Abstract
Background.
Persistent post-mastectomy pain (PPMP) is a significant negative outcome occurring after breast surgery, and understanding which individual women are most at risk is essential to targeting of preventive efforts. The biopsychosocial model of pain suggests that factors from many domains may importantly modulate pain processing and predict the progression to pain persistence.
Methods.
This prospective longitudinal observational cohort study used detailed and comprehensive psychosocial and psychophysical assessment to characterize individual pain-processing phenotypes in 259 women preoperatively. Pain severity and functional impact then were longitudinally assessed using both validated surgery-specific and general pain questionnaires to survey patients who underwent lumpectomy, mastectomy, or mastectomy with reconstruction in the first postsurgical year. An agnostic, multivariable modeling strategy identified consistent predictors of several pain outcomes at 12 months.
Results.
The preoperative characteristics most consistently associated with PPMP outcomes were preexisting surgical area pain, less education, increased somatization, and baseline sleep disturbance, with axillary dissection emerging as the only consistent surgical variable to predict worse pain. Greater pain catastrophizing, negative affect, younger age, higher body mass index (BMI), and chemotherapy also were independently predictive of pain impact, but not severity. Sensory disturbance in the surgical area was predicted by a slightly different subset of factors, including higher preoperative temporal summation of pain.
Conclusions.
This comprehensive approach assessing consistent predictors of pain severity, functional impact, and sensory disturbance may inform personalized prevention of PPMP and also may allow stratification and enrichment in future preventive studies of women at higher risk of this outcome, including pharmacologic and behavioral interventions and regional anesthesia.
Breast cancer is diagnosed for more than 250,000 women in the United States annually, and most of these women require at least one surgical procedure.1 Persistent post-mastectomy pain (PPMP) is increasingly recognized as an important problem2 after mastectomy and lumpectomy.3–8 Likely due to the lack of a widely accepted definition, the reported incidence of PPMP varies from 20 to 65%. Past studies have used yes/no dichotomization, have included any level of pain severity (≥ 1/10),9–12 or have included only moderately intense pain as PPMP (≥ 3/10 or ≥ 4/10 pain).5,13–17 Although dichotomizing pain is appealingly simple, it eliminates important information about pain severity and decreases the power to test associations sensitively with risk factors and treatments.
The biopsychosocial model of pain implicates a broad array of characteristics as important modulators of pain.18,19 In the context of postmastectomy pain, the contributions of disease characteristics, treatment differences, and individual biologic, psychological, and social factors have previously been investigated.20–24 Together, these factors may meaningfully contribute to the development, maintenance, and impact of persistent pain states,20 including PPMP.22 The use of a comprehensive biopsychosocial model may capture the complexities of pain and provide insight into why pain varies between individuals. Furthermore, it may serve as a useful taxonomy for investigating acute24,25 and chronic pain after breast surgery.
Some biologic variables previously associated with PPMP are younger age,3,26–35 genetics,9,36–38 and surgical factors such as type of procedure,39,40 especially axillary dissection.3,7,10,12,16,26,27,41 Psychophysical differences in pain processing between individuals can be assessed using quantitative sensory testing (QST).42 Previously, QST measures have predicted acute24,43,44 and persistent4,11,45,46 postsurgical pain.
Psychosocial factors also have been associated with PPMP.5,22 Anxiety and depression have been most commonly examined,2,47,48 but higher pain catastrophizing, somatization, negative affect, and sleep disturbance also have been associated with greater PPMP2,5,6,8,14,21,31,39,40,42,47,49–55 and psychological resilience with less PPMP.4,5,10,12,37 Social factors, although less well studied, may include sociodemographic variables including lower education56 and social engagement.
Importantly, few studies have simultaneously and preoperatively evaluated the comprehensive range of biopsychosocial variables in the prediction of PPMP. This study aimed to do just that using well-validated, brief measures at the time of surgical/anesthetic planning and then subsequently measuring multiple meaningful general and surgery-specific pain outcomes in the first year after surgery. The study aimed examine important associations between biopsychosocial predictors and PPMP that may help to explain the variation observed between individuals, and to develop and internally validate multivariable models for predicting measures of pain severity and impact 12 months after mastectomy.
METHODS
Description of the Cohort
This prospective, observational longitudinal cohort study was approved by the institutional review board, and patients were recruited from the preoperative anesthesia clinic from September 2014 to October 2017 at a single academic medical center. The eligibility criteria specified women 18–80 years old scheduled to undergo breast surgery, English proficiency, and no cognitive impairments interfering with questionnaire completion.
Data Collection
After providing informed consent, the patients underwent brief bedside QST in nonsurgical areas (hands, extensor forearm, and trapezius). Validated questionnaires assessing psychosocial phenotypes, demographics, and pain at surgical sites and other body areas were sent to patients via an emailed link to a secure data entry system (Redcap) for them to complete before their scheduled surgery. Previous reports from this cohort on acute postsurgical pain and opioid use (up to 2 weeks after surgery)24,57 and 6-month preliminary postsurgical outcomes58 have been published.
Surgical and Treatment Variables
Clinical and pathologic factors as well as procedure details including type, laterality, duration, reconstruction type, axillary procedure, and subsequent surgeries or complications were extracted from patient medical records 1 year postoperatively. Breast surgical extent was categorized as breast-conservation surgery (partial mastectomy or excisional biopsy), mastectomy, or mastectomy with reconstruction. Axillary surgical extent was evaluated independently and categorized as 0 (no axillary surgery), 1 (sentinel lymph node biopsy [SLNB]), or 2 (axillary lymph node dissection [ALND]). The patients who underwent ALND after index surgery were recategorized in the ALND category. Similarly, the patients who underwent subsequent total mastectomy after a lumpectomy were recategorized in the total mastectomy category. The patients electronically reported their use of other breast cancer treatment or treatments including radiation, chemotherapy, or endocrine therapy 1 year after surgery.
Perioperative Care and Analgesic Use
The majority of the patients received general anesthesia, and regional anesthesia (ultrasound-guided thoracic paravertebral block, proximal intercostal block, and/or pectoralis nerve block) was offered preoperatively to most of the patients undergoing total mastectomy depending on regional anesthesia availability and surgeons’ preferences. Additional intra- and postoperative analgesics including opioids, celecoxib, ketamine, and acetaminophen were administered according to anesthesia and surgical provider preference.
Psychosocial Assessment
Psychosocial measures previously associated with persistent pain in a retrospective cohort5 and those with strong psychometric properties and brevity were selected.24 The Pain Catastrophizing Scale (PCS),59 was used to measure pain-associated catastrophic thinking. Depressive symptoms, anxiety, and sleep disturbance were assessed using the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) short form.60 The Brief Symptom Index 18-Somatization Scale61 was used to measure somatization. The Positive Affect Negative Affect Scale (PANAS)62 was used to assess affect, and preferences for coping strategies were measured using the short-form Coping Strategies Questionnaire (CSQ).54,63
Psychophysical Assessment
Psychophysical assessment of baseline general pain sensitivity involved two brief, portable QSTs. Temporal summation of pain (TSP) and painful after-sensations (PAS) of mechanical pinprick pain were assessed with standardized weighted pinprick applicators using methods described by Rolke et al.42 and in our previous studies.4,24,25 Pressure pain threshold and tolerance were assessed using a digital pressure algometer (Wagner FDX, Greenwich, CT, USA) with a flat round transducer (probe area, 0.785 cm) bilaterally on the dorsal aspect of the proximal forearm approximately 3–4 cm distal to the elbow crease (extremity site) and over the trapezius muscle at the upper back approximately 2–3 cm above the scapular spine midway between the C7 prominence and humeral head (truncal site), as in previous studies.4,24,25
Pain Assessment
Persistent pain was measured at 2 weeks, then at 3, 6, and 12 months using the extended version of a surgery-specific questionnaire, the Breast Cancer Pain Questionnaire (BCPQ) (Appendix A), first developed by Gartner et al.3 and used in subsequent studies.47,49,64–71 The BCPQ queries patients about pain severity (scores 1–10) and frequency (scores 5 [constantly], 4 [daily], 3 [occasionally], 2 [weekly], 1 [monthly], and 0 [never]) in four surgically related body areas (breast, axilla, chest wall, arm). As in our previous studies,4,5,25 a Pain Severity Index (PSI) score was calculated using the following equation:
The BCPQ includes questions about the impact of surgical pain on physical activities relevant to the body area (Physical Impact of Pain), the impact of surgical pain on cognitive and emotional functioning (Cognitive & Emotional Impact of Pain), and sensory disturbance in the surgical area, including both negative (numbness) and positive (burning) alterations in sensation (Sensory Disturbance).17
To promote generalizability to other surgical and nonsurgical pain samples, the patients also completed the widely used and well-validated Brief Pain Inventory (BPI),72 wherein the average of the current, worst, least, and average pain ratings in the preceeding week produce the BPI Severity, and other questions evaluate pain intereference (BPI Interference).
Statistical Approach
Patient demographic, psychosocial, psychophysical, and pain outcome characteristics were summarized using frequencies and percentages, mean and standard deviations, or medians with interquartile ranges. To maximize power in the analyses, all pain outcomes were measured using a continuous scale reflective of the nonbinary nature of pain.
Uni- and multivariable analyses were modeled for several different pain outcomes, described earlier (Pain Severity Index, Physical Impact of Pain, Cognitive & Emotional Impact of Pain, Sensory Disturbance, BPI Severity, and BPI Interference). Candidate predictors were identical for all outcomes except the preoperative pain measure, which was provided from the corresponding baseline questionnaire (BCPQ or BPI).
In the univariable analysis, bivariable associations between all candidate predictors and each outcome were run using simple linear regression. Multivariable prediction models for outcomes were developed using linear regression with the least absolute shrinkage and selection operator (LASSO), a penalized regression method appropriate for preventing overfitting while creating a parsimonious model.73 The study assessed LASSO model discrimination via root mean square error (RMSE), a measure of the average magnitude of the difference between observed pain severity and impact scores 12 months after mastectomy and scores predicted by the model.
Internal model validation was performed using 100 bootstrap samples with the incorporation of multiple imputation (described later) to obtain optimism-corrected estimates of the RMSE and shrinkage factors while accounting for missing data.52,74 The shrinkage factor was estimated as the average slope obtained by regressing the observed scores for the original development sample on their predicted scores using models built on each bootstrap sample. Recalibration of the models using the shrinkage factor did not improve model RMSE or calibration, so original model coefficients are presented. Further, alternative modeling approaches (e.g., negative binomial regression) did not improve model discrimination or calibration or decrease heteroscedasticity of model residuals, so linear regression was chosen as the final approach.
To account for missing data, all models were built and internally validated using datasets imputed via the method of multivariate imputation by chained equations.75 Specifically, predictive mean matching and logistic regression were used to impute continuous and categorical variables, respectively, to create 40 complete datasets per original and bootstrap sample. To allow for a single set of model predictors to be selected across all imputed datasets, MI-LASSO, a group LASSO method, was used.76 Imputation models included corresponding outcome values measured at baseline, then at 6 months and 12 months.
Other variables included in the imputation model were selected based on maximizing the correlation with the variable imputed, as well as the proportion of cases with observed values on both the predictor and imputed variable. Beta coefficients, RMSE, and calibration metrics were calculated for each of the 40 imputed datasets and combined using Rubin’s rules.
To determine sample size, we used data from our previous study,4,5 in which approximately 35% of the patients experienced persistent post-mastectomy pain longer than 1 year after mastectomy (defined in that study as pain ≥ 3/10). We calculated effect sizes for predictor variables and determined that 200 patients would provide 80% power at a two-sided alpha level of 0.05 to detect effect sizes of 0.40 or greater. Statistical analyses were performed using the SAS software version 9.4 (SAS Institute Inc, Cary, NC, USA) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Participants
The baseline biopsychosocial assessments were completed by 259 patients, and 201 patients recruited from a single academic medical center completed the BCPQ at 1 year (Fig. 1). All the subjects were women, predominantly Caucasian (86.4%) with a mean age of 55.5, and 76% reported a college degree or higher (Table 1).
FIG. 1.
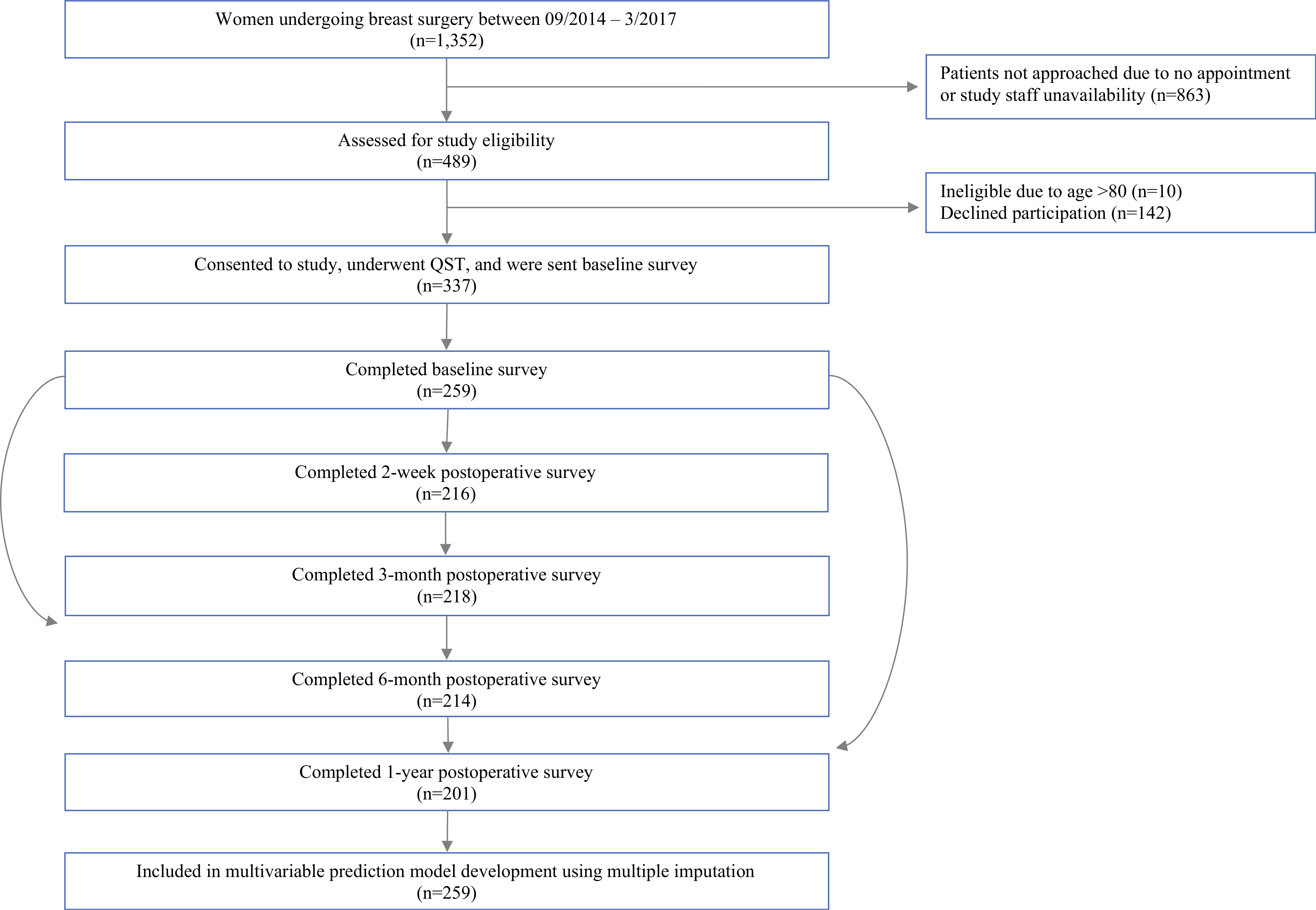
Study flow/consort diagram. Patients scheduled for breast surgery were approached at the anesthesia preoperative clinic and completed baseline and follow-up testing as indicated. The n listed is for the Pain Severity Index; n for completion of other pain outcomes. Physical Impact of Pain, Cognitive & Emotional Impact of Pain, Sensory Disturbance, Brief Pain Inventory (BPI) severity, and BPI interference ranged between 181 and 200, as indicated in Table 1. Subjects initially completing the baseline questionnaires who subsequently dropped out of the study did not differ from those not completing the 12-month questionnaires in terms of basic demographics or baseline psychosocial, psychophysical, or pain characteristics
TABLE 1.
Patient characteristics and univariate association with pain outcomes 12 months after surgery
| Variable | Baseline Values | Univariate Association with Pain Outcomes at 12 months after surgery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery Specific Pain Outcomes: Breast Cancer Pain Questionnaire (BCPQ) | General Pain Outcomes: Brief Pain Inventory (BPI) | |||||||||||||
| Pain Severity Index | Cognitive & Emotional Impact of Pain | Physical Impact of Pain | Sensory Disturbance | BPI Mean | BPI Impairment | |||||||||
| n | mean ± SD, median (Q1, Q3), n (%) | n=201 (0–200, higher is worse) | n=186 (14–56, higher is worse) | n=183 (0–38, higher is worse) | n=181 (0–8, higher is worse) | n=200 (0–10, higher is worse) | n=183 (0–100, higher is worse) | |||||||
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (99% CI) | P value | β (99% CI) | P value | |||
| Demographics | ||||||||||||||
| Age, mean ± SD | 259 | 55.5 (12.4) | −1.03 (−2.15, 0.09) | 0.071 | −0.77 (−1.17, −0.38) | < 0.001 | −0.33 (−0.58, −0.07) | 0.012 | −0.27 (−0.38, −0.17) | < 0.001 | −0.02 (−0.12, 0.07) | 0.626 | −0.31 (−1.3, 0.68) | 0.541 |
| Body Mass Index (BMI), mean ± SD | 259 | 27.4 (6.2) | 0.37 (−0.05, 0.79) | 0.085 | 0.19 (0.03, 0.35) | 0.023 | 0.1 (−0.01, 021) | 0.065 | 0.04 (−0.01, 0.08) | 0.097 | 0.06 (0.02, 0.1) | 0.005 | 0.71 (0.19, 1.23) | 0.007 |
| Education College graduate n (%) | 257 | 196 (76.3) | −6.81 (−14.41, 0.79) | 0.079 | −1.84 (−4.16, 0.48) | 0.121 | −2.96 (−4.82, −1.1) | 0.002 | −0.47 (−1.17, 0.23) | 0.187 | −1.08 (−1.69, −0.46) | 0.001 | −8.83 (−15.85, −1.8) | 0.014 |
| Race/ethnicity*, n (%) | 258 | |||||||||||||
| Caucasian | 223 (86.4) | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | |
| African American | 7 (2.7) | 29.78 (−4.43, 63.98) | 0.088 | 6.24 (0.07, 12.4) | 0.047 | 7.33 (1.55, 13.11) | 0.013 | 1.13 (−0.57, 2.82) | 0.192 | 1.56 (−0.45, 3.58) | 0.129 | 34.69 (9.16, 60.22) | 0.008 | |
| Hispanic/Latina | 5 (1.9) | −5.25 (−12.52, 2.01) | 0.156 | −1.71 (−7.84, 4.42) | 0.585 | −0.04 (−4.03, 3.94) | 0.982 | 1.18 (−1.13, 3.49) | 0.316 | −0.62 (−1.5, 0.26) | 0.169 | −4.82 (−15.6, 5.97) | 0.379 | |
| Asian | 11 (4.3) | −1.07 (−14.98, 12.83) | 0.88 | 3.5 (−1.16, 8.15) | 0.141 | 0.56 (−2.72, 3.84) | 0.736 | −0.53 (−2.09, 1.04) | 0.506 | −0.35 (−1.47, 0.77) | 0.544 | −2.64 (−14.54, 9.26) | 0.663 | |
| Mixed race | 8 (3.1) | −3.45 (−14.08, 7.19) | 0.524 | 3.26 (−2.6, 9.12) | 0.275 | 2 (−1.94, 5.94) | 0.319 | 1.06 (−0.55, 2.68) | 0.196 | −0.63 (−1.38, 0.13) | 0.104 | −5.33 (−11.17, 0.51) | 0.074 | |
| Other | 4 (1.6) | 1.47 (−20.66, 23.6) | 0.896 | −0.99 (−8.05, 6.07) | 0.782 | 2.36 (−3.92, 8.63) | 0.462 | −0.34 (−2.65, 1.98) | 0.775 | 0.85 (−1.59, 3.29) | 0.493 | 5.35 (−16.8, 27.5) | 0.635 | |
| Lifestyle | ||||||||||||||
| Alcoholic beverages per week, n (%) | 259 | |||||||||||||
| None | 97 (37.5) | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | |
| 1–4 | 105 (40.5) | −7.25 (−13.53, −0.96) | 0.024 | −0.73 (−2.79, 1.32) | 0.483 | −1.35 (−2.83, 0.13) | 0.075 | −0.61 (−1.2, −0.01) | 0.047 | −0.15 (−0.66, 0.36) | 0.554 | −1.04 (−6.45, 4.37) | 0.706 | |
| 5–10 | 48 (18.5) | −4.59 (−12.39, 3.2) | 0.248 | −1.36 (−3.82, 1.1) | 0.277 | −1.39 (−3.14, 0.36) | 0.118 | −0.68 (−1.45, 0.09) | 0.082 | −0.08 (−0.72, 0.56) | 0.808 | −1.6 (−8.34, 5.14) | 0.642 | |
| 10–20 | 9 (3.5) | −6.46 (−17.11, 4.2) | 0.235 | −2.16 (−7.12, 2.8) | 0.393 | −1.47 (−4.84, 1.89) | 0.389 | −0.73 (−2.17, 0.7) | 0.316 | 0.73 (−0.56, 2.02) | 0.266 | 8.31 (−5.54, 22.16) | 0.239 | |
| Weekly exercise amount, median (Q1, Q3); Self-reported, each instance >15 min, 1 point mild, 2 points moderate, 3 points heavy exercise | 259 | 12.0 (6.0, 19.5) | 0.1 (−0.16, 0.37) | 0.44 | −0.1 (−0.18, −0.01) | 0.031 | −0.01 (−0.07, 0.06) | 0.83 | −0.01 (−0.03, 0.02) | 0.499 | 0 (−0.02, 0.02) | 0.817 | −0.17 (−0.43, 0.09) | 0.207 |
| Surgical Variables | ||||||||||||||
| Previous breast surgery, n (%) | 259 | 66 (25.5) | 2.21 (−4.58, 8.99) | 0.524 | 0.5 (−1.56, 2.56) | 0.632 | 0.35 (−1.09, 1.79) | 0.634 | −0.3 (−0.89, 0.29) | 0.317 | 0.18 (−0.39, 0.75) | 0.531 | −0.34 (−5.9, 5.23) | 0.906 |
| Bilateral surgery, n (%) | 257 | 53 (20.6) | −1.78 (−8.18, 4.63) | 0.586 | 0.47 (−1.75, 2.69) | 0.676 | 0.14 (−1.39, 1.66) | 0.861 | 0.23 (−0.42, 0.88) | 0.492 | 0.12 (−0.42, 0.66) | 0.657 | 1.64 (−4.38, 7.67) | 0.592 |
| Surgery/reconstruction type, n (%) | 259 | |||||||||||||
| Breast conserving surgery (Lumpectomy) | 136 (52.5) | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | |
| Mastectomy | 34 (13.1) | 4.43 (−4.09, 12.94) | 0.308 | 2.06 (−0.68, 4.81) | 0.14 | 2.41 (0.27, 4.56) | 0.028 | 1.12 (0.24, 2) | 0.013 | 0.9 (0.18, 1.62) | 0.015 | 5.32 (−2.47, 13.12) | 0.181 | |
| Mastectomy with reconstruction - tissue expander | 68 (26.3) | 0.73 (−5.34, 6.8) | 0.814 | 0.48 (−1.61, 2.57) | 0.656 | 0.91 (−0.48, 2.3) | 0.198 | 0.37 (−0.25, 0.98) | 0.24 | 0.21 (−0.33, 0.75) | 0.447 | 2.17 (−3.51, 7.84) | 0.454 | |
| Mastectomy with reconstruction - autologous | 21 (8.1) | −0.72 (−10.49, 9.05) | 0.885 | 3.54 (0.19, 6.9) | 0.039 | 2.22 (−0.65, 5.09) | 0.13 | 1.17 (0.18, 2.17) | 0.021 | 0.23 (−0.62, 1.08) | 0.594 | 3.19 (−5.86, 12.24) | 0.489 | |
| Node surgery type, n (%) | 259 | |||||||||||||
| No auxillary surgery | 52 (20.1) | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | Reference | --- | |
| Sentinel lymph node procedure | 165 (63.7) | 1.28 (−4.48, 7.05) | 0.663 | 0.29 (−1.75, 2.34) | 0.78 | 0.79 (−0.59, 2.17) | 0.261 | 0.37 (−0.24, 0.99) | 0.235 | 0.29 (−0.27, 0.84) | 0.311 | 5.25 (0.46, 10.05) | 0.032 | |
| Axillary lymph node dissection | 42 (16.2) | 15.17 (5.2, 25.13) | 0.003 | 6.33 (2.96, 9.71) | < 0.001 | 4.03 (1.78, 6.29) | < 0.001 | 1.78 (0.95, 2.61) | < 0.001 | 0.7 (−0.05, 1.44) | 0.066 | 8.45 (1.04, 15.85) | 0.025 | |
| Medical Treatment within first year after surgery | ||||||||||||||
| Radiation therapy, n(%) | 256 | 146 (57.3) | 3.28 (−2, 8.55) | 0.223 | 1.57 (−0.2, 3.34) | 0.082 | 0.74 (−0.49, 1.98) | 0.236 | 0.25 (−0.27, 0.78) | 0.344 | −0.09 (−0.55, 0.37) | 0.711 | −0.22 (−5.03, 4.59) | 0.928 |
| Chemotherapy, n (%) | 256 | 92 (35.9) | 5.45 (0.1, 10.8) | 0.046 | 3.89 (2, 5.78) | < 0.001 | 2.61 (1.21, 4.01) | < 0.001 | 1.11 (0.59, 1.63) | < 0.001 | 0.38 (−0.07, 0.83) | 0.1 | 4.82 (−0.61, 10.25) | 0.082 |
| Hormone therapy, n (%) | 255 | 126 (49.6) | 4.01 (−1.16, 9.18) | 0.129 | −0.08 (−1.93, 1.77) | 0.931 | −0.21 (−1.46, 1.04) | 0.738 | 0.44 (−0.09, 0.97) | 0.102 | −0.05 (−0.49, 0.39) | 0.821 | 1.18 (−3.71, 6.06) | 0.637 |
| Baseline pain (Breast Cancer Pain questionnaire, BCPQ) | ||||||||||||||
| Pain Severity Index (PSI) (severity × frequency in 4 surgical areas, median) (Q1, Q3) | 258 | 0.0 (0.0, 4.0) | 0.95 (0.44, 1.45) | < 0.001 | 0.34 (0.21, 0.47) | < 0.001 | 0.18 (0.08, 0.28) | < 0.001 | 0.07 (0.04, 0.1) | < 0.001 | 0.62 (0.48, 0.75) | <0.001 | 5.67 (3.64, 7.7) | <0.001 |
| Highest Severity in surgical area median (Q1, Q3) | 259 | 0.0 (0.0, 2.0) | 3.77 (1.57, 5.97) | 0.001 | 1.35 (0.71, 1.99) | < 0.001 | 0.81 (0.41, 1.22) | < 0.001 | 0.35 (0.21, 0.49) | < 0.001 | ||||
| Physical Impact, median (Q1, Q3) | 226 | 0.0 (0.0, 0.0) | 3.06 (1.38, 4.74) | < 0.001 | 0.68 (0.2, 1.17) | 0.006 | 0.74 (0.41, 1.07) | < 0.001 | 0.21 (0.09, 0.32) | < 0.001 | 0.05 (0.03, 0.06) | <0.001 | 0.51 (0.33, 0.7) | <0.001 |
| Severity of chronic pain in other area(s), median (Q1, Q3) | 228 | 0.0 (0.0, 3.0) | 1.37 (0.06, 2.67) | 0.04 | 0.6 (0.15, 1.05) | 0.009 | 0.44 (0.13, 0.76) | 0.006 | 0.09 (−0.01, 0.19) | 0.087 | 0.33 (0.23, 0.43) | <0.001 | 3.39 (2.13, 4.65) | <0.001 |
| Opioid use, n (%) | 255 | 13 (5.1) | 8.22 (−5.15, 21.59) | 0.227 | 3.7 (−0.53, 7.93) | 0.087 | 3.74 (0.74, 6.73) | 0.015 | 0.1 (−1.19, 1.38) | 0.884 | 1.87 (0.74, 3) | 0.001 | 10.48 (−2.61, 23.57) | 0.116 |
| Psychosocial Variables | ||||||||||||||
| Pain Catastrophizing Scale (PCS total), median (Q1, Q3) | 246 | 4.0 (1.0, 9.0) | 0.29 (−0.2, 0.78) | 0.24 | 0.31 (0.14, 0.48) | < 0.001 | 0.12 (0, 0.24) | 0.059 | 0.05 (0.01, 0.09) | 0.008 | 0.06 (0.02, 0.09) | 0.002 | 0.76 (0.34, 1.18) | <0.001 |
| Anxiety (PROMIS SF), median (Q1, Q3) | 256 | 17.0 (13.0, 20.0) | 0.35 (−0.25, 0.94) | 0.254 | 0.45 (0.26, 0.64) | < 0.001 | 0.09 (−0.02, 0.2) | 0.126 | 0.06 (0, 0.11) | 0.034 | 0.02 (−0.02, 0.06) | 0.324 | 0.42 (−0.13, 0.98) | 0.133 |
| Depression (PROMIS SF), median (Q1, Q3) | 259 | 11.0 (9.0, 14.0) | 0.47 (−0.19, 1.13) | 0.163 | 0.61 (0.4, 0.81) | < 0.001 | 0.14 (0, 0.28) | 0.057 | 0.08 (0.02, 0.14) | 0.006 | 0.04 (−0.02, 0.09) | 0.18 | 0.52 (−0.11, 1.15) | 0.103 |
| Sleep Disturbance (PROMIS SF), median (Q1, Q3) | 250 | 21.0 (16.0, 26.0) | 0.85 (0.4, 1.31) | < 0.001 | 0.33 (0.19, 0.47) | < 0.001 | 0.18 (0.09, 0.28) | < 0.001 | 0.06 (0.03, 0.1) | 0.001 | 0.06 (0.03, 0.09) | <0.001 | 0.61 (0.24, 0.99) | 0.001 |
| Negative Affect (PANAS), median (Q1, Q3) | 246 | 17.0 (13.0, 20.0) | 0.33 (−0.12, 0.79) | 0.15 | 0.46 (0.28, 0.64) | < 0.001 | 0.13 (0.01, 0.26) | 0.035 | 0.07 (0.02, 0.11) | 0.009 | 0.04 (0, 0.07) | 0.065 | 0.61 (0.11, 1.12) | 0.018 |
| Positive Affect (PANAS), median (Q1, Q3) | 246 | 35.0 (30.0, 39.0) | −0.14 (−0.49, 0.2) | 0.409 | −0.28 (−0.4, −0.16) | < 0.001 | −0.1 (−0.19, −0.01) | 0.025 | −0.05 (−0.09, −0.02) | 0.004 | −0.02 (−0.05, 0.01) | 0.209 | −0.21 (−0.55, 0.12) | 0.207 |
| Somatization (BSI), median (Q1, Q3) | 248 | 7.0 (6.0, 8.0) | 2.84 (1.07, 4.61) | 0.002 | 1.11 (0.62, 1.6) | < 0.001 | 0.73 (0.39, 1.07) | < 0.001 | 0.23 (0.1, 0.35) | < 0.001 | 0.33 (0.23, 0.44) | <0.001 | 2.73 (1.22, 4.23) | <0.001 |
| Coping Strategies Questionnaire subscales, median (Q1, Q3) | ||||||||||||||
| Behavioral | 250 | 5.0 (3.0, 7.0) | −0.07 (−0.88, 0.75) | 0.871 | −0.09 (−0.36, 0.18) | 0.496 | 0.03 (−0.16, 0.22) | 0.776 | −0.03 (−0.11, 0.05) | 0.472 | −0.03 (−0.1, 0.04) | 0.433 | −0.31 (−1.1, 0.47) | 0.434 |
| Catastrophizing | 249 | 0.0 (0.0, 2.0) | 1.25 (−0.33, 2.83) | 0.12 | 0.43 (−0.05, 0.91) | 0.077 | 0.33 (−0.02, 0.068) | 0.063 | 0.06 (−0.08, 0.21) | 0.401 | 0.15 (0.02, 0.28) | 0.027 | 2.11 (0.57, 3.64) | 0.007 |
| Diverting | 251 | 4.0 (1.0, 6.0) | 0.17 (−0.71, 1.05) | 0.703 | −0.08 (−0.37, 022) | 0.612 | 0.02 (−0.19, 0.24) | 0.819 | −0.02 (−0.1, 0.07) | 0.734 | −0.02 (−0.1, 0.06) | 0.601 | −0.08 (−0.91,0.74) | 0.843 |
| Ignoring | 249 | 5.0 (2.0, 6.0) | 0.68 (−0.25, 1.6) | 0.151 | −0.22 (−0.52, 0.08) | 0.158 | 0.01 (−0.21, 0.23) | 0.917 | −0.02 (−0.11, 0.07) | 0.664 | 0.02 (−0.06, 0.1) | 0.63 | 0.08 (−0.79, 0.94) | 0.864 |
| Praying | 249 | 3.0 (0.0, 5.0) | 0.11 (−0.85, 1.06) | 0.826 | 0 (−0.33, 0.34) | 0.978 | 0.09 (−0.13, 0.32) | 0.427 | 0.03 (−0.07, 0.12) | 0.612 | −0.05 (−0.13, 0.03) | 0.258 | −0.16 (−1.01, 0.69) | 0.711 |
| Reinterpreting | 251 | 1.0 (0.0, 3.0) | −0.31 (−1.29, 0.66) | 0.526 | −0.09 (−0.43, 0.24) | 0.595 | 0.02 (−0.21, 0.26) | 0.838 | −0.02 (−0.12, 0.08) | 0.705 | −0.08 (−0.16, 0.01) | 0.083 | −0.17 (−1.08, 0.75) | 0.719 |
| Self Statements | 251 | 5.0 (3.0, 8.0) | 0.39 (−0.48, 1.27) | 0.38 | −0.08 (−0.36, 0.2) | 0.569 | 0 (−0.2, 0.2) | 0.975 | −0.03 (−0.11, 0.06) | 0.549 | 0 (−0.07, 0.07) | 0.992 | −0.1 (−0.89, 0.68) | 0.798 |
| Psychophysical Variables (Quantitative Sensor Testing, QST) | ||||||||||||||
| Territorial Summation of Pain, median (Q1, Q3) | 257 | 1.9 (1.0, 3.8) | 0.13 (−1.34, 1.6) | 0.861 | 0.27 (−0.2, 0.73) | 0.256 | 0.08 (−0.26, 0.41) | 0.643 | 0.13 (−0.01, 0.27) | 0.065 | 0.06 (−0.06, 0.18) | 0.343 | 0.36 (−1.01, 1.74) | 0.607 |
| Painful After-Sensations, median (Q1, Q3) | 256 | 0.0 (0.0, 0.2) | 0.81 (−6.28, 7.89) | 0.823 | −0.77 (−2.87, 1.34) | 0.475 | 0.03 (−1.5, 1.56) | 0.971 | 0.35 (−0.3, 1) | 0.288 | −0.28 (−0.83, 0.27) | 0.318 | −1.39 (−7.45, 4.67) | 0.653 |
| Pressure pain threshold-forearm, median (Q1, Q3) | 257 | 4.8 (3.7, 6.2) | −0.03 (−0.18, 0.12) | 0.714 | −0.02 (−0.07, 0.03) | 0.385 | −0.01 (−0.04, 0.03) | 0.678 | −0.01 (−0.02, 0.01) | 0266 | 0 (−0.01,0.01) | 0.768 | −0.03 (−0.17, 0.11) | 0.695 |
| Pressure pain threshold-trapezius, median (Q1, Q3) | 254 | 7.2 (5.0, 9.6) | 0.09 (−0.81, 1) | 0.837 | −0.12 (−0.4, 0.16) | 0.392 | 0.03 (−0.16, 0.22) | 0.759 | 0 (−0.08, 0.09) | 0.983 | 0.02 (−0.05, 0.09) | 0.573 | 0.04 (−0.72, 0.8) | 0.915 |
| Pressure pain tolerance-forearm median (Q1, Q3) | 257 | 7.4 (5.4, 9.4) | −0.01 (−0.98, 0.97) | 0.991 | −0.04 (−0.34, 0.25) | 0.774 | 0.04 (−0.17, 0.25) | 0.736 | 0.03 (−0.06, 0.12) | 0.539 | 0 (−0.08, 0.08) | 0.955 | 0.14 (−0.68, 0.97) | 0.738 |
| Pressure pain tolerance-trapezius, median (Q1, Q3) | 254 | 10.4 (7.3, 13.7) | −0.32 (−1.03, 0.4) | 0.383 | −0.13 (−0.37, 0.1) | 0.268 | −0.06 (−0.23, 0.1) | 0.449 | −0.02 (−0.09, 0.05) | 0.513 | 0 (−0.06, 0.06) | 0.999 | −0.03 (−0.69, 0.62) | 0.924 |
Beta coefficients, 95% confidence intervals, and p values were estimated using simple linear regression models of pain outcomes 12 months after surgery with multiple imputation to account for missing data
Darker colored cells indicate significance of univariate association at the 0.05 level, and lighter cells the 0.1 level; each outcome has an associated color which corresponds to colors in Fig. 4
PROMIS SF Patient-Reported Outcomes Measurement Information System Short Form, PANAS Positive And Negative Affect Schedule, BSI Brief Symptom Inventory
Considered in univariate but not included in LASSO
Surgical, Medical, and Anesthetic Treatment
The surgical indications were invasive cancer (77%), ductal carcinoma in situ (15%), prophylactic mastectomy (4.5%), and benign lesions (3.5%). The patients underwent a range of surgical procedures including breast-conserving surgery (54%) and mastectomy (46%), reconstruction involving tissue expander placement/implant (28%) or autologous reconstruction (8% deep inferior epigastric artery perforator [DIEP] or transverse rectus abdominis [TRAM] flap), sentinel lymph node biopsy (63%), and ALND (16%) (Table 1). Subsequent surgery after the index surgery was performed for 48 patients (18.5%), with 25 (52%) of these surgeries occurring within the first 3 months and 43 (90%) occurring within 6 months after the index surgery. Additional medical treatment of breast cancer included radiation (57.3%), chemotherapy (35.9%), and endocrine therapy (49.6%). The majority (96%) of the patients received general anesthesia for surgery. Of the patients who had total mastectomy, 44% also received regional anesthesia.
Pain Locations Over Time
Figure 2 depicts the number of surgically related body areas with pain reported at each time point (Fig. 2a). Similar to previous studies,3,41 pain in the breast, axilla, or both was most commonly reported (Fig. 2b). Mild preoperative breast pain was common (38%), but surgery-related locations had notably higher pain prevalence and severity after surgery.
FIG. 2.
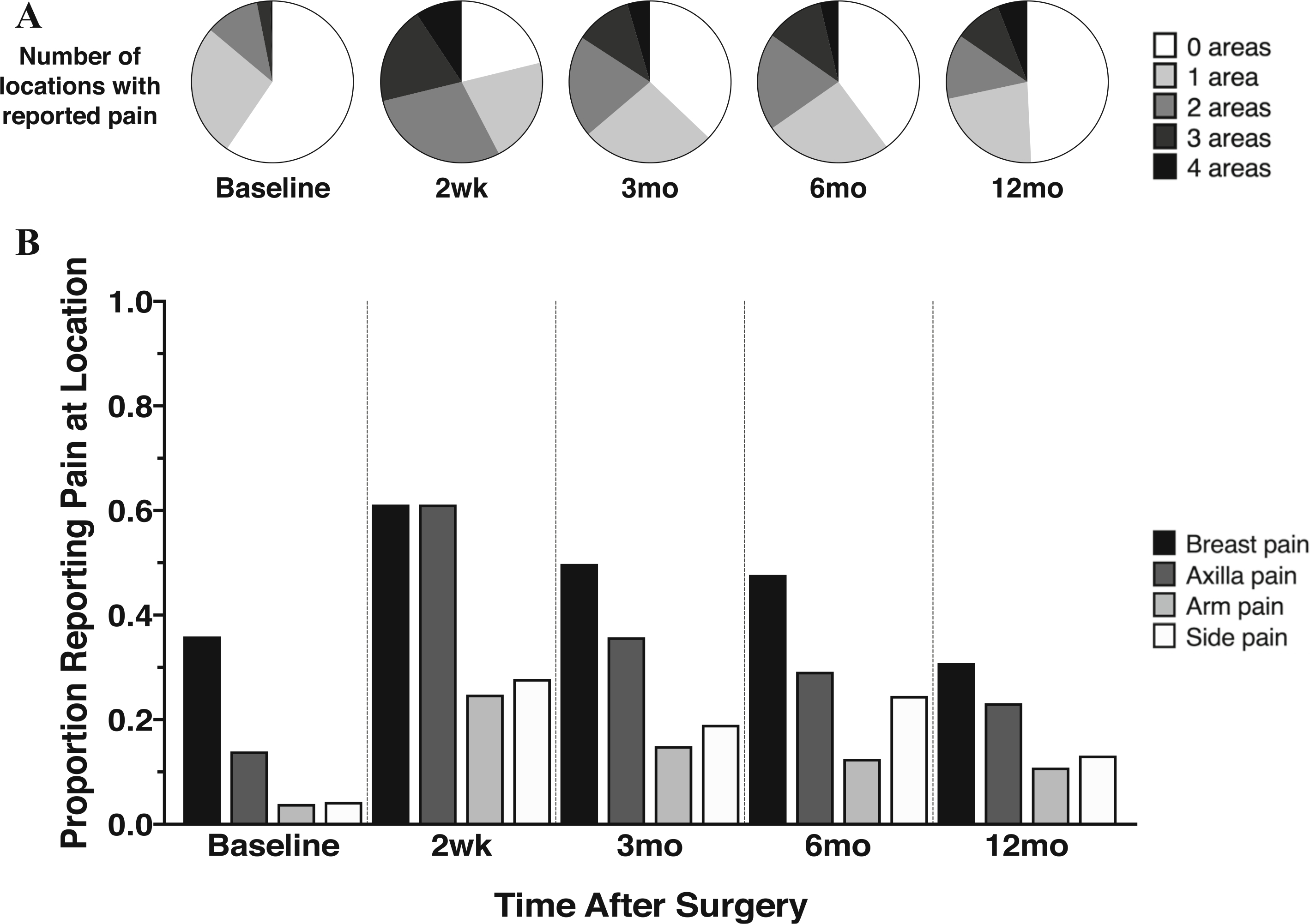
Longitudinal location and frequency of pain after breast surgery. Patients completed the Breast Cancer Pain Questionnaire at baseline and several times postoperatively. a Patient indication of pain in any of the four surgically related areas assessed. Pie charts show the number of surgically related body locations with some pain at each time point. b Proportion of patients reporting pain in each of the four specified surgically related areas across time
Incidence of PPMP and Pain Severity Index
To compare longitudinal prevalence of PPMP with rates reported in previous studies, we dichotomized PPMP using various cutoffs (≥ 1/10, ≥ 3/10, ≥ 5/10) to define PPMP (Fig. 3a). Approximately one third of the patients reported a pain level of 3/10 or higher in at least one body area, a proportion that remained constant at 3, 6, and 12 months.
FIG. 3.
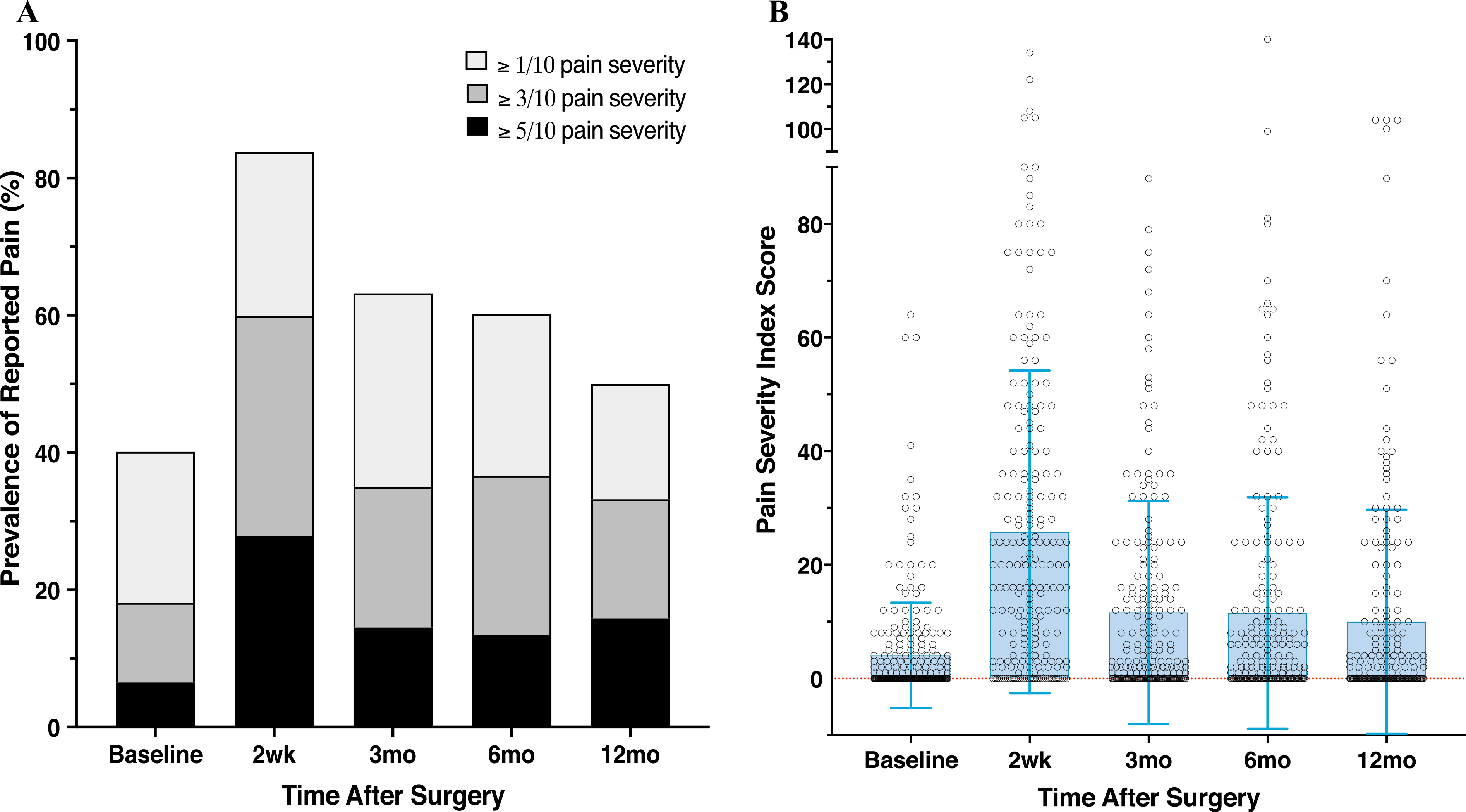
Pain Prevalence and Pain Severity Index. Patients completed the Breast Cancer Pain Questionnaire and indicated the severity and frequency of pain in surgically related body areas. a Patient rating of pain severity on a scale of 0–10. Nested bar graphs depict the prevalence of subjects reporting pain with various cutoffs (≥ 1, ≥ 3, ≥ 5/10) defining a clinically meaningful severity of pain. The highest rates and largest number of areas were reported at 2 weeks, and prevalence remained relatively consistent for 3 months and longer. b Distribution of subjects’ scores on the Pain Severity Index (PSI), which estimates the extent of surgical pain using severity, frequency, and area according to the following formula: PSI = Σ pain score at each site (0–10) × frequency (0–5). The frequency was scored as follows: 5 (constantly), 4 (daily), 3 (occasionally), 2 (weekly), 1 (monthly), and 0 (never), with a possible range of 0–200
To capture pain severity more thoroughly, we examined patients’ Pain Severity Index (PSI) scores, which encompass pain severity, frequency, and number of body areas affected.4,5,25 The magnitude and time course of PSI varied between patients, with the highest values observed 2 weeks after surgery and stable values after 3 months (Fig. 3b).
Assessment of Pain Impact and Sensory Disturbance
The physical, cognitive, and emotional impact of pain was highest 2 weeks after surgery, with lower, relatively stable mean impact scores across the 3-, 6-, and 12-month time points (Appendix 2, Fig. 6). Notably, the impact on cognitive and emotional functioning appeared more sustained beyond 2 weeks than the impact on physical functioning. As with PSI, a large amount of inter-individual variability in pain impact scores was observed, with a proportion of patients still reporting a substantial impact of pain at later time points (Appendix 2, Fig. 6b). Sensory disturbance remained relatively consistent across time (Appendix 2, Fig. 6c). Sensory disturbance scores were moderately correlated with pain severity and impact outcomes (Spearman’s rho, 0.41–0.68; p < 0.001). The BPI severity and interference scores were moderately to highly correlated with, but not identical to, the BCPQ Pain severity and impact scores (Appendix 3).
Association of Variables with Persistent Pain at 12 Months
Our prediction analysis focused on 12 months to avoid potentially confounding effects of radiation treatment (56% of the patients), subsequent surgical interventions (18.5% of the patients), or both that could lead to acute pain exacerbation, potentially confounding PPMP assessment at 3 and possibly 6 months.
Simple Univariable Associations of Preoperative Factors with Pain Outcomes at 12 Months
Factors were assessed for association of several PPMP outcomes at 12 months, including breast surgery-specific (BCPQ) and general (BPI) pain severity as well as impact measures (Table 1). The overlap of these associations is illustrated in a matrixed Venn diagram (Fig. 4). The factors associated with many outcomes (at the intersection of circles) included preexisting pain in surgical areas or elsewhere, ALND, chemotherapy, higher BMI, lower education, and higher sleep disturbance, somatization, pain catastrophizing, and negative affect. Several factors were associated with only some PPMP outcomes, including less exercise, radiation, higher depression and anxiety, younger age, greater weekly alcohol use, and baseline opioid use. The only QST associated with PPMP outcomes was temporal summation of pain, but only for sensory disturbance/neuropathic type pain in the surgical area.
FIG. 4.
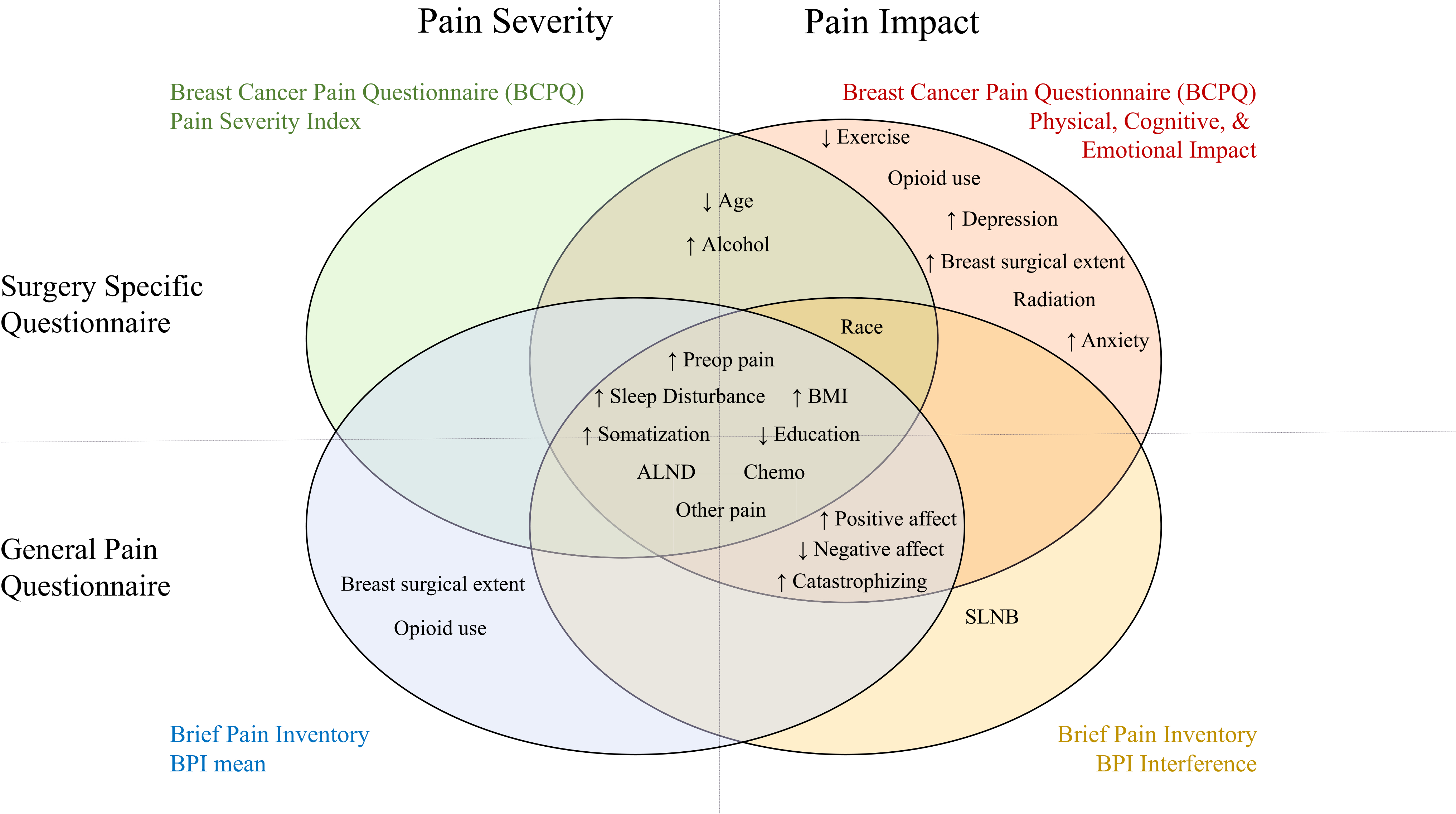
Univariate association of preoperative factors with pain outcomes. Factors significantly associated with at least one of the four main pain outcome types are shown, with factors associated with multiple outcomes falling within the overlapping areas. The top circles show the breast surgery-specific questionnaire (Breast Cancer Pain Questionnaire [BCPQ]) outcomes, and the bottom circles show the general pain questionnaire (BPI) outcomes. The circles on the left show the pain severity outcomes, and the circles on the right show the pain impact measures. ↑, higher value associated with higher pain; ↓, lower value associated with higher pain; ALND, axillary lymph node dissection; BMI, body mass index; SLNB, sentinel lymph node biopsy
Multivariable Prediction of Persistent Pain Outcomes at 12 Months
Given that many of the predictor variables were highly correlated, we next assessed them within a combined prediction model (multivariable prediction analysis) using LASSO (Table 2). The variables that independently and consistently contributed to prediction of all outcomes were preoperative pain, education, and sleep disturbance (Fig. 5). Other relatively consistent predictors were greater somatization, preoperative pain in other body areas, and baseline opioid use. Greater pain catastrophizing and negative affect, younger age, higher BMI, and chemotherapy were predictive of only pain impact, whereas ALND was predictive of both BCPQ pain severity and impact. Greater breast surgical extent was not predictive of pain severity or impact.
TABLE 2.
Multivariate prediction of pain outcomes using LASSO
| Variable | Multivariate Association with Pain Outcomes at 1 year | |||||
|---|---|---|---|---|---|---|
| Surgery Specific Pain Outcomes: Breast Cancer Pain Questionnaire (BCPQ) | General Pain Outcomes: Brief Pain Inventory (BPI) | |||||
| PSI (Pain Severity Index) | Cognitive Emotional Impact | Physical Impact | Sensory Disturbance | BPI Mean | BPI Impairment | |
| (0–200, higher is worse) | (14–56, higher is worse) | (0–38, higher is worse) | 0–8 (higher is worse) | (0–10, higher is worse) | (0–100, higher is worse) | |
| β | β | β | β | β | β | |
| Intercept | −1.0678 | 11.6409 | 0.8335 | 3.3270 | 0.3037 | 0.1875 |
| Age (per 5 years) | −0.0696 | −0.1202 | ||||
| BMI | 0.0553 | 0.1233 | ||||
| College graduate | −0.5023 | −1.4263 | −0.4801 | −1.2681 | ||
| Alcohol (per increase in category) | −0.0045 | |||||
| Total exercise | ||||||
| Bilateral surgery | ||||||
| SLNB vs. all other categories | ||||||
| ALND vs. all other categories | 5.0196 | 2.5070 | 1.0675 | 0.4625 | ||
| Mastectomy vs. all other categories | ||||||
| Mastectomy with reconstruction - tissue expanders vs. all other categories | ||||||
| Mastectomy with reconstruction - autologous vs. all other categories | ||||||
| Previous breast surgery | ||||||
| Radiation therapy | ||||||
| Chemotherapy | 1.3924 | 1.2587 | 0.3464 | 0.2378 | ||
| Hormone therapy | ||||||
| Opioid use | 1.2349 | 0.4485 | ||||
| Severity of other chronic pain | 0.2369 | 0.0859 | 0.1090 | 1.2203 | ||
| BSI - Total | 0.1220 | 0.0616 | 0.0715 | 0.0004 | 0.0953 | |
| Coping - Behavioral | ||||||
| Coping - Catastrophizing | 0.1096 | |||||
| Coping - Diverting | ||||||
| Coping - Ignoring | ||||||
| Coping - Praying | ||||||
| Coping - Reinterpreting | ||||||
| Coping - Self Statements | ||||||
| PANAS - Negative | 0.0738 | 0.0178 | ||||
| PANAS - Positive | −0.0331 | −0.0086 | ||||
| PCS - Total | 0.0334 | 0.0830 | ||||
| PROMIS - Anxiety | ||||||
| PROMIS - Depression | 0.2627 | |||||
| PROMIS - Sleep Disturbance | 0.3072 | 0.0510 | 0.0494 | 0.0057 | 0.0111 | 0.0944 |
| QST - Painful After-Sensations | ||||||
| QST - Temporal Summation of Pain | 0.0682 | |||||
| QST - Pressure pain tolerance - forearm | ||||||
| QST - Pressure pain threshold - forearm | ||||||
| QST - Pressure pain tolerance - trapezius | ||||||
| QST - Pressure pain threshold - trapezius | ||||||
| Preop Pain numerical severity in surgical area (BCPQ - Severity or BPI mean) | 0.0960 | 0.0934 | 0.2718 | 1.1109 | ||
| Preop severity index in surgical area (BCPQ - PSI) | 0.5094 | 0.2052 | 0.0574 | 0.0122 | ||
| Pain functional impact (BCPQ - Physical Impact or BPI - Impairment) | 1.2665 | 0.2870 | 0.0796 | 0.0033 | 0.2340 | |
Beta coefficients for models to predict pain outcomes one year after surgery were estimated as averages over 40 imputed datasets using MI-LASSO, a group LASSO method that attempts to avoid model overfitting while accounting for missing data. Where no coefficient is shown, the LASSO shrank the coefficient to zero because the corresponding variable did not contribute substantively to the model.
LASSO= Least Absolute Shrinkage and Selection Operator; BMI=Body Mass Index; SLND= Sentinel Lymph Node Procedure; ALND=Axillary Lymph Node Dissection; BSI=Brief Symptom Inventory (somatization); PCS=Pain Catastrophizing Scale; QST=Quantitative Sensory Testing; BCPQ=Breast Cancer Pain Questionnaire; BPI=Brief Pain Inventory; PSI=Pain Severity Index
FIG. 5.
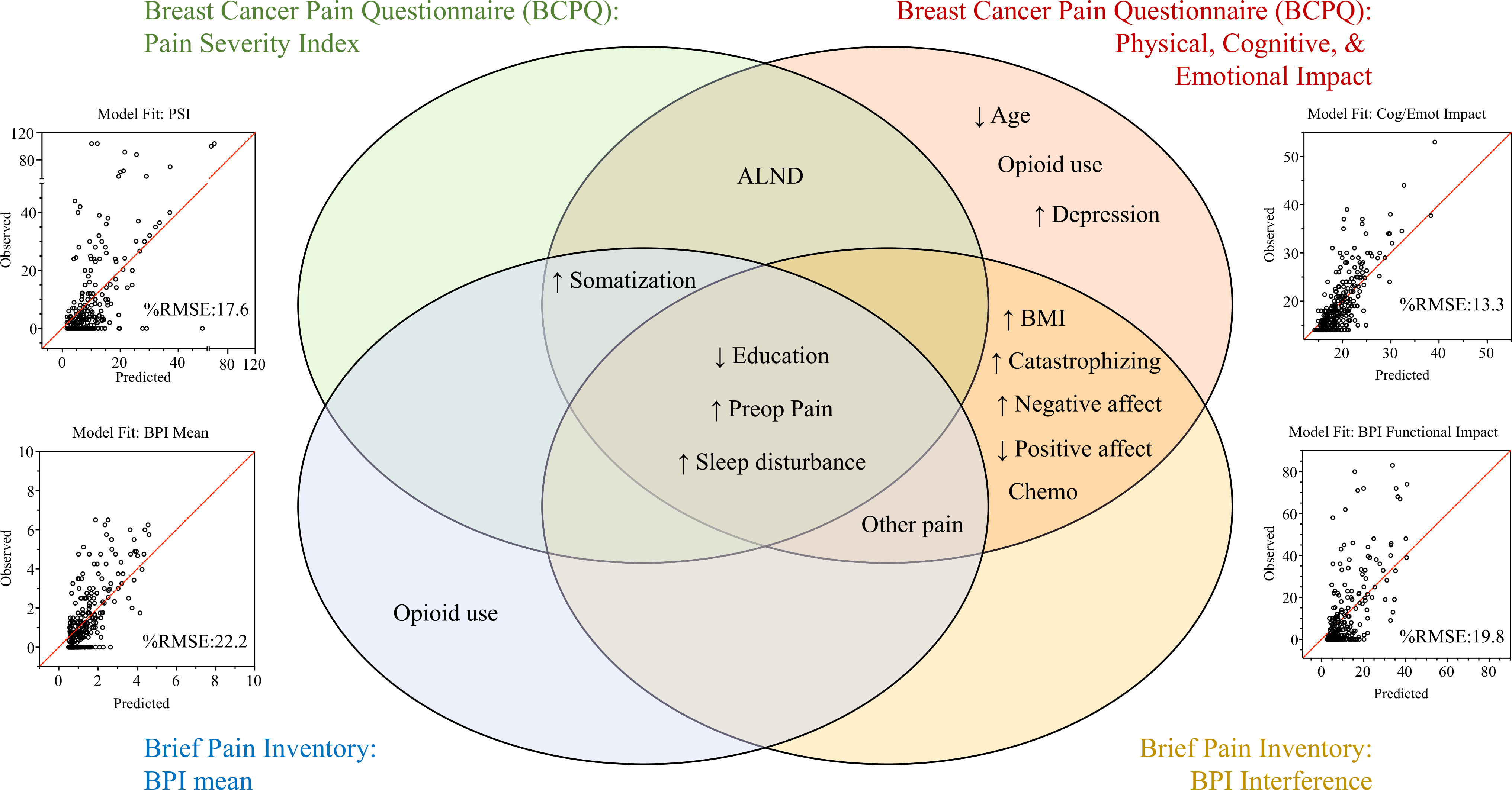
Predictors of persistent post-mastectomy pain (PPMP) at 12 months selected by least absolute shrinkage and selection operator (LASSO). Significant independent predictors of pain outcomes retained after multivariable regression with LASSO are shown. Surgery-specific questionnaire outcomes (top), general pain questionnaire outcomes (bottom), severity-related outcomes (left), and impact-related outcomes (right) are depicted, with factors most consistently associated across outcomes found in the intersection of circles. The diagonal line indicates perfect prediction. The average difference between the predicted and observed values for the subjects (%RMSE) was calculated, with lower values indicating better prediction. The model fit comparing predicted and observed values (scatterplots) is shown for each outcome, with the average percentage difference between predicted and observed values reported (%RMSE = RMSE/observed range of scores *100). The %RMSE was calculated by dividing the RMSE by the actual score range observed for the outcome. ↑, higher value associated with higher pain; ↓, lower value associated with higher pain; ALND, axillary lymph node dissection; BMI, body mass index; %RMSE, %Root mean squared error
Internal Validation of Models
Multivariable prediction models were internally validated via bootstrapping, and the accuracy of prediction (observed vs predicted values for each patient) was shown in scatterplots (Fig. 5). The most accurate prediction was observed for the BCPQ Cognitive and Emotional Impact of Pain (13% average difference between predicted and observed scores) compared with the less accurate predictions of pain severity (17% for PSI and 22% for BPI; Fig. 5) and sensory disturbance (22%; Appendix 4).
Analgesic Use
Opioid use 12 months after surgery was extremely uncommon. Only 8 (4%) of 201 patients reported taking any opioids, with only 5 (2.5%) of the 201 patients taking opioids for pain in the surgical area, thus precluding meaningful analysis of predictors of this outcome.
DISCUSSION
Previous studies have rarely evaluated all known pain modulators (demographic, biophysical, psychosocial) simulataneously and prospectively in a rigorous longitudinal assessment of diverse pain outcomes (severity, physical, cognitive and emotional functional impact, and sensory disturbance). This prospective longitudinal study examined associations between a comprehensive set of preoperative predictors and PPMP 12 months after surgery.
Using robust and agnostic modeling approaches, we developed reduced predictive models. The consistent independent predictors were preoperative pain, younger age, ALND, lower education, BMI, sleep disturbance, and the psychosocial variables somatization, catastrophizing, and depression. Notably, breast surgical extent (e.g., mastectomy vs breast-conserving surgery) or presence of reconstruction were absent from this list of predictors. Many predictors associated with greater pain severity and impact were consistent with those of previous studies.8,22,48
Our multivariable analysis yielded several notable findings. First, preoperative pain in the breast was found to be one of the strongest, most frequently selected predictors of PPMP. Preoperative surgery-specific site pain has rarely been reported in previous studies because it is seldom prospectively assessed with a rigorous, surgery-specific questionnaire. We observed a relatively high prevalence of at least mild pain among the participants at baseline (40%, Fig. 3a). Given that most of the participants in the study had a breast biopsy as part of their diagnostic workup in the month before surgery, it is conceivable that the high prevalence of breast pain may have resulted from the biopsy. However, future studies are needed to further explore the prevalence and duration of preoperative breast pain. Importantly, the severity, frequency, and number of pain locations increased substantially after surgery (Fig. 3b). Most, if not all, of the previous studies found that acute postoperative pain is a predictor of subsequent pain. Although inclusion of acute postoperative pain in prediction models may increase the accuracy of prediction, this pain score is not accesible preoperatively, making it a less useful predictor for surgical and anesthetic planning.
Second, the only surgical variable consistently associated with PPMP was ALND, congruent with previous findings,3 including our own studies, that did not detect a greater incidence of PPMP with mastectomy than with breast-conserving surgery, and a recent metaanalysis concluding that breast reconstruction was not associated with greater pain persistence.77 Axillary dissection has consistently been associated with persistent pain3,7,10,12,16,26,27,37,41 and sensory dysfunction,78 particularly in the distribution of the intercostobrachial nerve (ICBN).
Third, and somewhat surprisingly, baseline sleep disturbance was a consistent independent predictor of both pain severity and pain impact. Previous evaluations identified preoperative fatigue and sleep as important predictors of PPMP,55 which together with our findings bolsters the utility of sleep disturbance as a predictor and target of future study. The relationship between sleep and pain likely is bidirectional, with pain itself also disturbing sleep.50,79,80 Evidence for this self-reinforcing maladaptive spiral has been noted among patients with cancer,53,81 and both pharmacologic and behavioral interventions to improve sleep have been associated with chronic pain improvement.82
Fourth, psychosocial variables, including catastrophizing, anxiety, and depression, were more consistently predictive of pain impact than pain severity. Although this association does not constitute a causal link, some evidence is emerging that behavioral interventions directed at these factors in the peri- and postoperative period may improve pain.83–86 Morevover, although decisions about surgical procedure may not be negotiable, modification of the psychological health of patients with breast cancer through behavioral interventions and acquisition of coping strategies pre-surgically has essentially no downside. Similarly, social factors, including peer support and group interventions, have shown efficacy in lessening pain impact.87,88
Fifth, despite our previous findings that QST-assessed temporal summation of pain (TSP) predicts acute pain after mastectomy24 and total knee arthroplasty,43 TSP did not emerge as a significant associate or predictor of pain severity or pain impact in the current study. Our previous cross-sectional study observed an association of several QSTs (lower pressure pain threshold and higher TSP) with PPMP,4,11 similar to findings of a large cohort with orofacial pain.89 Mechanical pain sensitivity may increase for patients who experience persistent pain, such that cross-sectional studies observe these associations, whereas preoperative testing is less predictive.
Although some previous studies have examined pain impact, usually as a secondary outcome, it has rarely been used as a criterion to define which predictors are most important. Assessing the functional impact of pain has been recognized in consensus guidelines as crucial to the study of acute4 and chronic90 pain. Differential prediction of pain severity and impact may be an important consideration because most patients and clinicians are interested in whether post-surgical pain has a meaningful impact on patient quality of life, including physical and mental functioning, relationships, and employment. Interestingly, the independent predictors of the impact of pain included more psychosocial variables, preoperative opioid consumption, and younger age.
Some important limitations of this study should be noted. First, the patients who declined participation in ths study often cited feeling overwhelmed, perhaps leading to an underestimation of anxiety and catastrophizing, reflected by the lower scores on these measures than in previous cohorts. Second, the low number of non-white participants precluded a meaningful estimation of race as a risk factor. Third, the low rate of opioid use at 12 months, although generally encouraging, limited our ability to discern risk factors for this outcome. Previous work has demonstrated that higher anxiety and depression may be risk factors for opioid use,51 and our previous analysis showed sleep disturbace and TSP to be important predictors for opioid use at 2 weeks.24
The discernment of interindividual differences that predict risk of outcomes such as chronic pain is critically important to the development of personalized medicine. Even if perfect prediction is not possible, discernment of somewhat higher potential risk for persistent pain may help patients weigh the risks and benefits of surgery if other management options are available.
This study showed that the most consistent biopsychosocial predictors of PPMP are preoperative pain, lower education, sleep disturbance, and somatization, with axillary lymph node dissection and chemotherapy also playing an important role. Other important and potentially modifiable factors may include preoperative opioid use, sleep disturbance, and psychosocial state (catastrophizing, affect and depressive symptoms), many of which appear to predict the impact of pain better than severity. Recognition of these factors may help to identify patients most likely to benefit from preventive interventions that appear promising, including pharmacologic and behavioral interventions and regional anesthesia, and to inform definitive testing and efficient targetting of preventive therapies in future trials.
ACKNOWLEDGEMENTS
This study was supported by a grant from the NIH/NIGMS (K23 GM110540). We thank the patients who took the time to participate in this study, as well as the surgeons for their insight and feedback.
APPENDIX 1 BREAST CANCER PAIN QUESTIONNAIRE




APPENDIX 2
See Fig. 6.
FIG. 6.
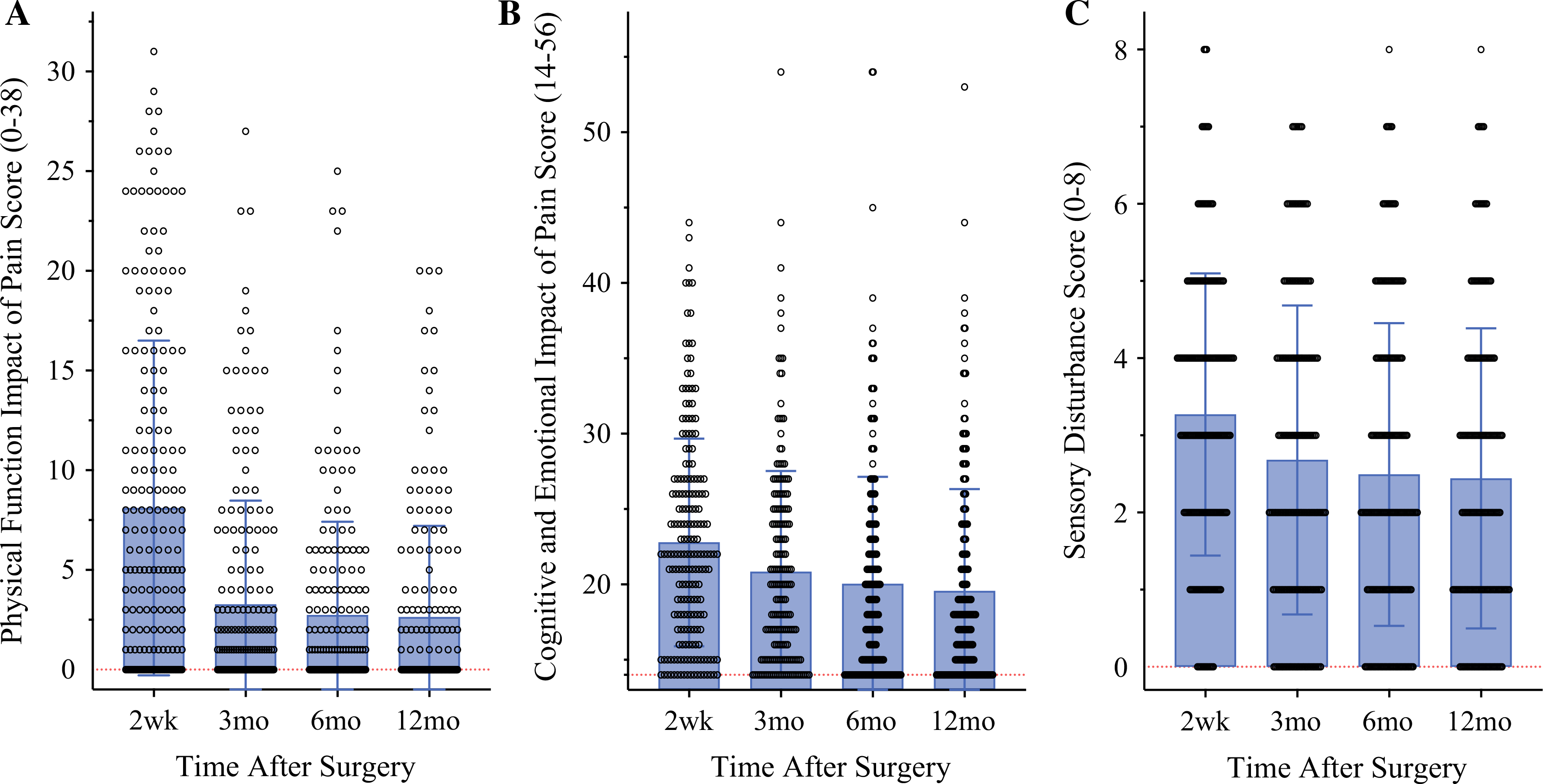
Longitudinal functional pain outcomes: functional impact of pain and sensory disturbance in the first year after breast surgery. a Extent of surgical area pain impact on patients’ daily physical functioning. b Extent of surgical area pain impact on patients’ cognitive and emotional functioning. c Extent of neuropathic-type sensory disturbance in surgical areas
APPENDIX 3
See Table 3.
TABLE 3.
Correlation between scores from the Breast Surgery Specific Questionnaire (BCPQ) and General Pain Questionnaire (BPI)
| General Pain Questionnaire | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks | 6 months | 1 year | |||||||
| BPI Mean | BPI Interference | BPI Mean | BPI Interference | BPI Mean | BPI Interference | BPI Mean | BPI Interference | |||
| Breast Surgery Specific Questionnaire | Baseline | Pain Severity Index | 0.335* | 0.331* | 0.402* | 0.341* | 0.357* | 0.243* | 0.273* | 0.215* |
| Cog/Emot Impact | - | - | - | - | - | - | - | - | ||
| Physical Impact | 0.451* | 0.419* | 0.276* | 0.206* | 0.355* | 0.325* | 0.407* | 0.388* | ||
| 2 weeks | Pain Severity Index | 0.189* | 0.247* | 0.710* | 0.623* | 0.383* | 0.405* | 0.365* | 0.349* | |
| Cog/Emot Impact | 0.145 | 0.233* | 0.533* | 0.745* | 0.299* | 0.447* | 0.315* | 0.407* | ||
| Physical Impact | 0.082 | 0.161 | 0.599* | 0.740* | 0.328* | 0.435* | 0.262* | 0.333* | ||
| 6 months | Pain Severity Index | 0.270* | 0.277* | 0.491* | 0.406* | 0.639* | 0.567* | 0.362* | 0.300* | |
| Cog/Emot Impact | 0.154 | 0.227* | 0.470* | 0.586* | 0.491* | 0.659* | 0.326* | 0.380* | ||
| Physical Impact | 0.302* | 0.333* | 0.466* | 0.511* | 0.614* | 0.727* | 0.394* | 0.469* | ||
| 1 year | Pain Severity Index | 0.232* | 0.273* | 0.427* | 0.355* | 0.518* | 0.425* | 0.602* | 0.408* | |
| Cog/Emot Impact | 0.267* | 0.352* | 0.449* | 0.528* | 0.435* | 0.582* | 0.528* | 0.606* | ||
| Physical Impact | 0.248* | 0.307* | 0.376* | 0.397* | 0.495* | 0.561* | 0.568* | 0.630* | ||
Scores from the breast surgery specific questionnaire (BCPQ) and the general pain questionnaire (BPI) were significantly correlated at each time point they were assessed. Cognitive/Emotional impact was not assessed at baseline.
Spearman correlation is significant at the 0.01 level (2-tailed)
BCPQ=Breast Cancer Pain Questionnaire; BPI=Brief Pain Inventory; Cog/Emot Impact= Cognitive & Emotional impact
APPENDIX 4
See Table 4.
TABLE 4.
Performance of multivariable linear regression models for predicting pain severity and impact outcomes 1 year after mastectomy
| Surgery specific pain outcomes: Breast Cancer Pain Questionnaire (BCPQ) |
General pain outcomes: Brief Pain Inventory (BPI) |
|||||
|---|---|---|---|---|---|---|
| Pain Severity Index | Cognitive emotional impact | Physical impact | Sensory disturbance | BPI mean | BPI impairment | |
| (0–200, higher is worse) | (14–56, higher is worse) | (0–38, higher is worse) | 0–8 (higher is worse) | (0–10, higher is worse) | (0–100, higher is worse) | |
|
| ||||||
| RMSE | ||||||
| Apparent | 16.69 | 4.85 | 3.75 | 1.65 | 1.30 | 14.87 |
| Optimism-corrected | 18.35 | 5.20 | 4.01 | 1.75 | 1.44 | 16.42 |
| %RMSE (error as % of reported scores range) | 17.64 | 13.34 | 20.06 | 21.91 | 22.16 | 19.78 |
| Calibration | ||||||
| Intercept | −0.47 | −4.08 | −0.48 | −0.57 | −0.16 | −0.71 |
| Slope | 1.04 | 1.21 | 1.14 | 1.22 | 1.11 | 1.05 |
RMSE is a measure of the average magnitude of the difference between observed vs. predicted scores. Apparent RMSE reflects predictive performance on the model development sample, while optimism-corrected RMSE (estimated via bootstrapping) is adjusted to better estimate performance on future samples. The shrinkage factor, a measure of model calibration, was estimated as the average slope of the regression line between the observed scores for the original development sample vs. their predicted scores using models built on each bootstrap sample
RMSE root mean square error
Footnotes
DISCLOSURE Andrea Pusic—co-developer of the Q-PROM portfolio and may receive royalties when these patient-reported outcome measures are used in for-profit, industry-sponsored clinical trials. There were no conflicts of interests.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2012;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- 3.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–92. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber KL, Martel MO, Shnol H, et al. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. 2013;154:660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain. 2013;14:1185–95. [DOI] [PubMed] [Google Scholar]
- 6.Meretoja TJ, Leidenius MHK, Tasmuth T, Sipila R, Kalso E. Pain at 12 months after surgery for breast cancer. JAMA. 2014;311:90–2. [DOI] [PubMed] [Google Scholar]
- 7.Meretoja TJ, Andersen KG, Bruce J, et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 2017;35:1660–7. [DOI] [PubMed] [Google Scholar]
- 8.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–46. [DOI] [PubMed] [Google Scholar]
- 9.Bortsov AV, Devor M, Kaunisto MA, et al. CACNG2 polymorphisms associate with chronic pain after mastectomy. Pain. 2019;160:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155:232–43. [DOI] [PubMed] [Google Scholar]
- 11.Edwards RR, Mensing G, Cahalan C, et al. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manag. 2013;46:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schou Bredal I, Smeby NA, Ottesen S, Warncke T, Schlichting E. Chronic pain in breast cancer survivors: comparison of psychosocial, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852–62. [DOI] [PubMed] [Google Scholar]
- 13.Baudic S, Jayr C, Albi-Feldzer A, et al. Effect of alexithymia and emotional repression on postsurgical pain in women with breast cancer: a prospective longitudinal 12-month study. J Pain. 2016;17:90–100. [DOI] [PubMed] [Google Scholar]
- 14.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- 15.Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113:1–4. [DOI] [PubMed] [Google Scholar]
- 16.Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156:2413–22. [DOI] [PubMed] [Google Scholar]
- 17.Mejdahl MK, Christoffersens KB, Andersen KG. Development and validation of a screening tool for surgery-specific neuropathic pain: neuropathic pain scale for postsurgical patients. Pain Physician. 2019;22:E81–90. [PubMed] [Google Scholar]
- 18.Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158(Suppl 1):S11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17(9 Suppl):T70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masselin-Dubois A, Attal N, Fletcher D, et al. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain. 2013;14:854–64. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber KL, Kehlet H, Belfer I, Edwards RR. Predicting, preventing, and managing persistent pain after breast cancer surgery: the importance of psychosocial factors. Pain Manag. 2014;4:445–59. [DOI] [PubMed] [Google Scholar]
- 23.Tait RC, Zoberi K, Ferguson M, et al. Persistent Post-mastectomy pain: risk factors and current approaches to treatment. J Pain. 2018;19:1367–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreiber KL, Zinboonyahgoon N, Xu X, et al. Preoperative psychosocial and psychophysical phenotypes as predictors of acute pain outcomes after breast surgery. J Pain. 2019;20:540–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber KL, Belfer I, Miaskowski C, Schumacher M, Stacey BR, Van De Ven T. AAAPT diagnostic criteria for acute pain following breast surgery. J Pain. 2019. 21(3–4):294–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alves Nogueira Fabro E, Bergmann A, do Amaral ESB, et al. Post-mastectomy pain syndrome: incidence and risks. Breast. 2012;21:321–5. [DOI] [PubMed] [Google Scholar]
- 27.Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80:39–48. [DOI] [PubMed] [Google Scholar]
- 28.Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol. 1999;17:143–9. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer. 2005;92:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peuckmann V, Ekholm O, Rasmussen NK, et al. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13:478–85. [DOI] [PubMed] [Google Scholar]
- 31.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith WC, Bourne D, Squair J, Phillips DO, Chambers WA. A retrospective cohort study of post mastectomy pain syndrome. Pain. 1999;83:91–5. [DOI] [PubMed] [Google Scholar]
- 33.Steegers MA, Wolters B, Evers AW, Strobbe L, Wilder-Smith OH. Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain. 2008;9:813–22. [DOI] [PubMed] [Google Scholar]
- 34.Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9:745–53. [DOI] [PubMed] [Google Scholar]
- 35.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer. 2008;99:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nissenbaum J, Devor M, Seltzer Z, et al. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Res. 2010;20:1180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustonen L, Aho T, Harno H, Sipila R, Meretoja T, Kalso E. What makes surgical nerve injury painful? A 4-year to 9-year follow-up of patients with intercostobrachial nerve resection in women treated for breast cancer. Pain. 2019;160:246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Reij RR, Joosten EA, van den Hoogen NJ. Dopaminergic neurotransmission and genetic variation in chronification of postsurgical pain. Br J Anaesth. 2019; 123(6), 853–64. [DOI] [PubMed] [Google Scholar]
- 39.Pereira S, Fontes F, Sonin T, et al. Neuropathic pain after breast cancer treatment: characterization and risk factors. J Pain Symptom Manag. 2017;54:877–88. [DOI] [PubMed] [Google Scholar]
- 40.Kulkarni AR, Pusic AL, Hamill JB, et al. Factors associated with acute postoperative pain following breast reconstruction. JPRAS Open. 2017;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juhl AA, Christiansen P, Damsgaard TE. Persistent pain after breast cancer treatment: a questionnaire-based study on the prevalence, associated treatment variables, and pain type. J Breast Cancer. 2016;19:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- 43.Abrecht CR, Cornelius M, Wu A, et al. Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: psychophysical and psychosocial factors. Pain Med. 2019;20:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortner C, Granot M, Richebe P, Cardoso M, Bollag L, Landau R. Preoperative scar hyperalgesia is associated with postoperative pain in women undergoing a repeat Caesarean delivery. Eur J Pain. 2013;17:111–23. [DOI] [PubMed] [Google Scholar]
- 45.Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015;156:55–61. [DOI] [PubMed] [Google Scholar]
- 46.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic postoperative pain: preoperative DNIC testing identifies patients at risk. Pain. 2008;138:22–8. [DOI] [PubMed] [Google Scholar]
- 47.Andersen KG, Jensen MB, Kehlet H, Gartner R, Eckhoff L, Kroman N. Persistent pain, sensory disturbances, and functional impairment after adjuvant chemotherapy for breast cancer: cyclophosphamide, epirubicin, and fluorouracil compared with docetaxel + epirubicin and cyclophosphamide. Acta Oncol. 2012;51:1036–44. [DOI] [PubMed] [Google Scholar]
- 48.Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2014;18:242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen KG, Jensen MB, Tvedskov TF, Kehlet H, Gartner R, Kroman N. Persistent pain, sensory disturbances, and functional impairment after immediate or delayed axillary lymph node dissection. Eur J Surg Oncol. 2013;39:31–5. [DOI] [PubMed] [Google Scholar]
- 50.Lautenbacher S, Kundermann B, Krieg J-C. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. [DOI] [PubMed] [Google Scholar]
- 51.Marcusa DP, Mann RA, Cron DC, et al. Prescription opioid use among opioid-naive women undergoing immediate breast reconstruction. Plast Reconstr Surg. 2017;140:1081–90. [DOI] [PubMed] [Google Scholar]
- 52.Musoro JZ, Zwinderman AH, Puhan MA, ter Riet G, Geskus RB. Validation of prediction models based on lasso regression with multiply imputed data. BMC Med Res Methodol. 2014;14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid GJ, Gilbert CA, McGrath PJ. The Pain Coping Questionnaire: preliminary validation. Pain. 1998;76:83–96. [DOI] [PubMed] [Google Scholar]
- 55.Lotsch J, Sipila R, Dimova V, Kalso E. Machine-learned selection of psychological questionnaire items relevant to the development of persistent pain after breast cancer surgery. Br J Anaesth. 2018;121:1123–32. [DOI] [PubMed] [Google Scholar]
- 56.Grol-Prokopczyk H Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain. 2017;158:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinboonyahgoon N, Vlassakov K, Lirk P, et al. Benefit of regional anaesthesia on postoperative pain following mastectomy: the influence of catastrophising. Br J Anaesth. 2019;123:e293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spivey TL, Gutowski ED, Zinboonyahgoon N, et al. Chronic pain after breast surgery: a prospective, observational study. Ann Surg Oncol. 2018;25:2917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524. [Google Scholar]
- 60.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dura E, Andreu Y, Galdon MJ, et al. Psychological assessment of patients with temporomandibular disorders: confirmatory analysis of the dimensional structure of the Brief Symptoms Inventory 18. J Psychosom Res. 2006;60:365–70. [DOI] [PubMed] [Google Scholar]
- 62.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. [DOI] [PubMed] [Google Scholar]
- 63.Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA. One- and two-item measures of pain beliefs and coping strategies. Pain. 2003;104:453–69. [DOI] [PubMed] [Google Scholar]
- 64.Ammitzboll G, Andersen KG, Bidstrup PE, et al. Effect of progressive resistance training on persistent pain after axillary dissection in breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2020;179:173–83. [DOI] [PubMed] [Google Scholar]
- 65.Andersen KG, Gartner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: a randomized trial. Breast. 2012;21:46–9. [DOI] [PubMed] [Google Scholar]
- 66.Andersen KG, Christensen KB, Kehlet H, Bidstup PE. The effect of pain on physical functioning after breast cancer treatment: development and validation of an assessment tool. Clin J Pain. 2015;31:794–802. [DOI] [PubMed] [Google Scholar]
- 67.Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment: a nationwide study of prevalence and associated factors. Breast. 2010;19:506–15. [DOI] [PubMed] [Google Scholar]
- 68.Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. 2018;167:157–69. [DOI] [PubMed] [Google Scholar]
- 69.Klit A, Mejdahl MK, Gartner R, Elberg JJ, Kroman N, Andersen KG. Breast reconstruction with an expander prosthesis following mastectomy does not cause additional persistent pain: a nationwide cross-sectional study. J Plast Reconstr Aesthet Surg. 2013;66:1652–8. [DOI] [PubMed] [Google Scholar]
- 70.Mejdahl MK, Andersen KG, Gartner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ. 2013;346:f1865. [DOI] [PubMed] [Google Scholar]
- 71.Mertz BG, Duriaud HM, Kroman N, Andersen KG. Pain, sensory disturbances, and psychological distress are common sequelae after treatment of ductal carcinoma in situ: a cross-sectional study. Acta Oncol. 2017;56:724–9. [DOI] [PubMed] [Google Scholar]
- 72.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. [DOI] [PubMed] [Google Scholar]
- 73.Tibshirani R Regression shrinkage and selection via the lasso. J Royal Stat Soc Series B Methodol. 1996;58:267–88. [Google Scholar]
- 74.Wahl S, Boulesteix AL, Zierer A, Thorand B, van de Wiel MA. Assessment of predictive performance in incomplete data by combining internal validation and multiple imputation. BMC Med Res Methodol. 2016;16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in RJ Stat. Softw. 2011;45:1–67. [Google Scholar]
- 76.Chen Q, Wang S. Variable selection for multiply-imputed data with application to dioxin exposure study. Stat Med. 2013;32:3646–59. [DOI] [PubMed] [Google Scholar]
- 77.Reghunathan M, Rahgozar P, Sbitany H, Srinivasa DR. Breast reconstruction does not increase the incidence of postmastectomy pain syndrome: results of a meta-analysis. Ann Plast Surg. 2020;84:611–7. [DOI] [PubMed] [Google Scholar]
- 78.Andersen KG, Duriaud HM, Aasvang EK, Kehlet H. Association between sensory dysfunction and pain 1 week after breast cancer surgery: a psychophysical study. Acta Anaesthesiol Scand. 2016;60:259–69. [DOI] [PubMed] [Google Scholar]
- 79.Afolalu EF, Ramlee F, Tang NK. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. 2018;39:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen ML, Araujo P, Frange C, Tufik S. Sleep disturbance and pain: a tale of two common problems. Chest. 2018;154:1249–59. [DOI] [PubMed] [Google Scholar]
- 81.Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5:132–6. [PMC free article] [PubMed] [Google Scholar]
- 82.Cheatle MD, Foster S, Pinkett A, Lesneski M, Qu D, Dhingra L. Assessing and managing sleep disturbance in patients with chronic pain. Anesthesiol Clin. 2016;34:379–93. [DOI] [PubMed] [Google Scholar]
- 83.Gorin SS, Krebs P, Badr H, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johannsen M, Farver I, Beck N, Zachariae R. The efficacy of psychosocial intervention for pain in breast cancer patients and survivors: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138:675–90. [DOI] [PubMed] [Google Scholar]
- 85.Darnall BD, Ziadni MS, Krishnamurthy P, et al. “My Surgical Success”: effect of a digital behavioral pain medicine intervention on time to opioid cessation after breast cancer surgery: a pilot randomized controlled clinical trial. Pain Med. 2019;20:2228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med. 2006;29:17–27. [DOI] [PubMed] [Google Scholar]
- 87.Ciechanowski P, Sullivan M, Jensen M, Romano J, Summers H. The relationship of attachment style to depression, catastrophizing, and health care utilization in patients with chronic pain. Pain. 2003;104:627–37. [DOI] [PubMed] [Google Scholar]
- 88.Davies KA, Macfarlane GJ, McBeth J, Morriss R, Dickens C. Insecure attachment style is associated with chronic widespread pain. Pain. 2009;143:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12(11 Suppl):T61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dworkin RH, Bruehl S, Fillingim RB, Loeser JD, Terman GW, Turk DC. Multidimensional diagnostic criteria for chronic pain: introduction to the ACTTION-American Pain Society Pain Taxonomy (AAPT). J Pain. 2016;17(9 Suppl):T1–9. [DOI] [PubMed] [Google Scholar]


