Medsger TA Jr, Lucas M, Michet CJ, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med 2005:118:2–10. [DOI] [PubMed] [Google Scholar]
- 6.Kahan A, Allanore Y. Primary myocardial involvement in systemic sclerosis. Rheumatology (Oxford) 2006;45 Suppl 4:iv14–7. [DOI] [PubMed] [Google Scholar]
- 7.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007;66:940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010;28(5 Suppl 62):S48–53. [PubMed] [Google Scholar]
- 9.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol 2012;30(2 Suppl 71):S30–37. [PMC free article] [PubMed] [Google Scholar]
- 11.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988. February;15:202–5. [PubMed] [Google Scholar]
- 12.Ciurzyński M, Bienias P, Lichodziejewska B, Szewczyk A, Glińska-Wielochowska M, Jankowski K, et al. Assessment of left and right ventricular diastolic function in patients with systemic sclerosis. Kardiol Pol 2008;66:269–76, discussion 277–8. [PubMed] [Google Scholar]
- 13.Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010;69:218–221. [DOI] [PubMed] [Google Scholar]
- 14.Blessberger H, Binder T. Two dimensional speckle tracking echocardiography: clinical applications. Heart 2010;96:2032–2040. [DOI] [PubMed] [Google Scholar]
- 15.Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction JACC Cardiovasc Imaging. 2018;11(2 Pt 1):260–274. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee M, Chung SE, Ton VK, Tedford RJ, Hummers LK, Wigley FM, et al. Unique Abnormalities in Right Ventricular Longitudinal Strain in Systemic Sclerosis Patients. Circ Cardiovasc Imaging 2016;9: 10.1161/CIRCIMAGING.115.003792 e003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee M, Mercurio V, Tedford RJ, Shah AA, Hsu S, Mullin CJ, et al. Right ventricular longitudinal strain is diminished in systemic sclerosis compared with idiopathic pulmonary arterial hypertension. Eur Respir J 2017;50: 1701436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercurio V, Mukherjee M, Tedford RJ, Zamanian RT, Khair RM, Sato T, et al. Improvement in Right Ventricular Strain with Ambrisentan and Tadalafil Upfront Therapy in Scleroderma Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2018;197:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercurio V, Tedford RJ, Mathai SC, Hassoun P, Hummers LK, Wigley FM, et al. Systemic Hypertension and the Scleroderma Heart: A Speckle Tracking Echocardiographic Study. J Am Soc Echocardiogr 2017:P2–133. [Google Scholar]
- 20.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 24.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351–369; quiz 453–355. [DOI] [PubMed] [Google Scholar]
- 25.Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging 2009;2:80–84. [DOI] [PubMed] [Google Scholar]
- 26.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013;26:493–8. [DOI] [PubMed] [Google Scholar]
- 27.Spethmann S, Dreger H, Schattke S, Riemekasten G, Borges AC, Baumann G, et al. Two-dimensional speckle tracking of the left ventricle in patients with systemic sclerosis for an early detection of myocardial involvement. Eur Heart J Cardiovasc Imaging 2012;13:863–870. [DOI] [PubMed] [Google Scholar]
- 28.Yiu KH, Schouffoer AA, Marsan NA, Ninaber MK, Stolk J, Vlieland TV, et al. Left ventricular dysfunction assessed by speckle-tracking strain analysis in patients with systemic sclerosis: relationship to functional capacity and ventricular arrhythmias. Arthritis Rheum 2011;63:3969–3978. [DOI] [PubMed] [Google Scholar]
- 29.Cusmà Piccione M, Zito C, Bagnato G, Oreto G, Di Bella G, Bagnato G, et al. Role of 2D strain in the early identification of left ventricular dysfunction and in the risk stratification of systemic sclerosis patients. Cardiovasc Ultrasound 2013;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadeddu C, Deidda M, Giau G, Lilliu M, Cadeddu F, Binaghi G, et al. Contractile reserve in systemic sclerosis patients as a major predictor of global cardiac impairment and exercise tolerance. Int J Cardiovasc Imaging 2015;31:529–536. [DOI] [PubMed] [Google Scholar]
- 31.Tadic M, Zlatanovic M, Cuspidi C, Stevanovic A, Celic V, Damjanov N, et al. Systemic sclerosis impacts right heart and cardiac autonomic nervous system. J Clin Ultrasound 2018;46:188–194. [DOI] [PubMed] [Google Scholar]
- 32.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 33.Bourji KI, Kelemen BW, Mathai SC, Damico RL, Kolb TM, Mercurio V, et al. Poor survival in patients with scleroderma and pulmonary hypertension due to heart failure with preserved ejection fraction. Pulm Circ 2017;7:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 35.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014:370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 36.Nelson MR, Hurst RT, Raslan SF, Cha S, Wilansky S, Lester SJ. Echocardiographic measures of myocardial deformation by speckle-tracking technologies: the need for standardization? J Am Soc Echocardiogr 2012;25:1189–1194. [DOI] [PubMed] [Google Scholar]
- 37.Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr 2015;28:1171–1181, e1172. [DOI] [PubMed] [Google Scholar]


