Abstract
Three versions of syncytiotrophoblast exist in the human placenta: an invasive type associated with the implanting conceptus, non-invasive villous type of definitive placenta, and placental bed giant cells. Syncytins are encoded by modified env genes of endogenous retroviruses (ERV), but how they contribute functionally to placental syncytial structures is unclear. A minimum of eight genes (ERVW1, ERVFRD-1, ERVV-1, ERVV-2, ERVH48–1, ERVMER34–1, ERV3–1, & ERVK13–1) encoding syncytin family members are expressed in human trophoblast, the majority from implantation to term. ERVW1 (Syncytin 1) and ERVFRD-1 (Syncytin 2) are considered the major fusogens, but, when the expression of their genes is analyzed by single cell RNAseq in first trimester placenta, their transcripts are distinctly patterned and also differ from those of their proposed binding partners, SLC1A5 and MFSD2A, respectively. ERVRH48–1 (suppressyn or SUPYN) and ERVMER34–1 are probable negative regulators of fusion and co-expressed, primarily in cytotrophoblast. The remaining genes and their products have been little studied. Syncytin expression is a feature of placental development in almost all eutherian mammals studied, in at least one marsupial, and in viviparous lizards, which lack the trophoblast lineage. Their expression has been inferred to be essential for pregnancy success in the mouse. All the main human ERV genes arose following independent retroviral insertion events, none of which trace back to the divergence of eutherians and metatherians (marsupials). While syncytins may be crucial for placental development, it seems unlikely that they helped orchestrate the divergence of eutherians and marsupials.
Keywords: endogenous retrovirus, extravillous trophoblast, fusogen, placental evolution, suppressyn, syncytiotrophoblast
1. Introduction
This review was written to address questions we had about human syncytins and their roles in promoting the fusion of human trophoblast cells and whether the genes that encode them might be involved in the evolution of the placenta. As this is a review, we made liberal use of the data of others, including extensive data mining on the single cell RNAseq results of Vento-Tormi et al.[1], which provide a user-friendly means of mapping the expression of any gene within the placenta and decidua of the pregnant human during the first trimester of pregnancy (https://maternal-fetal-interface.cellgeni.sanger.ac.uk/). Its use was invaluable and provided several surprises.
In view of the restrictions on numbers of citations, many deserving references could not be included. In addition, most genes and proteins listed have numerous acronyms. We have chosen to use the nomenclature provided by The GeneCards human gene database (genecards.org)
2. Cell Fusion and Syncytialization
Cell fusion to create a cellular structure with more than one nucleus is a not a rare process but is restricted to specialized cell types [2]. Among these are the cytotrophoblasts that fuse to form the syncytial covering of human placental villi. The molecular and cellular mechanisms that promote cell fusion are poorly understood. Perhaps the best appreciated is when enveloped viruses bind their target cell [3], a process that requires a membrane protein called a fusogen, of which the hemagglutinin subunit 2 (HA2) component on the envelope membrane of the influenza virus is probably the best understood. HA2 comprises a coiled bundle of alpha helices organized into a spring-loaded complex that, after binding to its “receptor”, inserts into the bilayer, changes its conformation, and pulls the virus and target cell together. Although the general features of the process may be conserved, the fusogens are structurally diverse. They all, however, possess the hallmark of promoting membrane fusion when inserted ectopically into the plasma membranes of cells that previously showed no inclination to fuse. The genes encoding the syncytins of placental trophoblasts have evolved from the env genes of endogenous retroviruses (ERV; see part 3) and are believed to operate in a somewhat analogous manner to HA2 to overcome the otherwise thermodynamically unfavorable process of membrane fusion.
3. Types of Human Syncytiotrophoblast
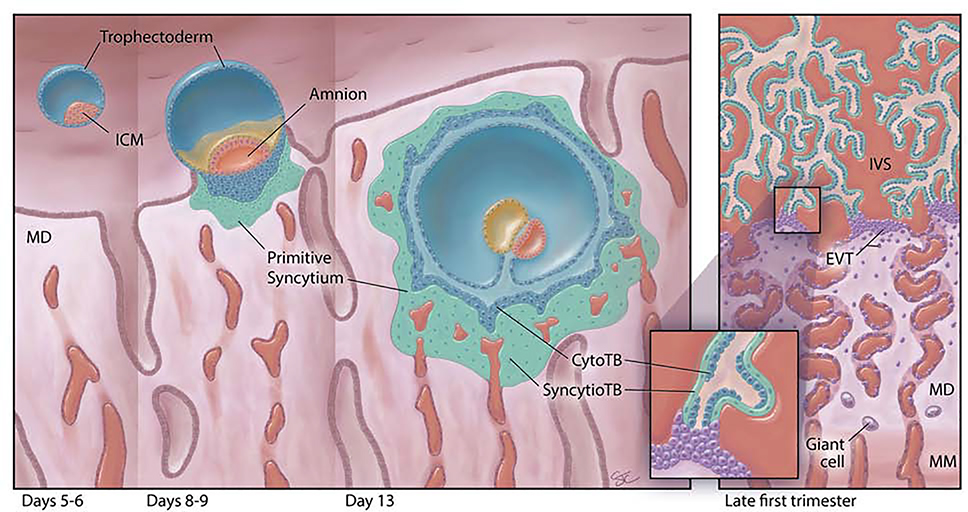
The first syncytiotrophoblast in the human placenta forms shortly after blastocyst formation by the fusion of trophoblasts derived from polar trophectoderm (Fig. 1). The latter carry a distinct set of upregulated genes that distinguish them from the mural trophectoderm, including ERVFRD-1, also known as Syncytin 2 [4]. This emerging structure, called primitive syncytium, enlarges and is present for approximately 7–9 days after polar trophectoderm initially attaches to the endometrium [5, 6], i.e. approximately week 3–4 since the last menstrual period. It forms the leading edge of the invading conceptus, breaching nearby capillaries and endometrial glands, and forming lacunae (Fig. 1). Additionally, syncytiotrophoblast at this stage produce high amounts of human chorionic gonadotrophin (hCG), which is required for this tiny structure to alert the mother to the presence of an embryo and prolong the lifespan of the corpus luteum of pregnancy [7]. RNA profiling of whole first trimester placentas shows that the transcriptome changes continuously during the first trimester [8], suggesting that the primitive syncytium might also be quite different from later emerging syncytial trophoblast populations in terms of gene expression, although definitive proof for this is lacking.
Fig. 1:
First trimester human placental development (left to right). The human blastocyst (far left), comprises an inner cell mass (ICM) completely surrounded by a layer of trophectoderm (TE) cells, and resides within the endometrial cavity just prior to implantation [days 5–6 post-fertilization (pf)]. At the time of implantation (days 8–9 pf), the ICM has developed the beginnings of an amniotic cavity (amnion) and the leading edge of the implanting embryo is characterized by an inner group of proliferative cytotrophoblast cells (CytoTB) and a deeper, non-proliferative, invasive, multinucleated, thickened mass of primitive syncytium. By day 13 pf, the embryo is fully implanted in the maternal decidua (MD). The primitive syncytium now completely surrounds the embryo. Primary villi have begun to form as invaginations of the cytotrophoblast layer. The primitive syncytium can invade the uterine glands (UG) and contacts maternal vessels. It contains lacunae that will grow and coalesce; they are filled with endometrial secretions and small amounts of maternal blood. By late in the first trimester (right panel), the villous placenta has been established with maternal blood now filling the intervillous space (IVS). Villi contain a discontinuous layer of proliferative CytoTB surrounded by multinucleated villous syncytiotrophoblast (SyncytioTB). The anchoring villi extend across the IVS to attach to the MD at their tips. Extending from these anchoring villi are the HLA-G-positive extravillous cytotrophoblast cells (EVTB). EVTB will invade deeply into the MD and remodel maternal spiral arteries. Although not depicted here, they will also invade into maternal veins, uterine glands and lymphatic spaces and reach well into the uterine myometrium. Multinucleated trophoblast giant cells (Giant cell) can be found deep in the MD and in the maternal uterine myometrium (MM).
What may be the in vitro equivalent of the primitive syncytium associated with embryo implantation arises when human pluripotent stem cells are directed along the TB lineage by the growth factor Bone Morphogenetic Protein-4 (BMP4) in presence of inhibitors of ACTIVIN/TGFB and FGF signaling [9, 10]. In this model system, human pluripotent stem cells quickly convert in their entirety to cytotrophoblasts [11]. Areas that express commonly recognized syncytiotrophoblast markers, including syncytins (Table 1), are present by day 7 but show no sign of villous structure formation. These syncytiotrophoblasts also carry a different transcript signature than villous syncytiotrophoblast and are also enriched in transcripts normally associated with invasive cells [12]
Table 1:
Relative Expression of ERV genes in trophoblast over the course of human pregnancy
| Gene Symbol | Chromosomal Location | d6–71 | d8–122 | 1st Trimester3 | Term4 | TB from ESC4 |
|---|---|---|---|---|---|---|
| ERVFRD-1 | chr6: 11102721–11112071 | + | + | +++ | ++ | ++ |
| ERVW-1 | chr7: 92097694–92107300 | NL | +++ | NL | +++++ | ++++ |
| ERVV-1 | chr19: 53517343–53519833 | +/− | +++ | + | ++ | +++ |
| ERVV-2 | chr19: 53044740–53051680 | +/− | ++ | + | +++ | ++ |
| ERVH48–1 | chr21: 44336913–44345756 | NL | +++ | NL | +++ | + |
| ERVMER 34–1 | chr4: 53609603–53617807 | + | + | ++ | + | + |
| ERV3–1 | chr7: 64450732–64467124 | +/− | + | NL | + | ++ |
| ERVK13–1 | chr16: 2708389–2723440 | NL | − | +/− | ++ | + |
The asterisks are intended to show only relative expressions of the genes at a particular stage of pregnancy; they do not indicate relative expression between stages, since the data were acquired on different platforms and processed differently. NL means that the gene was not present among the listed genes, either because it was not expressed (unlikely for ERVW-1) or simply missing. Note that the data for days 6–7 and 8–12 are from in vitro cultured embryos developing in absence of a surrounding maternal environment and therefore lacking the physiological cues that might help guide normal development and gene expression. This caveat also applies to Term trophoblast where term cytotrophoblasts were cultured in vitro to promote syncytiotrophoblast formation.
Petropoulos et al. [4]
West et al. [7]
Okae et al. [52]
Yabe et al. [9].
The syncytiotrophoblast of the mature, villous placenta, sometimes called “the definitive syncytium”[13], first forms after villi begin to project from the inner cytotrophoblast shell at around the end of the second week post coitum (Fig. 1). In contrast to the primitive syncytium, the villous syncytiotrophoblast forms a single layered shell over the maternal blood-facing surface of the villi [14]. In cultured villous explants in which the syncytiotrophoblast has been removed, cytotrophoblast spontaneously fuse to replace it [15]. Similarly, the spontaneously syncytialized villous cytotrophoblast cells in vitro culture upregulate syncytiotrophoblast markers as they differentiate [9], although it remains unclear whether in two-dimensional culture they provide a precise replica of the in vivo structures they mimic. Villous syncytiotrophoblast functions in production of hCG and other hormones of pregnancy, as well as in the exchange of nutrients, oxygen and waste between the maternal circulation and fetal blood vessels within the villi. Unlike the primitive syncytium, villous syncytiotrophoblast does not display features of invasiveness or migratory potential. It is terminally differentiated, and sloughs off debris into the maternal blood space as syncytial knots, while being continually replenished by cells from the underlying cytotrophoblast [16]. Villous syncytiotrophoblast also releases prolific quantities of exosomes and microvesicles whose contents may provide molecular information to maternal organs about the physiological state of the fetus [17]. Conceivably, syncytins, which are present in microvesicles shed from the placenta, contribute to fusion of these vesicles with their targets [18, 19].
There is a third, somewhat curious and poorly understood type of syncytiotrophoblast in the human placenta, called placental bed trophoblast giant cells. These are multinucleated and found within the interstitium of both deciduum and myometrium underlying the implantation site [20, 21]. They have been observed by both light and electron microscopy and contain six or so nuclei [20]. Unlike villous syncytiotrophoblast and the syncytiotrophoblast associated with the primitive placenta of the implanting embryo [7], they express the extravillous trophoblast marker HLA-G [22], as well as hCG and CSH2 (placental lactogen). The origin and function of the giant cells, are not clear. They may be remnants of the invasive primitive syncytium that remain in the implantation zone after villous formation[5, 23]. More likely, on the basis of detailed microscopic observation over the first half of pregnancy and the fact that they increase in number as the mature placenta is established, they are formed in the placental bed by the fusion of invaded extravillous trophoblasts [21].
Also unsettled is the extent to which these three syncytial populations, primitive syncytiotrophoblast, villous syncytiotrophoblast, and placental bed trophoblast giant cells, resemble each other in ontogeny, behavior and function. In particular, it remains unclear whether the fusogenic mechanisms that promote syncytialization are comparable. Most strikingly, while primitive syncytiotrophoblast is invasive, villous syncytiotrophoblast is certainly not. Also, while villous syncytiotrophoblast resembles a simple epithelial sheet, the other two forms comprise clumps of fused cells. All, however, appear to have an endocrine function, and have some means of resisting immune attack.
4. Endogenous Retroviruses (ERV) and their role in controlling placental cell fusion
Host cell entry for retroviruses occurs by fusion of the viral envelope with the host cell plasma membrane, a process that requires a fusogen (see Section 1, above). After infection, their single-stranded RNA is reverse-transcribed into double-stranded DNA, which becomes integrated into the host genome. There it can be transcribed to form new viral RNA genomes or, if the infected cell does not die, remain stably integrated as a permanent part of the host’s replicating genome. Retroviral genomes all contain three major coding regions: 1) gag encodes internal viral proteins, 2) pol encodes reverse transcriptase and integrase and 3) env encodes surface/transmembrane components of the viral envelope that allow the particle to become infectious [24]. Syncytins are encoded by modified env genes of endogenous retroviruses (ERV).
Although most retroviral infections are of somatic cells, in those rarer instances in which infection occurs in a germline cell, the retrovirus cDNA can be vertically transmitted to the next and subsequent generations. This has happened numerous times over the course of evolution to the extent that, in humans, endogenous retroviral genes comprise approximately 8% of the nuclear DNA[25]. Most of these viral genomic sequences have become truncated or mutated in ways that relegate them non-functional. However, a few env genes, although usually silent, have an open reading frame and the potential to generate a protein. Placental TBs appear to tolerate such promiscuous transcriptional activity from these elements more readily than most somatic tissues. Some of the best studied are those encoding syncytins, profusogenic proteins implicated in the formation of syncytiotrophoblast and in the evolution of the placenta [26, 27].
The first syncytin identified (now often called syncytin-1) [28–30] is encoded by a member of the HERV-W (Human Endogenous Retrovirus Group W Member 1, Envelope) family and properly named ERVW-1. A second, ERVFRD-1 (also known as syncytin-2), is encoded by the env gene of a different virus (Endogenous Retrovirus Group FRD member 1) (Table 1) [31, 32]. Although either ERVW-1 or ERVFRD-1 can promote cell-cell fusion in vitro, it is not clear whether both are necessary for TB syncytialization in vivo [33].
Like other envelope-expressed viral fusogens, the placental syncytins have co-opted cell surface proteins with unrelated physiologic function as cognate receptors to promote membrane fusion (see Section 1). For ERVW-1, that “receptor” is SLC1A5 (sometimes known as ASCT2 or Asyncytiotrophoblast-2), a neutral amino acid transporter. For ERVFRD-1, it is MFSD2A, a sodium-dependent lysophosphatidylcholine symporter [30, 34, 35]. However, there have been discrepancies in the literature regarding which TB subtypes express the human ERV proteins and their cognate receptors[29, 32, 34, 36–38]. ERVW-1 appears to be most strongly localized to villous syncytiotrophoblast, mainly in the basal membrane but also with the apical regions, and only weakly in villous cytotrophoblast, while its receptor SLC1A5 is expressed almost exclusively in villous cytotrophoblast. By contrast, ERVFRD-1 has been considered to be expressed in villous cytotrophoblast and its “receptor”, MFSD2, almost exclusively in syncytiotrophoblast. Consistent changes in ERV protein localization across pregnancy and associated with particular placental disease states have also been difficult to assess from the current literature.
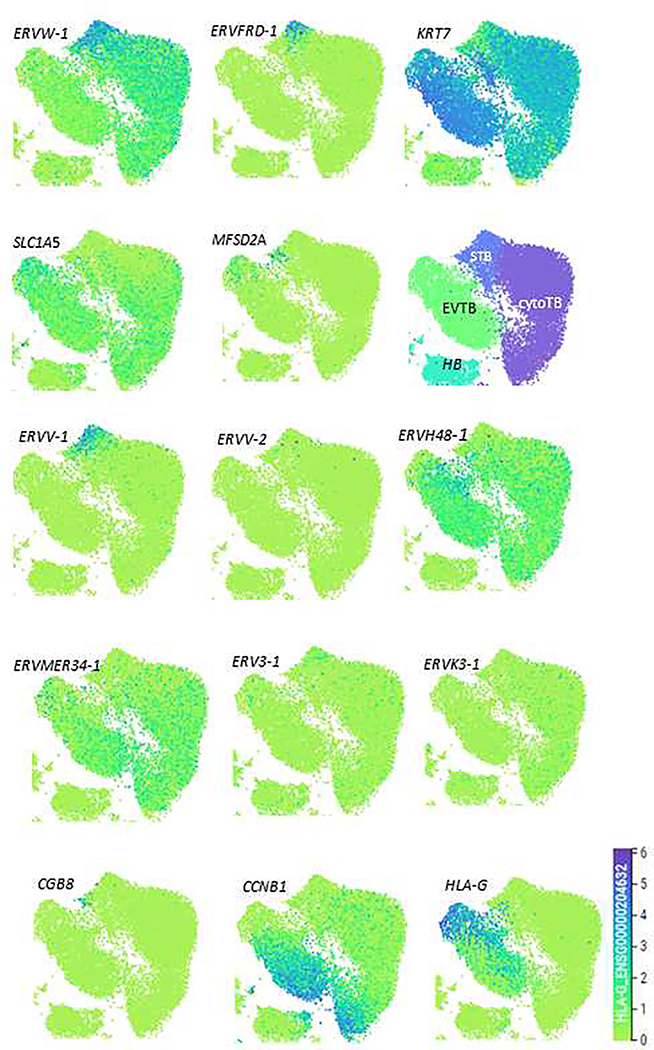
Single cell transcript patterns in placental tissue for ERVW-1 and SLC1A5 during the first trimester of pregnancy are consistent with the immunohistochemistry described above, namely ERVW-1 mRNA concentrated in syncytiotrophoblast and SLC1A5 mRNA in cytotrophoblast [1], raising questions as to how the two work in concert to promote cell fusion in the context of the placental villus. An additional puzzle is the observation that ERVFRD-1 transcript distribution contradicts the immunohistochemistry. Transcripts are primarily present in a discrete sub-population of syncytiotrophoblasts and barely at all in cytotrophoblast, while those for MFSD2A, which encode its presumed receptor, are found in a different cluster of presumed syncytiotrophoblast. We speculate that these two cell populations might comprise the immediate precursor cells that fuse to form the initiating syncytium. Indeed, we suggest that mature syncytium, which, in any case, is probably not regionally uniform in gene expression patterns, is not represented in either in Fig. 2 or in the data set of Vento-Tormo et al. [1] and suspect that syncytial structures with many nuclei are too large and too fragile to be collected for single RNA sequencing. Some evidence for this inference is evident from the patterning of CGB8 mRNA (Fig. 2), and many other syncytiotrophoblast markers, whose transcripts are known to be abundant in mature syncytiotrophoblast, but are barely represented in the single cell sequencing maps.
Fig. 2:
Placental cell clusters from 10x Genomics and Smart-seq2 (SS2) scRNA-seq analysis [1] derived from five first trimester placentas visualized by Uniform Manifold Approximation and Projection (UMAP), visualizing the distribution of syncytin gene transcripts for ERVW1, ERVFRD-1, ERVV-1, ERVV-2, ERVH48–1, ERVMER34–1, ERV3–1, & ERVK3–1, as well as for two putative receptors, SLC1A5 and MFSD2A, in the human placenta. Additionally, we map the location of the mRNA encoding the pan-TB marker KR7, the mature syncytiotrophoblast marker CGB8, and the EVT marker HLA-G, as well as CCNB1 (to indicate replicating cells). Relative expression of genes within individual cells (dots) is on an indigo-yellow scale (Bottom Right). Also shown is a map of cell types (HC: Hoffbauer cells). All data were derived by use of the web site provided by Vento-Tormo et al. https://maternal-fetal-interface.cellgeni.sanger.ac.uk/. Importantly, CGB8 expression is confined to a small cluster of cells in the syncytiotrophoblast region, a pattern consistent with the hypothesis that mature syncytiotrophoblast is probably poorly represented in the diagrams.
A range of other ERV-like genes, in addition to ERVW-1 and ERVFRD-1, are expressed in human placental trophoblast (Table 1). ERVV-1, ERVV-2, and ERV3–1 are little studied, but are expressed in syncytiotrophoblast from early human embryos [7], as well as at later stages and in in vitro differentiated placental TB [9]. ERVV-1 and ERVV-2 are located on the same chromosome, probably in tandem (Table 1), and likely arose by gene conversion following an initial single retroviral insertion [19]. They may not be fusogenic in humans [39]. In first trimester villous TB, there is a co-association of ERVV-1 transcripts with those for ERVFRD-1 (Fig. 2), while ERVV-2 and ERV3–1 appear to be poorly expressed in the single cell representation. Another ERV-like protein derived from an endogenous retroviral gene is ERVRH48–1 (often called suppressyn or SUPYN). In vitro, it inhibits rather than promotes TB fusion [36, 40], possibly by associating with SLC1A5 and blocking ERVW-1 binding [40]. Protein expression of ERVRH48–1 better mimics that of SLC1A5 rather than ERVW-1, i.e. expressed preferentially in unfused cytotrophoblast cells and in the non-syncytial extravillous cytotrophoblast (where ERVW-1 is also expressed). The observation is consistent with the single cell sequencing data in Fig 2. A fifth human ERV gene, ERVMER34–1, is expressed in TB generated from human pluripotent stem cells [9] and in the syncytiotrophoblast of cultured d 8–12 human embryos (Table 1). Its transcript distribution in first trimester placental cells, as well as in embryos [7], is similar to that of ERVH48–1, suggesting that it inhibits rather than promotes fusion. Finally, a sixth gene, ERVK13–1 encodes a long non-coding RNA transcript of unknown function. Together, the differences and similarities in these expression patterns are striking but also confusing. They need to be confirmed for different stages of pregnancy and with antibodies of confirmed specificity able to provide precise colocalization of different antigens.
5. Syncytins and Placental Evolution
Eutherians and metatherians (marsupials) diverged at least 130 million years ago [41]. Although the ancestral eutherian placenta has been inferred to have been invasive and hemochorial, a state where trophoblast is in direct contact with maternal blood, present day placental structures exist in a wide array of gross morphological forms and extent of invasiveness [42]. In a noteworthy example of convergent evolution, endogenous retrovirus-derived proteins with apparent analogous function but different retroviral origins to those of the human syncytin proteins have been found expressed in almost all mammalian placental types so far studied. The pig, which has a non-invasive, epitheliochorial placenta, may be one exception [43]. However, taxa that have a minimally invasive placenta, such as the pecoran ruminants that include cattle, sheep, and deer do express placental syncytins, which are believed to promote fusion between mononucleated TB cells to give rise to large binucleated cells that comprise around 20 % of total TB, and erode the integrity of local maternal uterine epithelium [44]. In the sheep, further fusions provide a syncytial plaque at the maternal fetal interface, although there has been uncertainty as to whether or not this syncytium is chimeric, i.e. part fetal TB, part maternal uterine epithelium [45].
Wherever placental syncytin genes are expressed, they appear to be associated with fusion events and often the formation of extensive syncytia. Sometimes these syncytia are invasive, as in the implanting human conceptus; in other instances, invasive features are absent. However, in only one species, the mouse, has there been an unequivocal demonstration that syncytins are required for both cell fusion and proper placentation. In the mouse, genetic ablation of either Syna and Synb, two genes that may serve functions analogous to those of the human ERVW-1 and ERVFRD-1, leads to defective placentation [46, 47]. Syna deletion leads to fetal death, apparently as a result of defects in trophoblast fusion of syncytiotrophoblast layer I. By contrast, loss of Synb targets fusion events required to form syncytiotrophoblast layer II, yet provides some live pups, albeit with growth retardation. These studies clearly establish that Syna and Synb contribute independently to the formation of syncytiotrophoblast in the mouse. No equivalent data are available for the human.
As a consequence of their almost ubiquitous association with the eutherian placenta, ERV-derived syncytins have been hypothesized to have had a key role in the evolutionary origins of that organ. However, most, if not all, of the assorted retroviral insertions that have given rise to present day ERV genes, occurred well after the eutherian-marsupial divergence. ERVW-1, for example arose 20–30 MYA and is absent in new world monkeys, whereas ERVFRD-1, which integrated approximately 20 million years earlier, is present in all primates except prosimians [43]. One possibility is that new retroviral insertions provided additional envelope genes, which were then “enslaved” and subsequently selected to replace already functioning ones existing at the time [48]. However, as no contemporary ERV gene has its origins stretching back to the earliest eutherians, a strong case linking acquisition of one or more syncytins to the one-time emergence of a eutherian placenta is unlikely ever to be made. In any case, placenta-associated syncytins are not unique to eutherian mammals and have also been described in the short-lived, superficial placentas of marsupials [49]. Another syncytin derived from an ERV envelope gene that can promote cell fusion is expressed in the placentas of viviparous lizards [50], species that develop a placenta-like structure from extra-embryonic mesoderm and not TB. The latter is a lineage unique to marsupials and eutherians. That is not to say that ERV genome insertions have not had a prominent role in guiding convergent placental evolution across distantly related vertebrates. Cell fusion is certainly an essential feature of placentation, but, additionally, there is some evidence in eutherians, at least, that sequences associated with ERV regulatory elements might control expression of multiple genes that, in combination, are hallmarks of the TB cell state [51]. In addition, many human syncytins carry an immunosuppressive domain that might assist in protecting the TB from maternal immune scrutiny [43]. In short, syncytin expression is intimately tied to the development, function, and evolution of the eutherian placenta, but it is far from clear whether or not the acquisition of one or more of these genes played a role in initiating the split that permitted eutherian mammals to diverge from their common ancestor with contemporary marsupials.
Acknowledgments
Funding Sources: The authors’ research is supported by grants 1R01HD094937 and 1R21A1145071 from the National Institutes of Health.
Abbreviations:
- RNAseq
RNA sequence analysis
- hCG
human chorionic gonadotropin
- ERV
endogenous retrovirus
Footnotes
Declarations of interest: none
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polanski K, Goncalves A, Gardner L, Holmqvist S, Henriksson J, Zou A, Sharkey AM, Millar B, Innes B, Wood L, Wilbrey-Clark A, Payne RP, Ivarsson MA, Lisgo S, Filby A, Rowitch DH, Bulmer JN, Wright GJ, Stubbington MJT, Haniffa M, Moffett A, Teichmann SA, Single-cell reconstruction of the early maternal-fetal interface in humans, Nature 563(7731) (2018) 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Petrany MJ, Millay DP, Cell Fusion: Merging Membranes and Making Muscle, Trends Cell Biol 29(12) (2019) 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hernandez JM, Podbilewicz B, The hallmarks of cell-cell fusion, Development 144(24) (2017) 4481–4495. [DOI] [PubMed] [Google Scholar]
- [4].Petropoulos S, Edsgard D, Reinius B, Deng Q, Panula SP, Codeluppi S, Reyes AP, Linnarsson S, Sandberg R, Lanner F, Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos, Cell 167(1) (2016) 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hertig AT, The primary human oocyte: some observations on the fine structure of Balbiani’s vitelline body and the origin of the annulate lamellae, Am J Anat 122(1) (1968) 107–37. [DOI] [PubMed] [Google Scholar]
- [6].Hamilton WJ, Boyd JD, Development of the human placenta in the first three months of gestation, J Anat 94 (1960) 297–328. [PMC free article] [PubMed] [Google Scholar]
- [7].West RC, Ming H, Logsdon DM, Sun J, Rajput SK, Kile RA, Schoolcraft WB, Roberts RM, Krisher RL, Jiang Z, Yuan Y, Dynamics of trophoblast differentiation in peri-implantation-stage human embryos, Proc Natl Acad Sci U S A 116(45) (2019) 22635–22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Soncin F, Khater M, To C, Pizzo D, Farah O, Wakeland A, Arul Nambi Rajan K, Nelson KK, Chang CW, Moretto-Zita M, Natale DR, Laurent LC, Parast MM, Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development, Development 145(2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yabe S, Alexenko AP, Amita M, Yang Y, Schust DJ, Sadovsky Y, Ezashi T, Roberts RM, Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas, Proc Natl Acad Sci U S A 113(19) (2016) E2598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roberts RM, Ezashi T, Sheridan MA, Yang Y, Specification of trophoblast from embryonic stem cells exposed to BMP4, Biol Reprod 99(1) (2018) 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Amita M, Adachi K, Alexenko AP, Sinha S, Schust DJ, Schulz LC, Roberts RM, Ezashi T, Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4, Proc Natl Acad Sci U S A 110(13) (2013) E1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jain A, Ezashi T, Roberts RM, Tuteja G, Deciphering transcriptional regulation in human embryonic stem cells specified towards a trophoblast fate, Scientific reports 7(1) (2017) 17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].James JL, Carter AM, Chamley LW, Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation?, Placenta 33(5) (2012) 327–34. [DOI] [PubMed] [Google Scholar]
- [14].Enders AC, Trophoblast differentiation during the transition from trophoblastic plate to lacunar stage of implantation in the rhesus monkey and human, Am J Anat 186(1) (1989) 85–98. [DOI] [PubMed] [Google Scholar]
- [15].Baczyk D, Dunk C, Huppertz B, Maxwell C, Reister F, Giannoulias D, Kingdom JC, Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi, Placenta 27(4–5) (2006) 367–74. [DOI] [PubMed] [Google Scholar]
- [16].Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P, Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation, Placenta 24(2–3) (2003) 181–90. [DOI] [PubMed] [Google Scholar]
- [17].Tannetta D, Collett G, Vatish M, Redman C, Sargent I, Syncytiotrophoblast extracellular vesicles - Circulating biopsies reflecting placental health, Placenta 52 (2017) 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vargas A, Zhou S, Ethier-Chiasson M, Flipo D, Lafond J, Gilbert C, Barbeau B, Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia, FASEB J 28(8) (2014) 3703–19. [DOI] [PubMed] [Google Scholar]
- [19].Lokossou AG, Toudic C, Barbeau B, Implication of human endogenous retrovirus envelope proteins in placental functions, Viruses 6(11) (2014) 4609–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].al-Lamki RS, Skepper JN, Burton GJ, Are human placental bed giant cells merely aggregates of small mononuclear trophoblast cells? An ultrastructural and immunocytochemical study, Hum Reprod 14(2) (1999) 496–504. [DOI] [PubMed] [Google Scholar]
- [21].Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I, The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy, Placenta 2(4) (1981) 303–16. [DOI] [PubMed] [Google Scholar]
- [22].Loke YW, King A, Burrows T, Gardner L, Bowen M, Hiby S, Howlett S, Holmes N, Jacobs D, Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody, Tissue antigens 50(2) (1997) 135–46. [DOI] [PubMed] [Google Scholar]
- [23].Robertson WB, Warner B, The ultrastructure of the human placental bed, J Pathol 112(4) (1974) 203–11. [DOI] [PubMed] [Google Scholar]
- [24].Coffin JM, Hughes SH, Varmus HE, Retroviral Virions and Genomes--Retroviruses, Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- [25].Harris JR, Placental endogenous retrovirus (ERV): structural, functional, and evolutionary significance, Bioessays 20(4) (1998) 307–316. [DOI] [PubMed] [Google Scholar]
- [26].Vance TD, Lee JE, Virus and eukaryote fusogen superfamilies, Current Biology 30(13) (2020) R750–R754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T, Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation, Philosophical Transactions of the Royal Society B: Biological Sciences 368(1626) (2013) 20120507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mi S, Lee X, Li X.-p., Veldman GM, Finnerty H, Racie L, LaVallie E, Tang X-Y, Edouard P, Howes S, Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis, Nature 403(6771) (2000) 785–789. [DOI] [PubMed] [Google Scholar]
- [29].Frendo J-L, Olivier D, Cheynet V, Blond J-L, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F, Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation, Molecular and cellular biology 23(10) (2003) 3566–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blond J-L, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset F-L, An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor, Journal of virology 74(7) (2000) 3321–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Blaise S, de Parseval N, Bénit L, Heidmann T, Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution, Proceedings of the National Academy of Sciences 100(22) (2003) 13013–13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Malassiné A, Blaise S, Handschuh K, Lalucque H, Dupressoir A, Evain-Brion D, Heidmann T, Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells, Placenta 28(2–3) (2007) 185–191. [DOI] [PubMed] [Google Scholar]
- [33].Pötgens A, Drewlo S, Kokozidou M, Kaufmann P, Syncytin: the major regulator of trophoblast fusion? Recent developments and hypotheses on its action, Human reproduction update 10(6) (2004) 487–496. [DOI] [PubMed] [Google Scholar]
- [34].Esnault C, Priet S, Ribet D, Vernochet C, Bruls T, Lavialle C, Weissenbach J, Heidmann T, A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2, Proceedings of the National Academy of Sciences 105(45) (2008) 17532–17537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sugimoto J, Schust DJ, Human endogenous retroviruses and the placenta, Reproductive Sciences 16(11) (2009) 1023–1033. [DOI] [PubMed] [Google Scholar]
- [36].Sugimoto J, Schust DJ, Kinjo T, Aoki Y, Jinno Y, Kudo Y, Suppressyn localization and dynamic expression patterns in primary human tissues support a physiologic role in human placentation, Scientific reports 9(1) (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset F-L, Kabat D, The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors, Journal of virology 76(13) (2002) 6442–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Soygur B, Sati L, The role of syncytins in human reproduction and reproductive organ cancers, Reproduction 152(5) (2016) R167–78. [DOI] [PubMed] [Google Scholar]
- [39].Esnault C, Cornelis G, Heidmann O, Heidmann T, Differential evolutionary fate of an ancestral primate endogenous retrovirus envelope gene, the EnvV syncytin, captured for a function in placentation, PLoS Genet 9(3) (2013) e1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sugimoto J, Sugimoto M, Bernstein H, Jinno Y, Schust D, A novel human endogenous retroviral protein inhibits cell-cell fusion, Scientific reports 3 (2013) 1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Griffith OW, Wagner GP, The placenta as a model for understanding the origin and evolution of vertebrate organs, Nat Ecol Evol 1(4) (2017) 72. [DOI] [PubMed] [Google Scholar]
- [42].Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R, Evolution of the mammalian placenta revealed by phylogenetic analysis, Proc Natl Acad Sci U S A 103(9) (2006) 3203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Denner J, Expression and function of endogenous retroviruses in the placenta, APMIS 124(1–2) (2016) 31–43. [DOI] [PubMed] [Google Scholar]
- [44].Hoffman LH, Wooding FB, Giant and binucleate trophoblast cells of mammals, J Exp Zool 266(6) (1993) 559–77. [DOI] [PubMed] [Google Scholar]
- [45].Seo H, Bazer FW, Burghardt RC, Johnson GA, Immunohistochemical Examination of Trophoblast Syncytialization during Early Placentation in Sheep, International journal of molecular sciences 20(18) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dupressoir A, Vernochet C, Harper F, Guégan J, Dessen P, Pierron G, Heidmann T, A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast, Proceedings of the National Academy of Sciences 108(46) (2011) E1164–E1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P, Heidmann T, Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene, Proc Natl Acad Sci U S A 106(29) (2009) 12127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Imakawa K, Nakagawa S, Miyazawa T, Baton pass hypothesis: successive incorporation of unconserved endogenous retroviral genes for placentation during mammalian evolution, Genes Cells 20(10) (2015) 771–88. [DOI] [PubMed] [Google Scholar]
- [49].Cornelis G, Vernochet C, Carradec Q, Souquere S, Mulot B, Catzeflis F, Nilsson MA, Menzies BR, Renfree MB, Pierron G, Zeller U, Heidmann O, Dupressoir A, Heidmann T, Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials, Proc Natl Acad Sci U S A 112(5) (2015) E487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cornelis G, Funk M, Vernochet C, Leal F, Tarazona OA, Meurice G, Heidmann O, Dupressoir A, Miralles A, Ramirez-Pinilla MP, Heidmann T, An endogenous retroviral envelope syncytin and its cognate receptor identified in the viviparous placental Mabuya lizard, Proc Natl Acad Sci U S A 114(51) (2017) E10991–E11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chuong EB, The placenta goes viral: Retroviruses control gene expression in pregnancy, PLoS Biol 16(10) (2018) e3000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T, Derivation of Human Trophoblast Stem Cells, Cell stem cell 22(1) (2018) 50–63 e6. [DOI] [PubMed] [Google Scholar]